Abstract
In 2019, a new coronavirus (2019-nCoV) infecting Humans has emerged in Wuhan, China. Its genome has been sequenced and the genomic information promptly released. Despite a high similarity with the genome sequence of SARS-CoV and SARS-like CoVs, we identified a peculiar furin-like cleavage site in the Spike protein of the 2019-nCoV, lacking in the other SARS-like CoVs. In this article, we discuss the possible functional consequences of this cleavage site in the viral cycle, pathogenicity and its potential implication in the development of antivirals.
Keywords: 2019-nCoV, SARS-CoV, Spike protein, Maturation protease, Furin, Antivirals
Highlights
-
•
The genomic sequence of 2019-nCoV indicates that the virus clusters with betacoronaviruses of lineage b.
-
•
2019-nCoV S-protein sequence has a specific furin-like cleavage site absent in lineage b CoV including SARS-CoV sequences.
-
•
The furin-like cleavage site in the S-protein of 2019-nCoV may have implications for the viral life cycle and pathogenicity.
-
•
Campaigns to develop anti-2019-nCoV therapeutics should include the evaluation of furin inhibitors.
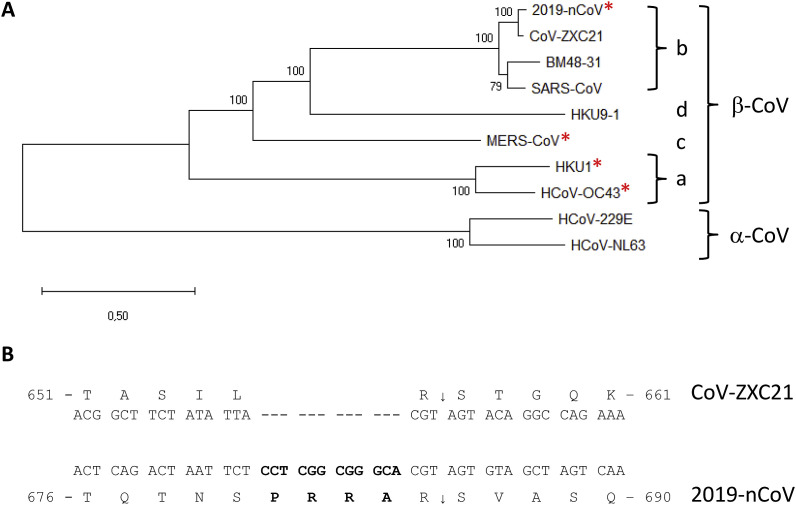
Human coronaviruses (CoV) are enveloped positive-stranded RNA viruses belonging to the order Nidovirales, and are mostly responsible for upper respiratory and digestive tract infections. Among them SARS-CoV and MERS-CoV that spread in 2002 and 2013 respectively, have been associated with severe human illnesses, such as severe pneumonia and bronchiolitis, and even meningitis in more vulnerable populations (de Wit et al., 2016). In December 2019, a new CoV (2019-nCoV) has been detected in the city of Wuhan, and this emerging viral infection was associated with severe human respiratory disease with a ~2–3% fatality rate (Li et al., 2020). The virus that was presumed to have initially been transmitted from an animal reservoir to humans possibly via an amplifying host. However human-to-human transmission has been reported, leading to a sustained epidemic spread with >31,000 confirmed human infections, including >640 deaths, reported by the WHO in early February 2020. The estimated effective reproductive number (R) value of ~2.90 (95%: 2.32–3.63) at the beginning of the outbreak raises the possibility of a pandemics (Zhao et al., 2020). This prompted WHO to declare it as a Public Health Emergency of International Concern. This is especially relevant because so far there are no specific antiviral treatments available or vaccine. Based on its genome sequence, 2019-nCoV belongs to lineage b of Betacoronavirus (Fig. 1 A), which also includes the SARS-CoV and bat CoV ZXC21, the latter and CoV ZC45 being the closest to 2019-nCoV. 2019-nCoV shares ~76% amino acid sequence identity in the Spike (S)-protein sequence with SARS-CoV and 80% with CoV ZXC21 (Chan et al., 2020). In this article, we focus on a specific furin-like protease recognition pattern present in the vicinity of one of the maturation sites of the S protein (Fig. 1B) that may have significant functional implications for virus entry.
Fig. 1.
