Abstract
More than 200 Chinese medicinal herb extracts were screened for antiviral activities against Severe Acute Respiratory Syndrome-associated coronavirus (SARS-CoV) using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) assay for virus-induced cytopathic effect (CPE). Four of these extracts showed moderate to potent antiviral activities against SARS-CoV with 50% effective concentration (EC50) ranging from 2.4 ± 0.2 to 88.2 ± 7.7 μg/ml. Out of the four, Lycoris radiata was most potent. To identify the active component, L. radiata extract was subjected to further fractionation, purification, and CPE/MTS assays. This process led to the identification of a single substance lycorine as an anti-SARS-CoV component with an EC50 value of 15.7 ± 1.2 nM. This compound has a CC50 value of 14980.0 ± 912.0 nM in cytotoxicity assay and a selective index (SI) greater than 900. The results suggested that four herbal extracts and the compound lycorine are candidates for the development of new anti-SARS-CoV drugs in the treatment of SARS.
Keywords: Severe Acute Respiratory Syndrome coronavirus (SARS-CoV), Drug screening, Natural products, Lycorine, Lycoris radiata herb
Severe Acute Respiratory Syndrome (SARS) is a respiratory illness caused by the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) (Drosten et al., 2003, Ksiazek et al., 2003, Peiris et al., 2003b, Poutanen et al., 2003). This febrile respiratory illness was initially described in early 2003 (Chan-Yeung and Yu, 2003, Donnelly et al., 2003, Lee et al., 2003, Peiris et al., 2003a, Tsang et al., 2003) and is life threatening and highly contagious. Currently, there are no approved or universally recommended therapies for SARS. Treatment for the disease is mainly supportive. Scientists worldwide have been vigorously trying to develop efficacious antiviral agents for the treatment of SARS in the event that SARS comes back in the future. Reports from several groups (Cinatl et al., 2003a, Cinatl et al., 2003b, Scagnolari et al., 2004) have suggested that some reagents, such as interferon and glycyrrhizin, pose anti-SARS-CoV activity.
In China, traditional herbal medicine has been frequently used in conjunction with conventional medicine to treat SARS. There is evidence showing that the herbal medicine is effective (Lin et al., 2003, Xiao et al., 2003, Zhao et al., 2003, Zhong and Zeng, 2003). However, the mechanisms of this treatment have not been clearly understood. It has been shown that natural plants contain antiviral activities to other coronaviruses (McCutcheon et al., 1995) and the mechanism of action of these herbal products is mainly through inhibition of viral replication (Vlietinck and Vanden Berghe, 1991, Jassim and Naji, 2003).
In this study, we selected over 200 in-house-made extracts of medicinal herbs that have been historically used for the treatment of virus-induced infectious diseases in China and tested their antiviral activities against SARS-CoV using a high throughput screening approach. The screening was based on a MTS assay (Cory et al., 1991, Khabar et al., 1996). The active samples from screening were then subjected to structure activity relationship (SAR) study to identify a single active chemical substance. The results of these studies and the potential usage of identified lead compounds in the treatment of SARS-CoV-induced infectious diseases are presented here.
In searching for new reagents for anti-SARS-CoV, collected herbs were extracted by refluxing with 95% ethanol or chloroform for 3 h. The extracted solvents were filtered and lyophilized and then re-dissolved in dimethyl sulphoxide (DMSO) (Sigma) and stored in 96-well sample plates at −80 °C for assays and screening. Two strains of SARS-CoV (BJ001, BJ006) used for antiviral compound screening were obtained from the Laboratory of Virology at the Academy of Military Medical Sciences in Beijing, China. The viruses were propagated in Vero E6 cells at 37 °C in a humidified atmosphere of 5% CO2. Vero E6 and HepG2 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen) containing 5% heat-inactivated fetal bovine serum (FBS) (Hyclone) and sodium bicarbonate, 3.7 g/l; glucose, 4.5 g/l; and 15 mM HEPES buffer. The virus-induced cytopathic effect (CPE) was determined by MTS method and by visualization of cellular morphology change. The number of viable cells is correlated with absorbance at 490 nm in MTS assay. Approximately, 4 × 103 Vero E6 cells/well were seeded onto Corning 96-well tissue culture plates (Corning Incorporated) with final volume of 100 μl and cultured for 24 h. Ten microliters of compounds or plant extracts at a concentration of 100 μg/ml were added into each well in duplicates before inoculating with virus stock. Interferon alpha (Hualida Biotech Company), proven to show antiviral activities against SARS-CoV (Cinatl et al., 2003b, Scagnolari et al., 2004), was used as the positive control. The viral titers were assessed by cytopathic effect (CPE) determined visually under the light-phase microscope 2–4 days post-infection (PI). The concentration to achieve 90% of cell lysis was used in antiviral compound screening. The infected cells with or without compound were incubated at 37 °C in a 5% CO2 atmosphere for 72 h. Then, 20 μl of MTS/phenazine methosulfate (PMS) (Promega) was added in each well. The cells were incubated for another 2 h in 37 °C. In the end, 50 μl 10% SDS was added to stop color reaction. The plates were measured at 490 nm using a VERSAMax microplate reader (Molecular Devices).
