Abstract
We found that the butanol fraction of Cinnamomi Cortex (CC/Fr.2) showed moderate inhibitory activity in wild-type severe acute respiratory syndrome coronavirus (wtSARS-CoV) and HIV/SARS-CoV S pseudovirus infections. The inhibition on pseudovirus was also seen in cells pretreated with the CC and CC/Fr.2 (IC50S, 283.4 ± 16.3 and 149.5 ± 13.5 μg/ml, respectively), however the highest activities on wtSARS-CoV were observed when the viruses were treated by the extracts before challenging (IC50S, 43.1 ± 2.8 and 7.8 ± 0.3 μg/ml; SIs, 8.4 and 23.1, respectively). Among the compounds fractionated from CC, procyanidin A2 and procyanidin B1 showed moderate anti-wtSARS-CoV activity (IC50S, 29.9 ± 3.3 and 41.3 ± 3.4 μM; SIs, 37.35 and 15.69, respectively). We also sought to determine whether they could interfere with the clathrin-dependent endocytosis pathway using transferrin receptor (TfR) as an indicator. CC/Fr.2 inhibited the internalization of TfR but the procyanidins did not. Taken together, CC/Fr.2 contains unknown substances, that could inhibit the infection, probably by interfering with endocytosis, and it also contains procyanidins that did not inhibit the internalization but inhibited the infection. Therefore, CC extracts contain anti-virus activities that act through distinct mechanisms according to differences in the compounds or mixtures.
Abbreviations: SARS-CoV, severe acute respiratory syndrome coronavirus; HIV, human immunodeficiency virus; VSV, vesicular stomatitis virus; Wild-type SARS-CoV, wtSARS-CoV; ACE2, angiotensin converting enzyme 2; IC50, 50% inhibitory concentration; CC50, 50% growth reduction of the cells; SI, selective index, CC50/IC50; TCID50, 50% tissue culture infectious dose; PBS, phosphate-buffered saline; BSA, bovine serum albumin; DMEM, Dulbecco's modified Eagle's medium; FITC, fluorescein isothiocyanate; PE, phycoerythrin; CHX, cycloheximide; MFI, mean fluorescence intensity
Keywords: SARS-CoV, Medicinal herbs, Cinnamomi Cortex, Pseudovirus, Transferrin receptor
1. Introduction
Severe acute respiratory syndrome (SARS) is an atypical type of contagious pneumonia with a high mortality rate, and is caused by SARS coronavirus (SARS-CoV) (Drosten et al., 2003, Ksiazek et al., 2003), which infected over 8000 patients of whom more than 700 died by July 2003 (Lingappa et al., 2004). Interferons (Haagmans et al., 2004, Paragas et al., 2005, Tan et al., 2004, Barnard et al., 2006), glycyrrhizin (Cinatl et al., 2003), 1,2,3,6-tetra-O-galloyl-β-d-glucose (TGG), luteolin (Yi et al., 2004), Lycoris radiate (S.Y. Li et al., 2005), emodin (Ho et al., 2006), N4-hydroxycytidine (Barnard et al., 2004) and protease inhibitor (Chen et al., 2005) have been reported to have anti SARS-CoV activities in vitro. However, the selectivity indices of these materials are as high as 100 and the mechanisms of their actions are not clear (De Clercq, 2004, De Clercq, 2006). Therefore, more research is necessary to discover drugs and to study their mechanisms for the practical interference of SARS-CoV infection. It was reported that medicinal herbs combined with western medicines improved the symptoms and decreased the required dosage of corticosteroids for SARS patients (Liu et al., 2006). To identify potential inhibitor(s) of SARS-CoV, we screened seven different medicinal herbs that were reported to be used for SARS patients (Zhang et al., 2004). We also fractionated the extracts to characterize the inhibitory molecule(s).
The spike proteins (S) on the surface of the SARS-CoV particle mediate the binding of the virus to its cell surface receptor, angiotensin converting enzyme 2 (ACE2) (Simmons et al., 2004, Li et al., 2003). We used a pseudovirus that consisted of luciferase encoded human immunodeficiency virus (HIV) (Yang et al., 2004) and SARS-CoV S protein for screening inhibitors. Because a pseudotyped virus does not replicate, the luciferase activities specifically reflect single-round infection. The inhibitory activities shown in the pseudotyped virus assay were also confirmed by the wild-type SARS-CoV (wtSARS-CoV) infection assay using SARS-CoV PUMC01 F5 (GenBank accession no. AY350750 B.J. Li et al., 2005).
Whether enveloped or nonenveloped, many viruses depend on the host cell endocytic pathways for entry. SARS-CoV S glycoprotein mediates viral entry through pH-dependent endocytosis, like vesicular stomatitis virus (VSV) (Sun et al., 2005). The endocytic pathways taken by viruses can be divided into clathrin-mediated and clathrin-independent pathways (Marsh and Helenius, 2006). Recently, we demonstrated that SARS-CoV mainly utilizes the clathrin-mediated endocytosis pathway for its entry to target cells (Inoue et al., 2007). Therefore, we examined whether effective herbs and their purified compounds may affect the endocytotic pathways.
2. Materials and methods
2.1. Cell lines
The following cell lines were obtained from Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University; HEK293T, HepG2, VeroE6 and Jurkat cells. The cells were cultured in either Dulbecco's modified Eagle's medium (DMEM) or RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin antibiotics.
2.2. Extracts of medicinal herbs and fractionated samples
The water extracts prepared from 7 kinds of dried medicinal herbs and four fractionated samples of Cinnamomi Cortex (CC) and Caryophylli Flos (CF) were provided by Tsumura & Co. (Tokyo, Japan). The dried medicinal herbs included Forsythiae Fructus, Scutellariae Radix, Astragali Radix, Bupleuri Radix, Glycyrrhizae Radix, CC and CF. Each dried medicinal herb (30 kg) was boiled under reflux in 360 l of distilled water for 1 h. The aqueous extract was separated from the residue by centrifugation followed by concentration under reduced pressure. The concentrated extracts were lyophilized. Four fractions of CC were obtained by the method shown in Fig. 1 . CC (114.9 g) was extracted with 700 ml of 50% aqueous ethanol (aq. EtOH/H2O under reflux for 2 h. The obtained extract was concentrated under reduced pressure to yield a 50% aq. EtOH/H2O extract (13.5 g, fraction 1). Most of this (11.2 g) could be dissolved in distilled water, and it was then extracted with ethylacetate (AcOEt, 200 ml × 2) and n-butanol (n-BuOH, 200 ml × 2). The n-BuOH layer, aq. layer and AcOEt layer were concentrated under reduced pressure to successively yield fractions 2 (CC/Fr.2, 3.5 g), 3 (CC/Fr.3, 3.7 g) and 4 (CC/Fr.4, 2.8 g), respectively. The four fractions of CF were also obtained by an essentially similar method as described above. These fractions were suspended in dimethyl sulfoxide (DMSO) at a concentration of 100 mg/ml and then serially diluted with DMEM.
