Abstract
In the current paper, we successfully developed and used metal–organic frameworks (MOFs) based on MIL-101(Cr)-NH2 with phosphorus acid functional groups MIL-101(Cr)-N(CH2PO3H2)2. The synthesized metal–organic frameworks (MOFs) as a multi-functional heterogeneous and nanoporous catalyst were used for the synthesis of N-amino-2-pyridone and pyrano [2,3-c]pyrazole derivatives via reaction of ethyl cyanoacetate or ethyl acetoacetate, hydrazine hydrate, malononitrile, and various aldehydes. The final step of the reaction mechanism was preceded by a cooperative vinylogous anomeric-based oxidation. Recycle and reusability of the described catalyst MIL-101(Cr)-N(CH2PO3H2)2 were also investigated.
1. Introduction
Metal–organic frameworks (MOFs) have been considered as a new category of nanoporous material. They have been used for various purposes such as storage and separation of gas, catalysts, and heavy-metal adsorption.1−5 Metal–organic frameworks (MOFs) based on the type of their ligands and functional groups on their surface exhibit different properties. Their functional groups may be initially present in the organic ligand structures or after synthesizing MOFs to create functional groups within the structure.6 Metal–organic frameworks (MOFs) exhibit a unique catalytic role in the preparation of hydrogen and methane gas and the synthesis of a wide range of chemical and pharmaceutical compounds. These compounds have shown a unique catalytic power due to their nanosize and porous structures with various functional groups.5,7,8 Developing Cr-based metal–organic frameworks (MOFs) is envisaged to achieve the goals such as: higher surface area, enhanced adsorption, water, and thermal stability in the course of reaction processes.9,10
Phosphorous acid and its derivatives are used as reagents, absorbents, catalysts for the preparation of food additives, precursor for the synthesis of phosphate fertilizers,11 and in pharmaceutical industries due to their nontoxicity as pH regulators. The design and synthesis of catalysts with phosphorous acid moieties are an attractive research proposal due to their biocompatibility. Recently, we have reported glycoluril, MIL-100(Cr)/En, and mesoporous SBA-15 with phosphorous acid tags.12−16 Design, synthesis, and use of metal–organic frameworks (MOFs) with phosphorous acid arms due to their properties of recovery, reuse, and high efficiency are suitable catalysts in the chemical processes.
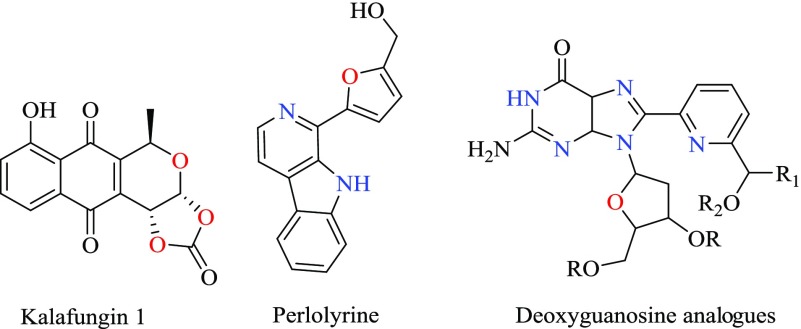
Heterocyclic moieties have been used as important building blocks within a wide range of medicinal and biologically active molecules.17−19 Two of the most important subclasses of heterocyclic chemistry are oxygen- and nitrogen-containing rings, which can be found in the skeletal structures of various types of biologically active and pharmaceutical compounds20,21 (Scheme 1). Among oxygen and nitrogen hetreocycles, the N-amino-2-pyridones and pyrano [2,3-c]pyrazoles have been shown to have anticancer, anticoagulant, anticonvulsant, antimicrobial, anti-HIV, antimalarial, antitumor, antibacterial, antifungal, and antitumor properties.
Scheme 1. Biological Compounds Containing Heterocyclic in the Structure.
In spite of large usage of N-amino-2-pyridones and pyrano [2,3-c]pyrazoles, only a few procedures have been developed for their synthesis, using piperidine, ZnO, sodium l-ascorbatea, and nano-MIIZr(PO4)6 as catalysts.22−25 Therefore, the development of new methodologies for the preparation of N-amino-2-pyridones and pyrano [2,3-c]pyrazoles is in great demand.
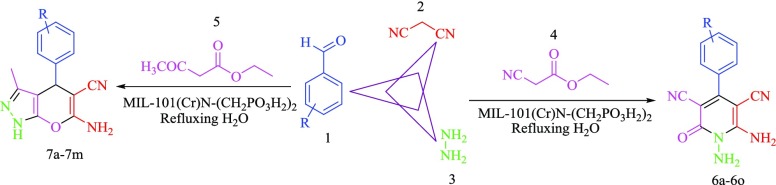
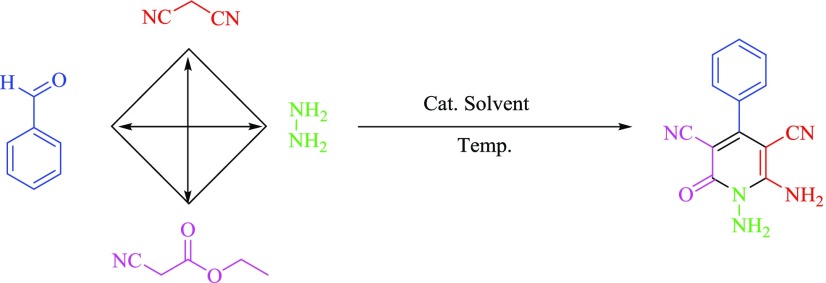
In the continuation of our previous investigation on the applications of catalysts with phosphorous acid functional groups, we have decided to design and synthesize a novel MIL-101(Cr)-N(CH2PO3H2)2 with phosphorous acidic arms as nanoporous MOFs and heterogeneous catalyst for the one-pot synthesis of pyrano [2,3-c]pyrazoles and N-amino-2-pyridones. The desired compounds were produced through the condensation of ethyl cyanoacetate or ethyl acetoacetate, hydrazine hydrate, malononitrile, and various aldehydes via a cooperative vinylogous anomeric-based oxidation mechanism and under solvent-free conditions (Scheme 2).
Scheme 2. Synthesis of N-Amino-2-pyridones and Pyrano [2,3-c]pyrazoles in Four-Component Reaction.
2. Results and Discussion
In this paper, we reported a clean method for the preparation of MIL-101(Cr)-N(CH2PO3H2)2 as a metal–organic framework (MOFs) by the one-pot reaction of MIL-101(Cr)-NH2, formaldehyde, phosphorous acid, and p-toluenesulfonic acid (p-TSA) under refluxing EtOH. This catalyst was fully characterized by Fourier transform infrared (FT-IR), X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX), elemental mapping analysis, the scanning electron microscopy (SEM), transmission electron microscopy (TEM), thermal gravimetric (TG), derivative thermal gravimetric (DTG), differential thermal analysis (DTA), and nitrogen adsorption–desorption isotherm Brunauer–Emmett–Teller (BET). Also, MIL-101(Cr)-N(CH2PO3H2)2 was tested for the synthesis of N-amino-2-pyridone and pyrano [2,3-c]pyrazole derivatives.
The FT-IR spectrum of MIL-101(Cr)-NH2 and MIL-101(Cr)-N(CH2PO3H2)2 is compared in Figure 1. The broad peak at 2600–3500 cm–1 was related to the OH of PO3H2 groups. Also, the absorption bands observed at 1021 and 1081 cm–1 are related to the P–O bond stretching and that at 1146 cm–1 is related to P=O.26 Furthermore, peaks of Cr–O of octahedral CrO6 appeared at 1391 cm–1 respectively27 (Figure 1). The FT-IR spectrum difference between MIL-101(Cr)-NH2 and MIL-101(Cr)-N(CH2PO3H2)2 confirmed the structure of the catalyst.
Figure 1.
