Abstract
Serious outbreaks of severe acute respiratory syndrome (SARS), caused by the newly discovered coronavirus SARS-CoV, occurred between late 2002 and early 2003 and there is an urgent need for effective antiviral agents. RNA interference in animals and post-transcriptional gene silencing plants is mediated by small double-stranded RNA molecules named small interfering RNA (siRNA). Recently, siRNA-induced RNA interference(RNAi) may provide a new approach to therapy for pathogenic viruses, e.g. HIV and HCV. In this study, the silencing potential of seven synthetic siRNAs against SARS-CoV leader, TRS, 3′-UTR and Spike coding sequence have been applied to explore the possibility for prevention of SARS-CoV infection. We demonstrate that siRNAs directed against Spike sequences and the 3′-UTR can inhibit the replication of SARS-CoV in Vero-E6 cells, and holds out promise for the development of an effective antiviral agent against SARS-CoV.
Keywords: SARS-CoV, siRNA, Spike protein, Antiviral agent
1. Main text
Severe acute respiratory syndrome (SARS) has recently created a serious health crisis and economic disruption in Canada and Southeast Asia (Lee et al., 2003). A previously unidentified coronavirus (SARS-CoV) isolated from SARS patients has been implicated in the etiology of the disease (Ksiazek et al., 2003, Peiris et al., 2003). No effective therapy is available and scientists worldwide are exploring various possible ways to fight SARS (Kontoyiannis et al., 2003, Anand et al., 2003).
RNA dependent gene silencing, a research hotspot in recent years, involves RNA interference (RNAi) in animals/mammalian cells and post-transcriptional gene silencing in plants. Transfection of 21-nt small interfering RNA (siRNA) into mammalian cells results in degradation of the target gene mRNA and silencing of its expression (Elbashir et al., 2001a). siRNA-induced RNAi may provide a new approach to therapy for pathogenic viruses. RNAi has been used to modulate the replication cycle of human immunodeficiency virus and hepatitis C virus in cultured human cells (Jacque et al., 2002, Wilson et al., 2003). SARS-CoV is a positive-stranded RNA virus, suggesting that siRNA treatment might be feasible for SARS-CoV. Coronavirus-infected cells contain a characteristic 3′ coterminal nested set of mRNAs, each of which has a capped leader sequence of approximate 70 nts derived from the 5′ end of the genome. At the 3′ end of the RNA genome is another UTR, followed by poly (A) of variable length. The sequences of both the 3′- and 5′-UTR are important for RNA replication and transcription. The synthesis of subgenomic negative sense RNA species by discontinuous RNA transcription is regulated by a core transcription-regulating sequence (TRS) found on the genome near the beginning of each ORF and at the 3′ end of the leader (Lai and Holmes, 2001). The consensus sequence, 5′-CUAAAC-3′, is found before each of the S and M genes and ORF 10 of SARS-CoV (Rota et al., 2003). The S glycoprotein is a large multifunctional protein which forms large petal-shaped spikes on the surface of the virions and plays a central role in the biology and pathogenesis of coronavirus (Lai and Holmes, 2001). The availability of the complete genome sequence of SARS-CoV (Rota et al., 2003, Marra et al., 2003) makes the design of siRNA aimed at SARS-CoV possible. We focused on four regions of the SARS-CoV genome, the leader sequence, TRS, 3′-UTR, and the coding sequence for the spike protein, to design specific siRNAs to test whether RNAi could selectively target SARS-CoV viral RNAs and inhibit viral protein expression and replication. Based on recent research (Elbashir et al., 2001a, Elbashir et al., 2001b) and the successful experience of the Ambion Corporation's researchers (Jacque et al., 2002); we designed seven siRNAs that were 21 nts in length and had a G/C content of 30–50%: these were siSARS-L (sense, AGCCAACCAACCTCGATCTdTdT), directed against the leader sequence region of each 5′ subgenomic mRNA; siSARS-TRS (sense, ATCTGTTCTCTAAACGAACdTdT), directed against the TRS region; siSARS-3′UTR1 (sense, TTTCGCAATTCCGTTTACGdTdT) and siSARS-3′UTR2 (sense, CGTAACTAAACAGCACAAGdTdT), directed against the 3′-UTR region; and siSARS-S1 (sense, TGTTGTTATACGAGCATGTdTdT), siSARS-S2 (sense, GGGCTATCAACCTATAGATdTdT), and siSARS-S3 (sense, CAAGGCGATTAGTCAAATTdTdT), directed against the spike protein region. The negative control siRNA, siJEV-M, (sense, CAACACGGACATTGCAGACdTdT) targets the gene for the membrane region of Japanese encephalitis virus. The selected siRNA sequences were subjected to BLAST analysis to ensure that only the intended gene was targeted. All siRNAs were purchased from Dharmacon Research, Inc. (Lafayette, Colo.). Inhibition of synthesis of viral RNA and antigen was measured by RT-PCR, Western blotting and Immunohistochemistry, respectively. In all experiments, Vero E6 cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. Vero E6 cells (4 × 104 cells/well) were seeded in 48-well plates for 24 h, transfected for 1 h with 100 pmol of the various siRNA duplexes using lipofectamine 2000 (Invitrogen Corp, Garlsbad, Calif), then infected with the Hong Kong strain of SARS-CoV at an M.O.I. of 0.01 and examined two days later. When the cells were examined for cytopathic effects by phase-contrast microscopy, cells transfected with siSARS-S2 or siSARS-S3 showed no marked morphologic changes, whereas those transfected with the other siSARSs rounded up and shrank (data not shown). To examine whether virus yield was inhibited by siRNAs, culture supernatants were collected and RT-PCR was employed to detect viral RNA. Two days post-infection, viral RNA was isolated from 140 μl of supernatant from SARS-CoV infected cells with a QIAamp® Viral RNA MINI kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions.One μl of extracted viral RNA was applied for RT-PCR by using Qiagen® One Step RT-PCR kit with SARS-specific primers Cor-p-F2 5′-CTAACATGCTTAGGATAATGG-3′ and Cor-p-R1 5′CAGGTAAGCGTAAAACTCATC3′. This primer set was designed to amplify a region in open reading frame 1b in the genome of SARS-CoV (Ksiazek et al., 2003). The RT-PCR conditions were: 50 °C, 30 min; 95 °C, 15 min; 25 cycles of (94 °C 30 s, 50 °C 30 s, 72 °C 1 min); 72 °C, 10 min. As shown in Fig. 1 , there was a consistent 90 and 85% reduction in viral genomic RNA copies in the supernatants of cells transfected with siSARS-S2 and siSARS-S3, respectively, but the reduction was less marked in siSARS3′UTR2-transfected cells (56%), and much lower in the other cells. This showed that viral RNA synthesis could be inhibited by certain specific siRNAs. As shown in Fig. 2 , when the transfected cells were lysed and subjected to Western blot analysis using a 1:500 dilution of a serum from a convalescent SARS patient (a gift from the Tri-Service General Hospital, Taipei, Taiwan), many prominent bands were seen; these were SARS-CoV viral antigens, as they were not present in non-infected cells. Virally-infected cells treated with siSARS-S2, siSARS-S3, or siSARS3′UTR2 showed, respectively, 64, 51, or 40% inhibition of viral antigen synthesis (Fig. 2). Immunohistochemistry was also used to examine the inhibitory effect of siRNA on viral antigen synthesis. The transfected cells were fixed in 10% formalin for 10 min, washed with PBS, air dried, and treated with acetone/methanol (1:1), then incubated with a 1:500 dilution of the same patient′s serum, washed, and probed with HRP-labeled goat anti-human IgG antibody, and the signal developed in DAB substrate solution (Pierce). Control SARS-CoV-infected cells were dark brown color, whereas siSARS-S2- or siSARS-S3-treated cells were paler, and siSARS-3′UTR2-treated cells were very pale brown (Fig. 3 ), indicating that siSARS-S2 and siSARS-S3 effectively blocked viral antigen synthesis. Taken together, our results showed that siSARS-S2 and siSARS-S3 treatment profoundly reduced the amount of RNA transcripts and viral antigens, while siSARS-3′UTR2 was less effective.
Fig. 1.
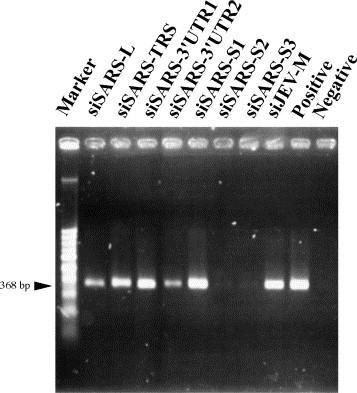
Effect of different siRNAs on viral yields in the culture supernatants from SARS-CoV-infected Vero E6 cells. Viral RNA was isolated from culture supernatants of SARS-CoV-infected Vero E6 cells and subjected to RT-PCR analysis. A marked reduction in the amount of 368 bp PCR product was detected in Vero E6 transfected with siSARS-S2 and siSARS-S3; siSRAS3′UTR2 had less effect, and the other siRNAs have much lower effect. Representative results from three separate experiments are shown. Positive: cells infected by SARS-CoV without siRNA treatment. Negative: cells without virus infection.
