Highlights
► Many traditional Chinese herbal medicines (TCHM) are used in China for the treatment of viral infections. ► These TCHM may contain drug-like molecules with antiviral activity. ► Novel antiviral compounds may potentially be identified in TCHM through activity-guided fractionation. ► Research on active molecules in TCHM is being aided by the development of large databases.
Abbreviations: TCHM, traditional Chinese herbal medicine; TCM, traditional Chinese medicine; HIV, human immunodeficiency virus; HSV, herpes simplex virus (type 1 and 2); Flu, influenza; HBV, hepatitis B virus; HCV, hepatitis C virus; HCMV, human cytomegalovirus; EVs, enteroviruses; EV71, enterovirus 71; SARS-CoV, SARS coronavirus; NV, norovirus; FMDV, foot-and-mouth disease virus; AdV, adenovirus; PIV, parainfluenza virus
Keywords: Traditional Chinese herbal medicine, Antiviral therapy, Antiviral drugs, Activity-guided fractionation
Abstract
Traditional Chinese herbal medicine (TCHM) is widely used in the prevention and treatment of viral infectious diseases. However, the operative mechanisms of TCHM remain largely obscure, mainly because of its complicated nature and the fragmented nature of research. In recent years, systematic methodologies have been developed to discover the active compounds in TCHM and to elucidate its underlying mechanisms. In this review, we summarize recent progress in TCHM-based antiviral research in China and other Asian countries. In particular, this review focuses on progress in targeting key steps in the viral replication cycle and key cellular components of the host defense system. Recent developments in centralized and standardized TCHM screening and databases are also summarized.
1. Introduction
Traditional Chinese herbal medicine (TCHM) is the most important component of the traditional Chinese medicine system, which has long been used for its multiple combinations of compounds in the form of processed natural products. Similar to conventional medicine, TCHMs are prescription or over-the-counter drugs. Today, TCHMs account for 10% of the prescription drugs in China.
Because of the long history of medical usage, from the drug discovery point of view, screening for active lead compounds from TCHMs extracts is considered more efficient compare to random screening from a standard combinatorial chemical library. More functional compounds (“hits”) are likely to be discovered from TCHM extracts in biological screening assays, and the chemical properties of these compounds are often more “drug-like” (e.g. with better pharmacokinetics and bioavailability). TCHM-derived active compounds are thus often better lead compounds for further chemical improvements. These characteristics of TCHMs offer major opportunities for finding novel chemical structures active against a variety of therapeutic targets.
However, even with these unique advantages, modernization and globalization of TCHM have been slow. Some of the most difficult issues have been understanding the operative mechanisms of TCHMs and identify their active components. This review summarizes recent progress and advantages of TCHM-based antiviral research in China. In particular, this paper follows the steps of the generalized virus life cycle and reports progress in assay development and in knowledge of the antiviral mechanisms of TCHMs or TCHM-derived compounds.
2. Evidence supporting the efficacy of TCHM
TCHMs are widely used for the prevention and treatment of viral infectious diseases in China and many other Asian countries. However, the international community remains uncertain about the efficacy of TCHMs, because of the lack of supporting clinical evidence collected under international standards (randomized, placebo-controlled, double-blind and multicentered clinical studies). Governments have put forward support aimed at international regulatory approval of TCHMs. Leading the pack is the compound T89 (also known as Dantonic®, a THCM product by Tasly Pharmaceuticals, China), which may become the first traditional Chinese medicine to receive Food and Drug Administration (FDA) approval in the United States. T89 is a TCHM used in China for the management of ischaemic heart disease. It is currently under a global phase III trial (ClinicalTrials.gov identifier: NCT01659580).
A growing number of TCHMs with antiviral activity is also garnering evidence of experimental and/or clinical efficacy. Table 1 shows a partial list of antiviral TCHMs approved by the China Food and Drug Administration (SFDA). TCHMs for respiratory viral infections represent the majority of drugs in the market.
Table 1.
Partial list of TCHM approved by the SFDA for the treatment of viral diseases.
| Herbs | Botanical names | Trade names | Virus | Diseases | References |
|---|---|---|---|---|---|
| Radix bupleuri | Bupleurum chinense, Bupleurum scorzonerifolium | Xiao-chai-hu capsule, Zheng-chai-hu-yin granule | Flu | Influenza, upper respiratory infection | Zhang et al., 2007, Zhao et al., 2007 |
| Fructus forsythiae | Forsythia suspensa | Yin-qiao-jie-du-wan (granule, tablet), Yin-qiao-san | Flu | Acute bronchitis, pneumonia, influenza | Li et al., 2008, Sun et al., 2006, Xie et al., 2006, Yang et al., 2005b |
| Flos lonicerae; Radix scutellariae | Lonicera japonica; Scutellaria baicalensis | Shuang-huang-lian-he-ji (granule, capsule, tablet), Yin-huang granule (tablet) | Flu, EVs, HSV, AdV, RSV, PIV | Influenza, tonsillitis, pharyngitis, upper respiratory infection, mumps, pneumonia | Chen et al., 2001, Chen et al., 2007, Shen et al., 2008, Sun et al., 2009, Wang et al., 2005, Wu et al., 2004, Wu et al., 2005 |
| Radix isatidis | Isatis tinctoria, Isatis indigotica, Baphicacanthus cusia | Ban-lan-gen granule, Li-zhu (Chuan-fang) kang-bing-du granule | Flu, HSV | Influenza, acute tonsillitis, mumps | Cao et al., 2006, Cao et al., 2007, Cao et al., 2010, Chen and Li, 2006, Fang et al., 2005, Hu and Zheng, 2003, Sun et al., 2010 |
| Panax ginseng; Radix ophiopogonis | Panax ginseng; Ophiopogon japonicus | Sheng-mai-yin (granule, capsule, injection) | EVs | Viral myocarditis | Zhang et al., 2005, Zhang and Zeng, 2009 |
| Radix sophorae Flavescentis | Sophora flavescens | Ku-shen tablet, Ku-shen-jian injection | HBV | Chronic hepatitis | Hou et al., 2005, Shi and Wang, 2012 |
| Spica prunellae; Flos chrysanthemi Indici; Folium mori | Prunella vulgaris; Chrysanthemum indicum, Chrysanthemum boreale, Chrysanthemum lavandulaefolium; Morus alba | Xia-sang-ju granule, Guang-yao-xing-qun-xia-sang-ju | Flu, RSV | Influenza | Huang et al., 2007, Zhan and Dong, 2006 |
3. Strategies for TCHM-based antiviral screening
The viral replication cycle includes attachment and entry into the host cell (Fig. 1 , 1–3), transcription of viral mRNA, viral genome replication (Fig. 1 and 4–6), protein synthesis and the assembly and budding of progeny virus particles (Fig. 1, 7 and 8). These steps provide targets for inhibitors of entry, replication (e.g., protease inhibitors, viral polymerase inhibitors, and integrase inhibitors, among others), assembly and budding. Such inhibitors are classified as direct antiviral agents. Previous studies have provided evidence of the direct antiviral activity of many medicinal herbs used in TCHMs (Sun, 2007, Wang et al., 2007, Wang et al., 2008, Zhao and Han, 2009).
