Abstract
A novel, real-time mortality recording system was designed to collect mortality data in companion animals from veterinary hospitals in Taiwan. This retrospective study aims to introduce the system, and to utilize the data collected for further investigation of the lifespan and mortality of the domesticated cat population stratified by demographic variables. Our data revealed that 1325 domesticated cats were acquired between 2012 and 2014. The median age of the study population was 8.0 years (IQR 3.0–13.0; range 0.0–22.7). Neutered and purebred cats lived longer. The most common causes of death were renal and urologic disorders, followed by neoplasia, infection, cardiovascular disorders, and trauma. Independent factors for common causes were surveyed. Advanced age and neutering was found to be associated with death due to renal and urologic disorders as well as with neoplasia. In contrast, younger age was found to be associated with death due to trauma and infection; being unneutered and living in the capital city were found to be associated with death due to trauma. Being male or purebred was found to be associated with death due to cardiovascular disorders.
Abbreviations: COD, cause of death; RFE, reason for euthanasia; MOI, multiple organ involvement
Keywords: Retrospective cohort study, Neuter status, Mortality surveillance, Euthanasia
1. Introduction
In humans, mortality statistics are highly valued and are available in most countries. Animal statistics, however, have not gained as much attention (Kircher and Anderson, 1987, O'Neill et al., 2014). A mortality survey on pets could improve in veterinary practices and advance the animal welfare. Nowadays, this issue is gaining more and more attention considering the current level of intimacy between owners and companion animals.
Previous studies have used a variety of databases to collect mortality data on animals. These include databases of insured animals, nationwide databases of primary care veterinary practices, and questionnaires (Proschowsky et al., 2003, Adams et al., 2010, Inoue et al., 2015a, O'Neill et al., 2015). The capabilities and limitations of these data collection methods have previously been discussed in detail (Moore and Lund, 2009, O'Neill et al., 2014). To date, limited studies have focused on the morbidity and mortality in domestic cats. In these studies, unique characteristics have been noted among the domesticated cat populations in Japan, Sweden, and England (Egenvall et al., 2009, Inoue et al., 2015b, O'Neill et al., 2015).
In Taiwan, there are approximately 568,000 pet cats and 1.7 million pet dogs, according to official statistics generated in 2015 by the Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Executive Yuan. However, an incomplete pet registration system and a scarcity of electronic medical record systems in veterinary practices limits our understanding of domesticated dogs and cats. To solve this problem, a real-time reporting system for causes of death (CODs) and reasons for euthanasia (RFEs) in cats and dogs was designed in 2011, and has been in operation since.
The primary aim of the current study was to elaborate on the real-time reporting system. The second objective was to understand the lifespan and the mortality of domesticated cats in Taiwan, and the third objective was to investigate the relationships between causes of death and multiple factors in this population.
2. Materials and methods
2.1. The real-time reporting system
To acquire real-time mortality data on companion dogs and cats, a web-based, veterinary-confirmed reporting system was developed by the authors’ research group. It was created independently of any veterinary hospitals in Taiwan and without any commercial consideration. The program “Investigations of CODs in Dogs and Cats” was started. To recruit veterinary clinics, the investigators attended regular meetings of regional veterinary medical associations where the system was introduced to the clinicians. Interested owners of veterinary clinics could voluntarily join the program by signing a contract; thereafter, they received specific permission for use of the website, instruction on use of the system, and technical support if needed. Only legally registered veterinary clinics with more than three licensed veterinarians qualified for use of the program. These veterinary clinics tended to offer full service and give more accurate diagnoses.
Clinicians used a clinic-specific ID and password to login to the website, and submitted each dog or cat death that occurred at their hospital or at their client’s home. For each case, clinicians were required to input data pertaining to the deceased animal, the owner’s personal information, the name of the clinician in charge, and information regarding the animal’s natural death or regarding euthanasia. The data pertaining to the deceased animal included age (year and month), breed, sex, and neuter status. The owner’s personal information, which included name, address and telephone number, was used to avoid duplicate or fake entries. The information regarding the animal’s natural death or regarding euthanasia included a case brief, clinical diagnosis, date of death, COD/RFE, and whether or not the animal was euthanized. To encourage clinicians to submit data, the authors’ research group offered free surgical pathology services and molecular diagnoses for common canine and feline infectious diseases.
To ensure the veracity of the data collected, a peer review system was established. All entries were reviewed instantly on submission (by two veterinarians at the authors’ institute). If the entries were duplicate, incomplete or irrational, the submissions were flagged and rejected. Clinicians were permitted to resubmit the entry after modifying it.
Statistical analyses were performed for annual reporting purposes and for further retrospective studies by one of the authors. Annual reports were released at the beginning of the following year by official authorities (Bureau of Animal and Plant Health Inspection and Quarantine, and the Taipei Animal Protection Office).
2.2. Data management
In this retrospective study, only records of deceased cats collected from the real-time reporting system between 2012 and 2014 were included. These served as an epidemiological model to elucidate the strengths and weaknesses of the real-time reporting system.
Demographic variables evaluated included biological meaningful and death-related factors. Biological meaningful factors included age, breed, sex, neuter status and whether or not the cat lived in the capital city. To study the difference between mixed (domestic shorthair) and purebred cats, the designated variable was whether the cat was purebred. Geographic information were accessed based on the owner’s address, which also represented the place where the cats lived. In Taiwan, there are huge differences in human population density and lifestyle between the capital, Taipei City, and other cities. This may be linked to the different lifestyles of the pet cats. In view of this, the variable of whether or not the cat lived in the capital city was applied to the current study in order to clarify the relationship between CODs/RFEs and the location of the cats. Death-related factors included the option for euthanasia and date of death. The option for euthanasia referred to whether or not the animal was chosen to be euthanized. Dates of death were subgrouped by season and year of death.
2.3. COD categories
To ensure accuracy and consistency, all cases were reviewed by both the first and the corresponding author. A final decision regarding COD/RFE was based on a comprehensive evaluation of the submitted case brief, clinical diagnosis, and provided COD/RFE.
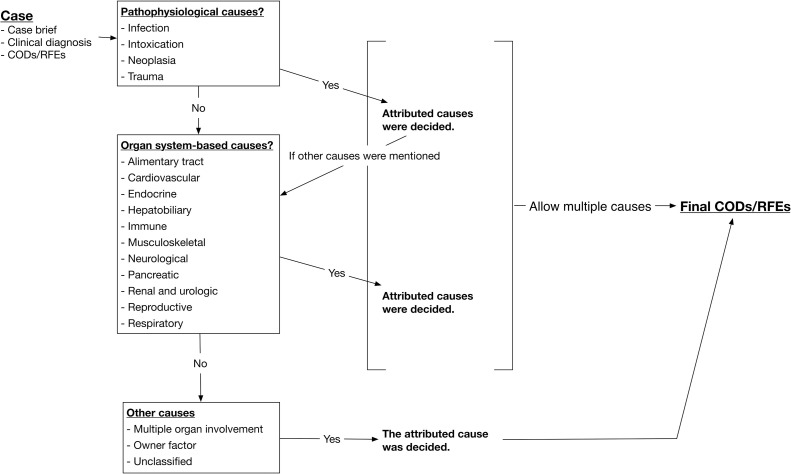
According to the information provided, many cases did not have a definitive diagnosis at the time of death; therefore, clinicians submitted multiple causes as COD/RFE. For accurate reflection of the comorbidities and their relationship to death (including euthanasia), multiple attributed causes were allowed for a single case. The CODs/RFEs were categorized into one or more of 18 groups according to following criteria (Fig. 1 ):
-
1.
