Abstract
Virologists have benefited from large-scale profiling methods to discover new host–virus interactions and to learn about the mechanisms of pathogenesis. One such technique, referred to as activity-based protein profiling (ABPP), uses active site-directed probes to monitor the functional state of enzymes, taking into account post-translational interactions and modifications. ABPP gives insight into the catalytic activity of enzyme families that does not necessarily correlate with protein abundance. ABPP has been used to investigate several viruses and their interactions with their hosts. Differential enzymatic activity induced by viruses has been monitored using ABPP. In this review, we present recent advances and trends involving the use of ABPP methods in understanding host–virus interactions and in identifying novel targets for diagnostic and therapeutic applications.
Deciphering host–viral interactions
Viruses constantly adapt to and transform their host environment to enable their replication and propagation 1, 2, 3, 4, 5, 6, 7, 8. They do so, in part, through molecular interactions with host proteins that often modify their regular endogenous function. Although viral genomes were the first to be completely sequenced – owing to their relatively small size [9] – their encoding proteins and the modified proteomes of their host are only now being elucidated 10, 11. These studies have revealed some fascinating intricacies such as multifunctional enzymes [12] and alternative open reading frames within viral genomes 13, 14. With the successful sequencing of the human genome and the genome of other model host organisms, the current challenge is to be able to monitor the effects of viral infections on the host proteome, to uncover novel host–virus interactions and to discover how these perturbations affect cell function, viral propagation and disease progression. Ultimately, new methods are being developed to obtain a better understanding of the mechanisms of viral pathogenesis.
Conventional abundance-based viral proteomics
Similar to microarray technology that enabled global gene expression analyses, advances in high-throughput mass spectrometry (MS) technologies have revolutionized both specific and global protein analyses. Coupled with 2D gel electrophoresis, LC-MS/MS (see Glossary) analysis of tryptic fragments derived from protein digests has been commonly used to detect abundance changes in both viral and host proteomes in response to viral infections 15, 16, 17, 18, 19, 20, 21. This technique has unveiled several proteins that play important roles during the pathogenesis of human immunodeficiency virus (HIV) [22], hepatitis C virus (HCV) [23], severe acute respiratory syndrome (SARS) [24], encephalomyocarditis virus [25], and simian virus 40 [26]. To overcome gel-to-gel variation, 2D differential gel electrophoresis technique (2D-DIGE) has been developed to study differences between two proteome samples (i.e. pathogenic versus non-pathogenic). In the 2D-DIGE approach, both samples are labeled with a unique fluorescent dye and are subsequently detected in the same 2D gel. Differentially expressed or sample-specific proteins are readily detected and analyzed based on their distinctive colored spots [27]. 2D-DIGE has been applied to study the host–virus interactions in several viral infections such as HIV [28], Dengue virus [19], and SARS [20].
Precise quantification of differentially expressed proteins is difficult to obtain by simply examining a gel. Alternatively, gel-free methods such as ‘stable isotope labeling with amino acids in cell culture’ (SILAC) have been used to quantify more accurately the intricate changes in host proteins caused by viral infections 29, 30, 31, 32, 33. Specifically, SILAC has been utilized to detect changes in both viral and host proteomes in response to many viral infections including those with coronaviruses [29], HIV [32], influenza A virus [31], pseudorabies virus [18], and HCV [34].
Despite the significant findings resulting from these abundance-based approaches, all of these examples fail to capture many dynamic changes such as post-translational modifications, proteolytic processing, and association with cofactors and regulatory proteins that influence the activity of enzymes and their zymogens. Applications of activity-based protein profiling (ABPP) for studying host–viral systems have identified host enzymes that are differentially regulated during viral propagation. For example, ABPP has been used to identify human carboxylesterase 1 (CES1) being essential for efficient HCV propagation [35], and has been used to aid characterization of the previously unknown ubiquitin protease catalytic active site of the tegument protein of several herpesviridae members 36, 37, 38, 39, 40. Also, ABPP has led the discovery of a new promising antiviral tetrahydroquinoline oxocarbazate cathepsin L inhibitor that abolishes SARS and Ebola virus entry [41] (Table 1 ).
Table 1.
Representative activity-based probes and their application in diagnostic/therapeutic virology
| Probe structure | Enzyme class | Virus | Applications | Refs |
|---|---|---|---|---|
|
Mechanism-based ABPs | ||||
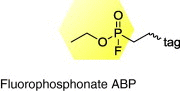 |
Serine hydrolases |
HCV |
CES1 influences cellular lipid metabolism during HCV replication and infection |
[35] |
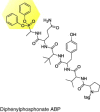 |
Kaposi-sarcoma-associated herpesvirus proteases |
HHV |
Active site inhibition regulates the binding affinity of monomer-dimer equilibrium at the spatially separate dimer interface of the protease |
[77] |
|
Substrate-based ABPs
| ||||
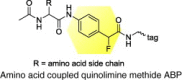 |
Proteases, hydrolases, oxidoreductases and isomerases |
HCV |
Identified several differentially active proteins during HCV replication |
[57] |
 |
USPs and UCHs |
EBV and HPV1 |
Activities of USP5, -7, -9, -13, -15 and -22 as well as UCH-L1 and -L3 are upregulated during viral infections |
[62] |
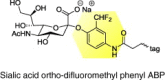 |
Neuraminidases |
Influenza A virus (H1N1) |
Mechanism-based ABPP of neuraminidases |
[71] |
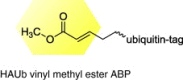 |
USP and UCH | HPV1 |
UCH-L1, UCH-L3, USP7, and USP9X show enhanced activity following transduction of HPV E6/E7 |
[63] |
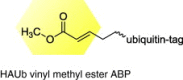 |
HSV-1 |
Identified UL36USP, encoded by HSV-1 genome. UL36USP activity peaks at late stages of viral replication and appears to require proteolytic processing from full-length UL36. |
[37] |
|
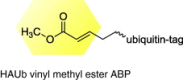 |
human cytomegalovirus |
Identified a high molecular weight protein as a functional deubiquitinase and that this enzyme activity was not absolutely essential for production of infectious virus. |
[39] |
|
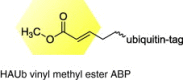 |
MDV |
Identification of the catalytic active cysteine of the MDV large tegument protein and the oncogenic potential of MDV in chickens |
[40] |
|
 |
Cysteine proteases |
Ebola virus and SARS-CoV |
Inhibitor of human cathepsin L that blocked SARS-CoV and Ebola pseudotype virus entry into human cells |
[41] |
 |
Caspase cysteine proteases |
murine norovirus (MNV-1) |
Identification of cathepsin B as upstream activator of the intrinsic apoptotic pathway induced by MNV |
[79] |
|
Non-directed ABPs
| ||||
 |
Isomerase, nucleotide binding, electron transport, structural, chaperone, hydrolase, transferase, oxidoreductase and protein binding | HCV | Identified nine differentially active enzymes during HCV propagation | [55] |
Abbreviations: ABP, activity-based probe; HAUb, hemagglutinin-tagged ubiquitin; HCV, hepatitis C virus; UCH, Ubiquitin C-terminal hydrolase; USP, Ubiquitin specific protease.
