Abstract
Hofmeister series (HS), ion specific effect, or lyotropic sequence acts as a pivotal part in a number of biological and physicochemical phenomena, e.g., changing the solubility of hydrophobic solutes, the cloud points of polymers and nonionic surfactants, the activities of various enzymes, the action of ions on an ion-channel, and the surface tension of electrolyte solutions, etc. This review focused on how ion specificity influences the critical micelle concentration (CMC) and how the thermoresponsive behavior of surfactants, and the dynamic transition of the aggregate, controls the aggregate transition and gel formation and tunes the properties of air/water interfaces (Langmuir monolayer and interfacial free energy). Recent progress of the ion specific effect in bulk phase and at interfaces in amphiphilic systems and gels is summarized. Applications and a molecular level theoretical explanation of HS are discussed comprehensively. This review is aimed to supply a fresh and comprehensive understanding of Hofmiester phenomena in surfactants, polymers, colloids, and interface science and to provide a guideline to design the microstructures and templates for preparation of nanomaterials.
1. Introduction
As a significant solution, electrolyte solutions play vital roles in human life and physicochemical science.1 When electrolytes dissolve in water, they dissociate into hydrated ions. Water molecules rearrange themselves in the hydration shells of the smaller ions due to the largely electric field around the small ions.2 Adding salts to protein aqueous solution affects the protein solubility and many other physicochemical properties of a protein solution.3 More than a century ago, Franz Hofmeister primary determined the capability of different salts to stabilize proteins from water, which depends on the types and concentration of the salts.4a At present, this effect is classified as the Hofmeister series (HS), lyotropic sequences, or ion specificity series and is a very widespread phenomenon.5 The difference of the influence of positive ions on precipitating proteins is smaller than negative charged ions.3 According to the ability of salts to influence the solubility of proteins in aqueous solution, ions can be divided into “salting-in” or “salting-out” ions.6
In recent decades, the ion specificity effects have aroused a new research upsurge because of their high impact in biological and physicochemical science, ranging from protein folding and precipitation to ordering of metal–organic frameworks (MOF) and macromolecules in bulk solutions.7 In addition, the HS is of great influence on polymers, colloids, and interface science. Extensive investigations have been carried out on macroscopic (bulk) thermodynamic behaviors including the CMC, solubility, and phase behavior of surfactants, the dynamic tansition of the aggregates, the swelling behavior of gels, and the cloud points of polymers, surface tension, and the interface property in the existence of different kinds of salts. Although being widely studied, the explanation of the underlying mechanism behind the effect is still under debating.1a,1d In this review, we presented a comprehensive explanation of the underlying mechanism of the HS at the molecular level, summarized the influence of specific ion effects in polymers, surfactants, gels, and the air/water interface, and described the application of the ion specificity effect, which aims to give a new insight of the HS and thus to provide a guideline for design of microstructures and templates for preparing nanostructure. The following parts are involved: (i) The background of the HS and the explanation of its mechanism (Supporting Information); (ii) the influence of the HS in polymer, micelles formation, phase transition of surfactant systems, gel property, lipid bilayers, and air/water interfaces, etc; (iii) the applications of ion specificity effects (Supporting Information).
2. Background of the Hofmeister Series
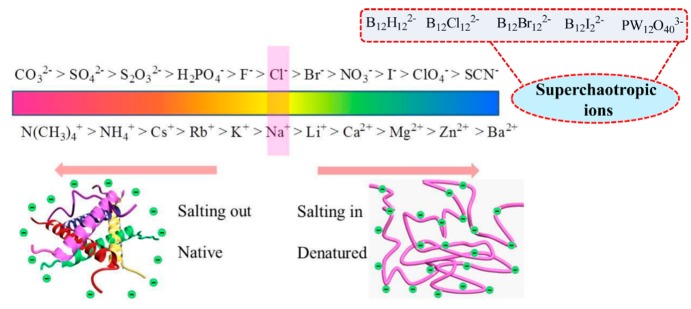
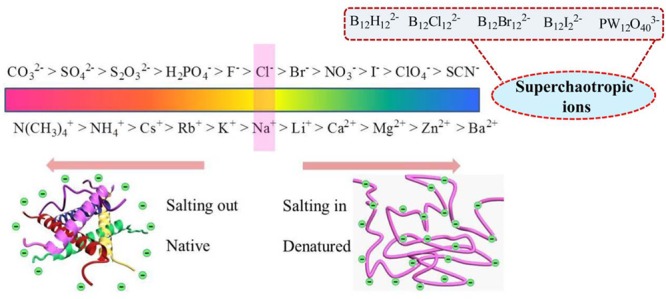
In 1888, Franz Hofmeister first reported that the salts to precipitating proteins and macromolecules out of aqueous solution generally follow a specific ions series, known as the HS, lyotropic sequences, or ion specificity.4a,4c In accordance of the capability of salts to stabilize proteins in solution, the classical Hofmeister ion series could be ranked. The overall order of the anion series is as follows: CO32– > SO42– > S2O32– > H2PO4– > F– > Cl– > Br– > NO3– > I– > ClO4– > SCN– (Figure 1).7 The series include two different groups: strongly hydrated ions and weakly hydrated ions. Anions on the left of Cl– are well hydrated, classified as kosmotrope ions (water structure maker), and tend to stabilize the native fold structure of proteins, leading to salting-out behavior.8a Meanwhile, anions on the right of Cl– are poorly hydrated, called chaotropes (water structure breaker), and tend to facilitate protein denaturation and unfolding, showing salting-in behavior. Cl– is the borderline between the two different kinds of ions, which has no obvious effect on protein stability.8b Recently, the reported larger anions such as anion borate cluster compounds (ABCCs, including dodecaborate anions, metallacarboranes, and carborane cluster) and polyoxometalates (POMs) have the propensity to associate with the hydrophobic and neutral polar phase, which is named the chaotropic effect.9a Simultaneously, theses ions are termed as superchaotropic ions,9b listed on the right of the HS (Figure 1), which extends the traditional HS. The superchaotropic ions do not exhibit hydrophobic properties but arouse special effects in molecular recognition with macrocyclic hosts and biological interaction with proteins,9c−9e which influence is much higher than the chaotropic ions (e.g., SCN–) (detailed discussion shown in section 3). The HS order for cations is N(CH3)4+ > NH4+ > Cs+ > Rb+ > Na+ > Li+ > Ca2+ > Mg2+ > Zn2+ > Ba2+ (Figure 1).1d The weakly hydrated cations on the left tend to stabilize the protein, whereas the divalent cations on the right can increase the solubility of protein and promote their unfolding. As is known, the HS for anions is more obvious than cations, and the position of cation in the HS is always changed in different phenomena.
Figure 1.
Conceptual sketch of the Hofmeister series (HS).
With the development of thermochemistry, many researchers attempt to decide the ion property (structure making or breaking), typically anions, via thermochemical functions, such as entropy (ΔS) and heat capacity (ΔCp).1b Marcus summarized the value of water-structural entropies of ionic hydration (ΔSstruc) based on the ion properties and judged the ion property (kosmotropic and chaotropic) using the ΔSstruc.1b,1e In the chemometric sense, with the value of ΔS > 60 (J·K–1·mol–1), the anions are classified as water structure breaker, while with the values <60 J·K–1·mol–1 the anions are constructed as water structure maker. For Cl– the value of ΔS is about 60, which is defined as the borderline. In the chaotropicity scale, the water structure arrounding the anions decreases, which can be transformed to the effective loss of hydrogen bonding in the solvation shell around the ions (ΔHB). Nau et al. suggested that the ΔHB provides an approach for assessment of ion chaotropic property.1f The anions are qualified as chaotropes, as ΔHB is lower than −1, and the anions are termed as superchaotropic anions when ΔHB < −2.
