Abstract
Diarrhea represents one of the most frequent disorders in dogs. In puppies, degradation of feces quality is associated with a reduced daily weight gain and an increased risk of death. Prevention of diarrhea in puppies requires a global approach encompassing enteropathogens, environment and management practices especially when housed in groups. The purpose of this study was to determine prevalence of enteropathogens in puppies in breeding kennels and to identify risk factors of diarrhea. Two hundred and sixty six puppies (between 5 and 14 weeks of age) from 29 French breeding kennels were included. For each kennel, data about environment, management of the kennel and puppies’ characteristics (age, sex and breed) were collected. For each puppy, fecal consistency and fecal excretion of enteropathogens (viruses and parasites) was evaluated. At least one enteropathogen was identified in 77.1% of puppies and 24.8% of puppies presented abnormal feces. The main risk factor of weaning diarrhea was fecal excretion of canine parvovirus type 2 (odds ratio = 5; confidence interval 95%: 1.7–14.7). A targeted sanitary and medical prophylaxis against canine parvovirus type 2 should be implemented to decrease risk of weaning diarrhea.
Keywords: Dog, Puppy, Enteropathogens, Diarrhea, Weaning, Parvovirus, Epidemiology
1. Introduction
Gastrointestinal and hepatic diseases in dogs are the third most frequent problem reported by owners in United States and Australia (Freeman et al., 2006). Diarrhea represents one of the most frequent disorders in dogs examined at private veterinary practice, with a prevalence of 2.2% (Lund et al., 1999), young dogs under 6 months of age being at a higher risk of diarrhea than adult dogs (Tupler et al., 2012). In puppies, degradation of feces quality is associated with a reduced daily weight gain and an increased risk of death (Grellet et al., 2012).
A great variety of parasites and viruses are described to be enteropathogens during the weaning period in puppies. Giardia duodenalis, Cryptosporidium parvum, Toxocara canis, Cystoisospora ohioensis complex, Cystoisospora canis, canine parvovirus type 2 (CPV2) and canine coronavirus (CCV) are the most prevalent (Hackett and Lappin, 2003). However, as in other species, diarrhea is multifactorial, involving factors intrinsic to the dog (breed size and age), nutritional factors (diet change without transition, food type and quality), together with lifestyle and environmental stressors (Weber et al., 2002, Weber et al., 2003, Sokolow et al., 2005, Hernot et al., 2006, Stavisky et al., 2011). Most studies on risk factors of diarrhea in young dogs focused on one single pathogen or a group of pathogens without taking into account environmental stressors (Finlaison, 1995, Buehl et al., 2006, Grellet et al., 2012, Tupler et al., 2012). Moreover most of the studies considering multiple enteropathogens infections were performed in shelters, in a context far different from that in breeding kennels (Sokolow et al., 2005, Tupler et al., 2012). The purpose of this epidemiological study was to determine prevalence of enteropathogens in puppies in breeding kennels and to perform a risk factors analysis for diarrhea during the weaning period including enteropathogens, environment and management procedures.
2. Materials and methods
2.1. Animals and breeding kennels
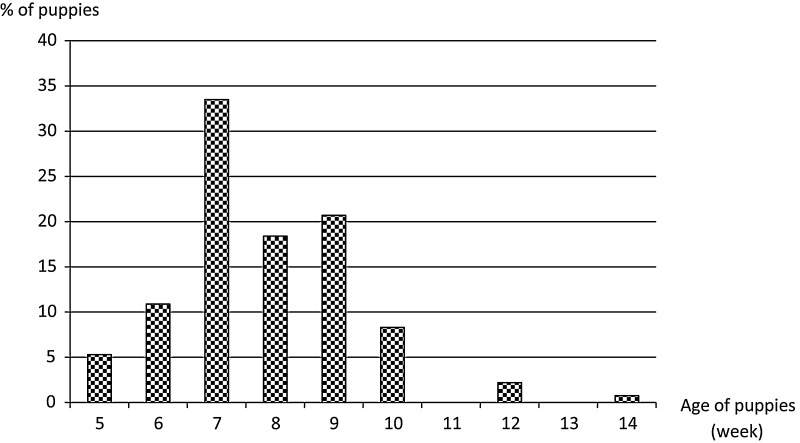
A total of 266 puppies (60 litters) from 29 French breeding kennels were included in this study between May and September 2009 (mean of 9 puppies included per kennel; range: 2–18). Puppies were between 5 and 14 weeks of age (mean: 7.8 weeks of age) (Fig. 1 ). These breeding kennels were randomly selected from a data base of breeders registered at Alfort Veterinary School for training programs. Only puppies with a normal clinical examination were included (puppies with clinical signs of prostration, dehydration and/or anorexia were excluded of this study). For each kennel, data concerning environmental factors (number of puppies sold per year, and litter size for each puppies included), management of the kennel and puppies (number of meals distributed per day, access to outdoor, vaccination) and puppies’ characteristics (age, breed, sex), were collected. Puppies vaccinated within the preceding 10 days before the visit were not included.
