Abstract
Edible bird's nest (EBN) is the nest of the swift that is made from its saliva. Although EBN has been widely used for enhancing immunocompetence, its antiviral efficacy has not been studied in detail. We found that EBN extract could strongly inhibit infection with influenza viruses in a host range-independent manner when it was hydrolyzed with Pancreatin F. Western blotting assay showed that the EBN extract bound to influenza virus. Furthermore, EBN extract could neutralize the infection of MDCK cells with influenza viruses and inhibit hemagglutination of influenza viruses to erythrocytes, but it could not inhibit the activity of influenza virus sialidase. Fluorometric HPLC indicated that the major molecular species of sialic acid in EBN is N-acetylneuraminic acid. The results suggest that EBN is a safe and valid natural source for the prevention of influenza viruses.
Keywords: Influenza, Bird nest, Sialic acid
1. Introduction
Edible bird's nest (EBN) is the nest of the swift that is made from its saliva, which contains sialylglycoconjugates. The composition of the swift's saliva resembles that of salivary mucin. Many studies have been carried out on the tonic effects of EBN, and it has been shown that EBN stimulates mitosis hormones and the growth factor for epidermal growth, resulting in repair of cells and stimulation of the immune system (Kong et al., 1987, Ng et al., 1986). Recently, several carbohydrate molecules, including new sialic acid-containing compounds and glycoconjugates, have been detected in EBN (Pozsgay et al., 1987, Reuter et al., 1989, Wieruszeski et al., 1987, Kakehi et al., 1994, Martin et al., 1977, Yu-Qin et al., 2000), but the importance of sialic acid residues in EBN is not clear.
Sialic acid residues of many glycoconjugates are involved in biologically important ligand–receptor interactions such as specific cell-to-cell, pathogen-to-cell, or drug-to-cell interactions. We found that an EBN extract could neutralize infection with influenza viruses in MDCK cells and inhibit the hemagglutination of influenza A viruses (such as human, avian, and porcine strains) to human erythrocytes.
2. Materials and methods
2.1. Edible bird's nest (EBN) extract
We used two kinds of samples from bird nests collected in Indonesia. Sample 1 (S1) was obtained from a bird's nest collected in a natural cave and sample 2 (S2) was obtained from a bird's nest that had been house-cultured. The bird's nests were dried for 16 h at 70 °C and then ground and sifted through a mesh (600 μM in pore size) for combing out plume and foreign substances. The grounded samples were kept in distilled water (5 g sample/200 ml water) at 5 °C for 16 h and then heated at 100 °C for 30 min. The suspension was treated with Pancreatin F (final concentration of 0.5 mg/ml, Amano Enzyme Inc., Japan) at 45 °C for 4 h at pH 8.5–9.0 and then heated at 90 °C for 5 min for enzyme deactivation. The treated extracts were filtrated with a filter paper (ADVANTEC filter paper no. 2), and the filtrate was freeze-dried. For neuraminidase treatment, the EBN extracts were incubated in PBS containing 200 mU/ml of neuraminidase from C. perfringens (N2876, Sigma) for 2 h at 37 °C and then heated at 90 °C for 5 min for enzyme deactivation.
2.2. Cells and viruses
Madin-Darby canine kidney (MDCK) cells were cultured in Eagle's minimum essential medium (EMEM; Nissui Pharmaceutical Co., Ltd., Japan) containing 5% (v/v) heat-inactivated fetal calf serum (FCS; Biological Industries). Human lung carcinoma A549 cells were purchased from ATCC and cultured in Dulbecco's modified eagle medium (DMEM; Nissui Pharmaceutical Co., Ltd.) supplemented with 10% (v/v) heat-inactivated FCS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycine (Gibco) at 37 °C in humidified air containing 5% CO2.
The influenza viruses used in this study (except strains A/Shizuoka/450/05 and A/Shizuoka/451/05, see Table 1 ) were propagated in the allantoic sacs of 11-day-old embryonated eggs and purified by sucrose density gradient centrifugation (Suzuki et al., 1980). A/Shizuoka/450/05 (H3N2) and A/Shizuoka/451/05 (H3N2) strains were grown with A549 cell monolayers in FCS-free DMEM supplemented with 2 μg/ml acetyl-trypsin, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycine at 34.5 °C in humidified air containing 5% CO2. Viral HA units were determined in micrometer plates using 0.5% human type O erythrocytes as described previously (Suzuki et al., 1983). Rabbit anti-influenza virus antibodies were raised by immunization of A/Memphis/1/71 (H3N2) virus grown in eggs as described previously (Suzuki et al., 1987).
Table 1.
