Highlights
-
•
Recombinant DNA technologies are leading the rapid expansion of bispecific antibody formats.
-
•
The therapeutic potential of bispecific antibodies is being realized through creative design.
-
•
Bispecific antibodies are potentially underutilized reagents for diagnostics.
Abstract
Artificial manipulation of antibody genes has facilitated the production of several unique recombinant antibody formats, which have highly important therapeutic and biotechnological applications. Although bispecific antibodies (bsAbs) are not new, they are coming to the forefront as our knowledge of the potential efficacy of antibody-based therapeutics expands. The next generation of bsAbs is developing due to significant improvements in recombinant antibody technologies. This review focuses on recent advances with a particular focus on improvements in format and design that are contributing to the resurgence of bsAbs, and in particular, on innovative structures applicable to next generation point-of-care (POC) devices with applicability to low resource environments.
Antibodies as bispecifics
Antibodies belong to a class of globular proteins, called immunoglobulins, that are produced by B lymphocytes, and are deployed by the immune system to identify and target foreign or ‘non-self’ molecules 1, 2. The structure of an antibody (Figure 1 ) determines its binding specificity and biological activity [3]. Antibodies are the most diverse proteins found in nature with the greatest variability in amino acid sequence contained within the hypervariable region or complementarity-determining regions (CDRs; see Glossary), located in the Fv region [4]. Thus, the antibody CDRs determines the specificity for its cognate antigen. The Fc region is essential for mediating effector functions including: antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis, antigen presentation to the immune system, degranulation, complement-mediated lysis, and regulation of cell activation and proliferation.
Figure 1.

Diagrammatic representations of antibody structure. (A) Classical Y-shaped IgG composed of two heavy (blue–red) and two light (gray–green) chains that are further divided into variable (VH in red and VL in green) and constant domains (CH in blue and CL in gray). The fragment variable (Fv) domain is the smallest fragment of an antibody required for binding and is composed of the VH and VL domains which house the complementarity determining regions (CDRs). (B) The introduction of a flexible linker to the VL–VH (or VH–VL) gives rise to the single chain fragment variable (scFv). (C) The fragment antigen binding (Fab) can be generated by both recombinant and enzymatic approaches as can the F(ab′)2 fragment (D), which is composed of two Fab fragments. The structures of the shark Ig-NAR (E) and camelid (F) VHH immunoglobulin differ from that of the IgG molecule and are composed of single variable heavy domains. The black circles indicate the antigen binding sites. Adapted from [4].
Nobel laureates (1984) Köhler and Milstein devised the first and what has become the most freely available and, hence, widely used method to generate monoclonal antibodies (mAbs) [5]. However, extensive antibody engineering and optimization were required before effective antibodies were produced. The first antitumor mAb, rituximab [6], was approved for use worldwide in 1997 and since then >35 mAbs have achieved regulatory approval for therapeutic use. Despite significant positive clinical results, especially in the case of hematological malignancies, adverse clinical outcomes and animal studies have highlighted underlying limitations of mAbs. Accordingly, many strategies have been developed in order to improve the specificity and control the functions of antibodies. One such important approach is the development of bsAbs and this review focuses on current and future avenues of research for bsAbs. In addition, the applicability of bsAbs for diagnostics is discussed and critically assessed.
bsAbs
bsAbs are antibody-derived proteins with the ability to bind to two different epitopes on the same or different antigens. The first bsAbs were produced by oxidative recombination of two univalent antibody preparations, however, it was not for another 20 years that bsAbs with therapeutic potential were produced [7]. The establishment of hybridoma technology in 1975 was a major advance paving the way for bsAb development [5]. bsAbs are produced by three main methods: (i) chemical conjugation, which involves chemical cross-linking; (ii) fusion of two different hybridoma cell lines; or (iii) genetic approaches involving recombinant DNA technology [7]. The fusion of two different hybridomas produces a hybrid-hybridoma (or quadroma, Figure 2A) secreting a heterogeneous antibody population including bispecific molecules [8]. Alternative approaches included chemical conjugation of two different mAbs and/or smaller antibody fragments [9]. Oxidative reassociation strategies to link two different antibodies or antibody fragments were found to be inefficient due to the presence of side reactions during reoxidation of the multiple native disulfide bonds [10]. Current methods for chemical conjugation focus on the use of homo- or hetero-bifunctional crosslinking reagents 7, 11. Recombinant DNA technology has yielded the greatest range of bsAbs, through artificial manipulation of genes and represents the most diverse approach for bsAb generation (45 formats in the past two decades) [11].
Figure 2.
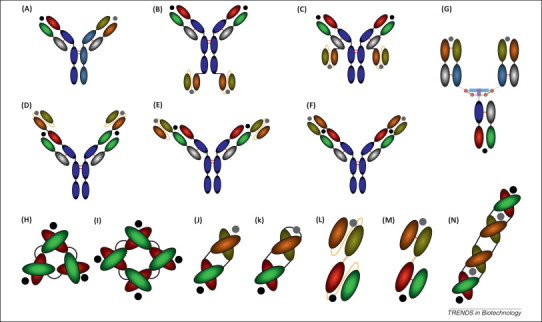
Bispecific antibody (bsAb) formats. Unique and diverse sets of bsAbs exist and can be described as IgG-like (A–G) and small bispecific (H–N) formats. The antigen-binding sites are indicated by the black and gray colored circles. A quadroma (A) is generated from the fusion of two different hybridomas and secrets a mixture of antibodies including a bispecific. Single chain fragment variable (scFv) can be linked to the Fc domain (B) and the constant light chain (C) of IgG molecules to generate the IgG–scFv format. Conversely, an scFv–IgG construct can be prepared through linkage of an scFv to the variable heavy (VH) (D) and variable light (VL) (E) of an IgG. The dual variable domain (DVD-Ig) bsAb (F) is created by fusion of a second VH–VL domain to the existing IgG VH–VL domain. The dock and lock (DNL) concept may be applied to multiple constructs through homodimerization dimerization of a DDD (blue) and AD (purple) sequence forming disulfide bonds (red circles) and a DNL-Fab is shown (G). A triabody (H) consists of three linked Fvs, whereas a tetrabody (I) consists of four Fv domains. The diabody (J) is a heterodimeric bsAb composed of two specificities and the stability the diabody is improved by encoding the construct as a single polypeptide chain (K). The bispecific T cell engager (BiTE) is a single polypeptide chain of two Fv molecules (TascFv) (L), whereas the tandem Fv (TaFv) (M) is a linked Fv molecule. The tandem diabody imparts avidity in addition to bispecificity (N). Adapted from [11].
Several bsAb formats can redirect cytotoxic effector cells against target cells that play key roles in disease processes. They can induce cytotoxicity, phagocytosis, and present antigens or directly suppress deregulated immune responses, depending on the nature of interaction between the bsAb and its target. Furthermore, they can deliver payloads including toxins, drugs, prodrugs and contrast agents [11]. bsAbs have proven to be of major therapeutic interest over the past 30 years, due to an array of potential capabilities. They also possess several advantageous characteristics for therapeutic development including design flexibility, modularity, optimal selectivity for activatory or downregulating molecules, oligoreactivity, and the delivery of therapeutic molecules [12]. The vast majority of bsAbs were initially designed to retarget effector cells towards tumor cells, and a variety of constructs were designed to retarget cells of the immune system by binding to and triggering Fc receptors on the surface of effector cells or by binding to T cell receptor (TCR) complexes [13]. These first generation bsAbs were predominantly generated using hybrid-hybridomas or by chemical crosslinking. Despite some significant biological effects elicited by these antibodies, there was no ongoing significant impact on the clinical course of a disease state. Issues associated with these first generation bsAbs included difficulty in large-scale production of homogeneous batches and a lack of efficacy of murine antibody fragments. Human anti-mouse antibody (HAMA) responses were observed in the majority of treated patients in addition to Fc-mediated side effects (cytokine-release syndrome, thrombocytopenia, and leukopenia). Subsequently, research has focused on approaches to overcome these limitations through novel antibody formats.
Recombinant bsAb (rBsAb) formats
With the advent of recombinant DNA technology, it is possible to ameliorate the shortcomings associated with traditional approaches for bsAb production. A plethora of different recombinant bsAb formats exist (Figure 2), ranging from whole IgG-like molecules (Figure 2A–E) to small recombinant formats (Figure 2F–N), such as tandem single chain variable fragment molecules (taFvs), diabodies (Dbs), single chain diabodies (scDbs), and various other derivatives of these. Bispecific tetravalent molecules are produced using Fc-mediated dimerization and possess two binding sites for each antigen, which impart increased avidity. A frequent approach to produce a tetravalent bispecific molecule is through the fusion of a single-chain Fv fragment to the C terminus of an antibody heavy chain or by substituting the Fab arm with a bispecific single-chain antibody fragment such as a tandem scFv or an scDb [14]. Other approaches fuse two different scFvs to the N terminus of constant heavy and light chain domains. It is also possible to fuse a second variable heavy (VH) and variable light (VL) domain to the heavy and light chains of an antibody, therefore leading to the production of a dual-variable-domain (DVD) antibody (Figure 2F) [15].