Fig. 1.
Distribution of puppies with age (n = 226).
Depending on the mean adult body weight of their respective breed, puppies were divided in two groups (small if mean adult body weight < 25 kg; large otherwise). Small breed dogs represented 25.6% (68/266) of the total number of dogs included. Based on the mean number of puppies sold per year (calculated over the last two years and considered as the size of the kennel), kennels were also separated into “small” (i.e. less than 30 puppies sold per year) and large kennels (i.e. more than 30 puppies sold per year). Puppies housed in breeding kennels producing 30 puppies or more per year represented 51.1% (136/266) of the total number dogs included. Puppies were divided into two groups according to the number of meal per day: puppies receiving less than 4 meals per day and puppies receiving 4 meals per days or more.
2.2. Evaluation of feces consistency
For each puppy, fecal consistency was evaluated by a single operator using a 13-point scale, based on the texture and shape of the feces (from liquid to hard and dry) (Grellet et al., 2012). Based on growth rate, thresholds for abnormal feces were previously validated and appeared to vary with breed stature and age (Grellet et al., 2012). Briefly, feces with a score ≤ 5 was classified as abnormal for large breed puppies whatever the age, for small breed puppies, fecal scores ≤6 and ≤7 were classified as abnormal for 4–5 weeks old puppies and for older puppies between 6 and 8 weeks old, respectively.
After collection, stools were separated in three samples, one being stored at +4 °C for coproscopy and other frozen (−20 °C) for Giardia intestinalis and Cryptosporidium parvum copro-antigens quantification.
A rectal swab was performed for each puppy immediately after stool collection for detection of canine parvovirus type 2 (CPV2) and canine coronavirus (CCV). The swabs were stored at −20 °C until DNA extraction.
2.3. Intestinal parasites
By the standard McMaster flotation technique using saturated magnesium sulphate solution (density: 1.28 g/ml) (Bauer et al., 2010), all eggs and oocysts were identified according to their morphological characteristics under light microscopy by a single operator (Levine and Ivens, 1965, Baek et al., 1993).
Copro-antigens of G. intestinalis and C. parvum were quantified on 100 mg of feces using respectively the ProSpecT-Giardia and the ProSpecT-Cryptosporidium Microplate Assay kit (Remel, France) (Decock et al., 2003, Mekaru et al., 2007, Rimhanen-Finne et al., 2007). An optical density value > 0.05 was considered positive according to the manufacturer's instructions.
2.4. Coronavirus and parvovirus fecal excretions
CPV2 and CCV detection were performed by qPCR and qRT-PCR respectively as already described (Grellet et al., 2012). Results from duplicate analyses (mean of two results) were expressed semi-quantitatively as viral load levels. Puppies were defined as excreting CPV2 and CCV for high viral loads over 1010.3 copies and 109.3 copies respectively (Grellet et al., 2012).
2.5. Data management and statistical analysis
Statistical analyses were performed with the SAS version 9.3 software (SAS Institute Inc., Cary, NC, USA).
2.5.1. Statistical analysis for prevalence of enteropathogens
Number of puppies with fecal positive and negative test results for each enteropathogen was tabled by different factors under study like age of puppies, size of the kennel, breed size, and litter size. Univariate analyses of the putative risk factors for each enteropathogen infection were performed. The significance of the univariate associations was determined using the χ 2-tests. A P value < 0.05 was considered statistically significant.
2.5.2. Statistical analysis for risk factors of abnormal feces
Correlation matrix of quantitative and dichotomous variables (excretion of CPV2, CCV, G. intestinalis, C. parvum, T. canis, C. ohioensis complex, and C. canis and number of meal per day, litter size, breeding kennel size) was determined with Kendall's Tau-b measure of correlation coefficient (Proc CORR). These highly correlated variables were defined as predictors in a partial least squares regression (Proc PLS) with fecal consistency as the response variable. The Variable Importance for Projection (VIP) statistic of Wold (1994) was used to assess the contribution of each predictor to the model (Wold, 1994). Only predictors with a VIP value over 0.8 were selected to be included in a new partial least squares regression (Wold, 1995). Variables of the final partial least squares regression with a VIP value over 0.8 were not collinear (r 2 < 0.10). These variables were subsequently integrated as independent variables and assessed as a fixed effect in a generalized linear mixed model (proc GLIMMIX) with fecal consistency as a binary outcome (logit transformation). As data on puppies were nested within naturally occurring hierarchies (puppies within litter, litters within breeding kennel), litter variable nested within breeding kennel, written as litter (breeding kennel), was defined as a random term. The respective influence of litter and breeding kennel as random effects was also determined.
