Abstract
Over 2.7 billion liters of pot ale is produced annually as a co-product of Scottish malt whisky, and apart from evaporation to pot ale syrup as a feed, it is primarily treated by anaerobic digestion or land/sea disposal. The aim of this study was to assess pot ale components and their potential applications. The insoluble solid fraction, mainly consisting of yeast, contained 55% protein, and as a protein feed ingredient, this could yield 32,400 tons of feed per annum, although the Cu content of this fraction would need to be monitored. The liquid fraction could yield 33,900 tons of protein per annum, and an SDS-PAGE profile of this fraction demonstrated that the proteins may be similar to those found in beer, which could extend their application as a food ingredient. This fraction also contained phosphorus, potassium, and polyphenols among other components, which could have added value. Overall, fractionation of pot ale could offer an alternative to evaporation to pot ale syrup while retaining the protein fraction in the food chain.
Introduction
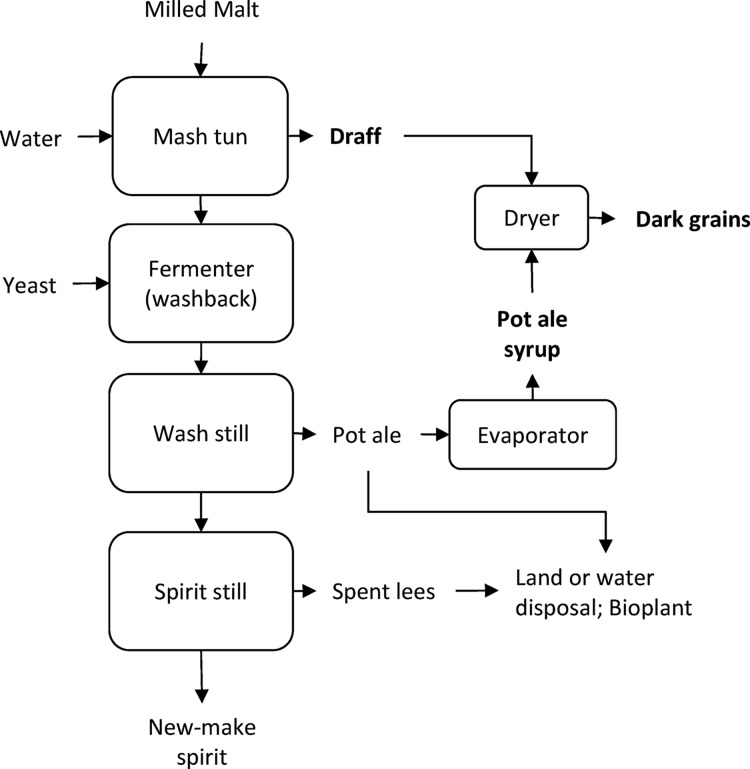
Scotland is famous for its malt whisky, and production is protected by the Scotch Whisky Regulations (2009)1 with malted barley, water, and yeast being the sole ingredients. Production is closely linked to a comprehensive environmental policy with the Scotch Whisky Association’s environmental strategy, updated in 2016, setting ambitious targets governing energy, water, and packaging sustainability.2 Integral to this is the generation and use of co-products. The main co-products from malt whisky are draff, the residual starch-depleted grains remaining after the mashing step, pot ale, the liquid residue from the first distillation step, and spent lees from subsequent distillations. An overview of the malt whisky production process including generation of co-products is illustrated in Figure 1. For every 1 L of alcohol produced, approximately 2.5 kg of draff, 8 L of pot ale, and 10 L of spent lees (and washings) were the resulting yield.3 Scottish distilleries have the capacity to produce over 400 million liters of alcohol per annum,4 which would yield almost 850,000 tons of draff, 2,720,000 tons of pot ale, and 3,400,000 tons of spent lees, assuming 85% production capacity as based on a 10 year average to 2017.5
Figure 1.
Malt whisky production and co-product generation. Co-products marketed as feeds are highlighted in bold.
The applications and disposal route for whisky co-products are diverse. Spent lees, a dilute solution of organic acids and alcohols with a low pH and biological oxygen demand (BOD) of 1500 mg/L,6 is generally treated by a conventional biological effluent treatment. It has significant levels of Cu in the range of 15–25 mg/L,7 and novel methods for Cu removal such as electrocoagulation or use of spent grain as an adsorbent have been suggested.7,8 Draff can be used directly as a feed or dried with pot ale syrup to form distillers dark grains, both of which are primarily used as cattle or sheep feed.9 Distilleries have also invested in combined heat and power plants with pressed draff co-combusted with wood. An example of this is the Rothes CoRDe facility in Speyside, which processes co-products from a number of surrounding distilleries and operates a biomass-fired combined heat and power (CHP) process with an annual input of 130,000 tons of wet draff and 40,000 tons of wood chips.10
Pot ale consists of yeast, yeast and barley residues, soluble protein and carbohydrate, and variable copper levels, and there is no single preferred application. As a feed, pot ale was traditionally fed to pigs with the earliest distilleries closely linked with agriculture6 and can be concentrated by evaporation to pot ale syrup, a nutrient-rich and palatable ruminant feed, listed under number 1.12.16 in the EU catalogue of feed materials.11 Pot ale syrup is typically 42% dry matter, 13.4% protein, and 41 mg/kg copper, according to commercial datasheet for pot ale syrup from Scottish malt whisky distilleries.12 The high copper content restricts its use in sheep, and the difficulties associated with handling and storing the highly viscous syrup means that it is not always an attractive option for farmers. Pot ale syrup currently sells at ∼£60/ton, and coupled with the high-energy demands in evaporation, it has a low return for distilleries (personal communication from distillers and feed merchants). There are 10 facilities now licensed for pot ale syrup feed production, according to the Agricultural Industries Confederation (AIC) trade insurance scheme checker, the official certification scheme for operating feed production facilities in the United Kingdom.13 This includes Tomintoul, Ardmore, Aberlour, Dalmunach, Glenlivet, Blair Athol, Dufftown, and Aberfeldy distilleries along with pot ale syrup production at the Glenlossie Dark Grains Plant, which processes pot ale from a number of Diageo-owned distilleries and Rothes CoRDe Ltd.
The other main uses of pot ale are consented land and sea disposal and treatment in anaerobic digestion (AD). Land spreading is at a cost to the distillery with a paid-for third party disposal. Consented discharge to the sea is permitted in certain circumstances, but this is only an option for coastal distilleries where specific discharge licenses are granted. For example, on Islay, an island off the west coast of Scotland with eight distilleries, pot ale from a number of the distilleries is discharged via Caol Ila distillery, which is located on the shore of the Sound of Isla. There are currently eight AD plants that the authors know of that are located at Scottish malt whisky distilleries, at Bruichladdich, Balmenach, Dailuiane, Glendullan, Roseisle, Glenmorangie, Alisa Bay and Glenfiddich distilleries, with two further distilleries (Dalmore and Tamnavulin) planning to send their pot ale to a new AD plant at the Invergordon grain distillery. With a high chemical oxygen demand (COD) and BOD, reported in the regions of 47 and 25 g/L, respectively,14 energy generation by anaerobic digestion is an attractive option. However, the drive toward energy generation, with CHP for draff and anaerobic digestion for pot ale, has met with resistance from Scottish farmers, with whisky co-products making an important contribution to local farming and economy.9 However, the two options are not necessarily incompatible with protein extraction from pot ale having a positive effect on the AD startup.15
The number of malt whisky distilleries in Scotland and capacity of existing distilleries are expanding with an increase from 234 to 362 MLPA between 2007 and 2017.5 This has led to increased volumes of pot ale and the drive for alternatives to current end routes. The first step in identifying new feed production methods is understanding the composition of pot ale, particularly with regards to the distribution of components between the solid and liquid fractions. There is a lack of consistent data on pot ale composition, and the main information is elucidated from commercial particulars of pot ale syrup products or studies on anaerobic digestion of pot ale. In the latter case, the focus is mainly on water quality parameters from a wastewater treatment point of view. The variation in BOD, COD, pH, volatile acids, and Cu in a distillery14 and impact of solid–liquid separation and pH adjustment on COD, phosphorus, ammonia, Cu, Ca, and Mg removal16,17 have been reported. However, the nutritional benefits are often overlooked, and further insight into this would expand the feed application options of this protein-rich co-product. The continued application of distillery co-products in feed is also important in satisfying the increasing demand for protein for animal and fish feeds. For 2017/2018, the European Union reported that 85 million tons of protein was used as feed of which 15% was sourced from soya bean meals.18 Soya bean meal is mainly imported with only 2% self-sufficiency on a protein basis, corresponding to annual imports of over 12.7 million tons of protein originating from soya bean meal. Similarly, the United Kingdom used 102,000 tons of soya cake and meal in animal feed in 2017.19 The continued use of pot ale in feed has the potential to provide nearly 36,000 tons of protein (estimated based on 33% protein on a dry matter basis and 2.7 million tons of pot ale with 4% dry matter produced per annum). In this study, the pot ale from a single distillery was assessed in terms of nutritive parameters and the implications for use in animal feed are discussed. Pot ale has two distinct fractions—an insoluble solid fraction, which mainly consists of yeast, and a fraction containing soluble carbohydrate and protein. The main aims of this study were to compare the distribution of components between the solid and liquid phases and to examine the protein fraction in more detail with particular focus on extending feed applications beyond the pot ale syrup for cattle and pigs.