Fig. 1.
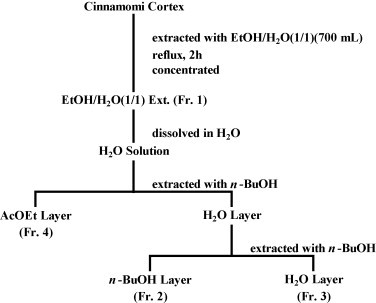
Fractionation procedure of Cinnamomi Cortex (see text).
2.3. Chemicals
2-Phenylethanol (phenethy alcohol), 2′-hydroxyacetophenone (o-hydroxyacetophenone), isoeugenol 2-hydroxycinnamic acid, 3,4-dimethoxycinnamic acid, 4-hydroxy-3-methoxycinnamic acid (ferulic acid), 4-hydroxycoumarin, 7-hydroxycoumarin, p-cymene, 4-allylanisole, ethylcinnamate and cinnamylacetate were purchased from Wako (Osaka, Japan). Trans-cinnamaldehyde, trans-cinnamic acid, licochalcone-A 4-hydroxy-3-methoxy cinnamaldehyde and cycloheximide (CHX) were purchased from Sigma (St. Louis, MO). The eight compounds procyanidin A2, procyanidin B1, procyanidin B2, procyanidin C1, procyanidin oligomer, (−)-epicatechin, (+)-catechin and cinnamtannin B1 were purified from CC as described previously (Nonaka et al., 1983). These chemicals were dissolved in DMSO at 100 mg/ml or 100 mM.
2.4. Infection of pseudoviruses
Pseudotyped lentiviruses were prepared as described previously (Yang et al., 2004). HEK293T cells were cotransfected with HIV-1-based lentiviral DNA and plasmids encoding full-length SARS-S (G.J. Nabel, National Institutes of Health, Bethesda, MD) or VSV-G (Naldini et al., 1996). The p24 Gag antigen levels were measured using an HIV-1 p24 Antigen Assay kit (ZeptoMetrix Corporation, New York). Five ng (p24) of the generated HIV/SARS-CoV S or HIV/VSVG were infected in HepG2 cells (1 × 104 cells/well in 96-well plates). After 12 h of cultivation, the cells were washed twice with DMEM which was then replaced with complete medium. Forty-eight hours after infection, the luciferase activity was measured by Mithras LB940 (Berthold Technologies GmbH & Co KG, Germany) according to the Manufacturer's instructions.
2.5. Inhibition of the pseudovirus infection
The supernatants containing 5 ng (p24) of the HIV/SARS-CoV S or HIV/VSVG pseudoviruses were mixed with or without the reagents at different concentrations and were then added to HepG2 cells. The DMSO solvent was also used for a control. After 12 h infection the cells were washed twice with DMEM which was then replaced with complete medium without the reagents. The relative luciferase activities were calculated as percentages of the control culture-background. The 50% inhibition concentration (IC50) of the reagents was determined from a curve relating the luciferase activities to the concentration of the reagents (Y. Xu et al., 2000).
To evaluate which step of virus replication was inhibited by the reagents, we examined the effects in an additional study in which the reagents were added on the cells at −1 or 12 h after HIV/SARS-CoV S infection because 12 h were required to obtain stable luciferase activities (Yang et al., 2004). Furthermore, the cells were pretreated with various concentrations of the reagents for 1 h followed by washing twice, and were challenged with pseudovirus to determine whether the reagents work on the cells or on the viruses.
2.6. Inhibition assay for wtSARS-CoV infection
The wtSARS-CoV strain PUMC01 F5 (GenBank accession no. AY350750) was used for the assay. We performed a plaque reduction assay in which 100 times the 50% cell culture infective doses (CCID50) of wtSARS-CoV was added with 100 μl of each reagent for 0 or 60 min at 37 °C and then transferred onto 6-well plates (1 × 106 cells/well) with the VeroE6 cells. The DMSO solvent was also used as a control. After 1 h of infection, the cells were washed twice by DMEM, and then 2% agarose was added onto the cell surface. A 2% secondary agarose layer with 0.05% neutral red dye was added on the third day after incubation. The numbers of plaques were counted after adding neutral red dye. The inhibitory concentrations for a 50% plaque reduction (IC50) were determined from a curve relating the plaque number to the concentrations of the reagents.
2.7. Cytotoxicity assay
In all experiments, we chose the optimal concentration of each reagent that yielded a cell viability of more than 90%. The cytotoxic analysis of the reagents for HepG2 was performed using a cell counting kit-8 (Dojindo Laboratories, Kumamoto, Japan) in the same conditions as described for the pseudovirus infection. For VeroE6 the extracts were incubated with the cells for 72 h and the viabilities were determined using Cell Titer 96 Aqueous Non-radioactive Cell Proliferation Assay Kits (Promega). The 50% growth reduction (CC50) was calculated. The viabilities of Jurkat cells were determined by the trypan blue dye exclusion method. Selective index was calculated as CC50/IC50 as described elsewhere (Baba et al., 1987).
2.8. Flow cytometric analysis
To investigate whether the compounds affect the expression of ACE2 on the surface of HepG2 cells, the cells were either left untreated or were pretreated with CCE, CC/Fr.2, CFE (0.1 mg/ml) or three compounds (procyanidin A2, procyanidin B1 and cinnamtannin B1 at 100 μM) in 37 °C for 60 min. The DMSO solvent was also used as a control. After washing with PBS, the cells were detached by treatment with 10 mM EDTA for 10 min. The detached cells were reacted with Biotinylated Anti-human ACE-2 ectodomain Antibody (R&D Systems). After reaction with phycoerythrin (PE) streptavidin (BD Pharmingen), the cells were washed and subjected to flow cytometry analysis.