3. Insights into Ion Specificity Effects from Assembly of Amphiphiles and Interface Property
Numerous phenomenas in surfactants, polymers, and interface science that involved salt exhibit obvious HS law. When being added into the surfactant solution, salts would influence the conformation, CMC, solubility, phase behavior of surfactants, and the dynamic transition of the aggregates. Moreover, they can regulate the self-assembly of surfactant systems and the swelling behavior of gels and can regulate the property of the air/water interface. Since the appearance of the HS in 1888, scientists have paid much attention to explore the ion specificity latent in colloids and interface science. Here we summarized these pieces of research and gave some typical examples of the ion specific effect on conformational change, aggregate transition of amphiphiles, gels, and air/water interface, etc., aiming to unfolding the fantastic Hofmeister series in colloid and surface science.
3.1. Insights from Polymers
The salts have at least four aspects of influence on polymers: (i) the variation of hydrophilicity/hydrophobicity; (ii) the shift of cloud point (CP); (iii) the change of molecule structure conformation; (iv) the alteration of lower critical solution temperature (LCST) or upper critical solution temperature (UCST).10a Well hydrated anions can reduce the solubility of polymer in solution, causing a salting-out effect, whereas the poorly hydrated anions increase the stability of polymer in solution, leading to the salting-in effect.10a For the nonionic surfactants, oligoethylene oxide oleyl ether (CnH2n+1(OCH2CH2)mH, CnEOm) is greatly influenced by anions in a sequence of HS (SO42– > HPO42– > CrO4– > CO32– > Cl– > Br– > NO3– > I– > ClO4– > SCN–).10b The kosmotropic anions, e.g., SO42– and CO32–, make CnEOm more hydrophobic and the chaotropic anions, such as NO3– and ClO4–, cause the nonionic surfactant to be more hydrophilic to facilitate the solubility of CnEOm.
The solubility of CnEOm decreases with temperature through the dehydration of −(O–CH2–CH2)x–OH (the hydrogen bonding vanishing between the −O–CH2–CH2– group and water) and conformational change (polar–nonpolar) of the −O–CH2–CH2– group.10c A liquid–liquid phase transition would occur in CnEOm aqueous solution with heating, and the corresponding temperature is known as the cloud point (Tc). The Tc would be shifted when added salt to CnEOm solutions, which depends on the salt types and property (kosmotrope or chaotrope). The Tc of C8EO4 at 60 mmol·L–1 is 313 K.11a The classical trend of anions in Tc evolution is WO42– > Cl– > SCN–, WO42– with SCN– acting as kosmotrope and chaotrope, by reducing and rising Tc of C8EO4 of ±5 °C, respectively. As important nanometric metal–oxide anions, POMs have large size of ∼1 nm, delocalization charge, and low charge density, classified as superchaotropic anions. The addition of POMs gives rise to a more pronounced increase in Tc than chaotropic anions in the HS at only very low concentrations. The Tc phenomenon of CnEOm is attributed to the change in micelle shape. Upon heating, a sphere to rod-like transition would occur because of the dehydration of the −(O–CH2–CH2)– group, accompanied by an enhancement in intermicellar attractions, giving rise to the phase demixion. The micellar size of nonionic surfactants is hardly affected by the traditional ions.11b However, since the adsorption of POM on CnEOm micelles is driven by the increase in entropy, the intermicellar repulsions and reduction in the micelles size appeared, leading to the remarkable increase in Tc. Recently, Pfitzner found that the emancipation of hydration water molecules from POMs to bulk phase caused the major entropic contribution for adsorption.9b During the adsorption, few water molecules are released, thus, stronger anion hydration caused a less efficient dehydration, i.e., lower entropy gain. The effect of anion polarizability on POMs is small as they scale with the dominant driven force in specific ion effect, dispersion forces.11c
The Hofmeister ion-specific effect on the conformational change of poly(3-alkoxy-4-methylthiophene) (PMNT), a water-soluble cationic polymer, was studied by Xia’s group through UV–vis spectroscopy, resonance Raman spectroscopy, and MD simulation.5a They found that the anions related to a bathochromic-shift absorption of PMNT in the anion sequence: SO42– < F– < Cl– < NO3– < Br– < I– < SCN–. When SO42– and F– were added, the UV–vis absorption of PMNT has no obvious variation, whereas when I– and SCN– were added, bathochromic-shift absorption is clearly observed. The absorption spectra results indicate that different anions addition could induce configuration changes of PMNT. MD evidence shows that this change can be ascribed to the direct interactions between anions and the PMNT backbone, as shown in Figure 2. The ab inito calculation together with MD simulation demonstrates that the PMNT backbone of PMNT would be more extended and ordered with bathochromic-shift absorption due to the strong suppression of I– inducing the hydrophobic collapse of the PMNT backbone. The results can be ascribed to two factors: (i) the electrical repulsion between two adjacent side chains of PMNT would be prevent, because the I- approaches the positively charged side chain; (ii) I– easily combines with the apolar moieties of PMNT. While for kosmotropic anions, e.g. F–, there does not combine with polymer, and since the hydrophobic collapse of PMNT moieties, a fold random-coiled arrangement of backbone with hardly absorption changes is obtained. Main in-plane skeleton Raman modes were performed to further confirm the backbone configurations of PMNT with different anions.5a The results do not supply an exhaustive explanation of the perplexing effect of the HS on cationic conjugated polymers, but they play a basic role of the HS on the structure–function relationships of biomacromolecules.
Figure 2.

Illustration of the interactions between anions and PMNT oligomer (20 repeat units) in water (light blue, F– anion (upper); brown, I– anion (lower)). Reprinted with permission from ref (5a). Copyright 2013 American Chemical Society.
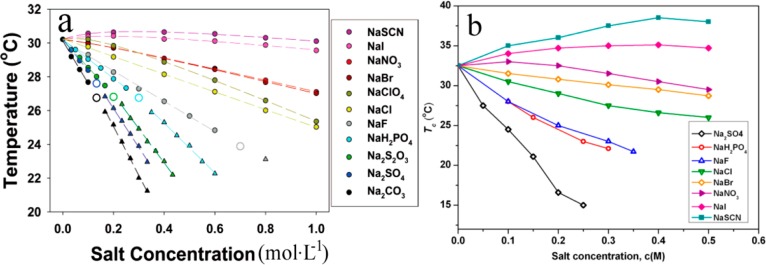
Thermoresponsive polymers have a reversible phase transition with the change in temperature, which have captured the collective imagination of researchers due to their important applications.10a,12a As a thermoresponsive polymer, poly(N-isopropylacrylamide), PNIPAM, exhibits a hydrophobic–hydrophilic transition at the LCST. Below the LCST, PNIPAM is soluble with an extended coil conformation.12b However, PNIPAM becomes insoluble with a folded arrangement above the LCST, causing the aqueous solution to turn to a cloudy state. The LCST of PNIPAM in water is 305 K.12b Bergbreiter and co-workers12c found that the Hofmeister anions have an obvious influence on the LCST of PNIPAM via a temperature gradient microfluidic apparatus and dark-field microscopy determination. Their study reveals that the anion effect on the LCST of PNIPAM is ascribed to three types of interactions of anions with polymer and its hydrated water molecules. The salt effects on the LCST can be modeled by eq 1:12d
| 1 |
where T0 is the LCST of PNIPAM in pure solution and [M] is the molar concentration of salt. The constant c has units of temperature divided by molar concentration. KA is the binding constant between anion and polymer. Bmax is the maximum increase in the LCST.
As shown in Figure 3, the ability of anions to decrease the LCST agrees with the HS sequence. The salting-out anions present a linear relationship at low salt concentration. When the concentration reaches a certain value, a two-step transition is observed, and the phase transition point is plotted. While for the chaotropic species such as ClO4– and SCN–, the effect of anions on the LCST is nonlinear. In Figure 3a, the data calculated from eq 1 are shown with dashed lines. The experimental data coincide with the theoretical calculation. The values of c, Bmax, and KA also fit the experimental data. Zhang et al. synthesized a novel thermoresponsive polymer, 2-hydroxy-3-isopropoxypropyl starches (HIPS), and studied the influence of sodium salts on its Tc.12a In the presence of kosmotropic salts, the hydrogen bonding between polymer and water would be destroyed; meanwhile, some water molecules released from the polymer chains, resulting in a reduction of polymer solubility and a dropping of Tc. On the contrary, chaotropic anions would increase the solubility of polymer and elevate the Tc with concentration, owing to the direct binding to polymer chains. At larger chaotrope concentrations, the Tc goes over a peak and then drops, due to the main driving force of hydrogen bonding between polymer and water, as shown in Figure 3b.