Fig. 1.
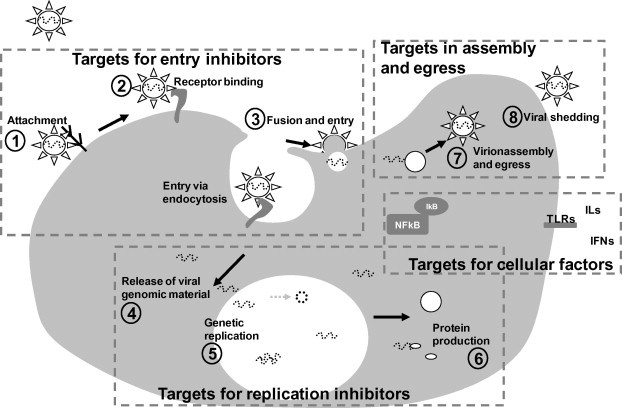
Major steps in the generalized viral life cycle. Potential targets for inhibitors of entry, replication, assembly and egress and cellular factors are indicated.
By definition, a virus depends on the cellular machinery to complete its replication cycle (e.g., cellular peptidase, transcription factors, and elongation factors). Following co-evolution with the host, many viruses have established sophisticated mechanisms to interact with the host immune system for immune evasion. These mechanisms provide cellular targets for antiviral drug intervention. Among the classes of antiviral agents, immunomodulators are the most abundant in TCHM.
Based on TCM theory, a remedy contains multiple active components (mainly herbs) with multiple targets. Some of these components work directly on the therapeutic targets, whereas others counteract drug toxicity or enhance the bioavailability of the medicine. Thus, a TCHM remedy is often composed of a hierarchy of different components, the so-called “monarch,” “minister,” “assistant,” and “guide components” (Yu et al., 2006). Considering the complicated nature of TCHM, experiments in laboratory animals have been considered the “gold standard” for pharmacological screening. The process is very important for medical evaluation, because it reflects the efficacy, side effects, and toxicity of medicines as a whole. In general, TCHM whole extracts are often tested first for their ability to protect animals against viral challenges (Fig. 2 ). However, such in vivo methods are costly and have low throughput. For TCHM testing, optimized cell-based assays are often carried out directly for the initial evaluation of whole extracts that show clinical evidence of antiviral activity. This practice is based on the assumption that compounds with direct antiviral activity are present in whole TCHM extracts. These compounds are measured by their ability to protect cells against virus-induced cytotoxicity (Fig. 2).
Fig. 2.
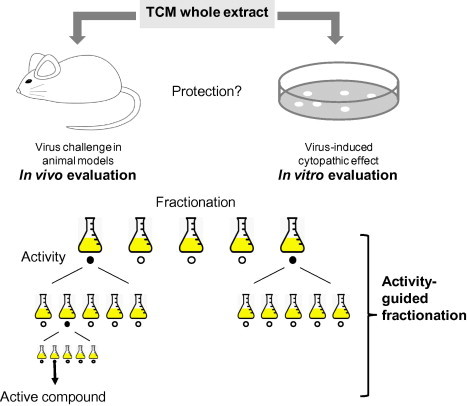
Schematic diagram of activity-guided fractionation. A TCHM whole extract is evaluated for its antiviral activity in laboratory animals and/or cell-based assays. To identify the active component, AGF is performed, and the fraction with antiviral activity is further fractionated until the active compound is identified.
Activity-guided fractionation (AGF) is often performed for subsequent identificaton of active fractions and further isolation of pure compounds (Koehn and Carter, 2005) (Fig. 2). The basic principle of AGF is that a TCHM fraction is further separated only when its antiviral activity is confirmed. In recent years, with improved understanding of viral replication mechanisms at the cellular and molecular level, highly specific assays with high-throughput capabilities have been developed (Fig. 3 ). These assays enhance the chances of success of AGF and provide data for understanding the mechanisms of action of the identified compounds.
Fig. 3.
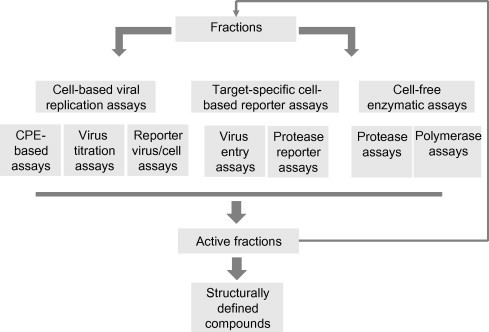
Target-specific assays used for active compound identification during AGF and for antiviral mechanism analysis.
In addition to classical bioscreening, computer-aided molecular design and docking-based virtual screening technologies are also being applied to the antiviral screening of TCHM. Progress in this area depends heavily on the availability of structural databases and bioinformatics. In the past, databases were scattered among individual laboratories, and included an insufficient number of compounds and limited associated information. However, several larger databases have recently been constructed. The TCM Database@Taiwan (http://tcm.cmu.edu.tw), built by a team led by Prof. Calvin Yu-Chian Chen from China Medical University in Taiwan contains the chemical structures of over 20,000 compounds (Chen, 2011). Using this database, the team has identified quinic acid, genipin, syringic acid, cucurbitine, fagarine, methyl isoferulate and their derivatives as potent anti-influenza compounds, through blocking of the viral M2 ion channel (Lin et al., 2011). Using the same approach, they also identified xynopine-2, rosmaricine-14 and rosmaricine-15 as strong antagonists of the binding of hemagglutinin subtype H1 to sialic acid (Chang et al., 2011b).
4. Viral entry inhibitors
Entry into host cells is the first step of the viral life cycle, and its machinery has been proven an excellent target for antiviral therapeutics. Advanced assays have been developed to identify compounds that inhibit this critical step of the viral life cycle (Peng, 2010). For many viruses, cell-surface attachment is accomplished through interaction with cell surface glycans. Polysaccharides have been observed to saturate the cell surface of viral attachment proteins and inhibit viral entry, as confirmed by antiviral TCM studies (Table 2 ).
Table 2.