ABPP
ABPP was developed to determine the changes in the catalytic state of enzymes in complex proteomes 42, 43, 44 and to ascribe previously unknown enzymatic functions to proteins [45]. ABPP uses active-site-directed covalent probes (activity-based probes, ABPs) consisting of a reactive group linked to a reporter tag (Figure 1a). Often, these ABPs are designed with the intention of exploiting conserved mechanistic features of their targeted enzyme superfamily 42, 46, 47, 48. The reaction between the ABP and its active protein target usually results in an irreversible covalent bond (Figure 1c), which facilitates subsequent analysis through the reporter tag. The reporter tag is generally a fluorophore for detection and visualization by in-gel fluorescence scanning, or biotin for purification of labeled enzymes (Figure 1a).
Figure 1.
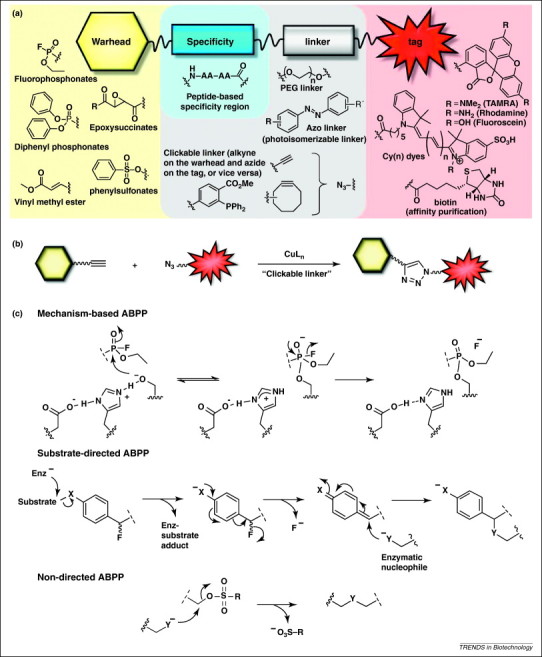
ABPP is a functional proteomics technique that uses ABPs to react covalently with the active site of mechanistically related classes of enzymes. (a) The chemical structure of an ABP consists of two essential components: a warhead reactive group (e.g. small molecule inhibitors, substrate-based scaffolds or protein-reactive molecules) that covalently targets the catalytic amino acid residue of an enzyme active site, and a reporter tag (fluorophore or biotin) for detection or purification. The linker region is a flexible chain of varying length and hydrophobicity that connects and acts as a spacer between the warhead and the bulky fluorophore tag. (b) A clickable linker most commonly exploits copper-catalyzed N3–alkyne cycloaddition to couple a chemically inert alkyne (≡) group on the ABP to an N3 group on the reporter tag. Click chemistry has been applied to the field of ABPP, allowing the active proteome to be labeled in situ and in vivo. (c) Examples of the three main classes of ABPs and reaction with their respective enzyme targets. Upper panel: mechanism-based ABPs are based on irreversible enzyme inhibitors, such as fluorophosphonate, and form a covalent bond with the catalytic nucleophile amino acid residue in the active site of the targeted enzyme. Depicted here is a fluorophosphonate ABP targeting a member of the serine hydrolase superfamily. Middle panel: substrate-directed ABPs depend on substrate-based scaffolds (usually an amino acid residue or a peptide, such as ubiquitin) that act as a specificity region targeting the probe to the catalytic active site of the enzyme. Following recognition of the substrate-based scaffold, the catalytic nucleophile of the enzyme (Enz–) cleaves the scissile bond in proximity of the substrate scaffold to generate anion (X–) that facilitates departure of the fluoride leaving group (F–), generating a highly reactive electrophilic quinolimine methide intermediate, that reacts with a nucleophilic residue (Y–) within the enzyme active site to bind covalently the probe to the enzyme. Lower panel: non-directed ABPs contain a mild reactive group of electrophiles, such as sulfonate ester, that have intermediate reactivity; enough to modify the catalytic nucleophile amino acids in their activated state, but low enough to prevent unspecific labeling of other nucleophile residues outside the active site. Depicted here is the sulfonate ester ABP that targets several mechanistically distinct classes of enzymes. A more detailed description of the different ABP classes, the history and discovery of warhead reactive groups and the ABP labeling mechanisms can be found elsewhere [42].
ABPs are divided into at least three different classes based on the nature of their warhead reactive groups: mechanism-based ABPs, substrate-based ABPs and non-directed ABPs (Figure 1c and Table 1). Mechanism-based ABPs (also known as directed ABPs) contain an electrophilic reactive group that forms a covalent bond to the catalytic amino acid residue of the target enzyme active site (Figure 1c). Examples of class-wide mechanism-based ABPP probes include fluorophosphonate, phosphonate esters, modified fluoroglycosides, and natural products like wortmannin and microcystin that target serine hydrolase, glycosidase, kinase and threonine phosphatase families, respectively 49, 50, 51, 52.