For the TfR and CD59 staining, the Jurkat cells and HepG2 cells were used for the assay. Jurkat cells were incubated with the extracts at different concentrations with or without 10 μg/ml of cycloheximide or at 100 μg/ml of each extract for various periods (or 5–60 min) at 37 or 4 °C followed by washing twice with cold PBS containing 1% BSA. Ten μl of PE-anti-TfR (BD Biosciences) and 10 μl of FITC-anti-CD59 (BD Biosciences) were incubated with 5 × 105 cells for 30 min at 4 °C. After washing, the cells were analyzed by Cytomics (Beckman Coulter, Fullerton, CA). Data were analyzed by using CXP Analysis software version 2.0. The HepG2 cells were cultured in 12-well plates one day before treatment. After the extracts were added for 60 min, the cells were washed twice by PBS then detached by treatment with 10 mM EDTA for 10 min. The recovered cells were reacted with PE-anti-TfR (BD Biosciences) and of FITC-anti-CD59 (BD Biosciences). After washing, the cells were analyzed by Cytomics (Beckman Coulter, Fullerton, CA).
2.9. Internalization of fluorescent transferrin-A488
In order to observe the internalization of transferrin, the Jurkat cells were washed twice with cold PBS and 5 × 105 cells were incubated in 200 μl PBS containing different concentrations of the extracts on ice for 30 min in polystyrene round-bottom tubes (Falcon 352058). Then, A488-labeled transferrin (Molecular Probes) was added at a final concentration of 50 μg/ml and incubated for another 30 min on ice. The treated cells were washed twice with cold PBS, resuspended at 107 cells/ml in cold PBS in the presence or absence of various concentrations of extracts and incubated at 37 °C for 15 min (Crotzer et al., 2004). Internalization was stopped by incubating the cells on ice and adding cold PBS. The cells were then fixed with 4% paraformaldehyde and plated on poly-l-lysine-treated cover slips (Sigma–Aldrich). Cells were viewed using a Zeiss LSM510 microscope, and a single section through the middle of the cell is shown.
3. Results
3.1. Inhibition of pseudovirus infection
First, we screened the extracts of the 7 medicinal herbs by HIV/SARS-CoV S pseudovirus infection and only two, CCE (Cinnamomi Cortex Extract) and CFE (Caryophylli Flos Extract), apparently showed low IC50 (<100 μg/ml) with slight and moderate SIs against HIV/SARS-CoV S pseudovirus (Table 1 ), and these inhibitory activities were dose-dependent (Fig. 2A). It is of note that both CCE and CFE also inhibited the infection of HIV/VSVG pseudovirus (Fig. 2B). To characterize the responsible substance(s), we separated the four fractions from CC and CF using aqueous ethanol (Fr.1), ethylacetate (Fr.4) and n-butanol (Fr.2) extraction methods (Fig. 1). The IC50 of CC/Fr.2 was the lowest among the four fractions of CC, although all fractions showed slight inhibitory activities (Table 1). The dose-dependent inhibition of CC/Fr.2 was also confirmed using both HIV/SARS-CoV S and HIV/VSVG pseudovirus infection (Fig. 2A and B). In the case of the CF fractions, CF/Fr.2, CF/Fr.3 and CF/Fr.4 showed moderate inhibitory activities (Table 1).
Table 1.
Inhibitory effects of medicinal herbs on infection of HIV/SARS-CoV S pseudovirus.
| Extracts | CC50 (μg/ml) | IC50 (μg/ml) | SI (CC50/IC50) |
|---|---|---|---|
| Forsythiae Fructus | 577.8 ± 79.7 | 401.4 ± 29.5 | 1.4 |
| Scutellariae Radix | 854.2 ± 76.2 | 853.2 ± 36.8 | 1.0 |
| Astragali Radix | 2726.5 ± 125.2 | 1623.7 ± 106.5 | 1.7 |
| Bupleuri Radix | 1213.6 ± 54.5 | 1643.2 ± 52.8 | <1 |
| Glycyrrhizae Radix | 741.2 ± 70.7 | 781.2 ± 65.4 | <1 |
| Cinnamomi Cortex (CCE) | 201.1 ± 17.1 | 30.3 ± 2.6 | 6.6 |
| Ethanol extract of CC (Fr.1) | 444.0 ± 13.7 | 85.3 ± 7.5 | 5.2 |
| Butanol fraction of CC (Fr.2) | 205.3 ± 11.2 | 37.3 ± 3.5 | 5.5 |
| Aqueous fraction of CC (Fr.3) | 267.7 ± 23.6 | 68.6 ± 5.7 | 3.9 |
| Ethylacetate fraction of CC (Fr.4) | 197.8 ± 14.1 | 58.1 ± 4.4 | 3.4 |
| Caryophylli Flos (CFE) | 757.2 ± 59.3 | 58.8 ± 5.6 | 12.9 |
| Ethanol extract of CF (Fr.1) | 553.4 ± 35.7 | 102.0 ± 7.3 | 5.4 |
| Butanol fraction of CF (Fr.2) | 1074.7 ± 82.0 | 51.3 ± 4.9 | 20.9 |
| Aqueous fraction of CF (Fr.3) | 1573.1 ± 88.2 | 67.1 ± 5.3 | 23.4 |
| Ethylacetate fraction of CF (Fr.4) | 441.5 ± 14.5 | 60.4 ± 5.5 | 7.3 |
Experiments were repeated three times, and the averages of IC50 (the inhibitory concentration of extract to inhibit the luciferase activity to 50% control value) and CC50 (the cytotoxic concentration of extract that reduced cell viability to 50%) are shown. The selectivity index (SI) corresponds to CC50/IC50.
Fig. 2.
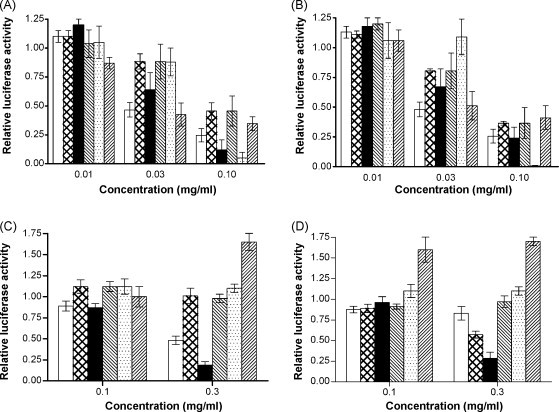
Inhibition of pseudovirus infection by medicinal herbs. Pseudoviruses were treated with the following extracts and then incubated on the target cells. HIV/SARS-CoV S pseudovirus (A) or HIV/VSVG pseudovirus (B) was mixed with or without the reagents and was then added to HepG2 cells for infection. Target cells were treated by the extracts for 1 h followed by infection of HIV/SARS-CoV S pseudovirus (C) or HIV/VSVG pseudovirus (D) infection. The extracts used were as follows: (□) CCE; ( ) CC/Fr.1; (■) CC/Fr.2; (
) CC/Fr.1; (■) CC/Fr.2; ( ) CC/Fr.3; (
) CC/Fr.3; ( ) CC/Fr.4; (
) CC/Fr.4; ( ) CFE. The experiment was repeated three times and the average is shown. The error bar indicates standard deviation (SD).