After primary screening, active compounds were cherry picked and a second round of test was performed for their antiviral effects. The pictures were taken to record cell morphology change caused by CPE and the inhibition effects of the compounds before MTS assay. As shown in Fig. 1 , four of the extracts, Lycoris radiata, Artemisia annua, Pyrrosia lingua, and Lindera aggregata exhibited significant inhibition effects on virus-induced CPE when SARS-CoV strain BJ001 was used in screening. A dose dependency of antiviral activities was determined by serial dilutions of compounds. The percentage of CPE reduction was calculated by subtracting the mean value of virus-infected cell control (0%) from the measured absorbance, and resulting number was divided by the measured absorbance of uninfected cell control (100%). The mean values and the standard deviation (S.D.) were taken for result analysis. The inhibition effects of all four natural product samples showed dose-dependent patterns (Fig. 1). The EC50 values were determined as the concentration of the compounds needed to achieve the inhibition of SARS-CoV-induced CPE to 50% of control value (cells without viral infection) and data analysis for the assays was performed using Prism™ version 3 software (Graphpad Software, Inc.). The EC50 values of inhibition are 2.4 ± 0.2, 34.5 ± 2.6, 43.2 ± 14.1, and 88.2 ± 7.7 μg/ml, respectively, much lower than previously identified compounds (Cinatl et al., 2003a). To check whether there is any significant strain variation, we used SARS-CoV strain BJ-006 and tested the inhibition activity of active compounds. The results were quite similar for two viral strains (Table 1 ). Viability of Vero cells measured by MTS assay was consistent with what we observed visually under the microscope (photos not shown). The addition of active compounds significantly blocked viral infection or replication and kept cells in a viable state. Interferon alpha also showed limited inhibition effects on virus-induced CPE, either judged by visual observation or MTS assay. The results were consistent with previous reports from Cinatl and Scagnolari's groups. The inhibition for all four compounds to virus infection/replication was apparently more potent than that of interferon alpha judged by visual observation and color absorbance in MTS assay (Fig. 1).
Fig. 1.
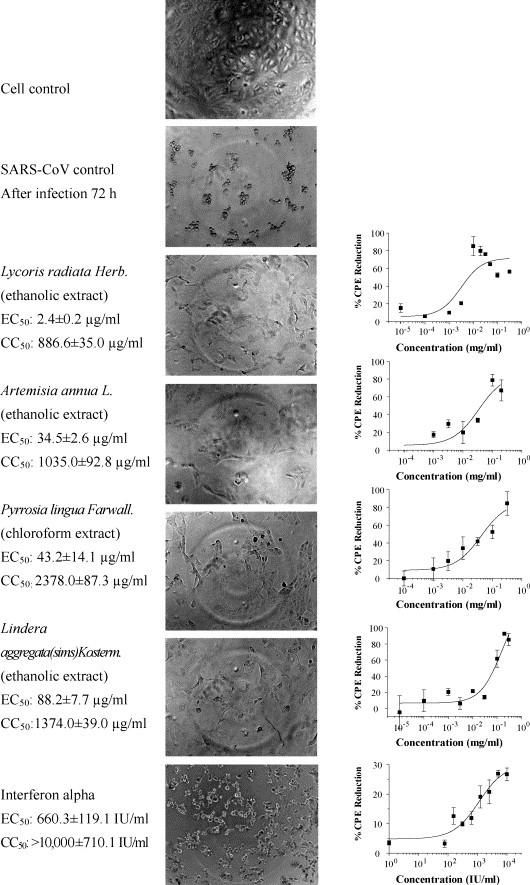
Effects of herb compound extracts on replication of SARS-CoV. The Vero E6 cell seeding, virus infection, compound addition, cell incubation, and measurement were described in the method. The percentage of CPE reduction was calculated by subtracting the mean of virus-infected cell control (0%) from the measured absorbance. The resulting number was divided by the uninfected cell control (100%). The mean values and the standard deviation (S.D.) are shown in the figures. Data presented are the average of duplicate values from three independent experiments. Magnification for visual observation: 200×.