3. Results
3.1. Prevalence of enteropathogens
77.1% (205/266) of the puppies were infected by at least one enteropathogen with 29.3% of them excreting 3 pathogens or more (Table 1, Table 2 ). Seven different viruses and parasites were identified. 14.7% of puppies (39/266) were infected by CPV2, 20.3% (54/266) by CCV, 41% (109/266) by Giardia sp., 25.9% (69/266) by C. parvum, 25.6% (68/266) by C. ohioensis complex, 22.2% (59/266) by T. canis, and 13.2% (35/266) by C. canis. All enteropathogens except T. canis presented a significantly higher prevalence in large breeding kennels. Puppies between 5 and 8 weeks of age presented a significantly higher prevalence of CPV2 and C. ohioensis complex and a lower prevalence of CCV and G. duodenalis than puppies between 9 and 14 weeks of age (Table 3 ).
Table 1.
Frequency of identification of enteropathogens in fecal samples.
| No. of viruses identified per puppy |
No. of parasites identified per puppy |
Total number of enteropathogens identified per puppy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 5 |
| 65.4 (174) | 34.2 (91) | 0.4 (1) | 25.6 (68) | 34.6 (92) | 28.6 (76) | 9.0 (24) | 2.3 (6) | 22.9 (61) | 21.8 (58) | 32 (85) | 17.7 (47) | 4.1 (11) | 1.5 (4) |
Data are given as % (number) of puppies.
Table 2.
Frequency of coinfection between enteropathogens in puppies.
| CPV2 | CCV | T. canis | C. ohioensis complex | C. canis | Giardia sp. | C. parvum |
|---|---|---|---|---|---|---|
| CPV2 | 0.4 (1) | 2.3 (6) | 1.5 (4) | 7.5 (20) | 11.3 (30) | 5.6 (15) |
| CCV | 1.9 (5) | 4.1 (11) | 3.8 (1) | 15.8 (42) | 4.9 (13) | |
| T. canis | 11.7 (31) | 4.1 (11) | 3.8 (10) | 9 (24) | ||
| C. ohioensiscomplex | 11.3 (3) | 3.8 (10) | 9.4 (25) | |||
| C. canis | 10.2 (27) | 4.5 (12) | ||||
| Giardiasp. | 11.7 (31) | |||||
| C. parvum |
Data are given as % (number) of puppies.
Table 3.
Prevalence of enteropathogens depending of puppies’ characteristics and environmental factors.
| Pathogens | Total prevalence | Age of puppies |
Size of the kennel |
Breed size |
Litter size |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5–6 weeks % ni/n |
7–8 weeks % ni/n |
9–14 weeks % ni/n |
Global P value | <30% ni/n |
≥30% ni/n |
P value | Large % ni/n |
Small % ni/n |
P value | ≤4% ni/n |
5–7% ni/n |
≥8% ni/n |
Global P value | ||
| CPV2 | 14.7 39/266 |
23.3a 10/43 |
16.7a 23/138 |
7.1b 6/85 |
0.032 | 0 0/130 |
28.7 39/136 |
<0.001 | 14.1 28/198 |
16.2 11/68 |
0.682 | 22.6a 14/62 |
20a 18/90 |
6.1b 7/114 |
0.003 |