If infection, intoxication, neoplasia, or trauma was mentioned as the definite pathophysiological cause of death, the case was categorized into that group.
-
2.
Cases that did not belong to any of the four major pathophysiological groups and those that had comorbid causes other than the pathophysiological causes were categorized based on the involvement of a specific organ system.
-
3.
For cases that did not satisfy any of the above criteria, they were categorized as multiple organ involvement (MOI), owner, or unclassified. The MOI group included cases reported simply as “multiple organ failure”. Causes of death reported as “weak” or “old” were also designated as MOI. The owner group included cases that were euthanized due to the owners personal reasons. Cases with insufficient information such as nonspecific clinical signs, which were minimally linked to death, were categorized into the unclassified group.
-
4.
We also searched for and recorded the origin of tumors and pathogens in cats that died from neoplasia or infection, for the purposes of statistical analysis.
Fig. 1.
Criteria for the final causes of death or reasons for euthanasia in the study on domesticated cats collected from the real-time system during 2012–2014 in Taiwan (n = 1325).
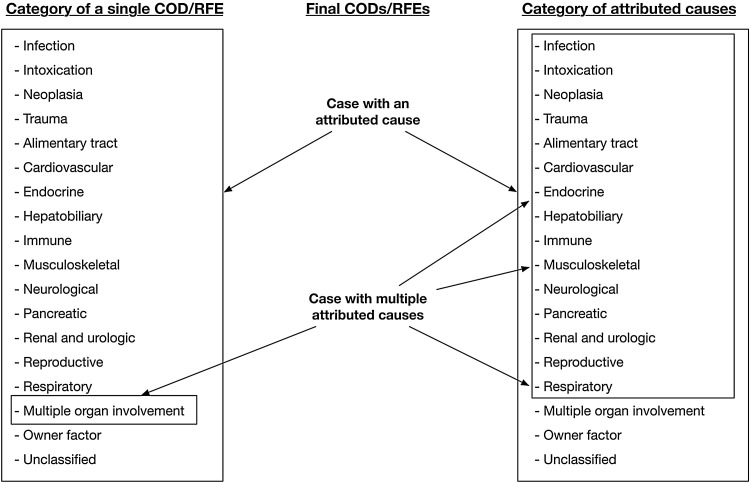
The authors reported the mortality of the study population in two ways: using the category of single COD/RFE and the category of causes that could be attributed to resulting in death (Fig. 2 ). Using the former categorization scheme, each case had only one COD/RFE, and cases with two or more causes were placed into the MOI group. Using the latter categorization scheme, each attributed cause was calculated separately. For further highlighting the comorbidities related to natural death or to euthanasia, results of the 18 groups determined by the first method were divided by the results determined by the second method. When the ratio was close to 1, the cause was more likely to be a primary COD/RFE. In contrast, a ratio <1 signified that the cause was more likely to have a comorbidity resulting in natural death or in euthanasia.
Fig. 2.
Diagram showing two categories of causes that may result in death in the study on domesticated cats collected from the real-time system during 2012–2014 in Taiwan (n = 1325).
2.4. Statistical analyses
All demographic variables were compared to each other. Age was the only continuous demographic variable and was expressed as mean ± standard deviation (SD) or as median, interquartile range and range. After performing a normality test for age, parametric or non-parametric inferential statistics were utilized. For age comparisons of two groups, independent t-test or Mann-Whitney U test were used. For age comparisons of multiple groups (over 2), we used one-way analysis of variance (ANOVA) or Kruskal-Wallis test, and Bonferroni correction test to control the family-wise error. In addition, when the two variables were categorical, they were compared using a chi-square test or Fisher’s exact test when cells had an expected count <5.
To clarify the relationship between demographic variables and causes of death and euthanasia, the effect of each attributed cause was determined by first creating dummy variables for each (where 1 = cat that died from that disease; 0 = cat that died from another disease). Age of cats that died of specific causes was expressed as median, interquartile range and range. All pathophysiological and organ system-based causes were compared with categorical demographic variables using a chi-square test or Fisher’s exact test when cells had an expected count <5.
Binary logistic regression models were utilized to examine the assumption that demographic factors might be independent factors associated with common attributed causes. The observed outcomes were dummy variables of the five most common attributed causes, and the explanatory factors were the demographic factors for each cat. For each attributed cause, univariable logistic regression models were carried out for each variable. Multivariable logistic regression models considering demographic factors were also performed to examine relevance among the factors. The results were reported as an odds ratio (OR) with a 95% confidence interval (CI), and the goodness-of-fit for logistic regression models were evaluated by likelihood ratio and Hosmer–Lemeshow test. To determine the independent variables for the attributed causes, the crude and adjusted ORs were compared; interactions between demographic variables were examined when demographic variables reached the significance in both univariable and multivariable logistic regression models.
A p value < 0.05 was considered to be statistically significant. All analyses were performed using SPSS Version 20 (SPSS Statistics, IBM Corp., Somers, NY).
3. Results
3.1. The reporting system
Two hundred and thirty-three veterinary hospitals were recruited for the “Investigations of CODs in Dogs and Cats ” program. One hundred and ten hospitals were located in the capital area and 123 were located in the non-capital area. According to official record, the coverage rate of veterinary hospitals in Taiwan as a whole was about 13%, and the coverage rate of veterinary hospitals in the capital city was 48%. A total of 1331 cases of deceased cats were submitted to the study, of these 6 cases were rejected without re-submission. The remaining 1325 cases were accepted and utilized for annual statistic reports; each of the three years (2012–2014) accounted for about one-third of caseload (Table 1 ). All 1325 cases were included in the COD/RFE categorization in the current retrospective study.
Table 1.
Number of domesticated cats and comparison of age among categorical demographic variables collected from the real-time system during 2012–2014 in Taiwan (n = 1325). The median, interquartile range (IQR) and range for age (years) at death are reported.