Figure 3.
(a) LCST values of PNIPAM determined in the presence of sodium salts at different concentrations (0 to 1.0 M). The dashed lines are curve fits to the data calculated from eq 1. (b) Change of the Tc of 10 g·L–1 HIPS solutions with the concentration of different sodium salts. Figure 3a was reprinted with permission from ref (12d). Copyright 2005 American Chemical Society. Figure 3b was reprinted with permission from ref (12a). Copyright 2013 American Chemical Society.
Compared to wide investigations of the specific anion effect in aqueous solutions, the study of the Hofmeister effect in nonaqueous solvent (organic or water–organic mixtures) has drawn little attention. Li’s group first investigated the ion specificity of the polymer system in water–organic solvent mixtures and demonstrated that the ranking of ions in the mixed solvent also followed the HS, which could be amplified by adding organic solvents (such as alcohols (EtOH) and dimethyl sulfoxide (DMSO)) to aqueous solution. The tendency of amplification of a specific anion effect can be monitored through the stability of the H2O–ethanol complex, which greatly depends on the structure of alcohols.13a Recently, they systematically studied the anionic specificity on LCST and UCST behavior of PNIPAM in mixed solvent (H2O–EtOH and H2O–DMSO).13b At low molar fraction of EtOH (xE) or DMSO (xD), PNIPAM has a LCST and at relatively high xE or xD, presents a UCST behavior. Turbidity and DSC studies showed that LCST for the anions is consistent with the HS at the molar fraction of xE or xD of 6%, since the solubility of PNIPAM in mixtures is mainly driven by the interactions among water, anion, and PNIPAM. Above the UCST, PNIPAM is soluble, while below the UCST it becomes insoluble. Thus, lower UCST suggested higher solubility. In H2O–EtOH mixtures, at xE = 20%, the anionic specificity on UCST follows the HS, since the dominant driving forces for the UCST are consistent with the LCST behavior. In H2O–DMSO mixtures, at xD= 70%, the anion sequence for the UCST abides by the following series: SCN– < ClO4– < NO3– < Br– > Cl– > CH3COO–. The reason might be that adsorption of the PNIPAM chain surface for anions and anionic polarization of hydrogen bonding between DMSO and PNIPAM have a converse influence on the ranking of HS for UCST. The results revealed that the combined effect of polymer surface adsorption for anions and the anionic polarization of hydrogen bonding between solvent and polymer has a significant role in the anionic specificity on the phase transition of PNIPAM in water–organic mixed solvent mixtures. Thus, we summarize that one can controllably regulate the LCST or Tc of polymer solution by adding different salts to the solution.
As the superchaotropic ions, ABCCs have an intrinsic amphiphilic nature, whose micellization is enthalpy-driven with dominant interactions of hydrophobic (metallacarboranes) or chaotropic (dodecaborate) effect. Due to the surface activity and aggregation mechanism, nanocomposites would be formed when these clusters mixed with polymers.14a The polymeric composites are formed through the spontaneous precipitation of a metallacarborane cluster with poly(ethylene oxide), PEO, in salts.14b The solid NMR results show that the spontaneous precipitation of cluster/PEO complex is dominated by the interaction of dihydrogen bonding between the B–H section of the cluster and the CH2 section of the polymer backbone, assisted by the complexation of cations by PEO. The nanocomposites have a distinct and highly ordered inner structure. Subsequently, the amorphous composites are produced via the coassembly of metallacarborane anion clusters and cationic poly(vinylpyridine) (PVP), in which the orientation of the cluster is dominated by the location of positive charges on the polymer chain.14c Recently, in the presence of Li+, Na+, and K+, the coassembly process of the metallacarborane cluster and double-hydrophilic block copolymer poly(ethylene oxide)-block-poly(2-alkyl oxazoline) (PEO–POX) was studied, aiming to estimate the preferred interaction of cluster with PEO or POX segments of the polymer.14b In the Na-medium, the ABCCs cluster has the same binding ability to PEO and POX section, leading to the formation of homogeneous nanostructures. Li+ has strong binding ability to the O-group in amidic compounds, and the combination of Li+ and clusters with POX is prioritized, which changes the equilibrium toward the complexation of cluster–POX leading to an obvious compartmentalization of POX segments within the hybrid nanoparticles. K+ can be strongly interacted with the PEG section, and simultaneously the PEO-compartments formed. The relatively simple self-assembly procedure of polymer and ABCCs could be used for the preparation of novel polymer–ABCCs complexes with desired functionalities, which provide an approach to build sophisticated supramolecular systems. However, the study of coassembly of ABCCs with polymers is in its infancy and requires further investigation.
3.2. Insights from Micelle Formation
In water, above the CMC, surfactants would spontaneously aggregate to spherical micelles, and at a higher concentration they grow to rodlike micelles. Packing parameter, P (P = v/a0l0, where v is the surfactant tail volume, a0 is the area per headgroup, and l0 is the surfactant chain length), can be used to predict the micellar shape.15a When P < 1/3, spherical micelles would form, while for 1/3 < P < 1/2, rod-like or worm-like micelles would be produced, and for P ∼ 1, a planar aggregate would form. The electrostatic repulsions between charged headgroups can be screened by adding inorganic salts to ionic surfactant solution, which is beneficial to aggregate, leading to the decreasing CMC, the increasing micelle size, and the transition from spherical to worm-like micelles.15b Sodium salts with simple anions to decrease the CMC of ionic surfactants correlate well with the HS law, because the large polarizability of anions enhances the binding of counterions at the micellar surface and screens the electrostatic repulsion among headgroups of surfactant.15bCMC variation dependence on the salt concentration can be expressed by the empiric eq 2:
| 2 |
where A and K0 are empirical constants, Z is the valence of counterions, and ci is the counterions concentration. Compared with the ionic surfactants, upon introduction of salts to nonionic surfactants solution the CMC is hardly changed. Adding salts to nonionic surfactant micellar solution mainly affects the “salting-out” or “salting-in” action. The CMC is decreased by “salting-out” anions and increased by “salting-in” anions.15c
The size of micelles formed by alklglycosides is systematically investigated by Ericsson et al.15d The decrease of micellar size follows the HS sequence: SO42– > Cl– > Br– > NO3– > I– > SCN–. I– and SCN– are chaotropic anions (salting-in) which cause a decrease of micellar size. They found that the salts’ effect on effective headgroup size has more influence on micellar shape than unimer solubility.
Regulating micellar shape characteristics is significant for surfactant function. Abezgauz et al. utilize cryogenic transmission electron microscopy (cryo-TEM), rheology, and scattering to investigate the influence of salts on cetylpyridinium chloride (CPyCl) micelles solution.15a They found that the nature and concentration of added anions and the place in the HS greatly affect the micellar structure. The salting-out anions, Br–, NO3–, and Cl–, induce a sphere → rod-like → worm-like micelles transition, whereas the salting-in anions, CO32–, PO43–, OH–, and SO42–, have no influence on the micellar shape and viscosity. The reason might be that pyridinium headgroups of CPyCl are a chaotropic species and fit the Collin’s law of matching affinities. The results provide a method to methodically tune the self-assembly process of cationic surfactants, including the micellar transition and micellar growth.15e
3.3. Insights from Gels
Organic or inorganic salts can greatly influence the solubility and the aggregates morphology of amphiphiles (polymers, surfactants, or lipids) in aqueous solution. Thus, the addition of sodium salts can effectively modulate the assembled structures of gelators, which provides a powerful approach to control the production of various aggregates from a single gelator. The influence of anions on the hydrophobic interaction of gelators and the more organized supramolecular structures decreases from kosmotrope to chaotrope, causing the decreasing in critical gelation concentrations (CGCs), stability, and the mechanical strength of gels in the order of the HS.16 Ulijn and co-workers found that different morphologies can be produced in gels with different sodium salts.17a Gels formed in PO43– consisted of a fibrous network corresponding to a higher strength. While for SCN–, spherical aggregates formed with a lower strength, for the chloride system, fibers were obtained with similar dimensions to phosphate samples but presented irregular and bendy morphology. The mechanism of an ion-specific effect on the gels’ morphology is the combination of electrostatic screening, ion binding, and hydrophobic interactions. The results open a novel approach to regulate molecular assembly in gel formation.