Results and Discussion
Pot Ale Composition and Distribution of Components between Yeast and Supernatant Fractions
Pot ale appeared as a brownish liquid and had two distinct layers with an insoluble solid, yeast fraction forming at the bottom of containers with settling as per previous descriptions (Table 122). The pH varied between 3.6 and 4.1, which is in line with a previous report where pH varied between 3.3 and 4.1 and was attributed to the concentration of volatile acids.14 The yeast content was approximately 2.9 × 108 cells/ml, and the total dry matter was 5.1% (Table 1). The concentration of yeast cells is as expected with pitching at 3–4 × 107 cells/ml, typically leading to approximately 2 × 108 cells/ml in the washback at the end of the fermentation,23 and this yeast would be concentrated up to 2-fold in the wash still. The dry matter content is typically between 4 and 4.5%,6 although the yeast settles out quite quickly, so batch variation would be expected depending on sampling procedures. The insoluble dry matter is not solely due to yeast concentration with other insoluble components such as grain particles and bacterial cells mainly from lactobacilli being present, in addition to precipitated protein.
Table 1. Characterization of Pot Ale from a Malt Whisky Distillerya.
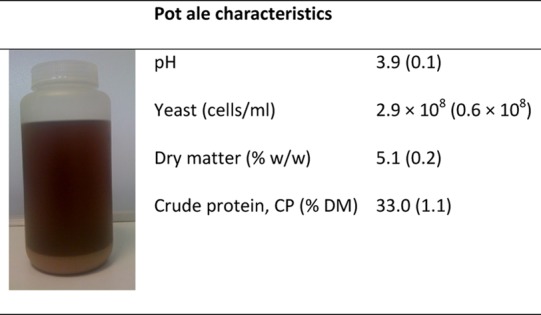
The image on the left is pot ale in a collection container after the yeast and other insoluble solids have settled out. A mean of six independent samples are shown with SEM in brackets.
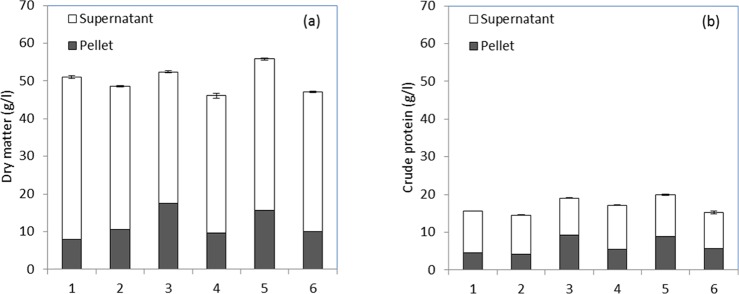
The crude protein content of pot ale varied between 14.6 and 20 g/L with a mean of 33% on a dry matter basis (Figure 2). This is similar to a commercially available pot ale syrup where the protein content of the Spey syrup from the AB Agri group is quoted between 30 and 35%.12 The distribution of dry matter and protein between the insoluble solids (pellet) and soluble (supernatant) fraction of pot ale is shown in Figure 2. The total dry matter varied between 47 and 58 g/L with 35–43 g/L soluble solids in the supernatant and 8–18 g/L as insoluble solids in the pellet fraction. The majority of the protein was in the supernatant fraction compared to the pellet (9.7–11.7 g protein/L compared to 4.5–9.2 g/L), although this was reflected by the variation in total dry matter. If these fractions were separated and dried separately, then this would yield products with 28 and 55% protein in the supernatant and yeast fractions, respectively. Separation of the yeast from a similar co-product stream from wheat bioethanol production has been suggested,24 and the potential of malt distillers yeast as a feed ingredient is explored in the following section.
Figure 2.
(a) Dry matter and (b) the crude protein content of pot ale samples (1–6) collected at different times from a malt whisky distillery and distribution between yeast solid (pellet) and soluble supernatant fractions. The mean of three determinations with SEM for the total sample are shown.
There is little information on the amino acid composition of pot ale and the contribution of yeast and soluble protein. For the commercial pot ale syrup, data for lysine, methionine, histidine, cysteine, and threonine is available with feeding recommended for cows, horses, and pigs but not sheep due to the copper content.12 For the pot ale samples analyzed here, lysine, and threonine were present in lower concentrations (4.1 and 3.1 compared to 6.5 and 5.6% crude protein, respectively), while histidine and methionine were present at a concentration similar to the pot ale syrup (3.5 compared to 3.2% and 0.9 compared to 1.1%) (Table 2). Cysteine was not analyzed in this study. The concentration and variability in amino acid components has implications for use in feed with animals and fish feed having specific minimal requirements for essential amino acids depending on age and species.25,26 For nonruminants, of the 22 amino acids, 12 are synthesized by the animal; the other 10 (arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine) must be provided in the diet for normal growth. The amino acid requirements of animals and fish is specific to species and stage of growth. A comparison of the amino acid profile of pot ale with requirements for Atlantic salmon and pigs is provided in Table 2. Inclusion of all animals and stages of growth are outside the scope of this report, and selected data for grower category of Atlantic salmon26 and mean data for pigs between 5 and 100 kg are provided.27 From Table 2, it is clear that most of the amino acids in the pot ale are present at less than the minimal concentration of requirements for Atlantic salmon and pigs. Arginine, methionine, and phenylalanine was less than required for both salmon and pigs, while isoleucine, leucine, lysine, and threonine were also below the minimal requirement for pigs. This would have implications for marketing the protein in pot ale as a complete animal or fish feed; however, as it only comprises a fraction of the total diet, other amino acids can be supplemented with other feeds, although this would impact on the economic value of pot ale as a feed ingredient.
Table 2. Composition of Pot Ale from Malt Whisky Distillery Compared to Commercial Pot Ale Syrup (Spey Syrup, AB Agri Ltd., Peterborough, UK)a.
| component | pot aleb | Spey syrupc | Atlantic salmond | pigse |
|---|---|---|---|---|
| dry matter (% w/w) | 5.1 (0.2) | 42.0 | ||
| crude protein, CP (% DM) | 33.0 (1.1) | 32.0 | ||
| total P (g/kg) | 13.4 (0.3) | 2.14 | 6.0 | 0.6 |
| Ca (g/kg) | 1.3 (0.1) | 1.5 | 0.9 | |
| Mg (g/kg) | 6.2 (0.1) | 6.0 | 0.5 | 0.04 |
| K (g/kg) | 23.1 (0.5) | 2.2 | 7 | 0.3 |
| Na (g/kg) | 0.7 (0.0) | 1.0 | 0.6 | 0.2 |
| Cu (mg/kg) | 101.4 (45) | 97.4 | 3 | 4.8 |
| Fe (mg/kg) | 37.0 (10.3) | nr | 60 | 77.8 |
| Mn (mg/kg) | 13.6 (2.3) | 35.7 | 15 | 3.0 |
| Zn (mg/kg) | 24.6 (2.8) | 22.6 | 50 | 77.8 |
| alanine | 3.6 (0.2) | |||
| arginine | 2.3 (0.6) | 3.7 | 2.7 | |
| aspartic acid | 6.1 (1.2) | |||
| glutamic acid | 7.3 (0.3) | |||
| glycine | 3.2 (0.0) | |||
| histidine | 3.5 (0.9) | 3.23 | 1.8 | 2.1 |
| isoleucine | 2.8 (0.4) | 1.8 | 3.2 | |
| leucine | 4.2 (0.6) | 3.2 | 6.1 | |
| lysine | 4.1 (0.6) | 6.47 | 4.1 | 6.0 |
| methionine | 0.9 (0.1) | 1.06 | 2.3 | 1.7 |
| phenylalanine | 2.4 (0.4) | 2.8 | 3.6 | |
| proline | 7.3 (0.9) | |||
| serine | 3.1 (0.4) | |||
| threonine | 3.1 (0.6) | 5.61 | 0.5 | 3.9 |
| tyrosine | 1.5 (0.5) | |||
| tryptophan | 0.5 | 1.0 | ||
| valine | 5 (0.3) | 3.0 | 3.9 |
Data is expressed on a dry matter basis with amino acid concentrations expressed as the percentage of crude protein. The protein amino acid and mineral requirements of atlantic salmon and pigs are shown for comparison.