| Categorical variables | Number of cats (%) | Median age (years) | IQR | Range | P value |
|---|---|---|---|---|---|
| Sex | 0.02 | ||||
| Male | 708 (53.4) | 8.0 | 3.0–12.3 | 0.0–22.7 | |
| Female | 617 (46.6) | 9.0 | 4.0–13.0 | 0.1–21.3 | |
| Neuter status | <0.01 | ||||
| Unneutered | 564 (42.6) | 5.0 | 0.5–11.0 | 0.0–22.0 | |
| Neutered | 761 (57.4) | 10.0 | 6.5–14.0 | 0.3–22.7 | |
| Breed | <0.01 | ||||
| Mixed | 709 (53.5) | 7.2 | 2.0–12.0 | 0.0–22.7 | |
| Purebred | 616 (46.5) | 10.0 | 5.0–14.0 | 0.1–22.0 | |
| Common breed | <0.01 | ||||
| Domestic shorthair (Mixed) | 709 (53.5) | 7.2 | 2.0–12.0 | 0.0–22.7 | |
| Persian | 413 (31.2) | 10.0 | 5.0–14.0 | 0.1–22.0 | |
| American shorthair | 63 (4.8) | 8.0 | 5.1–11.3 | 0.1–18.2 | |
| Scottish fold | 26 (2.0) | 6.0 | 0.3–14.0 | 0.9–9.2 | |
| Himalayan and Kashmir | 12 (0.9) | 13.0 | 2.0–18.0 | 11.0–16.1 | |
| Cats from capital area | 0.01 | ||||
| No | 828 (62.5) | 8.0 | 3.0–12.0 | 0.0–22.7 | |
| Yes | 497 (37.5) | 9.3 | 3.6–13.5 | 0.1–21.7 | |
| Option for Euthanasia | <0.01 | ||||
| No | 1016 (76.7) | 8.0 | 3.0–12.6 | 0.0–22.7 | |
| Yes | 309 (23.3) | 10.0 | 5.0–13.8 | 0.0–21.7 | |
| Year | 0.46 | ||||
| 2012 | 496 (37.4) | 8.0 | 3.0–13.0 | 0.0–22.7 | |
| 2013 | 408 (30.8) | 8.0 | 3.0–12.2 | 0.0–22.0 | |
| 2014 | 421 (31.8) | 9.0 | 4.0–13.0 | 0.1–21.4 | |
| Season | 0.10 | ||||
| Spring | 341 (25.7) | 7.5 | 2.0–13.0 | 0.1–22.7 | |
| Summer | 331 (25.0) | 8.9 | 3.0–13.0 | 0.0–21.7 | |
| Fall | 332 (25.1) | 8.0 | 3.0–12.1 | 0.0–20.6 | |
| Winter | 321 (24.2) | 9.0 | 4.0–13.0 | 0.1–22.0 | |
*P value represents the difference in median age among groups.
3.2. Demographic variable comparisons
Mean age of the study population was 8.4 ± 5.6 years, and median age was 8.0 years (IQR 3.0–13.0; range 0.0–22.7). Age variable in this study did not pass the normality test, so we analyzed related analysis using non-parametric inferential statistics. Comparisons of the cat age at death with all other demographic variables are reported in Table 1. Female, neutered, purebred cats and cats from the capital city displayed significantly greater median age than the opposite groups.
Domestic shorthair cats represented the majority of the study population, followed by Persian cats, American shorthair cats, Scottish fold cats, and Himalayan and Kashmir cats (Table 1). An additional 30 breeds were represented by ≤10 cats each. According to the Bonferroni correction test, the median longevity of Himalayan and Kashmir cats was significantly greater than the median longevity of Scottish fold cats (P = 0.04). The median longevity was also greater in Himalayan and Kashmir cats than in Persian, American shorthair, and domestic shorthair cats, but without reaching significance (P = 0.22, P = 0.08, and P = 0.05, respectively). In addition, the median longevity of domestic shorthair cats was significantly lower than the median longevity of Persian cats (P < 0.01). The percentage of purebred cats decreased significantly from 51% in 2012 to 40% in 2014 (P < 0.01). Purebred cats accounted for 49% of male cats and 43% of female cats, and the difference was significant (P = 0.04). The percentage of purebred cats was significantly higher in the non-capital area than in the capital city (54% versus 33%, P < 0.01).
The percentage of neutered cats among the study population increased significantly from 51% in 2012 to 66% in 2014 (P < 0.01), as did the percentage of female cats, increasing from 42% in 2012 to 52% in 2014 (P = 0.01). Among the study population, female cats displayed a significantly higher neuter rate than male cats (61% versus 54%, P = 0.01). Purebred cats displayed significantly lower neuter rate than mixed cats (53% versus 62%, P < 0.01). The percentage of neutered cats was significantly higher in the capital city than in the non-capital area (64% versus 54%, P < 0.01). Neutered cats were found to have a significantly higher chance of being euthanized (P < 0.01).
Euthanized cats comprised 23.3% of the study population (Table 1). The rates of euthanasia were found to increase significantly from 19% in 2012 to 26% in 2014 (P = 0.02). In addition, rates of euthanasia were found to be significantly higher among cats from the capital city versus cats from the non-capital area (P = 0.02). Rates of euthanasia were found to be significantly lower in purebred cats than in mixed cats (P < 0.01).
All other demographic variable comparisons were found to reach no significant difference.
3.3. Causes of death or reasons for euthanasia
The number, percentage, and ratio of each cause of death based on the category of a single COD/RFE and on the category of attributed causes are reported in Table 2 . By rank, the five most frequent single CODs/RFEs during 2012–2014 were renal and urologic disorders (25.1%), neoplasia (14.3%), infection (13.3%), multiple organ involvement (12.5%), and cardiovascular disorders (7.8%). The five most common attributed causes were renal and urologic disorders (31.9%), neoplasia (15.4%), infection (13.9%), cardiovascular disorders (9.9%), and trauma (7.6%).
Table 2.
Number and percentage of (a) category of a single cause of death/reason for euthanasia and (b) category of attributed causes of domesticated cats compared by year of death at veterinary clinics in Taiwan collected from the real-time system during 2012–2014 (n = 1325). The ratio of total number of the single cause of death/reason for euthanasia to the attributed causes is included in column (c). (<1.00 indicates that the cause was more likely to have a comorbidity resulting in natural death or in euthanasia.).
