Abstract
Based on the bioinformatics analysis of the gene encoding glycoprotein 5 (GP5) of porcine reproductive and respiratory syndrome virus (PRRSV) isolate HH08, two gene fragments were amplified by polymerase chain reaction (PCR), deleting the signal peptide and transmembrane sequences in GP5 gene. Both gene fragments were designated GP5a and GP5b, respectively. They were ligated with a linker and cloned into prokaryotic expression vector, pET-30a. Expression of the protein of interest was induced by isopropyl β-d-1-thiogalactopyranoside. The purified protein was used as an immunogen to elicit antibody in rabbit. The immunoreactivity of the protein was determined using ELISA and Western blot. Biologically active GP5 and anti-GP5 antibody inhibited cell infection by PRRSV. Moreover, the antibody produced in this study was capable of detecting the cell infection by PRRSV and distinguishing this virus from other viruses.
Keywords: PRRSV, GP5 protein, PCR, Prokaryotic expression
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is recognized as one of the most serious infectious diseases of swine, since its first appearance in North America in 1987 (Wootton et al., 1998). The disease is characterized by severe reproductive failure and respiratory distress (Benfield et al., 1992). The causative agent of PRRS is PRRS-virus (PRRSV) that was firstly identified in the Netherlands (Wensvoort et al., 1991). Shortly, another viral isolate of PRRS designated VR-2332 was identified in North America in 1992 (Collins et al., 1992). The European (EU) and North American (NA) isolates of PRRSV represent two distinct serotypes (Wensvoort et al., 1992, Nelson et al., 1993). At present, most identified PRRSV Chinese isolates belong to the NA-type.
PRRSV is an enveloped virus with a single-stranded positive-sense RNA genome. PRRSV, equine arteritis virus, lactate dehydrogenase-elevating virus of mice, and simian hemorrhagic fever virus belong to the family Arteriviridae (Meulenberg et al., 1993, Snijder and Meulenberg, 1998). Families Arteriviridae and Coronaviridae have been classified into order Nidovirales (Cavanagh, 1997). The viral genome is approximately 15 kb in length and consists of nine identified open reading frames (ORFs). ORFs 1a and 1b encode viral replicase polyproteins. ORFs 2a, 2b, 3 through 7 encode the viral structural proteins GP2, E, GP3, GP4, GP5, M and N, respectively (Stadejek et al., 2002).
The major envelope protein GP5 plays an important role in virus neutralization by inducing cellular and humoral responses (Faaberg et al., 2006, Jiang et al., 2007, Kim and Yoon, 2008). The GP5 is a glycoprotein that is composed of 200 amino acids. There are N-glycolysation sites and a hydrophilic signal peptide in the GP5, and the translated GP5 is retained in the endoplasmic reticulum (Plana Duran et al., 1997). There are three transmembrane domains, regions 62–83, 90–106, and 113–130, of the total residues encoding GP5. In addition, GP5 may induce cell apoptosis and interact with its potential cellular receptor (Delputte et al., 2002).
In the present paper, we cloned a truncated GP5 gene with the deletion of its signal peptide and transmembrane regions using a linker-based re-construction strategy. The high-level expression of the protein of interest was achieved in a prokaryotic system. Immunoreactivities of the GP5 and the specificity of anti-GP5 antibody were monitored using ELISA and immunoblotting. GP5 protein and anti-GP5 antibody inhibited PRRSV infection efficiently. The utility of the antibody in detecting PRRSV-infected cells was analyzed using immunofluorescence. Furthermore, the anti-GP5 antibody can be used as a diagnostic reagent for discriminating PRRSV from other viruses. Taken together, a reformed and biologically active GP5 protein expressed in a heterologous host was achieved at an inexpensive cost.
2. Materials and methods
2.1. Plasmids and bacterial strain
Recombinant plasmid, PMD-T-GP5 containing full-length GP5 gene of PRRSV Chinese isolate HH08 was prepared in our laboratory (Wang et al., 2009). The GP5 gene sequence deposited in GenBank database has been assigned an accession number GD 184821. Prokaryotic expression vector, pET-30a and E. coli strain BL21(DE3) pLysS were purchased from Novagen, USA.