Table 1.
Effects of plant extracts on SARS-CoV
| Plant extract | Family | Part used | Solvent | CC50a | Viral strain |
|||
|---|---|---|---|---|---|---|---|---|
| BJ-001 |
BJ-006 |
|||||||
| EC50b | SIc | EC50b | SIc | |||||
| Lycoris radiata | Amaryllis | Stem cortex | Ethanol | 886.6 (±35.0) | 2.4 (±0.2) | 370 | 2.1 (±0.2) | 422 |
| Artemisia annua | Compositae | Whole plant | Ethanol | 1053.0 (±92.8) | 34.5 (±2.6) | 31 | 39.2 (±4.1) | 27 |
| Pyrrosia lingua | Polypodiaceae | Leaf | Chloroform | 2378.0 (±87.3) | 43.2 (±14.1) | 55 | 40.5 (±3.7) | 59 |
| Lindera aggregate | Lauraceae | Root | Ethanol | 1374.0 (±39.0) | 88.2 (±7.7) | 16 | 80.6 (±5.2) | 17 |
| Interferon alpha | >100,000 (±710.1) | 660.3 (±119.1) | >151 | 587.2 (±39.0) | >170 | |||
Determined as the concentration of the compounds that reduced the cell viability to 50% of control (cells without addition of compound). Each represents the mean value from three independent experiments. The unit for CC50 values shown in the table is μg/ml except interferon alpha, which is IU/ml.
Determined as the concentration of the compounds needed to inhibit CPE to 50% of control value (cells without viral infection). Each represents the mean value from three independent experiments. The unit for EC50 values shown in the table is μg/ml except interferon alpha, which is IU/ml.
Selectivity index (CC50/EC50).
The cytotoxicity test for active compounds was based on the cell viability after cells were treated with various concentrations of compounds, and was determined by MTS method. Vero E6 and HepG2 cells in 96-well microplates incubated with serial 10-fold dilutions of testing compounds in DMEM containing 5% FBS. Cells were allowed to grow for an additional 72 h before the measurement. The CC50 values were determined as the concentration of the compounds that reducing the cell viability to 50% of control (cells without addition of compound). For the four active compounds, L. radiata, A. annua, P. lingua, and L. aggregata, the CC50 values range from 886.6 ± 35.0 up to 2378.0 ± 87.3 μg/ml in assays using Vero cells (Table 1). The selective index (SI), which was determined as the ratio of CC50 versus EC50 for one of the potent active compound extracts, L. radiata, is more than 300. Three others also showed good SI values, with the exception of L. aggregata. In order to examine compound toxicity to different cell types, we tested all four extracts on both Vero E6 and human HepG2 cell lines. The CC50 values of L. radiata, A. annua, P. lingua, and L. aggregata were 690.5 ± 21.0, 1022.9 ± 55.1, 2127.3 ± 178.9, and 1159.0 ± 93.3 μg/ml, respectively. The data obtained from these two cell lines were very similar (Table 1). The results suggested that there is no significant difference in compound toxicity against these two types of cell lines.