| (a) Category of a single cause of death/reason for euthanasia | (b) Category of attributed causes | (c) Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Year |
Year |
||||||||
| 2012 | 2013 | 2014 | Total | 2012 | 2013 | 2014 | Total | ||
| Number (%) | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) | Number (%) | ||
| Pathophysiological cause | |||||||||
| Infection | 71 (14.3) | 44 (10.8) | 61 (14.5) | 176 (13.3) | 76 (15.3) | 45 (11.0) | 63 (15.0) | 184 (13.9) | 0.96 |
| Intoxication | 11 (2.2) | 4 (1.0) | 3 (0.7) | 18 (1.4) | 11 (2.2) | 4 (1.0) | 3 (0.7) | 18 (1.4) | 1.00 |
| Neoplasia | 54 (10.9) | 61 (15.0) | 75 (17.8) | 190 (14.3) | 63 (12.7) | 64 (15.7) | 77 (18.3) | 204 (15.4) | 0.93 |
| Trauma | 33 (6.7) | 42 (10.3) | 25 (5.9) | 100 (7.5) | 34 (6.9) | 42 (10.3) | 25 (5.9) | 101 (7.6) | 0.99 |
| Organ system-based cause | |||||||||
| Alimentary tract | 8 (1.6) | 7 (1.7) | 7 (1.7) | 22 (1.7) | 9 (1.8) | 8 (2.0) | 9 (2.1) | 26 (2.0) | 0.85 |
| Cardiovascular | 33 (6.7) | 34 (8.3) | 36 (8.6) | 103 (7.8) | 39 (7.9) | 41 (10.0) | 51 (12.1) | 131 (9.9) | 0.79 |
| Endocrine | 10 (2.0) | 7 (1.7) | 10 (2.4) | 27 (2.0) | 17 (3.4) | 12 (2.9) | 24 (5.7) | 53 (4.0) | 0.51 |
| Hepatobiliary | 12 (2.4) | 13 (3.2) | 9 (2.1) | 34 (2.6) | 21 (4.2) | 21 (5.1) | 20 (4.8) | 62 (4.7) | 0.55 |
| Immune | 1 (0.2) | 7 (1.7) | 8 (1.9) | 16 (1.2) | 3 (0.6) | 8 (2.0) | 8 (1.9) | 19 (1.4) | 0.84 |
| Musculoskeletal | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1.00 |
| Neurological | 19 (3.8) | 11 (2.7) | 8 (1.9) | 38 (2.9) | 19 (3.8) | 11 (2.7) | 14 (3.3) | 44 (3.3) | 0.86 |
| Pancreatic | 10 (2.0) | 17 (4.2) | 14 (3.3) | 41 (3.1) | 23 (4.6) | 36 (8.8) | 35 (8.3) | 94 (7.1) | 0.44 |
| Renal and urologic | 145 (29.2) | 99 (24.3) | 89 (21.1) | 333 (25.1) | 178 (35.9) | 122 (29.9) | 123 (29.2) | 423 (31.9) | 0.79 |
| Reproductive | 2 (0.4) | 0 (0.0) | 1 (0.2) | 3 (0.2) | 2 (0.4) | 0 (0.0) | 1 (0.2) | 3 (0.2) | 1.00 |
| Respiratory | 13 (2.6) | 9 (2.2) | 12 (2.9) | 34 (2.6) | 15 (3.0) | 9 (2.2) | 16 (3.8) | 40 (3.0) | 0.85 |
| Others | |||||||||
| Weak, old, or multiple organ involvement | 57 (11.5) | 48 (11.8) | 61 (14.5) | 166 (12.5) | 16 (3.2) | 17 (4.2) | 9 (2.1) | 42 (3.2) | 3.95 |
| Unclassified | 14 (2.8) | 2 (0.5) | 2 (0.5) | 18 (1.4) | 14 (2.8) | 2 (0.5) | 2 (0.5) | 18 (1.4) | 1.00 |
| Owner | 2 (0.4) | 3 (0.7) | 0 (0.0) | 5 (0.4) | 2 (0.4) | 3 (0.7) | 0 (0.0) | 5 (0.4) | 1.00 |
| Total | 496 (100.0) | 408 (100.0) | 421 (100.0) | 1325 (100.0) | 543 (109.5) | 445 (109.1) | 480 (114.0) | 1468 (110.8) | |
Overall, multiple attributed causes were reported for approximately 10% of the cases. The ratio for the four pathophysiological causes was near one, while the ratio for the 11 organ system disorders varied by cause. Of the 18 possible causes, pancreatic, hepatobiliary, and endocrine disorders had the lowest ratios.
Table 3 shows the types of neoplasia and pathogen that were a COD or that led to euthanasia. The most common tumor type was reproductive tumor, which mostly included mammary gland tumors. This was followed by hematopoietic tumors and integumentary-musculoskeletal tumors. The most common pathogen was feline coronavirus (including most commonly feline infectious peritonitis virus), followed by feline parvovirus and feline immunodeficiency virus.
Table 3.
List of types of neoplasia and pathogen associated with death in domesticated cats collected from the real-time system during 2012–2014.
| (a) Types of neoplasia | Number | % |
|---|---|---|
| Reproductive | 48 | 24% |
| Hematopoietic | 37 | 18% |
| Integumentary and musculoskeletal | 33 | 16% |
| Respiratory and thoracic | 30 | 15% |
| Alimentary tract | 25 | 12% |
| Hepatopancreatobiliary | 11 | 5% |
| Cardiovascular | 5 | 2% |
| Ophthalmologic | 3 | 1% |
| Renal and urologic | 3 | 1% |
| Neurological | 1 | 0% |
| Unclassified | 8 | 4% |
| Total | 204 | 100% |
| (b) Types of pathogen | ||
| Feline coronavirus | 80 | 41% |
| Feline parvovirus | 49 | 25% |
| Feline immunodefiency virus | 15 | 8% |
| Feline leukemia virus | 7 | 4% |
| Hemobartonella felis | 4 | 2% |
| Feline calicivirus | 3 | 2% |
| Coccidia | 3 | 2% |
| Heart worms | 2 | 1% |
| Dermatophytes | 2 | 1% |
| Toxocara | 2 | 1% |
| Tapeworm | 1 | 1% |
| Escherichia coli | 1 | 1% |
| Unidentified | 25 | 13% |
| Total | 194 | 100% |
3.4. Demographic variable comparisons on attributed causes
The median ages of top 10 attributed causes are reported in Table 4 . The number and percentages of the five most common attributed causes grouped together by categorical demographic variables are reported in Table 5 , and the results of other causes are included in Table S1.
Table 4.
Age of domesticated cats of attributed causes of death and euthanasia (Top 10) collected from the real-time system during 2012–2014 in Taiwan. The median, interquartile range (IQR) and range for age (years) at death are reported.
| Attributed causes | Rank | Number (%) | Median (years) | IQR | Range |
|---|---|---|---|---|---|
| Renal and urologic disorders | 1 | 423 (31.9) | 11.0 | 7.3–14.0 | 1.0–22.0 |
| Neoplasia | 2 | 204 (15.4) | 12.0 | 8.0–14.1 | 0.2–21.0 |
| Infection | 3 | 184 (13.9) | 1.0 | 0.3–4.0 | 0.0–17.0 |
| Cardiovascular disorders | 4 | 131 (9.9) | 10.0 | 6.0–13.9 | 0.2–20.0 |
| Trauma | 5 | 101 (7.6) | 2.0 | 1.0–5.0 | 0.2–18.0 |
| Pancreatic disorders | 6 | 94 (7.1) | 10.0 | 7.0–13.0 | 1.0–20.6 |
| Hepatobiliary disorders | 7 | 62 (4.7) | 8.0 | 5.8–12.6 | 0.7–22.0 |
| Endocrine disorders | 8 | 53 (4.0) | 11.0 | 8.0–13.3 | 1.0–18.0 |
| Neurological disorders | 9 | 44 (3.3) | 8.4 | 1.7–12.0 | 0.1–20.0 |
| Respiratory disorders | 10 | 40 (3.0) | 5.5 | 1.1–11.8 | 0.1–21.4 |
Table 5.
Common causes attributed to causing death, and categorical demographic variables of domesticated cats collected from the real-time system during 2012–2014 in Taiwan. Only the five most common causes are present, and the data are reported as number and percent stratified by groups. The p value was calculated to compare the differences between groups by using Pearson’s chi-squared method.