Inhibitory effects of EBN extract treated with Pancreatin F on hemagglutination and infection of various influenza A viruses isolated from humans, ducks, and pigs
| Viruses | HAI (μg/ml) |
IC50 (μg/ml) |
||
|---|---|---|---|---|
| S1-Pan | S2-Pan | SI-Pan | S2-Pan | |
| Human strains | ||||
| A/PR/8/34 (H1N1) | 5 | 40 | 36 ± 6a | 182 ± 16 |
| A/Aichi/2/68 (H3N2) | 5 | 160 | 76 ± 9 | 464 ± 38 |
| A/HongKong/1/68 (H3N2) | 10 | 40 | 41 ± 8 | 260 ± 22 |
| A/Memphis/102/72 (H3N2) | 10 | 40 | NDb | ND |
| A/Tokyo/6/73 (H3N2) | 5 | 40 | ND | ND |
| A/Kumamoto/55/76 (H3N2) | 20 | 80 | ND | ND |
| A/Texas/1/77 (H3N2) | 5 | 80 | ND | ND |
| A/Yamanashi/2/77 (H3N2) | 5 | 40 | ND | ND |
| A/Bangkok/1/79 (H3N2) | 5 | 20 | ND | ND |
| A/Shizuoka/450/05 (H3N2) | 20 | 80 | 84 ± 12 | 232 ± 46 |
| A/Shizuoka/451/05 (H3N2) | 20 | 80 | 96 ± 10 | 244 ± 32 |
| Avian strains | ||||
| A/Dk/Alb/35/76 (H1N1) | 10 | 80 | 24 ± 6 | 156 ± 18 |
| A/Dk/HongKong/7/75 (H3N2) | 2.5 | 20 | 41 ± 4 | 124 ± 16 |
| A/Dk/HongKong/24/76 (H3N2) | 10 | 80 | 40 ± 6 | 246 ± 42 |
| A/Dk/Hokkaido/5/77 (H3N2) | 2.5 | 20 | ND | ND |
| A/Dk/Hokkaido/8/80 (H3N8) | 2.5 | 10 | 24 ± 5 | 98 ± 23 |
| A/Dk/HongKong/23/76 (H5N3) | 2.5 | 20 | 36 ± 8 | 142 ± 18 |
| Swine strains | ||||
| A/Sw/Iowa/15/30 (H1N1) | 2.5 | 10 | 10 ± 2 | 86 ± 8 |
| A/Sw/HongKong/126/82 (H3N2) | 2.5 | 40 | 10 ± 3 | 104 ± 12 |
The IC50 is the mean ± S.D. of three dependent experiments.
ND: Not detected.
2.3. Hemagglutination inhibition assay
Hemagglutination inhibition (HAI) assay was carried out using 96-well microtiter plates as described previously (Suzuki et al., 1983). Phosphate-buffered saline (PBS, pH 6.5) containing 0.01% gelatin was used as a dilution buffer. Human erythrocytes were used as indicator cells. Virus suspension (4 HA units in 0.025 ml of PBS) was added to each well containing the EBN extract in two-fold serial dilutions with the dilution buffer. The plates were incubated for 1 h at 4 °C. After 0.05 ml of 0.5% (v/v) human type O erythrocytes, in which the total sialic acid content for blood group O is 10-fold higher than that for blood group A or B (Bulai et al., 2003), in PBS had been added to the plates, the plates were kept for 1 h at 4 °C. The maximum dilution of the samples showing complete inhibition of hemagglutination was defined as the HAI titer.
2.4. Sialidase inhibition assay
A fluorometric assay was used to determine the inhibitory effect of the EBN extract on influenza virus sialidase activity (Suzuki et al., 1992). Five microliters of influenza virus suspension (500 ng protein) in 50 mM sodium acetate buffer (pH 5.5) containing two-fold serial dilution of the EBN extracts was stored at 37 °C for 30 min and then incubated with 5 μl of 4 mM 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (4-MU-Neu5Ac) (Sigma–Aldrich, St. Louis, MO) at 37 °C for 30 min. The reaction was stopped by the addition of 1 ml carbonate buffer (pH 10.7). The fluorescence of the released 4-methylumbelliferone was measured with a fluorescence spectrophotometer (F-4010; Hitachi, Tokyo) with excitation at 355 nm and emission at 460 nm.
2.5. Neutralization assay
Neutralization of influenza virus by the EBN extract was determined as previously described (Guo et al., 2002). Briefly, MDCK cell monolayers were maintained in EMEM containing 5% FCS in a 96-well plate. One hundred microliters of TCID50 (50% tissue culture infectious dose) of viruses in the presence of the EBN extract (0.5–4000 μg/ml) was inoculated at 34.5 °C for 2 h. After removal of the inoculum, the monolayers were washed three times with EMEM. The cells were examined using a light microscope for the progression of viral-induced cytopathic effects (CPE) after incubation at 34.5 °C for 20 h. Lactate dehydrogenase (LDH) that was released from MDCK cells was examined for virus neutralization by a slightly modified colorimetric assay. LDH activities in the medium were determined according to the manufacturer's instructions. Briefly, the medium (0.0125 ml) was diluted to 1:4 (v/v) with PBS and mixed with 0.05 ml of LDH reagent (Shinotest, Japan). The mixture was incubated at 37 °C for 10 min, and the reaction was stopped by the addition of 0.1 ml of 0.5N HCl. Absorbance was measured at 550 nm (reference at 630 nm). The assays were performed in triplicate for three independent experiments. As cytopathic effects appear on all cells, the LDH activity was indicated 100% as a relative activity.