Recombinant strategies can also be used to produce small bsAb fragments. The most common approach for this is the fusion of two different scFv molecules. This strategy forms the basis of the bispecific T cell engager (BiTE) developed for cancer immunotherapy (Figure 2L–M). A further expansion of this strategy is the fusion of an additional scFv fragment molecule, leading to the formation of a trivalent or trispecific antibody 14, 16. In an alternative approach, two bispecific Dbs (BS1.5 and BS1.5H) and two bispecific trivalent proteins (BS6 and BS8) were expressed within the same cell, produced and tested as potential agents for pretargeted delivery of radiolabeled bivalent haptens to tumors expressing carcinoembryonic antigen. These chains assembled in an antiparallel manner to form heterodimeric molecules 14, 17. ScFv fragments expressed in bacteria are known to exist in both monomeric and dimeric forms [18] and this can be exploited to form Dbs, which are generated by linking the VH domain of one antibody to the VL domain of another (Figure 2J). The linker is deliberately short (3–12 amino acids in length), which induces the two domains to pair with the complementary domain of another chain, thus creating two different antigen-binding sites. scDbs are a derivative of the Db approach (Figure 2K) and are produced by introducing an additional peptide linker to join the two antibody fragments, hence, the domains are expressed as a single polypeptide chain [14]. Although recombinant production of scFv fragments may circumvent the shortcomings associated with hybrid-hybridomas, the approach faces significant challenges. Certain forms of scFv have variable and unpredictable expression yields and the linker used can cause spontaneous aggregation of the recombinant material. Introducing shortened linkers to produce a Db does not always guarantee success, as the linker can induce deleterious conformational changes resulting in a reduction of antibody functionality [19]. To overcome solubility-related issues, several unique approaches have been undertaken to stabilize rbsAbs. Single domain antibodies (sdAbs) occur in the natural repertoire of both camelid and cartilaginous fish (Figure 1E,F). These single V domain constructs, known as VHH in camelids and V-NAR in sharks, are of minimal size (15 kDa). In addition, they demonstrate high expression levels, and exhibit high stability and solubility in vitro, which has made them attractive entities for bsAb generation [20]. SdAbs can be produced in bacteria (or yeast) and their properties support facile conversion to bispecific formats through linkage of two sdAbs directed against two different antigens. The resultant low molecular mass (≤30 kDa), although advantageous for biodistribution, can hamper therapeutic success due to rapid clearance in vivo by renal filtration and degradation. The positive attributes associated with sdAbs have made them a key point of therapeutic interest 20, 21, 22. The ‘dock-and-lock’ construction method involves homo- and heterodimerization of the dimerization and docking domain (DDD) of human cAMP-dependent protein kinase A (PKA) with the anchoring domain (AD) from A-kinase anchor protein (AKAP) 14, 23. Therefore, fusion of a Fab fragment directed against the first antigen to AD and fusion of a Fab fragment directed against the second antigen to the DDD domain (homodimer) and subsequent in vitro assembly of the two protein preparations results in a trivalent molecule composed of one Fab-AD and two Fab-DDD moieties (Figure 2G) [24]. Furthermore, a disulfide-stabilized scFv was fused to the C terminus of an IgG light chain creating an IgG–scFv bsAb (Figure 2C), expressed in mammalian cells and purified by one-step protein A chromatography. In this format, the bsAb exhibits IgG-like stability, and demonstrates IgG-like tumor targeting and blood clearance in vivo. This format is suggested as a standardized platform for the construction of functional bsAbs [25] and several such IgG–scFv formats are described in the literature (Figure 2B–E) [26].
rbsAbs for therapeutics
The monospecific nature of mAbs for therapy may often be a limitation for their effective use in complex human diseases. Cancer, HIV, inflammatory disease, infectious disease, and allergic disorders are multifaceted in nature and therapeutic resistance can result from engagement or upregulation of alternative receptor pathways. Current combination mAb therapies are undergoing clinical trials and show some efficacy in diseases, such as cancer, by simultaneous engagement of target antigens [11]. Although this approach shows promise, each mAb in the combination therapy may be required to achieve regulatory approval. Therefore, additional polyclonal strategies are under investigation for oligoreactive treatment of complex disease states 11, 27. rbsAbs have significant potential for application in multifaceted disease therapy, because they can be engineered to block or engage multiple sites on a single target simultaneously, or sites on different targets, within a single therapeutic entity. In addition, payloads can be delivered specifically to the target cell, and Fc effector engagement can recruit cells of the immune system. bsAbs are of particular therapeutic interest as strategies in cancer therapy, which is the predominant disease state to which bsAb are applied. Most current applications have focused on redirecting the cytotoxic activity of lymphocytic effector cells against tumor targets through binding to the T cell co-receptor molecule CD3. In addition to CD3, CD16 is the major activating receptor on natural killer (NK) cells and mediates low-affinity interactions with IgG. Several antigen targets have undergone evaluation with the majority of them representing tumor-associated antigens (TAAs) (Table 1 ).
Table 1.
rbsAbs for therapy
| Format | Target 1 | Target 2 | Refs |
|---|---|---|---|
| Cancer | |||
| TaFv | CD19 | CD3 (CTL) | 66, 67, 68, 69 |
| EpCAM | CD3 | 70, 71, 72 | |
| ErbB2 | CD3 | [72] | |
| Lewis Y | CD3 | [72] | |
| FAP | CD3 | [73] | |
| Wue | CD3 | [74] | |
| Melanoma proteoglycan | CD28 | [75] | |
| MHC complex | CD16 (NK cells) | [76] | |
| ErbB2 | CD16 | [77] | |
| EGFR | Adenovirus (Ad) | 78, 79, 80, 81, 82, 83 | |
| EpCAM | Ad | [83] | |
| CD40 | Ad | [84] | |
| CEA | Ad | [85] | |
| 3E10 (cell penetration) | P53 (apoptosis) | [86] | |
| Db | CD19 | CD3 | 87, 88 |
| CD20 | CD3 | [89] | |
| EGFR | CD3 | [90] | |
| MUC-1 | CD3 | [91] | |
| P glycoprotein | CD3 | [92] | |
| CD19 | CD16 | [93] | |
| HLA-DR | Y90 | [94] | |
| EGFR | IGFR | [95] | |
| VEGFR2 | VEGFR3 | [96] | |
| PSMA | CD3 | [97] | |
| scDb | CD19 | CD3 | [98] |
| Engodlin | CD3 | [99] | |
| Endoglin | Ad | [100] | |
| HMWMAA | Ad | [101] | |
| CEA | Ad | [85] | |
| CEA | Prodrug | [102] | |
| TaDb | CD19 | CD3 | 103, 104 |
| scFv-CH3 | ErbB2 | CD16 | [105] |
| IgG-scFv scFv-IgG scFv2- |
TRAIL-R2 | LTβR | [106] |
| EGFR | IGFR | [107] | |
| EGFR | IGFR | [108] | |
| IGFR | – | [109] | |
| IgG-scFv | CD123 | CD3 | [110] |
| IgG-scFv | CEA | DOTA | [111] |
| IgG-scFv | EGFR | Met | [112] |
| F(ab’) 2 | CD20 | CD22 | [113] |
| Multiple | EGFR | CD3 | [114] |
| TriMab | ErbB | IGF1R | [115] |
| bsFab (sdAb) | CEA | FcγRIIa | [116] |
| scbsAb | PSCA | CD3 | [117] |
| scFv-Fc-scFv | PDGFRβ | VEGF | [118] |
| BiTE | EGFRvIII | CD3 | 119, 120 |
| Allergic disease | |||
| IgG-like | FcɛRI | FcγRIIb (CD32B) | [121] |
| F(ab’)2 | IgE | FcγRIIb (CD32B) | [122] |
| IgG-like | CCR3 | CD300a | [123] |
| Infectious disease | |||
| IgG-scFv | HIV CCR5 epitope | HIV CCR5 epitope | [124] |
| VHH-CH | LukS-PV | LukF-PV | [125] |
| scFv | Malaria parasite (MSP) | CD3 | [126] |
| TaDb | MP65 | SAP-2 | [127] |
| Inflammatory disease | |||
| IgG-scFv scFv-Fc TascFv-Fc |
IL-17A | IL-23 | [128] |
| Db | FcγRIIb (CD32B) | CD79b | [129] |
| DVD-Ig | IL-1α | IL-1β | [130] |
| DVD-Ig | IL-12 | IL-18 | [15] |
| scAb | CCR5 | CD3 | [131] |
| scFv | IL-11β | IL-17A | [132] |
Abbreviations: Ad, adenovirus; Met, hepatocyte growth factor receptor; PDGFR, platelet-derived growth factor receptor; CCR, chemokine CC receptor; CEA, carcinoembryonic antigen; DOTA, 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid; EGFR, epidermal growth factor receptor; FAP, fibroblast activation protein; HMWMAA, high molecular weight melanoma-associated antigen; IGFR, insulin-like growth factor receptor; LTβR, leukotriene β receptor; MUC-1, Mucin 1; PSCA, prostate stem cell antigen; PSMA, prostate specific membrane antigen; SAP, secretory aspartyl proteinase; TRAIL-R2, TNF-related apoptosis inducing ligand receptor-2; VEGFR, vascular endothelial growth factor receptor.
Despite the ongoing development of various increasingly complex bsAb designs, only two formats, BiTES and Triomabs, have made a substantial impact. The Triomab format has proven to be the most successful due to its performance in clinical trials and the approval of catumaxomab by regulatory agencies. Those formats currently in clinical trials are listed in Table 2 .