| Renal and urologic cause |
Neoplasia |
Infection |
Cardiovascular cause |
Trauma |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||||||
| N (%) | N (%) | P value | N (%) | N (%) | P value | N (%) | N (%) | P value | N (%) | N (%) | P value | N (%) | N (%) | P value | |
| Sex | 0.16 | <0.01 | 0.56 | 0.03 | 0.14 | ||||||||||
| Male | 214 (30) | 494 (70) | 81 (11) | 627 (89) | 102 (14) | 606 (86) | 82 (12) | 626 (88) | 61 (9) | 647 (91) | |||||
| Female | 209 (34) | 408 (66) | 123 (20) | 494 (80) | 82 (13) | 535 (87) | 49 (8) | 568 (92) | 40 (6) | 577 (94) | |||||
| Neuter status | <0.01 | <0.01 | <0.01 | 0.07 | <0.01 | ||||||||||
| Intact | 123 (22) | 441 (78) | 51 (9) | 513 (91) | 124 (22) | 440 (78) | 46 (8) | 518 (92) | 74 (13) | 490 (87) | |||||
| Neuter | 300 (39) | 461 (61) | 153 (20) | 608 (80) | 60 (8) | 701 (92) | 85 (11) | 676 (89) | 27 (4) | 734 (96) | |||||
| Pure Breed | <0.01 | 0.35 | <0.01 | <0.01 | 0.54 | ||||||||||
| No | 204 (29) | 505 (71) | 103 (15) | 606 (85) | 120 (17) | 589 (83) | 55 (8) | 654 (92) | 57 (8) | 652 (92) | |||||
| Yes | 219 (36) | 397 (64) | 101 (16) | 414 (67) | 64 (10) | 552 (90) | 76 (12) | 540 (88) | 44 (7) | 572 (93) | |||||
| Cats from capital area | 0.87 | 0.10 | 0.14 | 0.43 | <0.01 | ||||||||||
| No | 263 (32) | 565 (68) | 117 (14) | 711 (86) | 106 (13) | 722 (87) | 86 (10) | 742 (90) | 79 (10) | 749 (90) | |||||
| Yes | 160 (32) | 337 (68) | 87 (18) | 410 (82) | 78 (16) | 419 (84) | 45 (9) | 452 (91) | 22 (4) | 475 (96) | |||||
| Option for Euthanasia | 0.11 | <0.01 | 0.27 | 0.32 | 0.02 | ||||||||||
| No | 313 (31) | 703 (69) | 124 (12) | 892 (88) | 147 (14) | 869 (86) | 105 (10) | 911 (90) | 87 (9) | 929 (91) | |||||
| Yes | 110 (36) | 199 (64) | 80 (26) | 228 (74) | 37 (12) | 272 (88) | 26 (8) | 283 (92) | 14 (5) | 295 (95) | |||||
| Year | 0.06 | 0.06 | 0.13 | 0.10 | 0.04 | ||||||||||
| 2012 | 178 (36) | 318 (64) | 63 (13) | 433 (87) | 76 (15) | 420 (85) | 39 (8) | 457 (92) | 34 (7) | 462 (93) | |||||
| 2013 | 122 (30) | 286 (70) | 64 (16) | 344 (84) | 45 (11) | 363 (89) | 41 (10) | 367 (90) | 42 (10) | 366 (90) | |||||
| 2014 | 123 (29) | 298 (71) | 77 (18) | 344 (82) | 63 (15) | 358 (85) | 51 (12) | 370 (88) | 25 (6) | 396 (94) | |||||
| Season | 0.75 | 0.91 | 0.34 | 0.92 | 0.28 | ||||||||||
| Spring | 109 (32) | 232 (68) | 54 (16) | 287 (84) | 55 (16) | 286 (84) | 33 (10) | 308 (90) | 33 (10) | 308 (90) | |||||
| Summer | 98 (30) | 233 (70) | 54 (16) | 277 (84) | 43 (13) | 288 (87) | 30 (9) | 301 (91) | 19 (6) | 312 (94) | |||||
| Fall | 110 (33) | 222 (67) | 48 (14) | 284 (86) | 49 (15) | 283 (85) | 34 (10) | 298 (90) | 24 (7) | 308 (93) | |||||
| Winter | 106 (33) | 215 (67) | 48 (15) | 273 (85) | 37 (12) | 284 (88) | 34 (11) | 287 (89) | 25 (8) | 296 (92) | |||||
Among top 10 attributed causes of death and euthanasia, cats that died of neoplasia displayed the greatest median age, while cats died from infection displayed the least median age. Female cats that died of neoplasia were observed at a higher rate than males; in contrast, male cats that died of cardiovascular disorders were observed at a higher rate than females. Intact cats that died from infection and trauma, were observed at a significantly higher rate than neutered cats. Conversely, neutered cats died from neoplasia, endocrine, pancreatic, renal, and urologic disorders at a significantly higher rate than unneutered cats. Compared to domestic shorthair cats, purebred cats died as a result of cardiovascular and renal and urologic disorders at a significantly higher rate, while purebred cats died from infection at a significantly lower rate. Death as a result of trauma showed a significantly higher rate in cats from the non-capital area than from the capital. For neoplasia and alimentary tract disorders, cats were euthanized significantly more often than they died of natural causes. However, cats euthanized due to trauma occurred significantly less often than did those dying of natural causes.
3.5. Relationship between common attributed causes and demographic variables
Demographic variables associated with the five most common attributed causes in univariable and multivariable logistic regression analyses are shown in Table 6 . The linear relation between continuous age and log odds among the five most common attributed causes were checked by linearity test; all of them passed the test (all P < 0.01).
Table 6.
Multivariable binary logistic regression analysis of demographic variables associated with the five most common attributed causes in domesticated cats collected from the real-time system during 2012–2014 in Taiwan.
| Renal and Urologic Disorders |
Neoplasia |
Neoplasia, reproductive neoplasia excluded |
Infection |
Cardiovascular Disorders |
Trauma |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Age | 1.1 (1.1–1.2) | <0.01 | 1.1 (1.1–1.1) | <0.01 | 1.1 (1.1–1.1) | <0.01 | 0.8 (0.7–0.8) | <0.01 | 1.0 (1.0–1.1) | 0.07 | 0.8 (0.8–0.9) | <0.01 |
| Sex | 0.37 | <0.01 | 0.55 | 0.90 | 0.01 | 0.47 | ||||||
| Male | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Female | 1.1 (0.9–1.4) | 1.8 (1.3–2.5) | 1.1 (0.8–1.6) | 1.0 (0.7–1.5) | 0.6 (0.4–0.9) | 0.8 (0.5–1.3) | ||||||
| Neutered | <0.01 | 0.01 | <0.01 | 0.30 | 0.17 | 0.01 | ||||||
| Intact | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Neutered | 1.7 (1.3–2.3) | 1.7 (1.2–2.4) | 2.1 (1.4–3.2) | 0.8 (0.5–1.2) | 1.3 (0.9–2.0) | 0.5 (0.3–0.8) | ||||||
| Purebred | 0.20 | 0.24 | 0.20 | 0.53 | 0.02 | 0.42 | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Yes | 1.2 (0.9–1.5) | 1.2 (0.9–1.7) | 1.3 (0.9–1.8) | 0.9 (0.6–1.3) | 1.6 (1.1–2.4) | 0.8 (0.5–1.3) | ||||||
| Cats from capital | 0.32 | 0.53 | 0.54 | 0.06 | 0.68 | <0.01 | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Yes | 0.9 (0.7–1.1) | 1.1 (0.8–1.5) | 0.9 (0.6–1.3) | 1.4 (1.0–2.1) | 0.9 (0.6–1.4) | 0.4 (0.3–0.8) | ||||||
| Euthanasia | 0.56 | <0.01 | <0.01 | 0.53 | 0.21 | 0.34 | ||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Yes | 1.1 (0.8–1.5) | 2.2 (1.6–3.1) | 2.1 (1.5–3.1) | 1.2 (0.7–1.8) | 0.7 (0.5–1.2) | 0.7 (0.4–1.4) | ||||||
| Year | 0.01 | 0.36 | 0.36 | 0.06 | 0.06 | 0.06 | ||||||
| 2012 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| 2013 | 0.7 (0.5–1.0) | 0.03 | 1.2 (0.8–1.8) | 0.37 | 1.3 (0.8–2.0) | 0.30 | 0.6 (0.4–1.0) | 0.05 | 1.4 (0.9–2.2) | 0.17 | 1.7 (1.1–2.9) | 0.03 |
| 2014 | 0.6 (0.5–0.8) | <0.01 | 1.3 (0.9–1.9) | 0.16 | 1.4 (0.9–2.1) | 0.16 | 1.1 (0.7–1.6) | 0.71 | 1.7 (1.1–2.7) | 0.02 | 1.0 (0.6–1.8) | 0.90 |
| Season | 0.58 | 0.82 | 0.90 | 0.94 | 0.96 | 0.21 | ||||||
| Spring | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Summer | 0.8 (0.6–1.2) | 0.26 | 1.0 (0.7–1.6) | 0.97 | 1.2 (0.7–1.9) | 0.53 | 0.9 (0.6–1.5) | 0.67 | 1.0 (0.6–1.7) | 0.94 | 0.5 (0.3–1.0) | 0.05 |
| Fall | 1.0 (0.7–1.5) | 0.88 | 0.9 (0.6–1.4) | 0.54 | 1.0 (0.6–1.7) | 0.88 | 1.0 (0.6–1.7) | 0.90 | 1.1 (0.7–1.9) | 0.67 | 0.7 (0.4–1.2) | 0.22 |
| Winter | 0.9 (0.6–1.3) | 0.63 | 0.9 (0.5–1.3) | 0.47 | 1.0 (0.6–1.6) | 0.98 | 0.9 (0.5–1.5) | 0.71 | 1.1 (0.6–1.8) | 0.80 | 0.9 (0.5–1.6) | 0.77 |
