Abstract
The activity of inosine-5′-monophosphate dehydrogenase (IMPDH) inhibitors, mizoribine and ribavirin, against severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) was determined by plaque reduction and yield reduction assays. Mizoribine and ribavirin selectively inhibited replication of SARS-CoV. The 50% inhibitory concentration (IC50) of mizoribine for SARS-CoV Frankfurt-1 and SARS-CoV HKU39849, as determined by plaque reduction was 3.5 μg/ml and 16 μg/ml, respectively, and the IC50 of ribavirin for SARS-CoV Frankfurt-1 and SARS-CoV HKU39849 was 20 μg/ml and 80 μg/ml, while the 50% cytotoxic concentration of mizoribine and ribavirin for Vero E6 cells exceeded 200 μg/ml. In a yield reduction assay, mizoribine (10 μg/ml) and ribavirin (40 μg/ml) inhibited the replication of SARS-CoV and reduced the infectious SARS-CoV titers to one-tenth or less. Mizoribine inhibited replication of SARS-CoV more strongly than ribavirin. However, neither drug could completely inhibit replication of SARS-CoV even at concentrations up to 100 μg/ml.
Keywords: SARS, Coronavirus, Ribavirin, Mizoribine
The first outbreak of severe acute respiratory syndrome (SARS) occurred in the Guangdong Province, in Southern China, in November 2002, and then spread through human-to-human infection from there to other areas of China, as well as Vietnam, Singapore, Canada and some 30 countries (Lee et al., 2003, Poutanen et al., 2003, Tsang et al., 2003). Approximately 8000 SARS patients have been reported and about 800 patients died in the outbreak from November 2002 and July 2003. SARS-associated coronavirus (SARS-CoV) has been identified as the causative agent for SARS (Drosten et al., 2003, Ksiazek et al., 2003).
In the present study, inhibitory effect of mizoribine (4-carbamoyl-1-β-d-ribofuranosylimidazolium-5-olate, M w 259.22) and ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide, M w 244.2) on the replication of SARS-CoV was evaluated. Mizoribine, an imidazole nucleoside, is an immunosuppressive agent used for renal transplantation, autoimmune diseases and steroid-resistant nephrotic syndrome in Japan. Mizoribine is phosphorylated by adenosine kinase and converted its active form, mizoribine 5′-monophosphate. This activated form of mizoribine acts as an inhibitor of the enzyme, inosine 5′-monophosphate dehydrogenase (IMPDH) and guanosine monophosphate synthetase, both enzymes being essential to the synthesis of guanosine monophosphate from inosine monophosphate through the de novo pathway (Yokota, 2002). Furthermore, mizoribine possesses in vitro anti-viral activities against herpes simplex virus, cytomegalovirus, respiratory syncytial virus, influenza viruses, and bovine viral diarrhea virus (Shiraki et al., 1990, Hosoya et al., 1993, Shigeta, 2000, Pancheva et al., 2002, Stuyver et al., 2002). Ribavirin is a well-known broad-spectrum antiviral agent. Ribavirin 5′-monophosphate, a metabolite of ribavirin, also acts as an inhibitor of IMPDH.
Mizoribine and ribavirin were supplied from Yamasa-shouyu Co., Ltd., Choshi, Chiba, Japan. The cytotoxic effect, cytotoxic concentration (CC) of mizoribine and ribavirin for Vero E6 cells (American Type Cell Collection, Manassas, VA.) was measured using a WST-1 cytotoxicity assay kit (Roche Diagnositics, Mannheim, Germany), according to the manufacturer's instructions. The CC50 and CC20 were defined as the concentration at which the viability of Vero E6 cells decreased to 50% and 80% of that of cells cultured without the addition of antiviral drugs, respectively. In the cytotoxicity assay, Vero E6 cells were cultured in Eagle's minimum essential medium (MEM) containing 2% fetal bovine serum (FBS), penicillin G, and streptomycin (MEM-2FBS), which was the same medium for growth of SARS-CoV. Briefly, approximately 104 of Vero E6 cells per well were inoculated in each well in a 96-well microplate and let stand for 4 h to allow the cells to adhere to the bottom of the wells. The culture medium was then replaced by the MEM-2FBS with or without drug. The cells were cultured for 3 days in a CO2 incubator under humidified condition, and then evaluated for cytotoxicity using the WST-1 kit. The level of Vero E6 cells’ viability at different concentration of either drug is shown in Fig. 1A. The CC50 of mizoribine and ribavirin exceed at 200 μg/ml, and the CC20 of mizoribine and ribavirin was 200 and 40 μg/ml, respectively.