Table 2.
rbsAbs in clinical trials
| Name (format) | Target 1 | Target 2 | Phase | Refs |
|---|---|---|---|---|
| MDX-447 [F(ab′) 2] | EGFR | FcγRI | I | [133] |
| MM-111 (trimeric scFv) | ErbB2 | ErbB3 | I–II | [134] |
| DT2219ARL (dimeric scFv) | CD19 | CD22/DT390 | I | [22] |
| TF2 (Tri-Fab) | CEA | HSG | I–II | [135] |
| rM28 (scAb) | Melanoma-associated proteoglycan | CD28 | I–II | [136] |
| MT103 (BiTE) | CD19 | CD3 | I–II | [137] |
| MT110 (BiTE) | EpCAM | CD3 | I | [138] |
| SAR156597 (Tetravalent bispecific tandem Ig) | IL-4 | IL-13 | I | [139] |
| AFM13 (TandAb) | CD30 | CD16A | I | [140] |
| MEHD-7945A (bsmAb) | EGFR | HER3 | I–II | [141] |
| Ozoralizumab (Trivalent bispecific nanobody) | TNF | HSA | II | [142] |
Adapted from [143].
Abbreviations: HSA, Human Serum Albumin; TNF, tumor necrosis factor.
Bispecific T cell engagers (BiTEs)
BiTEs combine a unique set of properties unreported for any other kind of bsAb (Box 1 ). The BiTE format potentially overcomes several limiting factors relating to the biological activity of tumor-directed bsAbs. BiTEs combine the minimal binding domains (Fv fragments) of two different mAbs fused together by a short flexible linker that allows free rotation of the two arms, and thus facilitates optimal antibody:antigen interaction [28]. They function by forming a link between T cells (CD3 or CD19) and TAAs, inducing T cell dependent cytotoxic activity by proteins including perforin and granzyme, independently of the presence of MHC I or co-stimulatory molecules [28], and these proteins enter tumor cells, initiating apoptosis. MT103 (blinatumomab, Table 2) a CD19-specific BiTE is currently in clinical trials for the treatment of non-Hodgkin's lymphoma and acute lymphoblastic leukemia [22]. MT110, an anti-human Epithelial cell adhesion molecule (EpCAM) × anti-human CD3 Tandem single chain fragment variable (taFv), was the second BiTE tested in clinical trials, and the first directed to a wide spectrum of solid tumors [29]. In vitro testing of MT110 reconfirmed the results obtained with MT103 on tumor cell lines, thereby demonstrating the modularity of the BiTE format. MT110 is currently being tested in a Phase I study with lung, colorectal, and gastrointestinal cancer. Initial results suggest that the pharmacokinetic properties and the risk of systemic activation associated with MT110 might require further molecular development in order to develop a safe and feasible treatment option [22].
Box 1. Advantages of the BiTE [144,145].
-
•
100–10 000-fold higher efficacy in tumor cell lysis relative to other CD3-bispecific formats and monoclonal IgG1 antibodies.
-
•
Induces target cell elimination by unstimulated peripheral T cells without the need for T cell co-stimuli or T cell preactivation regimens.
-
•
Strictly targets cell dependent, polyclonal activation of most CD4+ and CD8+ T cells.
-
•
High protein stability and homogeneity.
Trifunctional antibodies/Triomabs
Trifunctional antibodies (Triomabs) are intact IgG molecules characterized by their unique ability to engage three different cells types, typically, tumor cells, T cells, and accessory cells, such as, macrophages, dendritic cells, NK cells, and other Fc-receptor-expressing cells. Trifunctional antibodies have two different antigen-binding specificities, most commonly CD3 and a tumor antigen [22]. The presence of the intact Fc region facilitates interaction with receptors triggering several immune defense reactions (Box 2 ). Although trifunctional antibodies were initially perceived to be unfavorable for therapeutic development, due to retention of Fc effector functions, several of these antibodies were assessed in clinical trials. TRION Pharma and Fresenius Biotech developed the first successful trifunctional antibodies, composed of whole hybrid mouse/rat IgG molecules with specificity for CD3 and the tumor-associated antigens: HER2 (ertumaxomab) and EpCAM (catumaxomab). Catumaxomab was the first bsAb to reach the pharmaceutical marketplace (2009) for the treatment of malignant ascites in patients with EpCAM-positive carcinomas, by simultaneous engagement of type I, IIa, and III Fc receptors [30]. At present, an ongoing Phase II clinical trial for catumaxomab is underway for the treatment of ovarian and gastric cancer. Bi20 (Lymphomun) targets CD20, a B lymphocyte membrane antigen, which is a viable target for the treatment of B cell related malignancies. Bi20 is generated by chemical heteroconjugation of the CD-specific chimeric mAb, rituximab, and the CD3-specific murine mAb OKT-3 (muromonab). It differs from catumaxomab because it is composed of two whole immunoglobulins [12], and initial in vitro studies demonstrated that Bi20 mediated efficient and specific lysis of B cell lines compared to unarmed T cells or cell lines treated with rituximab alone. In a pilot study, consisting of six patients with recurrent B cell malignancies, promising results indicate the therapeutic potential of Bi20. Phase I clinical trials are currently underway to evaluate efficacy in patients with relapsed or refractory CD20+ non-Hodgkin's lymphoma (NHL) [31], in addition to other indications 32, 33, 34, 35.
Box 2. Immune defensive reactions.
-
•
Cytotoxic T cells, with their highly cytotoxic potential, abundance, and ‘search-and-destroy’ function, are the most potent killer cells of the human body. They are capable of effectively inducing tumor cell lysis and apoptosis.
-
•
Accessory cells target and eliminate tumor cells through processes such as phagocytosis or apoptosis. Additionally, they have the ability to release cytokines, which further stimulate the action of T cells.
-
•
Dendritic cells are capable of inducing long-lasting immunity against cancerous tumors by processing and presenting tumor cells and tumor cell derived material to the immune system.
Bispecific antibodies for diagnostics
The ability of bsAbs to bind simultaneously to a specific antigen and a given detection moiety enables them to function as excellent bifunctional immunoprobes in diagnostic assays (Figure 3 ). Associated advantages of bsAbs over traditional mAbs include: design flexibility and one-step addition of reagents compared to traditional multi-step procedures. Obviating the requirement to label a reagent directly, such as a secondary antibody, reduces the deleterious effects of chemical modification of either the enzyme or the antibody. Antibodies are extremely versatile and are incorporated into a variety of different immunodiagnostic assay platforms, such as: microtiter plate assay, swab, strip, filter disk, and ‘spinning-disc-type’ assays. bsAbs are attractive in such assays because they simplify the detection steps and are currently used for the development of simple, rapid, and highly sensitive immunoassays for the detection of bacterial and viral infectious diseases and in cancer diagnostics.
Figure 3.

Diagrammatic representation of a generalized assay format for a bispecific antibody (bsAb)-based immunoassay. The capture monoclonal antibody is immobilized on to a solid surface and binds to a specific epitope on its cognate antigen present in the test sample. Upon addition of the corresponding bsAb, one arm binds to a specific epitope on the antigen, while the other arm binds to a reporter molecule, such as horseradish peroxidase (HRPO), and converts the subsequently added substrate to a quantifiable signal or colored product. Adapted from [7].
In vivo cancer diagnostic imaging
In vivo cancer diagnostic imaging facilitates insight into the molecular and functional characteristics of cancerous tissue, which permits targeting, identifying, and assessing several different types of tumors. bsAbs in cancer diagnostic imaging have proven effective, particularly when implementing a pretargeting strategy 24, 36, 37. The pretargeting strategy enhances the sensitivity of imaging by elimination of background noise, as a consequence of untargeted radioactivity, and reduces unintended toxicity. In such a strategy, the bsAb is designed to bind to the target antigen and a radiometal–chelate or hapten–peptide complex (effector molecule). The pretargeting approach is a two-step imaging process in which the bsAb is injected initially, and once excess antibody has cleared from circulation, it is followed by injection of mono-divalent radiolabeled haptens [38]. Animal model experiments have shown that bsAbs are capable of an equivalent tumor uptake compared to conventional radiolabeled antibodies and have minimum accretion within a matter of minutes rather than several hours or days. Additionally, the radionucleotide is cleared rapidly from circulation and tissue retention in the kidneys and liver was low. Positron emission tomography (PET) imaging approaches use 131I -labeled haptens after pretargeting with a bsAb, however, the visualization of small malignant lesions remains problematic [39]. To improve the ability to image such lesions, an alternative strategy combines pretargeting with highly specific, high-activity radiotracer-labeled polymers 40, 41, 42. bsAb complexes were utilized to pretarget PC-3 human prostate cancer xenographs in SCID mice. These complexes consisted of intact anti-diethylenetriaminepentaacetic acid (DTPA) antibody or Fab-linked bombesin (Bom) coupled via thioether bonds (Bom-bsCx or Bom-bsFCx). Results indicated that the radiolabeled polymers accumulated at the pretargeted sites and enabled visualization of cancerous lesions as small as 1–2 mm. Additionally, metastatic melanoma lesions in the lungs of mice were imaged with a bsAb and 99mTc-DSPL (polylysine polymer conjugated to DTPA), succinylated, and labeled with 99mTc. The use of bsAbs in cancer imaging has significantly enhanced the quality and reliability of this technique. Furthermore, atherosclerotic lesions as small as 4 μm were successfully imaged in apolipoprotein E (apoE) knockout mice by use of surrogate antigen-coated polystyrene beads pretargeted with a bsAb F(ab′)2 × F(ab′)2 [43]. Within animal models, bsAbs have been proven to be superior to mAbs in terms of specificity, sensitivity, and signal intensity while maintaining minimal signal background.