For cats that died of renal and urologic disorders, increasing age and being neutered had significant higher odds ratio in both univariable and multivariable logistic regression models. The likelihood ratio of multivariable logit was 1491.752, and the Hosmer & Lemeshow test suggests the model was not a good fit to the data as P < 0.01. In addition, significant interaction between age and neuter status was noted (P < 0.01).
For cats that died of neoplasia, advanced age, female, neutering, and being euthanized had significant higher odds ratio in both univariable and multivariable logistic regression models. The likelihood ratio of multivariable logit was 1018.825, and the Hosmer & Lemeshow test suggests the model was a good fit to the data as P = 0.22.
To further clarify the relationships between explanatory variables and neoplasia other than reproductive tumors, the cats whose deaths were attributed to reproductive tumors were excluded from the univariable and multivariable models. Age, neuter status and option for euthanasia were still found to reach significance in both univariable and multivariable logistic regression models. The likelihood ratio of multivariable logit was 865.924, and the Hosmer & Lemeshow test suggests the model was a good fit to the data as P = 0.32. In addition, significant interactions between age and neuter status were noted (P = 0.02), while interactions between option for euthanasia and age or neuter status reached no significance.
For cats that died from infection, younger age displayed significantly higher odds ratio in both univariable and multivariable logistic regression models. The likelihood ratio of multivariable logit was 806.761, and the Hosmer & Lemeshow test suggests the model was a good fit to the data as P = 0.24.
For cats that died of cardiovascular disorders, male and purebred cats displayed significantly higher odds ratio in both univariable and multivariable logistic regression models. The likelihood ratio of multivariable logit was 827.239, and the Hosmer & Lemeshow test suggests the model was a good fit to the data as P = 0.45. No significant interaction between sex and whether or not the cat was purebred was noted.
For cats that died from trauma, younger age, being intact, and cats that lived in non-capital area displayed significantly higher odds ratio in both univariable and multivariable logistic regression models. The likelihood ratio of multivariable logit was 592.607, and the Hosmer & Lemeshow test suggests the model was a good fit to the data as P = 0.08. No significant interaction among age, neuter status and whether or not the cat lived in the capital city was noted.
4. Discussion
In the present study, we thoroughly inspect the three-year mortality data of domesticated cats, which was collected by the real-time reporting system. For the first time in Taiwan, the mortality and longevity of companion animals were investigated considering demographic factors. The strengths and weaknesses of the retrospective data collected from the real-time system will be discussed.
When compared to previous studies performed in England and Sweden, the overall median age of the cats in the present study was lower (Egenvall et al., 2009, O'Neill et al., 2015). In our study, purebred cats were found to live longer than mixed cats. The result is in contrast to the findings of a previous study performed in England, and might be due to the different breed distributions of purebred cats between two studies (O'Neill et al., 2015). Of the five most common breeds represented, we found that the Himalayan and Kashmir cats displayed the greatest median longevity. Similar to the study performed in England, longevity among specific breeds varied markedly in our study (O'Neill et al., 2015). Previous studies revealed that neutered cats lived longer than unneutered cats, and our findings were similar (Egenvall et al., 2009, O'Neill et al., 2015).
We applied the category of a single COD/RFE and the category of attributed causes, and then evaluated the ratio between them to reflect the prevalence and comorbidity of attributed diseases. Our results suggested that pancreatic, hepatobiliary, and endocrine disorders, which were associated with low ratios, are the three most common comorbidities. Previous studies have revealed that pancreatitis is often comorbid with other diseases in severely ill cats, such as hepatic lipidosis, inflammatory bowel disease, nephritis, and many other diseases (Ferreri et al., 2003, Simpson, 2015). Cats with endocrine diseases, such as diabetes mellitus and hyperadrenocorticism, were reported to develop subsequent hepatic abnormality and increased risk of infections (Dimski and Taboada, 1995, Bailiff et al., 2006, Lowe et al., 2008). In addition, hepatopathy was reported to be secondary to many diseases in cats (Dimski and Taboada, 1995, Lowe et al., 2008, Simpson, 2015). In the study population, the “weak, old, and MOI” and “unclassified” groups included 60 total cases, which may have consisted of mainly comorbidity cases. This could have reduced the numbers of some causes and created errors in the ratios.
In the present study, renal and urologic disorders were the most common attributed cause of natural death or euthanasia among domesticated cats in Taiwan. This is in accordance with the Swedish study (Egenvall et al., 2009). This may be related to insufficient water intake and a preference for feeding a dry diet in Taiwan. This has been suggested as an important cause of the development of feline lower urinary tract disease and the progression of feline chronic kidney disease (Elliott and Barber, 1998, Carciofi et al., 2005, Dru Forrester and Roudebush, 2007, White et al., 2011). In both univariable and multivariable logistic regression models, death due to renal and urologic disorders was associated with increased age and neuter status (higher in neutered cats), which has been broadly discussed in previous studies. Regarding feline chronic kidney diseases and lower urinary tract diseases, old age is a generally accepted risk factor, while breed predispositions have been variously observed (Buffington et al., 2006, White et al., 2011). It has been observed that the presence of feline lower urinary tract disease is associated with chronic kidney diseases (Mayer-Roenne et al., 2007, White et al., 2013). Because our reporting system was open with minimal interference in the clinicians’ opinions, we found that sometimes urologic disorders could not be separated from renal disorders based on the submitted information. Therefore, urologic and renal disorders were grouped together, and this may constitute a limitation. The interaction between the neuter status and age for the outcome of whether or not the cat died of the renal and urologic disorders was confirmed in our study population. The finding might be resulted from many possible reasons. For instance, neutered cats with longer lifespan have more time to develop renal and urologic disorders, or owners of neutered cats are more likely to take them to the veterinarian when they are sick. Main effects of age and neuter status should be interpreted carefully considering the existence of interaction effect. Further research is needed.