2.6. Western blot—virus binding assay
Western blotting was used to detect the binding activity of the viruses to the glycoprotein from the EBN extracts. Total amounts (100 μg) of proteins from the EBN extracts were subjected to SDS-PAGE through a 10–15% polyacrylamide running gel. Proteins were electrophoretically transferred to immune-blot PVDF membranes (Bio-Rad Lab., USA). The membranes were blocked for 1 h in PBS containing 3% skin milk and 0.1% Tween-20. Binding of proteins to influenza virus (A/Aichi/2/68 (H3N2), 256 HAU/ml) was carried out at 4 °C by incubation overnight (more than 12 h) and then probing with anti-H3N2 (anti-Mem) rabbit serum at a dilution of 1:1000, followed by horseradish peroxidase (HRP)-conjugated protein A (ICN Pharmaceuticals Inc.) at a dilution of 1:1000. Finally, the immunoreactive proteins were visualized using a solution containing 100 mM citrate buffer (pH 6.0), 60 mM N,N-dimethyl-p-phenylenediamine dihydrochloride in acetonitrile, 100 mM 4-chloro-1-naphthol in acetonitrile, and 3% H2O2 aqueous (5:1:1:0.005, by volume) for 15 min at room temperature.
2.7. Fluorometric HPLC method for determination of molecular species of sialic acids
Fluorometric determination of 5-N-acetyl-neuraminic acid (Neu5Ac) and 5-N-glycolylneuraminic acid (Neu5Gc) was conducted by a high-performance liquid chromatography (HPLC) method using 1,2-diamino-4,5-methylenedioxy-benzene (DMB) as previously described (Suzuki et al., 1996). The EBN extract was hydrolyzed with 200 μl of 25 mM sulfuric acid. The hydrolysate was reacted with DMB reagent and heated at 60 °C for 2.5 h in the dark to develop fluorescence for determination of sialic acids. A 10-μl aliquot of the resulting solution was used for determination of sialic acids. The fluorescence of DMB derivatives was detected at an excitation wavelength of 373 nm and an emission wavelength of 448 nm. For the establishment of calibration curves, standard mixtures of Neu5Ac and Neu5Gc (Sigma, USA) were used.
2.8. Lectin blotting method for determination of sialic acid linkages
To identify sialyoligosaccharides reactive with sialic acid (SA) α2,3galactose (Gal)- or SAα2,6Gal-specific lectins (glycan determination kit; Boehringer Mannheim Biochemicals, Mannheim, Germany), each section was incubated with 50 μl of digoxigenin (DIG)-labeled Sambucus nigra agglutinin (SNA) lectin [1 μg/ml; specific for SAα2,6Gal/N-acetylgalactosaminide (GalNac)] or Maackia amurensis agglutinin (MAA) lectin (5 μg/ml; specific for SAα2,3Gal) for 1 h at room temperature. After three washes with cold PBS, the membrane was incubated with HRP-conjugated anti-DIG antibody (Boehringer Mannheim Biochemicals) for 1 h at room temperature and then after three additional washes in cold PBS and buffered glycerol (pH 9.0) were visualized according to the protocol provided by the manufacturer (glycan determination kit; Boehringer Mannheim Biochemicals).
3. Results
3.1. Binding of the EBN extract to influenza virus
In order to check the reaction of EBN extract with influenza viruses, we used the human influenza virus strain A/Aichi/2/68 (H3N2), which bound to both sialyl α2-3 and α2-6 galactose linkages in salylglycoproteins and sialylglycolipids, and carried out an SDS-PAGE Western blotting assay to analyze the glycoprotein molecules. As shown in Fig. 1 , strain A/Aichi/2/68 could bind to the EBN extract both treated (molecular weight is lower than 25 kDa) and not treated (molecular weight is over than 50 kDa) with a pancreatic enzyme that hydrolyzes protein to polypeptide (Fig. 1A). The virus binding activity of EBN extract from S1 (from a bird's nest collected in a cave) was stronger than that of EBN extract from S2 (from a house-cultured nest). Furthermore, the effect of EBN extract from S1 was markedly reduced after treatment with neuraminidase (Fig. 1B), indicating that the virus binding is associated with sialic acid of the EBN.
Fig. 1.