) CFE. The experiment was repeated three times and the average is shown. The error bar indicates standard deviation (SD).
Pseudoviruses replicate in infected cells in a single cycle manner, and the luciferase activities are not solely dependent on the entry but also on transcription and protein synthesis. Therefore, the inhibitory activities of CCE and CFE were also examined at different times by adding them to the culture 1 h before infection or 12 h after infection by HIV/SARS-CoV S. The results showed that inhibition occurred only in the former case (data not shown) suggesting that they inhibited the HIV/SARS-CoV S infection only during the early steps of virus replication. Furthermore, pretreatment of HepG2 cells by CCE and CC/Fr.2 showed apparent inhibitory effects on HIV/SARS-CoV S pseudovirus (IC50S were 283.4 ± 16.3 and 149.5 ± 13.5 μg/ml, respectively), however the other fractions of CC, CFE and its fractions (data not shown) did not show inhibition after pretreatment. Also they showed similar effects on HIV/VSVG pseudovirus by pretreatment of HepG2 cells. Based on the above results, we hypothesized that CC/Fr.2 and CCE may inhibit virus entry by acting on the cells. The inhibitory activity of CC/Fr.2 was always more prominent than that of CCE indicating that CC/Fr.2 contained the entry inhibitor that acted on the cells.
3.2. Inhibition of wtSARS-CoV infection
We examined the inhibitory effects of the selected extracts, CCE and CFE, using a quantitative assay (plaque reduction assay) for the infection of wtSARS-CoV. In this assay CCE inhibited wtSARS-CoV infection when the viruses were mixed with the herbs and added onto the target cells, but CFE showed only weak inhibitory activity (Table 2 ). The IC50S counted by plaque numbers of the wtSARS-CoV, which were pretreated (1 h) with CCE, CC/Fr.2 or compounds (procyanidin A2, procyanidin B1 and cinnamtannin B1), were not different from those in which the viruses were added simultaneously with drugs (0 h) (P > 0.05). Therefore we could not prove virucidal effects of the compounds, and analyzed their inhibitory activities by adding the mixtures of compounds and the virus (0 h) to target cells. Among the four fractions of CC, CC/Fr.2 showed moderate inhibitory activities in the plaque reduction assay, suggesting that CC/Fr.2 contained more biologically active substances (Table 2). Unexpectedly, the four fractions of CF, as well as CFE, did not inhibit wtSARS-CoV infection (Table 2).
Table 2.
Inhibitory effects of medicinal herbs and their fractionated samples on infection by wild-type SARS-CoV.
| Extracts | CC50 (μg/ml) | IC50 (μg/ml) | SI |
|---|---|---|---|
| Cinnamomi Cortex extract (CCE) | 360.2 ± 23.0 | 43.1 ± 2.8 | 8.4 |
| Ethanol extract of CC (Fr.1) | 180.5 ± 5.8 | 10.7 ± 0.4 | 16.9 |
| Butanol fraction of CC (Fr.2) | 180.0 ± 6.0 | 7.8 ± 0.3 | 23.1 |
| Aqueous fraction of CC (Fr.3) | 360.0 ± 19.7 | 39.7 ± 2.1 | 9.1 |
| Ethylacetate fraction of CC (Fr.4) | 90.1 ± 4.6 | – | – |
| Caryophylli Flos extract (CFE) | 180.0 ± 5.2 | 50.1 ± 3.5 | 3.6 |
| Ethanol extract of CF (Fr.1) | 50.5 ± 4.7 | – | |
| Butanol fraction of CF (Fr.2) | 15.0 ± 0.8 | – | |
| Aqueous fraction of CF (Fr.3) | 310.0 ± 16.3 | – | |
| Ethylacetate fraction of CF (Fr.4) | 35.1 ± 2.8 | – | |
Experiments were repeated three times, and the data show the average IC50 (the inhibitory concentration of extracts for a 50% plaque reduction of the virus). SI corresponds to CC50/IC50. ‘–’ indicates that the extracts did not inhibit SARS-CoV infection at the highest optimal concentration.
3.3. The effects of chemicals
Because CCE may contain specific compounds that inhibit virus infection through clathrin-dependent endocytosis, we tried to identify the compounds. Several compounds reported to be present in CC and other derivatives have also been studied (Heide, 1972, Nonaka et al., 1983). Among these chemicals, trans-cinnamic acid, 2-phenylethanol (phenethy alcohol) and 2-hydroxycinnamic acid showed slight suppressive effects [selectivity index (SI) > 5] on HIV/SARS-CoV S pseudovirus infection (Table 3 ). However, these chemicals did not show any anti-SARS-CoV activities in wtSARS-CoV infection even at the highest optimal concentrations (data not shown). In the case of the eight compounds fractionated from CC, we examined all by pseudovirus and wtSARS-CoV infection. Among these compounds, procyanidin A2, procyanidin B1 and cinnamtannin B1 showed slight or moderate inhibitory effects in both assays (Table 4 ).
Table 3.