Pot ale data calculated as mean of six different samples from a single distillery with SEM shown in brackets. For P, Na, Mg, K, and Na, the analysis mean of the three samples are reported, and for the amino acid analysis, two different samples were analyzed.
The data for pot ale syrup was based on a commercial information for Spey syrup available from Trident, AB Agri Ltd.12 Mineral concentrations were recalculated to be expressed on a dry matter basis.
Data for grower Atlantic salmon from aquaculture feed and fertilizer resources information system.26
Data for pigs was calculated as a mean of data for categories between 5 and 100 kg of body weight.27
The concentration of minerals in pot ale has implications for its use either in feed or for disposal. For the macroelements, minimum feed requirements are often identified but upper levels are not necessarily set, whereas for trace elements, maximum allowable concentrations are controlled by regulations such as EC no. 1334/2003.28 The recommended levels of macroelements depend on species, stage of growth, and level of other dietary minerals. In terms of disposal, discharge of particular elements in to waterways or on land is governed by environmental regulations. Also, particular minerals can impact on process equipment; for example, calcium oxalate, calcium phosphate, and magnesium salts cause fouling of evaporators used for pot ale syrup production.29 Here, Ca, Mg, K, Na, and P were measured at mean values of 1.3, 6.2, 23.1, 0.7, and 13.4 g/kg dry matter, respectively, and the microelements at 101.4, 37.0, 13.6, and 24.6 mg/kg dry matter for Cu, Fe, Mn, and Zn, respectively (Table 2).
The concentrations of Ca, Mg, and Na were within the same range as that reported for Spey syrup (Table 2). However, P and K were 5 and 10 times that of Spey syrup with averages of 13.4 and 23.1 g/kg for P and K, respectively. Concentrations reported here are more in line with that of thin stillage (or distillers solubles) from corn bioethanol plants with 12.9 g/kg P and 17.6 g/kg K reported.30 The total P is similar to that previously reported for pot ale (0.7 g/L total P with 0.5 g/L as free P in the soluble fraction).16 The high P concentration is of particular concern, with excess P in the diet being excreted with subsequent pollution issues and accumulation in the environment, and there is also the wider question of P sustainability with future shortages as highlighted by the European sustainable phosphorus platform.31 For example, fish farming impacts phosphorus dynamics in lake sediments,32 one of the research areas of the European sustainable phosphorus platform is inefficient P cycling, and the environmental challenges of excess P in manure and potential to recover P from sewage or manure are well recognized.33
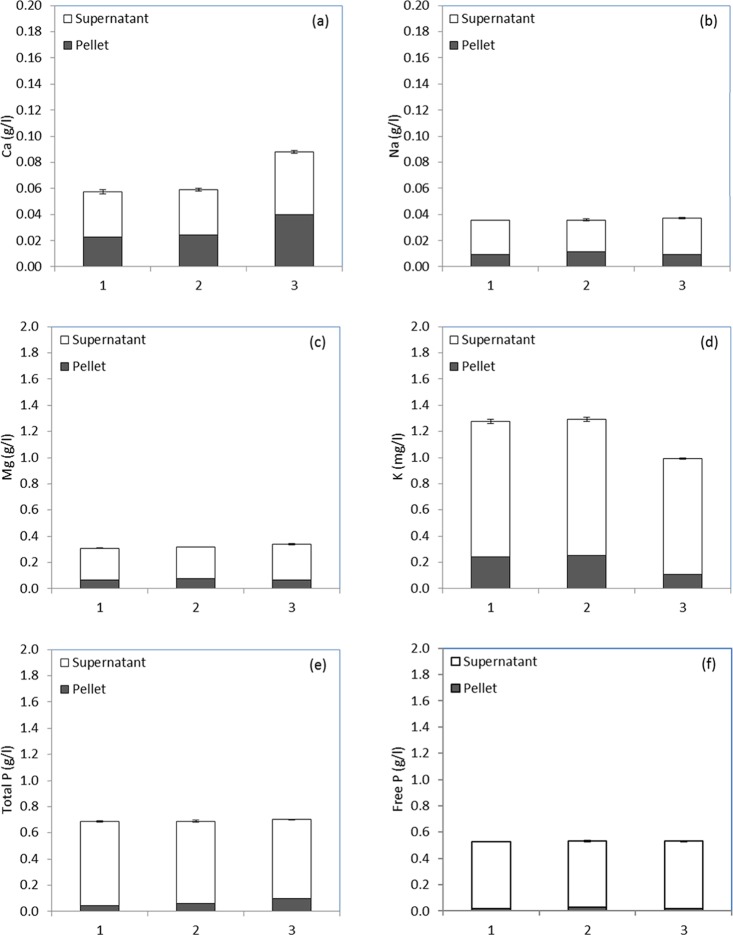
If new feed applications for pot ale are to be developed, then it is important to identify whether any process can be used to reduce the concentration of deleterious minerals in the final feed and to assess whether these elements are concentrated in the solid or liquid fractions. The distribution of Ca, K, Mg, Na, and free and total P between insoluble yeast and soluble, supernatant pot ale fractions were compared (Figure 3). There was very little variation in the P content between three distillery samples, and over 90% of it was associated with the supernatant fraction (Figure 3). This has implications for the use of this supernatant fraction as a feed component as, on a dry weight basis, the total P is 16.3 g/kg with 13.1 g/kg as free P. This makes the yeast fraction more attractive for feed applications. For the supernatant fraction, a process to reduce the P content may be required to extend feed applications and also to recover P as a potential product. Extraction of P from the supernatant has been demonstrated with a process using pH balancing to separate the Ca, P, N, and Mg as a solid precipitate with potential use as fertilizer16,17 and may offer an alternative to pot ale feed applications.
Figure 3.
(a) Ca, (b) Na, (c) M, (d) K, (e) total, and (f) free P of pot ale samples (1–3) collected at different times from a malt whisky distillery and distribution between yeast solid (pellet) and soluble supernatant fractions. The mean of three determinations with SEM for the total sample are shown. The total and supernatant fractions were analyzed with the pellet fraction estimated by difference.
Calcium, at 0.07 g/L, is similar to that of pot ale syrup, which has 1.5 g/kg on a dry matter basis corresponding to 0.06 g/L, assuming pot ale with 4.2% dry matter for the commercially available Spey syrup. This is sufficient for pigs, while for fish, Ca is often absorbed directly from the environment and minimum feed levels are not set.34 However, the high P compared to Ca may cause a Ca:P ratio imbalance in pot ale for feed applications, although most research is focused on the impact of high Ca on P bioavailability.35
Potassium was much higher at 1.19 g/L compared to 0.09 g/L quoted for pot ale syrup. Over 80% of this K was associated with the supernatant fraction, with 26 g/kg on a dry matter basis for this fraction. High levels of K have been implicated in negative impacts of increasing concentrations of corn steep liquor in liquid diets fed to pigs.36 In this case, K would be an issue for the supernatant fraction if used as a feed and would be expected to face the same issues as the corn steep liquor, which can be regarded as a similar by-product stream from corn-based distillery processes.