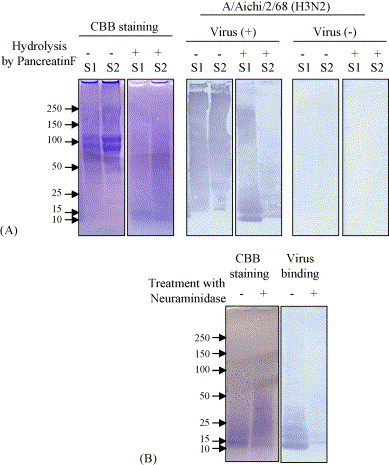
Binding of the EBN extract with influenza virus. (A) Binding of EBN extracts from S1 and S2 with strain A/Aichi/2/68 (H3N2) was detected by SDS-PAGE through a 10–15% polyacrylamide running gel and a Western blotting assay. The effects of Pancreatin F and neuraminidase treatment on EBN/virus binding activity were also determined. (A) Both EBN extracts were treated with Pancreatin F (final concentration of 0.5 mg/ml) at 45 °C for 4 h at pH 8.5–9.0. (B) Pancreatin F-treated EBN extract from S1 was further treated with 200 mU/ml of neuraminidase from C. perfringens (N2876, Sigma) to cleave terminal sialic acid residues in the EBN extract.
3.2. Inhibitory effect of EBN extract on influenza virus infection independent of virus strain
To measure the biological response of the EBN extract, we carried out an HAI assay and a neutralization assay (Suzuki et al., 1997). As shown in Fig. 2A, in case of no treatment with the pancreatic enzyme, neither the EBN extract from S1 nor that from S2 inhibited the hemagglutination of influenza A virus (strain A/Aichi/2/68) to human type O erythrocytes. However, EBN extracts (S1-Pan and S2-Pan) treated with Pancreatin F at 37 °C for 4 h had stronger HAI activities. The HAI activities of the extracts from S1-Pan and S2-Pan were 4 or 125 μg/ml, respectively. The difference in their HAI activities indicates that S1-Pan and S2-Pan contain different sialylconjugates, such as sialic acidic species and linkages of sialic acid to galactose. On the other hand, after treatment with both the pancreatic enzyme and neuraminidase, the EBN extracts from S1 (S1-Pan-NA) and S2 (S2-Pan-NA) had a reducing effect.
Fig. 2.
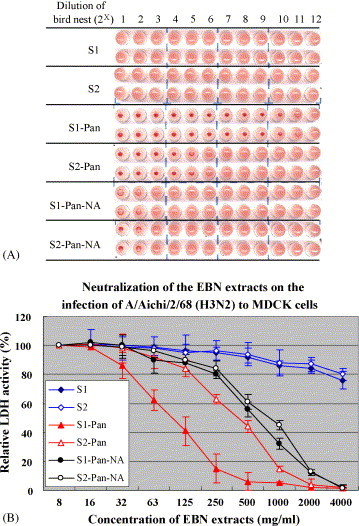
Effects of the EBN extracts from S1 and S2 treated with Pancreatin F (Pan) and/or a neuraminidase (NA) on the inhibitory effects of hemagglutinin of influenza virus (A/Aichi/2/68) (A) and the neutralization (B) of influenza virus infection as described in Section 2. S1 (the wild EBN) and S2 (the cultured EBN) are EBN extracts that were not treated with any enzyme. S1-Pan and S2-Pan are EBN extracts which were treated with Pancreatin F (final concentration of 0.5 mg/ml) at 45 °C for 4 h at pH 8.5–9.0. S1-Pan-NA and S2-Pan-NA are S1-Pan and S2-Pan that were further treated with 200 mU/ml of neuraminidase from C. perfringens (N2876, Sigma) at 37 °C for 2 h. The starting dilution concentration of the EBN extracts is 4 mg/ml.
We also investigated the neutralization of infection with influenza A virus (strain A/Aichi/2/68) in MDCK cells by the EBN extract. As shown in Fig. 2B, the results were similar to those of the HAI assay. The inhibitory effect of the EBN extract from S1 treated with Pancreatin F (S1-Pan) on strain A/Aichi/2/68 was stronger (IC50, ∼80 μg/ml) than that of the EBN extract from S2 (IC50, ∼400 μg/ml). After treatment with neuraminidase, the inhibitory effects of the EBN extracts (S1-Pan-NA and S2-Pan-NA) were 10-fold and 3-fold less than those of S1-Pan and S2-Pan, respectively.
Taken together, the results showed that the EBN extract from S1 after treatment with a pancreatic enzyme has stronger inhibitory activity than that of the EBN extract without enzyme treatment. The results indicate that the lower molecular peptides (10–25 kDa) compared with the higher molecular protein (over than 50 kDa) of the EBN extract are propitious to inhibit influenza A virus infection. On the other hand, the EBN extract (4 mg/ml) did not inhibit the neuraminidase activity of influenza viruses as using 4 mg/ml of their higher concentration (data not shown), indicating that the EBN extract acts on viral hemagglutinin but not viral neuramindase.