TCHM-derived ompounds inhibiting viral entry.
| Virus | Herbs | Compounds | Mechanism | References |
|---|---|---|---|---|
| HSV | Radix achyranthis bidentatae | Polysaccharide sulfuric ester derivatives | Binds to viral glycoproteins and interferes with viral attachment | Liu et al. (2004b) |
| Ganoderma lucidum, Spica prunellae | Polysaccharide | Inhibits viral attachment and penetration | Liu et al. (2004a) | |
| Euphorbia jolkini | Putranjivain A | Inhibits viral attachment and penetration | Cheng et al. (2004) | |
| Phyllanthus emblica | Pentagalloylglucose | Down-regulates cofilin1 to inhibit viral-induced rearrangements of actin cytoskeleton | Pei et al. (2011) | |
| Pericarpium granati | Tannin | Inhibits viral attachment | Zhang et al. (1995) | |
| HIV | Spica prunellae, Rhizoma cibotte | Tannin | Inhibits the gp41 six-helix bundle formation | Liu et al. (2002) |
| Flu | Fructus arctii | Arctigenin | Exhibits hemagglutination inhibition | Yang et al., 2005a, Yang et al., 2005b |
| EVs | Radix glycyrrhizae | Polysaccharide | Attaches to the cell surface and inhibits viral attachment and entry | Wang et al. (2001) |
| SARS-CoV | Radix et Rhizoma Rhei, Radix Polygoni Multiflori | Emodin | Blocks the S protein and ACE2 interaction | Ho et al. (2007) |
| Radix glycyrrhizae | Glycyrrhizin | Inhibits viral attachment and penetration | Chen et al. (2004) | |
| NV | Fructus schisandrae, Pomegranate | Tannin | Inhibits the binding to histo-blood group antigens (HBGAs) | Zhang et al. (2012) |
Polysaccharides and their derivatives are the most frequently found viral entry inhibitors. Mechanism studies show that these sugars target the viral attachment and/or internalization steps mediated by specific interactions with viral particles or cell-surface molecules, resulting in viral serotype- or host cell type-dependent activity (Baba et al., 1988, Marchetti et al., 1995). The composition of the sugar units and the diversity of the linkage chemistry are also factors that determine the functional properties and the target specificity of these compounds. Thus, while polysaccharides are considered to be broad-spectrum virus entry inhibitors, their derivatives display significant levels of virus-specific activity (Zhou and Meng, 1997). Because polysaccharides are also ligands for immunoregulatory cell-surface receptors such as the toll-like receptors, they might also function as immunomodulators (Takeda et al., 2003).
After attachment, viral surface proteins interact with cell-surface receptors, triggering conformational changes which initiated the entry process. Inhibition of formation of the entry machinery or of required conformational changes can prevent viral entry. As indicated in Table 2, aside from polysaccharides, tannins are the most identified entry inhibitors. Multiple mechanisms have been proposed for this activity, including the ability of tannins to interact with and precipitate proteins. Tannins have been shown to inhibit fusion completion in HIV infection (Liu et al., 2002). Although polysaccharides and tannins are not typical drug-like molecules, they display broad antiviral activity. Their development as topically applied medicines such as microbicides is actively pursued.
5. Replication inhibitors
Replication represents the core of the viral life cycle, and involves most viral protein functions. Inhibitors of viral proteases, polymerases, integrases (helicases), and reverse transcriptases of HIV, HCV, and herpesviruses have been clinically successful, and most current antiviral agents target this stage. Considering these unique scenarios, development of TCHMs with antiviral activity is focused principally on this stage of infection (Table 3 ). Compared with anti-entry TCHMs, compounds targeting replication are more chemically diverse and more virus-specific. Furthermore, considering that cellular machinery is required for viral replication, the mechanisms of many antiviral TCHMs involve cellular factors.
Table 3.
TCHM-derived compounds inhibiting viral replication.
| Virus | Herbs | Compounds | Mechanism | References |
|---|---|---|---|---|
| HSV | Chamaecyparis obtuse | Yatein | Inhibits HSV-1 ICP0 and ICP4 expression as well as viral DNA synthesis | Kuo et al. (2006) |
| Euphorbia jolkini | Putranjivain A | Affects the late stage of HSV-2 replication | Cheng et al. (2004) | |
| Limonium sinense | Samarangenin B | Inhibits viral replication | Kuo et al. (2002) | |
| Ranunculus sieboldii, Ranunculus sceleratus | Protocatechuyl aldehyde | Inhibits viral replication | Li et al. (2005) | |
| Limonium sinense | Isodihydrosyringetin, (−)-epigallocatechin 3-O-gallate, samarangenin B, myricetin, myricetin 3-O-α-rhamnopyranoside, quercetin 3-O-α-rhamnopyranoside, (−)-epigallocatechin, gallic acid, N-trans-caffeoyltyramine, N-trans-feruloyltyramine | Inhibits viral replication | Lin et al. (2000) | |
| Rhizoma coptidis | Berberine | Inhibits viral DNA synthesis | Chin et al. (2010) | |
| HIV | Chrysanthemum morifolium | Apigenin-7-O-β-D-g-lucopyranoside | Inhibits viral integrase | Lee et al. (2003) |
| Vatica cinerea | Vaticinone (23E)-27-nor-3-hydroxycycloart-23-en-25-one | Inhibits viral replication | Zhang et al. (2003) | |
| Aesculus chinensis | Triterpenoid saponins | Inhibits viral protease | Yang et al. (1999) | |
| Kadsura matsudai | Schizanrin B, C, D, and E | Inhibits viral replication | Kuo et al. (2001) | |
| Trichosanthes kirilowii | Trichosanthin | Inhibits viral replication | Wang et al. (2002) | |
| HBV | Radix scutellariae | Wogonin | Inhibits viral DNA polymerase | Guo et al. (2007) |
| Salvia miltiorrhiza | Protocatechuic aldehyde | Inhibits viral replication | Zhou et al. (2007) | |
| Ranunculus sieboldii, Ranunculus sceleratus | Apigenin 4′-O-α-rhamnopyranoside, apigenin 7-O-β-glucopyranosyl-4′-O-α-rhamnopyranoside, tricin 7-O-β-glucopyranoside, tricin, isoscopoletin | Inhibits viral replication | Li et al. (2005) | |
| Radix sophorae Flavescentis | Oxymatrine | Down-regulates the expression of heat-stress cognate 70 (HSC70) that is required for HBV DNA replication | Wang et al. (2011) | |
| Radix bupleuri | Saikosaponin C | Inhibits viral DNA replication and HBeAg production | Chiang et al. (2003) | |
| HCV | Saxifraga melanocentra | Polyphenolic compounds | Inhibits viral NS3 serine protease | Zuo et al. (2005) |
| Rhodiola kirilowii | 3,3′-Digalloylproprodelphinidin B2, 3,3′-Digalloylprocyanidin B2, (−)-Epigallocatechin-3-O-gallate, (−)-Epicatechin-3-O-gallate | Inhibits viral NS3 serine protease | Zuo et al. (2007) | |
| Flu | Fructus arctii | Arctigenin | Inhibits viral replication | Gao et al. (2002) |
| EV71 | Laggera pterodonta | Chrysosplenetin and penduletin | Inhibits viral RNA replication | Zhu et al. (2011) |
| HCMV | Allium sativum | Allitridin | Inhibits viral replication in earlier period of viral cycle before viral DNA synthesis | Zhen et al. (2006) |
| SARS-CoV | Radix glycyrrhizae | Glycyrrhizin | Inhibits viral replication | Chen et al. (2004) |
6. Inhibitors of packaging and assembly
The assembly and release of infectious virions is the final step in the viral life cycle. In this stage, vial structural proteins (often as pre-structural proteins such as P1 of enterovirus 71) mature until they are assembled into viral capsids. During this step, viral genomes are packaged into capsids for intracellular transport, enveloped (for enveloped viruses), then released. Despite the absolute requirement for sustained viral infection, no antiviral agents that target this stage have been developed. This limitation is partially due to limited knowledge of the packaging and assembly mechanisms of most viruses, resulting in a limited number of specific assays available. Studies of some TCHMs have revealed that their mechanisms of action involve viral packaging and assembly (summarized in Table 4 ), but the number remains limited, and the level of understanding is still preliminary.
