Highlights
-
•
Seroepidemiology of BVDV-1, BoHV-1, BRSV and BPIV-3 and risk of BRD was investigated.
-
•
For each virus, being seronegative at entry increased risk of BRD in feedlot cattle.
-
•
Animals seronegative for more than one virus were at progressively increased risk.
-
•
For each virus seroincrease resulted in increased risk of BRD in feedlot cattle.
-
•
Seroincrease for more than one virus further increased risk of BRD.
Keywords: Bovine respiratory disease, Seroepidemiology, Bovine viral diarrhoea virus 1, Bovine herpesvirus 1, Bovine respiratory syncytial virus, Bovine parainfluenza virus 3
Abstract
Bovine respiratory disease (BRD) is the most important cause of clinical disease and death in feedlot cattle. Respiratory viral infections are key components in predisposing cattle to the development of this disease. To quantify the contribution of four viruses commonly associated with BRD, a case-control study was conducted nested within the National Bovine Respiratory Disease Initiative project population in Australian feedlot cattle. Effects of exposure to Bovine viral diarrhoea virus 1 (BVDV-1), Bovine herpesvirus 1 (BoHV-1), Bovine respiratory syncytial virus (BRSV) and Bovine parainfluenza virus 3 (BPIV-3), and to combinations of these viruses, were investigated.
Based on weighted seroprevalences at induction (when animals were enrolled and initial samples collected), the percentages of the project population estimated to be seropositive were 24% for BoHV-1, 69% for BVDV-1, 89% for BRSV and 91% for BPIV-3.
For each of the four viruses, seropositivity at induction was associated with reduced risk of BRD (OR: 0.6–0.9), and seroincrease from induction to second blood sampling (35–60 days after induction) was associated with increased risk of BRD (OR: 1.3–1.5). Compared to animals that were seropositive for all four viruses at induction, animals were at progressively increased risk with increasing number of viruses for which they were seronegative; those seronegative for all four viruses were at greatest risk (OR: 2.4). Animals that seroincreased for one or more viruses from induction to second blood sampling were at increased risk (OR: 1.4–2.1) of BRD compared to animals that did not seroincrease for any viruses. Collectively these results confirm that prior exposure to these viruses is protective while exposure at or after feedlot entry increases the risk of development of BRD in feedlots. However, the modest increases in risk associated with seroincrease for each virus separately, and the progressive increases in risk with multiple viral exposures highlights the importance of concurrent infections in the aetiology of the BRD complex. These findings indicate that, while efficacious vaccines could aid in the control of BRD, vaccination against one of these viruses would not have large effects on population BRD incidence but vaccination against multiple viruses would be expected to result in greater reductions in incidence. The findings also confirm the multifactorial nature of BRD development, and indicate that multifaceted approaches in addition to efficacious vaccines against viruses will be required for substantial reductions in BRD incidence.
1. Introduction
Bovine respiratory disease (BRD) is the major cause of clinical disease and death in feedlot cattle (Smith, 1998) and has been estimated as causing more than 70% of clinical disease cases and 50% of deaths in Australian feedlots (Sackett et al., 2006). BRD has a multifactorial aetiology requiring pathogenic organisms, susceptible cattle and environmental stressors (Edwards, 2010). Of the viral infections commonly associated with BRD, most in isolation are thought to not result in severe clinical signs (Ellis, 2009). However under suitable conditions, viral induced immunosuppression and damage to the respiratory epithelia may lead to secondary bacterial respiratory tract infection and clinical signs of BRD (Ellis, 2009). Viruses are believed to play an important role in the pathogenesis of BRD, but the clinical presentation can develop with different combinations of viruses and bacteria so no particular infectious agents are known to be necessary causes of BRD (Confer, 2009, Ellis, 2009). The relative importance of specific pathogens in a particular population is likely to depend on dynamic relationships between the presence, virulence, and rate of transmission of pathogens, and prevalence of immunity amongst cattle in the population and the associated degree of herd immunity (Panciera and Confer, 2010).
Bovine herpesvirus 1 (BoHV-1), Bovine viral diarrhoea virus 1 (BVDV-1), Bovine respiratory syncytial virus (BRSV) and Bovine parainfluenza virus 3 (BPIV-3) have been frequently associated with BRD; BRSV and BPIV-3 are principally respiratory pathogens (Ellis, 2009, Ellis, 2010), while BoHV-1 and BVDV-1 can affect multiple systems (Panciera and Confer, 2010, Fulton, 2013). Evidence for associations between these viruses and BRD comes from serological studies and the detection of viruses in biological samples taken from animals diagnosed with BRD.
Seroprevalence studies indicate that the viruses implicated in BRD are ubiquitous in cattle populations. In unvaccinated populations, seroprevalences generally increase with the age of the animals (Taylor et al., 2006, Solís-Calderón et al., 2007). Reported seroprevalences for particular viruses at initial sampling around the time of feedlot entry have varied between populations. In North American studies the lowest consistently reported initial seroprevalence has been for BoHV-1 (1–18%), while seroprevalences to BVDV-1 (20–68%), BRSV (4–62%) and BPIV-3 (11–87%) have varied markedly (Martin and Bohac, 1986, Martin et al., 1989, Martin et al., 1990, O'Connor et al., 2001, Fulton et al., 2002a). In studies in which paired animal-level serum samples were tested, increasing serological titres (seroincreases) have been commonly reported, but vary markedly between populations, ranging from 2 to 6% for BoHV-1, 22 to 68% for BVDV-1, 8 to 86% for BRSV and 24 to 72% for BPIV-3 (Martin and Bohac, 1986, Martin et al., 1989, Martin et al., 1990, Martin et al., 1998, Martin et al., 1999, O'Connor et al., 2001).
Animals with low antibody titres to BoHV-1 at feedlot entry were at increased risk of BRD compared with animals with high titres in two studies (Martin and Bohac, 1986, Martin et al., 1989) but no such association was evident at the group level (Martin et al., 1990), nor at animal level where the risk of infection was low (Martin et al., 1999). Animals that were seropositive for BVDV-1 or had higher concentrations of BVDV-1 antibodies at initial sampling were at decreased risk of BRD in several studies (Martin et al., 1989, Martin et al., 1999, Durham et al., 1991, Booker et al., 1999, O'Connor et al., 2001). Seroincrease for BVDV-1 has been associated with increased risk of BRD at both the animal level and the group level (Martin and Bohac, 1986, Martin et al., 1990, O'Connor et al., 2001). In one study BVDV-1 was isolated from 26 of 90 BRD case samples and zero of nine control samples from lungs sampled at necropsy (Booker et al., 2008). Further studies demonstrated associations between both BVDV-1 serology and virus detection and occurrence of BRD (Fulton et al., 2000, Fulton et al., 2002b). Relationships between initial BRSV titres and BRD risk, and between seroincrease for BRSV and BRD risk, have been inconsistent (Martin et al., 1988, Martin et al., 1989, Martin et al., 1998, Martin et al., 1990, Martin et al., 1999, Allen et al., 1992, Fulton et al., 2002b). While BPIV-3 has been isolated from BRD cases in combination with other agents (Fulton et al., 2000), serological associations between BPIV-3 and BRD risk have also been inconsistent (Martin et al., 1988, Martin et al., 1989, Martin et al., 1990, Martin et al., 1998, Martin et al., 1999, Allen et al., 1992, Fulton et al., 2002b).
Viral genotypes/subtypes commonly associated with BRD and present in Australia differ from those reported elsewhere (Smith et al., 1995, Mahony et al., 2005, Horwood et al., 2008) so North American findings may not be generalisable to Australian feedlot populations. Australian seroprevalence surveys indicate that BoHV-1, BVDV-1, BRSV and BPIV-3 are ubiquitous in cattle herds (Dunn et al., 1995, Smith et al., 1995, Durham and Paine, 1997, Taylor et al., 2006). In one Australian study seroprevalences at feedlot entry for 500 sentinel cattle in 24 feedlot pens in six feedlots over 18 months were 13% for BoHV-1, 68% for BVDV-1, 27% for BRSV and 57% for BPI-3 (Dunn et al., 1995). In this population, seroconversion (i.e. changing from seronegative to seropositive) for BoHV-1 and BVDV-1 were associated with increased risk of BRD while seroconversion to BRSV and BPIV-3 were not (Dunn et al., 1995). This study lacked power to assess the effects of viruses for which seroprevalence at induction was high and the effects of combinations of viruses on BRD.
A limited number of commercial vaccines against viruses implicated in BRD were available in Australia at the time the current study was conducted. No vaccines against BRSV or BPIV-3 were available. Pestigard® is an Australian inactivated BVDV-1 vaccine registered to reduce reproductive losses due to BVDV-1. Rhinogard® is a modified-live intranasal BoHV-1 vaccine commonly administered at induction (initial processing) around the time of placement in a feedlot pen.
The aims of the current study were to determine the seroprevalence in Australian feedlot cattle at induction for four viruses commonly associated with BRD, to describe incidences of seroincrease during the first five to eight weeks after induction, and to investigate associations between both initial serostatus and seroincrease and risk of BRD. Further, the current study addresses the limitations of prior research by sampling animals from a large number of farms within a sufficient number of pens and feedlots across a range of time points and geographical regions, ensuring adequate power to investigate the effects of exposure to viruses and combinations of viruses on BRD across a large population with varying seroprevalences at feedlot entry. The measurement of seropositivity and serochange on an ordinal scale and knowledge of prior vaccination status for a subset of the population have also facilitated a more detailed investigation than has previously been reported.
2. Methods
2.1. Study design and study population
The National Bovine Respiratory Disease Initiative (NBRDI) was a prospective nationwide study conducted to investigate numerous putative risk factors for BRD (Hay et al., 2014). A total of 35,160 animals were enrolled at induction, between March 2009 and December 2011. At this time they were individually identified, animal characteristics recorded electronically and blood samples and nasal swabs collected prior to them being placed in a study cohort. A cohort consisted of all animals held and managed together in the same feedlot pen. A prospective unmatched risk-based case-control study was conducted nested within the NBRDI study population. Serological results were obtained for 7314 of these animals (3651 cases and 3663 controls) nested within 161 cohorts nested within 14 feedlots; these animals comprised the case-control study population.