Characterization of an nCoV-peculiar sequence at the S1/S2 cleavage site in the S-protein sequence, compared SARS-like CoV. (A) Phylogenetic tree of selected coronaviruses from genera alphacoronavirus (α-Cov) and betacoronavirus (β-CoV), lineages a, b, c and d: 2019-nCoV (NC_045512.2), CoV-ZXC21 (MG772934), SARS-CoV (NC_004718.3), SARS-like BM4821 (MG772934), HCoV-OC43 (AY391777), HKU9-1 (EF065513), HCoV-NL63 (KF530114.1), HCoV229E (KF514433.1), MERS-CoV (NC019843.3), HKU1 (NC_006577.2). The phylogenetic tree was obtained on the Orf1ab amino acid sequence using the Maximum Likelihood method by Mega X software. Red asterisks indicate the presence of a canonical furin-like cleavage motif at site 1; (B) Alignment of the coding and amino acid sequences of the S-protein from CoV-ZXC21 and 2019-nCoV at the S1/S2 site. The 2019-nCoV-specific sequence is in bold. The sequence of CoV-ZXC21 S-protein at this position is representative of the sequence of the other betacoronaviruses belonging to lineage b, except the one of 2019-nCoV. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
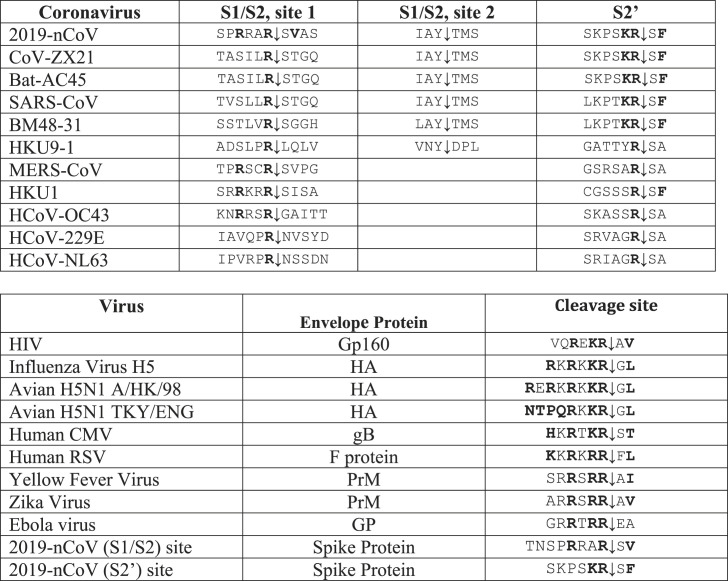
The proprotein convertases (PCs; genes PCSKs) constitute a family of nine serine secretory proteases that regulate various biological processes in both healthy and disease states (Seidah and Prat, 2012). By proteolysis, PCs are responsible for the activation of a wide variety of precursor proteins, such as growth factors, hormones, receptors and adhesion molecules, as well as cell surface glycoproteins of infectious viruses (Seidah and Chretien, 1999) (Table 1 ). Seven PCs cleave precursor proteins at specific single or paired basic amino acids (aa) within the motif (R/K)-(2X)n-(R/K)↓, where n = 0, 1, 2, or 3 spacer aa (Seidah and Chretien, 1999). Because of their role in the processing of many critical cell surface proteins PCs, especially furin, have been implicated in viral infections. They have the potential to cleave specifically viral envelope glycoproteins, thereby enhancing viral fusion with host cell membranes (Izaguirre, 2019; Moulard and Decroly, 2000). In the case of human-infecting coronaviruses such as HCoV-OC43 (Le Coupanec et al., 2015), MERS-CoV (Millet and Whittaker, 2014), and HKU1 (Chan et al., 2008) the spike protein has been demonstrated to be cleaved at an S1/S2 cleavage site (Fig. 2 ) generating the S1 and S2 subunits. The above three viruses display the canonical (R/K)-(2X)n-(R/K)↓ motif (Table 1). Additionally, it has been demonstrated that variation around the viral envelope glycoprotein cleavage site plays a role in cellular tropism and pathogenesis. For instance, the pathogenesis of some CoV has been previously related to the presence of a furin-like cleavage site in the S-protein sequence. For example, the insertion of a similar cleavage site in the infectious bronchitis virus (IBV) S-protein results in higher pathogenicity, pronounced neural symptoms and neurotropism in infected chickens (Cheng et al., 2019).
Table 1.