Table 4.
TCHM-derived compounds inhbiting viral packaging and assembly.
| Virus | Herbs | Compounds | Antiviral effect | References |
|---|---|---|---|---|
| HSV | Digitalis purpurea | Digitoxin | Inhibits viral release | Su et al. (2008) |
| Flu | Identified from TCM database@Taiwan (http://tcm.cmu.edu.tw) | Canavanine, α-(methylenecyclopropyl)glycine, quinic acid, 2-hydroxy-3-(3,4-dihydroxyphenyl)propanoic acid, β-d-fructofuranose | Binds to the M2 ion channel during simulation | Chang et al. (2011a) |
| Identified from TCM database@Taiwan (http://tcm.cmu.edu.tw) | Quinic acid, genipin, syringic acid, cucurbitine, fagarine, methyl isoferulate | Blocks the M2 channel activity | Lin et al. (2011) | |
| EVs | Phyllanthus emblica | Phyllaemblicin B | Inhibits viral infection both in in vitro and in vivo assays | Wang et al. (2009) |
7. Immunomodulators
As host cell invaders, viruses must escape the immune response to survive. Host innate and adaptive responses against viral infection and replication oppose viral strategies (escaping and blocking) against the host immune response. An excessive reaction of the host immune response may also lead to tissue damage and multi-organ injury (Ferrero-Miliani et al., 2007, La Gruta et al., 2007), which in turn may cause related diseases. TCHMs that enhance host antiviral immune responses or block viral immune escape mechanisms therefore display antiviral activity through immunoregulatory mechanisms.
Considering that many TCHMs have immunoregulatory activities (Table 5 ), many such remedies also display antiviral activities. This class of TCHMs includes multi-target compounds. For example, polysaccharides are potent interferon inducers and good viral entry inhibitors. Another example is glycyrrhizin, which has activity against entry, replication (Chen et al., 2004), and immunomodulation (Shinada et al., 1986).
Table 5.
TCHM-derived compounds with immunomodulatory activity.
| Virus | Herbs | Compounds | References |
|---|---|---|---|
| HSV | Rhizoma polygonati | Polysaccharide | Gu et al. (2003) |
| Herba houttuyniae | Quercetin, quercitrin or isoquercitrin | Chen et al. (2011) | |
| HBV | Radix sophorae Flavescentis | (+)-12a-Hydroxysophocarpine | Ding et al. (2006) and Liu et al. (2003) |
| Potentilla anserina | Total saponin | Cai et al. (2003) | |
| Flos caryophylli | Total saponin | (Gao et al., 2003) | |
| Kadsura japonica | C19 homolignans: taiwanschirins A, B, C; heteroclitin F; kadsurindutins A, kadsulignan L, and neokadsuranin | Kuo et al. (2005) and Ma et al. (2007) | |
| Ocimum basilicum | Pigenin | Chiang et al. (2005) | |
| Kadsura matsudai | Schizarin B, D, and E, | Kuo et al. (2001) | |
| Phyllanthus | Niranthin, hinokinin | Huang et al. (2003) | |
| Euphorbia humifusa | Humifusane A and humifusane B | Tian et al. (2011) | |
| FMDV | Raidx astragali | Polysaccharide | Li et al. (2011) |
8. Future directions
The major goal of current research is to meet international standards for the modernization of TCHMs. To achieve this goal, a TCHM must satisfy all requirements set by international standards, including evidence-supported efficacy (particularly through randomized, double-blind, placebo-controlled, multicenter clinical trials), safety assessment, and quality control. A centralized and standardized research system, aimed at achieving a better understanding of medicinal chemistry and the mechanism of action of TCHMs, is fundamental to achieving this goal.
8.1. Government support
Realizing these needs, the Twelfth Five-Year (2011–2016) Plan for the National Economic and Social Development of the People’s Republic of China laid out a national strategy for TCM development. Compared with former Plans, it reflects the equal importance of TCM and Western medicine at the national level. The project for “Supporting the Development of TCM” stipulates that “the protection, research, and rational utilization of Chinese materia medica resources, and establishment of quality evaluation and standardization system” has the highest priority in terms of government support (http://www.news.cn, 2011). This initiative shows a determination to solve the bottleneck of underdeveloped Chinese materia medica. Thus, based on the Plan, it is expected that TCM-based medical systems will be greatly enhanced through increased funding for basic research and improved education. This government support will undoubtedly result in advanced phytochemistry, assay development, and bioinformatics, which will in turn provide platform technologies and tools for the modernization and commercialization of TCM.
8.2. Centralized screening facilities
Supported by central and local governments, drug screening centers have been established in China in recent years (Table 6 ). These centers are operated by scientists with extensive experience in global pharmaceutical industries, and are equipped with state-of-the-art equipment, including robots capable of high-throughput screening. Large pharmaceutical companies such as Novartis have also set up research centers in China. Compounds originating from TCHMs are among their foci for drug discovery.
Table 6.