Detailed data were recorded by feedlot staff for each animal in the NBRDI project population (e.g. identification numbers, arrival date, induction date, sex, dentition, breed, induction weight) and supplied as animal-level electronic files. Further data were supplied for animals that were examined in the feedlots’ hospital crushes during the observation period. Each animal was monitored for clinical signs of disease from induction (defined as ‘day 0’) until it left the cohort for any reason, such as removal to the hospital pen or another pen separate from the cohort, death or feedlot exit. Blood samples were collected into 6 ml vacuum tubes and allowed to clot, and nasal swabs were obtained from all study animals at induction. Blood samples were collected from all study animals at follow-up, which was between 35 and 60 days inclusive after induction. Additional details about sample identification, verification and laboratory processing are provided in Supplementary Appendix A.
2.2. Case definition and inclusion criteria
The BRD case definition used in the NBRDI was based on the clinical signs recorded by feedlot staff in computerised hospital records after suspected ill animals were removed from their cohort for examination and treatment. Veterinarians servicing participating feedlots conducted regular training sessions for feedlot staff on the diagnosis of BRD, and seven of the fourteen participating feedlots were serviced by the same veterinary group. All animals with clinical signs indicating respiratory system involvement and presumptive diagnoses of “pneumonia”, “respiratory”, “BRD” and “IBR” (infectious bovine rhinotracheitis) were classified as having BRD for the NBRDI (Hay et al., 2014). A subset of these animals was eligible for inclusion as cases in the case-control study (the current study).
The outcome of interest in the case-control study was the development of BRD between days 7 and 35 inclusive from the ‘cohort close date’, defined as the latest date any animal was inducted into the cohort. Each cohort in the study was a closed population for 35 days from the cohort close date as no animals were added after that date and few animals (2.7%) were removed other than for BRD. Inclusion criteria for cases were (i) first clinical disease diagnosis was BRD and this was diagnosed between days 7 and 35 inclusive after the cohort close date and (ii) paired serum samples were available, the first collected at induction and the second collected at follow-up. Inclusion criteria for controls were (i) animal remained in the cohort from induction until at least 35 days after the cohort close date without being diagnosed with BRD or any other condition over that time and (ii) paired serum samples were available, the first collected at induction and the second collected at follow-up. Cases and controls were selected from the NBRDI project population in two batches (‘selection batch’) after animals had left the feedlot and their records completed. In total 7450 animals (3725 cases and 3725 controls) were randomly selected from animals eligible to be cases and controls respectively, and serology performed using stored serum samples. The sample size was determined by budgetary constraints. We targeted a 1:1 case:control ratio to maximise power for the given sample size.
2.3. Serological testing and exposure variables
Serum samples were individually tested using an indirect multiplex ELISA (BIOX K 284 ELISA®) to evaluate the humoral immune response to BoHV-1, BVDV-1, BRSV and BPIV-3. Tests were conducted according to manufacturer’s instructions. Raw optical density results for each test plate were exported to a Microsoft® Excel spreadsheet and formulae specified in the test kit algorithm were applied to adjust optical densities for control sample values, and to categorise these according to cut-points provided by the manufacturer. Each serological result was categorised as 0 (‘seronegative’, the category with the lowest optical densities), 1, 2, 3, 4, or 5 (where category 5 consisted of the highest optical densities). Cut-points varied slightly between plates with different batch numbers (‘test batches’). Plates with four different batch numbers were used in the study.
For each virus, serological exposure variables were labelled “induction”, “composite” and “seroconversion” (Table 1, Table 2 ). Induction variables described each animal’s serostatus at induction. Serological changes from induction to follow-up for each animal for each virus were described using composite and seroconversion variables; composite variables combined serostatus at induction with serological changes while seroconversion variables described whether an increase occurred only amongst those animals whose induction serostatus was category 0 (seronegative). Differences of one category between induction and follow-up were considered to reflect no change in antibody concentrations. Distributions of animals across categories of serological variables were examined and some categories were combined to ensure sufficient observations in each category. Large declines in serological status between induction and follow-up were considered not biologically plausible in the feedlot setting given the relatively short times between induction and follow-up sampling (Geraghty et al., 2012). Hence, animals displaying a decrease of two or more categories and changing from seropositive (category 1 or higher) to seronegative (category 0) were classified as having missing status for the composite variables (Table 2). For animals that decreased by two or more categories but remained seropositve at follow-up, optical density values were compared for all viruses. In most instances the optical density values were biologically plausible and these animals were classified as ‘no change’ (induction category 3) or ‘initially high’ (induction category 4 or 5). Where the decrease in optical density was not biologically plausible, values were set to missing for all composite variables.
Table 1.
Derivation of variables used in analyses (bolded) to investigate serological associations with BRD.
| Data/variable | Distribution/categories | Notes/usage in analyses |
|---|---|---|
| Virus-specific induction serology categorya (e.g. BoHV-1 induction) | 0 1 2 or 3 4 or 5 |
Variables included in analyses to measure associations between serostatus at induction and becoming a case |
| Virus-specific composite variablea (e.g. BoHV-1 composite) | No change Seroincrease Initially high Missing |
Derived from serology categories at induction and follow-up; used to describe and analyse change in serostatus between induction and follow-up See Table 2 for classification |
| Virus-specific collapsed compositea (e.g. BoHV-1 composite (collapsed)) | Collapsed version of composite variable | |
| No seroincrease | No change or initially high | |
| Seroincrease | Seroincrease | |
| Virus-specific seroconversiona (e.g. BoHV-1 seroconversion) | No Yes |
Derived from serology categories at induction and follow-up; used to describe and analyse change in serostatus between induction and follow-up but restricted to animals seronegative at induction |
| Number of viruses animal was seropositive to at induction | 0–4 | Derived from induction serology for all viruses: (BoHV-1, BVDV-1, BRSV & BPIV-3) |
| Number of viruses animal seroincreased for between induction and follow-up | 0–4 | Derived from virus-specific collapsed composite variable for all viruses: (BoHV-1, BVDV-1, BRSV & BPI-3) |
Separate variables for each of the four viruses investigated: bovine herpesvirus 1 (BoHV-1), bovine viral diarrhoea virus 1 (BVDV-1), bovine respiratory syncytial virus (BRSV) and bovine parainfluenza virus 3 (BPIV-3).
Table 2.
Scheme for deriving categories of variables describing serological changes from induction to follow-up for each animal for each virus based on the animal’s induction and follow-up statuses for the virusa.
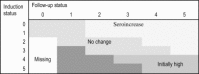 |
Dark shaded areas indicate combinations where optical density values were compared before classifying (see text for details).
In addition to these virus-specific variables, two variables combined data for all four viruses (Table 1). The number of viruses for which each animal was seropositive at induction was calculated as the number of the four viruses for which the induction serological category was at least 1. The number of viruses for which each animal seroincreased was calculated as the number of the four viruses for which the animal was categorised as ‘seroincrease’ for the composite variable. For this variable, animals classified as ‘initially high’ were pooled with those not seroincreasing for a particular virus.
2.4. Covariates and subsets
Six covariates, as determined by a hypothesised causal diagram (Fig. 1 ) were relevant to the analyses in this study. These were: mixing summary, the number of animals in the animal’s cohort, the presence of BVDV-1 in the animal’s cohort, the presence of an identified animal persistently infected with BVDV-1 in the animal’s group defined 28 days before induction, whether or not water troughs could be accessed by animals in adjoining pens and vaccination with a modified-live intranasal BoHV-1 vaccine at induction.
Fig. 1.

Postulated causal diagram showing variables relevant to the case-control analyses.
aRhinogard®: bovine herpesvirus 1 (BoHV-1) modified-live intranasal vaccine administered at induction.
bBoHV-1, bovine viral diarrhoea virus 1 (BVDV-1), bovine parainfluenza virus 3 (BPIV-3) and bovine respiratory syncytial virus (BRSV) “change” variables represent one of the three variables that measured change in serostatus between induction and follow-up, (e.g. BRSV composite, BRSV composite (collapsed) or BRSV seroconversion).
cCohort size: total number of NBRDI study animals enrolled into animal’s cohort.
dMixing summary: mixing prior to day-27 (yes, no) and number of group-28s forming cohort.
eBVDV-1 detected on quantitative real time-PCR testing of any pooled or individual sample from animal in the animal’s cohort.
fBVDV-1 persistently infected animal in group 28 days before induction.
Mixing summary was derived from the Australian National Livestock Identification System data as previously described (Hay et al., 2014). It categorised animals based on whether or not they had been mixed with animals from other farms prior to 27 days before induction and the number of groups defined 28 days before induction that formed the animal’s cohort (i.e. yes, <4; yes, ≥4; no, <4; and no, ≥4). ‘Cohort size’ (i.e. <200, ≥200) indicated the total number of animals enrolled into the animal’s cohort at induction. The presence of BVDV-1 in the cohort and the identification of animals persistently infected with BVDV-1 were determined from the results of pooled and selected animal-level quantitative real time-PCR testing of all animals inducted into the NBRDI (N = 35,160; Hay et al., 2016a). Any positive test from any pooled sample was used to classify the cohort-level variable, ‘BVDV in cohort’ (yes, no). This could have been due either to transiently or persistently infected animals. Further testing was performed to identify persistently infected animals and thus determine which groups defined 28 days prior to induction contained animals persistently infected with BVDV-1 (‘BVDV in group-28’: yes, no) (Hay et al., 2016a). ‘Shared pen water’ (yes, no) indicated if water troughs could be accessed by animals in adjoined pens. ‘Rhinogard’ (yes, no) indicated if animals were vaccinated with the modified-live intranasal BoHV-1 vaccine, Rhinogard®. Feedlots in the current study administered Rhinogard® to either all (ten feedlots) or none (four feedlots) of the animals they contributed to the study.