Fig. 1.
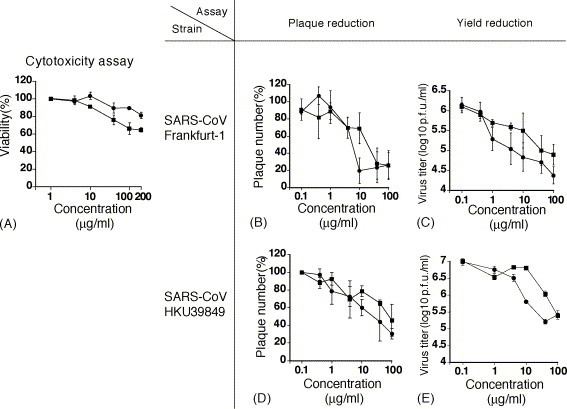
Cytotoxicity (A), inhibitory effect on plaque formation of SARS-CoV Frankfurt-1 (B) and HKU39849 (D) in a plaque reduction assay and inhibitory effect on replication of SARS-CoV Frankfurt-1 (C) and HKU39849 (E) in yield reduction assay. The symbols, “●” and “■” represent mizoribine and ribavirin, respectively. The vertical bar indicates 1 S.D. One hundred percent of plaque numbers in (B) and (D) correspond to the control (no compound). The SARS-CoV titers at 0 μg/ml of drugs are not shown in these figures, as these titers were not significantly different from those at a concentration of 0.1 μg/ml.
In the present study, two strains of SARS-CoV (Frankfurt-1 and HKU39849) were used. SARS-CoV (Frankfurt-1) and SARS-CoV (HKU39849) were kindly provided by Dr. John Ziebuhr of the Institute of Virology and Immunology, University of Wuerzburg, Wuerzburg, Germany, and Dr. J.S. Malik Peiris, Department of Microbiology, Hong Kong University, HKSAR, respectively. The anti-SARS-CoV activity of these drugs was firstly measured by a plaque reduction assay in Vero E6 cells. Vero E6 cell monolayers seeded in 24-well microplates were inoculated with 0.2 ml of SARS-CoV stock solution at 100 plaque formation unit (p.f.u.)/ml. After a 1-h incubation, the inocula were removed and the cells were washed with phosphate-buffered saline solution (PBS). The cells were then cultured in MEM-2FBS with a designated concentration of each compound and 0.5% methylcellulose for 48 h in a CO2 incubator under humidified conditions. The culture medium was then removed and the cells in the culture plate were fixed with a 10% formalin solution and stained with crystal violet solution. The plaque number was then counted. The incubation time was set at 48 h, because the plaque size became too large to be counted when the incubation time was set at 3 days or more. The experiment was performed in duplicate. The 50% inhibitory concentration (IC50) was defined as the concentration at which the plaque number decreased to half of that in cells cultured without addition of antiviral drugs. The same experiment was performed three times independently for each drug and the IC50 values were calculated as the average ± standard deviation (SD) of the three experiments. The 20% inhibitory concentration (IC20) and 80% inhibitory concentration (IC80) were determined as well. The inhibitory effect of mizoribine and ribavirin on SARS-CoV (Frankfurt-1 and HKU39849)-plaque formation is shown in Fig. 1B and D, respectively. The IC50, IC20 and IC80 values of mizoribine and ribavirin for SARS-CoV are shown in Table 1 , and so are the cytotoxic concentrations. Reduction in the number of plaques was demonstrated in cells cultured in medium to which mizoribine or ribavirn were added. However, IC80 values of both drugs could not be determined, because plaque formation by SARS-CoV Frankfurt-1 and HKU39849 was not completely inhibited even at a concentration of 100 μg/ml of each drug (Fig. 1B and D). As shown in Fig. 1B and D, mizoribine inhibited plaque formation by SARS-CoV more strongly than ribavirin in plaque reduction assay. The size of plaques was significantly decreased at a concentration of 10 μg/ml or greater for each drug (data not shown).