Drug screening and research centers focusing on TCHM and supported by central and local governments in China.
| Center Name | Affiliated Organization | Website | |
|---|---|---|---|
| The National Center for Drug Screening | Shanghai Institute of Materia Medica, Chinese Academy of Sciences | http://www.screen.org.cn | |
| National Engineering Research Center | National Engineering Research Center for TCM Pharmaceutical Technology | Yangtze River Pharmaceutical Group Nanjing Hailing Pharmaceutical Co., Ltd. | http://www.hailingyy.com/Center.asp |
| National Pharmaceutical Engineering Center for Solid Preparation in Chinese Herbal Medicine | Jiangxi Herbfine Hi-tech Co., Ltd. | http://www.herbfine.com | |
| National Engineering Research Center for Modernization of Extraction and Separation Process of TCM | Guangzhou Hanfang Pharmaceutical Co., Ltd. | http://www.hovfo.com | |
| National Engineering Research Center for TCM New Medicine (Compound) Development | Beijing Zhongyan TRT Medicine R&D Co., Ltd. | http://www.tongrentang.com/en/fellowsub/randd.php | |
| Chinese National Engineering Research Center | Chinese National Engineering Research Center for Modernization of TCM | Livzon Pharmaceutical Group, Inc. | http://www.livzon.com.cn/fzjg/zyyjzxView_214.Html |
| Chinese National Engineering Research Center for Gelatin | Shangdong Donggeejiao, Inc. | http://www.dongeejiao.com | |
| Chinese National Engineering Research Center for TCM, SHZJ | Shanghai Pharmaceutical Technology for TCM Co., Ltd. | http://www.nercmtcm.com | |
| National Center for Pharmaceutical Screening | Institute of Materia Medica, Chinese Academy of Medical Sciences | http://ncps.imm.ac.cn | |
| New Drug Screening Center, China Pharmaceutical University | China Pharmaceutical University | http://screen.cpu.edu.cn | |
| National Innovation Center of TCM Modernization in Shanghai | Shanghai Innovation Research Center of Traditional Chinese Medicine | http://www.sirc-tcm.sh.cn | |
8.3. Centralized databases
Information fragmentation poses a significant challenge to TCM research. Benefiting from strong financial support, large TCM-focused databases are now becoming available (Table 7 ). Comprehensively integrated databases are foreseen to greatly enhance TCHM-based drug discovery.
Table 7.
TCHM-focused databases in China.
| Names of databases | Data volume | Affiliated organization | Website |
|---|---|---|---|
| China traditional Chinese medicines database | 14,032 | Institute of Information on Traditional Chinese Medicine, China Academy of Chinese Medical Sciences | http://cowork.cintcm.com/engine/wdbintro.jsp |
| database of effective components in traditional Chinese medicines | 600 | Scientific Database of Chinese Academy of Sciences | http://www.medicine.csdb.cn/viewTable.jsp?ds=dataset@@medicine&tab=CMP |
| Traditional Chinese medicines database | 23,033 | NeoTrident Technology Co.,Ltd | http://www.neotrident.com/newweb/Product_View.asp?ProID=63 |
| Database of compounds from traditional Chinese medicine | 30,000 | Shanghai TCM Data Center | http://www.tcm120.com/1w2k/tcm_compound.asp |
| Database of compounds from traditional Chinese medicines metabolism | 1,741 | Shanghai TCM Data Center | http://temdb.sgst.cn/tcm_metabolize.asp |
| Database of compounds and components of traditional Chinese medicine | 3,500 | Shanghai TCM Data Center | http://temdb.sgst.cn/tcm_compcontent.asp |
| Traditional Chinese medicine and chemical components database | 19,700 | Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences | http://www.organchem.csdb.cn/scdb/main/tcm_introduce.asp |
Acknowledgments
This work was partially supported by the National Basic Research Program (973) (Grant Nos. 2009CB522300 and 2010CB530100), Department of Education of Guangdong Province (Grant No. GXZD0901).
References
- Baba M., Snoeck R., Pauwels R., de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrobial Agents and Chemotherapy. 1988;32:1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G., Zhao Y., Yuan H., Zhang X., Liu F., He C., Tang C., Li Z. Separation of Potentifla anserine active site (total saponin) and anti-DHBV DNA action in ducks. Central South Pharmacy (China) 2003;1:17–21. [Google Scholar]
- Cao, H., Tao, D.S., Zeng, Y.Q., Guan, Y., 2006. In vitro study on a TCM product (Kang Bing Du Granule) against highly pathogenic H5N1 avian influenza A virus (genotype E). In: The 5th Meeting of Consortium for Globalization of Chinese Medicine cum International Forum (Zhuhai) on Chinese Medicine Program & Abstract, 76–77.
- Cao, H., Tao, D.S., Zeng, Y.Q., Guan, Y., 2007. In vivo study on a TCM product (Kang Bing Du Granule) against highly pathogenic H5N1 avian influenza A virus (genotype E). In: The 6th Meeting of Consortium for Globalization of Chinese Medicine Program & Abstract, 69–70.
- Cao, H., Tao, D.S., Zeng, Y.Q., Guan, Y., 2010. In vitro study on Kang Bing Du Granule against swine-origin influenza A virus (A/H1N1). In: The 9th Meeting of Consortium for Globalization of Chinese Medicine Abstracts, 220.
- Chang T.T., Sun M.F., Chen H.Y., Tsai F.J., Lin J.G., Chen C.Y.C. Key features for designing M2 proton channel anti swine flu inhibitors. Journal of the Taiwan Institute of Chemical Engineers. 2011;42:701–708. [Google Scholar]
- Chang T.T., Sun M.F., Chen H.Y., Tsai F.J., Fisher M., Lin J.G., Chen C.Y. Screening from the world’s largest TCM database against H1N1 virus. Journal of Biomolecular Structure & Dynamics. 2011;28:773–786. doi: 10.1080/07391102.2011.10508605. [DOI] [PubMed] [Google Scholar]
- Chen C.Y. TCM Database@Taiwan: the world’s largest traditional Chinese medicine database for drug screening in silico. PLoS One. 2011;6:e15939. doi: 10.1371/journal.pone.0015939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.Q., Li B.C. Studies on antivirus of Banlangen buccal tablets. Journal of Henan University (Medical Science) 2006;25:67–69. [Google Scholar]
- Chen B.Q., Bao C.P., Xu Q.T. Antivirus of Shuanghuanglian buccal tablets. Journ al of Henan University (Medical Science) 2001;20:35–37. [Google Scholar]