Comparative sequences of envelope protein cleavage site(s) in coronaviruses (above) and in other RNA viruses (below). Empty boxes: no consensus motif detected..
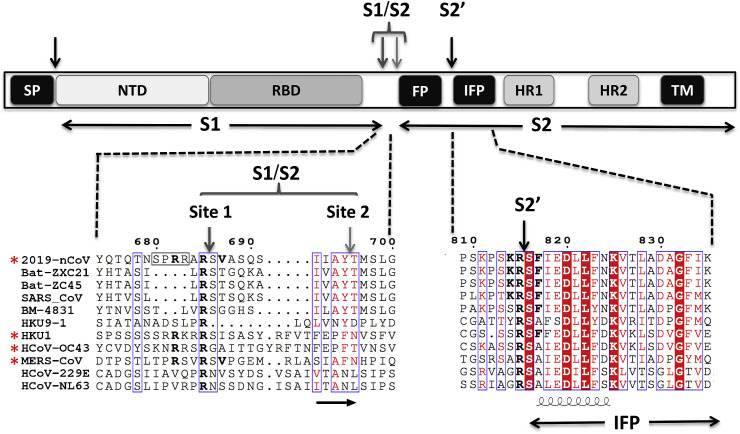
Fig. 2.
Schematic representation of the human 2019-nCoV S-protein with a focus on the putative maturation sites. The domains were previously characterized in SARS-CoV and MERS-CoV: Signal peptide (SP), N-terminal domain (NTD), receptor-binding domain (RBD), fusion peptide (FP), internal fusion peptide (IFP), heptad repeat 1/2 (HR1/2), and the transmembrane domain (TM). The SP, S1↓S2 and S2′ cleavage sites are indicated by arrows. The sequence of different CoV S1/S2 and S2′ cleavage sites were aligned using Multalin webserver (http://multalin.toulouse.inra.fr/multalin/) with manual adjustments and the figure prepared using ESPript 3 (http://espript.ibcp.fr/ESPript/ESPript/) presenting the secondary structure of SARS-CoV S-protein at the bottom of the alignment (PDB 5X58) (Yuan et al., 2017). Insertion of furin like cleavage site is surrounded by a black frame. Red asterisks indicate the presence of a canonical furin-like cleavage motif at the S1/S2 site. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Similarly, in the case of influenza virus, low-pathogenicity forms of influenza virus contain a single basic residue at the cleavage site, which is cleaved by trypsin-like proteases and the tissue distribution of the activating protease(s) typically restricts infections to the respiratory and/or intestinal organs (Sun et al., 2010). Conversely, the highly pathogenic forms of influenza have a furin-like cleavage site cleaved by different cellular proteases, including furin, which are expressed in a wide variety of cell types allowing a widening of the cell tropism of the virus (Kido et al., 2012). Furthermore the insertion of a multibasic motif RERRRKKR↓GL at the H5N1 hemagglutinin HA cleavage site was likely associated with the hyper-virulence of the virus during the Hong Kong 1997 outbreak (Claas et al., 1998). This motif exhibits the critical Arg at P1 and basic residues at P2 and P4, as well as P6 and P8 and an aliphatic Leu at P2’ positions (Table 1) (Schechter and Berger nomenclature (Schechter and Berger, 1968)), typical of a furin-like cleavage specificity (Braun and Sauter, 2019; Izaguirre, 2019; Seidah and Prat, 2012).