Inhibitory effects of chemical reagents on infection of HIV/SARS-CoV S pseudovirus.
| No. | Extracts | CC50 (mM) | IC50 (mM) | SI (CC50/IC50) |
|---|---|---|---|---|
| 1 | Trans-cinnamaldehyde | 0.2 ± 0.014 | 0.2 ± 0.016 | 1 |
| 2 | Trans-cinnamic acid | 22.4 ± 1.230 | 3.0 ± 0.178 | 7.4 |
| 3 | Licochalcone-A | 0.03 ± 0.001 | 0.03 ± 0.003 | 1 |
| 4 | 2-Phenylethanol (phenethy alcohol) | 24.5 ± 1.533 | 4.1 ± 0.199 | 6.0 |
| 5 | 2′-Hydroxyacetophenone (o-hydroxyacetophenone) | 1.8 ± 0.071 | 1.8 ± 0.102 | 1 |
| 6 | Isoeugenol | 3.9 ± 0.343 | 2.0 ± 0.142 | 2.0 |
| 7 | 2-Hydroxycinnamic acid | 2.5 ± 0.122 | 0.3 ± 0.013 | 8.3 |
| 8 | 3,4-Dimethoxycinnamic acid | 10.0 ± 0.620 | 5.0 ± 0.233 | 2 |
| 9 | 4-Hydroxy-3-methoxycinnamic acid (ferulic acid) | 10.0 ± 0.555 | 5.0 ± 0.312 | 2 |
| 10 | 4-Hydroxy-3-methoxy cinnamaldehyde | 0.7 ± 0.043 | 0.3 ± 0.013 | 2.3 |
| 11 | 4-Hydroxycoumarin | 10.3 ± 0.660 | 10.3 ± 0.500 | 1 |
| 12 | 7-Hydroxycoumarin | 10.7 ± 0.560 | 2.5 ± 0.172 | 4.3 |
| 13 | p-Cymene | 75.0 ± 4.350 | 75.0 ± 5.550 | 1 |
| 14 | 4-Allylanisole | 8.0 ± 0.563 | 8.4 ± 0.650 | <1 |
| 15 | Ethylcinnamate | 14.1 ± 1.220 | 7.1 ± 0.532 | 2.0 |
| 16 | Cinnamylacetate | 7.1 ± 0.420 | 7.1 ± 0.350 | 1 |
Experiments were repeated three times, and the averages of IC50 (the inhibitory concentration of extract to inhibit the luciferase activity to 50% control value) and CC50 (the cytotoxic concentration of extract that reduced cell viability to 50%) are shown. SI corresponds to CC50/IC50.
Table 4.
The effects of compounds fractionated from CC on HIV/SARS-CoV S and SARS-CoV wild-type.
| Compounds | MW | HIV/SARS-CoV S pseudovirus infection |
Plaque reduction assay on SARS-CoV |
||||
|---|---|---|---|---|---|---|---|
| CC50 (μM) | IC50 (μM) | SI | CC50 (μM) | IC50 (μM) | SI | ||
| Procyanidin A2 | 576.512 | 796.6 ± 63.7 | 120.7 ± 13.1 | 6.60 | 1116.7 ± 60.3 | 29.9 ± 3.3 | 37.35 |
| Procyanidin B1 | 578.528 | 656.2 ± 36.7 | 161.1 ± 20.3 | 4.08 | 648.2 ± 43.4 | 41.3 ± 3.4 | 15.69 |
| Cinnamtannin B1 | 864.769 | 242.3 ± 14.8 | 32.9 ± 2.8 | 7.36 | 184.7 ± 15.5 | 32.9 ± 3.9 | 5.61 |
Experiments were repeated three times, and the averages of IC50 and CC50 are shown. SI corresponds to CC50/IC50.
3.4. ACE2 expression after treatment by extracts and compounds
The HepG2 cells which were treated by CCE, CC/Fr.2, CFE (0.1 mg/ml) or three compounds (procyanidin A2, procyanidin B1 and cinnamtannin B1 at 100 μM) in 37 °C for 60 min were used for flow cytometry analysis. Both the positive percentages and MFI of PE-anti-ACE2 were analyzed. There was no significant difference for both parameters between treated cells and untreated control. The percentages and MFI of non-treated cells were 35.5 ± 2.6%, 635.7 ± 36.2, and those of treated cells were 34.3 ± 2.3% to 39.9 ± 2.8%, 601.3 ± 25.2 to 699.0 ± 40.4, respectively.
3.5. Influence on the expression of TfR on Jurkat cell surface by the herbal treatment.
Because both CCE and CFE inhibited not only the SARS-CoV infection but also that of HIV/VSVG, we suspected that they may act through shared endocytic pathways of the viruses, and both viruses are known to enter into cells via clathrin-dependent endocytic pathways (Sun et al., 2005, Inoue et al., 2007). TfR is considered to be a marker of clathrin-mediated endocytosis and CD59 is a marker of clathrin-independent endocytosis (Mellman, 1996, Naslavsky et al., 2004). As expected, CC/Fr.2 and CCE up-regulated the TfR expression on Jurkat cell in dose- and time-dependent manners (Fig. 3A and C). Furthermore, neither CC/Fr.3, CC/Fr.4 (Fig. 3) nor CFE and its fractions (data not shown) influenced the TfR expression. The enhanced expressions of TfR on Jurkat cells by CC/Fr.2 could not be explained by enhanced protein synthesis, because the up-regulation was also observed when the cells were incubated with CHX. No up-regulation of TfR was seen when the Jurkat cells were incubated with CCE at 4 °C. The up-regulation of only TfR was also found in treated HepG2 cells by CCE and CC/Fr.2 (MFI of untreated cells was 404.7 ± 25.5, and 628.3 ± 37.1 and 759.0 ± 42.0 for CCE and CC/Fr.2, respectively). In the case of the eight compounds fractionated from CC, we examined all by pseudovirus and wtSARS-CoV infection. Because procyanidin A2, procyanidin B1 and cinnamtannin B1 showed inhibitory effects on SARS virus infection (Table 4), the effects on the expressions of CD59 and TfR on Jurkat cells were examined. Flow cytometric analysis showed that procyanidin A2 and cinnamtannin B1 up-regulated the expression of TfR in dose- and time-dependent manners (Fig. 4 ). Procyanidin B1 did not affect the expression of CD59 and TfR, even though it inhibited the infection of wtSARS-CoV with a SI of about 15.
Fig. 3.