Infectious disease
Infectious-disease-causative agents include bacteria, viruses, parasites, and fungi. In the present ‘decade of the vaccine’, the World Health Organization (WHO) attributes 9.5 million deaths annually to infectious diseases, which is a significant socioeconomic burden on society. Global healthcare systems use surveillance measures to control the spread of infectious diseases, which are reliant on early detection. Tuberculosis (TB) is caused by the highly resistant bacterium Mycobacterium tuberculosis and particularly affects those with a weak immune system, for example, individuals infected with HIV. Although most infections are asymptomatic and latent, one in ten infections eventually progresses to active disease, which if untreated, kills >50% of those infected. The WHO reports that of the 8.7 million cases of TB in 2011, there were 1.4 million deaths worldwide [44]. A rapid, sensitive, specific, and inexpensive TB diagnostic for a POC setting is of considerable value and importance for TB control. Current methods of detection including sputum smear microscopy (SSM) and bacterial culture do not facilitate early diagnosis. The bacterial culture method is considered to be the gold standard for TB diagnosis, however, 2–6 weeks are required to obtain results. Through the development of molecular assays including the interferon gamma release assay (IGRA) [45] and nucleic acid amplification (NAA) [46], early detection has improved. These approaches require significant expertise and infrastructure and as a consequence are not suited for resource-limited areas. Lipoarabinomannan (LAM) is an important non-protein antigen of the bacterial cell wall, which is present in different body fluids of TB-infected patients. A bispecific mAb with specificity towards the LAM antigen and the reporter molecule, horseradish peroxidase (HRPO), was developed through hybrid-hybridoma technology. This bsAb was incorporated into a simple low cost immnuoswab-based assay to detect the LAM antigen [47]. The limit of detection of this assay for spiked synthetic LAM was 5.0 ng/ml in bovine urine, 0.5 ng/ml in rabbit serum, and 0.005 ng/ml in saline, and for bacterial LAM from M. tuberculosis H37Rv, it was found to be 0.5 ng/ml in rabbit serum [47]. The assay was further evaluated using 23 clinical serum samples collected from TB patients, of which 14 were positive and seven were negative in terms of anti-LAM antibody titer. The assay exhibited 100% specificity and 64% sensitivity (95% confidence interval) in a parallel comparison with laboratory culture. In addition to good specificity, visual results were obtained within 2 h of sample collection. This immunoassay was also evaluated in another format to test the sensitivity of the assay using a biotinylated CS-35 mAb. The transformation of the assay from a bispecific-based format to a monoclonal-based format reduced the sensitivity of the assay. The use of this bsAb, the first of its kind to detect any TB antigen, in the immnuoswab assay provided enhanced sensitivity and specificity [47], and the reported assay is particularly suited to resource-limited areas, as a rapid tool for detecting TB in resource constrained laboratory settings.
Hepatitis B
Hepatitis B is a significant health concern and viral hepatitis is a leading cause of liver cancer and commonly results in liver transplantation. Hepatitis B virus surface antigen (HBsAg) is the key screening target for detecting infected individuals. Current approaches for HBsAg screening rely on laboratory-based ELISA testing. A novel agglutination test for hepatitis B infection uses an anti-HBsAg × anti-human erythrocyte bsAb [48]. The Db format was developed by phage display, selecting scFv from red blood cells and HBsAg-specific libraries. In human blood samples (712 clinical specimens), agglutination was observed in samples containing HBsAg (100% specificity) and the test showed 97.7% sensitivity. The agglutination kinetics were HBsAg concentration dependent with high viral loads leading to agglutination in 1–2 min. Although the assay was specific and sensitive, it did not have equivalent limits of detection, compared to ELISA (10-fold difference), but operated within the observed HBsAg concentration range for actively infected individuals (5 ng/ml to 600 μg/ml) [48]. As a primary POC screening tool, the simple agglutination test is a powerful approach to assist in the rapid screening for hepatitis-B-infected individuals.
Escherichia coli
E. coli O157:H7 is considered to be a serious human pathogen and is associated with bloody diarrhea (hemorrhagic colitis) and hemolytic uremic syndrome (HUS). Current methods of detection of E. coli in food and water focus on enrichment and microbial culture [49], which may take 24–48 h to identify the organism. Other techniques focus on molecular methods such as PCR, which despite the sensitivity and rapid result retrieval capabilities, this technique is limited by the requirement to isolate DNA from samples. A more sensitive, rapid, and reliable diagnostic technique for the identification of E. coli is essential for appropriate detection and management to prevent disease outbreaks. To develop a highly sensitive bsAb-based assay for E. coli O157:H7 in water samples, a hybridoma secreting a mAb specific for E. coli O157:H7 whole bacteria and E. coli O157:H7 lipopolysaccharide (LPS) was fused with an anti-HRPO mAb-secreting hybridoma to generate a quadroma. The resulting bsAb was incorporated into a sandwich ELISA [50], allowing rapid, one-step detection of E. coli O157:H7. In this assay the anti-E. coli O157:H7 mAb was used as the capture reagent to enrich bacteria at the surface followed by bsAb mediated detection. The detection sensitivities of this assay were determined to be 100, 750, and 500 CFU/ml for tap water, lake water, and apple juice, respectively, in a microtiter-plate-based assay. A marked improvement in sensitivity for E. coli O157:H7 was achieved using both immunofilter and immunomagnetic ELISA formats, with detection limits of 1 CFU/ml and 10 CFU/ml, respectively. Additionally, the specificity of the assay was confirmed, because it did not detect other bacterial strains such as Salmonella, Pseudomonas, and non-pathogenic E. coli. This novel, bsAb-based assay had high specificity with low background and was both rapid and ultrasensitive. A major advantage of this assay, in comparison to current assays, was removal of the requirement for amplification steps.
Bordetella pertussis
B. pertussis is the causative bacterium of pertussis (whooping cough), which affects individuals of adolescent age, and there were an estimated 300 000 pertussis-related deaths in children (2008). Despite the availability of a vaccine, it has become a major health concern in recent years. Estimates from the WHO suggest that in 2008 there were 16 million cases of pertussis, of which 95% were in developing countries. Similar to many other bacterial infections, early detection is critical in order to prevent the spread of infection and to ensure correct treatment of infected individuals. Currently, there are two methods for detecting this bacterium. The first requires that clinical nasopharyngeal swab or aspirate samples are cultured for 3–7 days [51]. The second method involves the use of a fluorescently labeled mAb directed against an antigenic LPS molecule, which is present on the outer membrane of B. pertussis. Both assays have limitations regarding sampling, operator training, and sensitivity, which restrict their use in resource-constrained locations [52]. A ‘molecular velcro’ sandwich assay for the detection of B. pertussis was found to be useful for the analysis of clinical samples, immunochemical structural studies, and for the serological characterization of B. pertussis LPS [51]. The bsAb was produced by fusion of the anti-B. pertussis LPS mAb-secreting hybridoma with an anti-HRPO mAb-secreting hybridoma. The anti-LPS-specific mAb was capable of binding to heat-killed B. pertussis BP347 in both buffer and sample matrix (spiked nasopharyngeal aspirates) [51]. The assay proved highly sensitive with a lower limit of detection of ∼5 CFU. Interestingly, the binding of the B. pertussis to the anti-B. pertussis LPS mAb-coated solid phase was found to be irreversible, despite significant washing suggesting that the assay works as a form of ‘velcro molecular’ assay. This immunoassay achieved ultrasensitive detection of B. pertussis due to the availability of multiple LPS molecules on the bacterial surface for bsAb binding. Additionally, to facilitate ease of use and rapid detection, the immunoassay was tested in an immunoswab format permitting POC detection of B. pertussis in a clinical setting.
Staphylococcus aureus thermonuclease (TNase)
S. aureus is the most frequent cause of wound infection among hospitalized patients, with studies suggesting that it is present in 43% of infected leg ulcers [53]. The major biomedical problem of chronic wound healing and the continuing emergence of antibiotic-resistant species have become major health concerns. A novel fluorescence-based immunoassay enabled qualitative detection of S. aureus TNase to confirm the presence of S. aureus in vitro [53]. Rhodamine and fluorescein-labeled hemocyanin from Megathura crenulata (KLH) were prepared as immunoconjugates containing a sensitive fluorescent reporter moiety. A bsAb that both specifically quenched the fluorescence of the reporter conjugate and bound the TNase target antigen was produced using cell fusion techniques. In that study two KLH-fluorophore/TNase-specific antibody series were generated, with each member of the series specific to an epitope on either the KLH or the fluorescent moiety of the reporter conjugate. The decrease in fluorescence emission intensity at 580 nm/520 nm for the rhodamine and fluorescein conjugates, respectively, was analyzed with each bsAb of the series, and quenching was observed upon antibody–antigen binding [53]. Increased fluorescence intensity observed upon TNase binding was demonstrated with antibodies that have a higher affinity for the bacterial antigen than the fluorescent reporter. The specificity of the assay for S. aureus was also tested using the Pseudomonas aeruginosa LPS antigen as a control, which demonstrated that variations in the fluorescence quenching effect did not exceed 4%. These preliminary investigations suggest a highly sensitive assay with potential applications in biological specimens with a limited risk of false positive results.