| CCV | 20.3 54/266 |
7b 3/43 |
13.8b 19/138 |
37.6a 32/85 |
<0.001 | 0 0/130 |
39.7 54/136 |
<0.001 | 23.2 46/198 |
11.8 8/168 |
0.043 | 8.1b 5/62 |
32.2a 29/90 |
17.5b 20/114 |
0.001 |
| T. canis | 22.2 59/266 |
44.2a 19/43 |
22.5b 31/138 |
10.6c 9/85 |
<0.001 | 29.2 38/130 |
15.4 21/136 |
0.007 | 22.7 45/198 |
20.6 14/68 |
0.714 | 16.1 10/62 |
17.8 16/90 |
28.9 33/114 |
0.069 |
| C. ohioensis complex | 25.6 68/266 |
30.2a 13/43 |
31.9a 44/138 |
12.9b 11/85 |
0.005 | 23.8 31/130 |
27.2 37/136 |
0.002 | 29.3 58/198 |
14.7 10/68 |
0.017 | 19.4b 12/62 |
13.3b 12/90 |
38.5a 44/114 |
<0.001 |
| C. canis | 13.2 35/266 |
41.9a 18/43 |
6.8b 8/138 |
10.6b 9/85 |
<0.001 | 1.5 2/130 |
24.3 33/136 |
<0.001 | 17.2 34/198 |
1.5 1/68 |
0.001 | 4.8 3/62 |
16.7 15/90 |
14.9 17/114 |
0.081 |
| G. duodenalis | 41 109/266 |
32.6b 14/43 |
30.4b 42/138 |
62.4a 53/85 |
<0.001 | 17.7 23/130 |
63.2 86/136 |
<0.001 | 41.4 82/198 |
39.7 27/68 |
0.805 | 51.6a 32/62 |
46.7a 42/90 |
30.7b 35/114 |
0.011 |
| C. parvum | 25.9 69/266 |
37.2 16/43 |
22.5 31/138 |
25.9 22/85 |
0.156 | 20.8 27/130 |
30.9 42/136 |
0.06 | 26.3 52/198 |
25 17/68 |
0.838 | 27.4a 17/62 |
12.2b 11/90 |
36a 41/114 |
0.001 |
For each line, categories with different letters (a,b,c)were significantly different (P < 0.05).
ni/n = number of puppies infected for the category considered/total number of puppies in the category considered.
3.2. Risk factors of abnormal feces
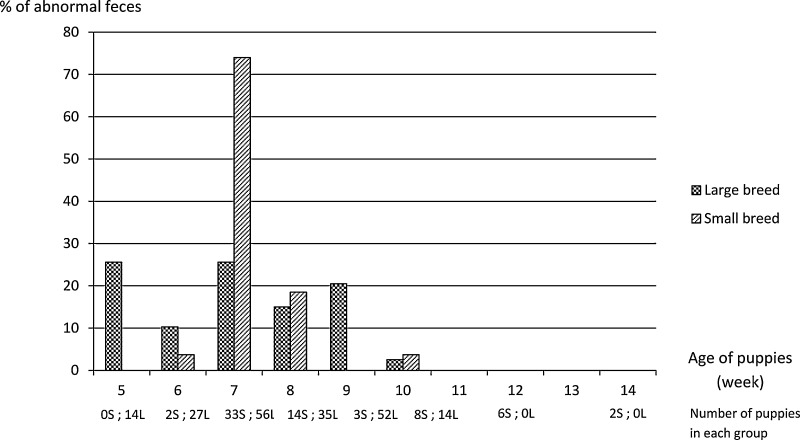
Sixty six out of 266 feces evaluated (24.8%) were classified as abnormal (Fig. 2 ). In the initial partial least squares regression CPV2, C. canis, G. intestinalis and the number of meal per day presented a VIP over 0.8. These four factors were included in a new partial least squares regression. CPV2 and number of meal per day were two factors keeping a significant impact on the incidence of abnormal feces with a VIP over 0.8 (VIP = 1.7 and VIP = 1.0 respectively). In the final model only fecal excretion of CPV2 increased risk of weaning diarrhea (P = 0.003, odds ratio = 5; confidence interval 95%: 1.7–14.7). 61.5% (24/39) of puppies infected by CPV2 presented abnormal feces compared to 15.2% (42/277) of puppies not infected by CPV2. A global significant effect of litter and breeding kennel was observed, with a significant effect of the litter level (P < 0.001), and no significant effect of the breeding kennel level (P = 0.101).
Fig. 2.
Incidence of abnormal feces depending on breed size (n = 266 puppies).