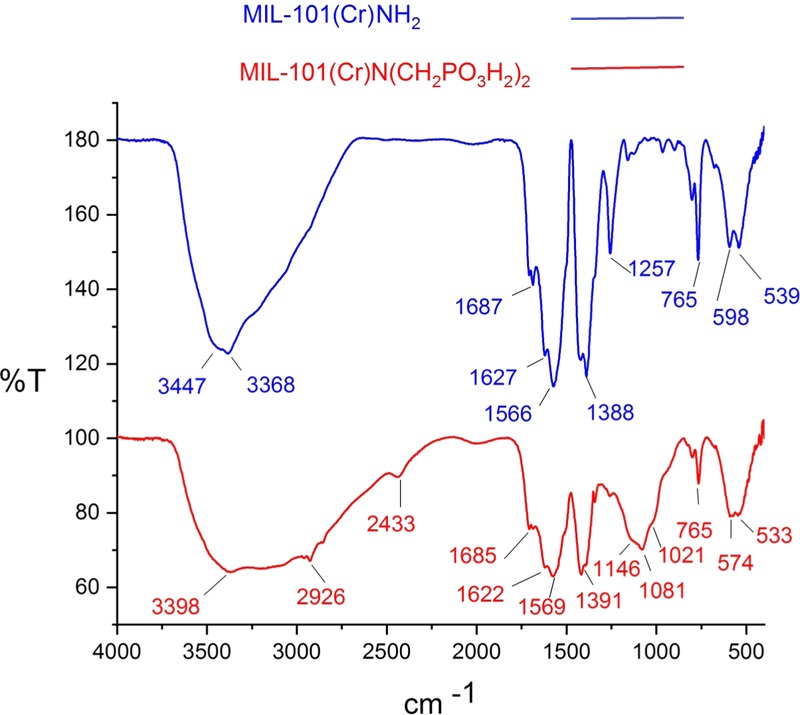
FT-IR spectra of MIL-101(Cr)-NH2 and MIL-101(Cr)-N(CH2PO3H2)2.
The presenting elements can be seen in the structure and morphology of MIL-101(Cr)-N(CH2PO3H2)2 using energy-dispersive X-ray spectroscopy (EDX), elemental mapping analysis, and scanning electron microscopy (SEM). Through energy-dispersive X-ray spectroscopy (EDX) and elemental mapping analysis, chrome, carbon, nitrogen, oxygen, and phosphor were confirmed in the structure of MIL-101(Cr)-N(CH2PO3H2)2 (Figure 2).
Figure 2.
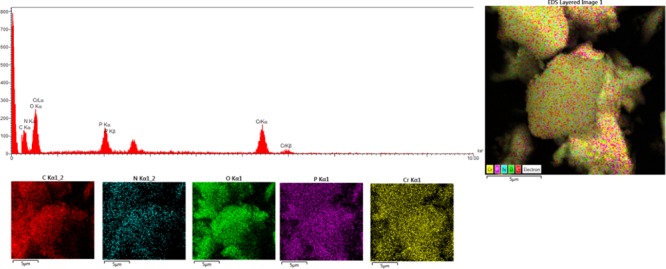
Energy-dispersive X-ray spectroscopy (EDX) and elemental mapping analysis of MIL-101(Cr)-N(CH2PO3H2)2.
MIL-101(Cr)-NH2 and MIL-101(Cr)-N(CH2PO3H2)2 structures were calculated in the range of 2–80° using XRD as shown in Figure 3. The XRD patterns of our synthesized MIL-101(Cr)-NH2 matched well with those reported in the literature,57,58 confirming the formation of MIL-101(Cr)-NH2. The main Bragg reflection around 2θ = 10 confirmed the successful synthesis of MIL-101(Cr)-NH2 as the MOF structure. The pattern of the phosphonic acid grafted MOF materials exhibits a very similar profile to the pattern of the as-synthesized MIL-101(Cr)-NH2 and is composed of main diffraction peaks of the MOF, confirming that the structures of MIL-101 (Cr)-NH2 remained intact with no apparent loss of crystallinity but with some slight decrease in peak intensities along with an increase in phosphonic acid contents due to the partial filling by the grafting guest molecules.
Figure 3.
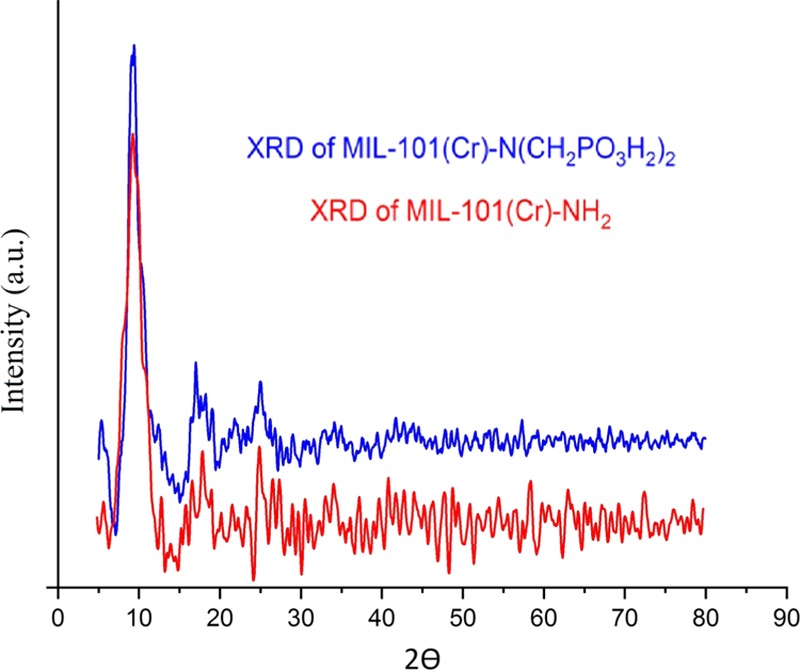
XRD of MIL-101(Cr)-NH2 and MIL-101(Cr)-N(CH2PO3H2)2 as MOFs catalyst.
The shape, morphology, and elements of MIL-101(Cr)-NH2 and MIL-101(Cr)-N(CH2PO3H2)2 were studied using SEM analysis. SEM images of MOFs catalyst reveal that the particles are cauliflower and the particle size is within the range of the nanoscales 13.77 and 30.55 (Figures 4 and 5). Figure 6 also shows the results of transmission electron microscopy (TEM) images of MIL-101(Cr)-N(CH2PO3H2)2. Morphology and topology of MOF catalysts were confirmed to be quasi-cube structure. The particles of MIL-101(Cr)-N(CH2PO3H2)2 were observed to of nano size (approximately 30–40 nm) with proper dispersion.
Figure 4.
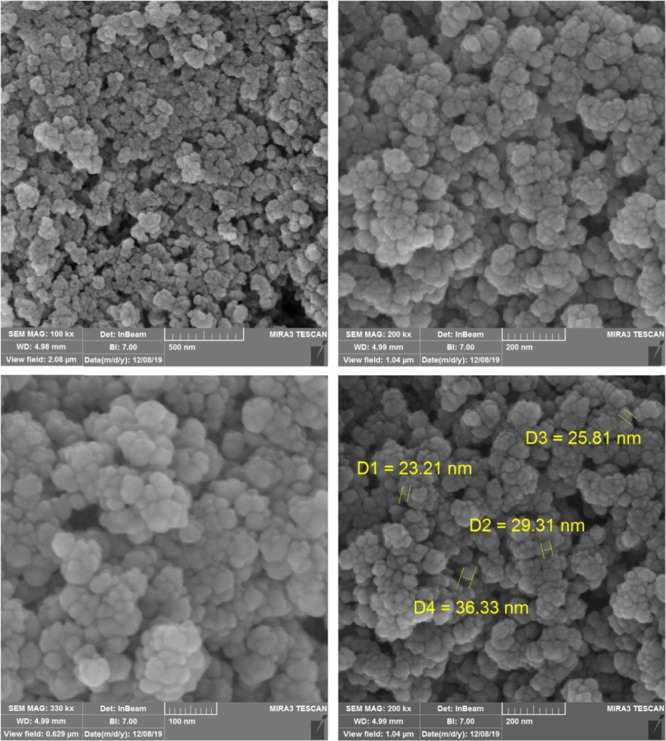
SEM images of MIL-101(Cr)-NH2.