Although mechanism-based ABPs are effective in targeting enzyme families with known covalent inhibitors, many enzyme classes do not use conserved active-site nucleophiles or electrophiles to catalyze their enzymatic reactions and thus cannot form covalent adducts with mechanism-based suicide inhibitors. To broaden the number of enzyme classes addressable by ABPP, non-directed ABPs that contain mild electrophilic reactive group were developed (Figure 1c) 53, 54. For example, non-directed sulfonate ester 53, 55, α-chloroacetamide [56], and spiroepoxide [10] probes have been used to successfully label different enzyme families with various cellular roles.
Substrate-based ABPs are another class of ABPs that exploit the versatility of chemical scaffolds that take advantage of substrate selectivities within certain families of proteins (usually an amino acid residue or peptide) (Figure 1a). Substrate-based scaffolds can be used as a specificity region to target the probe to the enzyme active site (Figure 1c) 57, 58, 59, 60, 61. By targeting a multitude of protein candidates with diverse range of functions and substrate preferences, this provides a better understanding of the degree to which viruses utilize host enzymes for replication and infection.
Several ABPP studies using these three ABP classes have recently been conducted in various viral systems (Table 1). Herein, we highlight these with a focus on the various applications of ABPP including identification of dysregulated enzyme functions as a result of viral infection, assigning catalytic functions to previously uncharacterized viral proteins, visualization of the altered active proteome in situ during viral disease progression, and developing new antiviral therapeutics and viral diagnostics.
Comparative ABPP during viral infection
Comparative ABPP (Figure 2a) is the most common use of ABPP in virology (Table 1). This approach, originally applied towards profiling applications, compares the differential activity between healthy and virus-infected host proteomes. Viral proteins and their altered host proteome are good candidates for comparative ABPP analyses, because viruses contain a manageable number of proteins, and viral infections involve coordinated host–virus interactions (e.g. attachment, cell entry, uncoating, replication, expression, virion assembly, and exit).
Figure 2.
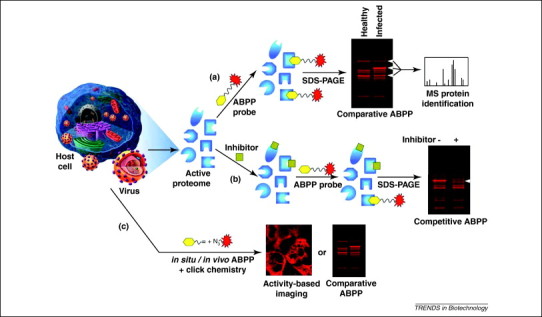
Applications of ABPP to study viral replication and infection. (a) Comparative ABPP has been extensively used to screen for differentially active enzymes during viral propagation with the potential to become therapeutic and diagnostic candidates. (b) Competitive ABPP is being used to screen inhibitor libraries and determine the specificity and sensitivity of potential therapeutics against both host and viral enzymes involved in the viral life cycle. (c) Comparative and imaging ABP probes have been used in situ by using the bioorthogonal click chemistry to monitor the variability of subcellular enzymatic activity induced by viral pathogenesis and the general experimental flow is depicted.
Several ABPs, such as the influenza hemagglutinin-tagged ubiquitin (HAUb)-derived bromoethylamine [62], HAUb vinylmethyl ester 62, 63 and fluorophosphonate [35] probes have been successfully used in comparative ABPP during viral infection (Table 1). The application of ABPP towards the deubiquitinating enzyme family is of significant importance, because unlike Ub conjugation, very little is known about the removal of Ub from Ub conjugates and how they influence cellular processes during an infection. The HAUb group of the probe (Table 1) is the substrate specificity region (Figure 1a) that targets the probe to the active site of ubiquitin-specific protease (USP). The catalytic cysteine residue undergoes 1,4-conjugate addition with the vinylmethyl ester warhead group (Figure 1a), creating a covalent bond with the probe (Figure 1c, middle panel) [64]. By comparing the activity profiles of these ABPs in presence or absence of Epstein–Barr virus (EBV) and human papilloma virus (HPV) infections, several deubiquitinating enzymes have been shown to be upregulated, such as USP-5, -7, -9, -13 and -15 [62], as well as Ub carboxyl-terminal hydrolases (UCH)-L1 and -L3 [63]. One identified target, UCH-L1, is only upregulated very late during viral infection, correlating with increased cellular growth. The use of ABPP in human lymphomas and cervical cancer biopsies to measure the increased activity of UCH-L1 gives a more realistic understanding of how UCH-L1 activity is linked to malignant cellular proliferation 62, 63, rather than simply monitoring the enzymatic activity in cell lines or animal models. Significant efforts are currently underway to characterize this differentially active deubiquitinating enzyme and its downstream targets to elucidate further the molecular details leading to EBV- and HPV-induced malignancy [65].
Another promising target of comparative ABPP is CES1, an endogenous liver enzyme identified with a fluorophosphonate mechanism-based ABP (Table 1, Figure 1c upper panel) to monitor the differential activity of serine hydrolases during HCV replication and infection (Figure 3a). CES1 activity not only correlates with HCV abundance, but also regulates HCV replication and infectivity in both cell culture and animal models (Figure 3b) [35]. CES1 is a multifunctional enzyme that modulates intracellular neutral lipid (e.g. triglycerides and cholesterol esters) biosynthesis by displaying triacylglycerol hydrolase [66] and acyl-CoA:acyl transferase [67] enzymatic activities. Coherent anti-Stokes Raman scattering microscopy [68] has also been used to demonstrate that CES1 overexpression gives rise to a significant increase in lipid droplet size (Figure 3b) [35]. CES1 is also involved in the transport of these neutral lipids into intracellular lipid droplets (LDs) for their subsequent secretion in very-low density lipoproteins (VLDLs) [66]. HCV is known to exploit the LD and VLDL cellular pathways for its own replication, virion assembly and budding 69, 70. Also, HCV modulates the host metabolic pathways by increasing the activity of CES1 to alter the intracellular environment for its efficient replication and propagation (Figure 3c) [35]. The ABPP discovery of CES1 and its role in increasing the intracellular lipid content during HCV propagation provides a better understanding of the development of liver steatosis (i.e. fatty liver), a phenotypic outcome found in many HCV-infected patients. Additionally, CES1 presents itself as a possible host target for the development of new antiviral therapeutics.