In addition, the anions in the HS have a strong influence on the swelling behavior of polymer hydrogels and have been extensively studied by Howse et al.17b The swelling degree of polymer hydrogels can be expressed by the expansion ratio (ER), defined as eq 3:
| 3 |
d is the equilibrium domain spacing and d0 is the dry domain spacing. The ER of ionic hydrogels depends on the balance between osmotic pressure (ions inside and outside of hydrogels), polymer–solvent combination, and elastic retractile force of polymers. Osmotic pressure is dominant in these variables; thus, the effect of salts on the expansion must be correlated with this interaction. The ion–water interactions also cause the swelling behavior of polymer gels. The effect of sodium salts on equilibrium ER of poly(methyl methacrylate) (PMMA) gels is shown in Figure 4. The ER value would decrease with the increase of salt concentration (Figure 4b). The decrease in swelling is attributed to the distribution of anions between gels and solution, at fixed salt concentration. The more chaotropic anions easily distribute in the gel, which increases the local ionic strength and causes considerable collapse in gel structure.17b Thus, the decrease of ER induced by anions is as follows: Ace– > Cl– > NO3– > Br– > I– > SCN–.
Figure 4.

(a) Effect of counterions on ER of PMMA, (b) effect of X– (Cl–, Br–, and I–) surface charge density on ER of PMMA at ionic strength of 0.1 mol·L–1 (black circle) and 0.3 mol·L–1 (red square). Reprinted with permission from ref (17b). Copyright 2010 American Chemical Society.
The thermoresponsive gelation behaviors are mainly driven by hydrogen bonding and hydrophobic interaction. Adding salts could change the hydrophobicity of gelators in water and shift the gel–sol transition temperature (Tgel).17c The ionic specific effect on Tgel of gels follows the HS law. The kosmotropic anions (e.g., CO32–, SO42–, F–) show a “salting-out” effect and enhance the gel–sol transition, leading to a lower Tgel, while the chaotropic anions (e.g., I–, SCN–, ClO4–) exhibit salting-in behavior, causing a higher Tgel. Compared to anions, cations have a weaker effect on the gel–sol transition, and the effect is ascribed to the electrostatic interaction between cations and gelators.18a In the cation HS, divalent cations and gelators have stronger electrostatic interactions than monovalent cations; thus, divalent cations have a more remarkable effect on Tgel.18a Moreover, the addition of X– into gels would form X . . .H hydrogen bonding and break the original hydrogen bonding, resulting in the disruption of gel and exhibiting a gel–sol transition. Thus, the addition of F–, AcO–, Cl–, and Br– into the gels formed by the low-molecular-weight gelators (LMWGs), d-gluconic acetal-based molecules, induced a gel–sol transition within several hours, while for the I–, a gel–sol transition was not observed, even after 48 h.17c The influence of anions on gel disruption also follows the HS: H2PO4– > F– > AcO– > Cl– > Br– > I–. The transition process was monitored by 1H NMR spectroscopy performed to monitor the transition process. Yin’s group explored the influence of ions on the gelation of LMWGs, l-glutamic acid Schiff base derives in nonaqueous solvent, DMSO. They found that the gelation capability of anions and the Tgel followed the sequence of SO42–, H2PO4–, AcO–, F–, NO3–, HSO4–, NO2–, which was consistent with the HS.18b That is to say, the gels produced with salting-out anions are more stable compared to the gels formed by salting-in anions. As the effective hydrogen bonding acceptors, anions are greatly competitive in the network structure formation of gels; therefore, the anion specificity has aroused a new research upsurge within the field of LMWGs gels.
3.4. Insight from Phase Transition
Because of the charged headgroups of surfactants, the introduction of salts has diverse influence on surfactant solution, such as aggregation transition and phase separation. The addition of salts to cationic–anionic surfactant systems would screen the electrostatic attractions between oppositely charged headgroups of surfactant, which affects the effective headgroup area and causes the aggregation transition.19
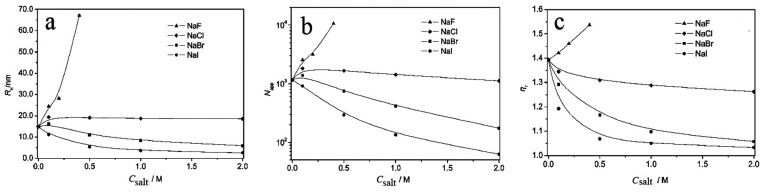
For the equimolar cationic–anionic surfactant mixtures, decyltriethylammonium bromide (C10NE) and sodium decylsulfonate (C10SO3Na), the HS anions shield the electrostatic repulsion, increase a0, and decrease P, giving rising to an aggregate transition from vesicle to micelle.19 The influence of NaX (X = F, Cl, Br, I) on C10NE-C10SO3 mixtures is shown in Figure 5. The addition of NaF can increase the size of C10NE-C10SO3 vesicles, and the concentration of NaF increases to a value where phase separation would occur. The surfactant–water interface can be stabilized by Br– and I– through preferential combination with surfactant, resulting in a “salting-in” effect. Thus, a decreasing of the size of C10NE-C10SO3 aggregates and the vesicle-to-micelle transition appears by the addition of NaBr or NaI. I– has higher ability in decreasing the aggregate size than Br–, which is consistent with the HS law. Since Cl– is the intermediate anion in the HS, the effect of NaCl on the C10NE-C10SO3 vesicles is small.20
Figure 5.
Effect of NaX (X = F–, Cl–, Br–, I–) on C10NE-C10SO3 mixtures with the total concentration of 30 mmol·L–1 at 25 °C. (a) Hydrodynamic radius (Rh) of C10NE-C10SO3 aggregates; (b) apparent aggregation number (Napp) of C10NE-C10SO3 aggregates; (c) relative viscosity (ηr) of C10NE-C10SO3 solutions. Reprinted with permission from ref (20). Copyright 2011 Royal Society of Chemistry.
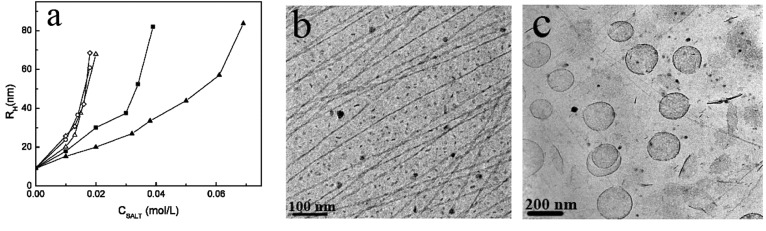
In cationic–anionic surfactant mixtures with excess anionic surfactant, the excess anions would compete with the headgroups, resulting in less hydration of headgroups with increasing ionic strength.19 The weaker hydration caused a smaller effective a0, resulting in a higher P and amicelle-to-vesicle transition. In dodecyltrimethylammonium bromide (DTAB) and excess sodium dodecyl sulfate (SDS) mixtures, an aggregate transition from rod-like micelles to vesicles occurred after addition of salts into the mixtures.. After the formation of vesicles, no anion specificity appeared upon adding salts. Whereas the nature of the cations had strong influence on the critical salt concentration of aggregate transition micelles-to-vesicles,21 the effect of cations on aggregate transition was studied by changing the concentration of alkali chloride salts. As shown in Figure 6a, the mean hydrodynamic radius (Rh) of micelles increases from 10 to 70 nm with increase in salt concentration. Based on the ability to increase the Rh of the aggregates, the cations can be ordered: Cs+ ∼ Rb+ ≥ K+ > Na+ > Li+. Cryo-TEM observation further confirms the transition from ribbon-like micelle to vesicles in the presence of salts (Figure 6b and c).21
Figure 6.