To further investigate the effects of BoHV-1 and BVDV-1, three subset analyses were conducted within subsets based on the animals’ vaccination statuses against these viruses. The first subset was restricted to animals known not to have received Rhinogard® and to have been inducted within seven days of arrival at the vicinity of the feedlot. The second was restricted to animals known to have received Rhinogard® at induction (96% of these were inducted within seven days of arrival). The third subset was restricted to animals that probably had not been vaccinated against BVDV-1 with Pestigard®. As part of the NBRDI, farmers (‘vendors’) who supplied groups of 20 or more study animals to participating feedlots were surveyed. Survey responses were used to identify a subset of animals that were bred on the vendor’s farm or purchased by 10 months of age and assumed to have not received Pestigard® prior to their induction and enrolment in the NBRDI.
2.5. Seroprevalence, percentages of animals that seroincreased and percentages of animals that seroconverted
Seroprevalences at induction, and proportions of animals that seroincreased and seroconverted, were estimated for the NBRDI project population as weighted averages of the observed values for these measures for cases and controls, weighted to account for the different proportions of animals eligible to be cases and controls that were included. Confidence intervals for estimated seroprevalences at induction, and percentages of animals that seroincreased and seroconverted for the NBRDI project population were calculated using the proportion command in the Stata® statistical software package (version 12) with weights specified using pweights. Confidence intervals were calculated using the logit transformation and t-distribution, but did not account for clustering of animals within group-28s within cohorts within feedlots. The sampling fraction for cases was 5.3 times that for controls (i.e. 3651/4442:3663/23,640). Therefore, each pooled serology estimate for the NBRDI project population was calculated as the sum of the observed proportion for cases multiplied by 0.16 (1/6.3) and the observed proportion for controls multiplied by 0.84 (5.3/6.3).
2.6. Statistical analyses
The unit of analysis was the individual animal. To estimate the effects of each serological predictor on the risk of BRD, models were fitted based on a priori constructed causal diagrams which explicitly considered biologically plausible pathways (Greenland et al., 1999, Shrier and Platt, 2008, Dohoo et al., 2009, Textor et al., 2011, Textor and Liskiewicz, 2011). One causal diagram depicted postulated causal relationships between measured exposure variables of interest in the case-control study and between exposure variables and being a BRD case (Fig. 1). A further diagram (Fig. 2 ) was constructed for use with the combined virus variables. Causal diagrams can be used to determine ‘adjustment sets’ of appropriate covariates to include in models to determine the effects of interest. A minimal sufficient adjustment set (Textor et al., 2011) is the minimal set of covariates which adequately controls confounding of the relationship between a specified exposure variable and the outcome variable. The causal diagrams (Fig. 1, Fig. 2) were used to determine minimal sufficient adjustment sets to include as covariates in models when estimating the effects of serological exposures on risk of BRD. The postulated causal diagrams were reproduced within the DAGitty® software (Textor et al., 2011) user interface to determine the minimal sufficient adjustment set to include in models to estimate the effects for each exposure of interest on the outcome of being a BRD case.
Fig. 2.
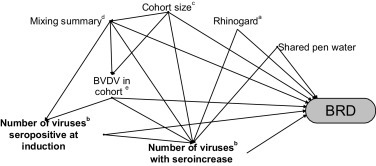
Postulated causal diagram showing variables relevant to estimating the effects of ‘number of virus’ variables in the case-control study.
aRhinogard®: bovine herpesvirus 1 (BoHV-1) modified-live intranasal vaccine administered at induction.
bNumber of viruses from the following: BoHV-1, bovine viral diarrhoea virus 1 (BVDV-1), bovine respiratory syncytial virus, bovine parainfluenza virus 3.
cCohort size: total number of NBRDI study animals enrolled into animal’s cohort.
dMixing summary: mixing prior to day-27 (yes, no) and number of group-28s forming cohort.
eBVDV-1 detected on quantitative real time-PCR testing of any pooled or individual sample from animal in the animal’s cohort.
The Stata® statistical software package (version 12) was used for all data management, preliminary analyses and to run the multilevel modelling software package, MLwiN® (version 2.27) which was used for modelling. For each exposure of interest, a model containing covariates determined by the minimal sufficient adjustment set was fitted using second-order penalised quasi-likelihood methods to produce starting values for the second model using Bayesian Markov chain Monte Carlo (MCMC) methods. Test batch and selection batch were included in most models as fixed effects; but they were not included in seroconversion models because models with sparse or empty categories for these variables failed to run. Three-level models were fitted, with random effects for feedlot and cohort nested within feedlot. Non-informative prior distributions were specified. Convergence was assessed by inspecting diagnostic trajectory plots and summary statistics (Browne, 2012) as previously described (Hay et al., 2014). MCMC chains were run for 50,000 iterations after a burn-in of 500 iterations. Posterior parameter estimates of mean odds ratios (ORs) and 95% credible intervals were obtained.
Correlations between each paired combination of categorical serological variables (i.e. induction and composite) were assessed by using pair-wise Spearman’s rank correlation coefficients. Correlations between each paired combination of binary serological variables (i.e. induction serostatus and seroconversion; e.g. BVDV-1 seroconversion and BRSV seroconversion) were assessed using pair-wise tetrachoric correlation coefficients. This method assumes a latent bivariate normal distribution for each pair of variables (Bonett and Price, 2005). It is not possible to validate this assumption using empirical data and there may be some departure from these distributional assumptions for some pairs of variables.
To assess interactions in associations between serological variables and BRD risk, each possible two-way interaction between variables for each pair of viruses was investigated by examining the multiple (i.e. overall) Wald p-value following the fitting of a second-order penalised quasi-likelihood model. Interactions between each combination of variables describing induction serostatus (e.g. BVDV induction and BRSV induction), composite variables (e.g. BVDV composite and BRSV composite) and between each combination of collapsed composite variables (e.g. BVDV composite (collapsed) and BRSV composite (collapsed)) were assessed. Where the overall p-value for the joint interaction terms was <0.05, model estimation using MCMC methods was planned but none of these p-values were <0.05.
3. Results
A flow chart indicating the relationship between the NBRDI project population and the case-control population is shown in Fig. 3 . Intervals between each animal’s induction date and their cohort’s close date ranged from zero to 15 days, but for 75% the interval was three days or less and for 92% the interval was six days or less. All of the 7314 selected animals (3651 cases and 3663 controls) with serological results were included in the analyses investigating serostatus at induction, but 313 (4%; 134 cases and 179 controls) were excluded from the analyses of the composite serochange variables because their change in serostatus between induction and follow-up was implausible for at least one virus. Descriptive results for seroprevalences at induction and changes in serostatus from induction to follow-up for each of the four viruses are shown in Tables 3, 4 and 5 . The results of subset analyses for BoHV-1 stratified by Rhinogard® vaccination are presented in Table 3 and results for the subset analyses for BVDV-1 restricted to animals not vaccinated with Pestigard® are presented in Table 4. Based on the pooled weighted seroprevalences, an estimated 76% of animals were seronegative for BoHV-1 at induction, 48% seroincreased and 54% of seronegative animals seroconverted during the 35–60 day follow-up period (Table 3). The majority (79%) of animals in the NBRDI project population were vaccinated with Rhinogard® at induction and 87% of animals in the case-control study received Rhinogard®. An estimated 31% of animals were seronegative for BVDV-1 at induction, 23% seroincreased and 55% of susceptible animals seroconverted during the 35–60 day follow-up period (Table 4). In the subset of animals known to have not been previously vaccinated against BVDV-1 with Pestigard®, distributions of animals across categories for the BVDV-1 variables were similar to those estimated from the entire case-control study population, but the proportion that was seronegative at induction (34%) and the proportion that seroincreased (27%) were slightly higher (Table 4). An estimated 11% of animals were seronegative for BRSV at induction, 29% seroincreased and 65% of susceptible animals seroconverted during the 35–60 day follow-up period (Table 5). Lastly, an estimated 9% of animals were seronegative for BPIV-3 at induction, 17% seroincreased and 54% of susceptible animals seroconverted during the 35–60 day follow-up period (Table 5).
Fig. 3.
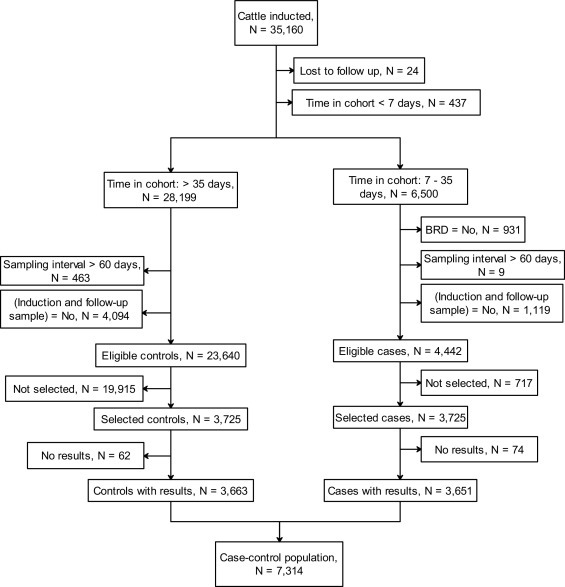
Flowchart showing selection of animals for inclusion in the case-control study.
Table 3.