Figure 3.
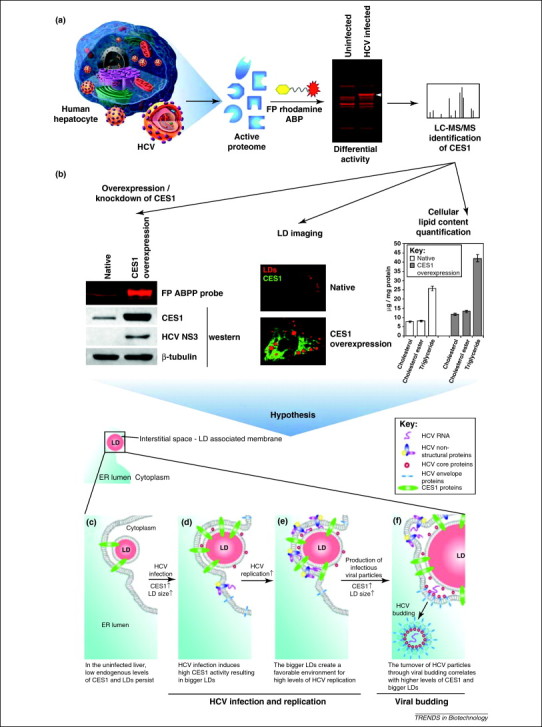
Schematic stepwise example of how the altered function of a differentially active protein identified by ABPP is linked to viral pathogenesis, such as HCV [35]. (a) The activity profile of serine hydrolases during HCV replication was obtained with a fluorophosphonate (FP) ABP. Following gel separation and comparison of the labeled proteomes from non-infected and HCV-infected hepatocytes, a differentially labeled protein was identified by LC-MS/MS to be human CES1. (b) To determine the effect of CES1 activity on HCV propagation, the expression of CES1 was knocked down and overexpressed while monitoring the levels of HCV. As CES1 activity was found to influence the HCV life cycle, its specific role during HCV propagation was narrowed down to the metabolism of inert lipids [i.e. cholesterol esters (CEs) and triglycerides (TGs)] and their storage into intracellular LDs. (c–f) Based on the findings that CES1 favors CE and TG loading in LDs, a hypothetical synergy between CES1 and HCV has been suggested: the HCV-induced high expression of the endoplasmic reticulum (ER) protein CES1 loads TGs and CEs in LDs, increasing their size and number (d). (e) Upon LD saturation with these inert lipids, the ER–LD interstitial space increases, creating a favorable environment where high HCV replication occurs. (f) At the viral budding stage of HCV, an increase in the turnover dynamics of HCV particles allows CES1 and HCV to occupy the abundant LD–ER interface abundantly.
Non-directed comparative viral ABPP
Although mechanism-based ABPP is effective in analyzing enzymes with known covalent inhibitors, substrate-based and non-directed probes have also been applied to broaden the activity-based profiling of host enzyme families that could be involved in viral replication and infection (Table 1). By applying a non-directed sulfonate ester and a variety of substrate-based amino acid coupled quinolimine methide ABPs, we have identified several differentially active proteins during HCV replication that encompass many enzyme families 55, 57. The broader specificity of the substrate-based ABP is likely attributed to the single amino acid composition of the probe and the electrophilic properties of the quinolimine methide intermediate [57]. The specificity of this probe would be improved by substituting the single amino acid with a di- or oligopeptide substrate, as shown with a tripeptide epoxide [41] and a hexapeptide diphenylphosphonate [71] ABPs discussed further below. Of the identified ABPP targets, heat shock protein 70, nuclear distribution gene C homolog, chaperonin containing TCP1, protein disulfide isomerase 1 and 6, cathepsin D and isochorismatase domain containing 1 have been observed to have altered activity during HCV replication, with virtually unchanged abundance. These differential activities, which are probably attributed to post-translational modifications or cofactor/inhibitor association, would have been undetected by other conventional proteomic techniques. The identification of these non-directed ABPP targets suggests that both combinatorial and mechanism-based ABPP methods can accelerate the discovery of protein activities associated with the pathogenic states of viral infections.
In situ/in vivo viral ABPP applications through click chemistry
The early versions of ABPs were designed with bulky reporter groups (i.e. fluorophore or biotin), which limited their uptake and distribution in cells and tissues. Hence, the initial ABPP protocols restricted the usage of ABPs to in vitro experiments from homogenized cells or tissues. This resulted in a loss of cellular compartmentalization and altered the biodistribution of cofactors, inhibitors and binding partners essential for enzymatic regulation and probably did not represent the true functional state of the targeted enzymes 72, 73. To provide a more physiologically relevant enzymatic profile, the enzymes needed to be assessed within the context of their native subcellular environments. To accomplish this, in vivo ABPs containing small bio-orthogonal reporter groups, typically terminal alkyne (≡) or azide (N3) (Figure 1b), were developed. The small size of the bio-orthogonal reporter group greatly improves the ABP cell permeability and distribution in cells for in situ or in vivo proteome labeling. To visualize or isolate the enzymes bound to the alkyne ABPs, the alkyne-labeled proteome is subsequently reacted with N3 containing a reporter tag via a copper-catalyzed azide–alkyne cycloaddition, commonly referred to as a ‘click chemistry’ reaction (Figure 1b) [74]. The in situ ABPP labeling technique has been shown by our group using a non-directed sulfonate ester alkyne (PS4≡) ABP during HCV replication and delivered significant labeling disparities between in vitro and in situ ABPP [55]. The number of protein candidates labeled in situ with the PS4≡ ABP doubled when compared to the in vitro labeling of the cell homogenate [55]. This demonstrates that catalytic activity is greatly influenced by the structural integrity of the enzyme and its native subcellular environment, and illustrates a key advantage of in situ and in vivo ABPP.