(a) Effect of chloride salts on the growth of Rh of DTAB/SDS aggregates: CsCl (○), RbCl (◇), KCl (Δ), NaCl (■), and LiCl (▲). Cryo-TEM photographs of DTAB/SDS mixtures at a total concentration of 1 wt % and a molar ratio of 1:2.5 without NaCl (b) and with 44 mmol·L–1 NaCl (c). Reprinted with permission from ref (21). Copyright 2007 American Chemical Society.
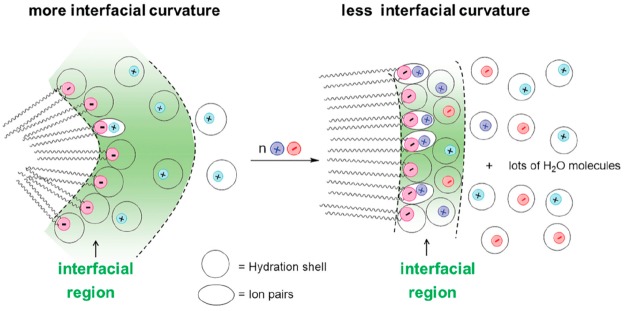
Interfacial molarities variation during the micelles to vesicles transition in sodium bis(2-ethylhexyl) sulfosuccinate (AOT) solution after adding salts was monitored by chemical trapping (CT).22a The transition from micelle to vesicle has specificity to cation and obeys the HS (Cs+ > Rb+ > K+ > Na+ > Li+), no matter the anion type. A great reduction in interfacial water molarity and an increase in interfacial headgroup molarity concurrently appeared, as shown in Figure 7. The added salts increase the interfacial molarities causing an increase in P and resulting in a series phase transition (micelles to vesicles to lamellar structures). When salts were added into the AOT micelles solutions, an ion-pair would form in the interfacial regions and some water would be released. Due to the diffusion of water molecules into the bulk phase the solution entropy increases, which also contributes to the micelle-to-vesicle transition.
Figure 7.
Added salt, MXn, influence on the interfacial composition of AOT micelles (left) and vesicles (right). Reprinted with permission from ref (22a). Copyright 2019 Royal Society of Chemistry.
Compared with the halogens and oxoacids in the HS, POMs are negatively charged metal oxide clusters with large size of about 1 nm. Due to strong electrostatic interaction, POMs can be strongly adsorbed onto the zwitterionic or cationic lipid bilayer, causing fluid-to-gel phase transition and structure breaking of the bilayer. POM adsorption on the lipid bilayer could release energy, which comes from the enthalpy related to gelation and the entropy relevant to local surface rebuilding of the bilayer.22b The liposome shrinkage and morphological breaking further confirmed the reconstruction of the lipid bilayer induced by POMs.
Saha found that KClO4, KI, KCl, and KF could affect the interaction between host and guest molecule.16 At a lower salts concentration, host–guest complexes are more stable, and the fluorescence intensity enhances. The fluorescence property can be regulated by adding different potassium salts. Regulating the optical properties of the dye in β-cyclodextrin nanochannels could promote researchers to synthesize more supramolecular materials.22c,22d
At a given temperature, adding anions can also induce aggregate transition. Increase of kosmotropic anion concentration can decrease the transition temperature, whereas increase of the chaotropic anions concentration increases the transition temperature. Koynova and co-workers derived a general thermodynamic equation to give a quantitative description of the anions effect on transition temperature, which is similar to the Clausius–Claperyron equation interrelating transition temperature and pressure, as shown in eq 4:22e
| 4 |
where x is the fraction of interfacial water per surfactant molecule, c is the solute concentration in interfacial water, ΔH is the molar transition heat, and R is the gas constant; the prime and second signs denote the high and low temperature phase, respectively.
The specific anions effect on phase transition temperature was investigated by Zhang et al.8b PEO–PPO–PEO polymer solution contains weakly hydrated anions and shows two different phase transitions. The first phase transition involves the dehydration of PPO block at lower temperature, which represents the critical micellization temperature. The second phase transition refers to the dehydration of PEO block, which exhibits cloud point temperature. The transition temperature presents a complex dependence on salts nature and concentration. The phase transition modulation of PEO–PPO–PEO by adding salts can be described by eqs 1 and (5). In the presence of weakly hydrated anions, the first phase transition is fit with eq 1. The second phase transition can be described by eq 5:
| 5 |
where c, the value for strongly hydrated and weakly hydrated anions, correlates with different properties of anions.8b
3.5. Insights from Langmuir Monolayer
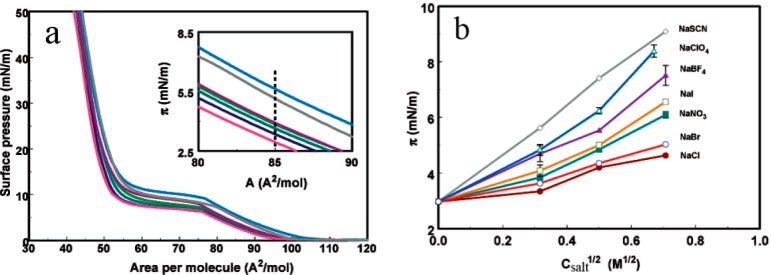
Lenotids’s group studied the effect of sodium salts on the Langmuir monolayer of a zwitterionic lipid, 1,2-dipalmitoylphosphatidylcholine (DPPC).23 Since DPPC is zwitterionic, the interaction between DPPC and anions is not mainly driven by Coulomb interactions. Adding salts to the subphase of the DPPC monolayer can increase the pressure at fixed molecular area, which suggests the stabilization of the liquid-expanded phase (LEP) of the monolayer. The surface pressure–molecular area (π–A) isotherms of DPPC on the water surface and 0.1 mol·L–1 sodium salt solutions are shown in Figure 8a. In the range between 80 to 90 Å2, the monolayer is in irregular LEP.24 In the area range of 80 to 90 Å2, all isotherms are general parallel to water, which suggests that the change of π is ascribed to the absorption of salts at the DPPC monolayer. Different anions increase the π following the sequence: Cl– < Br– < NO3– < I– < BF4– < ClO4– < SCN–, coinciding with classical HS law. Figure 8b shows a direct illustration of different anions on the DPPC Langmuir monolayer. A straight line is obtained with the slopes being consistent with the HS law.
Figure 8.
(a) π–A isotherms of the subphase of DPPC monolayer with water and 0.1 mmol·L–1 sodium salts solution. From top to bottom: DPPC on NaSCN, NaClO4, NaBF4, NaI, NaNO3, NaBr, NaCl solutions and on water. (b) Monolayer π as a function of the square root of salt concentration for a lipid area of 85 Å2. Reprinted with permission from ref (23). Copyright 2004 American Chemical Society.