The results described above were based on the assays and screening of herb extracts, which are mixtures of many compounds. It would be quite interesting to identify effective component(s) with antiviral activities. One of the active samples which showed better inhibition effect in anti-SARS-CoV screening, L. radiata extract, was chosen for further identification of the active component in it. We first isolated the total alkaloid from L. radiata according to previous reports (Hong and Ma, 1964). We then examined its antiviral activity. This was based on previous findings that the majority of bioactive components of L. radiata were from its alkaloid fraction (Takagi et al., 1968; Miyasaka and Hiramatsu, 1980; Cortese et al., 1983). From CPE/MTS assays, the isolated alkaloid showed potent inhibitory activity against SARS-CoV infection (Table 2 ). Further separation of the total alkaloid compounds by RP-HPLC (MeOH–H2O, 5–95% gradient) using a Shimadzu 10A-VP HPLC system (Shimadzu Co.) generated four fractionated samples, A, B, C, and D. The four fractions were collected and re-examined for their anti-SARS-CoV activity. The results from this round of testing indicated that fraction B had significant inhibitory activity against SARS-CoV while others had none (Fig. 2 ). Visual observation also showed that the CPE induced by virus infection on Vero E6 cells was blocked by addition of either alkaloid sample or fractioned sample B (data not shown). The results suggested that fraction B contained a substance with activity against SARS-CoV, which might be what we were searching for. To identify an active antiviral substance from the fraction B of the alkaloid sample isolated from L. radiata, a LC–MS/MS on a Thermo Finnigan LCQ DECA XP was applied for compound analysis. The mass analysis results revealed one major peak with m/z 287, which suggested only a dominant single compound in the fraction. Further analysis found that the mass of this substance matched the mass of lycorine [C16H17NO4] (Fig. 3 ) (Ali et al., 1981). A chromatogram of lycorine showed the same elution profile on RP-HPLC as that of the main peak of fraction B (data not shown). We then tested commercially available lycorine (NICBP, Beijing) in CPE/MTS assay to check inhibition effect on the CPE induced by the virus. The results (Table 2) showed that commercial lycorine and isolated lycorine possessed similar antiviral activities against SARS-CoV. The EC50 values for commercial and isolated lycorine are 48.8 ± 3.6 and 15.7 ± 1.2 nM, respectively. The cytotoxicity assays also generated results indicating the similarity. The SI values for the two compounds are around 900. All these demonstrated that lycorine was the effective component in L. radiata to inhibit the CPE induced by SARS-CoV.
Table 2.
Effects of components isolated from L. radiata on SARS-CoV (BJ-001)
| Samples | EC50a | CC50b | SIc |
|---|---|---|---|
| Total alkaloid from Lycoris radiata | 1.0 (±0.1) | 93.9 (±7.4) | 94 |
| Commercial lycorine | 48.8 (±3.6) | 43210.0 (±2101.0) | 885 |
| Isolated lycorine from Lycoris radiata | 15.7 (±1.2) | 14980.0 (±912.0) | 954 |
Determined as the concentration of the compounds needed to inhibit CPE to 50% of control value (cells without viral infection). Each value represents the mean ± S.D. from three independent experiments. The unit for EC50 values shown in the table is μg/ml for total alkaloid from L. radiata, and is nM for both forms of lycorine.
Determined as the concentration of the compounds that reducing the cell viability to 50% of control (cells without addition of compound). Each value represents the mean ± S.D. from three independent experiments. The unit for CC50 values shown in the table is μg/ml for total alkaloid from L. radiata, and is nM for both forms of lycorine.
Selectivity index (CC50/EC50).
Fig. 2.
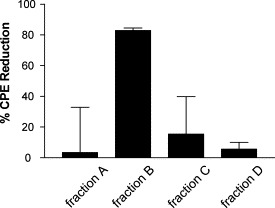
Effect of fraction A, B, C, and D of L. radiata on inhibition of CPE caused by SARS-CoV in Vero E6 cells. OD value was measured in a CPE assay as described under experimental procedures. The percentage of CPE reduction was calculated by subtracting the mean of infected cell control (0%) from the measured absorbance. The resulting number was divided by the uninfected cell control (100%). The mean values and the standard deviation (S.D.) are shown in the figures. Data presented are the average of duplicate values from three independent experiments.
Fig. 3.
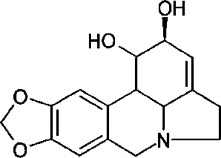
Chemical structure of lycorine.