Severe acute respiratory syndrome (SARS)
SARS is a serious form of pneumonia and affected >8000 people worldwide, spreading to 30 countries across five continents, in the 2002–2003 outbreak. SARS coronavirus (SARS-CoV) is the responsible agent and was transmitted from wild animals to the human population 54, 55, and delayed identification of this virus aided contagion. The SARS virus is detected in humans by RT-PCR 56, 57 indirect fluorescence assay, which detects anti-SARS-CoV antibodies in body fluids [58] and by isolation of SARS-CoV from clinical samples 59, 60. Such viral culturing is time consuming, tedious and insensitive, therefore, PCR- and antibody-based detection methods are the predominant techniques adopted for surveillance. A highly sensitive and rapid bsAb-based immunoswab assay was developed for the early detection of SARS-CoV. This assay was superior to the traditional monoclonal ELISA-based assay [61] and detected the viral antigen nucleoplasmid protein (NP). Three different mAbs that recognize various epitopes on the NP antigen and the anti-HRPO mAb were utilized to generate the anti-SARS-CoV NP × anti-HRPO bsAbs. This immunoswab assay showed NP detection limits of 10 pg/ml in saline, 20–200 pg/ml in pig nasopharyngeal aspirates, and 500 pg/ml in rabbit serum, thus, demonstrating the bispecific detection approach as superior in terms of sensitivity and specificity. The immunoswab assay facilitated rapid detection (∼45 min) [61] and was robust, potentially permitting screening of numerous infected individuals within a short period of time, and hence, assisting to contain viral infection. Furthermore, by targeting the SARS-CoV spike protein (S1) in both mAb- and bsAb-based assays, to identify SARS infected individuals, it was also demonstrated that the bsAb detection reagent could improve the sensitivity of the assay from 37 to 19 ng/ml [62].
Although these examples demonstrate the utility of bsAbs for diagnostic applications, there are several shortcomings related to the use of quadromas including yield, format uniformity, and purification. The advent of recombinant formats is of considerable value to overcome these limitations. It is anticipated that bsAb formats in the therapeutic arena can be meaningfully applied to diagnostics as the development of next generation diagnostic devices for POC, decentralized, and aged care applications demand superior diagnostic reagents.
Concluding remarks
The bsAb stable is large with big pharmaceutical companies investing over US$7.5 billion in bsAbs since 2009 [27]. The front-runners have yet to clearly emerge but the increasing confidence in bsAbs over the past few years has resulted in a plethora of bsAb formats. In combination with highly innovative molecular biological approaches, the momentum gained through clinical success is prompting the emergence of additional formats. In many cases these may prove ineffective for therapeutic applications, resulting in a high attrition rate. However, the therapeutic arena has seen significant advancements in bsAb-based agents, which culminating in a single bsAb achieving regulatory approval. This bsAb, catumaxomab, does not extend far beyond the traditional IgG format but to access truly unique functions many more original and groundbreaking approaches will be required. Similar to the development of human mAb therapies, the efficacy and safety of simultaneous engagement of multiple disease targets will take considerable research and potentially, considerable time, to show marketplace dominance [63]. Current clinical trials are in progress and larger numbers of entities are expected to enter clinical evaluation in the future. It is still unclear if such smart retargeting strategies will be successful but bsAbs are backed as ‘hot stock’ at present due to their real potential to have a significant impact on human disease treatment and management [27]. Although the next big blockbuster therapy may well be a bsAb, the application of such constructs to diagnostics is an attractive alternative to current strategies, which has real potential to pave the way for improved, next-generation diagnostics with applications in low-resource and POC settings. bsAbs in simple immunodiagnostic tests are applicable to such settings and can assist in ensuring these tests meet the ASSURED principles [64]. Regardless of the application there are some common issues that will need to be addressed (Box 3 ) including manufacturability, scale, and stability, with issues relating to immunogenicity, pharmacokinetics, and biodistribution being of particular concern for therapeutics [65]. The bsAb tale is moving beyond two specificities and into chapters that will vastly improve their therapeutic applications and will also significantly impact on current diagnostic challenges.
Box 3. Outstanding questions.
-
•
Will the multitude of bsAbs formats provide comparable preclinical information?
-
•
Is the Triomab or BiTE or sdAb the way forward for human therapeutics?
-
•
Will the paucity of diagnostic bispecifics be improved by a single bispecific format coming to the fore in the therapeutic arena?
-
•
Can bispecific antibody deliver superior, simplified diagnostics with application in low resource settings, through simplification of the assay capture and/or detection steps?
Acknowledgments
H.B. and R.J.O’K. are funded under the program for Research in Third Level Institutions (PRTLI) Cycle 5. R.J.O’K. is supported by Science Foundation Ireland under CSET Grant no. 10/CE/B1821. J.C.W. and P.J.C. are supported by Australian Research Council (ARC) and National Health and Medical Research Council (NHMRC) grants. J.C.W. is an ARC Fellow and an Honorary NHMRC Principal Research Fellow.
Glossary
- Bispecific antibody (bsAb)
antibody-derived proteins with the ability to bind to two different epitopes.
- Bispecific T cell engager (BiTE)
a class of bsAbs that is capable of directing a host immune system.
- Complementarity determining region (CDR)
the hypervariable region of an antibody, which exhibits a high level of sequence diversity and is involved in antigen binding.
- Diabody (Db)
a heterodimeric bsAb.
- Human anti-mouse antibody (HAMA)
the most common human anti-animal antibody interferent.
- Monoclonal antibody (mAb)
monospecific antibodies produced by immortalization of a specific antibody-secreting B cell.
- Recombinant bispecific antibody (rbsAb)
a bsAb produced by genetic manipulation of genes.
- Single chain diabody (scDb)
a derivative of the Db approach where a peptide linker joins the two antibody fragments and the domains are expressed as a single polypeptide chain.
- Single chain fragment variable (scFv)
antibody format where the variable regions of the heavy (VH) and light chains (VL) are expressed with a linker sequence connecting them.
- Single domain antibody (sdAb)
an antibody fragment consisting of a single monomeric variable antibody domain.
- Tandem single chain fragment variable (taFv)
a linked fragment variable (Fv) molecule.
- Trifunctional antibody (Triomab)
intact IgG molecules that can engage three different cells types.
References
- 1.Ohlin M., Zouali M. The human antibody repertoire to infectious agents: implications for disease pathogenesis. Mol. Immunol. 2003;40:1–11. doi: 10.1016/s0161-5890(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 2.Nelson P.N. Monoclonal antibodies. Mol. Pathol. 2000;53:111–117. doi: 10.1136/mp.53.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch J.R., Racher A.J. Antibody production. Adv. Drug Deliv. Rev. 2006;58:671–685. doi: 10.1016/j.addr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Conroy P.J. Antibody production, design and use for biosensor-based applications. Semin. Cell Dev. Biol. 2009;20:10–26. doi: 10.1016/j.semcdb.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 6.Pescovitz M.D. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am. J. Transplant. 2006;6:859–866. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 7.Parashar A. Bispecific antibodies for diagnostic applications. In: Kontermann R.E., editor. Bispecific Antibodies. 1st edn. Springer; 2011. pp. 349–367. [Google Scholar]
- 8.Milstein C., Cuello A.C. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305:537–540. doi: 10.1038/305537a0. [DOI] [PubMed] [Google Scholar]
- 9.Graziano R.F., Guptill P. Chemical production of bispecific antibodies. Methods Mol. Biol. 2004;283:71–85. doi: 10.1385/1-59259-813-7:071. [DOI] [PubMed] [Google Scholar]
- 10.Nisonoff A., Rivers M.M. Recombination of a mixture of univalent antibody fragments of different specificity. Arch. Biochem. Biophys. 1961;93:460–462. doi: 10.1016/0003-9861(61)90296-x. [DOI] [PubMed] [Google Scholar]
- 11.Kontermann R. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182–197. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Spriel A.B. Immunotherapeutic perspective for bispecific antibodies. Immunol. Today. 2000;21:391–397. doi: 10.1016/s0167-5699(00)01659-5. [DOI] [PubMed] [Google Scholar]
- 13.Stein C. Natural killer (NK)- and T-cell engaging antibody-derived therapeutics. Antibodies. 2012;1:88–123. [Google Scholar]
- 14.Müller D., Kontermann R.E. Bispecific antibodies for cancer immunotherapy: current perspectives. BioDrugs. 2010;24:89–98. doi: 10.2165/11530960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Wu C. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat. Biotechnol. 2007;25:1290–1297. doi: 10.1038/nbt1345. [DOI] [PubMed] [Google Scholar]