2.2. Bioinformatics analysis and primer design
The molecular characteristics including the hydrophilicity of the GP5 were analyzed using DNAstar software (USA). Two pairs of primers were designed. P1: 5′-GGGGGATATCAACAGCAACAGC-3′; P2: 5′-CCCCGGATCCACCACCACCAGAACCACCACCACCAAATTTGTTAGC-3′; P3: 5′GGGGGGATCCGGTGGCGGTGGTTCTTGCATGTCCTGG3′; and P4: 5′CCCCAAGCTTCTAGAGACGACC3′. There are EcoR V and Hind III restriction enzyme sites in P1 and P4, respectively (underlined parts). A linker sequence encoding three repeated amino acid sequences (GGGGS) was introduced into primers P2 and P3 (The linker sequence was in black bold and underlined parts are BamH I sites).
2.3. Construction of recombinant plasmid
The N terminal part of GP5 gene was amplified using primers P1 and P2. The resulting PCR product named GP5a was digested with EcoRV and BamH I. The purified fragment was cloned into pET-30a vector using standard molecular cloning technique. The recombinant plasmid was designated as pET-GP5a. At the same time, the C terminal part of the GP5 gene designated as GP5b was amplified using primers P3 and P4. The purified PCR product was cloned into the BamH I and Hind III sites of pET-GP5a, resulting in a recombinant plasmid pET-GP5ab. The insert was then subjected to DNA sequencing.
2.4. Expression and purification of PRRSV GP5
Recombinant plasmid pET-GP5ab was purified with a plasmid purification kit (Keygen, Nanjing, China) and transformed into host cells, Escherichia coli (E. coli), BL21(DE3) pLysS. Positive clones were identified on Luria-Bertani (LB) agar plates containing Kanamycin (50 μg/ml). Protein expression was optimized according to a recent reference with minor modification (Liu et al., 2009). Briefly, the E. coli harboring pET-GP5ab was cultured in LB liquid medium at 37 °C with shaking until the optical density (OD) of the culture at 600 nm reached 0.6. Then, isopropyl β-d-thiogalactoside (IPTG) was added to a final concentration of 0.5 or 1 mM to induce expression at 37 °C for 7 h. The empty vector transformed bacteria were used as control. The bacteria were pelleted at 10,000 rpm, at 4 °C for 10 min and re-suspended in TE buffer (50 mM Tris and 1 mM EDTA, pH 8.0). Then, they were digested with lysozyme at a final concentration of 100 μg/L at room temperature for 30 min. The cell suspension was sonicated on ice for 30 min. Then the lysate was centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant and the pellets were mixed with sodium dodecyl sulfate (SDS)-loading buffer, respectively. Both samples were subjected to 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Gel-purification of inclusion bodies and renaturation of fusion protein by dialysis were basically performed according to the reference (Liu et al., 2009). The protein of interest was designated as GP5.
2.5. Western blot
The bacteria bearing either GP5 gene or empty vector were subjected to IPTG induction and SDS-PAGE. The proteins were transferred to a nitrocellulose membrane. The membrane was blocked with blocking buffer (5% non-fat dry milk and 0.05% Tween-20 in PBS) at 4 °C overnight. The next day, the membrane was incubated with a polyclonal antibody against PRRSV (1:500 diluted in PBS-0.05% Tween 20, PBST) at 37 °C for 1 h. After three times washing with PBST, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000 diluted in PBST, BOSTER, China). The protein bands were visualized via diaminobenzidine enzyme-based color development in the dark.
2.6. Preparation of polyclonal antibody
To achieve anti-GP5 antibody, a New Zealand rabbit was inoculated with 1 ml purified GP5 (1 mg/ml) emulsified with equal amount of Freund's complete adjuvant via subcutaneous injection. Fourteen days later, the rabbit was injected with the same antigen (1 ml) mixed with the equal volume of Freund's incomplete adjuvant at 10 days’ interval for four times. Finally, blood was collected from neck artery of the immunized rabbit to isolate antiserum.