Table 1.
Inhibitory concentrations of mizoribine and ribavirin on SARS-CoV Frankfurt-1 and HKU39849 strains
| Drug | SARS-CoV strain | Inhibitory concentration (μg/ml) |
Cytotoxic concentration (μg/ml) |
|||
|---|---|---|---|---|---|---|
| IC20 | IC50 | IC80 | CC20 | CC50 | ||
| Mizoribine | Frankfurt-1 | 2.2 ± 2.0 | 3.5 ± 2.9 | >100 | 200 | >200 |
| HKU39849 | 1.4 ± 0.6 | 16 ± 2.8 | >100 | |||
| Ribavirin | Frankfurt-1 | 8.9 ± 6.3 | 20 ± 15 | >100 | 40 | >200 |
| HKU39849 | 15 ± 1.4 | 80 ± 28 | >100 | |||
The inhibitory effects of mizoribine and ribavirin on SARS-CoV replication were further evaluated by a yield reduction assay. Vero E6 cells seeded in 6-well microplates were inoculated with SARS-CoV solution for 1 h to allow infection with SARS-CoV at a multiplicity of infection (m.o.i.) of 0.01 p.f.u./cell. After 1 h, the inocula were removed and the cells were washed twice with PBS and cultured in MEM-2FBS with or without drug for 20 h. The time between inoculation of SARS-CoV and harvesting samples was 20 h, because the preliminary study revealed that the growth of SARS-CoV was so fast that 2 or more days-incubation made it difficult to assess an inhibitory effect of mizoribine and ribavirin on SARS-CoV replication. Therefore, a 20-h incubation time was set in the yield reduction assays. The medium was then collected and stored in −80 °C until use. At this stage, no obvious specific cytopathic effect was observed (data not shown). In each experiment, two wells were used at each concentration of the antiviral drugs. The infectious dose of SARS-CoV was determined by a plaque assay in Vero E6 cells. The same experiment was conducted three times independently and the infectious dose was calculated as the average ± S.D. of the three independent experiments. Mizoribine inhibited replication of SARS-CoV (Frankfurt-1 and HKU39849), and the infectious viral dose at the concentration of 10 μg/ml decreased to one-tenth or less of the control (Fig. 1C and E). The inhibitory effect of mizoribine on SARS-CoV replication was greater than that of ribavirin (Fig. 1C and E).
In both plaque reduction and yield reduction assays, it was demonstrated that mizoribine inhibited replication of SARS-CoV more strongly than ribavirin. In order to determine whether the difference in the degree of inhibitory effect between mizoribine and ribavirin on SARS-CoV was a phenomenon observed only for SARS-CoV, the inhibitory effect of these drugs on replication of vaccinia virus (Lister strain) and herpes simplex virus type 1 (VR-3 strain), which were stored in the National Institute of Infectious Diseases, Tokyo, Japan, was measured as a control by plaque reduction assay in Vero E6 cells. It has been reported that ribavirin inhibits replication of vaccinia virus (Kirsi et al., 1983). The plaque reduction assay for vaccinia virus and HSV-1 was carried out as described above for SARS-CoV except for the length of the culture period. In the plaque reduction assay for vaccinia virus and HSV-1, the cells were cultured for 4 days. As shown in Fig. 2A, ribavirin inhibited replication of vaccinia virus to the same extent as mizoribine. However, ribavirin did not show anti-HSV-1 activity in Vero E6 cells, while mizoribine showed an inhibitory effect on the replication of HSV-1 (Fig. 2B). These results suggested that the difference in degree of inhibitory effect on viral replication between mizoribine and ribavirin was dependent on the type of virus and that the mode of antiviral action of mizoribine was not the same as that of ribavirin, although both drugs act as IMPDH inhibitors.