- Chen F., Chan K.H., Jiang Y., Kao R.Y., Lu H.T., Fan K.W., Cheng V.C., Tsui W.H., Hung I.F., Lee T.S., Guan Y., Peiris J.S., Yuen K.Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. Journal of Clinical Virology. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.J., Ge L., Xiao S.H., Gu L., Liu J. Experimental studies on anti-flu virus effect of Yinhuang injection in vivo and in vitro. Lishizhen Medicine and Materia Medica Research. 2007;18:591–592. [Google Scholar]
- Chen X., Wang Z., Yang Z., Wang J., Xu Y., Tan R.X., Li E. Houttuynia cordata blocks HSV infection through inhibition of NF-kappaB activation. Antiviral Research. 2011;92:341–345. doi: 10.1016/j.antiviral.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lin T., Yang C., Wang K., Lin L., Lin C. Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. Journal of Antimicrobial Chemotherapy. 2004;53:577–583. doi: 10.1093/jac/dkh136. [DOI] [PubMed] [Google Scholar]
- Chiang L.C., Ng L.T., Liu L.T., Shieh D.E., Lin C.C. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Medica. 2003;69:705–709. doi: 10.1055/s-2003-42797. [DOI] [PubMed] [Google Scholar]
- Chiang L.C., Ng L.T., Cheng P.W., Chiang W., Lin C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clinical and Experimental Pharmacology and Physiology. 2005;32:811–816. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- Chin L.W., Cheng Y.W., Lin S.S., Lai Y.Y., Lin L.Y., Chou M.Y., Chou M.C., Yang C.C. Anti-herpes simplex virus effects of berberine from Coptidis rhizoma, a major component of a Chinese herbal medicine, Ching-Wei-San. Archives of Virology. 2010;155:1933–1941. doi: 10.1007/s00705-010-0779-9. [DOI] [PubMed] [Google Scholar]
- Ding P., Liao Z., Huang H., Zhou P., Chen D. (+)-12α-Hydroxysophocarpine, a new quinolizidine alkaloid and related anti-HBV alkaloids from Sophora flavescens. Bioorganic & Medicinal Chemistry Letters. 2006;16:1231–1235. doi: 10.1016/j.bmcl.2005.11.073. [DOI] [PubMed] [Google Scholar]
- Fang J.G., Tang J., Yang Z.Q., Hu Y., Liu Y.H., Wang W.Q. Effect of Radix isatidis against herpes simplex virus type I in vitro. Chinese Traditional and Herbal Drugs. 2005;36:242–244. [Google Scholar]
- Ferrero-Miliani L., Nielsen O.H., Andersen P.S., Girardin S.E. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clinical and Experimental Immunology. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Dong X., Kang T., Zhao C., Huang Z., Zhang X. Activity of in vitro anti-influenza virus of arctigenin. Chinese Traditional and Herbal Drugs. 2002;33:724–726. [Google Scholar]
- Gao S., Chi B., Wang F., Liu X., Jin Z., He H. Effects ofthe extract of Syringa on the secretions of HBsAg and HBeAg in HepG2.2.15 cells. Journal of the Fourth Military Medical University (China) 2003;24:1234–1235. [Google Scholar]
- Gu H., Meng Y., Pu Q. Polysaccharide from Polygonatum cyrtonema hua against herpes simplex virus in vitro. Chinese Journal of Applied & Environmentol Biology. 2003;9:21–23. [Google Scholar]
- Guo Q., Zhao L., You Q., Yang Y., Gu H., Song G., Lu N., Xin J. Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Research. 2007;74:16–24. doi: 10.1016/j.antiviral.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ho T., Wu S., Chen J., Li C., Hsiang C. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Research. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z.H., Tan D.M., Xie Y.T., Lu M.H., Xie J.P., Liu G.Z., Wang L. Therapeutic effect of Kurarinone in patients with chronic hepatitis B. Practical Preventive Medicine. 2005;12:824–826. [Google Scholar]
- Hu X.C., Zheng W.Q. Resistance to Lsatia indigotica fort lectin inhibit influenza virus. Journal of Shanghai Teachers University (Natural Sciences) 2003;32:62–65. [Google Scholar]
- Huang R.L., Huang Y.L., Ou J.C., Chen C.C., Hsu F.L., Chang C. Screening of 25 compounds isolated from Phyllanthus species for anti-human hepatitis B virus in vitro. Phytotherapy Research. 2003;17:449–453. doi: 10.1002/ptr.1167. [DOI] [PubMed] [Google Scholar]
- Huang X.J., Hou W., Zhao Y.L., Luo F., Yang Z.Q. An experimental study on anti-respiratory syncytial virus with Xiasangju extract. Chinese Journal of Modern Drug Application. 2007;1:11–14. [Google Scholar]
- Koehn F.E., Carter G.T. The evolving role of natural products in drug discovery. Nature Reviews Drug Discovery. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- Kuo Y.H., Li S.Y., Huang R.L., Wu M.D., Huang H.C., Lee K.H. Schizanrins B, C, D, and E, four new lignans from Kadsura matsudai and their antihepatitis activities. Journal of Natural Products. 2001;64:487–490. doi: 10.1021/np000261m. [DOI] [PubMed] [Google Scholar]
- Kuo Y.C., Lin L.C., Tsai W.J., Chou C.J., Kung S.H., Ho Y.H. Samarangenin B from Limonium sinense suppresses herpes simplex virus type 1 replication in vero cells by regulation of viral macromolecular synthesis. Antimicrobial Agents and Chemotherapy. 2002;46:2854–2864. doi: 10.1128/AAC.46.9.2854-2864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y.H., Wu M.D., Huang R.L., Kuo L.M., Hsu Y.W., Liaw C.C., Hung C.C., Shen Y.C., Ong C.W. Antihepatitis activity (anti-HBsAg and anti-HBeAg) of C19 homolignans and six novel C18 dibenzocyclooctadiene lignans from Kadsura japonica. Planta Medica. 2005;71:646–653. doi: 10.1055/s-2005-871271. [DOI] [PubMed] [Google Scholar]
- Kuo Y.C., Kuo Y.H., Lin Y.L., Tsai W.J. Yatein from Chamaecyparis obtusa suppresses herpes simplex virus type 1 replication in HeLa cells by interruption the immediate-early gene expression. Antiviral Research. 2006;70:112–120. doi: 10.1016/j.antiviral.2006.01.011. [DOI] [PubMed] [Google Scholar]
- La Gruta N.L., Kedzierska K., Stambas J., Doherty P.C. A question of self-preservation: immunopathology in influenza virus infection. Immunology and Cell Biology. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Kim H.J., Lee Y.S. A new anti-HIV flavonoid glucuronide from Chrysanthemum morifolium. Planta Medica. 2003;69:859–861. doi: 10.1055/s-2003-43207. [DOI] [PubMed] [Google Scholar]