In the HAI and neutralization experiments, the EBN extract at 4 mg/ml had no side effects, such as hemolysis and cytolysis, on human erythrocytes and MDCK cells.
We further tested the effects of the EBN extracts (S1-Pan and S2-Pan) against on 19 strains isolated from humans, ducks, and pigs. As shown in Table 1, the EBN extracts (S1-Pan and S2-Pan) had strong effects on the HAI and neutralization of all influenza viruses used in this test, indicating that its effect is independent of virus strain.
3.3. Analysis of sialic acid molecular species and linkages in the EBN extract
Many studies have indicated that various bird nests have abundant sialic acid-containing sugar chains (Pozsgay et al., 1987, Reuter et al., 1989, Wieruszeski et al., 1987, Kakehi et al., 1994, Martin et al., 1977). In order to determine the mechanism underlying the antiviral effect of EBN extract, we carried out fluorometric HPLC and a lectin binding assay for analyzing the molecular species of sialic acid and the linkage between sialic acid and galactose.
Neu5Ac was detected as the major molecular species of sialic acid in the EBN extract from S1 (96.8% of total sialic acid) (Fig. 3A-a, b). Peak 2 in panel c is much higher than that in panel b. After alkali hydrolysis with 1N NaOH for 3 h at 37 °C, peak 2 became lower and peak 1 became higher (panel d), indicating that the EBN extract from S2 has an O-acetyl sialic acid species. We further examined the linkages between non-reducing terminal sialic acid to Gal in the EBN extract using sialic acid-Gal linkage-specific lectins by SDS-PAGE (Fig. 3B). Since MAA (specificity for sialic acid terminally linked 2-3 to Gal in N-glycan and O-glycan) bound strongly to the EBN extract from S1 and weakly to the EBN extract from S2, it is possible that MAA can not bind to O-acetyl sialic acid to Gal linkage. On the other hand, SNA [specificity for sialic acid terminally linked 2-6 to Gal (or GalNAc) in N-glycan] did not react with either of the EBN extracts. These findings suggest that the sialyl-glycoconjugates of the EBN extract from S1 have Neu5Acα2-3Gal linkages and that the EBN extract from S2 also have an O-acetylated Neu5Ac, while Neu5Ac is the major sialic acid species. These sialyl glycoconjugates containing Neu5Ac2-3Gal linkages may act as the main inhibitor of influenza virus infection.
Fig. 3.
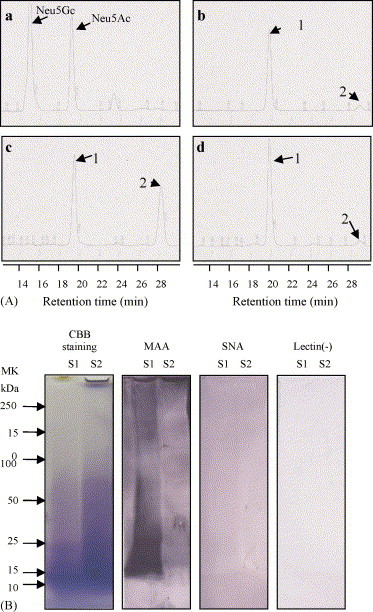
Detection of molecular species of sialic acid and sialyl-Gal linkages in the EBN extracts. (A) Chromatograms of DMB derivatives of Neu5Ac and Neu5Gc obtained from the EBN extracts. Standard mixtures of Neu5Ac and Neu5Gc (a) and of the EBN extracts from S1 (b) and S2 (c and d) were treated as described in the Section 2. The fluorescence of DMB derivatives was detected at an excitation wavelength of 373 nm and an emission wavelength of 448 nm. (Panel d) EBN extract from S2 treated for 3 h at 37 °C with 1N NaOH, which hydrolyzes the O-acetyl residue of O-acetylated sialic acids. (B) Lectin blotting of the EBN extracts. EBN extracts from S1 and S2 (0.2 mg/lane) were examined. SDS-PAGE was performed with 10–15% polyacrylamide gels. Nitrocellulose transfers were incubated with MAA lectin specific for sialic acid α2-3 Gal or with SNA lectin specific for sialic acid α2-6 Gal on the N-glycan. That was visualized according to the protocol provided by the manufacturer (glycan determination kit; Boehringer Mannheim Biochemicals, Mannheim, Germany).