- 16.Kellner C. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J. Immunother. 2008;31:871–884. doi: 10.1097/CJI.0b013e318186c8b4. [DOI] [PubMed] [Google Scholar]
- 17.Rossi E.A. Development of new multivalent-bispecific agents for pretargeting tumor localization and therapy. Clin. Cancer Res. 2003;9:3886S–3896S. [PubMed] [Google Scholar]
- 18.Griffiths A.D. Human anti-self antibodies with high specificity from phage display libraries. EMBO J. 1993;12:725–734. doi: 10.1002/j.1460-2075.1993.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auf der Maur A. Antigen-independent selection of stable intracellular single-chain antibodies. FEBS Lett. 2001;508:407–412. doi: 10.1016/s0014-5793(01)03101-5. [DOI] [PubMed] [Google Scholar]
- 20.Els Conrath K. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J. Biol. Chem. 2001;276:7346–7350. doi: 10.1074/jbc.M007734200. [DOI] [PubMed] [Google Scholar]
- 21.Holliger P., Hudson P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 22.Chames P., Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs. 2009;1:539–547. doi: 10.4161/mabs.1.6.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi E.A. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6841–6846. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold D.V. A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Res. 2008;68:4819–4826. doi: 10.1158/0008-5472.CAN-08-0232. [DOI] [PubMed] [Google Scholar]
- 25.Orcutt K.D. A modular IgG-scFv bispecific antibody topology. Protein Eng. Des. Sel. 2010;23:221–228. doi: 10.1093/protein/gzp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kontermann R.E. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol. Sin. 2005;26:1–9. doi: 10.1111/j.1745-7254.2005.00008.x. [DOI] [PubMed] [Google Scholar]
- 27.Holmes D. Buy buy bispecific antibodies. Nat. Rev. Drug Discov. 2011;10:798–800. doi: 10.1038/nrd3581. [DOI] [PubMed] [Google Scholar]
- 28.Wolf E. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov. Today. 2005;10:1237–1244. doi: 10.1016/S1359-6446(05)03554-3. [DOI] [PubMed] [Google Scholar]
- 29.Brischwein K. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol. Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 30.Seimetz D. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer Treat. Rev. 2010;36:458–467. doi: 10.1016/j.ctrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Buhmann R. Immunotherapy of recurrent B-cell malignancies after allo-SCT with Bi20 (FBTA05), a trifunctional anti-CD3 × anti-CD20 antibody and donor lymphocyte infusion. Bone Marrow Transplant. 2009;43:383–397. doi: 10.1038/bmt.2008.323. [DOI] [PubMed] [Google Scholar]
- 32.Ruf P. Ganglioside GD2-specific trifunctional surrogate antibody Surek demonstrates therapeutic activity in a mouse melanoma model. J. Transl. Med. 2012;10:219. doi: 10.1186/1479-5876-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eissler N. Trifunctional bispecific antibodies induce tumor-specific T cells and elicit a vaccination effect. Cancer Res. 2012;72:3958–3966. doi: 10.1158/0008-5472.CAN-12-0146. [DOI] [PubMed] [Google Scholar]
- 34.Dettmar K. Transient lymphocyte decrease due to adhesion and migration following catumaxomab (anti-EpCAM × anti-CD3) treatment in vivo. Clin. Transl. Oncol. 2012;14:376–381. doi: 10.1007/s12094-012-0811-5. [DOI] [PubMed] [Google Scholar]
- 35.Hess J. Cancer therapy with trifunctional antibodies: linking innate and adaptive immunity. Future Oncol. 2012;8:73–85. doi: 10.2217/fon.11.138. [DOI] [PubMed] [Google Scholar]
- 36.Cardillo T.M. Improved targeting of pancreatic cancer: experimental studies of a new bispecific antibody, pretargeting enhancement system for immunoscintigraphy. Clin. Cancer Res. 2004;10:3552–3561. doi: 10.1158/1078-0432.CCR-03-0340. [DOI] [PubMed] [Google Scholar]
- 37.Goldenberg D.M. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J. Clin. Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 38.Sharkey R.M. Bispecific antibody pretargeting of radionuclides for immuno single-photon emission computed tomography and immuno positron emission tomography molecular imaging: an update. Clin. Cancer Res. 2007;13:5577s–5585s. doi: 10.1158/1078-0432.CCR-07-1087. [DOI] [PubMed] [Google Scholar]
- 39.McBride W.J. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J. Nucl. Med. 2006;47:1678–1688. [PubMed] [Google Scholar]
- 40.Khaw B.A. Imaging experimental atherosclerotic lesions in ApoE knockout mice: enhanced targeting with Z2D3-anti-DTPA bispecific antibody and 99mTc-labeled negatively charged polymers. J. Nucl. Med. 2006;47:868–876. [PubMed] [Google Scholar]
- 41.Tekabe Y. Targeting very small model lesions pretargeted with bispecific antibody with 99mTc-labeled high-specific radioactivity polymers. Nucl. Med. Commun. 2010;31:320–327. doi: 10.1097/MNM.0b013e32833576e8. [DOI] [PubMed] [Google Scholar]
- 42.Patil V. Imaging small human prostate cancer xenografts after pretargeting with bispecific bombesin-antibody complexes and targeting with high specific radioactivity labeled polymer-drug conjugates. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:824–839. doi: 10.1007/s00259-011-2050-3. [DOI] [PubMed] [Google Scholar]
- 43.Gada K.S. Pretargeted gamma imaging of murine metastatic melanoma lung lesions with bispecific antibody and radiolabeled polymer drug conjugates. Nucl. Med. Commun. 2011;32:1231–1240. doi: 10.1097/MNM.0b013e32834af77b. [DOI] [PubMed] [Google Scholar]
- 44.WHO . 2012. Global Tuberculosis Report.http://www.who.int/topics/tuberculosis/en/ WHO. [Google Scholar]
- 45.Cattamanchi A. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J. Acquir. Immune Defic. Syndr. 2011;56:230–238. doi: 10.1097/QAI.0b013e31820b07ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis J.L. Nucleic acid amplification tests for diagnosis of smear-negative TB in a high HIV-prevalence setting: a prospective cohort study. PLoS ONE. 2011;6:e16321. doi: 10.1371/journal.pone.0016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarkar S. A bispecific antibody based assay shows potential for detecting tuberculosis in resource constrained laboratory settings. PLoS ONE. 2012;7:e32340. doi: 10.1371/journal.pone.0032340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y.P. Rapid detection of hepatitis B virus surface antigen by an agglutination assay mediated by a bispecific diabody against both human erythrocytes and hepatitis B virus surface antigen. Clin. Vaccine Immunol. 2007;14:720–725. doi: 10.1128/CVI.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manafi M., Kremsmaier B. Comparative evaluation of different chromogenic/fluorogenic media for detecting Escherichia coli O157:H7 in food. Int. J. Food Microbiol. 2001;71:257–262. doi: 10.1016/s0168-1605(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 50.Guttikonda S. Monospecific and bispecific antibodies against E. coli O157 for diagnostics. J. Immunol. Methods. 2007;327:1–9. doi: 10.1016/j.jim.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Tang X.L. Use of bispecific antibodies in molecular velcro assays whose specificity approaches the theoretical limit of immunodetection for Bordetella pertussis. Clin. Diagn. Lab. Immunol. 2004;11:752–757. doi: 10.1128/CDLI.11.4.752-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tilley P.A. Detection of Bordetella pertussis in a clinical laboratory by culture, polymerase chain reaction, and direct fluorescent antibody staining; accuracy, and cost. Diagn. Microbiol. Infect. Dis. 2000;37:17–23. doi: 10.1016/s0732-8893(00)00117-6. [DOI] [PubMed] [Google Scholar]
- 53.Wagstaffe S.J. Bispecific antibody-mediated detection of the Staphylococcus aureus thermonuclease. Anal. Chem. 2012;84:5876–5884. doi: 10.1021/ac203403d. [DOI] [PubMed] [Google Scholar]
- 54.Guan Y. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 55.Wang L.F. Review of bats and SARS. Emerg. Infect. Dis. 2006;12:1834–1840. doi: 10.3201/eid1212.060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poon L.L. The aetiology, origins, and diagnosis of severe acute respiratory syndrome. Lancet Infect. Dis. 2004;4:663–671. doi: 10.1016/S1473-3099(04)01172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yam W.C. Evaluation of reverse transcription-PCR assays for rapid diagnosis of severe acute respiratory syndrome associated with a novel coronavirus. J. Clin. Microbiol. 2003;41:4521–4524. doi: 10.1128/JCM.41.10.4521-4524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan P.K. Immunofluorescence assay for serologic diagnosis of SARS. Emerg. Infect. Dis. 2004;10:530–532. doi: 10.3201/eid1003.030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keyaerts E. Growth kinetics of SARS-coronavirus in Vero E6 cells. Biochem. Biophys. Res. Commun. 2005;329:1147–1151. doi: 10.1016/j.bbrc.2005.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamashita M. Susceptibility of human and rat neural cell lines to infection by SARS-coronavirus. Biochem. Biophys. Res. Commun. 2005;334:79–85. doi: 10.1016/j.bbrc.2005.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kammila S. A rapid point of care immunoswab assay for SARS-CoV detection. J. Virol. Methods. 2008;152:77–84. doi: 10.1016/j.jviromet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sunwoo H.H. Quantitative and sensitive detection of the SARS-CoV spike protein using bispecific monoclonal antibody-based enzyme-linked immunoassay. J. Virol. Methods. 2013;187:72–78. doi: 10.1016/j.jviromet.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reichert J.M. Bispecific antibodies and ADCs: once and future kings? MAbs. 2011;3:329–330. doi: 10.4161/mabs.3.4.16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pang T., Peeling R.W. Diagnostic tests for infectious diseases in the developing world: two sides of the coin. Trans. R. Soc. Trop. Med. Hyg. 2007;101:856–857. doi: 10.1016/j.trstmh.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 65.Hemmerle T. A critical evaluation of the tumor-targeting properties of bispecific antibodies based on quantitative biodistribution data. Protein Eng. Des. Sel. 2012;25:851–854. doi: 10.1093/protein/gzs061. [DOI] [PubMed] [Google Scholar]