2.7. ELISA
To evaluate the immunoreactivity between the GP5 and its polyclonal antibody, an indirect ELISA was performed. Briefly, ELISA plates were coated with 100 μl GP5 (100 μg/ml) at 4 °C overnight in carbonate–bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6). The wells were blocked with blocking buffer at 37 °C for 2 h. After three times washing with PBST, the wells were incubated with the serially diluted polyclonal antiserum at 37 °C for 1 h. After three times washing with PBST, the wells were incubated with HRP-conjugated goat anti-rabbit IgG (BOSTER, China 1:2000 diluted in PBST) at 37 °C for 1 h. OPD (o-phenylenediamine dihydrochloride) substrate was added and incubated for 15 min after washing with PBST. The OD value was read at 490 nm using an ELISA reader, after stopping the reaction with 50 μl stop buffer (2 M H2SO4). At the same time, PRRSV, pseudorabies virus (PRV), transmissible gastroenteritis coronavirus (TGEV), porcine epidemic diarrhea coronavirus (PEDV) and infectious bronchitis coronavirus (IBV) were used as coating antigens (2 μg/well) for discrimination ELISA as above.
2.8. Virus binding blocking assay
To analyze the blocking ability of GP5 protein to virus infection, Marc-145 cells (a monkey kidney cell line) in 24-well plates were incubated with purified GP5 serially diluted in serum-free medium at 37 °C for 2 h, followed by three times washing with PBS. Then, the cells were infected with PRRSV at an MOI (multiplicity of infection) of 2 at 37 °C for 48–72 h. To investigate the antiviral effect of anti-GP5 antibody, PRRSV was pre-incubated with serially diluted anti-GP5 sera or control rabbit sera at 37 °C for 1 h. Then, the viruses at an MOI of 2 was used to infect Marc-145 cells at 37 °C for 48–72 h. The wells were subjected to plaque assays using crystal violet staining (Li et al., 2009, Sui et al., 2010).
2.9. Immunofluorescence
Marc-145 cells were cultured in Dulbecco's modified essential medium (DMEM) supplemented with 10% newborn bovine serum (ExCell Biology, Inc. Shanghai, China) at 37 °C in a CO2 incubator. About 90% confluent cell monolayers were infected with PRRSV isolate HH08 at 37 °C for 48–72 h. Then, the cells were subjected to indirect immunofluorescence according to previous references with minor modification (Ren et al., 2006, Schwegmann-Wessels et al., 2009). Briefly, the cells were fixed 4% (w/v) formalin in PBS for 20 min and then incubated with the anti-GP5 antibody (1:500 dilution in 1% BSA) at 37 °C for 1 h. After washing with PBS, fluoresceine isothiocyanate (FITC) labeled goat anti-rabbit IgG (1:1000 dilution in 1% BSA) was added for another 1 h in the dark. After three times washing with PBS, the green fluorescence signals were detected by fluorescence microscope (Leica, Germany).
3. Results
3.1. Bioinformatics analysis of GP5
The molecular characteristics of the GP5 were analyzed using DNAstar software. The signal peptide (residues 1–30) and three transmembrane regions (residues 62–83, 90–106, and 113–130) showed strong hydrophilicity. These regions had poor antigenic index and surface probability inferred from the predicted plot (Fig. 1 ).
Fig. 1.
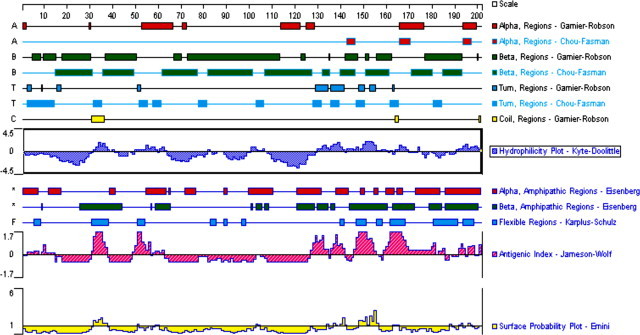
Molecular characteristics of GP5. The molecular characteristics of the GP5 were analyzed and indicated using DNAstar software (USA).
3.2. Construction of a recombinant plasmid bearing fused GP5 gene
The amplified PCR products were 121 bp and 248 bp, respectively. Both fragments were inserted into the multiple cloning sites of the expression vector using linker-based fusion gene cloning strategy. The DNA sequencing results indicated that the sequence of the insert was identical with template sequence.