The coronavirus S-protein is the structural protein responsible for the crown-like shape of the CoV viral particles, from which the original name “coronavirus” was coined. The ~1200 aa long S-protein belongs to class-I viral fusion proteins and contributes to the cell receptor binding, tissue tropism and pathogenesis (Lu et al., 2015; Millet and Whittaker, 2014). It contains several conserved domains and motifs (Fig. 2). The trimetric S-protein is processed at the S1/S2 cleavage site by host cell proteases, during infection. Following cleavage, also known as priming, the protein is divided into an N-terminal S1-ectodomain that recognises a cognate cell surface receptor and a C-terminal S2-membrane-anchored protein involved in viral entry. The SARS-CoV S1-protein contains a conserved Receptor Binding Domain (RBD), which recognises the angiotensin-converting enzyme 2 (ACE2) (Li et al., 2003). The SARS-CoV binds to both bat and human cells, and the virus can infect both organisms (Ge et al., 2013; Kuhn et al., 2004). The RBD surface of S1/ACE2 implicates 14 aa in the S1 of SARS-CoV (Li et al., 2005). Among them, 8 residues are strictly conserved in 2019-nCoV, supporting the hypothesis that ACE2 is also the receptor of the newly emerged nCoV (Wan et al., 2020). The S2-protein contains the fusion peptide (FP), a second proteolytic site (S2′), followed by an internal fusion peptide (IFP) and two heptad-repeat domains preceding the transmembrane domain (TM) (Fig. 2). Notably, the IFPs of the 2019-nCoV and SARS-CoV are identical, displaying characteristics of viral fusion peptides (Fig. 2). While the molecular mechanism involved in cell entry is not yet fully understood, it is likely that both FP and IFP participate in the viral entry process (Lu et al., 2015) and thus the S-protein must likely be cleaved at both S1/S2 and S2′ cleavage sites for virus entry. The furin-like S2′ cleavage site at KR↓SF with P1 and P2 basic residues and a P2′ hydrophobic Phe (Seidah and Prat, 2012), downstream of the IFP is identical between the 2019-nCoV and SARS-CoV (Fig. 2). In the MERS-CoV and HCoV-OC43 the S1/S2 site is replaced by RXXR↓SA, with P1 and P4 basic residues, and an Ala (not aliphatic) at P2′, suggesting a somewhat less favourable cleavage by furin. However, in the other less pathogenic circulating human CoV, the S2′ cleavage site only exhibits a monobasic R↓S sequence (Fig. 2) with no basic residues at either P2 and/or P4 needed to allow furin cleavage, suggesting a less efficient cleavage or higher restriction at the entry step depending on the cognate proteases expressed by target cells. Even though processing at S2′ in 2019-nCoV is expected to be a key event for the final activation of the S-protein, the protease(s) involved in this process have not yet been conclusively identified. Based on the 2019-nCoV S2′ sequence and the above arguments, we propose that one or more furin-like enzymes would cleave the S2′ site at KR↓SF. In contrast to the S2′, the first cleavage between the RBD and the FP (S1/S2 cleavage site, Fig. 2) has been extensively studied for many CoVs (Lu et al., 2015). Interestingly the S1/S2 processing site exhibits different motifs among coronaviruses (Fig. 2, site 1 & site 2), with many of them displaying cleavage after a basic residue. It is thus likely that the priming process is ensured by different host cell proteases depending on the sequence of the S1/S2 cleavage site. Accordingly the MERS-CoV S-protein, which contains a RSVR↓SV motif is cleaved during virus egress, probably by furin (Mille and Whittaker, 2014). Conversely the S-protein of SARS-CoV remains largely uncleaved after biosynthesis, possibly due to the lack of a favourable furin-like cleavage site (SLLR-ST). In this case, it was reported that following receptor binding the S-protein is cleaved at a conserved sequence AYT↓M (located 10 aa downstream of SLLR-ST) by target cells’ proteases such as elastase, cathepsin L or TMPRSS2 (Bosch et al., 2008; Matsuyama et al., 2010, 2005; Millet and Whittaker, 2015). As the priming event is essential for virus entry, the efficacy and extent of this activation step by the proteases of the target cells should regulate cellular tropism and viral pathogenesis. In the case of the 2019-nCoV S-protein, the conserved site 2 sequence AYT↓M may still be cleaved, possibly after the preferred furin-cleavage at the site 1 (Fig. 2).
Since furin is highly expressed in lungs, an enveloped virus that infects the respiratory tract may successfully exploit this convertase to activate its surface glycoprotein (Bassi et al., 2017; Mbikay et al., 1997). Before the emergence of the 2019-nCoV, this important feature was not observed in the lineage b of betacoronaviruses. However, it is shared by other CoV (HCoV-OC43, MERS-CoV, MHV-A59) harbouring furin-like cleavage sites in their S-protein (Fig. 2; Table 1), which were shown to be processed by furin experimentally (Le Coupanec et al., 2015; Mille and Whittaker, 2014). Strikingly, the 2019-nCoV S-protein sequence contains 12 additional nucleotides upstream of the single Arg↓ cleavage site 1 (Fig. 1, Fig. 2) leading to a predictively solvent-exposed PRRAR↓SV sequence, which corresponds to a canonical furin-like cleavage site (Braun and Sauter, 2019; Izaguirre, 2019; Seidah and Prat, 2012). This furin-like cleavage site, is supposed to be cleaved during virus egress (Mille and Whittaker, 2014) for S-protein “priming” and may provide a gain-of-function to the 2019-nCoV for efficient spreading in the human population compared to other lineage b betacoronaviruses. This possibly illustrates a convergent evolution pathway between unrelated CoVs. Interestingly, if this site is not processed, the S-protein is expected to be cleaved at site 2 during virus endocytosis, as observed for the SARS-CoV.