Figure 5.
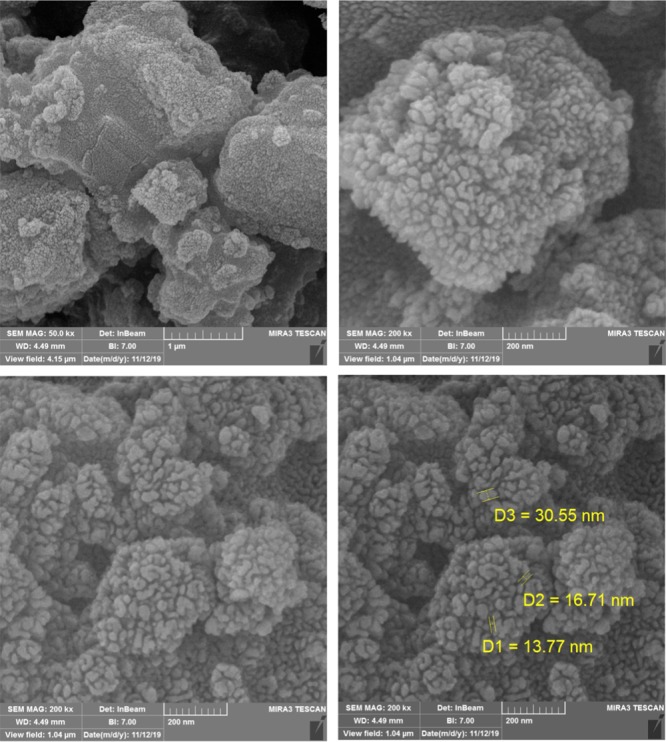
SEM images of MIL-101(Cr)-N(CH2PO3H2)2.
Figure 6.
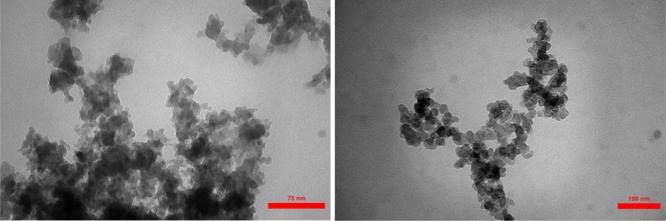
TEM of MIL-101(Cr)-N(CH2PO3H2)2.
Thermal gravimetric (TG) analysis and differential thermal analysis (DTA) of MIL-101(Cr)-N(CH2PO3H2)2 are shown in Figure 7. Two declining stages were observed for MIL-101(Cr)-N(CH2PO3H2)2 in the TG pattern. The amount of weight loss is estimated to be 5–7%, which can be attributed to the decreases in solvent (organic and water). The results show that the catalyst can be used up to 220 °C, which can be related to the departure of PO3H2 arms and decomposition of the structure of MIL-101(Cr)-N(CH2PO3H2)2 (Figure 7).
Figure 7.
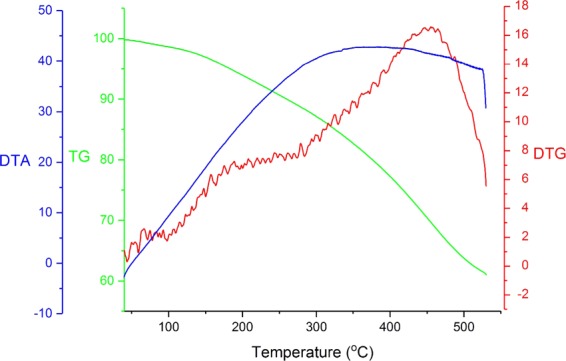
TG analysis, DTG analysis, and DTA of MIL-101(Cr)-N(CH2PO3H2)2.
Nitrogen adsorption–desorption isotherms of MIL-101(Cr)-NH2 and MIL-101(Cr)-N(CH2PO3H2)2 were measured and the results are presented in Figure 8a,c. Observation of hysteresis loops for both of them means that the prepared catalysts are mesoporous. The obtained BET surface areas of MIL-101(Cr)-NH2 and MIL-101(Cr)-N(CH2PO3H2)2 are 1708 and 528 m2 g–1, respectively. Their total pore volumes are 1.46 and 0.38 cm3 g–1, respectively. The Barrett–Joyner–Halenda (BJH) pore size distribution data are presented in Figure 8b,d, showing that most of the pores for both samples are smaller than 10 nm.
Figure 8.
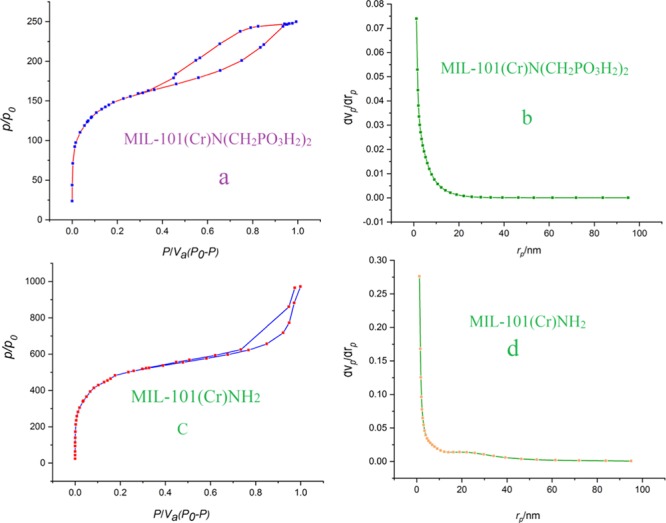
N2 adsorption/desorption isotherm for (a) MIL-101(Cr)-NH2 and (c) IL-101(Cr)-N(CH2PO3H2)2. (b, d) Their pore size distribution plots.
After the structure of MIL-101(Cr)-N(CH2PO3H2)2 was approved, it was used as a MOFs catalyst for the synthesis of N-amino-2-pyridones and pyrano [2,3-c]pyrazoles. To synthesizeN-amino-2-pyridones, the four-part reaction between ethyl cyanoacetate (1 mmol, 0.113 g), hydrazine hydrate (1.2 mmol, 0.060 g), malononitrile (1.1 mmol, 0.072 g) and benzaldehyde (1 mmol, 0.106 g) under refluxing solvents such as: water, ethanol, acetonitrile, n-hexane solvents and solvent-free in the presence a catalytic amount of MIL-101(Cr)-N(CH2PO3H2)2 was tested, the results of which are shown in Table 1. The results are summarized in Table 1. The results show that refluxing water is best of choice for producing N-amino-2-pyridones (Table 1, entry 1). Increasing the amount of catalyst did not show any increase in efficiency (Table 1, entry 9). By decreasing the amounts of catalysts, a decrease in efficiency was observed (Table 1, entries 7 and 8). Reducing the temperature has increased the reaction time and reduced the efficiency of the product (Table 1, entries 10–13).