3.3. Expression and purification of GP5
The expression of GP5 was induced by addition of IPTG to the medium. SDS-PAGE indicated that the expression of GP5 was detectable at 1 h post-induction, and the stable expression was persisted up to at least 7 h. Most of the protein was expressed in the inclusion body and its molecular weight was about 18 kDa as expected (Fig. 2 ).
Fig. 2.
SDS-PAGE analysis on the GP5. The bacteria bearing GP5 gene or empty vector were induced by IPTG and then the proteins were subjected to SDS-PAGE. Lane 1: Un-induced bacteria; Lane 2: Induced bacteria bearing the empty vector; Lanes 3–9: Bacteria bearing the GP5 gene induced at 1–7 h post-IPTG induction; Lane M: Protein molecular weight marker
3.4. Immunoblotting
To analyze the biological activity of the GP5, a porcine polyclonal antibody against PRRSV was used as a primary antibody in Western blot. The result showed that the bacterially expressed fused GP5 was able to react with the specific antibody, in contrast, the protein from bacteria bearing the empty vector did not react with the same antibody (Fig. 3 ).
Fig. 3.
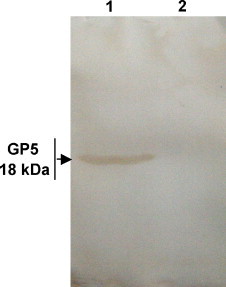
Immunoblotting. The bacteria bearing GP5 gene or empty vector were induced by IPTG and then the protein was subjected to Western blot analysis using the antibody against PRRSV. Lane 1: Bacteria bearing GP5 gene; Lane 2: Control bacteria bearing empty vector
3.5. Efficacy of antibody produced in this study
Anti-GP5 antibody was prepared by immunizing a rabbit with the GP5. Our result showed that the antibody titration was 1:99052. To further analyze the utility of the antibody, various viruses were used as coating antigens in indirect ELISA. The results indicated that the anti-GP5 antibody recognized PRRSV specifically, and no cross-reaction between the antibody and other viruses was detected (Fig. 4 ).
Fig. 4.
ELISA. The PRRSV, TGEV, PEDV, IBV and PRV were used as coating antigens. The purified GP5 and BSA were used as control antigens. Polyclonal antibody against the GP5 protein was used as the primary antibody in the indirect ELISA. The concentration of viruses and proteins was 20 μg/ml and 100 μg/ml, respectively. Number 1–6 in the horizontal axis is PRRSV, TGEV, PEDV, IBV, PRV, GP5 and BSA, respectively. The vertical axis is the OD490 value.
3.6. Inhibitory effect of GP5 and its specific antibody on infection in vitro
The effect of GP5 and its specific antibody on Marc-145 cell infection by PRRSV was analyzed. The results indicated that both proteins inhibited cell infection efficiently. At a concentration of 60 μg, the GP5 blocked PRRSV infection completely (Fig. 5A). When the anti-GP5 antibody was diluted at a proportion of 1:32 in medium, the virus infection was totally inhibited, in contrast, the serum isolated from unvaccinated rabbit had no inhibitory effect (Fig. 5B). The inhibitory effect of the proteins to PRRSV infection was in a dose-dependent fashion.
Fig. 5.
Inhibitory effect of GP5 and anti-GP5 serum on PRRSV infection invitro. Marc-145 cells were pre-incubated serially diluted GP5 protein (5−1 dilution is equal to 12 μg protein) and then the cells were infected with PRRSV. The inhibition rate of the protein to virus infection is shown in Panel A. In parallel, PRRSV was pre-treated with serially diluted anti-GP5 antibody, and then the viruses were used to infect Marc-145 cells. The inhibition rate of the antibody to virus infection is shown in Panel B. Values along the horizontal axis indicate protein or antibody dilution and the infection inhibition rates are shown in vertical axis.
3.7. Detection of virus-infected cells
The polyclonal antibody produced in this study was used as a primary antibody to detect the virus-infection cells. Immunofluorescence results showed that the green fluorescence signals were detectable on the surface of virus-infected cells, in contrast, there was no positive signal on the mock infection cells (Fig. 6 ).
Fig. 6.