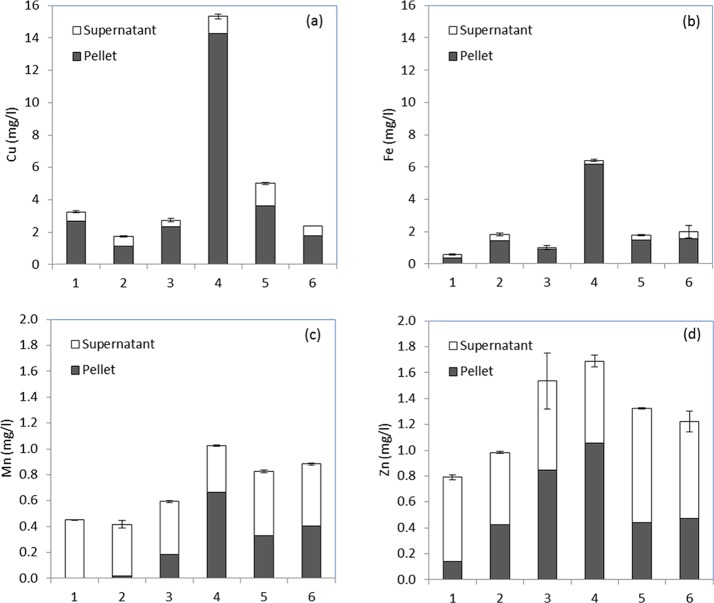
In terms of trace elements, Cu is the most problematic in the use of pot ale syrup in animal feed. The maximum permitted levels of trace elements in feeds is controlled by EC regulation 2831/2003 on feed additives with the maximum content of Cu, as mg/kg of complete feedingstuff (with 88% dry matter) listed in regulation EC no. 1334/2003 and amended in 2112/2003.28,37 Maximum Cu levels in complete feedingstuff for pigs is 25 mg/kg, 35 mg/kg for bovine (15 mg/kg before the start of rumination), 15 mg/kg for ovine, 50 mg/kg for crustaceans, and 25 mg/kg for fish and other species. In the pot ale tested here, the Cu content was quite variable, 1.7–15.3 mg/L, corresponding to an average of 101 mg/kg pot ale. This is similar to previous reports where the Cu content of pot ale varied between 2 and 6 mg/L over an 8 week period.14 Cu in pot ale originates from the distinctive pot still, which is composed of copper and has an essential role to play in whisky aroma.38 The Cu content in feed is controlled particularly for sheep where feeds must include the following label when copper exceeds 10 mg/kg: “the level of copper in this feedingstuff may cause poisoning in certain breeds of sheep”.28 The copper level in pot ale on a dry matter basis exceeds the maximum allowable level for all animals if used as a complete feedingstuff, and the variability in the content is of concern when predicting how much to mix with rations. With this in mind, separation processes that can reduce the Cu content of the feed fraction would be particularly desirable. Cu was predominantly associated with the yeast fraction. With approximately 80% of the Cu, this fraction contained 410 mg/kg, whereas the supernatant fraction contained 20 mg/kg on a dry matter basis. In all cases, the content in the yeast fraction was higher than recommended feed levels, and this would impact on the proportion of yeast that could be incorporated into feed. Separation of the yeast fraction automatically removes Cu from the supernatant fraction, and this may open up new applications for the use of the supernatant fraction. Binding of Cu to the yeast cell wall is expected with the action of yeast as a metal biosorbent being well known.39
The concentrations of Fe and Mn were less than the maximum allowable levels in feed according to EU regulations.28,37 Fe ranged from 0.6 to 3.9 mg/L (11–83 mg/kg on a dry matter basis) and was mainly associated with the yeast fraction with an average of 81% located in this phase. The concentration of Fe in pot ale was well below the maximum allowable level for feed, which ranges from the lowest level of 500 mg/kg for ovine to the highest level of 1250 mg/kg for pet animals.37 Mn ranged from 0.4 to 1.0 mg/L (7.7–21.6 mg/kg on a dry matter basis) in the total pot ale, which is less than the maximum allowable level of 100 mg/kg for fish and 150 mg/kg for other species.
The EU has recently reduced Zn levels in feeds for farmed animals (EU regulation no. 2016/1095) due to concerns about elevated Zn in drainage systems and surface water.40 This regulation sets the upper limit for a complete feed at 180 mg of Zn per kg of complete feed for salmonids and calf milk replacers; 150 mg/kg for piglets, sows, rabbits, and other fish; and 120 mg/kg for other species and classes of animals. On a dry matter basis, the Zn content ranged from 15.7 to 35.6 mg/kg in the pot ale, which is less than the maximum allowable levels. For the supernatant fraction, the Zn concentration was less than the regulated levels with an average value of 18.2 mg/kg. The Zn content of the yeast fraction was higher and ranged from 18.0 to 108.8 mg/kg, although this is still less than the maximum allowable level.
One of the main issues with the use of distillation residues for feed is the inherent variation in the composition.41 One way to enhance the quality and composition of pot ale-based feed products would be to separate the feed-valuable components. Separation of the yeast would address the variability in yeast concentration and the greater value, and expanded applications may result from using individual ingredients rather than concentrating all the components as seen with products such as pot ale syrup. Separation of yeast as a product from bioethanol distilleries has been suggested with the yeast tested as a feed ingredient for nonruminants.24,42,43 However, such processes will only truly be economically viable and sustainable if all fractions are valorized and have an end route. From the data in Figures 2 to 4, the composition of insoluble solid and liquid fractions from pot ale was calculated and potential applications assessed.
Figure 4.
(a) Cu, (b) Fe, (c) Mn, and (d) Zn content of pot ale samples (1–6) collected at different times from a malt whisky distillery and distribution between yeast solid (pellet) and soluble supernatant fractions. The mean of three determinations with SEM for the total sample is shown. The total and supernatant fractions were analyzed with the pellet fraction estimated by difference.
Characterization of the Insoluble Solids and Supernatant Fractions and Potential Applications
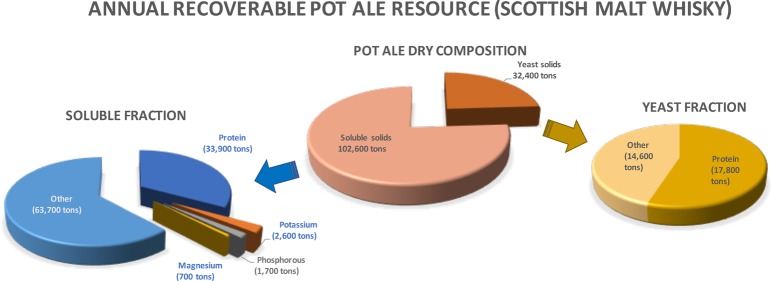
On average, 1.2% of pot ale was insoluble solids on a weight volume basis. This fraction mainly consists of yeast and yeast debris and also contains some lactobacilli and cereal residues, including proteins precipitated during distillation. The yeast fraction contained 55% protein on a dry matter basis. Based on Scottish malt whisky distilleries having the capacity to produce 2.7 million tons of pot ale per annum, this would yield 32,400 tons of dried yeast (corresponding to 17,820 tons of protein) per annum. Scottish malt whisky distilleries do not currently separate the yeast fraction from pot ale for utilization as a feed ingredient. There is the potential to separate this fraction as a slurry and use as is, for example, as a pig feed as per brewer’s yeast44 or dried and used as a component in other feeds.
To assess the value of pot ale yeast, the amino acid profile of the pellet fraction was analyzed and additional composition was estimated based on the data in Figures 2 to 4 and Table 1, and compared to that of dried brewer’s yeast, a yeast protein concentrate from a wheat-based bioethanol plant and a single-cell protein product, Feedkind, from Calysta, Inc. (Table 3). For pot ale yeast amino acid analysis, two batches were analyzed. Overall, the pot ale yeast had a similar profile to the brewer’s yeast. Surprisingly, it had a lower protein concentration compared to the bioethanol yeast (55% compared to 67.6%, respectively), although this may be related to the fact that, in this report, the pellet fraction was washed with water before analysis and would have only reflected the protein in the yeast and other centrifuged solids in pot ale. The concentration of essential amino acids in pot ale yeast, apart from methionine, was in excess of that required for salmon and pig feed, as indicated in Table 1. In particular, lysine concentration, which is regarded as one of the limiting amino acids in plant-based proteins, was present at similar concentrations to the brewer’s dried yeast and soya bean meal at 6.4% and well in excess of lysine concentration of YPC and bacterial protein meal. Cu was concentrated in the pot ale yeast fraction with 107–340 mg/kg, which is over 10 times that allowed in animal feed, including fish.28 Based on this, inclusion of pot ale yeast in feeds would be restricted to a maximum of 10% of the feed ration. The Zn content of the pot ale yeast varied between 18 and 109 mg/kg. A higher Zn content may be attractive for feed applications with the use of Zn supplementation in EU feeds by addition of salts such as zinc oxide being recently restricted. In this case, being able to add a feed ingredient with an elevated Zn content, which is still below the recommended levels, would be attractive. Supplementation of pig diets with zinc and copper has been suggested as an alternative to antibiotics.45 Similarly, copper supplementation also plays an important role in egg-laying hens and may contribute to lower egg yolk cholesterol.46 It may be that the pot ale yeast could have additional benefits, although the bioavailability of the mineral components would need to be confirmed.