ABPP function assignment of uncharacterized viral proteins
The relatively large DNA genomes of herpes viruses with 100–200 open-reading frames have made them a popular virus group for characterization by ABPP of novel catalytic functions of expressed viral gene products. Important advances have recently been made in characterizing the multifunctional activities of the highly variable tegument protein of several herpesviridae members using an HAUb vinylmethyl ester ABP (Table 1) 36, 37, 38, 39, 40. Despite sharing only 15% sequence similarity between some herpesviridae members [38], ABPP is able to identify an important conserved deubiquitinating enzymatic activity in the N-terminal segment of the murine [38] and human [39] cytomegaloviruses, EBV [38], herpes simplex virus 1 (HSV-1) [37], chicken Marek's disease virus (MDV) [40] and murine gammaherpesvirus 68 [36]. In one of these studies, the use of both mutagenesis and the HAUb vinylmethyl ester ABP led to the identification of the catalytically active cysteine of the MDV large tegument protein, and linked it to the oncogenic potential of MDV in chickens [40]. The deubiquitinating activity of these herpesviridae tegument proteins is believed to not only be implicated in viral gene transcription, cell cycle regulation and virion budding, but also in tumor development. Despite the immense complexity of the herpes genome, the highly conserved deubiquitinating catalytic site of the tegument protein among alpha-, beta-, and gamma-herpesviruses is of great potential for antiviral therapeutic development [37].
Similarly, another group has used a hexapeptide diphenylphosphonate ABP to study the activity of a poorly understood Kaposi-sarcoma-associated herpesvirus (human herpesvirus 8; HHV-8) protease (Table 1) [71]. By characterizing the active site of HHV-8 protease through ABPP, its catalytic activity has been shown to depend on the quaternary structure of the resultant dimer, and that the active sites of the monomer are catalytically independent. The discovered link between the HHV-8 protease quaternary structure and catalytic activity offers an alternate inhibition strategy that could use the hexapeptide diphenylphosphonate ABP for developing and screening of substoichiometric inhibitors that could destabilize the dimeric interface [71].
Competitive ABPP for antiviral inhibitor screening
Given that viruses require a host for their propagation, they are more vulnerable to inhibition of cellular pathways. Conventional antiviral drugs have focused on selectively targeting viral gene products to minimize side effects experienced by the patient. The limited number of viral targets and the rapid emergence of drug-resistant viral mutations necessitate the search for host cell targets as alternatives for antiviral therapeutic development. As host–virus interactions become more apparent, ABPP can be used to screen for inhibitors of novel host targets through competitive assays. In these assays, relatively more potent inhibitors have been identified by their ability to block access of the ABP to the enzyme active site (Figure 2b).
Competitive ABPP has recently been applied for screening and testing antiviral drugs against SARS coronavirus (CoV) and the Ebola pseudotype virus. A biotin tripeptide epoxide ABP (biotin-Lys-C5 alkyl linker-Tyr-Leu-epoxide or DCG-04) (Table 1) has been used to determine the efficacy of a novel tetrahydroquinoline oxocarbazate CID 23631927 inhibitor against its host human cell cathepsin L target [41]. The endosomal cathepsin L is an appealing target for antiviral development, because it mediates viral entry by triggering the fusion of the plasma membrane with endosomes via proteolysis 75, 76. The DCG-04 ABP revealed that the tetrahydroquinoline oxocarbazate inhibitor was cell permeable and reduced the activity of cathepsin L by 38% [41]. These findings demonstrate a potential role for DCG-04 ABP for identifying novel inhibitors, such as oxocarbazate inhibitor CID 23631927 that is a subnanomolar, slow-binding, reversible inhibitor of human cathepsin L, which prevents SARS and Ebola virus entry into human cells [41]. Further applications of the DCG-04 ABP could be to explore the mechanisms of viral entry and screen for other potent inhibitors.
Similarly, ABPs that target extracellular membrane proteins have been used in viral diagnostic development. A substrate-based sialic-acid-containing ABP probe incorporating an ortho-difluoromethylphenyl trapping moiety (Table 1) has been immobilized to the surface of a microplate well and has successfully captured influenza virion particles through their neuraminidase surface glycoproteins [77]. The immobilization of influenza virion particles to functionalized surfaces via ABPs has the potential to accelerate the simultaneous screening of hundreds of antiviral antibodies and drugs. Furthermore, ABPs, such as substrate-based probes containing sialic acid and ortho-difluoromethylphenyl trapping moiety, could also influence viral diagnostics by generating unique activity profiles for dozens of viral species and strains to be matched against unknown specimens for identification.
ABPP for viral pathogenesis imaging
The combination of live cell imaging with ABPP gives researchers the ability to monitor rapidly changes of subcellular enzymatic activity during viral pathogenesis in real time (Figure 2c). Although not based on click chemistry (Figure 1b), the sulforhodamine valylalanylaspartic acid fluoromethyl ketone (SR-VAD-fmk) poly caspase ABP (Table 1) is cell-membrane-permeable [78], making it suitable for in situ and in vivo ABPP. The in situ application of SR-VAD-fmk on norovirus-infected mouse macrophage cells has shown that activation of caspase cysteine proteases occurred within 2 h of viral infection [79]. In vitro ABPP characterization studies have identified cathepsin B as a novel host enzyme that has increased activity during early viral infection [79]. This implies that the murine norovirus could take advantage of the cathepsin-B-induced apoptosis for its transfer in fragmented membrane bodies to neighboring cells.