- Li H., Zhou C., Pan Y., Gao X., Wu X., Bai H., Zhou L., Chen Z., Zhang S., Shi S., Luo J., Xu J., Chen L., Zheng X., Zhao Y. Evaluation of antiviral activity of compounds isolated from Ranunculus sieboldii and Ranunculus sceleratus. Planta Medica. 2005;71:1128–1133. doi: 10.1055/s-2005-873169. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Yan Q.B., Sun Y., Tong H.M. Study on Yinqiao San against avian influenza virus in vitro. Journal of Northeast Asricultural University. 2008;39:90–93. [Google Scholar]
- Li J., Zhong Y., Li H., Zhang N., Ma W., Cheng G., Liu F., Liu F., Xu J. Enhancement of Astragalus polysaccharide on the immune responses in pigs inoculated with foot-and-mouth disease virus vaccine. International Journal of Biological Macromolecules. 2011;49:362–368. doi: 10.1016/j.ijbiomac.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Lin L.C., Kuo Y.C., Chou C.J. Anti-herpes simplex virus type-1 flavonoids and a new flavanone from the root of Limonium sinense. Planta Medica. 2000;66:333–336. doi: 10.1055/s-2000-8540. [DOI] [PubMed] [Google Scholar]
- Lin C.H., Chang T.T., Sun M.F., Chen H.Y., Tsai F.J., Chang K.L., Fisher M., Chen C.Y. Potent inhibitor design against H1N1 swine influenza: structure-based and molecular dynamics analysis for M2 inhibitors from traditional Chinese medicine database. Journal of Biomolecular Structure & Dynamics. 2011;28:471–482. doi: 10.1080/07391102.2011.10508589. [DOI] [PubMed] [Google Scholar]
- Liu S., Jiang S., Wu Z., Lv L., Zhang J., Zhu Z., Wu S. Identification of inhibitors of the HIV-1 gp41 six-helix bundle formation from extracts of Chinese medicinal herbs Prunella vulgaris and Rhizoma cibotte. Life Sciences. 2002;71:1779–1791. doi: 10.1016/s0024-3205(02)01939-2. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhu M., Shi R., Yang M. Radix Sophorae flavescentis for chronic hepatitis B: a systematic review of randomized trials. American Journal of Chinese Medicine. 2003;31:337–354. doi: 10.1142/S0192415X03001107. [DOI] [PubMed] [Google Scholar]
- Liu Y., He K., Yang M., Pu Q., Zhang J. Antiviral activity against HSV-2 of sulfated polysaccharide from Cyathula officinalis Kuan in vitro. Chinese Journal of Applied & Environmental Biology. 2004;10:46–50. [Google Scholar]
- Liu J., Yang F., Ye L.B., Yang X.J., Timani K.A., Zheng Y., Wang Y.H. Possible mode of action of antiherpetic activities of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. Journal of Ethnopharmacology. 2004;95:265–272. doi: 10.1016/j.jep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Ma W., Ma X., Huang H., Zhou P., Chen D. Dibenzocyclooctane lignans from the stems of Kadsura induta and their antiviral effect on hepatitis B virus. Chemistry & Biodiversity. 2007;4:966–972. doi: 10.1002/cbdv.200790087. [DOI] [PubMed] [Google Scholar]
- Marchetti M., Pisani S., Pietropaolo V., Seganti L., Nicoletti R., Orsi N. Inhibition of herpes simplex virus infection by negatively charged and neutral carbohydrate polymers. Journal of Chemotherapy. 1995;7:90–96. doi: 10.1179/joc.1995.7.2.90. [DOI] [PubMed] [Google Scholar]
- Pei Y., Xiang Y.F., Chen J.N., Lu C.H., Hao J., Du Q., Lai C.C., Qu C., Li S., Ju H.Q., Ren Z., Liu Q.Y., Xiong S., Qian C.W., Zeng F.L., Zhang P.Z., Yang C.R., Zhang Y.J., Xu J., Kitazato K., Wang Y.F. Pentagalloylglucose downregulates cofilin1 and inhibits HSV-1 infection. Antiviral Research. 2011;89:98–108. doi: 10.1016/j.antiviral.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Peng T. Strategies for antiviral screening targeting early steps of virus infection. Virologica Sinica. 2010;25:281–293. doi: 10.1007/s12250-010-3135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S.Y., Liu J.H., Tian Y.R., Guo J., Feng J.Z., Liu S.D., Zeng X.J., Dong X.H., Mei L. Antiviral activity of Shuanghuanglian tablet against influenza A1 virus FM 1 and adenovirus ADV3 in mice. China Practical Medicine. 2008;3:50–52. [Google Scholar]
- Shi J., Wang L.X. Observation of therapeutic effects of Ku-shen-jian injection on hepatitis B. Chinese Remedies & Clinics. 2012;12:515–516. [Google Scholar]
- Shinada M., Azuma M., Kawai H., Sazaki K., Yoshida I., Yoshida T., Suzutani T., Sakuma T. Enhancement of interferon-gamma production in glycyrrhizin-treated human peripheral lymphocytes in response to concanavalin A and to surface antigen of hepatitis B virus. Proceedings of the Society for Experimental Biology and Medicine. 1986;181:205–210. doi: 10.3181/00379727-181-42241. [DOI] [PubMed] [Google Scholar]
- Su C.T., Hsu J.T.A., Hsieh H.P., Lin P.H., Chen T.C., Kao C.L., Lee C.N., Chang S.Y. Anti-HSV activity of digitoxin and its possible mechanisms. Antiviral Research. 2008;79:62–70. doi: 10.1016/j.antiviral.2008.01.156. [DOI] [PubMed] [Google Scholar]
- Sun J. Antiviral active ingredients in plants. Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai. 2007;21:75–79. [Google Scholar]
- Sun J., Chu Y.L., Zheng J.W., Jiang F.L., Shao L.Q., Chai C.B. Experimental study on Yinqiaoganmao granule against influenza A in vitro. Journal of Shanxi College of Traditional Chinese Medicine. 2006;29:49–50. [Google Scholar]
- Sun J., Wang N.R., Yang B., He S.Q. Study on Shuanghuanglian inhibiting effect of influenza virus A1 gene. Journal of Clinical Pulmonary Medicine. 2009;14:298–300. [Google Scholar]
- Sun H.H., Deng W., Zhan L.J., Xu L.L., Li F.D., Lv Q., Zhu H., Liu Y., Ma C.M., Bao L.L. Effect of Banlangen granule on mice challenged with A/ California/7/2009. Chinese Journal of Comparative Medicine. 2010;20:53–57. [Google Scholar]
- Takeda K., Kaisho T., Akira S. Toll-like receptors. Annual Review of Immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tian Y., Sun L.M., Li B., Liu X.Q., Dong J.X. New anti-HBV caryophyllane-type sesquiterpenoids from Euphorbia humifusa Willd. Fitoterapia. 2011;82:251–254. doi: 10.1016/j.fitote.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang H., Shi Y., Wang W. Inhibition of glycyrrhiza polysaccharide on virus. Acta Scientiarum Naturalium Universitatis Nankaiensis. 2001;34:126–128. [Google Scholar]
- Wang J., Nie H., Tam S., Huang H., Zheng Y. Anti-HIV-1 property of trichosanthin correlates with its ribosome inactivating activity. FEBS Letters. 2002;531:295–298. doi: 10.1016/s0014-5793(02)03539-1. [DOI] [PubMed] [Google Scholar]