Table 3. Characterization of Yeast from Pot Ale from a Malt Whisky Distillery and Comparison to Brewer’s Dried Yeast,44 Yeast Protein Concentrate (YPC), that is, Bioethanol Yeast,58 Bacterial Protein Meal,59 and Soya Bean Meal60.
| component | pot ale yeasta | Brewer’s dried yeast | YPC (bioethanol yeast)b | bacterial protein meal | soya bean meald |
|---|---|---|---|---|---|
| crude protein (%) | 55 | 48.9 | 67.6 | 69.2 | 53.5 |
| amino acids (% CP) | |||||
| alanine | 4.7 (0.1) | 5.9 | 3.4 | 4.3 | |
| arginine | 4.3 (0.3) | 4.4 | 4.1 | 6.2 | 7.3 |
| aspartic acid | 8.5 (0.4) | 9.0 | 4.7 | 8.5 | 11.4 |
| glutamic acid | 10.5 (1.0) | 14.7 | 28.6c | 10.3 | 17.9 |
| glycine | 3.3 (0.1) | 4.0 | 3.2 | 4.9 | 4.2 |
| histidine | 2.7 (0.0) | 2.0 | 2.3 | 2.3 | 2.7 |
| isoleucine | 4.5 (0.3) | 4.6 | 4.0 | 4.5 | 4.6 |
| leucine | 7.1 (0.5) | 6.2 | 7.4 | 7.5 | 7.7 |
| lysine | 6.4 (0.2) | 6.3 | 2.7 | 5.8 | 6.3 |
| methionine | 1.6 (0.1) | 1.5 | 1.5 | 2.7 | 1.4 |
| phenylalanine | 4.5 (0.1) | 3.6 | 5.0 | 4.2 | 5.1 |
| proline | 3.4 (0.5) | 3.4 | 9.6 | 4.0 | 5.0 |
| serine | 4.7 (0.5) | 4.3 | 4.0 | 3.6 | 4.6 |
| threonine | 4.6 (0.4) | 4.4 | 2.9 | 4.4 | 3.8 |
| tyrosine | 2.7 (0.1) | 2.7 | 3.0 | 3.6 | 3.5 |
| valine | 5.1 (0.3) | 4.9 | 4.9 | 5.8 | 4.8 |
| macroelement (g/kg) | |||||
| Ca | 2.3–2.9 | 2.9 | 3.6 | ||
| K | 6.3–30.7 | nr | 25.0 | ||
| Mg | 3.8–8.4 | 2.4 | 3.4 | ||
| Na | 0.5–1.2 | 1.8 | 0.1 | ||
| P | 5.5–7.2 | 13.1 | 7.6 | ||
| microelements (mg/kg) | |||||
| Cu | 107–340 | 23 | 18 | ||
| Fe | 44–635 | 78 | 169 | ||
| Mn | 0–69 | 34 | 40 | ||
| Zn | 18–109 | 114 | 57 |
For amino acid analysis, two different pellet fractions were analyzed (mean data ± SD shown); for the other components, characterization is based on the data in Figures 2 to 4 and Table 1.
Amino acid concentration assumed to be mg/kg feed and calculated as % CP.
Reported as glutamate.
Data for high protein, dehulled soya bean meal.
One issue with the use of yeast as a feed product is the variation in amino acid profile with components varying with strains, growth conditions, and additional process steps. It would be expected that the pot ale yeast would be suitable as a feed ingredient similar to brewer’s yeast, which is used for pigs, ruminant, poultry, and fish.44 However, there are a number of inherent differences that may be advantageous to the use of the distillery yeast. Scottish malt whisky distilleries typically use four types of Saccharomyces cerevisae—distillers M and MX yeast, Pinnacle Yeast, and DistilaMax yeast.47 In contrast to breweries where different yeast strains may be used depending on beer style, a distillery will tend to utilize the same yeast strain and propagation strategy, so variation within a distillery would be reduced. Also, compared to brewer’s yeast, the pot ale yeast would not have the bittering effect, originating from hops, which causes issues with palatability.48 Another difference is that brewer’s yeast is still viable and needs to be inactivated, either by heating or acid treatment, whereas distiller’s yeast is already inactivated after distillation. However, the effect of distillation on yeast integrity and quality is unknown, although based on the research on bioethanol yeast,24,42 there is the potential to use yeast from pot ale from malt whisky as feed ingredients.
If yeast is separated from pot ale as a feed ingredient, then new end routes for the resulting supernatant fraction also need to be realized. On average, 3.8% of the pot ale was solids in the supernatant fraction and it contained 28% protein on a dry matter basis. For the pot ale supernatant analyzed here, it had particularly high concentrations of Mg (6.6 g/kg), P (16.2 g/kg), and K (25.5 g/kg). With high COD (values between 46 and 54 g for centrifuged pot ale from different distilleries),15 it is suitable for anaerobic digestion, although the high phosphorus concentration in particular can be problematic, remaining in the treated water after digestion and requiring an additional treatment step.49 The insoluble yeast fraction is often seen as deleterious to anaerobic digestion, with yeast settling out at the bottom of reactors,22 and enzymatic pretreatment is suggested for lysing cells to improve AD.50 Separation of the yeast fraction as a feed ingredient may therefore improve anaerobic digestion or even open up new application routes for the soluble pot ale fraction.
As discussed previously, the elevated P and K levels may impede the application of this fraction as a feed ingredient. In addition, the majority of the supernatant fraction is carbohydrates and it can be assumed that it is a complex mixture of nonfermentative dextrins remaining after yeast fermentation in addition to solubilized fiber components (originating from cellulose, hemicellulose, and lignin), organic acids such as acetic and lactic acid, polyphenols, and glycerol. For the pot ale assessed in this report, the carbohydrate fraction was not characterized. However, this fraction would be deleterious for new feed applications such as the use in aquaculture, with carbohydrate levels minimized for salmon feed in particular with a maximum level of 10–12% carbohydrate and 2–3% max fiber.26 The use of the supernatant fraction as a fermentation medium for ethanol production by Kluyveromyces marxianus has been suggested, although without prior concentration or addition of other components, yields are low, impacting on the potential to commercialize such a process.51,52
Additional value may be added by separating individual components. For example, research has focused on a pot ale supernatant as a source of polyphenols as assessed under the EU-sponsored project “Process for Upgrade and recovery of polyphenol extracts”(PUReOPE) and also on separation of the nitrogen, phosphorus, and magnesium as fertilizer with evaporation of the remaining fraction to produce a concentrate for anaerobic digestion.17 In addition, the protein component of this fraction may also be of value. The pot ale supernatant contains approximately 33% protein on a dry matter basis, and based on 2.7 million tons of pot ale per annum of which 3.8% is the supernatant fraction, this would indicate that this fraction from malt whisky distilleries in Scotland could yield approximately 34,000 tons of protein per annum. The authors have developed a process to separate this protein from pot ale,53 and if linked with other valorization steps, then it could be applied as a biorefinery process for whisky co-products. In addition, there is considered to be an urgent need for diverse sources of protein as a raw material for bioplastic production54 to further reduce reliance on fossil fuel-based materials.
In addition to the application of protein from the supernatant fraction as a protein feed ingredient, it may also be assumed that the proteins are similar to those present in beer, which are involved in foam formation and stability55 and therefore may have interesting characteristics. The soluble proteins in pot ale were assessed by SDS-PAGE with pot ale either analyzed directly or after concentration using 3 or 10 K NMWL tubes (Figure 5). For the direct pot ale sample, a band at 12–14 kDa was apparent. This band was also present in retentate samples with an additional band at 38–42 kDa visible for all dilutions. The two bands are within the size range of LTP1 and protein Z as reported for beer samples.56 It should be highlighted, however, that the molecular weights reported here are only approximations as a prestained protein marker was used. In addition to these two bands, two other protein bands were also visible at the higher protein loading levels; one at 72–80 kDa and the other with a molecular weight higher than the largest Mw marker (175 kDa) were visible at higher protein loading (lanes 2 and 7). Proteins with a Mw higher than protein Z have not been routinely reported in beer. This is mainly due to the fact that large proteins are often precipitated and removed during wort preparation. It is also possible that these are of yeast origin and may correspond to one of the proteins detected in malt beer.57 It is difficult to conclude whether hordein-derived bands, as reported for beer between 15 and 32 kDa,56 are absent in pot ale. The high concentration of the low Mw protein resulted in quite a dark-stained broad band on the gel, and this may have masked other proteins. It may be that the additional value can be exploited from pot ale proteins if individual proteins such as LTP1 and protein Z can be separated and tested for potential application as functional proteins in food or feed.