Obviously much more work is needed to demonstrate experimentally our assertion, but the inhibition of such processing enzyme(s) may represent a potential antiviral strategy. Indeed, it was recently shown that in an effort to limit viral infections, host cells that are infected by a number of viruses provoke an interferon response to inhibit the enzymatic activity of furin-like enzymes. It was also demonstrated that HIV infection induces the expression of either the protease activated receptor 1 (PAR1) (Kim et al., 2015) or guanylate binding proteins 2 and 5 (GBP2,5) (Braun and Sauter, 2019) that restrict the trafficking of furin to the trans Golgi network (PAR1) or to early Golgi compartments (GBP2,5) where the proprotein convertase remains inactive. Altogether, these observations suggest that inhibitors of furin-like enzymes may contribute to inhibiting virus propagation.
A variety of approaches have been proposed to inhibit furin activity to limit tumour growth, viral and bacterial infection. Thus, a variant of the naturally occurring serine protease inhibitor α-1 antitrypsin harbouring a consensus furin cleavage, called α-1 antitrypsin Portland (α1-PDX), inhibits furin and prevents the processing of HIV-1 Env (Anderson et al., 1993). The addition of a chloromethylketone (CMK) moiety to the C-terminus of a polybasic cleavage motif and a decanoyl group at the N-terminus to favour cell penetration (dec-RVKR-cmk) irreversibly blocked the enzymatic activity of furin, PC7, PC5, PACE4 and PC7 (Decroly et al., 1996, Garten et al., 1994). Finally, the elucidation of the crystal structure of furin resulted in the design of a 2,5-dideoxystreptamine-derived inhibitor, where two molecules of the inhibitor form a complex with furin (Dahms et al., 2017). As furin-like enzymes are involved in a multitude of cellular processes, one important issue would be to avoid systemic inhibition that may result in some toxicity. Accordingly, it is likely that such small molecule inhibitors, or other more potent orally active ones, possibly delivered by inhalation and exhibiting a slow dissociation rate from furin to allow for sustained inhibition, deserve to be rapidly tested to assess their antiviral effect against 2019-nCoV.
Acknowledgments
This work was supported by a CIHR Foundation grant # 148363 (NGS), a Canada Research Chairs in Precursor Proteolysis (NGS; # 950-231335), and by the European Virus Archive Global (BCo; EVA GLOBAL) funded by the European Union's Horizon 2020 research and innovation programme under grant agreement No 871029.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2020.104742.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Anderson E.D., Thomas L., Hayflick J.S., Thomas G. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed α1-antitrypsin variant. J. Biol. Chem. 1993;268:24887–24891. [PubMed] [Google Scholar]
- Bassi D.E., Zhang J., Renner C., Klein-Szanto A.J. Targeting proprotein convertases in furin-rich lung cancer cells results in decreased in vitro and in vivo growth. Mol. Carcinog. 2017;56:1182–1188. doi: 10.1002/mc.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., Bartelink W., Rottier P.J.M. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008;82:8887–8890. doi: 10.1128/jvi.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun E., Sauter D. Furin-mediated protein processing in infectious diseases and cancer. Clin. Transl. Immunol. 2019;8:e1073. doi: 10.1002/cti2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.M., Woo P.C., Lau S.K., Tse H., Chen H.L., Li F., Zheng B.J., Chen L., Huang J.D., Yuen K.Y. Spike protein, S, of human coronavirus HKU1: role in viral life cycle and application in antibody detection. Exp. Biol. Med. 2008;233:1527–1536. doi: 10.3181/0806-RM-197. [DOI] [PubMed] [Google Scholar]
- Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microb. Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Zhao Y., Xu G., Zhang K., Jia W., Sun Y., Zhao J., Xue J., Hu Y., Zhang G. The S2 subunit of QX-type infectious bronchitis coronavirus spike protein is an essential determinant of neurotropism. Viruses 11. 2019 doi: 10.3390/v11100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas E.C., Osterhaus A.D., Van Beek R., De Jong J.C., Rimmelzwaan G.F., Senne D.A., Krauss S., Shortridge K.F., Webster R.G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- Dahms S.O., Jiao G.-S., Than M.E. Structural studies revealed active site distortions of human furin by a small molecule inhibitor. ACS Chem. Biol. 2017;12:2474. doi: 10.1021/acschembio.7b00633. [DOI] [PubMed] [Google Scholar]