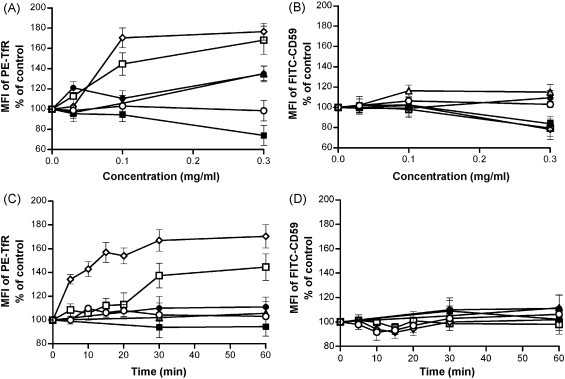
Effect of medicinal herbs on TfR or CD59 expression on Jurkat cells. A dose-dependent effect was observed (A and B). Jurkat cells were treated with various concentrations of the following extracts for 1 h followed by two-color staining with PE-anti-TfR (A) and FITC-anti-CD59 (B). A time-dependent effect was also observed (C and D). Jurkat cells were treated with the various extracts for 0–60 min and were analyzed with PE-anti-TfR (C) and FITC-anti-CD59 (D). (□) CCE; (▵) CC/Fr.1; (♢) CC/Fr.2; (●) CC/Fr.3; (■) CC/Fr.4; (○) DMSO. Y-axis indicates MFI (mean fluorescence intensity) % of control. The experiment was repeated three times and the average is shown. The error bar indicates standard deviation (SD).
Fig. 4.
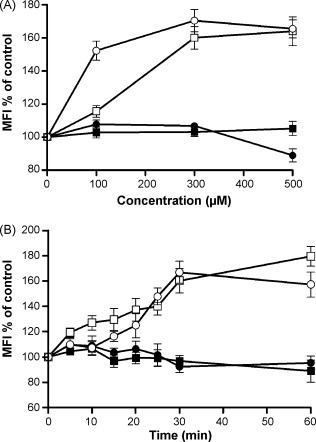
The effects of procyanidin A2 and cinnamtannin B1 on TfR or CD59 expression on Jurkat cells. The dose- and time-dependent effects of procyanidin A2 and cinnamtannin B1 on Jurkat cells were examined. Jurkat cells were treated with various concentrations of procyanidin A2 and cinnamtannin B1 for 1 h (A) or were treated with 300 μM of procyanidin A2 and 100 μM of cinnamtannin B1 for various periods (B), following PE-anti-TfR and FITC-anti-CD59 staining flow cytometry was applied. (■) FITC-anti-CD59-procyanidin A2; (●) FITC-anti-CD59-cinnamtannin B1; (□) PE-anti-TfR-procyanidin A2; (○) PE-anti-TfR-cinnamtannin B1. The experiment was repeated three times and the average is shown. The error bar indicates standard deviation (SD).
3.6. Internalization of fluorescent transferrin-A488
Because TfR is a recycling molecule in cells, we considered that interference by CCE and CC/Fr.2 with the recycling of TfR resulted in the enhanced expression of TfR on Jurkat cells. The Tfn-A488 was used for detection of the internalization, which is a step of endocytosis. At 37 °C incubation the Tfn-A488 was internalized into Jurkat cells in the absence of the herbs after 15 min (Fig. 5 , middle panel). We examined whether the extracts interfered with the internalization of Tfn-A488 in Jurkat cells. CCE and all its fractions except for CC/Fr.4 blocked the internalization of Tfn-A488 at 100 μg/ml (Table 5 ), and no internalization was observed when CC/Fr.2 was added at a relatively low concentration (10 μg/ml), which is close to the IC50 for the wild-type infection (7.8 μg/ml). CFE and the other fractions had no effect on the internalization (Table 5) even at a concentration (100 μg/ml) higher than the IC50 for HIV/SARS-CoV S pseudovirus infection (Table 5). Finally, no procyanidins inhibited the internalization at the optimal concentration.
Fig. 5.
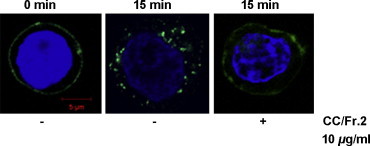
CCE and CC/Fr.2 affect the internalization of Tfn-A488 at the plasma membrane. Jurkat cells were treated with different concentration of CCE or CC/Fr.2 at 4 °C for 30 min. Then A488-conjugated transferrin was added and incubated at 4 °C for 30 min. After washing the Jurkat cells were incubated at 37 °C for 0 or 15 min in the presence or absence of the extracts. The nucleus is stained with DAPI. The results are representative of at least three independent experiments and numbers are provided in Section 3. Bar, 5 μm for all panels.
Table 5.
The internalization of Tfn-A488 in Jurkat cells after treatment with the extracts.
| Extracts | Internalization of Tfn-A488 |
||
|---|---|---|---|
| 100 μg/ml | 10 μg/ml | 6 μg/ml | |
| CCE | − | + | + |
| Ethanol extract of CC (Fr.1) | − | + | + |
| Butanol fraction of CC (Fr.2) | − | − | + |
| Aqueous fraction of CC (Fr.3) | − | + | + |
| Ethylacetate fraction of CC (Fr.4) | + | + | + |
| CFE | + | + | + |
| Ethanol extract of CF (Fr.1) | + | + | + |
| Butanol fraction of CF (Fr.2) | + | + | + |
| Aqueous fraction of CF (Fr.3) | + | + | + |
| Ethylacetate fraction of CF (Fr.4) | + | + | + |
‘−’ indicates that no internalization of Tfn-A488 was observed in 30 fields of observation. ‘+’ indicates that the internalization of Tfn-A488 was observed in 30 fields of observation. The results are representative of at least three independent experiments.
3.7. Discussion
We used a pseudovirus to evaluate the anti-SARS-CoV activities of medicinal herbs using luciferase as a reporter. The 7 kinds of medicinal herbs which were recommended to be used for SARS patients (Liu et al., 2006) were examined for their effects on HIV/SARS-CoV S pseudovirus infection. Two herbal extracts (CCE and CFE) showed slightly or moderate inhibitory activities on both HIV/SARS-CoV S and HIV/VSVG pseudovirus infection.