4. Discussion
The present study represents the first investigation of the prevalence of weaning diarrhea in puppies living in a breeding kennel. Prevalence of this clinical sign, affecting both growth and survival was high with 24.8% of puppies between 5 and 14 weeks concerned. Among the seven different enteropathogens (2 viruses and 5 parasites) tested in this study, 77.1% of puppies were infected by at least one virus or parasite, and 55.3% carried multiple organisms. Prevalence of parasites was higher than the prevalence of viruses (74.4% vs 34.6%). This high prevalence of multiple infections is in accordance with a previous study on dogs entering animal shelters in which 55% of them presented multiple digestive infections (Tupler et al., 2012). In our study, 14.7% of puppies were excreting CPV2, 20.3% by CCV. However, only 0.4% of puppies presented a mixed infection by these two viruses. Prevalence of these viruses depends on age, lifestyle and health status of dogs. Serological and virological investigations demonstrated that CCV and CPV2 are highly prevalent in kennels and animal shelters compared to single owned dogs (Rimmelzwaan et al., 1991, Tennant et al., 1993, Bandai et al., 1999, Naylor et al., 2001b, Schulz et al., 2008). Moreover a higher prevalence of CCV and CPV2 was described in young animals under 6 months of age compared to adult dogs (Sakulwira et al., 2003, Gates and Nolan, 2009a, Gates and Nolan, 2009b, Epe et al., 2010). In addition to the wide distribution and contagiosity of these viruses, the methods used for detection of these viruses can also contribute to this high prevalence. PCR assays have been proven to be up to 4 × 104 times more sensitive than electronic microscopy and virus isolation for detection of CCV (Naylor et al., 2001a). This method is able to detect virus in the feces of low-grade shedding animals below 106 particles per gram of unprocessed feces, which is considered the detection limit for electronic microscopy. In our study, a higher prevalence of CPV2, CCV was observed in breeding kennels producing 30 puppies per year or more. The higher prevalence in this sub-population could be linked to the contagiousness of these pathogens and their stability in the environment (Terpstra et al., 2007, Eterpi et al., 2010). The close contact between animals and the density of puppies could promote the environmental contamination and subsequently the spread of the infection.
17.9% of puppies in our study were found excreting a high load of CPV2. This virus is well described as inducing hemorrhagic diarrhea associated with vomiting, anorexia, dehydration and depression (Meunier et al., 1985, Prittie, 2004). However, in our study, CPV2 also increased risk of weaning diarrhea but without systemic signs, as already described, and 12.5% of dogs without gastrointestinal disease excreted this virus (Grellet et al., 2012). Thus our study demonstrated that puppies can excrete high viral loads of CPV2 without any systemic sign. This observation is in accordance with one previous study in which fecal excretion was also quantified by PCR (Schmitz et al., 2009). However studies using less sensitive methods (fecal antibody-based antigen tests, immune-electron microscopy) did not observed this healthy carrier status (Hackett and Lappin, 2003, Desario et al., 2005, Sokolow et al., 2005, Schulz et al., 2008, Schmitz et al., 2009). The lack of systemic clinical signs on these puppies could be linked either to an efficient systemic immunity or to local intestinal immunity (Rice et al., 1982, Macartney et al., 1988). Fecal IgA, endogenous or provided by milk, protect intestinal mucosa by inhibiting the adherence of pathogens, thereby preventing adhesion of these pathogens. Canine milk was found rich in IgA (Heddle and Rowley, 1975) with high levels of CPV2 antibodies (Decaro et al., 2004). These antibodies, repeatedly ingested in large quantities by the puppies during the first weeks of life, could provide some protection to the intestinal mucosa against CPV2 deleterious effects decreasing the systemic clinical signs associated to CPV2 infection (septicaemia, dehydration). Nevertheless these clinically healthy animals probably represent major sources of virus for other animals and for the environmental contamination. Interestingly, in our study, young puppies (between 5 and 8 weeks of age) presented a higher infection rate by CPV2 than older ones. This result highlights the interest of vaccination before 8 weeks of age in breeding kennels to limit CPV2 spreading. The interference with maternally derived antibodies considered as one of the most important causes of immunization failure in puppies (Macartney et al., 1988), can be overcome by the use of high titer CPV2 vaccines (De Cramer et al., 2010).
Other infectious agents tested were not associated with weaning diarrhea. In our study, 20.3% of puppies were infected by CCV, but this virus was not identified as a risk factor of abnormal feces. Implications of CCV in acute dog diarrhea are controversial. No relation between coronavirus and diarrhea was observed in different studies, with more healthy dogs infected by this virus than dogs with diarrhea in some of these studies (Sokolow et al., 2005, Schulz et al., 2008, Tupler et al., 2012). However, CCV was also described as a virus inducing severe gastroenteritis, lethal in some cases (Evermann et al., 2005, Buonavoglia et al., 2006, Decaro et al., 2008, Decaro et al., 2009). These variations in clinical signs could be linked to variations in pathogenicity between strains (Escutenaire et al., 2007), to the age of infected dogs (Decaro et al., 2009), to the number of genotypes infecting puppies simultaneously (Decaro et al., 2005) or to the association of the coronavirus with other enteropathogens (Appel, 1988). Neither C. Ohioensis complex nor C. canis were associated with weaning diarrhea in our study. Impact of these parasites on weaning diarrhea is still controversial (Buehl, 2006). This difference of clinical signs observed between studies may be explained by differences in the age of infected dogs, the environmental conditions and the virulence of species.