In the logistic regression model for neoplasia-attributed deaths, independent variables were age, sex, neuter status and option for euthanasia initially. However, sex became non-significant when reproductive tumors were excluded. This finding was consistent with the current knowledge that feline mammary gland tumors are frequently associated with a poor prognosis in females (Seixas et al., 2011). Sterilization in cats was observed to contribute to an increased risk of dying from neoplasia, irrespective of whether reproductive tumors were considered. The interaction between age and neuter status was confirmed in the study population, which may also be associated with many possible reasons and should be considered. To date, no studies have reviewed the association between gonadectomy and increased risk of developing neoplasia in cats; further investigation is needed.
The most common infections in this study were viral in origin. These results are compatible with those obtained from previous studies performed in England and Sweden (Egenvall et al., 2009, O'Neill et al., 2015). We found that young cats had higher risk of death due to infection. This may be related to incomplete vaccination programs in young cats, or the high prevalence of feline infectious peritonitis in the study population. The age predilection for feline infectious peritonitis has been studied extensively (Pesteanu-Somogyi et al., 2006, Worthing et al., 2012).
Regarding death related to cardiovascular disorders, the finding that male or purebred cats were at higher risk than female or mixed cats was supported by a previous study on insured Japanese cats and their morbidity patterns (Inoue et al., 2015b). Trauma was significantly associated with age, neuter status, and the variable of whether the cat was from the capital city. Young or unneutered cats tended to have a higher risk of dying from trauma, similar to cat populations in Japan, England and Sweden (Egenvall et al., 2009, Inoue et al., 2015b, O'Neill et al., 2015). As for location, cats from the capital city having a low risk of trauma may imply a possible difference in lifestyle between the capital and non-capital area. This is similar to the results obtained from a study on Swedish dogs (Bonnett et al., 2005, Egenvall et al., 2005). More evidence is needed to confirm the correlation between lifestyle and this geographic factor.
The investigation of explanatory factors for attributed causes offers a convenient way to compare this study population to other populations of cats. To allow for a better prediction model, more independent variables should be included in the future, such as sterilization timing and factors on the effect of the clinical practice.
One of the distinguishing features of our system is the inclusion of both euthanized and naturally deceased cases. A similar design was used in the English study (O'Neill et al., 2015). However, euthanasia was responsible for about one-fourth of our study population, which was hugely different from the composition of the study population in England (O'Neill et al., 2015). The difference may be related to how the owners perceive their pets, how the veterinarians suggest for specific diseases, different cost and different animal welfare strategies between the countries. In addition, strong correlations were noticed between the variables of whether the cat/owner was from the capital city and neuter status, option for euthanasia, and neoplasia as attributing causes. The difference between countries and correlations among geographic variables suggests that spatial variations may be related to different philosophies in veterinary practice, animal welfare issues, cost concerns, and other decision-making issues. Future studies on relevant issues will help in the targeted promotion and enhancement of animal welfare, as has been discussed in several reviews (Moore and Lund, 2009, O'Neill et al., 2014).
This system displayed the ability to reflect dynamic changes in a targeted population. For example, the second most common COD in cats in 2012 was infection, which shifted to neoplasia in 2013 and 2014. Such changes may sometimes correlate to epidemiological events. From October 2012 to February 2013, an outbreak of thiamine deficiency in cats due to defective dry food occurred in Taiwan. In severe cases, cats appear to have died from neural disorders (Chang et al., 2016). Based on data collected by our system, a notably higher incidence of neurological disorders attributed to CODs or RFEs occurred during this period as compared to the following two years (data not shown). This correlation suggests that this system may have the potential for epidemic surveillance in companion animals. Future direction should focus on long-term data collection to establish a baseline reference for the study population with the goal of population surveillance.
When compared to the VetCompass and Small Animal Veterinary Surveillance Network (SAVSNET) systems used in English studies (Radford et al., 2011, Jones et al., 2014, O'Neill et al., 2015), our system has some similarities and differences. Similar to our system, the two English systems also targeted domesticated animals frequenting primary care veterinary hospitals and also utilized nationwide, veterinarian-based, web-based data from veterinary hospitals. However, VetCompass and SAVSNET were similar to an electronic medical records system that could access the complete data on both living and deceased animals. Furthermore, SAVSNET also collected real-time data from veterinary diagnostic laboratories, which realized the purpose of real-time disease surveillance. With many more variables, these studies had a greater capacity for morbidity and mortality research.
Our system, in contrast, could only access mortality data, and this constituted the primary limitation of the present study: mortality rates were not available. A second limitation of our system was the relatively fewer variables evaluated as compared to VetCompass, especially dynamic demographic factors such as the timing of sterilization and the approach taken to determine a definitive diagnosis. This limitation may, to a certain extent, influence the categorization of COD/RFE and the assumption of logistic models, as discussed above.
There were several other limitations in this study. First, it may be disputed whether the reported data was representative of the domesticated cat population in Taiwan. The higher percentage of participating veterinary hospitals in the capital area (which accounted for approximately two-thirds of the reported cases) meant that cats frequenting veterinary hospitals in the capital city were over represented in our study and that the potential effects of clinical practice variations or cost concerns may exist. Secondly, a reporting bias may have been created because of variations among veterinarians and veterinary hospitals, the accuracy of the diagnosis (which may be influenced by many factors), and whether each veterinary hospital submitted all of their decreased animals to the database.
5. Conclusions
This study presented a novel approach for recording the real-time mortality of a pet population. This system is not perfect and can be improved further. However, to our knowledge, this is the first large-scale retrospective study on mortality of pet cats in Asia, and the data provides a valuable insight into the overall mortality of the domesticated cat population in Taiwan. We hope to bring more focus on the mortality of companion animals, and encourage further research in this field.
Unexpectedly, by discovering associations between common attributed causes and explanatory variables, we found that neutered cats had a significantly higher risk of dying from neoplasia and renal and urologic disorders than unneutered cats. Further research is now required to investigate the relationship between these diseases and neuter status in cats.