Table 1. Effect of Different Amounts of Catalysts, Temperature, and Solvent (5 mL) in the Synthesis of N-Amino-2-pyridones.
| entry | catalysts (mg) | temperature (°C) | solvent | time (min) | yield (%) |
|---|---|---|---|---|---|
| 1 | 10 | reflux | H2O | 40 | 90 |
| 2 | 10 | reflux | EtOH | 60 | 72 |
| 3 | 10 | reflux | n-hexane | 90 | trace |
| 4 | 10 | reflux | CH3CN | 45 | 76 |
| 5 | 10 | 110 | toluene | 65 | 55 |
| 6 | 10 | 100 | solvent-free | 60 | 43 |
| 7 | 7 | 80 | H2O | 50 | 81 |
| 8 | 5 | reflux | H2O | 60 | 80 |
| 9 | 15 | reflux | H2O | 40 | 90 |
| 10 | 10 | 75 | H2O | 55 | 78 |
| 11 | 10 | 50 | H2O | 65 | 66 |
| 12 | 10 | r.t. | H2O | 85 | 35 |
| 13 | r.t. | H2O | 120 | trace |
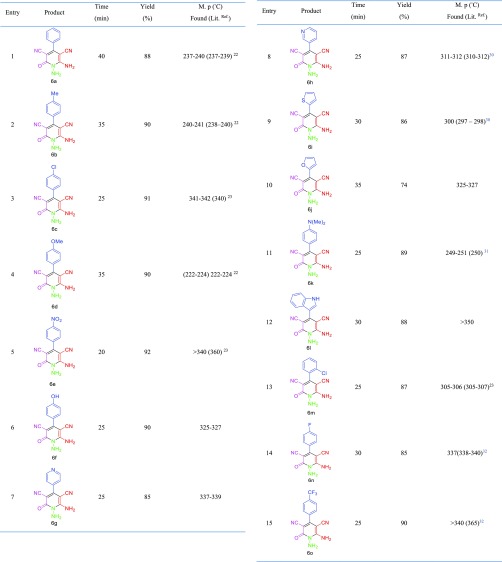
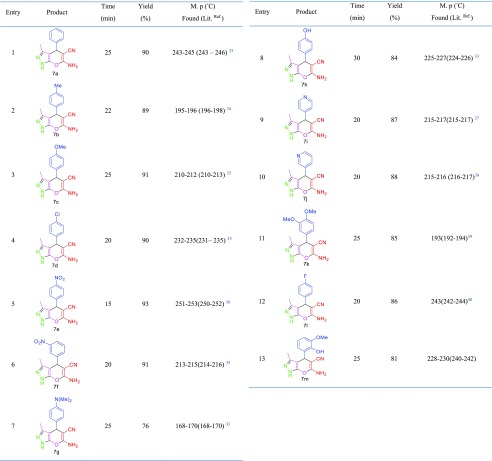
After optimization of the reaction conditions for the synthesis of N-amino-2-pyridones, a wide range of aromatic aldehydes, including electron withdrawal, electron release and heterocyclic rings have been synthesized (Table 2). Then, to investigate the effect of the functional group, ethyl acetoacetate replaced ethyl cyanoacetate, and it was observed that the product of pyrano [2,3-c]pyrazoles was synthesized by changing the cyano group to the carbonyl group. Under optimal conditions for N-amino-2-pyridones, a wide range of pyrano [2,3-c]pyrazoles were also synthesized (Table 3). The results reveal that the described catalyst can produce N-amino-2-pyridones and pyrano [2,3-c]pyrazoles derivatives in short reaction time and high efficiency.
Table 2. Synthesis of N-Amino-2-pyridones Using MIL-101(Cr)-N(CH2PO3H2)2.
Table 3. Synthesis of Pyrano [2,3-c]pyrazoles Using MIL-101(Cr)-N(CH2PO3H2)2.
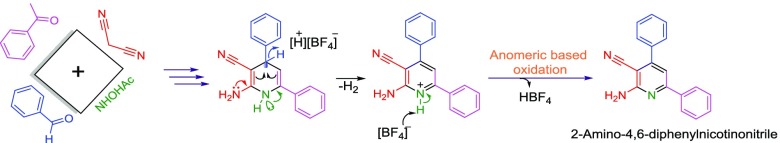
Alabugin has recently comprehensively reviewed the stereoelectronic effects as a bridge between structure and reactivity.39−41 On the basis of Alabugin’s concept, we have recently introduced a new term “vinylogous anomeric-based oxidation”.42−46 Vinylogous anomeric effect has been approved by Katritzky.47 In the cooperative vinylogous anomeric-based oxidation mechanism, through a multicomponent reaction strategy, starting materials interact with each other to yield a suitable intermediate. The vinylogous anomeric effect is the major driving force of oxidation and/or aromatization of intermediate for preparing the desired product. For example, in the case of 2-amino-4,6-diphenylnicotinonitrile a cooperative vinylogous anomeric-based oxidation mechanism occurs at the related intermediate (Scheme 3).48 A wide range of aromatized molecules through the described mechanism have been reported.49
Scheme 3. Cooperative Vinylogous Anomeric-Based Oxidation Leads to the Production of 2-Amino-4,6-diphenylnicotinonitrile.
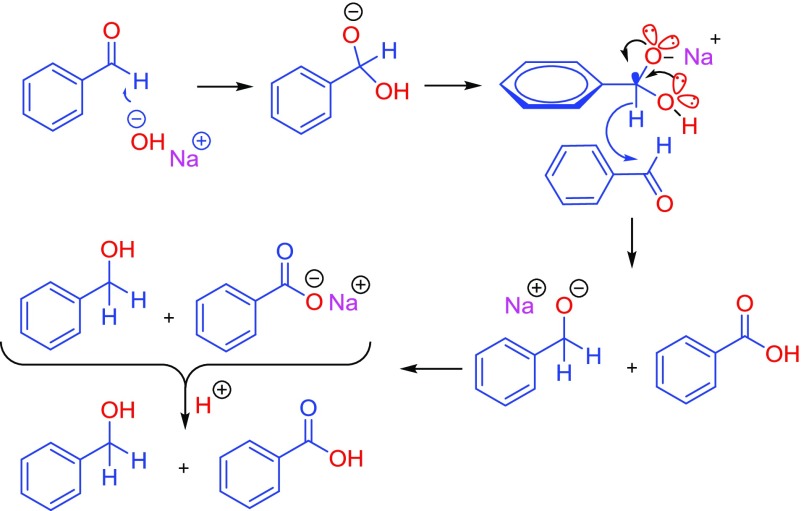
The anomeric effect can lead to bond weakening in a wide range of organic reactions. For example, recently we have suggested that in the Cannizzaro reaction after the addition of hydroxide (OH–) to the carbonyl group of aldehydes which did not have α-hydrogen (Scheme 4), both the lone pairs of electrons of oxygen atoms within the tetrahedral carbon shared their electrons in the antibonding orbital of the C–H bond (nN → σ*C–H) and weakened it. The resulting labile hydride acts as a powerful nucleophile that attacks the second molecule of aldehyde. Finally, this reaction produced equal amounts of corresponding alcohol and acid.50
Scheme 4. Anomeric-Based Oxidation Leads to Hydride Transfer in the Mechanism of Cannizzaro Reaction.