Fig. 2.
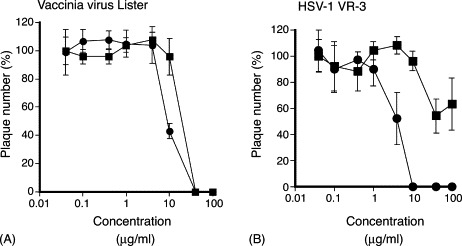
Inhibitory effect of mizoribine (●) and ribavirin (■) on vaccinia virus (Lister strain) (A) and HSV-1 VR-3 (B) determined by plaque reduction assay. The vertical bar indicates 1 S.D. calculated from the data obtained by independent three experiments. One hundred percent of plaque numbers correspond to the control (no compound).
During the outbreak of SARS in 2003, several therapeutic strategies for the treatment of SARS were tried, such as administration of ribavirin, lopinavir/ritonavir (a protease inhibitor), interferon, steroids, and so on, alone or in combination with each other (Ho et al., 2003, Loutfy et al., 2003, Zhao et al., 2003, Zhaori, 2003, Chu et al., 2004). However, no efficacious treatments of SARS with antiviral agents have been developed. Several compounds, such as ribavirin, lopinavir/ritonavir, 6-azauridine, pyrazofurin, interferon-α, interferon-β, glycyrrhizin, niclosamide (antihelminthic drug), aurintricarboxylic acid, nelfinavir (HIV protease inhibitor), S-nitroso-N-acetylpenicillamine (a nitric oxide donor), and chloroquine (antimalaria drug) were demonstrated to show anti-SARS-CoV activity in vitro (Cinatl et al., 2003, Chu et al., 2004, He et al., 2004, Hensley et al., 2004, Keyaerts et al., 2004, Sainz et al., 2004, Stroher et al., 2004, Wu et al., 2004, Yamamoto et al., 2004).
Cinatl et al. (2003) reported that ribavirin did not have anti-SARS-CoV activity in vitro. Also in the present study ribavirin did not completely inhibit replication of SARS-CoV even at a concentration of 100 μg/ml. However, ribavirin did possess an inhibitory effect on replication of SARS-CoV, as demonstrated in our present study as well as that of Chu et al. (2004). Scoring of cytopathogenicity was performed in the former study to determine the antiviral activity of the tested compounds (Cinatl et al., 2003), while plaque reduction assay was performed in the present studies and those of Chu et al. (2004). The reason why an inhibitory effect of ribavirin was demonstrated in the present study and not in the previous study by Cinatl et al. (2003) may be attributed to difference in the procedures used, and, in particular, to the duration of the incubation times of the cells in the presence of ribavirin.
Ribavirin is phosphorylated to form ribavirin mono-, di- and triphosphate. Ribavirin-monophosphate inhibits the enzymatic activity of IMPDH, resulting in decreased intracellular guanosine triphosphate and deoxyguanosine triphosphate pool levels (Streeter et al., 1973). This alteration in guanosine triphosphate pool levels causes suppression in cellular DNA, mRNA and protein synthesis. Possible mechanisms of antiviral activity of ribavirin were reported for rotavirus, vesicular stomatitis virus, influenza virus and vaccinia virus (Eriksson et al., 1977, Lowe et al., 1977, Muller et al., 1977, Goswami et al., 1979, Smee et al., 1982, Kirsi et al., 1983, Toltzis et al., 1988). As described above, the mode of antiviral action of mizoribine is considered to be similar, but not identical to that of ribavirin, although both drugs act as IMPDH inhibitors. The mechanism of anti-SARS-CoV activity of mizoribine and ribavirin should be addressed in future studies.