We evaluated the anti-virus activities of the fractionated extracts and found that CC/Fr.1, CC/Fr.2 and all four fractions of CF showed slightly or moderate inhibitory activities against pseudovirus in the infection assay (Table 1). Because HIV-based pseudovirus RNA should be reverse transcribed followed by integration to make the reporter gene product, we examined whether these inhibitors could inhibit wild-type infection. In the plaque reduction assay of wtSARS-CoV infection, the SIs of CC/Fr.1 and CC/Fr.2 were found to be moderate; however, CFE and its fractions did not show significant inhibitory activities. We believe CCE may inhibit the early steps of SARS-CoV infection, based on the time of addition study and on the plaque reduction assay in the wild-type infection. Furthermore, the extract appeared to act on both cells and the virus because it inhibited infection of the cells by pretreatment of the cells or viruses. The IC50S (283.4 ± 16.3 μg/ml for CCE and 149.5 ± 13.5 μg/ml for CC/Fr.2) for pre-treatment of the cells were much higher than those obtained by the experiments in which the reagent was mixed with the virus first (IC50S, 43.1 ± 2.8 μg/ml for CCE and 7.8 ± 0.3 μg/ml for CC/Fr.2), although we could not demonstrate virucidal activities in our assay. However, the mechanisms of the inhibitory activities by pre-treatment were studied. Because CCE inhibited not only SARS-CoV but also HIV/VSVG pseudovirus infection, we suspected that they might interact with molecules that are important for the entry of both viruses. It is known that VSV and many enveloped viruses must be endocytosed for infection, and they are then transported to early endosomes, late endosomes, and lysosomes using the endocytic pathway. VSV is well-known to infect cells in a pH-dependent manner and was shown to utilize the clathrin-mediated endocytic pathway upon infection. We have also reported that SARS-CoV entry is clathrin-dependent. We therefore examined whether CCE or CFE could interfere with the recycling of TfR.
As we expected, CCE and CC/Fr.2 but not CFE up-regulated the TfR expression in time- and dose-dependent manners despite the presence of CHX (Fig. 3), and the TfR-inducing activity of CC/Fr.2 was higher than that of CCE. These results suggested that the anti-virus activity of CC/Fr.2, but not that of CFE, might be related to the endocytic pathway of viruses. TfR is a well-known clathrin-mediated recycling molecule, and interference with its recycling may enhance the TfR expression on the cell surface. Therefore, we hypothesized that CC/Fr.2 may have both biological activities via its effect on clathrin pathways. In fact, CC/Fr.2 inhibited the internalization of TfR at a relatively low concentration 10 μg/ml (Table 5).
It is well-known that CC contains large amounts of proanthocyanidins (Prior and Gu, 2005), also known as condensed tannin, which has been accredited with a variety of beneficial effects on health (Beecher, 2004). Tannins and related compounds were reported to possess anti-HIV activity (Lee et al., 1992, H.X. Xu et al., 2000). Proantocyanidins are reported to have anti-HIV and anti-HSV activities (De Bruyne et al., 1999). Therefore, we examined 8 compounds isolated from CC for various biological activities and found that three compounds that were structurally very similar showed inhibitory activities on SARS-CoV infection (Fig. 6 ) and two of them caused up-regulation of TfR expression. The structures of procyanidins B1 and B2 are closely related to each other except for the configuration at the C-3′ position, but procyanidins B2 did not inhibit SARS-CoV infection nor up-regulate TfR expression (data not shown). These data strongly suggest that the anti-SARS-CoV activities in CC/Fr.1 and CC/Fr.2 could be explained by procyanidins. In fact, procyanidins were detected in Fr.1 and Fr.2 but not in Fr.3 (Tanaka, unpublished observation March 12, 2008). However, none of the procyanidins inhibited the internalization of TfR at the optimal concentration (data not shown), suggesting they may not act on the TfR recycling pathway directly. It was also demonstrated that the compounds did not affect ACE2 expression which is a SARS-CoV receptor. A previous study indicated that procyanidin A2 has the highest SI (24) against HIV suggesting that it has a virucidal effect (De Bruyne et al., 1999). Therefore it is possible that Fr.2 may contain both endocytosis inhibitor(s) and procyanidins, although we could not identify the substances which could inhibit endocytosis. The up-regulation of TfR by procyanidin is a puzzling phenomenon discovered in this experiment. It is known that proanthocyanidins and their related compounds have chelating activities (Scalbert et al., 2000) and depletion of Fe in cells or medium causes up-regulation of the TfR transcript. However, the up-regulation of mRNA was observed 24 h after the chelators were added (Xu et al., 2008). Iron chelation by clinically relevant anthracyclines causes an alteration in the expression of iron-regulated genes and atypical changes in intracellular iron distribution and trafficking (Xu et al., 2008). We could see the up-regulation within 15 min after the addition of procyanidins (Fig. 4) and it is also known that transferrin are the principal plasma proteins that bind wine catechins (Brunet et al., 2002), therefore, the swift up-regulation of TfR may be caused by depletion of Tfn in the medium. It is of note that α-interferon induces depletion of the intracellular iron content and up-regulation of functional TfR on human epidermoid cancer KB cells (Caraglia et al., 1994), and has also anti-SARS activities (Paragas et al., 2005, Tan et al., 2004, Barnard et al., 2006). Although, the relationships between these two activities are not clear, the higher SI of procyanidin A2 could be explained by its up-regulation of TfR.
Fig. 6.
Procyanidin A2, procyanidin B1 and cinnamtannin B1 are structurally very similar.
In this study we found that CC/Fr.2 contains inhibitors of SARS virus infection. The fraction also inhibited the internalization of TfR indicating the interference of clathrin-dependent endocytosis. The fraction also contained procyanidins which could have a virucidal effect and some of them showed up-regulation of TfR, probably by depleting transferrin and/or iron from the culture. The novel and complex biological activities of CC could lead to the amelioration of symptoms of a number of virus-infected diseases, including SARS.
Acknowledgements
This work was supported by Grant-in-Aid for Special Educational Grant from Ministry of Education, Culture, Sports, Science and Technology and Grants-in-Aid from the Scientific Research Expenses for Health and Welfare Program from the Ministry of Health and Welfare, Japan and also by Foundation of Heilongjiang Education Committee No. 1153h09. M. Zhuang is a recipient of a grant Japan Health Sciences Foundation. We are grateful to TSUMURA & Co. for providing the extracts. We acknowledge Dr. Hiroaki Nishimura for critical reading of the manuscript. We also acknowledge Prof. Yoshiteru Oshima for continuous encouragement.