4. Discussion
In this study, we found that the EBN extract had a potent inhibitory activity against infection of host cells with human, avian, and porcine influenza viruses. The effect is mediated by the Neu5Ac residues of sialyl-sugar chains in the EBN extract. We also found that the inhibitory activity of EBN extract against influenza virus was markedly enhanced by treatment with a pancreatic enzyme (Pancreatin F) that contains protease to hydrolyze glycoproteins to glycopeptides. We noted the EBN extracts that were not treated with a pancreatic enzyme could be bound by influenza viruses but had no effect on HAI or the neutralization of influenza virus infection in MDCK cells, indicating small molecular of the EBN extract was more propitious for its anti-viral effect.
In a general way, the hemagglutination of viruses propagated in chicken eggs preferentially binds to receptors with a silaic acid bond to the adjacent sugar via α2-3 linkage, whereas the HA of human MDCK-grown viruses preferentially binds to receptors with a silaic acid bond to the adjacent sugar via α2-6 linkage. We determined the effects of the EBN extract on viruses propagated in chicken eggs and also on recent viruses [strains A/Shizuoka/450/05 and A/Shizuoka/451/05 (H3N2)] isolated from influenza patients in February 2005 and cultured in A549 cells, human lung carcinoma cells that express both sialyl α2-3 and α2-6 linkages. The inhibitory effects of the EBN extract on strains A/Shizuoka/450/05 and A/Shizuoka/451/05 (H3N2) were similar to those on other strains used in this study.
The data obtained from lectin blotting showed that Neu5Acα2-3Gal linkage was the major molecular species of sialic acid in the EBN extract. Why could the EBN extract inhibit infection of recently isolated influenza viruses? We have recently reported that sialic acid-free components from bacteria (Guo et al., 2001, Nakata et al., 2000) and sulphatide (Suzuki et al., 1996, Suzuki et al., 2003) also have some inhibitory effects on influenza A virus infection. The EBN extract may contain some Neu5Acα2-6Gal linkages since the lectin SNA can not detect the Neu5Acα2-6Gal linkage in O-glycan and glycolipids (Dalziel et al., 1999).
We do not know why there is a difference between EBNs from the wild and cultured environments. One possible reason is that the saliva of the wild swift and that of the cultivated swift are different. Another possibility is that the O-acetylated sialic acid residue in the EBN from the wild environment or in its harvest process is destroyed. Further investigation of this issue is needed.
Chinese have been consuming bird nests for hundreds of years, and most of them believe that EBN consists of several proteins and minerals that promote the generation and growth of human cells, rejuvenate human skin, and strengthen the immune system. Although Ou et al. (2001) reported that bird's nest is the common cause of food-induced anaphylaxis in children, which could lead to potentially life-threatening allergenic reactions, and the presence of a major allergen of 66 kDa in EBN (Goh et al., 2001). In this study, the proteins or peptides which had the best anti-influenza effect were some 10–25 kDa sialylglycoproteins of the EBN extract after with Pancreatin F. Although the proteins of over than 50 kDa (including a major allergen of 66 kDa) untreated with pancreatic enzyme could bind to influenza A virus, the neutralization against the virus infection not detected or was very low. Furthermore, the EBN extract showed no side effects, such as hemolysis and cytolysis, on erythrocytes and MDCK cells even at a higher concentration (4 mg/ml). Therefore, we think that the EBN extract (lower than 25 kDa) treated with Pancreatin F will become an effective and secure agent for anti-virus.
Many studies have validated several different sialyl linkages to galactose or N-acetylgalactosamine (Pozsgay et al., 1987, Reuter et al., 1989, Wieruszeski et al., 1987, Kakehi et al., 1994, Martin et al., 1977) in bird nests. Not only influenza A viruses but also other microbes, such as influenza B and C viruses, parainfluenza virus, Newcastle disease virus, rotavirus, coronavirus and bacteria (cholera), recognize sialic acid and its derivatives, O-acetylated sialic acid-containing sugar chains as its receptor (Guo et al., 1998, Schultze et al., 1990, Suzuki et al., 2001a, Suzuki et al., 2001b, Sillerud et al., 1981). Although the O-acetylated sialic acid-containing sugar chains in the EBN are not a good source of the inhibitory activity for influenza A viruses, they may have an effective roles against other viruses which recognize O-acetylated sialic acid, such as influenza C virus and coronavirus.
We have searched the way of the harvest of the cultured house and wild cave bird nests and also their processing in the market, but could not obtain any more detailed information on them. Therefore, we have no clear answer on the difference of the binding and inhibitory activities between EBN from the wild and from cultured environments. One reason of the low binding and inhibitory activity in S2 rather than S1 may due to in the presence of O-acetylated sialyl sugar chains in S2 which do not bind and inhibit the influenza A and B viruses.
Taken together, the results suggested that EBN is a safe and valid natural source for the prevention of influenza viruses in vitro, however, the detailed in vivo effect of the inhibition of the influenza viruses by EBN should be evaluated.