Distribution of serological variables and estimated odds ratios for the effects of bovine herpesvirus 1 (BoHV-1) serological variables on the odds of being a BRD casea.
| Variable & category | Number of animals | Number (%) of controls | Number (%) of cases | Pooled weighted % (95% CI)i | Adjusted odds ratio (95% credible interval) |
|---|---|---|---|---|---|
| BoHV-1 inductionb | |||||
| 0 | 5681 | 2760 (75.5) | 2921 (80.0) | 76.2 (75.0–77.4) | Ref. cat. |
| 1 | 906 | 447 (12.2) | 459 (12.6) | 12.3 (11.4–13.2) | 1.0 (0.8–1.2) |
| 2 or 3 | 606 | 379 (10.4) | 227 (6.2) | 9.7 (8.9–10.5) | 0.7 (0.6–0.9) |
| 4 or 5 | 113 | 69 (1.9) | 44 (1.2) | 1.8 (1.4–2.2) | 1.0 (0.5–1.6) |
| BoHV-1 induction serostatusb | |||||
| Negative | 5681 | 2760 (75.5) | 2921 (80.0) | 76.2 (75.0–77.4) | Ref. cat. |
| Positive | 1625 | 895 (24.5) | 730 (20.0) | 23.7 (22.6–25.0) | 0.9 (0.7–1.0) |
| BoHV-1 compositec | |||||
| No change | 3101 | 1847 (53.1) | 1254 (35.7) | 50.2 (48.8–51.7) | Ref. cat. |
| Seroincrease | 3805 | 1581 (45.4) | 2224 (63.2) | 48.2 (46.8–49.6) | 1.4 (1.2–1.6) |
| Initially high | 95 | 56 (1.6) | 39 (1.1) | 1.5 (1.2–1.9) | 1.5 (0.8–2.5) |
| BoHV-1 seroconversiond | |||||
| No | 2267 | 1332 (48.3) | 935 (32.0) | 45.7 (44.1–47.3) | Ref. cat. |
| Yes | 3414 | 1428 (51.7) | 1986 (68.0) | 54.3 (52.7–55.9) | 1.3 (1.1–1.5) |
| Vaccinated with Rhinogard® | |||||
| BoHV-1 seroconversione | |||||
| No | 1742 | 852 (40.6) | 890 (31.1) | 39.1 (37.3–40.8) | Ref. cat. |
| Yes | 3217 | 1248 (59.4) | 1969 (68.9) | 60.9 (59.2–62.7) | 1.3 (1.1–1.6) |
| Not vaccinated with Rhinogard® | |||||
| BoHV-1 induction serostatusf | |||||
| Negative | 484 | 458 (75.5) | 26 (89.7) | 77.7 (74.3–81.1) | Ref. cat. |
| Positive | 152 | 149 (24.5) | 3 (10.3) | 22.3 (18.9–25.7) | 0.7 (0.1–2.1) |
| BoHV-1 compositeg | |||||
| No change | 475 | 459 (79.1) | 16 (59.3) | 76.0 (71.9–80.0) | Ref. cat. |
| Seroincrease | 122 | 111 (19.1) | 11 (40.7) | 22.6 (18.6–26.6) | 1.4 (0.4–3.4) |
| Initially high | 10 | 10 (1.7) | 0 (0.0) | 1.5 (0.6–2.3) | excluded |
| BoHV-1 seroconversionh | |||||
| No | 368 | 353 (77.1) | 15 (57.7) | 74.0 (69.5–78.4) | Ref. cat. |
| Yes | 116 | 105 (22.9) | 11 (42.3) | 26.0 (21.6–30.5) | 1.3 (0.3–3.4) |
Models fitted using three-level mixed effects logistic regression; N: number of observations in model; this may differ from the numbers in the descriptive results because of missing values for covariates.
Covariates: mixing summary, test batch, selection batch; N = 7302.
Covariates: mixing summary, test batch, selection batch, cohort size, Rhinogard, shared pen water; N = 6997.
Covariates: mixing summary, cohort size, Rhinogard, shared pen water; N = 2841.
Covariates: mixing summary, cohort size, shared pen water; N = 4633.
Covariates: mixing summary; N = 636 animals sampled within 7 days of arrival.
Covariates: mixing summary, cohort size, shared pen water; N = 607 animals sampled within 7 days of arrival.
Covariates: mixing summary, cohort size, shared pen water; N = 484 animals sampled within 7 days of arrival.
Estimated seroprevalences and seroincidences for the NBRDI project population, the source population for the current case-control study; calculated as pooled weighted averages (0.84 × observed percentage in controls + 0.16 × observed percentage in cases) based on the ratio of the sampling fractions for selection from the NBRDI project population as a case versus as a control (5.3:1).
Table 4.
Distribution of serological variables and estimated odds ratios for the effects of bovine viral diarrhoea virus 1 (BVDV-1) serological variables on the odds of being a BRD casea.
| Variable & category | Number of animals | Number (%) of controls | Number (%) of cases | Pooled weighted % (95% CI)h | Adjusted odds ratio (95% credible interval) |
|---|---|---|---|---|---|
| BVDV-1 inductionb | |||||
| 0 | 2469 | 1073 (29.3) | 1396 (38.2) | 30.7 (29.5–32.0) | Ref. cat. |
| 1 | 376 | 160 (4.4) | 216 (5.9) | 4.6 (4.0–5.2) | 1.3 (1.0–1.7) |
| 2 or 3 | 1058 | 609 (16.6) | 449 (12.3) | 15.9 (14.9–17.0) | 0.8 (0.6–1.0) |
| 4 or 5 | 3411 | 1821 (49.7) | 1590 (43.6) | 48.7 (47.3–50.1) | 0.8 (0.7–0.9) |
| BVDV-1 induction serostatusb | |||||
| Negative | 2469 | 1073 (29.3) | 1396 (38.2) | 30.7 (29.5–32.0) | Ref. cat. |
| Positive | 4845 | 2590 (70.7) | 2255 (61.8) | 69.3 (68.0–70.5) | 0.8 (0.7–1.0) |
| BVDV-1 compositec | |||||
| No change | 1777 | 1006 (28.9) | 771 (21.9) | 27.8 (26.5–29.0) | Ref. cat. |
| Seroincrease | 1948 | 745 (21.4) | 1203 (34.2) | 23.4 (22.3–24.6) | 1.3 (1.1–1.6) |
| Initially high | 3276 | 1733 (49.7) | 1543 (43.9) | 48.8 (47.4–50.2) | 1.0 (0.8–1.1) |
| BVDV-1 seroconversiond | |||||
| No | 921 | 513 (47.8) | 408 (29.2) | 44.8 (42.3–47.4) | Ref. cat. |
| Yes | 1548 | 560 (52.2) | 988 (70.8) | 55.2 (52.6 –57.7) | 1.6 (1.3–2.1) |
| No prior vaccination with Pestigard® | |||||
| BVDV-1 induction serostatuse | |||||
| Negative | 574 | 207 (31.6) | 367 (46.9) | 34.0 (31.0–37.1) | Ref. cat. |
| Positive | 864 | 449 (68.4) | 415 (53.1) | 66.0 (62.9–69.0) | 0.6 (0.4–0.9) |
| BVDV-1 compositef | |||||
| No change | 345 | 185 (30.3) | 160 (21.1) | 28.8 (25.7–31.9) | Ref. cat. |
| Seroincrease | 483 | 148 (24.2) | 335 (44.3) | 27.4 (24.5–30.3) | 1.4 (0.9–2.1) |
| Initially high | 540 | 278 (45.5) | 262 (34.6) | 43.8 (40.4–47.1) | 0.8 (0.6–1.2) |
| BVDV-1 seroconversiong | |||||
| No | 184 | 108 (52.2) | 76 (20.7) | 47.1 (41.4–52.9) | Ref. cat. |
| Yes | 390 | 99 (47.8) | 291 (79.3) | 52.9 (47.1–58.6) | 2.9 (1.6–5.0) |
Models fitted using three-level mixed effects logistic regression; N: number of observations in model; this may differ from the numbers in the descriptive results because of missing values for covariates.
Covariates: mixing summary, test batch, selection batch, BVDV PI animal in group-28; N = 7314.
Covariates: mixing summary, test batch, selection batch, cohort size, BVDV in cohort, shared pen water; N = 6997.
Covariates: mixing summary, cohort size, BVDV in cohort, shared pen water; N = 2469.
Covariates: mixing summary, PI animal in group-28; N = 1438.
Covariates: mixing summary, cohort size, BVDV in cohort, shared pen water; N = 1321.
Covariates: mixing summary, cohort size, BVDV in cohort, shared pen water; N = 574.
Estimated seroprevalences and seroincidences for the NBRDI project population, the source population for the current case-control study; calculated as pooled weighted averages (0.84 × observed percentage in controls + 0.16 × observed percentage in cases) based on the ratio of the sampling fractions for selection from the NBRDI project population as a case versus as a control (5.3:1).
Table 5.