5. Conclusion
Based on this study, CPV2 infection was the major risk factors of weaning diarrhea. Some central strategies can be suggested like a targeted sanitary and medical prophylaxis against CPV2, particularly in large breeding kennels.
References
- Appel M.J.G. Does canine coronavirus augment the effects of subsequent parvovirus infection? Vet. Med. 1988:360–366. [Google Scholar]
- Baek B.K., Kim C.S., Kim J.H., Han K.S., Kim Y.G. Studies on isosporosis in dogs. I: isolation and sporulation of Isospora ohioensis. Korean J. Parasitol. 1993;31:201–206. doi: 10.3347/kjp.1993.31.3.201. [DOI] [PubMed] [Google Scholar]
- Bandai C., Ishiguro S., Masuya N., Hohdatsu T., Mochizuki M. Canine coronavirus infections in Japan: virological and epidemiological aspects. J. Vet. Med. Sci. 1999;61:731–736. doi: 10.1292/jvms.61.731. [DOI] [PubMed] [Google Scholar]
- Bauer B.U., Pomroy W.E., Gueydon J., Gannac S., Scott I., Pfister K. Comparison of the FLOTAC technique with the McMaster method and the Baermann technique to determine counts of Dictyocaulus eckerti L1 and strongylid eggs in faeces of red deer (Cervus elaphus) Parasitol. Res. 2010;107:555–560. doi: 10.1007/s00436-010-1893-z. [DOI] [PubMed] [Google Scholar]
- Buehl I.E., Prosl H., Mundt H.C., Tichy A.G., Joachim A. Canine isosporosis – epidemiology of field and experimental infections. J. Vet. Med. B: Infect. Dis. Vet. Public Health. 2006;53:482–487. doi: 10.1111/j.1439-0450.2006.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C., Castagnaro M., Tempesta M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006;12:492–494. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cramer K.G., Stylianides E., van Vuuren M. Efficacy of vaccination at 4 and 6 weeks in the control of canine parvovirus. Vet. Microbiol. 2010;149:126–132. doi: 10.1016/j.vetmic.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Decaro N., Campolo M., Lorusso A., Desario C., Mari V., Colaianni M.L., Elia G., Martella V., Buonavoglia C. Experimental infection of dogs with a novel strain of canine coronavirus causing systemic disease and lymphopenia. Vet. Microbiol. 2008;128:253–260. doi: 10.1016/j.vetmic.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Desario C., Campolo M., Cavalli A., Ricci D., Martella V., Tempesta M., Buonavoglia C. Evaluation of lactogenic immunity to canine parvovirus in pups. New Microbiol. 2004;27:375–379. [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Campolo M., Mari V., Desario C., Lucente M.S., Lorusso E., Kanellos T., Gibbons R.H., Buonavoglia C. Immunity after natural exposure to enteric canine coronavirus does not provide complete protection against infection with the new pantropic CB/05 strain. Vaccine. 2009;28:724–729. doi: 10.1016/j.vaccine.2009.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Ricci D., Elia G., Desario C., Campolo M., Cavaliere N., Di Trani L., Tempesta M., Buonavoglia C. Genotype-specific fluorogenic RT-PCR assays for the detection and quantitation of canine coronavirus type I and type II RNA in faecal samples of dogs. J. Virol. Methods. 2005;130:72–78. doi: 10.1016/j.jviromet.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decock C., Cadiergues M.C., Larcher M., Vermot S., Franc M. Comparison of two techniques for diagnosis of giardiasis in dogs. Parasite. 2003;10:69–72. doi: 10.1051/parasite/2003101p69. [DOI] [PubMed] [Google Scholar]
- Desario C., Decaro N., Campolo M., Cavalli A., Cirone F., Elia G., Martella V., Lorusso E., Camero M., Buonavoglia C. Canine parvovirus infection: which diagnostic test for virus? J. Virol. Methods. 2005;126:179–185. doi: 10.1016/j.jviromet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Epe C., Rehkter G., Schnieder T., Lorentzen L., Kreienbrock L. Giardia in symptomatic dogs and cats in Europe – results of a European study. Vet. Parasitol. 2010;173:32–38. doi: 10.1016/j.vetpar.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Escutenaire S., Isaksson M., Renstrom L.H., Klingeborn B., Buonavoglia C., Berg M., Belak S., Thoren P. Characterization of divergent and atypical canine coronaviruses from Sweden. Arch. Virol. 2007;152:1507–1514. doi: 10.1007/s00705-007-0986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eterpi M., McDonnell G., Thomas V. Virucidal activity of disinfectants against parvoviruses and reference viruses. Appl. Biosaf. 2010;15:165–171. [Google Scholar]