Acknowledgments
Support for this work was provided by a grant of the JSPS Postdoctoral Fellowship for Foreign Researchers (15 03131) from the Japan Society for the Promotion of Science, and a grant-in-aid (17390022) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Bulai T., Bratosin D., Pons A., Montreuil J., Zanetta J.P. Diversity of the human erythrocyte membrane sialic acids in relation with blood groups. FEBS Lett. 2003;534(1–3):185–189. doi: 10.1016/s0014-5793(02)03838-3. [DOI] [PubMed] [Google Scholar]
- Dalziel M., McFarlane I., Axford J.S. Lectin analysis of human immunoglobulin G N-glycan sialylation. Glycoconj. J. 1999;6:801–807. doi: 10.1023/a:1007183915921. [DOI] [PubMed] [Google Scholar]
- Goh D.L., Chua K.Y., Chew F.T., Liang R.C., Seow T.K., Ou K.L., Yi F.C., Lee B.W. Immunochemical characterization of edible bird's nest allergens. J. Allergy Clin. Immunol. 2001;107:1082–1087. doi: 10.1067/mai.2001.114342. [DOI] [PubMed] [Google Scholar]
- Guo C.T., Ohta S., Yoshimoto A., Nakata R., Shortridge K.F., Takahashi T., Suzuki T., Miyamoto D., Hidari K.I.P.J., Suzuki Y. A unique phosphatidylinositol bearing a novel branched-chain fatty acid from Rhodococcus equi binds to influenza virus hemagglutinin and inhibits the infection of cells. J. Biochem. 2001;130:377–384. doi: 10.1093/oxfordjournals.jbchem.a002996. [DOI] [PubMed] [Google Scholar]
- Guo C.T., Sun X.L., Kanie O., Shortridge K.F., Suzuki T., Miyamoto D., Hidari K.I., Wong C.H., Suzuki Y. An O-glycoside of sialic acid derivative that inhibits both hemagglutinin and sialidase activities of influenza viruses. Glycobiology. 2002;12:183–190. doi: 10.1093/glycob/12.3.183. [DOI] [PubMed] [Google Scholar]
- Guo C.T., Wong C.H., Kajimoto T., Miura T., Ida Y., Juneja L.R., Kim M.J., Masuda H., Suzuki T., Suzuki Y. Synthetic sialylphosphatidylethanolamine derivatives bind to human influenza A viruses and inhibit viral infection. Glycoconj. J. 1998;15:1099–1108. doi: 10.1023/a:1006961912465. [DOI] [PubMed] [Google Scholar]
- Kakehi K., Susami A., Taga A., Suzuki S., Honda S. High-performance capillary electrophoresis of O-glycosidically linked sialic acid-containing oligosaccharides in glycoproteins as their alditol derivatives with low-wavelength UV monitoring. J. Chromatogr. A. 1994;680:209–215. doi: 10.1016/0021-9673(94)80069-3. [DOI] [PubMed] [Google Scholar]
- Kong Y.C., Keung W.M., Yip T.T., Ko K.M., Tsao S.W., Ng M.H. Evidence that epidermal growth factor is present in swiftlet's (Collocalia) nest. Comp. Biochem. Physiol. B. 1987;87:221–226. doi: 10.1016/0305-0491(87)90133-7. [DOI] [PubMed] [Google Scholar]
- Martin J.E., Tanenbaum S.W., Flashner M. A facile procedure for the isolation of N-acetylneuramic acid from edible bird's-nest. Carbohydr. Res. 1977;56:423–425. doi: 10.1016/s0008-6215(00)83368-6. [DOI] [PubMed] [Google Scholar]
- Nakata K., Guo C.T., Matsufuji M., Yoshimoto A., Inagaki M., Higuchi R., Suzuki Y. Influenza A virus-binding activity of glycoglycerolipids of Aquatic Bacteria. J. Biochem. 2000;127:191–198. doi: 10.1093/oxfordjournals.jbchem.a022594. [DOI] [PubMed] [Google Scholar]
- Ng M.H., Chan K.H., Kong Y.C. Potentiation of mitogenic response by extracts of the swiftlet's (Collocalia) nest. Biochem. Int. 1986;13:521–531. [PubMed] [Google Scholar]
- Ou K., Seow T.K., Liang R.C., Lee B.W., Goh D.L., Chua K.Y., Chung M.C. Identification of a serine protease inhibitor homologue in Bird's Nest by an integrated proteomics approach. Electrophoresis. 2001;22:3589–3595. doi: 10.1002/1522-2683(200109)22:16<3589::AID-ELPS3589>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Pozsgay V., Jennings H., Kasper D.L. 4,8-anhydro- N-acetylneuraminic acid. Isolation from edible bird's nest and structure determination. Eur. J. Biochem. 1987;162:445–450. doi: 10.1111/j.1432-1033.1987.tb10622.x. [DOI] [PubMed] [Google Scholar]
- Reuter G., Schauer R., Szeiki C., Kamerling J.P., Vliegenthart J.F. A detailed study of the periodate oxidation of sialic acids in glycoproteins. Glycoconj. J. 1989;6:35–44. doi: 10.1007/BF01047888. [DOI] [PubMed] [Google Scholar]