Distribution of serological variables and estimated odds ratios for the effects of bovine respiratory syncytial virus (BRSV) and bovine parainfluenza virus 3 (BPIV-3) serological variables on the odds of being a BRD casea.
| Variable & category | Number of animals | Number (%) of controls | Number (%) of cases | Pooled weighted % (95% CI)f | Adjusted odds ratio (95% credible interval) |
|---|---|---|---|---|---|
| BRSV inductionb | |||||
| 0 | 919 | 397 (10.8) | 522 (14.3) | 11.4 (10.5–12.3) | Ref. cat. |
| 1 | 1719 | 829 (22.6) | 890 (24.4) | 22.9 (21.8–24.1) | 0.8 (0.6–1.0) |
| 2 or 3 | 3487 | 1820 (49.7) | 1667 (45.7) | 49.0 (47.7–50.4) | 0.7 (0.6–0.8) |
| 4 or 5 | 1189 | 617 (16.8) | 572 (15.7) | 16.7 (15.6–17.7) | 0.8 (0.6–1.0) |
| BRSV induction serostatusb | |||||
| Negative | 919 | 397 (10.8) | 522 (14.3) | 11.4 (10.5–12.3) | Ref. cat. |
| Positive | 6395 | 3266 (89.2) | 3129 (85.7) | 88.6 (87.7–89.5) | 0.8 (0.6–0.9) |
| BRSV compositec | |||||
| No change | 3650 | 1920 (55.1) | 1730 (49.2) | 54.2 (52.7–55.6) | Ref. cat. |
| Seroincrease | 2212 | 977 (28.0) | 1235 (35.1) | 29.2 (27.9–30.5) | 1.5 (1.3–1.7) |
| Initially high | 1139 | 587 (16.9) | 552 (15.7) | 16.7 (15.6–17.7) | 1.2 (1.0–1.5) |
| BRSV seroconversiond | |||||
| No | 282 | 146 (36.8) | 136 (26.0) | 35.1 (31.0–39.1) | Ref. cat. |
| Yes | 637 | 251 (63.2) | 386 (74.0) | 64.9 (60.9–69.0) | 1.5 (0.9–2.2) |
| BPIV-3 inductionb | |||||
| 0 | 713 | 311 (8.5) | 402 (11.0) | 8.9 (8.1–9.7) | Ref. cat. |
| 1 | 1114 | 557 (15.2) | 557 (15.3) | 15.2 (14.2–16.2) | 0.6 (0.5–0.8) |
| 2 or 3 | 3525 | 1769 (48.3) | 1756 (48.1) | 48.3 (46.9–49.6) | 0.6 (0.5–0.7) |
| 4 or 5 | 1962 | 1026 (28.1) | 936 (25.6) | 27.6 (26.4–28.9) | 0.6 (0.5–0.8) |
| BPIV-3 induction serostatusb | |||||
| Negative | 713 | 311 (8.5) | 402 (11.0) | 8.9 (8.1–9.7) | Ref. cat. |
| Positive | 6601 | 3352 (91.5) | 3249 (89.0) | 91.1 (90.3–91.9) | 0.6 (0.5–0.7) |
| BPIV-3 compositec | |||||
| No change | 3798 | 1982 (57.9) | 1816 (51.6) | 56.0 (54.6–57.5) | Ref. cat. |
| Seroincrease | 1352 | 545 (15.6) | 807 (23.0) | 16.8 (15.8–17.8) | 1.4 (1.1–1.6) |
| Initially high | 1851 | 957 (27.5) | 894 (25.4) | 27.1 (25.9–28.4) | 1.1 (0.9–1.2) |
| BPIV-3 seroconversione | |||||
| No | 278 | 151 (48.6) | 127 (31.6) | 45.8 (41.1–50.6) | Ref. cat. |
| Yes | 435 | 160 (51.4) | 275 (68.4) | 54.2 (49.4–58.9) | 1.5 (0.9–2.2) |
Models fitted using three-level mixed effects logistic regression; N: number of observations in model; this may differ from the numbers in the descriptive results because of missing values for covariates.
Covariates: mixing summary, test batch, selection batch; N = 7314.
Covariates: mixing summary, test batch, selection batch, cohort size, shared pen water; N = 6997.
Covariates: mixing summary, cohort size, shared pen water; N = 919.
Covariates: mixing summary, cohort size, shared pen water; N = 713.
Estimated seroprevalences and seroincidences for the NBRDI project population, the source population for the current case-control study; calculated as pooled weighted averages (0.84 × observed percentage in controls + 0.16 × observed percentage in cases) based on the ratio of the sampling fractions for selection from the NBRDI project population as a case versus as a control (5.3:1).
The distribution of the BoHV-1 serological variables in the case-control population and the estimated odds ratios for the effects are displayed in Table 3. Odds ratios for the subsets stratified by Rhinogard® vaccination were similar to those obtained using the full case-control population. Across the case-control population, being seropositive for BoHV-1 at induction was associated with a slightly reduced risk of BRD (OR 0.9, 95% credible interval: 0.7–1.0), with animals in induction categories 2 or 3 at moderately reduced risk (OR 0.7, 95% credible interval: 0.6–0.9) relative to induction category 0. BoHV-1 seroconversion occurred much more frequently in vaccinated (61%) than in unvaccinated (26%) animals. Seroconversion (i.e. an increase of at least two categories) in initially seronegative animals vaccinated with Rhinogard® at induction was associated with increased risk of BRD (OR 1.3, 95% credible interval: 1.1–1.6).
The distributions of the serological variables relating to BVDV-1 in the case-control population and the estimated odds ratios for the effects are displayed in Table 4. Being seropositive for BVDV-1 at induction was associated with reduced risk of BRD (OR 0.8, 95% credible interval: 0.6–1.0 and 0.7–0.9) for animals in induction categories 2 or 3 and 4 or 5, respectively. Seroincrease for BVDV-1 was associated with an increase in risk of BRD (OR 1.3, 95% credible interval: 1.1–1.6) as was seroconversion (OR 1.6, 95% credible interval: 1.3–2.1). In the subset of animals assumed to have not been previously vaccinated with Pestigard® prior to feedlot entry, the estimated effects were similar to those observed in the full case-control population, but with a more marked protective effect in animals that were seropositive at induction (OR 0.6, 95% credible interval: 0.4–0.9 compared to 0.8 for the full case-control study population). Amongst animals not previously vaccinated, those that seroconverted to BVDV-1 were at much higher risk of BRD than animals not seroconverting (OR 2.9, 95% credible interval: 1.6–5.0).
The distribution of the serological variables relating to BRSV in the case-control population and the estimated odds ratios for the effects are displayed in Table 5. Prior exposure to BRSV was associated with reduced risk of BRD relative to that for induction category 0 (OR 0.8, 95% credible intervals: 0.6–0.9), with similar effect estimates across categories. Seroincrease to BRSV was associated with an increase in risk of BRD (OR 1.5, 95% credible interval: 1.3–1.7) as was seroconversion (OR 1.5, 95% credible interval: 0.9–2.2).
The distribution of the serological variables relating to BPIV-3 in the case-control population and the estimated odds ratios for the effects are displayed in Table 5. Prior exposure to BPIV-3 was associated with reduced risk of BRD relative to induction category 0 (OR 0.6, 95% credible intervals: 0.5–0.7) with consistent effect estimates across categories. Seroincrease to BPIV-3 was associated with an increase in risk of BRD (OR 1.4, 95% credible interval: 1.1–1.6) as was seroconversion (OR 1.5, 95% credible interval: 0.9–2.2).
Correlations in both induction category and composite serochange variables between all pairs of viruses were weak (Spearman’s rho <0.20) (results not shown). The estimated tetrachoric correlation coefficients for binary variables are displayed in Table 6 . The closest correlations observed were only modest; these were correlations between seroconversion for BRSV and BPIV-3 (rho = 0.36) and between seroconversion for BRSV and BVDV-1 (rho = 0.34).
Table 6.
Correlation coefficients for associations between binary serological variables measuring induction serostatus and seroconversion.
| Induction serostatus | Seroconversion | |||||
|---|---|---|---|---|---|---|
| BoHV-1a | BVDV-1b | BRSVc | BoHV-1 | BVDV-1 | BRSV | |
| Induction serostatus | ||||||
| BoHV-1 | 1.00 | |||||
| BVDV-1 | 0.20 | 1.00 | ||||
| BRSV | 0.10 | 0.21 | 1.00 | |||
| BPIV-3d | 0.16 | 0.17 | 0.29 | |||
| Seroconversion | ||||||
| BoHV-1 | 1.00 | |||||
| BVDV-1 | 0.07 | 1.00 | ||||
| BRSV | 0.28 | 0.34 | 1.00 | |||
| BPIV-3 | 0.22 | 0.28 | 0.36 | |||
BoHV-1: bovine herpesvirus 1.
BVDV-1: bovine viral diarrhoea virus 1.
BRSV: bovine respiratory syncytial virus.
BPIV-3: bovine parainfluenza virus 3.
The induction serological profiles of animals in the current study together with the estimated distributions across the NBRDI project population are presented in Table 7 . The vast majority of animals had antibodies to at least one virus at induction, with only an estimated 1.1% of animals being seronegative for all four viruses. The most common pattern, in an estimated 43% of the project population was for animals to be seropositive for BVDV-1, BRSV and BPIV-3 and seronegative for BoHV-1 at induction. About 16% of the NBRDI project population were estimated to be seropositive for all four viruses investigated and 25% were estimated to be seronegative for two or more viruses at induction; 73% of these were seronegative for both BoHV-1 and BVDV-1. Table 8 presents the distributions of profiles for seroincreases. An estimated 73% of animals in the NBRDI project population seroincreased for at least one of the four viruses investigated.
Table 7.
Serological profiles of 7306 study animals (cases and controls pooled) at induction for four viruses and estimated pooled weighted seroprevalences across the project populationa,b.
| BoHV-1 | BVDV-1 | BRSV | BPIV-3 | Number of animals | Number of controls | Number of cases | Pooled weightedc % |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 99 | 35 | 64 | 1.1 |
| 1 | 0 | 0 | 0 | 10 | 5 | 5 | 0.1 |
| 0 | 1 | 0 | 0 | 64 | 22 | 42 | 0.7 |
| 0 | 0 | 1 | 0 | 193 | 65 | 128 | 2.1 |
| 0 | 0 | 0 | 1 | 268 | 109 | 159 | 3.2 |
| 1 | 1 | 0 | 0 | 7 | 6 | 1 | 0.1 |
| 1 | 0 | 1 | 0 | 20 | 9 | 11 | 0.3 |
| 1 | 0 | 0 | 1 | 54 | 18 | 36 | 0.6 |
| 0 | 1 | 1 | 0 | 253 | 126 | 127 | 3.5 |
| 0 | 1 | 0 | 1 | 325 | 151 | 174 | 4.2 |
| 0 | 0 | 1 | 1 | 1506 | 662 | 844 | 18.9 |
| 1 | 1 | 1 | 0 | 65 | 41 | 24 | 1.0 |
| 1 | 1 | 0 | 1 | 89 | 48 | 41 | 1.3 |
| 1 | 0 | 1 | 1 | 312 | 163 | 149 | 4.4 |
| 0 | 1 | 1 | 1 | 2973 | 1590 | 1383 | 42.6 |
| 1 | 1 | 1 | 1 | 1068 | 605 | 463 | 15.9 |
Seronegative at induction was designated 0 and any positive value was designated 1.