Future virus ABPP perspectives
While gel-based ABPP approaches are robust enough to identify abundantly expressed proteins, they lack the ability to identify low-abundance targeted proteins. A recent ABPP gel-free approach uses 2D liquid chromatography to enrich and identify ABPP-labeled protein targets in the presence of highly abundant unlabeled host protein contaminants (ABPP–MudPIT). This high-throughput characterization of labeled proteins, combined with the simultaneous identification of the probe-labeled sites (TOP–ABPP), could speed up the active site characterization of newly identified antiviral targets for therapeutic inhibitor screening [80]. Modern virology and antiviral drug discovery are thus expected to be progressively enhanced by new research involving viral ABPP. Activity-based technology currently targets over a dozen classes of enzymes [48]. As more covalent inhibitors are discovered, the repertoire of addressable enzyme families will increase to cover a larger portion of the active proteome. ABPP is a valuable technique for providing unique information on the enzymatically active proteome, which complements other large-scale profiling methods, such as abundance-based proteomics, SILAC, microarray, and systems biology approaches. The future challenges for the application of ABPP in virology will be to determine which of the myriad of host enzymes are essential for virus infection. These newly identified ABPP candidates will represent novel targets for the development of viral diagnostics and antiviral therapeutics.
Glossary
- ABPP-multidimensional protein identification (ABPP-MudPIT)
a high-throughput gel-free ABPP approach involving biotinylated ABPs to label the active proteome. Purification of the biotinylated ABP-labeled proteins with (strept)avidin beads allows for their enrichment to identify less abundant protein targets.
- Bio-orthogonal functional group
A chemical moiety that is unreactive towards all functional groups found in biological systems but may undergo other reactions in the presence of specific molecules.
- Comparative ABPP
an ABPP application that compares two or more proteomes and analyzes their differing activities.
- Competitive ABPP
a competitive mode of ABPP in which specific inhibitors are identified by their ability to block access of the ABP to the enzyme active site.
- LC-MS/MS
liquid chromatography–tandem mass spectrometry: an analytical technique where hydrolyzed protein fragments are separated by liquid chromatography and assessed for abundance through an initial mass spectrometer. The peptides are subsequently fragmented by collision induced dissociation to determine the original peptide sequence in a second mass spectrometer.
- Proteome
an entire set of proteins expressed by a genome, cell, tissue or organism at a given time and biological state.
- Stable isotope labeling with amino acids in cell culture (SILAC)
a quantitative proteomic technique that uses non-radioactive isotope labeling and LC-MS/MS to determine protein abundance.
- Tandem-orthogonal proteolysis-ABPP (TOP-ABPP)
ABPP method to enrich specifically ABP-labeled peptide fragments while discarding the remaining digested proteome. The use of a tobacco etch virus proteolytic site in the ABP eliminates false positives to determine accurately the probe-labeled active site in the protein target.
References
- 1.Jenner R.G., Young R.A. Insights into host responses against pathogens from transcriptional profiling. Nat. Rev. Microbiol. 2005;3:281–294. doi: 10.1038/nrmicro1126. [DOI] [PubMed] [Google Scholar]
- 2.Katze M.G. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 3.Olsen B. Global patterns of influenza a virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- 4.Randall R.E., Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 5.Rehermann B., Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 6.Tortorella D. Down-regulation of MHC class I antigen presentation by HCMV; lessons for tumor immunology. Immunol. Invest. 2000;29:97–100. doi: 10.3109/08820130009062289. [DOI] [PubMed] [Google Scholar]
- 7.Sagan S.M. The influence of cholesterol and lipid metabolism on host cell structure and hepatitis C virus replication. Biochem. Cell Biol. 2006;84:67–79. doi: 10.1139/o05-149. [DOI] [PubMed] [Google Scholar]
- 8.Pezacki J.P. Host–virus interactions during hepatitis C virus infection: a complex and dynamic molecular biosystem. Mol. Biosyst. 2010;6:1131–1142. doi: 10.1039/b924668c. [DOI] [PubMed] [Google Scholar]
- 9.Sanger F. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977;265:687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- 10.Bernhard O.K. New insights into viral structure and virus–cell interactions through proteomics. Expert Rev. Proteomics. 2005;2:577–588. doi: 10.1586/14789450.2.4.577. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell K.L., Frappier L. Viral proteomics. Microbiol. Mol. Biol. Rev. 2007;71:398–411. doi: 10.1128/MMBR.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenbach B.D., Rice C.M. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 13.Lo S.Y. Comparative studies of the core gene products of two different hepatitis C virus isolates: two alternative forms determined by a single amino acid substitution. Virology. 1994;199:124–131. doi: 10.1006/viro.1994.1104. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z. Synthesis of a novel hepatitis C virus protein by ribosomal frameshift. EMBO J. 2001;20:3840–3848. doi: 10.1093/emboj/20.14.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vester D. Quantitative analysis of cellular proteome alterations in human influenza A virus-infected mammalian cell lines. Proteomics. 2009;9:3316–3327. doi: 10.1002/pmic.200800893. [DOI] [PubMed] [Google Scholar]
- 16.Pastorino B. Identification of cellular proteome modifications in response to West Nile virus infection. Mol. Cell Proteomics. 2009;8:1623–1637. doi: 10.1074/mcp.M800565-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cilia M. Genetics coupled to quantitative intact proteomics links heritable aphid and endosymbiont protein expression to circulative polerovirus transmission. J. Virol. 2011;85:2148–2166. doi: 10.1128/JVI.01504-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skiba M. Quantitative whole-cell proteome analysis of pseudorabies virus-infected cells. J. Virol. 2008;82:9689–9699. doi: 10.1128/JVI.00995-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tchankouo-Nguetcheu S. Differential protein modulation in midguts of Aedes aegypti infected with chikungunya and dengue 2 viruses. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0013149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Diepen A. Quantitative proteome profiling of respiratory virus-infected lung epithelial cells. J. Proteomics. 2010;73:1680–1693. doi: 10.1016/j.jprot.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Wang J. Proteome responses to stable hepatitis B virus transfection and following interferon alpha treatment in human liver cell line HepG2. Proteomics. 2009;9:1672–1682. doi: 10.1002/pmic.200800621. [DOI] [PubMed] [Google Scholar]