- Evermann J.F., Abbott J.R., Han S. Canine coronavirus-associated puppy mortality without evidence of concurrent canine parvovirus infection. J. Vet. Diagn. Invest. 2005;17:610–614. doi: 10.1177/104063870501700618. [DOI] [PubMed] [Google Scholar]
- Finlaison D.S. Faecal viruses of dogs – an electron microscopy study. Vet. Microbiol. 1995;46:295–305. doi: 10.1016/0378-1135(95)00094-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L.M., Abood S.K., Fascetti A.J., Fleeman L.M., Michel K.E., Laflamme D.P., Bauer C., Kemp B.L., Van Doren J.R., Willoughby K.N. Disease prevalence among dogs and cats in the United States and Australia and proportions of dogs and cats that receive therapeutic diets or dietary supplements. J. Am. Vet. Med. Assoc. 2006;229:531–534. doi: 10.2460/javma.229.4.531. [DOI] [PubMed] [Google Scholar]
- Gates M.C., Nolan T.J. Endoparasite prevalence and recurrence across different age groups of dogs and cats. Vet. Parasitol. 2009;166:153–158. doi: 10.1016/j.vetpar.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates M.C., Nolan T.J. Risk factors for endoparasitism in dogs: retrospective case–control study of 6578 veterinary teaching hospital cases. J. Small Anim. Pract. 2009;50:636–640. doi: 10.1111/j.1748-5827.2009.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grellet A., Feugier A., Chastant-Maillard S., Carrez B., Boucraut-Baralon C., Casseleux G., Grandjean D. Validation of a fecal scoring scale in puppies during the weaning period. Prev. Vet. Med. 2012;106:315–323. doi: 10.1016/j.prevetmed.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett T., Lappin M.R. Prevalence of enteric pathogens in dogs of North-Central Colorado. J. Am. Anim. Hosp. Assoc. 2003;39:52–56. doi: 10.5326/0390052. [DOI] [PubMed] [Google Scholar]
- Heddle R.J., Rowley D. Dog immunoglobulins. I. Immunochemical characterization of dog serum, parotid saliva, colostrum, milk and small bowel fluid. Immunology. 1975;29:185–195. [PMC free article] [PubMed] [Google Scholar]
- Hernot D.C., Dumon H.J., Biourge V.C., Martin L.J., Nguyen P.G. Evaluation of association between body size and large intestinal transit time in healthy dogs. Am. J. Vet. Res. 2006;67:342–347. doi: 10.2460/ajvr.67.2.342. [DOI] [PubMed] [Google Scholar]
- Levine N.D., Ivens V. Isospora species in the dog. J. Parasitol. 1965;51:859–864. [PubMed] [Google Scholar]
- Lund E.M., Armstrong P.J., Kirk C.A., Kolar L.M., Klausner J.S. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J. Am. Vet. Med. Assoc. 1999;214:1336–1341. [PubMed] [Google Scholar]
- Macartney L., Thompson H., McCandlish I.A., Cornwell H.J. Canine parvovirus: interaction between passive immunity and virulent challenge. Vet. Rec. 1988;122:573–576. doi: 10.1136/vr.122.24.573. [DOI] [PubMed] [Google Scholar]
- Mekaru S.R., Marks S.L., Felley A.J., Chouicha N., Kass P.H. Comparison of direct immunofluorescence, immunoassays, and fecal flotation for detection of Cryptosporidium spp. and Giardia spp. in naturally exposed cats in 4 Northern California animal shelters. J. Vet. Intern. Med. 2007;21:959–965. doi: 10.1892/0891-6640(2007)21[959:codiia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Meunier P.C., Cooper B.J., Appel M.J., Slauson D.O. Pathogenesis of canine parvovirus enteritis: the importance of viremia. Vet. Pathol. 1985;22:60–71. doi: 10.1177/030098588502200110. [DOI] [PubMed] [Google Scholar]
- Naylor M.J., Harrison G.A., Monckton R.P., McOrist S., Lehrbach P.R., Deane E.M. Identification of canine coronavirus strains from feces by S gene nested PCR and molecular characterization of a new Australian isolate. J. Clin. Microbiol. 2001;39:1036–1041. doi: 10.1128/JCM.39.3.1036-1041.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M.J., Monckton R.P., Lehrbach P.R., Deane E.M. Canine coronavirus in Australian dogs. Aust. Vet. J. 2001;79:116–119. doi: 10.1111/j.1751-0813.2001.tb10718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prittie J. Canine parvoviral enteritis: a review of diagnosis, management, and prevention. J. Vet. Emerg. Crit. Care. 2004;14:167–176. [Google Scholar]