The surface hydrophobicity can be quantified by interfacial free energy (γ). For macroscopic surfaces, γ is referred to as the energy needed to increase the exposure area of water or solution surfaces, which can be measured by the work of adhesion, Wad. The Wad is defined as the energy required to divide the two surfaces surrounded with water or aqueous solution. For two chemically identical surfaces:
| 6 |
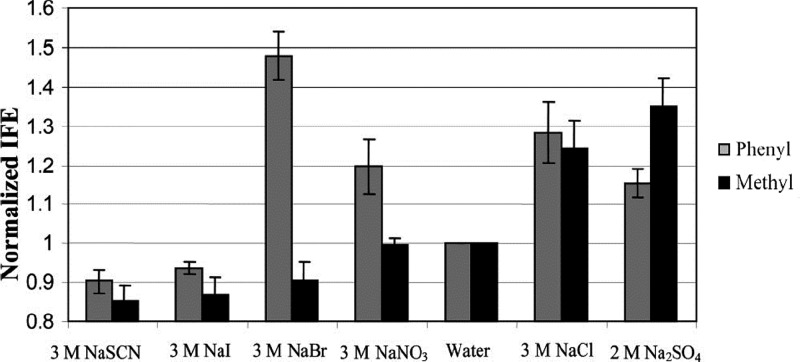
Serafin and co-workers studied the Hofmeister-type anion effect on γ of hydrophobic monolayers through Wad determination in chemical force microscopy (CFM).24,25 The selected anions span the range of the HS, from kosmostropic Na2SO4 to chaotropic NaSCN. The specific-ion dependence on the γ of aliphatic (terminal methyl) and aromatic (terminal phenyl) monolayers was studied and shown in Figure 9. The general trend for the aliphatic monolayer agrees well with the HS law: SO42– > Cl– > NO3– > I– > SCN–, i.e., chaotropic ions decrease γ, whereas kosmotropic ions increase it. However, for the aromatic monolayer, the specific-ion behavior substantially deviates from the HS law. Especially, NaBr and NaNO3 solutions present a great increase in γ compared to water. The differences between aromatic and aliphatic surface are attributed to the metal−π (or metal−π–π) interactions and the “normal” hydrophobic specific-ion effect. The specific ion effect on an aromatic surface provides novel perception into the cosolvent effect on biologically relevant processes.25
Figure 9.
Normalized γ values for aliphatic and aromatic monolayers in different sodium salts. The salts aqueous solution concentrations are inserted. Reprinted with permission from ref (25). Copyright 2009 American Chemical Society.
4. Conclusions and Outlook
Many physicochemical and biological phenomena are relevant to the electrolytes solution properties, especially water. Numerous phenomena in colloids, polymers, and interface science that involved salts exhibit dramatic ion specificity. At present, ABCCs and POMs greatly extend the HS and promote the progress of the HS in colloid and interfaces science. This review systematically described the counterions and superchaotropic ions (ABCCs and POMs) effects on colloids and interface field, including the thermoresponsiveness of polymers, the CGC and the swelling behavior of gels and gel–sol transition, the phase transition of cationic–anionic systems, the micelles formation, and the Langmuir monolayer, which aims to provide a novel perception into ion specificity from the assembly and the interface properties of amphiphiles. Two model mechanisms, indirect and direct mechanisms of action, including the molecular level explanation, were elaborately discussed to explain the mechanism behind the Hofmeister effect. In general, the ions specific effect on the variation of hydrophilicity/hydrophobicity, the shift of cloud point, the alteration of LCST or UCST of polymers, on decreasing the CMC of surfactants and decreasing in CGCs, stability, the mechanical strength and the Tgel of gels, and the increase in surface pressure are coincident with the direct HS. The variation of shift of cloud point, change of molecule structure conformation of polymers, the decrease of ER and Tgel of gels, phase transition, can be explained by the direct actions mechanism. The variation of hydrophilicity/hydrophobicity of polymers can be explained by the indirect actions mechanism. The alteration of LCST or UCST of polymer can be explained by the combination of indirect actions and direct actions. However, the actual solution systems are inevitably complicated by a delicate balance of all the interactions (water–water, water–ions, water–solutes, ions–solutes, cations–anions), which requires much more strenuous effort to give a molecular level interpretation. Moreover, many experimental techniques should be developed to determine the microscopic structures of solutes and solvents and to provide deep insight into the involved mechanisms dominant in the dynamics of water and ions.
In the past several decades, a number of ion specific effects on surfactant systems were reported. However, the investigation mainly focused on the anion effect, and few cation effects were considered. In order to give an overall understanding of the ion specific effect in colloidal systems, the effect of cations on the amphiphile assembly and air/water interface property should be undertaken in the next investigation. The ion–dipole and nonelectrostatic interactions play important roles in the Hofmeister effect; thus, the HS is not restricted to aqueous solution but can exist in organic solvents, e.g., ethanol, DMSO, and ethylene carbonate, etc., which should be paid much attention in further research. In addition, since the Hofmeister effect could modulate the aggregates transition via imparting different salts, it is envisaged to be used for constructing microstructures and templates for different practical applications in the future.
Acknowledgments
This work is financially supported by the NSFC (21603123), the Opening Project of Oil & Gas Field Applied Chemistry Key Laboratory of Sichuan Province (KF201405).
Biographies

Beibei Kang received her Bachelor of Engineering degree in Applied Chemistry from Shandong University of Technology, Zibo, P. R. China, in 2019. Lately, Kang continues her Ms.D. under the supervision of Doctor Song at Shandong University of Technology. Her research direction is “Surfactant hydrogels” and she has published 3 original articles.

Huicheng Tang received his Bachelor of Engineering degree from Shandong University of Technology, Zibo, P. R. China, in 2019. Subsequently, Tang continues his Ms.D. under the supervision of Doctor Song at Shandong University of Technology. His research direction is “Self-healing materials”, and he has published 2 original articles.

Zengdian Zhao received his Ms.D. from Nanjing University of Technology, Nanjing, P. R. China, in 2004. In July, 1987, he joined the Shandong University of Technology, where he became Professor in 2001.

Shasha Song received her Ph.D. from Shandong University, in 2015. In July, 2015, she joined Shandong University of Technology. Her research focused on viscoelastic hydrogels: structures and functions and she has published 13 original articles.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00237.