Figure 5.
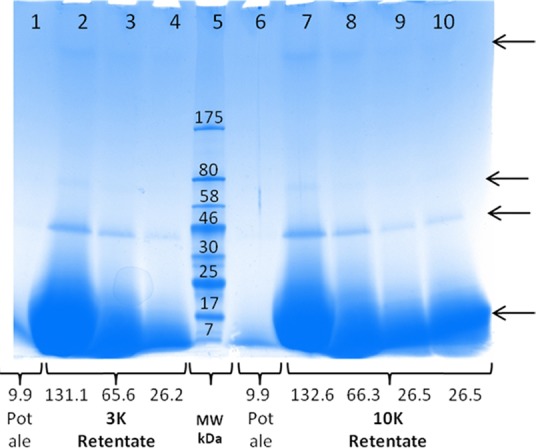
SDS-PAGE of pot ale proteins separated on a 4–20% Tris-glycine gel. Samples were either run directly without treatment (lanes 1 and 6) or after concentration using either 3 (lanes 2, 3, and 4) or 10 K (lanes 7, 8, 9, and 10) nominal molecular weight tubes. The protein concentration (μl) in each sample is shown, and a broad range prestained molecular weight ladder was in lane 5. The arrows indicate the main bands that were visible on the gel.
Overall, there are a number of components in pot ale that can be valorized. Overall, approximately 2.7 million tons of pot ale is produced per annum by the malt whisky industry in Scotland. If the insoluble solids and supernatant fractions are separated, then this has the potential to yield 32,400 tons of dry yeast containing 17,820 tons of protein as a product stream. If protein is recovered from the supernatant fraction (102,600 tons of total dry matter), then 33,858 tons of protein could be obtained for food or feed, while minerals such as 2616 tons of K, 1662 tons of P, and 677 tons of Mg can also be reutilized and the remaining stream is concentrated or used as is in biological processes. Any system developed for pot ale valorization may also be applicable to other similar co-product streams. In particular, they may be applied to liquid fraction from other whiskey distilleries and grain bioethanol plants where thin stillage/solubles is one of the main products and typically concentrated by evaporation and added to spent grains to form dried distillers grains with solubles (DDGS). With approximately 40% of the energy use of a bioethanol plant used in producing DDGS,58 processes that reduce the energy requirements of DDGS production or add value to the co-products are required to ensure the continued sustainability of co-product markets.3
Materials and Methods
Sample Collection and Storage
Pot ale was collected from a Scottish lowland malt whisky distillery on six different occasions over a 3 year period (2012–2015). In Scotland, two-row Spring malting barley is the only cereal used for malt whisky production, and in this case, the pot ale samples would have been sourced from three different harvests. Samples were refrigerated and analyzed within 1 week or stored at −20 °C. For analysis, either the total sample was analyzed or it was separated into a supernatant and pellet fractions by centrifuging at 4000 rpm for 5 min (Heraeus Multifuge 3SR). For nitrogen and amino acid analyses, samples were freeze-dried and stored in a dessicator. Both total aliquots and centrifuged solids were freeze-dried (Edwards Super Modulyo Freeze Dryer) to less than 15% moisture. It was not possible to freeze dry the supernatant fractions as these remained quite viscous and syrup-like, and in this case, where freeze drying was required for analysis, only the total and centrifuged solids were analyzed and the supernatant composition was determined as the difference between these two samples. In all cases, results were reported as a mean of three independent analyses unless otherwise stated.
Dry Matter, pH, and Yeast Content
The dry matter content (total solids) of pot ale samples was determined by drying preweighed samples in an oven at 105 °C for 24 h and expressing the dry weight on a % (w/w) basis. The solid content of the centrifuged solids and supernatant was also determined and expressed as % (w/w) of the original pot ale. Where the concentration refers to % (w/w) of the individual fraction, that is, solids or supernatant fraction, this is specified. The concentration of intact yeast cells in pot ale was determined by direct counting using a hemocytometer following suitable dilution in water. In all samples, rod-shaped bacteria in long chains, assumed to be lactobacilli, were visible, but these were not quantified. The pH was analyzed directly using a pH meter (Hanna Instruments, pH 201 microprocessor pH meter).
Micro- and Macroelement Characterization
Flame atomic absorption spectroscopy (AAS) (PerkinElmer AAnalyst 200) was used to determine the concentration of Ca, Na, Mg, K, Cu, Fe, Mn, and Zn. Total pot ale samples were digested with nitric acid, whereas supernatants were acidified with 200 μL of 69% nitric acid added to 10 mL of samples prior to analysis. For nitric acid digestion, 0.4 mL of 69% HNO3 was added to 0.6 mL of pot ale in a boiling tube and heated on a heating block at 105 °C for 1 h. After cooling, 6 mL of distilled deionized water was added. Samples that were diluted with water were required. For Ca and Mg analysis, La was added to 1% (from a 50 g/L La stock solution prepared using La2O3) and Cs was added to 0.1% (from a 10 g/L Cs stock solution prepared using CsCl) for K and Na analysis. Metal concentrations were determined with reference to the corresponding standards, prepared by diluting commercial AAS standard stock solutions available from Sigma-Aldrich (1000 ppm in all cases apart from 10 g/L Na; TraceCERT AAS, Sigma-Aldrich Co. LLC., Dorset, England). The metal concentrations of the pellet fraction were calculated as the difference between the supernatant and total fractions.
Phosphorus (Total and Available) Analysis
Free and total phosphorus were analyzed using the Megazyme phytic acid (Phytate)/Total phosphorus kit (K-PHYT, Megazyme, Co. Wicklow, Ireland). The total phosphorus was measured as phosphorus released by phytase and alkaline phosphatase with free phosphorus determined before enzyme treatment. Total and supernatant fractions of pot ale were analyzed, according to the instructions provided with the kit with pellet determined by difference. Samples were extracted prior to analysis by mixing 10 mL of total pot ale or supernatant samples with 33 mL of 1 M HCl and 7 mL of distilled deionized water in a 100 mL duran bottle and incubating on a shaker overnight. After extraction, 1 mL of the extract was added to a 1.5 mL of a microcentrifuge tube and centrifuged at 13,000 rpm for 10 min. Then, 0.5 mL of the supernatant was added to a microcentrifuge tube and neutralized by addition of 0.5 mL of 0.75 M NaOH. The enzymatic dephosphorylation reaction was followed as per the Megazyme standard assay procedure according to the instructions and reagents provided with the kit. Subsequently, the phosphorus content of free and total samples were analyzed using the colorimetric method outlined in the kit with reference to a phosphorus calibration curve prepared at the same time.
Protein and Amino Acid Analysis
Crude protein of freeze-dried samples (total and pellet, with supernatant determined by difference) was determined by combustion using an Exeter CE-440 elemental analyzer (Exeter Analytical UK Ltd., Coventry, UK). Crude protein was calculated as N × 6.25.
Total amino acid analysis of freeze-dried samples (total and pellet, with supernatant determined by difference) was conducted by acid hydrolysis followed by ion exchange chromatography with ninhydrin detection and was conducted by AltaBioscience Ltd., Redditch, UK. For this method, asparagine and glutamine are converted to aspartic and glutamic acid, and tryptophan and cysteine need to be determined separately as they are destroyed during the acid hydrolysis step. The content of specific amino acids was reported as the percentage of the crude protein.
SDS-PAGE Electrophoresis
Pot ale from a single batch was centrifuged at 4000 rpm for 5 min (Heraeus Multifuge 3SR), and the protein concentration of the supernatant was determined by Bradford Assay.20 The supernatant was either analyzed directly by SDS-PAGE or concentrated using Amicon Ultra-15 Centrifugal filter tubes (3 and 10 K devices, UFC900308 and UFC901008, Merck Millipore Ltd. Cork, Ireland) where 15 mL of the supernatant was added to the filter cup of either 3 or 10 K tubes and centrifuged at 4000g and 4 °C. After 3 h, the volume of retentate (the fraction retained in the filter cup by the ultrafiltration membrane) was reduced by over 90%. The concentrated protein sample in the retentate was further dialyzed by adding 15 mL of water to the filter cup and centrifuging at 4000g for 3 h at 4 °C. The retentate was then transferred to an eppendorf tube, the filter cup was washed with 200 μL of water, and this wash fraction was combined with the retentate. The protein concentration was determined before diluting samples with distilled water and analyzing by SDS-PAGE.