- 66.Dreier T. T Cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3-bispecific single-chain antibody construct. J. Immunol. 2003;170:4397–4402. doi: 10.4049/jimmunol.170.8.4397. [DOI] [PubMed] [Google Scholar]
- 67.Dreier T. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int. J. Cancer. 2002;100:690–697. doi: 10.1002/ijc.10557. [DOI] [PubMed] [Google Scholar]
- 68.Loffler A. Efficient elimination of chronic lymphocytic leukaemia B cells by autologous T cells with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Leukemia. 2003;17:900–909. doi: 10.1038/sj.leu.2402890. [DOI] [PubMed] [Google Scholar]
- 69.Gruen M. T-cell-mediated lysis of B cells induced by a CD19 × CD3 bispecific single-chain antibody is perforin dependent and death receptor independent. Cancer Immunol. Immunother. 2004;53:625–632. doi: 10.1007/s00262-003-0496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren-Heidenreich L. Redirected T-cell cytotoxicity to epithelial cell adhesion molecule-overexpressing adenocarcinomas by a novel recombinant antibody, E3Bi, in vitro and in an animal model. Cancer. 2004;100:1095–1103. doi: 10.1002/cncr.20060. [DOI] [PubMed] [Google Scholar]
- 71.Maletz K. Bispecific single-chain antibodies as effective tools for eliminating epithelial cancer cells from human stem cell preparations by redirected cell cytotoxicity. Int. J. Cancer. 2001;93:409–416. doi: 10.1002/ijc.1348. [DOI] [PubMed] [Google Scholar]
- 72.Wimberger P. Efficient tumor cell lysis by autologous, tumor-resident T lymphocytes in primary ovarian cancer samples by an EP-CAM-/CD3-bispecific antibody. Int. J. Cancer. 2003;105:241–248. doi: 10.1002/ijc.11056. [DOI] [PubMed] [Google Scholar]
- 73.Wuest T. Construction of a bispecific single chain antibody for recruitment of cytotoxic T cells to the tumour stroma associated antigen fibroblast activation protein. J. Biotechnol. 2001;92:159–168. doi: 10.1016/s0168-1656(01)00355-8. [DOI] [PubMed] [Google Scholar]
- 74.Honemann D. A novel recombinant bispecific single-chain antibody, bscWue-1 × CD3, induces T-cell-mediated cytotoxicity towards human multiple myeloma cells. Leukemia. 2004;18:636–644. doi: 10.1038/sj.leu.2403264. [DOI] [PubMed] [Google Scholar]
- 75.Grosse-Hovest L. Cloned transgenic farm animals produce a bispecific antibody for T cell-mediated tumor cell killing. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6858–6863. doi: 10.1073/pnas.0308487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruenke J. A recombinant bispecific single-chain Fv antibody against HLA class II and FcgammaRIII (CD16) triggers effective lysis of lymphoma cells. Br. J. Haematol. 2004;125:167–179. doi: 10.1111/j.1365-2141.2004.04893.x. [DOI] [PubMed] [Google Scholar]
- 77.McCall A.M. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. J. Immunol. 2001;166:6112–6117. doi: 10.4049/jimmunol.166.10.6112. [DOI] [PubMed] [Google Scholar]
- 78.Haisma H.J. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther. 2000;7:901–904. doi: 10.1038/sj.cgt.7700198. [DOI] [PubMed] [Google Scholar]
- 79.Grill J. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin. Cancer Res. 2001;7:641–650. [PubMed] [Google Scholar]
- 80.Witlox M.A. Epidermal growth factor receptor targeting enhances adenoviral vector based suicide gene therapy of osteosarcoma. J. Gene Med. 2002;4:510–516. doi: 10.1002/jgm.308. [DOI] [PubMed] [Google Scholar]
- 81.Dirven C.M. Gene therapy for meningioma: improved gene delivery with targeted adenoviruses. J. Neurosurg. 2002;97:441–449. doi: 10.3171/jns.2002.97.2.0441. [DOI] [PubMed] [Google Scholar]
- 82.Beusechem V.W.V. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. J. Virol. 2002;76:2753–2762. doi: 10.1128/JVI.76.6.2753-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heideman D.A. Selective gene transfer into primary human gastric tumors using epithelial cell adhesion molecule-targeted adenoviral vectors with ablated native tropism. Hum. Gene Ther. 2002;13:1677–1685. doi: 10.1089/104303402760293529. [DOI] [PubMed] [Google Scholar]
- 84.Brandão J.G. CD40-targeted adenoviral gene transfer to dendritic cells through the use of a novel bispecific single-chain Fv antibody enhances cytotoxic T cell activation. Vaccine. 2003;21:2268–2272. doi: 10.1016/s0264-410x(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 85.Korn T. Recombinant bispecific antibodies for the targeting of adenoviruses to CEA-expressing tumour cells: a comparative analysis of bacterially expressed single-chain diabody and tandem scFv. J. Gene Med. 2004;6:642–651. doi: 10.1002/jgm.555. [DOI] [PubMed] [Google Scholar]
- 86.Weisbart R.H. Construction and expression of a bispecific single-chain antibody that penetrates mutant p53 colon cancer cells and binds p53. Int. J. Oncol. 2004;25:1113–1118. [PubMed] [Google Scholar]
- 87.Cochlovius B. Treatment of human B cell lymphoma xenografts with a CD3 × CD19 diabody and T cells. J. Immunol. 2000;165:888–895. doi: 10.4049/jimmunol.165.2.888. [DOI] [PubMed] [Google Scholar]
- 88.Kipriyanov S.M. Synergistic antitumor effect of bispecific CD19 × CD3 and CD19 × CD16 diabodies in a preclinical model of non-Hodgkin's lymphoma. J. Immunol. 2002;169:137–144. doi: 10.4049/jimmunol.169.1.137. [DOI] [PubMed] [Google Scholar]
- 89.Xiong D. Efficient inhibition of human B-cell lymphoma xenografts with an anti-CD20 × anti-CD3 bispecific diabody. Cancer Lett. 2002;177:29–39. doi: 10.1016/s0304-3835(01)00758-3. [DOI] [PubMed] [Google Scholar]
- 90.Hayashi H. A highly effective and stable bispecific diabody for cancer immunotherapy: cure of xenografted tumors by bispecific diabody and T-LAK cells. Cancer Immunol. Immunother. 2004;53:497–509. doi: 10.1007/s00262-003-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takemura S. A mutated superantigen SEA D227A fusion diabody specific to MUC1 and CD3 in targeted cancer immunotherapy for bile duct carcinoma. Cancer Immunol. Immunother. 2002;51:33–44. doi: 10.1007/s00262-001-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao Y. Efficient inhibition of multidrug-resistant human tumors with a recombinant bispecific anti-P-glycoprotein × anti-CD3 diabody. Leukemia. 2004;18:513–520. doi: 10.1038/sj.leu.2403267. [DOI] [PubMed] [Google Scholar]
- 93.Schlenzka J. Combined effect of recombinant CD19 × CD16 diabody and thalidomide in a preclinical model of human B cell lymphoma. Anticancer Drugs. 2004;15:915–919. doi: 10.1097/00001813-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 94.DeNardo D.G. Anti-HLA-DR/anti-DOTA diabody construction in a modular gene design platform: bispecific antibodies for pretargeted radioimmunotherapy. Cancer Biother. Radiopharm. 2001;16:525–535. doi: 10.1089/10849780152752128. [DOI] [PubMed] [Google Scholar]
- 95.Lu D. The effect of variable domain orientation and arrangement on the antigen-binding activity of a recombinant human bispecific diabody. Biochem. Biophys. Res. Commun. 2004;318:507–513. doi: 10.1016/j.bbrc.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 96.Leonard J.P. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113:2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 97.Buhler P. A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells. Cancer Immunol. Immunother. 2008;57:43–52. doi: 10.1007/s00262-007-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kipriyanov S.M. Effect of domain order on the activity of bacterially produced bispecific single-chain Fv antibodies. J. Mol. Biol. 2003;330:99–111. doi: 10.1016/s0022-2836(03)00526-6. [DOI] [PubMed] [Google Scholar]
- 99.Korn T. Bispecific single-chain diabody-mediated killing of endoglin-positive endothelial cells by cytotoxic T lymphocytes. J. Immunother. 2004;27:99–106. doi: 10.1097/00002371-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 100.Nettelbeck D.M. Targeting of adenovirus to endothelial cells by a bispecific single-chain diabody directed against the adenovirus fiber knob domain and human endoglin (CD105) Mol. Ther. 2001;3:882–891. doi: 10.1006/mthe.2001.0342. [DOI] [PubMed] [Google Scholar]
- 101.Nettelbeck D.M. Retargeting of adenoviral infection to melanoma: combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int. J. Cancer. 2004;108:136–145. doi: 10.1002/ijc.11563. [DOI] [PubMed] [Google Scholar]
- 102.Sabine Brüsselbach T.K. Enzyme recruitment and tumor cell killing in vitro by a secreted bispecific single-chain diabody. Tumor Target. 1999;4:115–123. [Google Scholar]