Theoretical explanation of the Hofmeister series and application of the Hofmeister effect (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Xie W.; Gao Y. A simple theory for the Hofmeister series. J. Phys. Chem. Lett. 2013, 4, 4247–4252. 10.1021/jz402072g. [DOI] [PubMed] [Google Scholar]; b Ferreira L.; Uversky V.; Zaslavsky B. Effects of the Hofmeister series of sodium salts on the solvent properties of water. Phys. Chem. Chem. Phys. 2017, 19, 5254–5261. 10.1039/C6CP08214A. [DOI] [PubMed] [Google Scholar]; c Mazzini V.; Craig V. What is the fundamental ion-specific series for anions and cations? Ion specificity in standard partial molar volumes of electrolytes and electrostriction in water and non-aqueous solvents. Chem. Sci. 2017, 8, 7052–7065. 10.1039/C7SC02691A. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Oncsik T.; Desert A.; Trefalt G.; Borkovec M.; Szilagyi I. Charging and aggregation of latex particles in aqueous solutions of ionic liquids: towards an extended Hofmeister series. Phys. Chem. Chem. Phys. 2016, 18, 7511–7520. 10.1039/C5CP07238G. [DOI] [PubMed] [Google Scholar]; e Marcus Y. ViscosityB-coefficients, structural entropies and heat capacities, and the effects of ions on the structure of water. J. Solution Chem. 1994, 23, 831–848. 10.1007/BF00972677. [DOI] [Google Scholar]; f Assaf K.; Nau W. The chaotropic effect as an assembly motif in chemistry. Angew. Chem., Int. Ed. 2018, 57, 13968–13981. 10.1002/anie.201804597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Marcus Y. Effect of ions on the structure of water: structure making and breaking. Chem. Rev. 2009, 109, 1346–1370. 10.1021/cr8003828. [DOI] [PubMed] [Google Scholar]; b Lee E.; Choi J.; Cho M. The effect of Hofmeister anions on water structure at protein surfaces. Phys. Chem. Chem. Phys. 2017, 19, 20008–20015. 10.1039/C7CP02826A. [DOI] [PubMed] [Google Scholar]; c Okur H.; Hladílková J.; Rembert K.; Cho Y.; Heyda J.; Dzubiella J.; Cremer P.; Jungwirth P. Beyond the Hofmeister series: ion-specific effects on proteins and their biological functions. J. Phys. Chem. B 2017, 121, 1997–2014. 10.1021/acs.jpcb.6b10797. [DOI] [PubMed] [Google Scholar]
- Aoki K.; Shiraki K.; Hattori T. Salt effects on the picosecond dynamics of lysozyme hydration water investigated by terahertz time-domain spectroscopy and an insight into the Hofmeister series for protein stability and solubility. Phys. Chem. Chem. Phys. 2016, 18, 15060–15069. 10.1039/C5CP06324H. [DOI] [PubMed] [Google Scholar]
- a Hofmeister F. On the understanding of the effects of salts. Naunyn-Schmiedeberg's Arch. Pharmacol. 1888, 24, 247–260. 10.1007/BF01918191. [DOI] [Google Scholar]; b Li K.; Yang J.; Gu J. Salting-in species induced self-assembly of stable MOFs. Chem. Sci. 2019, 10, 5743–5748. 10.1039/C9SC01447K. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Pérez-Fuentes L.; Bastos-González D.; Faraudo J.; Drummond C. Effect of organic and inorganic ions on the lower critical solution transition and aggregation of PNIPAM. Soft Matter 2018, 14, 7818–7828. 10.1039/C8SM01679H. [DOI] [PubMed] [Google Scholar]
- a Qiu M.; Long S.; Li B.; Yan L.; Xie W.; Niu Y.; Wang X.; Guo Q.; Xia A. Toward an understanding of how the optical property of water-soluble cationic polythiophene derivative is altered by the addition of salts: The Hofmeister effect. J. Phys. Chem. C 2013, 117, 21870–21878. 10.1021/jp407430y. [DOI] [Google Scholar]; b Rouster P.; Pavlovic M.; Szilagyi I. Destabilization of titania nanosheet suspensions by inorganic salts: Hofmeister series and Schulze-Hardy rule. J. Phys. Chem. B 2017, 121, 6749–6758. 10.1021/acs.jpcb.7b04286. [DOI] [PubMed] [Google Scholar]
- Klaus A.; Tiddy G.; Rachel R.; Trinh A.; Maurer E.; Touraud D.; Kunz W. Hydrotrope-induced inversion of salt effects on the cloud point of an extended surfactant. Langmuir 2011, 27, 4403–4411. 10.1021/la104744e. [DOI] [PubMed] [Google Scholar]
- Wang L.; Guo Y.; Li P.; Song Y. Anion-specific effects on the assembly of collagen layers mediated by magnesium ion on mica surface. J. Phys. Chem. B 2014, 118, 511–518. 10.1021/jp405035x. [DOI] [PubMed] [Google Scholar]
- a Janc T.; Lukšič M.; Vlachy V.; Rigaud B.; Rollet A.; Korb J. Ion-specificity and surface water dynamics in protein solutions. Phys. Chem. Chem. Phys. 2018, 20, 30340–30350. 10.1039/C8CP06061D. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Deyerle B.; Zhang Y. Effects of Hofmeister anions on the aggregation behavior of PEO–PPO–PEO triblock copolymers. Langmuir 2011, 27, 9203–9210. 10.1021/la201463g. [DOI] [PubMed] [Google Scholar]
- a Assaf K.; Ural M.; Pan F.; Georgiev T.; Simova S.; Rissanen K.; Gabel D.; Nau W. Water structure recovery in chaotropic anion recognition: high-affinity binding of dodecaborate clusters to γ-cyclodextrin. Angew. Chem., Int. Ed. 2015, 54, 6852–6856. 10.1002/anie.201412485. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Buchecker T.; Schmid P.; Renaudineau S.; Diat O.; Proust A.; Pfitzner A.; Bauduin P. Polyoxometalates in the Hofmeister series. Chem. Commun. 2018, 54, 1833–1836. 10.1039/C7CC09113C. [DOI] [PubMed] [Google Scholar]; c Sakamoto A.; Unoura K.; Nabika H. Molecular scale insights into activity of polyoxometalate as membrane-targeting nanomedicine from single-molecule observations. J. Phys. Chem. C 2018, 122, 1404–1411. 10.1021/acs.jpcc.7b11251. [DOI] [Google Scholar]; d Assaf K.; Hennig A.; Peng S.; Guo D.; Gabel D.; Nau W. Hierarchical host–guest assemblies formed on dodecaborate-coated gold nanoparticles. Chem. Commun. 2017, 53, 4616–4619. 10.1039/C7CC01507K. [DOI] [PubMed] [Google Scholar]; e Okur H.; Hladílková J.; Rembert K.; Cho Y.; Heyda J.; Dzubiella J.; Cremer P.; Jungwirth P. Beyond the Hofmeister series: Ion-specific effects on proteins and their biological functions. J. Phys. Chem. B 2017, 121, 1997–2014. 10.1021/acs.jpcb.6b10797. [DOI] [PubMed] [Google Scholar]
- a Umapathi R.; Reddy P. Influence of additives on thermoresponsive polymers in aqueous media: a case study of poly (N-isopropylacrylamide). Phys. Chem. Chem. Phys. 2018, 20, 9717–9744. 10.1039/C7CP08172C. [DOI] [PubMed] [Google Scholar]; b Dag Ö.; Alayoǧlu S.; Uysal İ. Effects of ions on the liquid crystalline mesophase of transition-metal salt: surfactant (CnEOm). J. Phys. Chem. B 2004, 108, 8439–8446. 10.1021/jp049716x. [DOI] [Google Scholar]; c Dong R.; Hao J. Complex fluids of poly (oxyethylene) monoalkyl ether nonionic surfactants. Chem. Rev. 2010, 110, 4978–5022. 10.1021/cr9003743. [DOI] [PubMed] [Google Scholar]
- a Naskar B.; Diat O.; Nardello-Rataj V.; Bauduin P. Nanometer-size polyoxometalate anions adsorb strongly on neutral soft surfaces. J. Phys. Chem. C 2015, 119, 20985–20992. 10.1021/acs.jpcc.5b06273. [DOI] [Google Scholar]; b Bauer C.; Bauduin P.; Girard L.; Diat O.; Zemb T. Hydration of sugar based surfactants under osmotic stress: A SAXS study. Colloids Surf., A 2012, 413, 92–100. 10.1016/j.colsurfa.2012.03.006. [DOI] [Google Scholar]; c Ninham B.; Yaminsky V. Ion binding and ion specificity: the Hofmeister effect and Onsager and Lifshitz theories. Langmuir 1997, 13, 2097–2108. 10.1021/la960974y. [DOI] [Google Scholar]
- a Ju B.; Cao S.; Zhang S. Effect of additives on the cloud point temperature of 2-hydroxy-3-isopropoxypropyl starch solutions. J. Phys. Chem. B 2013, 117, 11830–11835. 10.1021/jp404083r. [DOI] [PubMed] [Google Scholar]; b Du H.; Wickramasinghe R.; Qian X. Effects of Salt on the Lower Critical Solution Temperature of Poly (N-Isopropylacrylamide). J. Phys. Chem. B 2010, 114, 16594–16604. 10.1021/jp105652c. [DOI] [PubMed] [Google Scholar]; c Shechter I.; Ramon O.; Portnaya I.; Paz Y.; Livney Y. Microcalorimetric study of the effects of a chaotropic salt, KSCN, on the lower critical solution temperature (LCST) of aqueous poly(N-isopropylacrylamide) (PNIPA) solutions. Macromolecules 2010, 43, 480–487. 10.1021/ma9018312. [DOI] [Google Scholar]; d Zhang Y.; Furyk S.; Bergbreiter D.; Cremer P. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. 10.1021/ja0546424. [DOI] [PubMed] [Google Scholar]