In summary, mizoribine and ribavirin possess an inhibitory effect on the replication of SARS-CoV in vitro, but their anti-SARS-CoV activity is virustatic rather than virucidal. The findings obtained in the present study do not legitimate the use of ribavirin or mizoribine for the treatment of patients with SARS, but may contribute to the further development of antiviral agents for SARS-CoV and of therapeutic strategies for SARS.
Acknowledgements
We thank Dr. J. Ziebuhr of the Institute of Virology and Immunology, University of Wuerzburg, Wuerzburg, Germany, and Dr. J.S. Malik Peiris, Department of Microbiology, Hong Kong University, HKSAR, for kindly providing with SARS-CoV Frankfult-1 strain and HKU39849 strain, respectively. We also thank Dr. H. Machida and Dr. N. Ashida, of the Yamasa-shouyu Co., Ltd., Choshi, Chiba, Japan, for kindly providing mizoribine and ribavirin. We also thank Ms. M. Ogata for her technical and official assistance. The study was partly supported by a Grants-in-Aids from the Ministry of Health, Labor and Welfare of Japan.
References
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Eriksson B., Helgstrand E., Johansson N.G., Larsson A., Misiorny A., Noren J.O., Philipson L., Stenberg K., Stening G., Stridh S., Öberg B. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob. Agents Chemother. 1977;11:946–951. doi: 10.1128/aac.11.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami B.B., Borek E., Sharma O.K., Fujitaki J., Smith R.A. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem. Biophys. Res. Commun. 1979;89:830–836. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- He R., Adonov A., Traykova-Adonova M., Cao J., Cutts T., Grudesky E., Deschambaul Y., Berry J., Drebot M., Li X. Potent and selective inhibition of SARS coronavirus replication by aurintricarboxylic acid. Biochem. Biophys. Res. Commun. 2004;320:1199–1203. doi: 10.1016/j.bbrc.2004.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley L.E., Fritz L.E., Jahrling P.B., Karp C.L., Huggins J.W., Geisbert T.W. Interferon-beta 1a and SARS coronavirus replication. Emerg. Infect. Dis. 2004;10:317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J.C., Ooi G.C., Mok T.Y., Chan J.W., Hung I., Lam B., Wong P.C., Li P.C., Ho P.L., Lam W.K., Ng C.K., Ip M.S., Lai K.N., Chan-Yeung M., Tsang K.W. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2003;168:1449–1456. doi: 10.1164/rccm.200306-766OC. [DOI] [PubMed] [Google Scholar]
- Hosoya M., Shigeta S., Ishii T., Suzuki H., De Clercq E. Comparative inhibitory effects of various nucleoside and nonnucleoside analogues on replication of influenza virus types A and B in vitro and in ovo. J. Infect. Dis. 1993;168:641–646. doi: 10.1093/infdis/168.3.641. [DOI] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsi J.J., North J.A., McKernan P.A., Murray B.K., Canonico P.G., Huggins J.W., Srivastava P.C., Robins R.K. Broad-spectrum antiviral activity of 2-beta-d-ribofuranosylselenazole-4-carboxamide, a new antiviral agent. Antimicrob. Agents Chemother. 1983;24:353–361. doi: 10.1128/aac.24.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., Pham D.H., Deif H., LaMere E.A., Chang M., Kain K.C., Farcas G.A., Ferguson P., Latchford M., Levy G., Dennis J.W., Lai E.K., Fish E.N. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- Lowe J.K., Brox L., Henderson J.F. Consequences of inhibition of guanine nucleotide synthesis by mycophenolic acid and virazole. Cancer Res. 1977;37:736–743. [PubMed] [Google Scholar]