BoHV-1: bovine herpesvirus 1, BVDV-1: bovine viral diarrhoea virus 1, BRSV: bovine respiratory syncytial virus, BPIV-3: bovine parainfluenza virus 3.
Pooled weighted seroprevalences calculated as (0.84 × observed percentage in controls + 0.16 × observed percentage in cases) based on the ratio of the sampling fractions for selection from the NBRDI project population as a case versus as a control (5.3:1).
Table 8.
Distribution of combinations of seroincreases between induction and follow-up in the 7001 animals (cases and controls pooled) with non-missing values for the combined seroincrease variablea,b.
| BoHV-1 | BVDV-1 | BRSV | BPIV-3 | Number of animals | Number of controls | Number of cases | Pooled weightedc % |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 1559 | 1033 | 526 | 27.3 |
| 1 | 0 | 0 | 0 | 1522 | 763 | 759 | 21.8 |
| 0 | 1 | 0 | 0 | 432 | 213 | 219 | 6.1 |
| 0 | 0 | 1 | 0 | 498 | 329 | 169 | 8.7 |
| 0 | 0 | 0 | 1 | 225 | 116 | 109 | 3.3 |
| 1 | 1 | 0 | 0 | 505 | 177 | 328 | 5.8 |
| 1 | 0 | 1 | 0 | 623 | 249 | 374 | 7.7 |
| 1 | 0 | 0 | 1 | 310 | 119 | 191 | 3.7 |
| 0 | 1 | 1 | 0 | 180 | 72 | 108 | 2.2 |
| 0 | 1 | 0 | 1 | 109 | 44 | 65 | 1.4 |
| 0 | 0 | 1 | 1 | 106 | 60 | 46 | 1.7 |
| 1 | 1 | 1 | 0 | 330 | 103 | 227 | 3.5 |
| 1 | 1 | 0 | 1 | 127 | 42 | 85 | 1.4 |
| 1 | 0 | 1 | 1 | 210 | 70 | 140 | 2.3 |
| 0 | 1 | 1 | 1 | 87 | 36 | 51 | 1.1 |
| 1 | 1 | 1 | 1 | 178 | 58 | 120 | 1.9 |
Seroincrease denoted 0 for ‘no’ and 1 for ‘yes’.
BoHV-1: bovine herpesvirus 1; BVDV-1: bovine viral diarrhoea virus 1; BRSV: bovine respiratory syncytial virus; and BPIV-3: bovine parainfluenza virus 3.
Pooled weighted averages (0.84 × observed percentage in controls + 0.16 × observed percentage in cases) based on the ratio of the sampling fractions for selection odds of being selected from the NBRDI project population as a case versus as a control (5.3:1).
The distributions of the two combined virus variables (i.e. number of viruses seropositive at induction and number of viruses animals seroincreased for between induction and follow-up) are shown in Table 9 along with the weighted estimated seroprevalences in the NBRDI project population and their effects on risk of BRD. High percentages of the NBRDI project population were estimated to be seropositive for two (28%) or three (49%) viruses at induction. Compared to animals that were seropositive for all four viruses at induction, those seropositive for less than four viruses were at increased risk of BRD, with risk progressively increasing with seropositivity to fewer viruses (Table 9). Those seronegative for all of the viruses were at highest risk BRD (OR 2.4, 95% credible interval: 1.3–4.3). Based on weighted averages of seroincreases between induction and follow-up, seroincrease for one (40%) or two (23%) viruses was common in the NBRDI project population. Those animals seroincreasing for one virus were at increased risk (OR 1.4, 95% credible interval: 1.2–1.7) compared to those not seroincreasing for any viruses (Table 9), with those seroincreasing for two, three or four viruses at moderate to markedly increased risk (OR 2.0, 95% credible interval: 1.6–2.4, OR 2.1, 95% credible interval: 1.6–2.6 and OR 1.6, 95% credible interval: 1.0–2.4).
Table 9.
Distribution and estimated odds ratios for the total effects of number of viruses for which animals were seropositive at induction and number of viruses for which animals had a seroincrease (increase of at least two categories) between induction and follow-up on the odds of being a BRD case.
| Variable & category | Number of animals | Number (%) of controls | Number (%) of cases | Pooled weighted % (95% CI) | Adjusted odds ratio (95% credible interval) |
|---|---|---|---|---|---|
| Number of viruses animal was seropositive for at inductiona | |||||
| 0 | 99 | 35 (1.0) | 64 (1.7) | 1.1 (0.8–1.4) | 2.4 (1.3–4.3) |
| 1 | 535 | 201 (5.5) | 334 (9.2) | 6.1 (5.4–6.7) | 1.9 (1.4–2.5) |
| 2 | 2165 | 972 (26.6) | 1193 (32.7) | 27.6 (26.3–28.8) | 1.3 (1.1–1.6) |
| 3 | 3439 | 1842 (50.4) | 1597 (43.7) | 49.3 (47.9–50.7) | 1.1 (0.9–1.3) |
| 4 | 1068 | 605 (16.5) | 463 (12.7) | 15.9 (14.9–17.0) | Ref. cat. |
| Number of viruses animal seroincreased for between induction and follow-upb | |||||
| 0 | 1559 | 1033 (29.7) | 526 (15.0) | 27.2 (26.0–28.6) | Ref. cat. |
| 1 | 2677 | 1421 (40.8) | 1256 (35.7) | 40.0 (38.6–41.4) | 1.4 (1.2–1.7) |
| 2 | 1833 | 721 (20.7) | 1112 (31.6) | 22.5 (21.3–23.6) | 2.0 (1.6–2.4) |
| 3 | 754 | 251 (7.2) | 503 (14.3) | 8.4 (7.6–9.1) | 2.1 (1.6–2.6) |
| 4 | 178 | 58 (1.7) | 120 (3.4) | 1.9 (1.6–2.3) | 1.6 (1.0–2.4) |
Covariates: mixing summary, BVDV in cohort, test batch, selection batch; 3 level; N = 7232.
Covariates: mixing summary, test batch, selection batch, cohort size, shared pen water, BVDV in cohort, Rhinogard, number of viruses seropositive for at induction; 3 level; N = 6997.
4. Discussion
The current study has several notable strengths compared with prior research. In sampling a large population of animals from a wide geographical area throughout a three year timeframe, and then randomly selecting animals for inclusion in the case-control study, the study had high statistical power to detect effects of viruses and strong external validity of the findings for extrapolating to moderate to large feedlots in Australia. We were able to appropriately adjust for numerous confounders because high quality data were available for study animals. Being able to adjust for prior mixing history in the analyses was an important strength because serostatus at induction would be expected to vary depending on both if and when prior mixing occurred.
Although feedlot staff were trained in the diagnosis of BRD by the feedlots’ veterinarians. it was unlikely that sensitivity and specificity of diagnoses were 100%; further, these probably differed between staff and between feedlots. BRD misclassification errors may have led to some misclassification bias. However, any such bias would be expected to be towards the null and confounding due to differences in misclassification amongst feedlots would be expected to be at least partially controlled by fitting random effects for feedlots. Collection of a large number of biological samples within the constraints of commercially operating feedlots presented challenges in the current study. While management personnel of the participating feedlots had agreed to the project protocols prior to enrolment, implementation required some operational discretion in the field due to commercial imperatives. An example of this was the timing of collection of the follow-up sera. While an optimal time, day 42, was stipulated, the actual day of collection was at the discretion of feedlot managers, based on availability of resources such as staffing, as long as it was within 35–60 days. Further, in the commercial setting, it was not feasible to repeat sample collection at a particular stage (i.e. induction or follow-up) if samples collected were unsuitable. Animals from nine cohorts were ineligible for inclusion in the case-control study because the time between initial and follow-up samples was more than 60 days (three cohorts), no samples were collected (five cohorts) or all samples from a particular stage were unsuitable for testing (one cohort). About 4% of case-control animals were excluded from analysis of the combined virus serochange variable because results for at least one virus were deemed biologically implausible. Incorrectly paired samples due, for example, to animal or sample identification errors could cause these. However few animals returned highly implausible results for paired samples for more than one virus. Thus, whilst a few samples may have been of poor quality and some may have been mismatched between animals, the majority of samples appeared to have been correctly paired.
The results of this study support prior research that indicates that BoHV-1, BVDV-1, BRSV, and BPIV-3 are ubiquitous in Australian cattle populations (Dunn et al., 1995, Smith et al., 1995, Durham and Paine, 1997, Taylor et al., 2006). Based on weighted seroprevalence, an estimated 24% of NBRDI study animals were seropositive for BoHV-1 at induction compared to 13% in a previous Australian study (Dunn et al., 1995) and 1–18% in North American studies (Martin and Bohac, 1986, Martin et al., 1989, Martin et al., 1990, Martin et al., 1999). At induction, 69% of the NBRDI project population were estimated to have antibodies to BVDV-1; this is consistent with a prior Australian study that reported a seroprevalence of 68% (Dunn et al., 1995). In the current study, 89% of animals were seropositive for BRSV and 91% were seropositive for BPIV-3 at induction, both of which are much higher than that reported in North American studies (Martin and Bohac, 1986, Martin et al., 1989, Martin et al., 1990, Martin et al., 1999) and seroprevalences of 27% and 57% for BRSV and BPIV-3, respectively, at initial sampling of cattle entering Australian feedlots (Dunn et al., 1995). Overall, it is apparent that high proportions of cattle entering Australian feedlots are seropositive for BVDV-1, BRSV, and BPIV-3 with fewer seropositive for BoHV-1. The generally higher seroprevalence compared to those reported in North American studies are not unexpected because cattle entering feedlots in North America are generally younger and lighter (Snowder et al., 2007, Sanderson et al., 2008). Although age of study animals at induction was unknown, median weight at induction for cattle enrolled in the NBRDI was 438 kg (Ref: Hay et al., 2016b) compared to a median of 335 kg in a large North American study (Sanderson et al., 2008). Animals entering Australian feedlots have more extensive mixing histories (Hay et al., 2014); older animals which have previously been commingled with animals from other farms would be more likely have been exposed to and developed immunological memory to a wider range of pathogens compared to younger animals. Some differences between studies may also be attributable to differences in serological testing methods and classification cut-points. For example, in the current study, substantial proportions of animals had low antibody levels (category 1), especially for BRSV (23%) and BPIV-3 (15%), and we classified these as seropositive; this may have resulted in higher seroprevalences compared with previous studies.