Conflict of interest
None of the authors of this paper have a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgements
The research was funded by Taipei City Animal Protection Office under Grant no. 101010, 102049, 103027, by Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Executive Yuan under Grant no. 103AS-10.1.1-BQ-B1(2), 102AS-10.1.1-BQ-B1(2), 101AS-10.1.2-BQ-B1(2), and by Ministry of Science and Technology, Taiwan, R.O.C. under Grant no. MOST103-2313-B-002-041-MY3. The authors gratefully acknowledge Kendy Tzu-Yun Teng and Jhih-Jyun Yang for statistic consultation.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.prevetmed.2016.12.011.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adams V.J., Evans K.M., Sampson J., Wood J.L. Methods and mortality results of a health survey of purebred dogs in the UK. J. Small Anim. Pract. 2010;51:512–524. doi: 10.1111/j.1748-5827.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Bailiff N.L., Nelson R.W., Feldman E.C., Westropp J.L., Ling G.V., Jang S.S., Kass P.H. Frequency and risk factors for urinary tract infection in cats with diabetes mellitus. J. Vet. Intern. Med. 2006;20:850–855. doi: 10.1892/0891-6640(2006)20[850:farffu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bonnett B.N., Egenvall A., Hedhammar A., Olson P. Mortality in over 350,000 insured Swedish dogs from 1995 to 2000: I. Breed- gender-, age- and cause-specific rates. Acta Vet. Scand. 2005;46:105–120. doi: 10.1186/1751-0147-46-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington C.A., Westropp J.L., Chew D.J., Bolus R.R. Risk factors associated with clinical signs of lower urinary tract disease in indoor-housed cats. J. Am. Vet. Med. Assoc. 2006;228:722–725. doi: 10.2460/javma.228.5.722. [DOI] [PubMed] [Google Scholar]
- Carciofi A.C., Bazzoli R., Zanni A. Influence of water content and the digestibility of pet foods on the water balance of cats. Braz. J. Vet. Res. Anim. 2005;42:429–434. [Google Scholar]
- Chang Y.P., Chiu P.Y., Lin C.T., Liu I.H., Liu C.H. Outbreak of thiamine deficiency in cats associated with the feeding of defective dry food. J. Feline Med. Surg. 2016 doi: 10.1177/1098612X15625353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimski D.S., Taboada J. Feline idiopathic hepatic lipidosis. Vet. Clin. North Am. Small Anim. Pract. 1995;25:357–373. doi: 10.1016/s0195-5616(95)50031-2. [DOI] [PubMed] [Google Scholar]
- Dru Forrester S., Roudebush P. Evidence-based management of feline lower urinary tract disease. Vet. Clin. North Am. Small Anim. Pract. 2007;37:533–558. doi: 10.1016/j.cvsm.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Egenvall A., Bonnett B.N., Hedhammar A., Olson P. Mortality in over 350,000 insured Swedish dogs from 1995 to 2000: II. Breed-specific age and survival patterns and relative risk for causes of death. Acta Vet. Scand. 2005;46:121–136. doi: 10.1186/1751-0147-46-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egenvall A., Nodtvedt A., Haggstrom J., Strom Holst B., Moller L., Bonnett B.N. Mortality of life-insured Swedish cats during 1999–2006: age, breed, sex, and diagnosis. J. Vet. Intern. Med. 2009;23:1175–1183. doi: 10.1111/j.1939-1676.2009.0396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Barber P. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J. Small Anim. Pract. 1998;39:78–85. doi: 10.1111/j.1748-5827.1998.tb03598.x. [DOI] [PubMed] [Google Scholar]
- Ferreri J.A., Hardam E., Kimmel S.E., Saunders H.M., Van Winkle T.J., Drobatz K.J., Washabau R.J. Clinical differentiation of acute necrotizing from chronic nonsuppurative pancreatitis in cats: 63 cases (1996–2001) J. Am. Vet. Med. Assoc. 2003;223:469–474. doi: 10.2460/javma.2003.223.469. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hasegawa A., Hosoi Y., Sugiura K. A current life table and causes of death for insured dogs in Japan. Prev. Vet. Med. 2015;120:210–218. doi: 10.1016/j.prevetmed.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hasegawa A., Sugiura K. Morbidity pattern by age, sex and breed in insured cats in Japan (2008–2013) J. Feline Med. Surg. 2015 doi: 10.1177/1098612X15616433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.H., Dawson S., Gaskell R.M., Coyne K.P., Tierney A., Setzkorn C., Radford A.D., Noble P.J. Surveillance of diarrhoea in small animal practice through the Small Animal Veterinary Surveillance Network (SAVSNET) Vet. J. 2014;201:412–418. doi: 10.1016/j.tvjl.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Kircher T., Anderson R.E. Cause of death. Proper completion of the death certificate. J. Am. Med. Rec. Assoc. 1987;58:47–51. [PubMed] [Google Scholar]
- Lowe A.D., Campbell K.L., Graves T. Glucocorticoids in the cat. Vet. Dermatol. 2008;19:340–347. doi: 10.1111/j.1365-3164.2008.00717.x. [DOI] [PubMed] [Google Scholar]
- Mayer-Roenne B., Goldstein R.E., Erb H.N. Urinary tract infections in cats with hyperthyroidism, diabetes mellitus and chronic kidney disease. J. Feline Med. Surg. 2007;9:124–132. doi: 10.1016/j.jfms.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G.E., Lund E. Disease reporting and surveillance: where do companion animal diseases fit in? Vet. Clin. North Am. Small Anim. Pract. 2009;39:225–240. doi: 10.1016/j.cvsm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- O'Neill D.G., Church D.B., McGreevy P.D., Thomson P.C., Brodbelt D.C. Approaches to canine health surveillance. Canine Genet. Epidemiol. 2014;1:2. doi: 10.1186/2052-6687-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill D.G., Church D.B., McGreevy P.D., Thomson P.C., Brodbelt D.C. Longevity and mortality of cats attending primary care veterinary practices in England. J. Feline Med. Surg. 2015;17:125–133. doi: 10.1177/1098612X14536176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesteanu-Somogyi L.D., Radzai C., Pressler B.M. Prevalence of feline infectious peritonitis in specific cat breeds. J. Feline Med. Surg. 2006;8:1–5. doi: 10.1016/j.jfms.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proschowsky H.F., Rugbjerg H., Ersboll A.K. Mortality of purebred and mixed-breed dogs in Denmark. Prev. Vet. Med. 2003;58:63–74. doi: 10.1016/s0167-5877(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Noble P.J., Coyne K.P., Gaskell R.M., Jones P.H., Bryan J.G., Setzkorn C., Tierney A., Dawson S. Antibacterial prescribing patterns in small animal veterinary practice identified via SAVSNET: the small animal veterinary surveillance network. Vet. Rec. 2011;169:310. doi: 10.1136/vr.d5062. [DOI] [PubMed] [Google Scholar]
- Seixas F., Palmeira C., Pires M.A., Bento M.J., Lopes C. Grade is an independent prognostic factor for feline mammary carcinomas: a clinicopathological and survival analysis. Vet. J. 2011;187:65–71. doi: 10.1016/j.tvjl.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Simpson K.W. Pancreatitis and triaditis in cats: causes and treatment. J. Small Anim. Pract. 2015;56:40–49. doi: 10.1111/jsap.12313. [DOI] [PubMed] [Google Scholar]
- White J.D., Malik R., Norris J.M. Feline chronic kidney disease: can we move from treatment to prevention? Vet. J. 2011;190:317–322. doi: 10.1016/j.tvjl.2010.12.011. [DOI] [PubMed] [Google Scholar]
- White J.D., Stevenson M., Malik R., Snow D., Norris J.M. Urinary tract infections in cats with chronic kidney disease. J. Feline Med. Surg. 2013;15:459–465. doi: 10.1177/1098612X12469522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthing K.A., Wigney D.I., Dhand N.K., Fawcett A., McDonagh P., Malik R., Norris J.M. Risk factors for feline infectious peritonitis in Australian cats. J. Feline Med. Surg. 2012;14:405–412. doi: 10.1177/1098612X12441875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.