- Wang G.T., Song Y.Y., Ren G.J., Wang Z.Y., Xu H.Z. Antiviral activity of Yinhuang for injection on respiratory syncytial virus in vitro. Chinese Journal of New Drugs and Clinical Remedies. 2005;24:887–889. [Google Scholar]
- Wang Y., Wang R., Hou W. Anti-viral components of natural products. Natural Product Reports and Development. 2007;19:179–182. [Google Scholar]
- Wang Y., Qin L., Wang Y., Yue Q. Research progress in antiviral effects of traditional Chinese medicine. Medical Recapitulate. 2008;14:3488–3490. [Google Scholar]
- Wang Y.F., Wang X.Y., Ren Z., Qian C.W., Li Y.C., Kaio K., Wang Q.D., Zhang Y., Zheng L.Y., Jiang J.H., Yang C.R., Liu Q., Zhang Y.J., Wang Y.F. Phyllaemblicin B inhibits Coxsackie virus B3 induced apoptosis and myocarditis. Antiviral Research. 2009;84:150–158. doi: 10.1016/j.antiviral.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Wang Y.P., Zhao W., Xue R., Zhou Z.X., Liu F., Han Y.X., Ren G., Peng Z.G., Cen S., Chen H.S., Li Y.H., Jiang J.D. Oxymatrine inhibits hepatitis B infection with an advantage of overcoming drug-resistance. Antiviral Research. 2011;89:227–231. doi: 10.1016/j.antiviral.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Wu H.B., Li H.M., Lu C.A., Gao Y.J., Li X.Q., Zhou A.X. Experimental study on Shuanghuanglian dispersible tablets against viruses. Chinese Journal of Experimental Traditional Medical Formulae. 2004;10:48–50. [Google Scholar]
- Wu C.L., Yang Z.Q., Hou W., Du C.X., Luo F. Experimental study on Shuanghuanglian oral liquid against respiroviruses. Journal of Mathernatical Medicine. 2005;18:592–594. [Google Scholar]
- Xie B., Yang Z.F., Chen Q.Y., Liu N., Huang B.S., Zhu Y.T. In vitro experimental study on the effect of Lonicerae and Forsythlae powder against several kinds of respiroviruses. China Tropical Medicine. 2006;6:16–17. [Google Scholar]
- Yang X.W., Zhao J., Cui Y.X., Liu X.H., Ma C.M., Hattori M., Zhang L.H. Anti-HIV-1 protease Triterpenoid saponins from the seeds of Aesculus chinensis. Journal of Natural Products. 1999;62:1510–1513. doi: 10.1021/np990180u. [DOI] [PubMed] [Google Scholar]
- Yang Z., Liu N., Huang B., Wang Y., Hu Y., Zhu Y. Effect of anti-influenza virus of Arctigenin in vivo. Zhong Yao Cai (China) 2005;28:1012–1014. [PubMed] [Google Scholar]
- Yang Z.F., Huang B.S., Liu N., Wang Y.F., Zhu Y.T. Experimental study on the action of Yinqiaosan against influenza A1. China Tropical Medicine. 2005;5:1423–1425. [Google Scholar]
- Yu L., Luo J., Tan X. The basic research ideas of herbal prescriptions composing principles. Traditional Chinese Drug Research & Clinical Pharmacology (China) 2006;16:43–45. [Google Scholar]
- Zhan, H.Q., Dong, T.X., 2006. Active part and preparation method of anti-flu traditional Chinese medicine. Chinese Patent Number 200610005382.2.
- Zhang Q.X., Zeng F.Z. Observation of the therapeutic effect of Huangqi injection and Shengmai injection for pediatric viral myocarditis. Journal of Emergency in Traditional Chinese Medicine. 2009;18:556–557. [Google Scholar]
- Zhang J., Yan B., Yao X., Gao Y., Song J. Tannin from Pericarpium granati inhibites herpes simplex virus type 2. China Journal of Chinese Materia Medica. 1995;20:556–560. [PubMed] [Google Scholar]
- Zhang H.J., Tan G.T., Hoang V.D., Hung N.V., Cuong N.M., Soejarto D.D., Pezzuto J.M., Fong H.H. Natural anti-HIV agents. Part IV. Anti-HIV constituents from Vatica cinerea. Journal of Natural Products. 2003;66:263–268. doi: 10.1021/np020379y. [DOI] [PubMed] [Google Scholar]
- Zhang F.Y., Gao Y.F., Song H.R. Study on the effect against CVB3 by ShengmaiYin and abstracts from stems and leaves of Scutellaria baicalensis in vivo. Tianjin Medical Journal. 2005;33:717–719. [Google Scholar]
- Zhang B., Huang F., Dai Y., Mi J.Y. Experimental study of antiviral effect of compound Chaihu capsule on mice infected with influenza virus. Chinese Journal of Clinical Pharmacology and Therapeutics. 2007;12:173–176. [Google Scholar]
- Zhang X.F., Dai Y.C., Zhong W., Tan M., Lv Z.P., Zhou Y.C., Jiang X. Tannic acid inhibited norovirus binding to HBGA receptors, a study of 50 Chinese medicinal herbs. Bioorganic & Medicinal Chemistry. 2012;20:1616–1623. doi: 10.1016/j.bmc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Han X. Research progress in antiviral effects of traditional Chinese medicine and active ingredients. Journal of Practical Traditonal Chinese Medicine. 2009;25:428–430. [Google Scholar]
- Zhao P., Liu A.P., Liu N., Li Q.Y. Studies on Zhengchaihuyin inhibit influenza A and B in vitro. Journal of Practical Medical Techniques. 2007;14:2155–2156. [Google Scholar]
- Zhen H., Fang F., Ye D.Y., Shu S.N., Zhou Y.F., Dong Y.S., Nie X.C., Li G. Experimental study on the action of allitridin against human cytomegalovirus in vitro: Inhibitory effects on immediate-early genes. Antiviral Research. 2006;72:68–74. doi: 10.1016/j.antiviral.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Zhou L., Meng Y. Studies on antiviral activities of polysaccharides and their derivatives. Chinese Journal of Applied & Environmental Biology. 1997;3:82–90. [Google Scholar]
- Zhou Z., Zhang Y., Ding X.R., Chen S.H., Yang J., Wang X.J., Jia G.L., Chen H.S., Bo X.C., Wang S.Q. Protocatechuic aldehyde inhibits hepatitis B virus replication both in vitro and in vivo. Antiviral Research. 2007;74:59–64. doi: 10.1016/j.antiviral.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Zhu Q.C., Wang Y., Liu Y.P., Zhang R.Q., Li X., Su W.H., Long F., Luo X.D., Peng T. Inhibition of enterovirus 71 replication by chrysosplenetin and penduletin. European Journal of Pharmaceutical Sciences. 2011;44:392–398. doi: 10.1016/j.ejps.2011.08.030. [DOI] [PubMed] [Google Scholar]
- Zuo G., Li Z., Chen L., Xu X. Short communication in vitro anti-HCV activities of Saxifraga melanocentra and its related polyphenolic compounds. Antiviral Chemistry & Chemotherapy. 2005;16:393–398. doi: 10.1177/095632020501600606. [DOI] [PubMed] [Google Scholar]
- Zuo G., Li Z., Chen L., Xu X. Activity of compounds from Chinese herbal medicine Rhodiola kirilowii (Regel) Maxim against HCV NS3 serine protease. Antiviral Research. 2007;76:86–92. doi: 10.1016/j.antiviral.2007.06.001. [DOI] [PubMed] [Google Scholar]