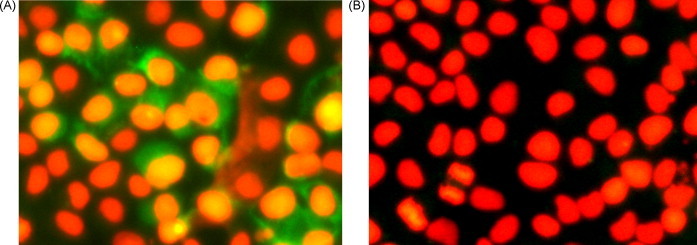
Immunofluorescence analysis. The polyclonal antibody against the GP5 was used as primary antibody to detect PRRSV-infected Marc-145 cells. Panel A: Virus-infected cells; Panel B: Mock-infected cells. The nuclei were stained with propidium iodide.
4. Discussion
Nowadays, PRRSV infection has been recognized as one severe threatening to the pig industry. It has been indicated that serum neutralization of PRRSV correlates with antibody response to the GP5 (Gonin et al., 1999). The production of anti-GP5 antibody is associated with disappearance of viremia in host and the viral glycoprotein can induce potent humoral immune response (Plagemann, 2004). In several previous reports, PRRSV GP5 has been expressed in mammalian cells, recombinant viruses and host animals (Lee et al., 2004, Yun et al., 2009, Pirzadeh and Dea, 1998). It is documented that adenoviral-expressed PRRSV GP5 can maintain its known biological functions, but its cellular maturation differs from that of the authentic viral protein (Gagnon et al., 2003). Therefore, selection of gene expression systems for functional analysis of GP5 protein still needs to be investigated. Nevertheless, as far as the high-level expression is concerned, the E. coli-based prokaryotic expression system is a powerful host cell system for expressing heterologous genes (Yin et al., 2007).
Many glycoproteins from cells or viruses produced in this system have shown certain biological activities (Liu et al., 2009, Gomaa et al., 2009, Schwanke et al., 2009, Li et al., 2010). In this study, a N-terminal signal peptide (31 amino acids in length) was identified in the protein using bioinformatics analysis. The hydrophobic peptide putatively influences the protein expression in prokaryotic expression system. Therefore, we tried to express a truncated GP5 gene without signal peptide sequence in E. coli, however, the protein of interest was not achieved at various inductive expression conditions (data not shown). We supposed that the predicted transmembrane regions (residues 60–130) might have impacted the expression of GP5. Using site-directed PCR, two fragments were amplified and inserted into the same expression vector. Both fragments were ligated with a linker sequence in order to maintain the spatial conformation of GP5. Our results indicated that high-level and stable expression of the fused GP5 was achieved at 1 h post-induction.
The fused GP5 can be purified via a Ni-NTA affinity column, because it contains a Histidine (His)-tag. Nevertheless, the harvested amount of purified protein was very limited using affinity purification (data not shown). Therefore, the GP5 was purified using gel-purification (Liu et al., 2009). Our results showed that gel-purified protein was able to react with anti-PRRSV antibody, indicating that the protein is biological activity. The titration of anti-GP5 antibody isolated from the GP5-immunized rabbit reached 1:99052, confirming the biological activity of the bacterially expressed GP5. When we used PRRSV as the coating antigen in ELISA, the antibody was able to differentiate between the virus and controls, indicating that the antibody is a feasible diagnostic reagent. Moreover, the GP5 and its antibody had an inhibitory effect on PRRSV infection in vitro, confirming that both are useful and active.
To further evaluate the utility of the antibody produced in this study, the antibody was used to detect the virus-infected cells. Immunofluorescence results showed that the PRRSV was detectable using the anti-GP5 antibody and the positive signal distributed on the cell membrane predominantly. Most importantly, the antibody can discriminate PRRSV from other viruses efficiently. Because the concentration of the GP5 protein used in ELISA was higher that that of coating viruses, the binding value between PRRSV and the antibody was lower than that between GP5 and the antibody. To our knowledge, this is the first example of successful expression of a functional PRRSV GP5 in prokaryotic system. The bacterially expressed GP5 has excellent biological activity. Expression of GP5 and achievement of anti-GP5 antibody provide important materials for further studies on PRRSV biology. They are useful diagnostic reagents in clinic surveillance for PRRS. Moreover, the designing for expressing fusion genes used in this study is an attractive strategy for other transmembrane protein.