- Muller W.E., Maidhof A., Taschner H., Zahn R.K. Virazole (1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide); a cytostatic agent. Biochem. Pharmacol. 1977;26:1071–1075. doi: 10.1016/0006-2952(77)90246-5. [DOI] [PubMed] [Google Scholar]
- Pancheva S., Dundarova D., Remichkova M. Potentiating effect of mizoribine on the anti-herpes virus activity of acyclovir. Z. Naturforsch. [C] 2002;57:902–904. doi: 10.1515/znc-2002-9-1024. [DOI] [PubMed] [Google Scholar]
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Sainz B., Jr., Mossel E.C., Peters C.J., Garry R.F. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) Virology. 2004;329:11–17. doi: 10.1016/j.virol.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta S. Recent progress in antiviral chemotherapy for respiratory syncytial virus infections. Expert Opin. Investig. Drugs. 2000;9:221–235. doi: 10.1517/13543784.9.2.221. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Ishibashi M., Okuno T., Kokado Y., Takahara S., Yamanishi K., Sonoda T., Takahashi M. Effects of cyclosporine, azathioprine, mizoribine, and prednisolone on replication of human cytomegalovirus. Transplant. Proc. 1990;22:1682–1685. [PubMed] [Google Scholar]
- Smee D.F., Sidwell R.W., Clark S.M., Barnett B.B., Spendlove R.S. Inhibition of rotaviruses by selected antiviral substances: mechanisms of viral inhibition and in vivo activity. Antimicrob. Agents Chemother. 1982;21:66–73. doi: 10.1128/aac.21.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter D.G., Witkowski J.T., Khare G.P., Sidwell R.W., Bauer R.J., Robins R.K., Simon L.N. Mechanism of action of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc. Natl. Acad. Sci. U.S.A. 1973;70:1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroher U., DiCaro A., Li Y., Strong J.E., Aoki F., Plummer F., Jones S.M., Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-alpha. J. Infect. Dis. 2004;189:1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuyver L.J., Lostia S., Patterson S.E., Clark J.L., Watanabe K.A., Otto M.J., Pankiewicz K.W. Inhibitors of the IMPDH enzyme as potential anti-bovine viral diarrhoea virus agents. Antiviral Chem. Chemother. 2002;13:345–352. doi: 10.1177/095632020201300602. [DOI] [PubMed] [Google Scholar]
- Toltzis P., O’Connell K., Patterson J.L. Effect of phosphorylated ribavirin on vesicular stomatitis virus transcription. Antimicrob. Agents Chemother. 1988;32:492–497. doi: 10.1128/aac.32.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Wu C.J., Jan J.T., Chen C.M., Hsieh H.P., Hwang D.R., Liu H.W., Liu C.Y., Huang H.W., Chen S.C., Hong C.F., Lin R.K., Chao Y.S., Hsu J.T. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004;48:2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Yang R., Yoshinaka Y., Amari S., Nakano T., Cinatl J., Rabenau H., Doerr H.W., Hunsmann G., Otaka A., Tamamura H., Fujii N., Yamamoto N. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S. Mizoribine: mode of action and effects in clinical use. Pediatr. Int. 2002;44:196–198. doi: 10.1046/j.1328-8067.2002.01536.x. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Zhang F., Xu M., Huang K., Zhong W., Cai W., Yin Z., Huang S., Deng Z., Wei M., Xiong J., Hawkey P.M. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J. Med. Microbiol. 2003;52:715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
- Zhaori G. Antiviral treatment of SARS: can we draw any conclusions. CMAJ. 2003;169:1165–1166. [PMC free article] [PubMed] [Google Scholar]