- 22.Misumi S. Three isoforms of cyclophilin A associated with human immunodeficiency virus type 1 were found by proteomics by using two-dimensional gel electrophoresis and matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Virol. 2002;76:10000–10008. doi: 10.1128/JVI.76.19.10000-10008.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takashima M. Overexpression of alpha enolase in hepatitis C virus-related hepatocellular carcinoma: association with tumor progression as determined by proteomic analysis. Proteomics. 2005;5:1686–1692. doi: 10.1002/pmic.200401022. [DOI] [PubMed] [Google Scholar]
- 24.Chen J.H. Plasma proteome of severe acute respiratory syndrome analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17039–17044. doi: 10.1073/pnas.0407992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meurs E.F. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J. Virol. 1992;66:5805–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gluck U. Suppression of tumorigenicity in simian virus 40-transformed 3T3 cells transfected with alpha-actinin cDNA. Proc. Natl. Acad. Sci. U.S.A. 1993;90:383–387. doi: 10.1073/pnas.90.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unlu M. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–2077. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 28.Ringrose J.H. Proteomic studies reveal coordinated changes in T-cell expression patterns upon infection with human immunodeficiency virus type 1. J. Virol. 2008;82:4320–4330. doi: 10.1128/JVI.01819-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emmott E. Quantitative proteomics using stable isotope labeling with amino acids in cell culture reveals changes in the cytoplasmic, nuclear, and nucleolar proteomes in Vero cells infected with the coronavirus infectious bronchitis virus. Mol. Cell. Proteomics. 2010;9:1920–1936. doi: 10.1074/mcp.M900345-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emmott E. Elucidation of the avian nucleolar proteome by quantitative proteomics using SILAC and changes in cells infected with the coronavirus infectious bronchitis virus. Proteomics. 2010;10:3558–3562. doi: 10.1002/pmic.201000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emmott E. Quantitative proteomics using SILAC coupled to LC-MS/MS reveals changes in the nucleolar proteome in influenza A virus-infected cells. J. Proteome Res. 2010;9:5335–5345. doi: 10.1021/pr100593g. [DOI] [PubMed] [Google Scholar]
- 32.Pathak S. HIV induces both a down-regulation of IRAK-4 that impairs TLR signalling and an up-regulation of the antibiotic peptide dermcidin in monocytic cells. Scand. J. Immunol. 2009;70:264–276. doi: 10.1111/j.1365-3083.2009.02299.x. [DOI] [PubMed] [Google Scholar]
- 33.Vogels M.W. Identification of host factors involved in coronavirus replication by quantitative proteomics analysis. Proteomics. 2011;11:64–80. doi: 10.1002/pmic.201000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannova P. Modification of host lipid raft proteome upon hepatitis C virus replication. Mol. Cell. Proteomics. 2006;5:2319–2325. doi: 10.1074/mcp.M600121-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Blais D.R. Activity-based protein profiling identifies a host enzyme, carboxylesterase 1, which is differentially active during hepatitis C virus replication. J. Biol. Chem. 2010;285:25602–25612. doi: 10.1074/jbc.M110.135483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gredmark S. A functional ubiquitin-specific protease embedded in the large tegument protein (ORF64) of murine gammaherpesvirus 68 is active during the course of infection. J. Virol. 2007;81:10300–10309. doi: 10.1128/JVI.01149-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kattenhorn L.M. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell. 2005;19:547–557. doi: 10.1016/j.molcel.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Schlieker C. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J. Virol. 2005;79:15582–15585. doi: 10.1128/JVI.79.24.15582-15585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J. High-molecular-weight protein (pUL48) of human cytomegalovirus is a competent deubiquitinating protease: mutant viruses altered in its active-site cysteine or histidine are viable. J. Virol. 2006;80:6003–6012. doi: 10.1128/JVI.00401-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarosinski K. A herpesvirus ubiquitin-specific protease is critical for efficient T cell lymphoma formation. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20025–20030. doi: 10.1073/pnas.0706295104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah P.P. A small-molecule oxocarbazate inhibitor of human cathepsin L blocks severe acute respiratory syndrome and ebola pseudotype virus infection into human embryonic kidney 293T cells. Mol. Pharmacol. 2010;78:319–324. doi: 10.1124/mol.110.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans M.J., Cravatt B.F. Mechanism-based profiling of enzyme families. Chem. Rev. 2006;106:3279–3301. doi: 10.1021/cr050288g. [DOI] [PubMed] [Google Scholar]
- 43.Kaschani F. Diversity of serine hydrolase activities of unchallenged and botrytis-infected Arabidopsis thaliana. Mol. Cell. Proteomics. 2009;8:1082–1093. doi: 10.1074/mcp.M800494-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kocks C. Functional proteomics of the active cysteine protease content in Drosophila S2 cells. Mol. Cell. Proteomics. 2003;2:1188–1197. doi: 10.1074/mcp.M300067-MCP200. [DOI] [PubMed] [Google Scholar]
- 45.Barglow K.T., Cravatt B.F. Activity-based protein profiling for the functional annotation of enzymes. Nat. Methods. 2007;4:822–827. doi: 10.1038/nmeth1092. [DOI] [PubMed] [Google Scholar]
- 46.Speers A.E., Cravatt B.F. Chemical strategies for activity-based proteomics. Chembiochem. 2004;5:41–47. doi: 10.1002/cbic.200300721. [DOI] [PubMed] [Google Scholar]