Acknowledgements
We acknowledge Prof. Ulrich Neumann of Clinic for Poultry, University of Veterinary Medicine Hannover, Germany for providing the Ni-NTA affinity column system. The research work of the authors was supported by funds from National Natural Science Foundation of China (30700590; 30700591; 30972195), Heilongjiang Provincial Science and Technology Department (ZJN0702-01; QC07C32), China.
References
- Benfield D.A., Nelson E., Collins J.E., Harris L., Goyal S.M., Robison D., Christianson W.T., Morrison R.B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J. Vet. Diagn. Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Collins J.E., Benfield D.A., Christianson W.T., Harris L., Hennings J.C., Shaw D.P., Goyal S.M., McCullough S., Morrison R.B., Joo H.S. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- Delputte P.L., Vanderheijden N., Nauwynck H.J., Pensaert M.B. Involvement of the matrix protein in attachment of porcine reproductive and respiratory syndrome virus to a heparinlike receptor on porcine alveolar macrophages. J. Virol. 2002;76:4312–4320. doi: 10.1128/JVI.76.9.4312-4320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faaberg K.S., Hocker J.D., Erdman M.M., Harris D.L., Nelson E.A., Torremorell M., Plagemann P.G. Neutralizing antibody responses of pigs infected with natural GP5 N-glycan mutants of porcine reproductive and respiratory syndrome virus. Viral. Immunol. 2006;19:294–304. doi: 10.1089/vim.2006.19.294. [DOI] [PubMed] [Google Scholar]
- Gagnon C.A., Lachapelle G., Langelier Y., Massie B., Dea S. Adenoviral-expressed GP5 of porcine respiratory and reproductive syndrome virus differs in its cellular maturation from the authentic viral protein but maintains known biological functions. Arch. Virol. 2003;148:951–972. doi: 10.1007/s00705-002-0943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa M.H., Yoo D., Ojkic D., Barta J.R. Use of recombinant S1 spike polypeptide to develop a TCoV-specific antibody ELISA. Vet. Microbiol. 2009;138:281–288. doi: 10.1016/j.vetmic.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonin P., Pirzadeh B., Gagnon C.A., Dea S. Seroneutralization of porcine reproductive and respiratory syndrome virus correlates with antibody response to the GP5 major envelope glycoprotein. J. Vet. Diagn. Invest. 1999;11:20–26. doi: 10.1177/104063879901100103. [DOI] [PubMed] [Google Scholar]
- Jiang W., Jiang P., Wang X., Li Y., Wang X., Du Y. Influence of porcine reproductive and respiratory syndrome virus GP5 glycoprotein N-linked glycans on immune responses in mice. Virus Genes. 2007;35:663–671. doi: 10.1007/s11262-007-0131-y. [DOI] [PubMed] [Google Scholar]
- Kim W.I., Yoon K.J. Molecular assessment of the role of envelope-associated structural proteins in cross neutralization among different PRRS viruses. Virus Genes. 2008;37:380–391. doi: 10.1007/s11262-008-0278-1. [DOI] [PubMed] [Google Scholar]
- Lee C., Rogan D., Erickson L., Zhang J., Yoo D. Characterization of the porcine reproductive and respiratory syndrome virus glycoprotein 5 (GP5) in stably expressing cells. Virus Res. 2004;104:33–38. doi: 10.1016/j.virusres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Li G., Zeng Y., Yin J., Lillehoj H.S., Ren X. Cloning, prokaryotic expression, and biological analysis of recombinant chicken IFN-gamma. Hybridoma. 2010;29:1–6. doi: 10.1089/hyb.2009.0053. [DOI] [PubMed] [Google Scholar]
- Li J., Yin J., Sui X., Li G., Ren X. Comparative analysis of the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro. Avian Pathol. 2009;38:215–221. doi: 10.1080/03079450902912184. [DOI] [PubMed] [Google Scholar]
- Liu B., Li G., Sui X., Yin J., Wang H., Ren X. Expression and functional analysis of porcine aminopeptidase N produced in prokaryotic expression system. J. Biotechnol. 2009;141:91–96. doi: 10.1016/j.jbiotec.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J., Hulst M.M., de Meijer E.J., Moonen P.L., den Besten A., de Kluyver E.P., Wensvoort G., Moormann R.J. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.A., Christopher-Hennings J., Drew T., Wensvoort G., Collins J.E., Benfield D.A. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J. Clin. Microbiol. 1993;31:3184–3189. doi: 10.1128/jcm.31.12.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirzadeh B., Dea S. Immune response in pigs vaccinated with plasmid DNA encoding ORF5 of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1998;79:989–999. doi: 10.1099/0022-1317-79-5-989. [DOI] [PubMed] [Google Scholar]