For SDS-PAGE analysis, samples were mixed with equal volumes of the Laemmli sample buffer (Sigma-Aldrich Ltd., Dorset UK) heated at 90 °C for 5 min, cooled on ice, and centrifuged prior to loading on to 4–20% precast polyacrylamide gels (Bio-Rad Mini-PROTEAN Tris-glycine (TGX) precast gels, Bio-Rad Laboratories, Herts, UK). Gels were run using a Bio-Rad Mini-PROTEAN Tetra cell system for mini precast gels with Tris/glycine/SDS running buffer (10× Tris/glycine/SDS running buffer, Bio-Rad Laboratories). A prestained, broad range (7−175 KDa) protein marker (P7708 New England Biolabs Ltd. Hertz., UK) were run with all gels to estimate the protein molecular weight. Electrophoresis was at 180 V for approximately 40 min and stopped when the dye front reached the bottom of the gel. After electrophoresis, gels were rinsed with water and stained overnight with a Colloidal Coomassie Blue stain21 consisting of 5% (w/v) aluminium sulphate hydrate, 10% (v/v) ethanol, 0.02% (w/v) Coomassie Brilliant Blue G-250, and 8% (v/v) orthophosphoric acid. Gels were rinsed and destained (10% ethanol and 2% phosphoric acid) for 1–2 h and rinsed with water until the background stain was removed. The SDS-PAGE gels were scanned with a Bio-Rad GelDoc EZ imaging system (Bio-Rad Laboratories), and images were analyzed using the Bio-Rad’s Image Lab software program to estimate the molecular weight of protein bands.
The authors gratefully acknowledge the financial support of the Scottish Funding Council and Scottish Enterprise during the course of this work.
The authors declare no competing financial interest.
References
- The Scotch Whisky Regulations, 2009. URL (http://www.legislation.gov.uk/uksi/2009/2890/made) (20/11/2019)
- Scotch Whisky Association , 2017. Environmental strategy. URL (https://www.scotch-whisky.org.uk/insights/sustainability/environmental-strategy/) (20/11/2019)
- White J. S.; Traub J. E.; Maskell D. L.; Hughes P. S.; Harper A. J.; Willoughby N. A.. Recovery and applications of proteins from distillery by-products. In Protein By products; Dhillon G.S., Ed.; Academic Press: 2016; pp 235–253. [Google Scholar]
- Ronde I.Malt whisky yearbook 2019; MagDig Media Ltd: Shropshire, UK, 2019. [Google Scholar]
- Gray A. S.The Scotch whisky industry review; Pagoda: Aberdeen, UK, 2017. [Google Scholar]
- Pass B.; Lambert I.. Coproducts. In Handbook of Alcoholic Beverages Series. Whisky: Technology, Production and Marketing; Russell I. Ed.; Academic Press: San Diego, CA, 2003. [Google Scholar]
- Lu S.; Gibb S. W. Copper removal from wastewater using spent-grain as biosorbent. Bioresour. Technol. 2008, 99, 1509–1517. 10.1016/j.biortech.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Jack F.; Bostock J.; Tito D.; Harrison B.; Brosnan J. Electrocoagulation for the removal of copper from distillery waste streams. J. Inst. Brew. 2014, 120, 60–64. 10.1002/jib.112. [DOI] [Google Scholar]
- Bell J.; Morgan C.; Dick G.; Reid G.. Distillery feed by-products briefing. An AA211 Special Economic Study for the Scottish Government; SAC Consulting, 2012. URL (http://www.sruc.ac.uk/download/downloads/id/1057/distillery_feed_by-products_briefing) (20/11/2019)
- Sherrard A. Future proofing the ‘spirit of Speyside’. Bioethanol Int. 2014, 72, 10–13. [Google Scholar]
- Feed Materials Register URL (http://www.feedmaterialsregister.eu) (20/11/2019)
- AB Agri Group , UK. Spey syrup URL (https://www.tridentfeeds.co.uk/products/spey-syrup/) (20/11/2019)
- Agricultural Industries Confederation, UK. URL (https://www.aictradeassurance.org.uk/home/) (20/11/2019)
- Graham J.; Peter B.; Walker G. M.; Wardlaw A.; Campbell E.. Characterisation of the pot ale profile from a malt whisky distillery. In Distilled spirits: science and sustainability; Fotheringham R., Goodall I., Murray D. Eds.; Nottingham University Press/The Institute of Brewing and Distilling: 2012. [Google Scholar]
- Barrena R.; Traub J. E.; Gil C. R.; Goodwin J. A.; Harper A. J.; Willoughby N. A.; Sánchez A.; Aspray T. J. Batch anaerobic digestion of deproteinated malt whisky pot ale using different source inocula. Waste Manag. 2018, 71, 675–682. 10.1016/j.wasman.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Dionisi D.; Bruce S. S.; Barraclough M. J. Effect of pH adjustment, solid–liquid separation and chitosan adsorption on pollutants’ removal from pot ale wastewaters. J. Environ. Chem. Eng. 2014, 2, 1929–1936. 10.1016/j.jece.2014.08.013. [DOI] [Google Scholar]
- Uzukwu C. C.; Barraclough M. J.; Dionisi D. Experimental investigation of a new process for treatment and valorisation of pot ale wastewaters. Water Sci. Technol. 2017, 75, 1194–1203. 10.2166/wst.2016.614. [DOI] [PubMed] [Google Scholar]
- EU Crops Market Observatory ; 2019. EU Feed Protein Balance Sheet. URL (https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/farming/documents/eu-feed-protein-balance-sheet_2017-18_en.pdf) (20/11/2019)
- DEFRA , 2018. GB animal feed, – final dataset 08/02/2018. URL (https://www.gov.uk/government/statistics/animal-feed-production) (20/11/2019)
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Pink M.; Verma N.; Rettenmeier A. W.; Schmitz-Spanke S. CBB staining protocol with higher sensitivity and mass spectrometric compatibility. Electrophoresis 2010, 31, 593–598. 10.1002/elps.200900481. [DOI] [PubMed] [Google Scholar]
- Goodwin J. A. S.; Stuart J. B. Anaerobic digestion of malt whisky distillery pot ale using upflow anaerobic sludge blanket reactors. Bioresour. Technol. 1994, 49, 75–81. 10.1016/0960-8524(94)90175-9. [DOI] [Google Scholar]
- Russell I.; Stewart G.. Distilling yeast and fermentation. In Whisky; Russell I., Bamforth C., Stewart G., Eds.; Academic Press, 2014; pp 123–146. [Google Scholar]
- Burton E. J.; Scholey D. V.; Williams P. E. V. Use of cereal crops for food and fuel–characterization of a novel bioethanol coproduct for use in meat poultry diets. Food Energy Secur. 2013, 2, 197–206. 10.1002/fes3.30. [DOI] [Google Scholar]
- Miller E. L.Protein nutrition requirements of farmed livestock and dietary supply. In Protein sources for the animal feed industry, expert consultation and workshop, Bangkok; Food and Agriculture Organization of the United Nations: Rome, 2004; pp 29–76. [Google Scholar]
- FAO 2019, Aquaculture Feed and Fertilizer Resources Information System. URL (http://www.fao.org/fishery/affris/species-profiles/atlantic-salmon/atlantic-salmon-home/en/) (20/11/2019)
- Cromwell G. L.Nutritional requirements of pigs; In MSD Veterinary Manual; 2019. URL (https://www.msdvetmanual.com/management-and-nutrition/nutrition-pigs/nutritional-requirements-of-pigs) (20/11/2019)
- European Commission (2003a) Regulation No 1334/2003 amending the conditions for authorisation of a number of additives in feedingstuffs belonging to the group of trace elements. URL (https://eur-lex.europa.eu) (20/11/2019)
- Stewart D.Co-products. In Whisky; Russell I.; Bamforth C.; Stewart G., Eds.; Academic Press, 2014; pp 271–289. [Google Scholar]