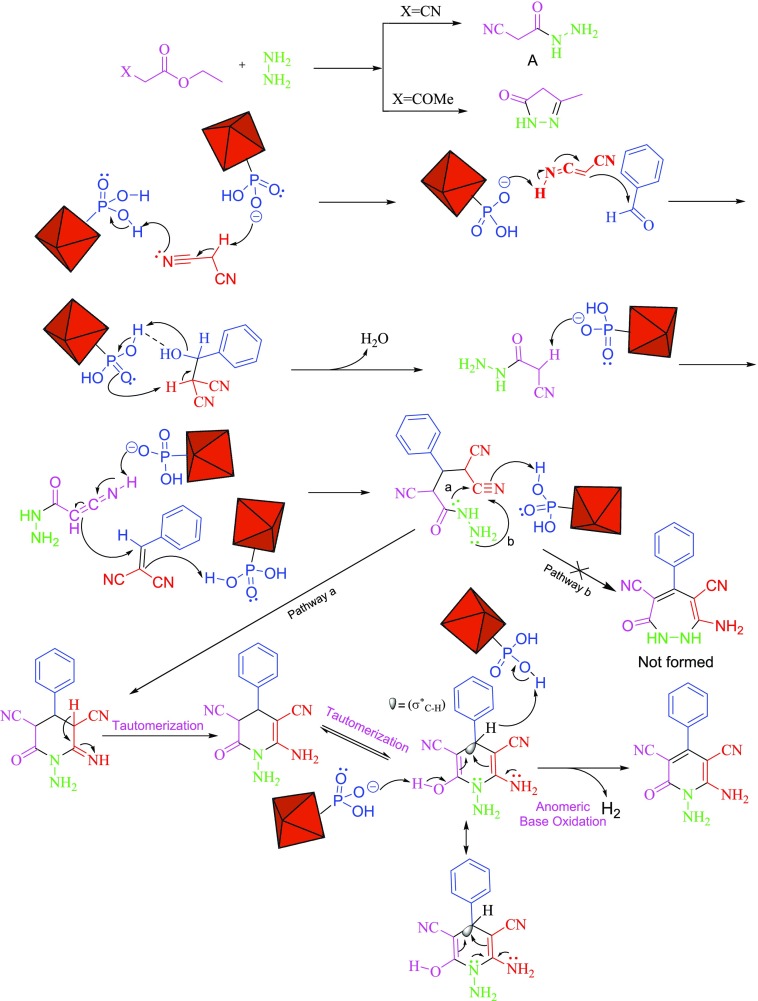
On the basis of the above-mentioned background, a rational mechanism for the synthesis of N-amino-2-pyridones based on a cooperative vinylogous anomeric-based oxidation for the final step is suggested in Scheme 5. At first, the carbonyl group of aldehyde is activated by the acidic group of MIL-101(Cr)-N(CH2PO3H2)2. Malononitrile reacts with the carbonyl group of aldehyde to afford an intermediate I by removing one molecule of H2O. Then, compound (A) attacks intermediate I as a Michael acceptor to give intermediate II. Further, intermediate II with intramolecular cyclocondensation reacts to give III. Finally, intermediate III was derived via a cooperative vinylogous anomeric-based oxidation (−H2) to give the desired product (Scheme 5). It should be noted that the target reaction was also carried out under argon and nitrogen atmospheres to make sure that the final desired product was not produced via an aerobic oxidation pathway. The reaction was performed successfully under the air atmosphere. Thus, our evidence shows that the target reaction was preceded via a cooperative vinylogous anomeric-based oxidation mechanism.
Scheme 5. Proposed Mechanism for the Synthesis N-Amino-2-pyridones Using MIL-101(Cr)-N(CH2PO3H2)2.
To evaluate the performance of MIL-101(Cr)-N(CH2PO3H2)2 as a catalyst for the preparation of N-amino-2-pyridones, we have used various organic and inorganic acid catalysts for the condensation reaction between 4-nitro benzaldehyde (1 mmol, 0.151 g), ethyl cyanoacetate (1 mmol, 0.113 g), hydrazine hydrate (1.2 mmol, 0.060 g), and malononitrile (1.1 mmol, 0.072 g) (Table 3). As indicated in Table 3, MIL-101(Cr)-N(CH2PO3H2)2 is the best catalyst for the synthesis of N-amino-2-pyridone derivatives (Table 4).
Table 4. Evaluation of Various Catalysts for the Synthesis of N-Amino-2-pyridones in Comparison with MIL-101(Cr)-N(CH2PO3H2)2 in Ethanol under Reflux Conditions.
| entry | catalyst | amount of catalyst (mol %) | time (min) | yield (%) |
|---|---|---|---|---|
| 1 | MIL-101(Cr)-N(CH2PO3H2)2 | 5 mg | 20 | 92 |
| 2 | MIL-101(Cr)-NH2 | 5 mg | 60 | 24 |
| 3 | [Py-SO3H]Cl51 | 10 | 35 | 82 |
| 4 | Fe3O4@SiO2@PrNH252 | 5 mg | 60 | 25 |
| 5 | SSA53 | 10 | 25 | 85 |
| 6 | TrBr | 15 | 60 | 65 |
| 7 | p-TSA | 10 | 45 | 65 |
| 8 | nano-SB-[PSIM]Cl54 | 10 | 35 | 78 |
| 9 | TrCl | 10 | 45 | 72 |
| 10 | GTBSA55 | 10 | 30 | 85 |
| 11 | [Fe3O4@SiO2@Pr-DABCO-SO3H]Cl256 | 10 mg | 35 | 82 |
| 12 | FeCl3 | 10 | 50 | 75 |
| 13 | Al(HSO4)3 | 10 | 45 | 75 |
| 14 | H3[p(W3O10)4]·xH2O | 15 | 60 | 75 |
| 15 | Fe3O4 | 10 mg | 55 | 68 |
| 16 | trichloroisocyanuric acid | 10 | 55 | 70 |
| 17 | Et3N | 20 | 60 | trace |
| 18 | NaHSO4 | 10 | 60 | 35 |
| 19 | H3PO3 | 10 | 20 | 75 |
| 20 | H3PO3 + MIL-101(Cr)-NH2 | 5 + 5 | 20 | 90 and without recycling |
According to the results in Figure 9, MIL-101(Cr)-N(CH2PO3H2)2 can be separated by centrifugation and reused without significantly reducing its catalytic activity. For this purpose, recyclability of the catalyst was tested in the reaction between 4-nitro benzaldehyde (1 mmol, 0.151 g), ethyl cyanoacetate (1 mmol, 0.113 g), hydrazine hydrate (1.2 mmol, 0.060 g), and malononitrile (1.1 mmol, 0.072 g) as a model reaction under the above-mentioned optimized reaction conditions. Therefore, MIL-101(Cr)-N(CH2PO3H2)2 can be reused up to six runs without noticeable changes in the catalytic activity.
Figure 9.
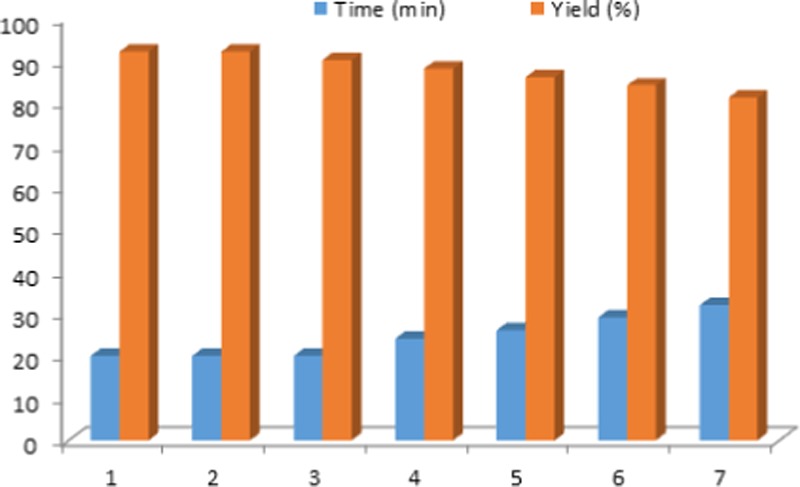
Recyclability of MIL-101(Cr)-N(CH2PO3H2)2 for the synthesis of N-amino-2-pyridone compounds.
3. Conclusions
After the preparation of metal–organic framework MIL-101(Cr)-NH2 with phosphorous acid functional groups, its structure was approved. The presented MIL-101(Cr)-N(CH2PO3H2)2 was successfully applied for producing a wide range of pyrano [2,3-c]pyrazole and N-amino-2-pyridone derivatives via cooperative vinylogous anomeric-based oxidation mechanism. Short reaction times, clean profile of reaction, easy work-up, recyclability, and reusability of catalyst are advantages of the presented methodology.