- a Xu Y.; Liu G. Amplification of Hofmeister effect by alcohols. J. Phys. Chem. B 2014, 118, 7450–7456. 10.1021/jp504317j. [DOI] [PubMed] [Google Scholar]; b Liu L.; Wang T.; Liu C.; Lin K.; Liu G.; Zhang G. Specific anion effect in water–nonaqueous solvent mixtures: interplay of the interactions between anion, solvent, and polymer. J. Phys. Chem. B 2013, 117, 10936–10973. 10.1021/jp406215c. [DOI] [PubMed] [Google Scholar]
- a Dordovic V.; Tosner Z.; Uchman M.; Zhigunov A.; Reza M.; Ruokolainen J.; Pramanik G.; Cigler P.; Kalikova K.; Gradzielski M.; Matejicek P. Stealth amphiphiles: self-assembly of polyhedral boron clusters. Langmuir 2016, 32, 6713–6722. 10.1021/acs.langmuir.6b01995. [DOI] [PubMed] [Google Scholar]; b Dordovic V.; Uchman M.; Reza M.; Ruokolainen J.; Zhigunov A.; Ivankov O.; Matějíček P. Cation-sensitive compartmentalization in metallacarborane containing polymer nanoparticles. RSC Adv. 2016, 6, 9884–9892. 10.1039/C5RA27588A. [DOI] [Google Scholar]; c Brus J.; Zhigunov A.; Czernek J.; Kobera L.; Uchman M.; Matějíček P. Control over the self-assembly and dynamics of metallacarborane nanorotors by the nature of the polymer matrix: A solid-state NMR study. Macromolecules 2014, 47, 6343–6354. 10.1021/ma501117a. [DOI] [Google Scholar]
- a Abezgauz L.; Kuperkar K.; Hassan P.; Ramon O.; Bahadur P.; Panino D. Effect of Hofmeister anions on micellization and micellar growth of the surfactant cetylpyridinium chloride. J. Colloid Interface Sci. 2010, 342, 83–92. 10.1016/j.jcis.2009.08.045. [DOI] [PubMed] [Google Scholar]; b Jiang N.; Li P.; Wang Y.; Wang J.; Yan H.; Thomas R. Micellization of cationic gemini surfactants with various counterions and their interaction with DNA in aqueous solution. J. Phys. Chem. B 2004, 108, 15385–15391. 10.1021/jp0488057. [DOI] [Google Scholar]; c Khatory A.; Lequeux F.; Ken F.; Candau S. Linear and nonlinear viscoelasticity of semidilute solutions of wormlike micelles at high salt content. Langmuir 1993, 9, 1456–1464. 10.1021/la00030a005. [DOI] [Google Scholar]; d Ericsson C.; Söderman O.; Garamus V.; Bergström M.; Ulvenlund S. Effects of Temperature, Salt, and Deuterium Oxide on the Self-aggregation of alkylglycosides in dilute solution. 1. n-Nonyl-β-D-glucoside. Langmuir 2004, 20, 1401–1408. 10.1021/la035613e. [DOI] [PubMed] [Google Scholar]
- Nebot V.; Ojeda-Flores J.; Smets J.; Fernández-Prieto S.; Escuder B.; Miravet J. Rational design of heat-set and specific-ion-responsive supramolecular hydrogels based on the hofmeister effect. Chem. - Eur. J. 2014, 20, 14465–14472. 10.1002/chem.201402547. [DOI] [PubMed] [Google Scholar]
- a Roy S.; Javid N.; Sefcik J.; Halling P.; Ulijn R. Salt-induced control of supramolecular order in biocatalytic hydrogelation. Langmuir 2012, 28, 16664–16670. 10.1021/la303388s. [DOI] [PubMed] [Google Scholar]; b Swann J.; Bras W.; Topham P.; Howse J.; Ryan A. Effect of the Hofmeister anions upon the swelling of a self-assembled pH-responsive hydrogel. Langmuir 2010, 26, 10191–10197. 10.1021/la100339f. [DOI] [PubMed] [Google Scholar]; c Guan X.; Fan K.; Gao T.; Ma A.; Zhang B.; Song J. A novel multi-stimuli responsive gelator based on D-gluconic acetal and its potential applications. Chem. Commun. 2016, 52, 962–965. 10.1039/C5CC08615A. [DOI] [PubMed] [Google Scholar]
- a Ding F.; Tang Z.; Ding B.; Xiong Y.; Cai J.; Deng H.; Du Y.; Shi X. Tunable thermosensitive behavior of multiple responsive chitin. J. Mater. Chem. B 2014, 2, 3050–3056. 10.1039/c4tb00067f. [DOI] [PubMed] [Google Scholar]; b Sun J.; Liu Y.; Jin L.; Chen T.; Yin B. Coordination-induced gelation of an L-glutamic acid Schiff base derivative: the anion effect and cyanide-specific selectivity. Chem. Commun. 2016, 52, 768–771. 10.1039/C5CC07903A. [DOI] [PubMed] [Google Scholar]
- Vlachy N.; Jagoda-Cwiklik B.; Vácha R.; Touraud D.; Jungwirth P.; Kunz W. Hofmeister series and specific interactions of charged headgroups with aqueous ions. Adv. Colloid Interface Sci. 2009, 146, 42–47. 10.1016/j.cis.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Chen L.; Xing H.; Yan P.; Ma J.; Xiao J. Anomalous effects of concentrated salts on the equimolarly mixed cationic–anionic surfactant mixtures. Soft Matter 2011, 7, 5365–5372. 10.1039/c1sm05115f. [DOI] [Google Scholar]
- Renoncourt A.; Vlachy N.; Bauduin P.; Drechsler M.; Touraud D.; Verbavatz J.; Dubois M.; Kunz W.; Ninham B. Specific alkali cation effects in the transition from micelles to vesicles through salt addition. Langmuir 2007, 23, 2376–2381. 10.1021/la062837z. [DOI] [PubMed] [Google Scholar]
- a Liu C.; Wang Y.; Gao Y.; Zhang Y.; Zhao L.; Xu B.; Romsted L. Effects of interfacial specific cations and water molarities on AOT micelle-to-vesicle transitions by chemical trapping: the specific ion-pair/hydration model. Phys. Chem. Chem. Phys. 2019, 21, 8633–8644. 10.1039/C8CP05987J. [DOI] [PubMed] [Google Scholar]; b Jing B.; Hutin M.; Connor E.; Cronin L.; Zhu Y. Polyoxometalate macroion induced phase and morphology instability of lipid membrane. Chem. Sci. 2013, 4, 3818–3826. 10.1039/c3sc51404h. [DOI] [Google Scholar]; c Sowmiya M.; Tiwari A.; Eranna S.; Sharma A.; Saha S. Study of the binding interactions of a hemicyanine dye with nanotubes of β-cyclodextrin and effect of a hofmeister series of potassium salts. J. Phys. Chem. C 2014, 118, 2735–2748. 10.1021/jp406533u. [DOI] [Google Scholar]; d Medda L.; Barse B.; Cugia F.; Boström M.; Parsons D.; Ninham B.; Monduzzi M.; Salis A. Hofmeister challenges: ion binding and charge of the BSA protein as explicit examples. Langmuir 2012, 28, 16355–16363. 10.1021/la3035984. [DOI] [PubMed] [Google Scholar]; e Koynova R.; Brankov J.; Tenchov B. Modulation of lipid phase behavior by kosmotropic and chaotropic solutes. Eur. Biophys. J. 1997, 25, 261–274. 10.1007/s002490050038. [DOI] [PubMed] [Google Scholar]
- Aroti A.; Leontidis E.; Maltseva E.; Brezesinski G. Effects of Hofmeister anions on DPPC Langmuir monolayers at the air- water interface. J. Phys. Chem. B 2004, 108, 15238–15245. 10.1021/jp0481512. [DOI] [Google Scholar]
- Leontidis E.; Belloni A. Liquid expanded monolayers of lipids as model systems to understand the anionic Hofmeister series: 1. A tale of models. J. Phys. Chem. B 2009, 113, 1447–1459. 10.1021/jp809443d. [DOI] [PubMed] [Google Scholar]
- Patete J.; Petrofsky J.; Stepan J.; Waheed A.; Serafin J. Hofmeister effect on the interfacial free energy of aliphatic and aromatic surfaces studied by chemical force microscopy. J. Phys. Chem. B 2009, 113, 583–588. 10.1021/jp807876s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.