- de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Publ. Gr. 2016 doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroly E., Wouters S., Di Bello C., Lazure C., Ruysschaert J.-M., Seidah N.G. Identification of the Paired Basic Convertases Implicated in HIV gp160 Processing Based on in Vitro Assays and Expression in CD4+ Cell Lines. J. Biol. Chem. 1996;271:30442–30450. doi: 10.1074/jbc.271.48.30442. [DOI] [PubMed] [Google Scholar]
- Garten W., Hallenberger S., Ortmann D., Schäfer W., Vey M., Angliker H., Shaw E., Klenk H.D. Processing of viral glycoproteins by the subtilisin-like endoprotease furin and its inhibition by specific peptidylchloroalkylketones. Biochimie. 1994;76:217–225. doi: 10.1016/0300-9084(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Ge X.-Y., Li J.-L., Yang X.-L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., Zhang Y.-J., Luo C.-M., Tan B., Wang N., Zhu Y., Crameri G., Zhang S.-Y., Wang L.-F., Daszak P., Shi Z.-L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre G. The proteolytic regulation of virus cell entry by furin and other proprotein convertases. Viruses 11. 2019 doi: 10.3390/v11090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido H., Okumura Y., Takahashi E., Pan H.Y., Wang S., Yao D., Yao M., Chida J., Yano M. Role of host cellular proteases in the pathogenesis of influenza and influenza-induced multiple organ failure. Biochim. Biophys. Acta Protein Proteonomics. 2012 doi: 10.1016/j.bbapap.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Kim W., Zekas E., Lodge R., Susan-Resiga D., Marcinkiewicz E., Essalmani R., Mihara K., Ramachandran R., Asahchop E., Gelman B., Cohen É.A., Power C., Hollenberg M.D., Seidah N.G. Neuroinflammation-Induced interactions between protease-activated receptor 1 and proprotein convertases in HIV-associated neurocognitive disorder. Mol. Cell Biol. 2015;35:3684–3700. doi: 10.1128/mcb.00764-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J.H., Li W., Choe H., Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell. Mol. Life Sci. 2004;61:2738–2743. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Coupanec A., Desforges M., Meessen-Pinard M., Dubé M., Day R., Seidah N.G., Talbot P.J. Cleavage of a neuroinvasive human respiratory virus spike glycoprotein by proprotein convertases modulates neurovirulence and virus spread within the central nervous system. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Li M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T.K., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. NEJMoa2001316. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasllieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greeneugh T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Wang Q., Gao G.F. Bat-to-human: spike features determining “host jump” of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015 doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Ujike M., Morikawa S., Tashiro M., Taguchi F. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12543–12547. doi: 10.1073/pnas.0503203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbikay M., Sirois F., Yao J., Seidah N.G., Chrétien M. Comparative analysis of expression of the proprotein convertases furin, PACE4, PC1 and PC2 in human lung tumours. Br. J. Canc. 1997;75:1509–1514. doi: 10.1038/bjc.1997.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mille J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. U.S.A. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. U.S.A. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulard M., Decroly E. Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochim. Biophys. Acta Rev. Biomembr. 2000 doi: 10.1016/S0304-4157(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the active site of proteases. 3. Mapping the active site of papain; specific peptide inhibitors of papain. Biochem. Biophys. Res. Commun. 1968;32:898–902. doi: 10.1016/0006-291x(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Seidah N.G., Chretien M. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999;848:45–62. doi: 10.1016/S0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- Seidah N.G., Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012 doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- Sun X., Tse L.V., Ferguson A.D., Whittaker G.R. Modifications to the hemagglutinin cleavage site control the virulence of a neurotropic H1N1 influenza virus. J. Virol. 2010;84:8683–8690. doi: 10.1128/JVI.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J. Virol. 2020 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Cao D., Zhang Y., Ma J., Qi J., Wang Q., Lu G., Wu Y., Yan J., Shi Y., Zhang X., Gao G.F. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 2017 doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Ran J., Musa S.S., Yang G., Wang W., Lou Y., Gao D., Yang L., He D., Wang M.H. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020:30053–30059. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.