Our results indicate that induction serostatus categories of 1 or higher for BRSV and BPIV-3 at induction offered similar degrees of protection regardless of category. In contrast, induction serostatus category of 2 or higher for BVDV-1 was required for any protection from BRD, while for BoHV-1, category 2 and 3 was protective but 4 and 5 was associated with increased risk of BRD. It is possible that those animals in category 1 at initial sampling were recently exposed and had not yet developed maximal immunity. Similarly, categories 4 or 5 were possibly also more recently exposed animals whereas those in antibody categories 2 or 3 at induction may have been exposed a sufficient time prior to induction to produce a more effective immunity. Nevertheless, seropositivity at induction was associated with reduced risk of BRD for all four viruses. While findings from previous studies generally also indicate that seropositivity to BVDV-1 (Martin and Bohac, 1986, Martin et al., 1989, Martin et al., 1990, Dunn et al., 1995, O'Connor et al., 2001) and BoHV-1 (Martin and Bohac, 1986, Martin et al., 1989, Dunn et al., 1995) at initial sampling was associated with decreased risk of BRD, effects of BRSV and BPIV-3 serostatus at induction have been inconsistent (Martin and Bohac, 1986, Martin et al., 1989, Martin et al., 1990, Allen et al., 1992). Seroepidemiological studies are subject to limitations in external validity because not all viruses of interest may be circulating in any particular feedlot population, the virulence of particular strains of virus may vary in different locations and over time, and the effects of particular viruses are likely to depend on the prevalence of immunity amongst cattle in the population and the associated degree of herd immunity, and on non-infectious factors. Limitations are more likely to impact on results of studies involving small or localised populations. These limitations are less likely to have influenced the external validity of results of the current study for extrapolating to the Australian feedlot population due to the large study population, the enrolment of a substantial number of geographically widely dispersed feedlots, and the fact that those feedlots obtained animals from a wide geographic area.
Animals that seroincreased (OR: 1.3–1.5; Table 3, Table 4, Table 5) or seroconverted (OR: 1.3–1.6; Table 3, Table 4, Table 5) for any of the four viruses were at similarly increased risk of being diagnosed with BRD. This indicated that each of these viruses modestly to moderately increased the risk of BRD. Our results also indicated that these risk increases were cumulative. The risk of BRD increased as the number of the four viruses that the animal had antibodies to at induction decreased and those animals seroincreasing to one virus were at increased risk compared to those not seroincreasing to any viruses, with those seroincreasing to at least two viruses at markedly increased risk (Table 8). This accumulating risk demonstrates the complexity of BRD development and suggests additive effects of exposure to additional viruses. Importantly, 15% of cases did not seroincrease for any of the four viruses studied. Viruses are generally accepted as playing an important role in initiating BRD, but not all putative viruses or other infectious pathogens that have been associated with BRD were investigated in the current study. For example, Mycoplasma bovis (Horwood et al., 2014) and Bovine coronavirus (Moore et al., 2014) may play important roles. Although biological synergism between infectious agents is believed to play an important role in the development of BRD (Ellis, 2009), close correlations in serological variables and significant interactions between effects of specific viruses on BRD risk were not observed in the current study.
For each of the viruses studied, seroincrease was associated with similarly increased risk of BRD. In determining the relative importance of these viruses at the population level, we need to consider the proportions of animals that seroincrease and the proportion of animals initially at risk of seroincrease (i.e. induction serostatus category 3 or less). In the NBRDI project population, the largest percentage (98%) of animals was susceptible to seroincrease for BoHV-1. Of animals not vaccinated at induction, 23% seroincreased, while across the NBRDI population an estimated 48% seroincreased. Thus, assuming similar odds ratios for BRD due to seroincrease to each virus, BoHV-1 probably had the greatest impact in the study population, contributing the largest increase in BRD incidence at the population level. Interestingly amongst animals vaccinated with Rhinogard® at induction, those seroconverting to BoHV-1 during the time on feed were at increased risk of BRD (OR 1.3, 95% CI 1.1–1.6) compared with those not seroconverting, with a similar effect size to that observed with seroconversion or seroincrease for each of the other viruses, raising uncertainty about the efficacy of this vaccine in preventing BRD.
The effects of using modified live vaccines at induction into feedlots are unclear; immunologically stressed animals may be unable to mount an effective immune response following vaccination (Richeson et al., 2008). There is a need to conduct a randomised controlled trial to evaluate the efficacy of Rhinogard® administered at induction.
Results from the subset analyses restricted to those animals known to have not been previously vaccinated with Pestigard® provide further insight into the role of BVDV-1. The high seroprevalence at induction (66%; Table 4) in this subset provides evidence that natural exposure to BVDV-1 is widespread in Australian cattle prior to feedlot entry. Of animals initially seronegative, more than half were estimated to seroconvert between induction and follow-up and seroconversion was associated with a markedly increased risk of BRD. This suggests that BVDV-1 infection is an important risk factor for BRD in populations of immunologically naïve animals. Implications of this should be considered if a large-scale BVDV-1 control program is to be implemented in the Australian beef industry. If the proportion of cattle arriving at feedlots without immunity to BVDV-1 is decreased without substantial reductions in probability of exposure of feedlot cattle to BVDV-1, risk of BRD may be increased.
5. Conclusions
The current study has provided support for previously published evidence which indicates that prior exposure resulting in measurable antibody levels to either BoHV-1 or BVDV-1 at feedlot induction is associated with reduced risk of BRD at the feedlot, while exposure to either of these viruses after induction increases risk of BRD. Our results, unlike other studies, provide strong evidence that being seropositive for BRSV or BPIV-3 at induction is associated with reduced risk, while exposure to BRSV and BPIV-3 at the feedlot increases risk of BRD. The roles of individual viruses will be more important in populations with low seroprevalences at induction. Although not all viruses implicated in the BRD complex were investigated in the current study, for each virus investigated, seroincrease was associated with only a modest increase in risk, indicating that each virus in isolation has only a modest effect on BRD risk. However, seroincrease for multiple viruses further increased risk, indicating that exposure to multiple viruses resulted in markedly increased risk of BRD. These findings confirm the multifactorial nature of BRD development. In addition, for animals not vaccinated against BVDV-1 that seroconverted to that agent, BRD risk was markedly increased. Collectively these results indicate that, while efficacious vaccines could aid in the control of BRD, vaccination against one of these viruses would not have large effects on population BRD incidence but vaccination against multiple viruses would be expected to result in greater reductions in incidence. While viruses have a critical role in initiating BRD, there is also a need to focus on the multifactorial nature of the disease and consider other risk factors in addition to viral pathogens when planning BRD control strategies.