Some previous research has provided good evidence that natural herbs are good sources for antiviral compounds. Glycyrrhizin, an active component of licorice roots, has been reported to show antiviral activity against SARS-CoV in vitro (Cinatl et al., 2003a), but the EC50 for the inhibition of viral infection is very high (300 μg/ml). Plant extracts of eucalyptus, Lonicera japonica, and Ginsenoside-Rb1, one of the pharmacologically active components of Panax ginseng, were also recently reported to show activity against the SARS-CoV at the concentration of 100 μM (Wu et al., 2004). In our experiments, we screened hundreds of natural products and found that four extracts of Chinese herbs used in traditional Chinese medicine exhibited anti-SARS-CoV activity in Vero cell-based assays. The EC50 values range from 2.4 ± 0.2 to 88.2 ± 7.7 μg/ml, which is much better than the value reported for Glycyrrhizin. Among the four of them, L. radiata shows the best potency (EC50: 2.4 ± 0.2 μg/ml) and has great SI value (>300). The results suggest that these herbs or herb extracts, especially from L. radiata, might be good candidates for antiviral medicine. Furthermore, the RP-HPLC purification of L. radiata extract and LC–MS analysis of samples, in combination with CPE/MTS assays, led to the identification of lycorine as a potent antiviral compound against SARS-CoV. The EC50 value (15.7 ± 1.2 nM) from viral inhibition assay was lower that of original crude extract, which was expected for a single active component. Several previous reports have demonstrated that lycorine inhibits the Poliomyelitis virus and the Herpes Simplex virus (type I) (Ieven et al., 1982; Renard-Nozaki et al., 1989). Our study provides new evidence that this compound also has antiviral activity against SARS-CoV. Altogether, lycorine seems to be a compound with broad antiviral activities. Lycorine inhibits SARS-CoV in CPE inhibition assays at a concentration well below that of Glycyrrhizin. The CC50 of lycorine in the Vero E6 and HepG2 cell lines tested are 14980.0 ± 912.0 and 18810.0 ± 1322.0 nM, respectively. The SI value is higher than 900. All these results suggest that lycorine has properties that meet the needs to be an antiviral drugs. However, the mechanism of antiviral activity of these active compounds is not clear. In the future, it could be worthwhile to investigate how these compounds, especially lycorine, interact with expressed viral proteins and antigens. It is also valuable to further evaluate these compounds with other antiviral assays, such as virus yield reduction and/or real time PCR for viral RNA replication.
In conclusion, the compounds extracted from A. annua, L. radiata, P. lingua, and L. aggregata have been identified to show antiviral activity against SARS-CoV in Vero cell-based CPE/MTS screening. Further structure and activity study has determined that lycorine is an active component in the alkaloid portion of the herbal plant L. radiata. The results from our study provide strong support for the usage of these herbs to treat SARS-CoV infectious diseases. Our results also demonstrated that lycorine is a good candidate for the development of new antiviral medicine.
Acknowledgements
We thank Dr. Qingyu Zhu (the Academy of Military Medical Sciences, Beijing) for providing us the virus samples. This study was supported by funding from the National High Technology Research and Development Program of China (863 program; No: 2003AA208217) and the One Hundred Scientist Plan of Chinese Academy of Sciences.
References
- Ali A.A., Kating H., Frahm A.W., El-moghazi A.M., Ramadan M.A. Two non-hydroxylated alkaloids in Crinum augustum. Phytochemistry. 1981;20:1121–1123. [Google Scholar]
- Chan-Yeung M., Yu W.C. Outbreak of severe acute respiratory syndrome in Hong Kong Special Administrative Region: case report. BMJ. 2003;326:850–852. doi: 10.1136/bmj.326.7394.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese I., Renna G., Siro-Brigiani G., Poli G., Cagiano R. Pharmacology of lycorine. 1. Effect on biliary secretion in the rat. Boll. Soc. Ital. Biol. Sper. 1983;59:1261–1264. [PubMed] [Google Scholar]