- Belyea R.; Eckhoff S.; Wallig M.; Tumbleson M. Variability in the nutritional quality of distillers solubles. Bioresour. Technol. 1998, 66, 207–212. 10.1016/S0960-8524(98)00062-5. [DOI] [Google Scholar]
- European Sustainable Phosphorous Platform URL (www.phosphorusplatform.eu) (20/11/2019)
- Jia B.; Tang Y.; Tian L.; Franz L.; Alewell C.; Huang J. H. Impact of fish farming on phosphorus in reservoir sediments. Sci. Rep. 2015, 5, 16617. 10.1038/srep16617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster M.; Reyer H.; Ball E.; Fornara D.; McKillen J.; Sørensen K. U.; Poulsen H. D.; Andersson K.; Ddiba D.; Arata L.; Sckokai P.; Magowan E.; Wimmers K. Bridging gaps in the agricultural phosphorus cycle from an animal husbandry perspective—The case of pigs and poultry. Sustainability 2018, 10, 1825. 10.3390/su10061825. [DOI] [Google Scholar]
- National Research Council (NRC) . Nutrient requirements of fish; National Academy Press: Washington DC, 1993. [Google Scholar]
- González-Vega J. C.; Walk C. L.; Murphy M. R.; Stein H. H. Requirement for digestible calcium by 25 to 50 kg pigs at different dietary concentrations of phosphorus as indicated by growth performance, bone ash concentration, and calcium and phosphorus balances. J. Anim. Sci. 2016, 94, 5272–5285. 10.2527/jas.2016-0751. [DOI] [PubMed] [Google Scholar]
- De Lange C. F. M.; Zhu C. H.Liquid feeding corn-based diets to growing pigs: practical considerations and use of co-products. In Feed efficiency in swine; Patience J.F., Ed.; Wageningen Academic Publishers: Wageningen, 2012; pp 63–80. [Google Scholar]
- European Commission (2003b) Regulation No 2112/2003 correcting Regulation (EC) No 1334/2003 amending the conditions for authorisation of a number of additives in feedingstuffs belonging to the group trace elements URL (https://eur-lex.europa.eu) (20/11/2019)
- Harrison B.; Fagnen O.; Jack F.; Brosnan J. The impact of copper in different parts of malt whisky pot stills on new make spirit composition and aroma. J. Inst. Brew. 2011, 117, 106–112. 10.1002/j.2050-0416.2011.tb00450.x. [DOI] [Google Scholar]
- Huang C.-p.; Huang C.-p.; Morehart A. L. The removal of Cu(II) from dilute aqueous solutions by Saccharomyces cerevisiae. Water Res. 1990, 24, 433–439. 10.1016/0043-1354(90)90225-U. [DOI] [Google Scholar]
- European Commission (2016) Commission Implementing Regulation (EU) 2016/1095 concerning the authorisation of Zinc acetate dihydrate, Zinc chloride anhydrous, Zinc oxide, Zinc sulphate heptahydrate, Zinc sulphate monohydrate, Zinc chelate of amino acids hydrate, Zinc chelate of protein hydrolysates, Zinc chelate of glycine hydrate (solid) and Zinc chelate of glycine hydrate (liquid) as feed additives for all animal species and amending Regulations URL (https://eur-lex.europa.eu) (20/11/2019)
- Böttger C.; Südekum K. H. European distillers dried grains with solubles (DDGS): Chemical composition and in vitro evaluation of feeding value for ruminants. Anim. Feed Sci. Technol. 2017, 224, 66–77. 10.1016/j.anifeedsci.2016.12.012. [DOI] [Google Scholar]
- Omar S. S.; Merrifield D. L.; Kühlwein H.; Williams P. E. V.; Davies S. J. Biofuel derived yeast protein concentrate (YPC) as a novel feed ingredient in carp diets. Aquaculture 2012, 330-333, 54–62. 10.1016/j.aquaculture.2011.12.004. [DOI] [Google Scholar]
- Williams P. E. V.; Williams P. G.. Protein Recovery. U.S. Patent 9,963,671, May 8 2018.
- Heuzé V.; Thiollet H.; Tran G.; Edouard N.; Lessire M.; Lebas F.. Brewers yeast. In Feedipedia 2018; a programme by INRA, CIRAD, AFZ and FAO. URL (https://www.feedipedia.org/node/72) (20/11/2019)
- Liu Y.; Espinosa C. D.; Abelilla J. J.; Casas G. A.; Lagos L. V.; Lee S. A.; Kwon W. B.; Mathai J. K.; Navarro D. M. D. L.; Jaworski N. W.; Stein H. H. Non-antibiotic feed additives in diets for pigs: a review. Anim. nutr. 2018, 4, 113–125. 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegede A. V.; Oso A. O.; Fafiolu A. O.; Sobayo R. A.; Idowu O. M. O.; Odguva O. Effects of dietary copper on performance, serum and egg yolk cholesterol and copper residues in yolk of laying chickens. Slovak J. Anim. Sci. 2015, 48, 29–36. [Google Scholar]
- Walker G. M.; Hill A. E. Saccharomyces cerevisiae in the production of whisk (e) y. Beverages 2016, 2, 38. 10.3390/beverages2040038. [DOI] [Google Scholar]
- Priest F. G.; Stewart G. G.. Handbook of Brewing; 2nd edition., CRC Press, 2006. [Google Scholar]
- Tokuda M.; Fujiwara Y.; Kida K. Pilot plant test for removal of organic matter, N and P from whisky pot ale. Process Biochem. 1999, 35, 267–275. 10.1016/S0032-9592(99)00063-1. [DOI] [Google Scholar]
- Mallick P.; Akunna J. C.; Walker G. M. Anaerobic digestion of distillery spent wash: Influence of enzymatic pre-treatment of intact yeast cells. Bioresour. Technol. 2010, 101, 1681–1685. 10.1016/j.biortech.2009.09.089. [DOI] [PubMed] [Google Scholar]
- Barron N.; Mulholland H.; Boyle M.; McHale A. P. Ethanol production by Kluyveromyces marxianus IMB3 during growth on straw-supplemented whiskey distillery spent wash at 45 C. Bioprocess Eng. 1997, 17, 383–386. 10.1007/PL00008971. [DOI] [Google Scholar]
- Ferguson P.; Mulholland H.; Barron N.; Brady D.; McHale A. P. Sucrose-supplemented distillery spent wash as a medium for production of ethanol at 45°C by free and alginate-immobilized preparations of Kluyveromyces marxianus IMB3. Bioprocess Eng. 1998, 18, 257–259. 10.1007/PL00008987. [DOI] [Google Scholar]
- Traub J. E., White J. S., Maskell D. L., Harper A. J., Hughes P. S., Willoughby N. A.. Protein Recovery. U.S. Patent 10,214,559, February 26, 2019.
- Capezza A. J.; Wu Q.; Newson W. R.; Olsson R. T.; Espuche E.; Johansson E.; Hedenqvist M. S. Superabsorbent and Fully Biobased Protein Foams with a Natural Cross-Linker and Cellulose Nanofibers. ACS Omega 2019, 4, 18257–18267. 10.1021/acsomega.9b02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. E.; Bamforth C. W.. Beer foam: achieving a suitable head. In Beer: a quality perspective; Bamforth C. W., Ed.; Academic Press, 2008; pp 1–60. [Google Scholar]
- Leiper K. A.; Stewart G. G.; McKeown I. P. Beer polypeptides and silica gel part II. polypeptides involved in foam formation. J. Inst. Brew. 2003, 109, 73–79. 10.1002/j.2050-0416.2003.tb00595.x. [DOI] [Google Scholar]
- Fasoli E.; Aldini G.; Regazzoni L.; Kravchuk A. V.; Citterio A.; Righetti P. G. Les Maîtres de l’Orge: The Proteome Content of Your Beer Mug. J. Proteome Res. 2010, 9, 5262–5269. 10.1021/pr100551n. [DOI] [PubMed] [Google Scholar]
- Burton E. J.; Scholey D. V.; Williams P. E. V.. Types, properties and processing of bio-based animal feed. In Advances in Biorefineries; Waldron K. W., Ed.; Woodhead Publishing, 2014; pp 771–802. [Google Scholar]
- Øverland M.; Skrede A.; Matre T. Bacterial Protein Grown on Natural Gas as Feed for Pigs. Acta Agric. Scand., Sect. A 2001, 51, 97–106. 10.1080/090647001750193422. [DOI] [Google Scholar]
- Heuzé V.; Tran G.; Kaushik S.; 2019. Soybean meal. Feedipedia, a programme by INRA, CIRAD, AFZ and FAO. URL (https://www.feedipedia.org/node/674)(20/11/2019)