4. Experimental Section
4.1. General Procedure for the Preparation of MIL-101(Cr)-NH2
MIL-101(Cr)-NH2 was synthesized according to our previously reported experimental procedure.57,58 A mixture of Cr(NO3)3·9(H2O) (2 mmol, 0.8 g), 2-aminoterephthalic acid (2 mmol, 0.362 g), and sodium hydroxide (5 mmol, 0.2 g) was dispersed and then stirred in 20 mL of water for 10 min. The resulting solution was heated in a Teflon-lined autoclave at 150 °C for 12 h. After cooling the reaction mixture to room temperature, the green solid was collected, washed with dimethylformamide (DMF), and then further purified by solvothermal treatment in ethanol at 100 °C for 24 h. Finally, the desired product was dried at 80 °C in a vacuum oven.
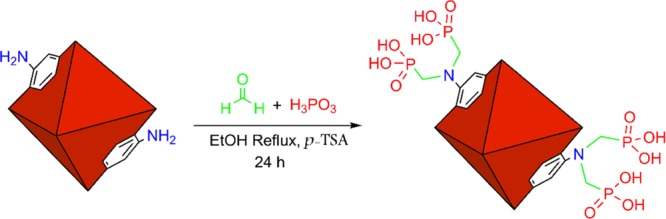
4.2. General Procedure for the Preparation of MIL-101(Cr)-N(CH2PO3H2)2
In a 50 mL round-bottom flask, MIL-101(Cr)-NH2 (1 g), formaldehyde (0.33 mL, 9 mmol), phosphorus acid (0.665 mL, 9 mmol), p-TSA (10 mol %, 0.017 g), and ethanol (25 mL) were refluxed for 8 h. Then, it was washed three times with ethanol and dried in a vacuum oven at 70 °C to obtain MIL-101(Cr)-N(CH2PO3H2)2 (1.7 g) (Scheme 6). The amount of phosphorus is 4.56% of the MOFs component of MIL-101(Cr)-N(CH2PO3H2)2.
Scheme 6. Synthesis of MIL-101(Cr)-N(CH2PO3H2)2 Containing Phosphorous Acid Groups.
4.3. General Procedure for the Synthesis of Pyrano [2,3-c]pyrazole and N-Amino-2-pyridone Derivatives (10a–10t) Using MIL-101(Cr)-N(CH2PO3H2)2
In a four-component reaction in water reflux conditions, including ethyl cyanoacetate or ethyl acetoacetate (1 mmol), hydrazine hydrate (1.2 mmol, 0.060 g), malononitrile (1.1 mmol, 0.072 g), aldehyde (1 mmol), and MIL-101(Cr)-N(CH2PO3H2)2 (10 mg) were stirred in a 25 mL round-bottom flask (time for any case has been presented in Tables 2 and 3). Thin-layer chromatography (TLC) technique was used to assess the reaction progress. After the reaction was completed, the solvent was evaporated and then 2 mL of ethylene glycol was added to the reaction mixture. The catalyst was removed from the reaction using centrifugation at 1000 rpm. The product was purified using 96% ethanol (Scheme 2).
Acknowledgments
We thank the Bu-Ali Sina University and Iran National Science Foundation (INSF) (Grant No. 98001912) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b02133.
FT-IR; 1H NMR; and 13C NMR spectroscopy details (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Li H.; Eddaoudi M.; O’Keeffe M.; Yaghi O. M. Design and synthesis of an exceptionally stable and highly porous metal–organic framework. Nature 1999, 402, 276–279. 10.1038/46248. [DOI] [Google Scholar]
- Lee J. Y.; Farha O. K.; Roberts J.; Scheidt K. A.; Nguyen S. T.; Hupp J. T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. 10.1039/b807080f. [DOI] [PubMed] [Google Scholar]
- Goetjen T. A.; Zhang X.; Liu J.; Hupp J. T.; Farha O. K. Metal–Organic Framework supported single site chromium (III) catalyst for ethylene oligomerization at low pressure and temperature. ACS Sustainable Chem. Eng. 2019, 7, 2553–2557. 10.1021/acssuschemeng.8b05524. [DOI] [Google Scholar]
- Nowacka A. E.; Briantais P.; Prestipino C.; Llabrés i Xamena F. X. Selective aerobic oxidation of cumene to cumene hydroperoxide over mono-and bimetallic trimesate metal–organic frameworks prepared by a facile “green” aqueous synthesis. ACS Sustainable Chem. Eng. 2019, 7, 7708–7715. 10.1021/acssuschemeng.8b06472. [DOI] [Google Scholar]
- Cao C.-C.; Chen C.-X.; Wei Z.-W.; Qiu Q.-F.; Zhu N.-X.; Xiong Y.-Y.; Jiang J.-J.; Wang D.; Su C.-Y. Catalysis through dynamic spacer installation of multivariate functionalities in metal–organic frameworks. J. Am. Chem. Soc. 2019, 141, 2589–2593. 10.1021/jacs.8b12372. [DOI] [PubMed] [Google Scholar]
- Huang X. C.; Lin Y. Y.; Zhang J. P.; Chen X. M. Ligand-directed strategy for zeolite-type metal–organic frameworks: zinc (II) imidazolates with unusual zeolitic topologies. Angew. Chem., Int. Ed. 2006, 45, 1557–1559. 10.1002/anie.200503778. [DOI] [PubMed] [Google Scholar]
- Doustkhah E.; Lin J.; Rostamnia S.; Len C.; Luque R.; Luo X.; Bando Y.; Wu K. C. W.; Kim J.; Yamauchi Y.; Ide Y. Development of sulfonic-acid-functionalized mesoporous materials: synthesis and catalytic applications. Chem. - Eur. J. 2019, 25, 1614–1635. 10.1002/chem.201802183. [DOI] [PubMed] [Google Scholar]
- Alamgholiloo H.; Rostamnia S.; Hassankhani A.; Banaei R. Synthesis of a zeolitic imidazolate–zinc metal–organic framework and the combination of its catalytic properties with 2, 2, 2-trifluoroethanol for N-formylation. Synlett 2018, 26, 1593–1596. 10.1055/s-0037-1610159. [DOI] [Google Scholar]
- Jhung S. H.; Khan N. A.; Hasan Z. Analogous porous metal–organic frameworks: synthesis, stability and application in adsorption. CrystEngComm 2012, 14, 7099–7109. 10.1039/c2ce25760b. [DOI] [Google Scholar]
- Yang C.-X.; Yan X.-P. Metal–organic framework MIL-101 (Cr) for high-performance liquid chromatographic separation of substituted aromatics. Anal. Chem. 2011, 83, 7144–7150. 10.1021/ac201517c. [DOI] [PubMed] [Google Scholar]
- Mitsunobu O.; Yamada M. Preparation of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380–2382. 10.1246/bcsj.40.2380. [DOI] [Google Scholar]
- Jalili F.; Zarei M.; Zolfigol M. A.; Rostamnia S.; Moosavi-Zare A. R. SBA-15/PrN (CH2PO3H2)2 as a novel and efficient mesoporous solid acid catalyst with phosphorous acid tags and its application on the synthesis of new pyrimido [4, 5-b] quinolones and pyrido [2, 3-d] pyrimidines via anomeric based oxidation. Microporous Mesoporous Mater. 2019, 294, 109865 10.1016/j.micromeso.2019. [DOI] [Google Scholar]
- Afsar J.; Zolfigol M. A.; Khazaei A.; Zarei M.; Gu Y.; Alonso D. A.; Khoshnood A. Synthesis and application of melamine-based nano catalyst with phosphonic acid tags in the synthesis of (3′-indolyl) pyrazolo [3, 4-b] pyridines via vinylogous anomeric based oxidation. Mol. Catal. 2019, 482, 110666 10.1016/j.mcat.2019.110666. [DOI] [Google Scholar]