- Cory A.H., Owen T.C., Barltrop J.A., Cory J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- Donnelly C.A., Ghani A.C., Leung G.M., Hedley A.J., Fraser C., Riley S., Abu-Raddad L.J., Ho L.M., Thach T.Q., Chau P., Chan K.P., Lam T.H., Tse L.Y., Tsang T., Liu S.H., Kong J.H., Lau E.M., Ferguson N.M., Anderson R.M. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Hong S.H., Ma G.N. Studies on the alkaloids of Amaryllidaceae III: alkaloids and new alkaloids – Squamigerine isolated from Lycoris squamigera Maxim and two other Amaryllidaceae species. Acta Pharm. Sin. 1964;11:1–5. [PubMed] [Google Scholar]
- Ieven M., Vlietinck A.J., Vanden Berghe D.A., Totte J., Dommisse R., Esmans E., Alderweireldt F. Plant antiviral agents. III. Isolation of alkaloids from Clivia miniata Regel (Amaryllidaceae) J. Nat. Prod. 1982;45:564–573. doi: 10.1021/np50023a009. [DOI] [PubMed] [Google Scholar]
- Jassim S.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- Khabar K.S., al-Zoghaibi F., Dzimiri M., Taha M., al-Tuwaijri A., al-Ahdal M.N. MTS interferon assay: a simplified cellular dehydrogenase assay for interferon activity using a water-soluble tetrazolium salt. J. Interferon Cytokine Res. 1996;16:31–33. doi: 10.1089/jir.1996.16.31. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Lin L., Han Y., Yang Z.M. Clinical observation on 103 patients of severe acute respiratory syndrome treated by integrative traditional Chinese and Western medicine. Zhong guo Zhong Xi Yi Jie He Za Zhi. 2003;23:409–413. [PubMed] [Google Scholar]
- McCutcheon A.R., Roberts T.E., Gibbons E., Ellis S.M., Babiuk L.A., Hancock R.E., Towers G.H. Antiviral screening of British Columbian medicinal plants. J. Ethnopharmacol. 1995;49:101–110. doi: 10.1016/0378-8741(95)90037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka K., Hiramatsu Y. Pharmacological studies of lycorenine, an alkaloid of Lycoris radiata Herb: II. Effects of blood pressure in rats and dogs and the mechanism of tachyphylaxis to the vasodepressor action of lycorenine in rats. Jpn. J. Pharmacol. 1980;30:655–664. doi: 10.1254/jjp.30.655. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Renard-Nozaki J., Kim T., Imakura Y., Kihara M., Kobayashi S. Effect of alkaloids isolated from Amaryllidaceae on herpes simplex virus. Res. Virol. 1989;140:115–128. doi: 10.1016/s0923-2516(89)80089-5. [DOI] [PubMed] [Google Scholar]
- Scagnolari C., Vicenzi E., Bellomi F., Stillitano M.G., Pinna D., Poli G., Clementi M., Dianzani F., Antonelli G. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antiviral Ther. 2004;9:1003–1011. [PubMed] [Google Scholar]
- Takagi S., Katagi T., Takebayashi K. Gas liquid chromatography of alkaloids. II. Quantitative analysis of alkaloids of Lycoris radiata herb. Chem. Pharm. Bull. (Tokyo) 1968;16:1121–1123. doi: 10.1248/cpb.16.1121. [DOI] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Vlietinck A.J., Vanden Berghe D.A. Can ethnopharmacology contribute to the development of antiviral drugs? J. Ethnopharmacol. 1991;32:141–153. doi: 10.1016/0378-8741(91)90112-q. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Li Y., Chen R., Li S., Zhong S., Zhong N. A retrospective study of 78 patients with severe acute respiratory syndrome. Chin. Med. J. 2003;116:805–810. [PubMed] [Google Scholar]
- Wu C.-Y., Jan J.-T., Ma S.-H., Kuo C.-J., Juan H.-F., Cheng Y.-S.E., Hsu H.-H., Huang H.-C., Wu D., Brik A., Liang F.-S., Liu R.-S., Fang J.-M., Chen S.-T., Liang P.-H., Wong C.-H. Small molecules targeting severe acute respiratory syndrome human coronavirus. PNAS. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.H., Guo Y.B., Wu H., Li X.H., Guo X.H., Jin R.H., Dign H.G., Meng Q.H., Lang Z.W., Wang W., Yan H.P., Huang C., Liu D.G. Clinical manifestation, treatment, and outcome of severe acute respiratory syndrome: analysis of 108 cases in Beijing. Zhonghua Yi Xue Za Zhi. 2003;83:897–901. [PubMed] [Google Scholar]
- Zhong N.S., Zeng G.Q. Our strategies for fighting severe acute respiratory syndrome (SARS) Am. J. Respir. Crit. Care Med. 2003;168:7–9. doi: 10.1164/rccm.200305-707OE. [DOI] [PubMed] [Google Scholar]