Acknowledgment
This study was supported by grant B.FLT.0224 from Meat and Livestock Australia with matching funds provided by the Australian Government.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.prevetmed.2016.01.024.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Allen J.W., Viel L., Bateman K.G., Nagy E., Rosendal S., Shewen P.E. Serological titers to bovine herpesvirus-1, bovine viral diarrhea virus, parainfluenza-3 virus, bovine respiratory syncytial virus and pasteurella-haemolytica in feedlot calves with respiratory-disease—associations with bacteriological and pulmonary cytological variables. Can. J. Vet. Res. 1992;56:281–288. [PMC free article] [PubMed] [Google Scholar]
- Bonett D.G., Price R.M. Inferential methods for the tetrachoric correlation coefficient. J. Educ. Behav. Stat. 2005;30:213–225. [Google Scholar]
- Booker C.W., Guichon P.T., Jim G.K., Schunicht O.C., Harland R.J., Morley P.S. Seroepidemiology of undifferentiated fever in feedlot calves in western Canada. Can. Vet. J. 1999;40:40–48. [PMC free article] [PubMed] [Google Scholar]
- Booker C.W., Abutarbush S.M., Morley P.S., Jim G.K., Pittman T.J., Schunicht O.C., Perrett T., Wildman B.K., Fenton R.K., Guichon P.T., Janzen E.D. Microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in Western Canada. Can. Vet. J. 2008;49:473–481. [PMC free article] [PubMed] [Google Scholar]
- Browne W.J. Centre for Multilevel Modelling, University of Bristol; Bristol: 2012. MCMC Estimation in MLwiN, Version 2.26. [Google Scholar]
- Confer A.W. Update on bacterial pathogenesis in BRD. Anim. Health Res. Rev. 2009;10:145–148. doi: 10.1017/S1466252309990193. [DOI] [PubMed] [Google Scholar]
- Dohoo I., Martin W., Stryhn H. VER Inc.; Charlottetown, Canada: 2009. Veterinary Epidemiologic Research. [Google Scholar]
- Dunn S., Godwin J., Hoare R., Kirkland P.D. Meat Research Corporation; North Sydney: 1995. Diseases of Feedlot Cattle. [Google Scholar]
- Durham P.J.K., Hassard L.E., Van Donkersgoed J. Serological studies of infectious bovine rhinotracheitis, parainfluenza 3, bovine viral diarrhea, and bovine respiratory syncytial viruses in calves following entry to a bull test station. Can. Vet. J. 1991;32:427–429. [PMC free article] [PubMed] [Google Scholar]
- Durham P.J.K., Paine G.D. Serological survey for antibodies to infectious agents in beef cattle in northern South Australia. Aust. Vet. J. 1997;75:139–140. doi: 10.1111/j.1751-0813.1997.tb14176.x. [DOI] [PubMed] [Google Scholar]
- Edwards T.A. Control methods for bovine respiratory disease for feedlot cattle. Vet. Clin. North Am.—Food Anim. Pract. 2010;26:273–284. doi: 10.1016/j.cvfa.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Ellis J.A. Update on viral pathogenesis in BRD. Anim. Health Res. Rev. 2009;10:149–153. doi: 10.1017/S146625230999020X. [DOI] [PubMed] [Google Scholar]
- Ellis J.A. Bovine Parainfluenza-3 Virus. Vet. Clin. North Am.—Food Anim. Pract. 2010;26:575–593. doi: 10.1016/j.cvfa.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Fulton R.W. Host response to bovine viral diarrhea virus and interactions with infectious agents in the feedlot and breeding herd. Biologicals. 2013;41:31–38. doi: 10.1016/j.biologicals.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton R.W., Purdy C.W., Confer A.W., Saliki J.T., Loan R.W., Briggs R.E., Burge L.J. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can. J. Vet. Res. 2000;64:151–159. [PMC free article] [PubMed] [Google Scholar]
- Fulton R.W., Cook B.J., Step D.L., Confer A.W., Saliki J.T., Payton M.E., Burge L.J., Welsh R.D., Blood K.S. Evaluation of health status of calves and the impact on feedlot performance: assessment of a retained ownership program for postweaning calves. Can. J. Vet. Res. 2002;66:173–180. [PMC free article] [PubMed] [Google Scholar]
- Fulton R.W., Ridpath J.F., Saliki J.T., Briggs R.E., Confer A.W., Burge L.J., Purdy C.W., Loan R.W., Duff G.C., Payton M.E. Bovine viral diarrhea virus (BVDV) 1b: predominant BVDV subtype in calves with respiratory disease. Can. J. Vet. Res. 2002;66:181–190. [PMC free article] [PubMed] [Google Scholar]
- Geraghty T., O’Neill R., More S., O’Grady L. Dynamics of individual animal Bovine Herpes Virus-1 antibody status on 9 commercial dairy herds. Res. Vet. Sci. 2012;93:143–149. doi: 10.1016/j.rvsc.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Greenland S., Pearl J., Robins J.M. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- Hay K.E., Barnes T.S., Morton J.M., Clements A.C.A., Mahony T.J. Risk factors for bovine respiratory disease in Australian feedlot cattle: use of a causal diagram-informed approach to estimate effects of animal mixing and movements before feedlot entry. Prev. Vet. Med. 2014;117:160–169. doi: 10.1016/j.prevetmed.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Hay K.E., Ambrose R.C.K., Morton J.M., Horwood P.F., Gravel J.L., Waldron S., Commins M.A., Fowler E.V., Clements A.C.A., Barnes T.S., Mahony T.J. Effects of exposure to Bovine viral diarrhoea virus 1 on risk of bovine respiratory disease in Australian feedlot cattle. Prev. Vet. Med. 2016 doi: 10.1016/j.prevetmed.2016.01.025. [DOI] [PubMed] [Google Scholar]
- Hay K.E., Morton J.M., Mahony T.J., Clements A.C.A., Barnes T.S. Associations between animal characteristic and environmental risk factors and bovine respiratory disease in Australian feedlot cattle. Prev. Vet. Med. 2016 doi: 10.1016/j.prevetmed.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Horwood P.F., Gravel J.L., Mahony T.J. Identification of two distinct bovine parainfluenza virus type 3 genotypes. J. Gen. Virol. 2008;89:1643–1648. doi: 10.1099/vir.0.2008/000026-0. [DOI] [PubMed] [Google Scholar]
- Horwood P.F., Schibrowski M.L., Fowler E.V., Gibson J.S., Barnes T.S., Mahony T.J. Is Mycoplasma bovis a missing component of the bovine respiratory disease complex in Australia? Aust. Vet. J. 2014;92:185–191. doi: 10.1111/avj.12184. [DOI] [PubMed] [Google Scholar]
- Mahony T.J., McCarthy F.M., Gravel J.L., Corney B., Young P.L., Vilcek S. Genetic analysis of bovine viral diarrhoea viruses from Australia. Vet. Microbiol. 2005;106:1–6. doi: 10.1016/j.vetmic.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Martin S.W., Bateman K.G., Shewen P.E., Rosendal S., Bohac J.E. The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can. J. Vet. Res. 1989;53:355–362. [PMC free article] [PubMed] [Google Scholar]
- Martin S.W., Bateman K.G., Shewen P.E., Rosendal S., Bohac J.G., Thorburn M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can. J. Vet. Res. 1990;54:337–342. [PMC free article] [PubMed] [Google Scholar]
- Martin S.W., Bohac J.G. The association between serological titers in infectious bovine-rhinotracheitis virus, bovine virus diarrhea virus, parainfluenza-3 virus, respiratory syncytial virus and treatment for respiratory-disease in Ontario feedlot calves. Can. J. Vet. Res. 1986;50:351–358. [PMC free article] [PubMed] [Google Scholar]
- Martin S.W., Darlington G., Bateman K., Holt J. Undifferentiated bovine respiratory disease (shipping fever): is it communicable? Prev. Vet. Med. 1988;6:27–35. [Google Scholar]
- Martin S.W., Harland R.J., Bateman K.G., Nagy E. The association of titers to Haemophilus somnus, and other putative pathogens, with the occurrence of bovine respiratory disease and weight gain in feedlot calves. Can. J. Vet. Res. 1998;62:262–267. [PMC free article] [PubMed] [Google Scholar]
- Martin S.W., Nagy E., Armstrong D., Rosendal S. The associations of viral and mycoplasmal antibody titers with respiratory disease and weight gain in feedlot calves. Can. Vet. J. 1999;40:560–570. [PMC free article] [PubMed] [Google Scholar]
- Moore S.J., O’Dea M.A., Perkins N., Barnes A., O’Hara A.J. Mortality of live export cattle on long-haul voyages: pathologic changes and pathogens. J. Vet. Diagn. Investig. 2014;26:252–265. doi: 10.1177/1040638714522465. [DOI] [PubMed] [Google Scholar]
- O'Connor A., Martin S.W., Nagy E., Menzies P., Harland R. The relationship between the occurrence of undifferentiated bovine respiratory disease and titer changes to bovine coronavirus and bovine viral diarrhea virus in 3 Ontario feedlots. Can. J. Vet. Res. 2001;65:137–142. [PMC free article] [PubMed] [Google Scholar]
- Panciera R.J., Confer A.W. Pathogenesis and pathology of bovine pneumonia. Vet. Clin. North Am.—Food Anim. Pract. 2010;26:191–214. doi: 10.1016/j.cvfa.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeson J.T., Beck P.A., Gadberry M.S., Gunter S.A., Hess T.W., Hubbell D.S., Jones C. Effects of on-arrival versus delayed modified live virus vaccination on health, performance, and serum infectious bovine rhinotracheitis titers of newly received beef calves. J. Anim. Sci. 2008;86:999–1005. doi: 10.2527/jas.2007-0593. [DOI] [PubMed] [Google Scholar]
- Sackett, D., Holmes, P., Abbott, K., Jephcott, S., Barber, M., 2006. Assessing the economic cost of endemic disease on the profitability of Australian beef cattle and sheep producers. Final Report: Project AHW.087. Sydney.
- Sanderson M.W., Dargatz D.A., Wagner B.A. Risk factors for initial respiratory disease in United States’ feedlots based on producer-collected daily morbidity counts. Can. Vet. J. 2008;49:373–378. [PMC free article] [PubMed] [Google Scholar]
- Shrier I., Platt R.W. Reducing bias through directed acyclic graphs. BMC Med. Res. Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.A., Young P.L., Reed K.C. Emergence of a new bovine herpesvirus-1 strain in australian feedlots. Arch. Virol. 1995;140:599–603. doi: 10.1007/BF01718435. [DOI] [PubMed] [Google Scholar]
- Smith R.A. Impact of disease on feedlot performance: a review. J. Anim. Sci. 1998;76:272–274. doi: 10.2527/1998.761272x. [DOI] [PubMed] [Google Scholar]
- Snowder G.D., Van Vleck L.D., Cundiff L.V., Bennett G.L., Koohmaraie M., Dikeman M.E. Bovine respiratory disease in feedlot cattle: phenotypic, environmental, and genetic correlations with growth, carcass, and longissimus muscle palatability traits. J. Anim. Sci. 2007;85:1885–1892. doi: 10.2527/jas.2007-0008. [DOI] [PubMed] [Google Scholar]
- Solís-Calderón J.J., Segura-Correa J.C., Aguilar-Romero F., Segura-Correa V.M. Detection of antibodies and risk factors for infection with bovine respiratory syncytial virus and parainfluenza virus-3 in beef cattle of Yucatan, Mexico. Prev. Vet. Med. 2007;82:102–110. doi: 10.1016/j.prevetmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Taylor L.F., Black P.F., Pitt D.J., Mackenzie A.R., Johnson S.J., Rodwell B.J. A seroepidemiological study of bovine pestivirus in Queensland beef and dairy herds conducted in 1994/95. Aust. Vet. J. 2006;84:163–168. doi: 10.1111/j.1751-0813.2006.tb12771.x. [DOI] [PubMed] [Google Scholar]
- Textor J., Hardt J., Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22 doi: 10.1097/EDE.0b013e318225c2be. 745–745. [DOI] [PubMed] [Google Scholar]
- Textor J., Liskiewicz M. Adjustment criteria in causal diagrams: an algorithmic perspective. Proceedings of the 27th Conference on Uncertainty in Artificial Intelligence; Corvallis, OR; 2011. pp. 681–688. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