- Schultze B., Gross H.J., Brossmer R., Klenk H.D., Herrler G. Hemagglutinating encephalomyelitis virus attaches to N-acetyl-9-O-acetylneuraminic acid-containing receptors on erythrocytes: comparison with bovine coronavirus and influenza C virus. Virus Res. 1990;16:185–194. doi: 10.1016/0168-1702(90)90022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillerud L.O., Prestegard J.H., Yu R.K., Konigsberg W.H., Schafer D.E. Observation by 13C NMR of interactions between cholera toxin and the oligosaccharide of ganglioside GM1. J. Biol. Chem. 1981;256:1094–1097. [PubMed] [Google Scholar]
- Suzuki S., Sawa H., Komagome R., Orba Y., Yamada M., Okada Y., Ishida Y., Nishihara H., Tanaka S., Nagashima K. Broad distribution of the JC virus receptor contrasts with a marked cellular restriction of virus replication. Virology. 2001;286:100–112. doi: 10.1006/viro.2001.0972. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Horiike G., Yamazaki Y., Kawabe K., Masuda H., Miyamoto D., Matsuda M., Nishimura S.I., Yamagata T., Ito T., Kida H., Kawaoka Y., Suzuki Y. Swine influenza virus strains recognize sialylsugar chains containing the molecular species of sialic acid predominantly present in the swine tracheal epithelium. FEBS Lett. 1997;404:192–196. doi: 10.1016/s0014-5793(97)00127-0. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Portner A., Scroggs R.A., Uchikawa M., Koyama N., Matsuo K., Suzuki Y., Takimoto T. Receptor specificities of human respiroviruses. J. Virol. 2001;75:4604–4613. doi: 10.1128/JVI.75.10.4604-4613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Sometani A., Yamazaki Y., Horiike G., Mizutani Y., Masuda H., Yamada M., Tahara H., Xu G., Miyamoto D., Oku N., Okada S., Kiso M., Hasegawa A., Ito T., Kawaoka Y., Suzuki Y. Sulphatide binds to human and animal influenza A viruses, and inhibits the viral infection. Biochem. J. 1996;318:389–393. doi: 10.1042/bj3180389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Takahashi T., Nishinaka D., Murakami M., Fujii S., Hidari K.I., Miyamoto D., Li Y.T., Suzuki Y. Inhibition of influenza A virus sialidase activity by sulfatide. FEBS Lett. 2003;553:355–359. doi: 10.1016/s0014-5793(03)01045-7. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nagao Y., Kato H., Suzuki T., Matsumoto M., Murayama J. The hemagglutinins of the human influenza viruses A and B recognize different receptor microdomains. Biochim. Biophys. Acta. 1987;903:417–424. doi: 10.1016/0005-2736(87)90048-4. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nakao T., Ito T., Watanabe N., Toda Y., Xu G., Suzuki T., Kobayashi T., Kimura Y., Yamada A. Structural determination of gangliosides that bind to influenza A, B, and C viruses by an improved binding assay: strain-specific receptor epitopes in sialo-sugar chains. Virology. 1992;189:121–131. doi: 10.1016/0042-6822(92)90687-k. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Morioka T., Matsumoto M. Action of ortho- and paramyxovirus neuraminidase on gangliosides. Hydrolysis of ganglioside GM1 by Sendai virus neuraminidase. Biochim. Biophys. Acta. 1980;619:632–639. [PubMed] [Google Scholar]
- Suzuki Y., Suzuki T., Matsumoto M. Isolation and characterization of receptor sialoglycoprotein for hemagglutinating virus of Japan (Sendai virus) from bovine erythrocyte membrane. J. Biochem. 1983;93:1621–1633. doi: 10.1093/oxfordjournals.jbchem.a134301. [DOI] [PubMed] [Google Scholar]
- Wieruszeski J.M., Michalski J.C., Montreuil J., Strecker G., Peter-Katalinic J., Egge H., van Halbeek H., Mutsaers J.H., Vliegenthart J.F. Structure of the monosialyl oligosaccharides derived from salivary gland mucin glycoproteins of the Chinese swiftlet (genus Collocalia) J. Biol. Chem. 1987;262:6650–6657. [PubMed] [Google Scholar]
- Yu-Qin Y., Liang X., Hua W., Hui-Xing Z., Xin-Fang Z., Bu-Sen L. Determination of edible bird's nest and its products by gas chromatography. J. Chromatogr. Sci. 2000;38:27–32. doi: 10.1093/chromsci/38.1.27. [DOI] [PubMed] [Google Scholar]