Fig. 2.
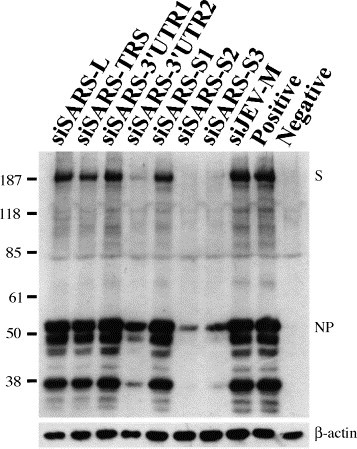
Effect of siRNAs on viral antigen synthesis in SARS-CoV-infected Vero E6 cells. Western blot analysis showed that siSARS-S2 and siSARS-S3 significantly inhibited the synthesis of SARS-CoV antigens in Vero E6 cells. The bottom panel is a Western blot stained with anti-β-actin antibody as an internal control for the amount of protein loaded. The viral antigens spike protein (S) and nucleocapsid protein (NP) are indicated.
Fig. 3.
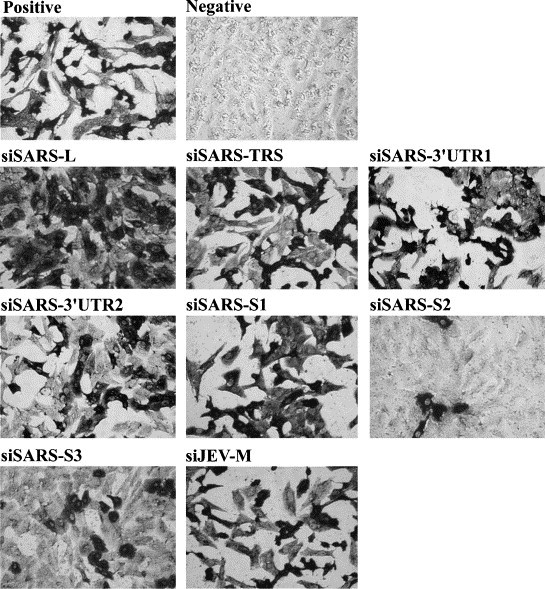
Inhibition of SARS-CoV replication by siRNA in SARS-CoV-infected Vero E6 cells. Vero E6 cells were treated with various siRNA. Immunohistochemistry was performed at 48 h post-infection. Vero E6 were fixed and stained with the same anti-serum directed against SARS-CoV as used in Western blot analysis. Positive: cells infected by SARS-CoV without siRNA treatment. Negative: cells without virus infection.
These data show that, in our test system, 100 pmol of siSARS-S2, siSARS-S3, or siSARS-3′UTR2 siRNA could inhibit SARS-CoV replication. However, further studies are required to optimize the amount of siRNAs and to evaluate the feasibility of using siRNAs in SARS-CoV-infected animals before use in humans.
Acknowledgements
This work was supported by grant NSC 92-2751-B-016-002-Y from the National Science Council, Taipei, Taiwan.
References
- Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M., Martinez J., Patkaniowska A., Lendeckel W., Tuschl T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 2001;20:6877–6888. doi: 10.1093/emboj/20.23.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacque J.M., Triques K., Stevenson M. Modulation of HIV-1 replication by RNA interference. Nature. 2002;418:435–438. doi: 10.1038/nature00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiannis D.P., Pasqualini R., Arap W. Aminopeptidase N inhibitors and SARS. Lancet. 2003;361:1558. doi: 10.1016/S0140-6736(03)13186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., SARS Working Group. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lai M.M., Holmes K.V. Coronaviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincott-Williams and Wilkins; Philadelphia: 2001. pp. 1163–1185. [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y., SARS study group. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Wilson J.A., Jayasena S., Khvorova A., Sabatinos S., Rodrigue-Gervais I.G., Arya S., Sarangi F., Harris-Brandts M., Beaulieu S., Richardson C.D. RNA interference blocks gene expression and RNA synthesis from hepatitis C replicons propagated in human liver cells. Proc. Natl. Acad. Sci. USA. 2003;100:2783–2788. doi: 10.1073/pnas.252758799. [DOI] [PMC free article] [PubMed] [Google Scholar]