- Rice J.B., Winters K.A., Krakowka S., Olsen R.G. Comparison of systemic and local immunity in dogs with canine parvovirus gastroenteritis. Infect. Immun. 1982;38:1003–1009. doi: 10.1128/iai.38.3.1003-1009.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimhanen-Finne R., Enemark H.L., Kolehmainen J., Toropainen P., Hanninen M.L. Evaluation of immunofluorescence microscopy and enzyme-linked immunosorbent assay in detection of Cryptosporidium and Giardia infections in asymptomatic dogs. Vet. Parasitol. 2007;145:345–348. doi: 10.1016/j.vetpar.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., Groen J., Egberink H., Borst G.H., UytdeHaag F.G., Osterhaus A.D. The use of enzyme-linked immunosorbent assay systems for serology and antigen detection in parvovirus, coronavirus and rotavirus infections in dogs in the Netherlands. Vet. Microbiol. 1991;26:25–40. doi: 10.1016/0378-1135(91)90039-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakulwira K., Vanapongtipagorn P., Theamboonlers A., Oraveerakul K., Poovorawan Y. Prevalence of canine coronavirus and parvovirus infections in dogs with gastroenteritis in Thailand. Vet. Med. – Czech. 2003;48:163–167. [Google Scholar]
- Schmitz S., Coenen C., Konig M., Thiel H.J., Neiger R. Comparison of three rapid commercial canine parvovirus antigen detection tests with electron microscopy and polymerase chain reaction. J. Vet. Diagn. Invest. 2009;21:344–345. doi: 10.1177/104063870902100306. [DOI] [PubMed] [Google Scholar]
- Schulz B.S., Strauch C., Mueller R.S., Eichhorn W., Hartmann K. Comparison of the prevalence of enteric viruses in healthy dogs and those with acute haemorrhagic diarrhoea by electron microscopy. J. Small Anim. Pract. 2008;49:84–88. doi: 10.1111/j.1748-5827.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolow S.H., Rand C., Marks S.L., Drazenovich N.L., Kather E.J., Foley J.E. Epidemiologic evaluation of diarrhea in dogs in an animal shelter. Am. J. Vet. Res. 2005;66:1018–1024. doi: 10.2460/ajvr.2005.66.1018. [DOI] [PubMed] [Google Scholar]
- Stavisky J., Radford A.D., Gaskell R., Dawson S., German A., Parsons B., Clegg S., Newman J., Pinchbeck G. A case–control study of pathogen and lifestyle risk factors for diarrhoea in dogs. Prev. Vet. Med. 2011;99:185–192. doi: 10.1016/j.prevetmed.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant B.J., Gaskell R.M., Jones R.C., Gaskell C.J. Studies on the epizootiology of canine coronavirus. Vet. Rec. 1993;132:7–11. doi: 10.1136/vr.132.1.7. [DOI] [PubMed] [Google Scholar]
- Terpstra F.G., van den Blink A.E., Bos L.M., Boots A.G., Brinkhuis F.H., Gijsen E., van Remmerden Y., Schuitemaker H., van’t Wout A.B. Resistance of surface-dried virus to common disinfection procedures. J. Hosp. Infect. 2007;66:332–338. doi: 10.1016/j.jhin.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Tupler T., Levy J.K., Sabshin S.J., Tucker S.J., Greiner E.C., Leutenegger C.M. Enteropathogens identified in dogs entering a Florida animal shelter with normal feces or diarrhea. J. Am. Vet. Med. Assoc. 2012;241:338–343. doi: 10.2460/javma.241.3.338. [DOI] [PubMed] [Google Scholar]
- Weber M., Martin L., Biourge V., Nguyen P., Dumon H. Influence of age and body size on the digestibility of a dry expanded diet in dogs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2003;87:21–31. doi: 10.1046/j.1439-0396.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- Weber M.P., Stambouli F., Martin L.J., Dumon H.J., Biourge V.C., Nguyen P.G. Influence of age and body size on gastrointestinal transit time of radiopaque markers in healthy dogs. Am. J. Vet. Res. 2002;63:677–682. doi: 10.2460/ajvr.2002.63.677. [DOI] [PubMed] [Google Scholar]
- Wold S. Chernornetric Methods in Molecular Design. VCH; 1994. PLS for multivariate linear modeling; p. 359. [Google Scholar]
- Wold S. Umetrics Inc.; Winchester, MA: 1995. Multivariate analysis (3-day course) [Google Scholar]