- Sepehrmansouri H.; Zarei M.; Zolfigol M. A.; Moosavi-Zare A. R.; Rostamnia S.; Moradi S. Multilinker phosphorous acid anchored En/MIL-100 (Cr) as a novel nanoporous catalyst for the synthesis of new N-heterocyclic pyrimido [4, 5-b] quinolines. Mol. Catal. 2019, 481, 110303 10.1016/j.mcat.2019.01.023. [DOI] [Google Scholar]
- Zarei M.; Zolfigol M. A.; Moosavi-Zare A. R.; Noroozizadeh E.; Rostamnia S. Three-Component Synthesis of Spiropyrans Using SBA-15/En Bonded Phosphorous Acid [SBA-15/Pr-NH1-y (CH2PO3H2)y-Et-NH2-x (CH2PO3H2)x] as a New Nanoporous Heterogeneous Catalyst. ChemistrySelect 2018, 3, 12144–12149. 10.1002/slct.201802525. [DOI] [Google Scholar]
- Moradi S.; Zolfigol M. A.; Zarei M.; Alonso D. A.; Khoshnood A. Synthesis of a Biological-Based Glycoluril with Phosphorous Acid Tags as a New Nanostructured Catalyst: Application for the Synthesis of Novel Natural Henna-Based Compounds. ChemistrySelect 2018, 3, 3042–3047. 10.1002/slct.201702544. [DOI] [Google Scholar]
- Dalvie D. K.; Kalgutkar A. S.; Khojasteh-Bakht S. C.; Obach R. S.; O’Donnell J. P. Biotransformation Reactions of Five-Membered Aromatic Heterocyclic Rings. Chem. Res. Toxicol. 2002, 15, 269–299. 10.1021/tx015574b. [DOI] [PubMed] [Google Scholar]
- Mikhailopulo I. A.; Poopeiko N.; Prikota T.; Sivets G.; Kvasyuk E. I.; Balzarini J.; De Clercq E. Synthesis and antiviral and cytostatic properties of 3′-deoxy-3′-fluoro-and 2′-azido-3′-fluoro-2′, 3′-dideoxy-D-ribofuranosides of natural heterocyclic bases. J. Med. Chem. 1991, 34, 2195–2202. 10.1021/jm00111a040. [DOI] [PubMed] [Google Scholar]
- Tietze L. F.; Rackelmann N. Domino reactions in the synthesis of heterocyclic natural products and analogs. Pure Appl. Chem. 2004, 76, 1967–1983. 10.1351/pac200476111967. [DOI] [Google Scholar]
- Johnson L. E.; Dietz A. Kalafungin, a new antibiotic produced by Streptomyces tanashiensis strain Kala. Appl. Environ. Microbiol. 1968, 16, 1815–1821. 10.1128/AEM.16.12.1815-1821.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.; Li L.; Sun B.; Gao Y.; Song W.; Zhao X.; Gao Y.; Xie Z.; Zhang N.; Ji J.; Yuan H.; Lou H. Design and synthesis of furyl/thineyl pyrroloquinolones based on natural alkaloid perlolyrine, lead to the discovery of potent and selective PDE5 inhibitors. Eur. J. Med. Chem. 2018, 150, 30–38. 10.1016/j.ejmech.2018.02.039. [DOI] [PubMed] [Google Scholar]
- Safaei-Ghomi J.; Saberimoghadam M. R.; Shahbazialavi H.; Asgarikheirabadi M. An efficient method for the synthesis of N-amino-2-pyridones using reusable catalyst ZnO nanoparticles. J. Chem. Res. 2014, 38, 583–585. 10.3184/174751914X14109743944636. [DOI] [Google Scholar]
- Hosseini H.; Bayat M. An efficient and ecofriendly synthesis of highly functionalized pyridones via a one-pot three-component reaction. RSC Adv. 2018, 8, 27131–27143. 10.1039/C8RA05690K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei-Ghomia J.; Asgari-Kheirabadia M.; Shahbazi-Alavia H.; Ziaratib A. Synthesis of methyl 6-amino-5-cyano-4-aryl-2, 4-dihydropyrano [2, 3-c] pyrazole-3-carboxylates using nano-ZnZr4(PO4)6 as an efficient catalyst. Iran. J. Catal. 2016, 6, 319–324. [Google Scholar]
- Kiyani H.; Bamdad M. Sodium ascorbate as an expedient catalyst for green synthesis of polysubstituted 5-aminopyrazole-4-carbonitriles and 6-amino-1, 4-dihydropyrano [2, 3-c] pyrazole-5-carbonitriles. Res. Chem. Intermed. 2018, 44, 2761–2778. 10.1007/s11164-018-3260-0. [DOI] [Google Scholar]
- Jagodić V. Infrared Spectra of Organophosphorus Compounds. III. Croat. Chem. Acta 1977, 49, 127–133. [Google Scholar]
- Zhou M.; Andrews L. Infrared spectra and density functional calculations of the CrO2–, MoO2–, and WO2– molecular anions in solid neon. J. Chem. Phys. 1999, 111, 4230–4238. 10.1063/1.479721. [DOI] [Google Scholar]
- Férey G.; Mellot-Draznieks C.; Serre C.; Millange F.; Dutour J.; Surblé S.; Margiolaki I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. 10.1126/science.1116275. [DOI] [PubMed] [Google Scholar]
- Rostamnia S.; Alamgholiloo H.; Jafari M. Ethylene diamine post-synthesis modification on open metal site Cr-MOF to access efficient bifunctional catalyst for the Hantzsch condensation reaction. Appl. Organomet. Chem. 2018, 32, e4370 10.1002/aoc.4370. [DOI] [Google Scholar]
- Abdel Latif F. F.; Mekheimer R.; Ahmed E. K.; Abdel Aleem T. A simple route for the synthesis of pyrano [2, 3-c] pyrazole and pyridine-2-one derivatives. Pharmazie 1993, 48, 736–738. [Google Scholar]
- Soto J. L.; Seoane C.; Zamorano P.; Cuadrado F. J. A convenient synthesis of N-amino-2-pyridones. Synthesis 1981, 1981, 529–530. 10.1055/s-1981-29512. [DOI] [Google Scholar]
- Assiria M. A.; Abdel-Kariemc S. M.; Alic T. E.; Yahiad I. The Free Internet Journal for Organic Chemistry. Org. Chem. 2018, 240–253. 10.24820/ark.5550190.p010.478. [DOI] [Google Scholar]
- Upadhyay A.; Sharma L. K.; Singh V. K.; Dubey R.; Kumar N.; Singh R. K. P. Electrochemically induced one pot synthesis of 1, 4-dihydropyrano [2, 3-c]-pyrazole-5-carbonitrile derivatives via a four component-tandem strategy. Tetrahedron Lett. 2017, 58, 1245–1249. 10.1016/j.tetlet.2017.01.049. [DOI] [Google Scholar]
- Maleki B.; Eshghi H.; Barghamadi M.; Nasiri N.; Khojastehnezhad A.; Ashrafi S. S.; Pourshiani O. Silica-coated magnetic NiFe2O4 nanoparticles-supported H3PW12O40; synthesis, preparation, and application as an efficient, magnetic, green catalyst for one-pot synthesis of tetrahydrobenzo [b] pyran and pyrano [2, 3-c] pyrazole derivatives. Res. Chem. Intermed. 2016, 42, 3071–3093. 10.1007/s11164-015-2198-8. [DOI] [Google Scholar]
- Survase D.; Bandgar B.; Helavi V. Polyethylene glycol-promoted synthesis of pyrimido [1, 2-a] benzimidazole and pyrano [2, 3-c] pyrazole derivatives in water. Synth. Commun. 2017, 47, 680–687. 10.1080/00397911.2017.1278774. [DOI] [Google Scholar]
- Safaei-Ghomi J.; Shahbazi-Alavi H.; Heidari-Baghbahadorani E. ZnFe2O4 nanoparticles as a robust and reusable magnetically catalyst in the four component synthesis of [(5-hydroxy-3-methyl-1H-pyrazol-4yl)(phenyl) methyl] propanedinitriles and substituted 6-amino-pyrano [2, 3-c] pyrazoles. J. Chem. Res. 2015, 39, 410–413. 10.3184/174751915X14358475706316. [DOI] [Google Scholar]