- Plagemann P.G. GP5 ectodomain epitope of porcine reproductive and respiratory syndrome virus, strain Lelystad virus. Virus Res. 2004;102:225–230. doi: 10.1016/j.virusres.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Plana Duran J., Climent I., Sarraseca J., Urniza A., Cortés E., Vela C., Casal J.I. Baculovirus expression of proteins of porcine reproductive and respiratory syndrome virus strain Olot/91. Involvement of ORF3 and ORF5 proteins in protection. Virus Genes. 1997;14:19–29. doi: 10.1023/a:1007931322271. [DOI] [PubMed] [Google Scholar]
- Ren X., Glende J., Al-Falah M., de Vries V., Schwegmann-Wessels C., Qu X., Tan L., Tschernig T., Deng H., Naim H.Y., Herrler G. Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J. Gen. Virol. 2006;87:1691–1695. doi: 10.1099/vir.0.81749-0. [DOI] [PubMed] [Google Scholar]
- Schwanke R.C., Renard G., Chies J.M., Campos M.M., Junior E.L., Santos D.S., Basso L.A. Molecular cloning, expression in Escherichia coli and production of bioactive homogeneous recombinant human granulocyte and macrophage colony stimulating factor. Int. J. Biol. Macromol. 2009;45:97–102. doi: 10.1016/j.ijbiomac.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Schwegmann-Wessels C., Glende J., Ren X., Qu X., Deng H., Enjuanes L., Herrler G. Comparison of vesicular stomatitis virus pseudotyped with the S proteins from a porcine and a human coronavirus. J. Gen. Virol. 2009;90:1724–1729. doi: 10.1099/vir.0.009704-0. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., Meulenberg J.J. The molecular biology of arteriviruses. J. Gen. Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- Stadejek T., Stankevicius A., Storgaard T., Oleksiewicz M.B., Belák S., Drew T.W., Pejsak Z. Identification of radically different variants of porcine reproductive and respiratory syndrome virus in Eastern Europe: towards a common ancestor for European and American viruses. J. Gen. Virol. 2002;83:1861–1873. doi: 10.1099/0022-1317-83-8-1861. [DOI] [PubMed] [Google Scholar]
- Sui X., Yin J., Ren X. Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res. 2010;85:346–353. doi: 10.1016/j.antiviral.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Li G., Yin J., Ren X. Phylogenetic characterization of genes encoding for glycoprotein 5 and membrane protein of PRRSV isolate HH08. J. Vet. Sci. 2009;10:309–315. doi: 10.4142/jvs.2009.10.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensvoort G., de Kluyver E.P., Luijtze E.A., den Besten A., Harris L., Collins J.E., Christianson W.T., Chladek D. Antigenic comparison of Lelystad virus and swine infertility and respiratory syndrome (SIRS) virus. J. Vet. Diagn. Invest. 1992;4:134–138. doi: 10.1177/104063879200400203. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Pol J.M., ter Laak E.A., Bloemraad M., de Kluyver E.P., Kragten C., van Buiten L., den Besten A., Wagenaar F. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Wootton S.K., Nelson E.A., Yoo D. Antigenic structure of the nucleocapsid protein of porcine reproductive and respiratory syndrome virus. Clin. Diagn. Lab. Immunol. 1998;5:773–779. doi: 10.1128/cdli.5.6.773-779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Li G., Ren X., Herrler G. Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J. Biotechnol. 2007;127:335–347. doi: 10.1016/j.jbiotec.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Yun T., Ni Z., Yu B., Chen L., Hua J., Wang G., Liu G. Construction and immunogenicity of recombinant adenovirus co-expressing the GP5 and M protein of porcine reproduction and respiratory syndrome virus in mice. Sheng Wu Gong Cheng Xue Bao. 2009;25:488–495. [PubMed] [Google Scholar]