- 103.Kipriyanov S.M. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J. Mol. Biol. 1999;293:41–56. doi: 10.1006/jmbi.1999.3156. [DOI] [PubMed] [Google Scholar]
- 104.Reusch U. Effect of tetravalent bispecific CD19×CD3 recombinant antibody construct and CD28 costimulation on lysis of malignant B cells from patients with chronic lymphocytic leukemia by autologous T cells. Int. J. Cancer. 2004;112:509–518. doi: 10.1002/ijc.20417. [DOI] [PubMed] [Google Scholar]
- 105.Shahied L.S. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J. Biol. Chem. 2004;279:53907–53914. doi: 10.1074/jbc.M407888200. [DOI] [PubMed] [Google Scholar]
- 106.Michaelson J.S. Anti-tumor activity of stability-engineered IgG-like bispecific antibodies targeting TRAIL-R2 and LTbetaR. MAbs. 2009;1:128–141. doi: 10.4161/mabs.1.2.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu D. Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J. Biol. Chem. 2004;279:2856–2865. doi: 10.1074/jbc.M310132200. [DOI] [PubMed] [Google Scholar]
- 108.Dong J. A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs. 2011;3:273–288. doi: 10.4161/mabs.3.3.15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dong J. Combination of two insulin-like growth factor-I receptor inhibitory antibodies targeting distinct epitopes leads to an enhanced antitumor response. Mol. Cancer Ther. 2010;9:2593–2604. doi: 10.1158/1535-7163.MCT-09-1018. [DOI] [PubMed] [Google Scholar]
- 110.Kuo S.R. Engineering a CD123 × CD3 bispecific scFv immunofusion for the treatment of leukemia and elimination of leukemia stem cells. Protein Eng. Des. Sel. 2012;25:561–569. doi: 10.1093/protein/gzs040. [DOI] [PubMed] [Google Scholar]
- 111.Yazaki P.J. A series of anti-CEA/anti-DOTA bispecific antibody formats evaluated for pre-targeting: comparison of tumor uptake and blood clearance. Protein Eng. Des. Sel. 2013;26:187–193. doi: 10.1093/protein/gzs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Castoldi R. A novel bispecific EGFR/Met antibody blocks tumor-promoting phenotypic effects induced by resistance to EGFR inhibition and has potent antitumor activity. Oncogene. 2013 doi: 10.1038/onc.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tuscano J.M. The Bs20 × 22 anti-CD20-CD22 bispecific antibody has more lymphomacidal activity than do the parent antibodies alone. Cancer Immunol. Immunother. 2011;60:771–780. doi: 10.1007/s00262-011-0978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Asano R. Domain order of a bispecific diabody dramatically enhances its antitumor activity beyond structural format conversion: the case of the hEx3 diabody. Protein Eng. Des. Sel. 2013;26:359–367. doi: 10.1093/protein/gzt009. [DOI] [PubMed] [Google Scholar]
- 115.Castoldi R. Molecular characterization of novel trispecific ErbB-cMet-IGF1R antibodies and their antigen-binding properties. Protein Eng. Des. Sel. 2012;25:551–559. doi: 10.1093/protein/gzs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rozan C. Single domain antibody-based and linker-free bispecific antibodies targeting FcgammaRIII induce potent anti-tumor activity without recruiting regulatory T cells. Mol. Cancer Ther. 2013;12:1481–1491. doi: 10.1158/1535-7163.MCT-12-1012. [DOI] [PubMed] [Google Scholar]
- 117.Feldmann A. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T cells. J. Immunol. 2012;189:3249–3259. doi: 10.4049/jimmunol.1200341. [DOI] [PubMed] [Google Scholar]
- 118.Mabry R. A dual-targeting PDGFRbeta/VEGF-A molecule assembled from stable antibody fragments demonstrates anti-angiogenic activity in vitro and in vivo. MAbs. 2010;2:20–34. doi: 10.4161/mabs.2.1.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choi B.D. A novel bispecific antibody recruits T cells to eradicate tumors in the “immunologically privileged” central nervous system. Oncoimmunology. 2013;2:e23639. doi: 10.4161/onci.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi B.D. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc. Natl. Acad. Sci. U.S.A. 2013;110:270–275. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jackman J. Development of a two-part strategy to identify a therapeutic human bispecific antibody that inhibits IgE receptor signaling. J. Biol. Chem. 2010;285:20850–20859. doi: 10.1074/jbc.M110.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tam S.W. A bispecific antibody against human IgE and human FcgammaRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy. 2004;59:772–780. doi: 10.1111/j.1398-9995.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 123.Munitz A. Reversal of airway inflammation and remodeling in asthma by a bispecific antibody fragment linking CCR3 to CD300a. J. Allergy Clin. Immunol. 2006;118:1082–1089. doi: 10.1016/j.jaci.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 124.Schanzer J. Development of tetravalent, bispecific CCR5 antibodies with antiviral activity against CCR5 monoclonal antibody-resistant HIV-1 strains. Antimicrob. Agents Chemother. 2011;55:2369–2378. doi: 10.1128/AAC.00215-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Laventie B.J. Heavy chain-only antibodies and tetravalent bispecific antibody neutralizing Staphylococcus aureus leukotoxins. Proc. Natl. Acad. Sci. U.S.A. 2011;108:16404–16409. doi: 10.1073/pnas.1102265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoshida S. T-cell activation and cytokine production via a bispecific single-chain antibody fragment targeted to blood-stage malaria parasites. Blood. 2003;101:2300–2306. doi: 10.1182/blood-2002-03-0831. [DOI] [PubMed] [Google Scholar]
- 127.De Bernardis F. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J. Infect. Dis. 2007;195:149–157. doi: 10.1086/509891. [DOI] [PubMed] [Google Scholar]
- 128.Mabry R. Engineering of stable bispecific antibodies targeting IL-17A and IL-23. Protein Eng. Des. Sel. 2010;23:115–127. doi: 10.1093/protein/gzp073. [DOI] [PubMed] [Google Scholar]
- 129.Veri M.C. Therapeutic control of B cell activation via recruitment of Fcgamma receptor IIb (CD32B) inhibitory function with a novel bispecific antibody scaffold. Arthritis Rheum. 2010;62:1933–1943. doi: 10.1002/art.27477. [DOI] [PubMed] [Google Scholar]
- 130.Wu C. Molecular construction and optimization of anti-human IL-1alpha/beta dual variable domain immunoglobulin (DVD-Ig) molecules. MAbs. 2009;1:339–347. doi: 10.4161/mabs.1.4.8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bruhl H. Depletion of CCR5-expressing cells with bispecific antibodies and chemokine toxins: a new strategy in the treatment of chronic inflammatory diseases and HIV. J. Immunol. 2001;166:2420–2426. doi: 10.4049/jimmunol.166.4.2420. [DOI] [PubMed] [Google Scholar]
- 132.Qi J. A bispecific antibody against IL-1beta and IL-17A is beneficial for experimental rheumatoid arthritis. Int. Immunopharmacol. 2012;14:770–778. doi: 10.1016/j.intimp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 133.Fury M.G. A phase-I trial of the epidermal growth factor receptor directed bispecific antibody MDX-447 without and with recombinant human granulocyte-colony stimulating factor in patients with advanced solid tumors. Cancer Immunol. Immunother. 2008;57:155–163. doi: 10.1007/s00262-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McDonagh C.F. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol. Cancer Ther. 2012;11:582–593. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 135.Hammer O. CD19 as an attractive target for antibody-based therapy. MAbs. 2012;4:571–577. doi: 10.4161/mabs.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stamova S. Cancer immunotherapy by retargeting of immune effector cells via recombinant bispecific antibody constructs. Antibodies. 2012;1:172–198. [Google Scholar]
- 137.Portell C.A. Clinical and pharmacologic aspects of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia. Clin. Pharmacol. 2013;5:5–11. doi: 10.2147/CPAA.S42689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cioffi M. EpCAM/CD3-Bispecific T-cell engaging antibody MT110 eliminates primary human pancreatic cancer stem cells. Clin. Cancer Res. 2012;18:465–474. doi: 10.1158/1078-0432.CCR-11-1270. [DOI] [PubMed] [Google Scholar]
- 139.Dhimolea E., Reichert J.M. Poster Sessions: September 27–28, 2011. MAbs. 2012;4:14–16. doi: 10.4161/mabs.4.1.18821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reiners K.S. Rescue of impaired NK cell activity in hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol. Ther. 2013;21:895–903. doi: 10.1038/mt.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang S. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2013;73:824–833. doi: 10.1158/0008-5472.CAN-12-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kratz F., Elsadek B. Clinical impact of serum proteins on drug delivery. J. Control. Release. 2012;161:429–445. doi: 10.1016/j.jconrel.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 143.Dhimolea E., Reichert J.M. World Bispecific Antibody Summit, September 27–28, 2011, Boston, MA. MAbs. 2012;4:4–13. doi: 10.4161/mabs.4.1.18821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mack M. Biologic properties of a bispecific single-chain antibody directed against 17-1A (EpCAM) and CD3: tumor cell-dependent T cell stimulation and cytotoxic activity. J. Immunol. 1997;158:3965–3970. [PubMed] [Google Scholar]
- 145.Loffler A. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]