- 47.Jessani N., Cravatt B.F. The development and application of methods for activity-based protein profiling. Curr. Opin. Chem. Biol. 2004;8:54–59. doi: 10.1016/j.cbpa.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Cravatt B.F. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y. Activity-based protein profiling: the serine hydrolases. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yee M.C. A cell-permeable, activity-based probe for protein and lipid kinases. J. Biol. Chem. 2005;280:29053–29059. doi: 10.1074/jbc.M504730200. [DOI] [PubMed] [Google Scholar]
- 51.Vocadlo D.J., Bertozzi C.R. A strategy for functional proteomic analysis of glycosidase activity from cell lysates. Angew. Chem. Int. Ed. Engl. 2004;43:5338–5342. doi: 10.1002/anie.200454235. [DOI] [PubMed] [Google Scholar]
- 52.Shreder K.R. Design and synthesis of AX7574: a microcystin-derived, fluorescent probe for serine/threonine phosphatases. Bioconjug. Chem. 2004;15:790–798. doi: 10.1021/bc0499580. [DOI] [PubMed] [Google Scholar]
- 53.Adam G.C. Profiling the specific reactivity of the proteome with non-directed activity-based probes. Chem. Biol. 2001;8:81–95. doi: 10.1016/s1074-5521(00)90060-7. [DOI] [PubMed] [Google Scholar]
- 54.Adam G.C. Proteomic profiling of mechanistically distinct enzyme classes using a common chemotype. Nat. Biotechnol. 2002;20:805–809. doi: 10.1038/nbt714. [DOI] [PubMed] [Google Scholar]
- 55.Singaravelu R. Activity-based protein profiling of the hepatitis C virus replication in Huh-7 hepatoma cells using a non-directed active site probe. Proteome Sci. 2010;8 doi: 10.1186/1477-5956-1188-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barglow K.T., Cravatt B.F. Discovering disease-associated enzymes by proteome reactivity profiling. Chem. Biol. 2004;11:1523–1531. doi: 10.1016/j.chembiol.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 57.Blais D.R. Activity-based proteome profiling of hepatoma cells during hepatitis C virus replication using protease substrate probes. J. Proteome Res. 2010;9:912–923. doi: 10.1021/pr900788a. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Q. Developing novel activity-based fluorescent probes that target different classes of proteases. Chem. Commun. 2004;7:1512–1513. doi: 10.1039/b404471a. [DOI] [PubMed] [Google Scholar]
- 59.Kato D. Activity-based probes that target diverse cysteine protease families. Nat. Chem. Biol. 2005;1:33–38. doi: 10.1038/nchembio707. [DOI] [PubMed] [Google Scholar]
- 60.Sieber S.A. Proteomic profiling of metalloprotease activities with cocktails of active-site probes. Nat. Chem. Biol. 2006;2:274–281. doi: 10.1038/nchembio781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berger A.B. Identification of early intermediates of caspase activation using selective inhibitors and activity-based probes. Mol. Cell. 2006;23:509–521. doi: 10.1016/j.molcel.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 62.Ovaa H. Activity-based ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2253–2258. doi: 10.1073/pnas.0308411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rolen U. Activity profiling of deubiquitinating enzymes in cervical carcinoma biopsies and cell lines. Mol. Carcinog. 2006;45:260–269. doi: 10.1002/mc.20177. [DOI] [PubMed] [Google Scholar]
- 64.Hemelaar J. Chemistry-based functional proteomics: Mechanism-based activity-profiling tools for ubiquitin and ubiquitin-like specific proteases. J. Proteome Res. 2004;3:268–276. doi: 10.1021/pr0341080. [DOI] [PubMed] [Google Scholar]
- 65.Lindner H.A. Deubiquitination in virus infection. Virology. 2007;362:245–256. doi: 10.1016/j.virol.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilham D. Triacylglycerol hydrolase is localized to the endoplasmic reticulum by an unusual retrieval sequence where it participates in VLDL assembly without utilizing VLDL lipids as substrates. Mol. Biol. Cell. 2005;16:984–996. doi: 10.1091/mbc.E04-03-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Becker A. Purification, cloning, and expression of a human enzyme with acyl coenzyme A: cholesterol acyltransferase activity, which is identical to liver carboxylesterase. Arterioscler. Thromb. 1994;14:1346–1355. doi: 10.1161/01.atv.14.8.1346. [DOI] [PubMed] [Google Scholar]
- 68.Pezacki J.P. Chemical contrast for imaging living systems: molecular vibrations drive CARS microscopy. Nat. Chem. Biol. 2011;7:137–145. doi: 10.1038/nchembio.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang H. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyanari Y. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 71.Marnett A.B. Communication between the active sites and dimer interface of a herpesvirus protease revealed by a transition-state inhibitor. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6870–6875. doi: 10.1073/pnas.0401613101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Speers A.E. Activity-based protein profiling in vivo using a copper(i)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 2003;125:4686–4687. doi: 10.1021/ja034490h. [DOI] [PubMed] [Google Scholar]
- 73.Speers A.E., Cravatt B.F. Profiling enzyme activities in vivo using click chemistry methods. Chem. Biol. 2004;11:535–546. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Kolb H.C. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 75.Simmons G. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaletsky R.L. Proteolysis of the Ebola virus glycoproteins enhances virus binding and infectivity. J. Virol. 2007;81:13378–13384. doi: 10.1128/JVI.01170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu C.P. Design of a mechanism-based probe for neuraminidase to capture influenza viruses. Angew. Chem. Int. Ed. Engl. 2005;44:6888–6892. doi: 10.1002/anie.200501738. [DOI] [PubMed] [Google Scholar]
- 78.Lee B.W. In vitro and in vivo apoptosis detection using membrane permeant fluorescent-labeled inhibitors of caspases. Methods Mol. Biol. 2008;414:109–135. doi: 10.1007/978-1-59745-339-4_10. [DOI] [PubMed] [Google Scholar]
- 79.Furman L.M. Cysteine protease activation and apoptosis in murine norovirus infection. Virol. J. 2009;6 doi: 10.1186/1743-1422X-1186-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weerapana E. Tandem orthogonal proteolysis-activity-based protein profiling (TOP-ABPP) - a general method for mapping sites of probe modification in proteomes. Nat. Protoc. 2007;2:1414–1425. doi: 10.1038/nprot.2007.194. [DOI] [PubMed] [Google Scholar]