References
- Baba M., Konno K., Shigeta S., De Clercq E. In vitro activity of (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine against newly isolated clinical varicella-zoster virus strains. Eur. J. Clin. Microbiol. 1987;6:158–160. doi: 10.1007/BF02018198. [DOI] [PubMed] [Google Scholar]
- Barnard D.L., Hubbard V.D., Burton J., Smee D.F., Morrey J.D., Otto M.J., Sidwell R.W. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and β-d-N4-hydroxycytidine. Antivir. Chem. Chemother. 2004;15:15–22. doi: 10.1177/095632020401500102. [DOI] [PubMed] [Google Scholar]
- Barnard D.L., Day C.W., Bailey K., Heiner M., Montgomery R., Lauridsen L., Chan P.K., Sidwell R.W. Evaluation of immunomodulators, interferons and known in vitro SARS-CoV inhibitors for inhibition of SARS-CoV replication in BALB/c mice. Antivir. Chem. Chemother. 2006;17:275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- Beecher G.R. Proanthocyanidins: biological activities associated with human health. Pharm. Biol. 2004;42:2–20. [Google Scholar]
- Brunet M.J., Bladé C., Salvadó M.J., Arola L. Human apo A-I and rat transferrin are the principal plasma proteins that bind wine catechins. J. Agric. Food Chem. 2002;50:2708–2712. doi: 10.1021/jf011257z. [DOI] [PubMed] [Google Scholar]
- Caraglia M., Libroia A.M., Corradino S., Coppola V., Guarrasi R., Barile C., Genua G., Bianco A.R., Tagliaferri P. Alpha-interferon induces depletion of intracellular iron content and upregulation of functional transferrin receptors on human epidermoid cancer KB cells. Biochem. Biophys. Res. Commun. 1994;203:281–288. doi: 10.1006/bbrc.1994.2179. [DOI] [PubMed] [Google Scholar]
- Chen L., Gui C., Luo X., Yang Q., Günther S., Scandella E., Drosten C., Bai D., He X., Ludewig B., Chen J., Luo H., Yang Y., Yang Y., Zou J., Thiel V., Chen K., Shen J., Shen X., Jiang H. Cinanserin is an inhibitor of the 3C-like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro. J. Virol. 2005;79:7095–7103. doi: 10.1128/JVI.79.11.7095-7103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotzer V.L., Mabardy A.S., Weiss A., Brodsky F.M. T cell receptor engagement leads to phosphorylation of clathrin heavy chain during receptor internalization. J. Exp. Med. 2004;199:981–991. doi: 10.1084/jem.20031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyne T., Pieters L., Witvrouw M., De Clercq E., Vanden Berghe D., Vlietinck A.J. Biological evaluation of proanthocyanidin dimers and related polyphenols. J. Nat. Prod. 1999;62:954–958. doi: 10.1021/np980481o. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antivirals and antiviral strategies. Nat. Rev. Microbiol. 2004;2:704–720. doi: 10.1038/nrmicro975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev. Anti. Infect. Ther. 2006;4:291–302. doi: 10.1586/14787210.4.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguière A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G., van Riel D., de Jong T., Itamura S., Chan K.H., Tashiro M., Osterhaus A.D. Pegylated IFN-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide R.T. Qualitative analysis of the essential oil of cassia (cinnamomum cassia blume) J. Agric. Food Chem. 1972;20:747–752. [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2006;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. SARS Working Group. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Kashiwada Y., Nonaka G.I., Nishioka I., Nishizawa M., Yamagishi T., Bodner A.J., Kilkuskie R.E., Chen Y.C. In: Tannis and Related Compounds as Anti-HIV Agents. Natural Products as Antiviral Agents. Chu C.K., Cutler H.G., editors. Plenum Press; New York: 1992. pp. 69–90. [Google Scholar]
- Li B.J., Tang Q., Cheng D., Qin C., Xie F.Y., Wei Q., Xu J., Liu Y., Zheng B.J., Woodle M.C., Zhong N., Lu P.Y. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat. Med. 2005;11:944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., Zhang X., Hua S.N., Yu J., Xiao P.G., Li R.S., Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa J.R., McDonald L.C., Simone P., Parashar U.D. Wresting SARS from uncertainty. Emerg. Infect. Dis. 2004;10:167–170. doi: 10.3201/eid1002.031032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang M., He L., Li Y., Kang Y. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS) Cochrane Database Syst. Rev. 2006 doi: 10.1002/14651858.CD004882.pub2. CD004882. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Naldini L., Blömer U., Gage F.H., Trono D., Verma I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N., Weigert R., Donaldson J.G. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol. Biol. Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka G., Morimoto S., Nishioka I. Tannins and related compounds. Part 13. Isolation and structures of trimeric, tetrameric and pentameric proanthicyanidins from cinnamon. J. Chem. Soc., Perkin Trans. 1983;1:2139–2145. [Google Scholar]
- Paragas J., Blatt L.M., Hartmann C., Huggins J.W., Endy T.P. Interferon alfacon1 is an inhibitor of SARS-corona virus in cell-based models. Antiviral Res. 2005;66:99–102. doi: 10.1016/j.antiviral.2005.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior R.L., Gu L. Occurrence and biological significance of proanthocyanidins in the American diet. Phytochemistry. 2005;66:2264–2280. doi: 10.1016/j.phytochem.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Scalbert A., Déprez S., Mila I., Albrecht A.M., Huneau J.F., Rabot S. Proanthocyanidins and human health: systemic effects and local effects in the gut. Biofactors. 2000;13:115–120. doi: 10.1002/biof.5520130119. [DOI] [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Yau V.K., Briggs B.J., Whittaker G.R. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology. 2005;338:53–60. doi: 10.1016/j.virol.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Tan E.L., Ooi E.E., Lin. C.Y., Tan H.C., Ling A.E., Lim B., Stanton L.W. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.X., Wan M., Dong H., But P.P.H., Foo L.Y. Inhibitory activity of flavonoids and tannis against HIV-1 protease. Biol. Pharm. Bull. 2000;23:1072–1076. doi: 10.1248/bpb.23.1072. [DOI] [PubMed] [Google Scholar]
- Xu X., Sutak R., Richardson D.R. Iron chelation by clinically relevant anthracyclines: alteration in expression of iron-regulated genes and atypical changes in intracellular iron distribution and trafficking. Mol. Pharmacol. 2008;73:833–844. doi: 10.1124/mol.107.041335. [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhang X., Matsuoka M., Hattori T. The possible involvement of CXCR4 in the inhibition of HIV-1 infection mediated by DP178/gp41. FEBS Lett. 2000;487:185–188. doi: 10.1016/s0014-5793(00)02336-x. [DOI] [PubMed] [Google Scholar]
- Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L., Shen Y., Luo M., Zuo G., Hu J., Duan D., Nie Y., Shi X., Wang W., Han Y., Li T., Liu Y., Ding M., Deng H., Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.M., Liu X.M., He L. Effect of integrated traditional Chinese and Western medicine on SARS: a review of clinical evidence. World J. Gastroenterol. 2004;10:3500–3505. doi: 10.3748/wjg.v10.i23.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]