- Bakherad M.; Keivanloo A.; Gholizadeh M.; Doosti R.; Javanmardi M. Using magnetized water as a solvent for a green, catalyst-free, and efficient protocol for the synthesis of pyrano [2, 3-c] pyrazoles and pyrano [4′, 3′: 5, 6] pyrazolo [2, 3-d] pyrimidines. Res. Chem. Intermed. 2017, 43, 1013–1029. 10.1007/s11164-016-2680-y. [DOI] [Google Scholar]
- Kamel M. M. Convenient synthesis, characterization, cytotoxicity and toxicity of pyrazole derivatives. Acta Chim. Slov. 2015, 62, 136–141. 10.17344/acsi.2014.828. [DOI] [PubMed] [Google Scholar]
- Shaterian H. R.; Azizi K. Mild, four-component synthesis of 6-amino-4-aryl-3-methyl-1, 4-dihydropyrano [2, 3-c] pyrazole-5-carbonitriles catalyzed by titanium dioxide nano-sized particles. Res. Chem. Intermed. 2014, 40, 661–667. 10.1007/s11164-012-0991-1. [DOI] [Google Scholar]
- Vekariya R. H.; Patel K. D.; Patel H. D. Fruit juice of Citrus limon as a biodegradable and reusable catalyst for facile, eco-friendly and green synthesis of 3, 4-disubstituted isoxazol-5 (4H)-ones and dihydropyrano [2, 3-c]-pyrazole derivatives. Res. Chem. Intermed. 2016, 42, 7559–7579. 10.1007/s11164-016-2553-4. [DOI] [Google Scholar]
- Alabugin I. V.Stereoelectronic Effects: A Bridge between Structure and Reactivity; John Wiley & Sons, 2016. [Google Scholar]
- Alabugin I. V.; dos Passos Gomes G.; Abdo M. A. Hyperconjugation. WIREs Comput. Mol. Sci. 2019, 9, e1389 10.1002/wcms.1389. [DOI] [Google Scholar]
- Alabugin I. V.; Gilmore K. M.; Peterson P. W. Hyperconjugation. WIREs Comput. Mol. Sci. 2011, 1, 109–141. 10.1002/wcms.6. [DOI] [Google Scholar]
- Torabi M.; Yarie M.; Zolfigol M. A. Synthesis of a novel and reusable biological urea based acidic nanomagnetic catalyst: Application for the synthesis of 2-amino-3-cyano pyridines via cooperative vinylogous anomeric based oxidation. Appl. Organomet. Chem. 2019, 33, e4933 10.1002/aoc.4933. [DOI] [Google Scholar]
- Karimi F.; Zolfigol M. A.; Yarie M. A novel and reusable ionically tagged nanomagnetic catalyst: Application for the preparation of 2-amino-6-(2-oxo-2H-chromen-3-yl)-4-arylnicotinonitriles via vinylogous anomeric based oxidation. Mol. Catal. 2019, 463, 20–29. 10.1016/j.mcat.2018.11.009. [DOI] [Google Scholar]
- Noura S.; Ghorbani M.; Zolfigol M. A.; Narimani M.; Yarie M.; Oftadeh M. Biological based (nano) gelatoric ionic liquids (NGILs): Application as catalysts in the synthesis of a substituted pyrazole via vinylogous anomeric based oxidation. J. Mol. Liq. 2018, 271, 778–785. 10.1016/j.molliq.2018.09.023. [DOI] [Google Scholar]
- Zolfigol M. A.; Kiafar M.; Yarie M.; Taherpour A. A.; Fellowes T.; Hancok A. N.; Yari A. A convenient method for preparation of 2-amino-4, 6-diphenylnicotinonitrile using HBF4 as an efficient catalyst via an anomeric based oxidation: A joint experimental and theoretical study. J. Mol. Struct. 2017, 1137, 674–680. 10.1016/j.molstruc.2017.02.083. [DOI] [Google Scholar]
- Babaee S.; Zolfigol M. A.; Zarei M.; Zamanian J. 1, 10-Phenanthroline-Based Molten Salt as a Bifunctional Sulfonic Acid Catalyst: Application to the Synthesis of N-Heterocycle Compounds via Anomeric Based Oxidation. ChemistrySelect 2018, 3, 8947–8954. 10.1002/slct.201801476. [DOI] [Google Scholar]
- Katritzky A. R.; Steel P. J.; Denisenko S. N. X-Ray crystallographic evidence for a vinylogous anomeric effect in benzotriazole-substituted heterocycles. Tetrahedron 2001, 57, 3309–3314. 10.1016/S0040-4020(01)00218-6. [DOI] [Google Scholar]
- Kalhor S.; Yarie M.; Rezaeivala M.; Zolfigol M. A. Novel magnetic nanoparticles with morpholine tags as multirole catalyst for synthesis of hexahydroquinolines and 2-amino-4, 6-diphenylnicotinonitriles through vinylogous anomeric-based oxidation. Res. Chem. Intermed. 2019, 45, 3453–3480. 10.1007/s11164-019-03802-7. [DOI] [Google Scholar]
- Yarie M. Catalytic anomeric based oxidation. Iran. J. Catal. 2017, 7, 85–88. [Google Scholar]
- Kiafar M.; Zolfigol M. A.; Yarie M.; Taherpour A. The first computational study for the oxidative aromatization of pyrazolines and 1,4-dihydropyridines using 1,2,4-triazolinediones: an anomeric-based oxidation. RSC Adv. 2016, 6, 102280–102291. 10.1039/C6RA20929G. [DOI] [Google Scholar]
- Moosavi-Zare A. R.; Zolfigol M. A.; Zarei M.; Zare A.; Khakyzadeh V.; Hasaninejad A. Design, characterization and application of new ionic liquid 1-sulfopyridinium chloride as an efficient catalyst for tandem Knoevenagel–Michael reaction of 3-methyl-1-phenyl-1H-pyrazol-5 (4H)-one with aldehydes. Appl. Catal., A 2013, 467, 61–68. 10.1016/j.apcata.2013.07.004. [DOI] [Google Scholar]
- Zolfigol M. A.; Moosavi-Zare A. R.; Zarei M.; Zare A.; Noroozizadeh E.; Karamian R.; Asadbegy M. Synthesis of β-phthalimido-alcohols via regioselective ring opening of epoxide by using reusable basic magnetic nano particles and their biological investigation. RSC Adv. 2016, 6, 62460–62466. 10.1039/C6RA07660B. [DOI] [Google Scholar]
- Zolfigol M. A. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron 2001, 57, 9509–9511. 10.1016/S0040-4020(01)00960-7. [DOI] [Google Scholar]
- Zare A.; Merajoddin M.; Moosavi-Zare A. R.; Zarei M.; Beyzavi M. H.; Zolfigol M. A. Design and characterization of nano-silica-bonded 3-n-propyl-1-sulfonic acid imidazolium chloride {nano-SB-[PSIM]Cl} as a novel, heterogeneous and reusable catalyst for the condensation of arylaldehydes with β-naphthol and alkyl carbamates. Res. Chem. Intermed. 2016, 42, 2365–2378. 10.1007/s11164-015-2154-7. [DOI] [Google Scholar]
- Zarei M.; Sepehrmansourie H.; Zolfigol M. A.; Karamian R.; Farida S. H. M. Novel nano-size and crab-like biological-based glycoluril with sulfonic acid tags as a reusable catalyst: its application to the synthesis of new mono-and bis-spiropyrans and their in vitro biological studies. New J. Chem. 2018, 42, 14308–14317. 10.1039/C8NJ02470G. [DOI] [Google Scholar]
- Rajabi-Salek M.; Zolfigol M. A.; Zarei M. Synthesis of a novel DABCO-based nanomagnetic catalyst with sulfonic acid tags: application to the synthesis of diverse spiropyrans. Res. Chem. Intermed. 2018, 44, 5255–5269. 10.1007/s11164-018-3421-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.