Abstract
Signal-dependent targeting of proteins into and out of the nucleus is mediated by members of the importin (IMP) family of transport receptors, which recognise targeting signals within a cargo protein and mediate passage through the nuclear envelope-embedded nuclear pore complexes. Regulation of this process is paramount to processes such as cell division and differentiation, but is also critically important for viral replication and pathogenesis; phosphorylation appears to play a major role in regulating viral protein nucleocytoplasmic trafficking, along with other posttranslational modifications. This review focuses on viral proteins that utilise the host cell IMP machinery in order to traffic into/out of the nucleus, and in particular those where trafficking is critical to viral replication and/or pathogenesis, such as simian virus SV40 large tumour antigen (T-ag), human papilloma virus E1 protein, human cytomegalovirus processivity factor ppUL44, and various gene products from RNA viruses such as Rabies. Understanding of the mechanisms regulating viral protein nucleocytoplasmic trafficking is paramount to the future development of urgently needed specific and effective anti-viral therapeutics. This article was originally intended for the special issue “Regulation of Signaling and Cellular Fate through Modulation of Nuclear Protein Import”. The Publisher apologizes for any inconvenience caused.
Abbreviations: BPV, bovine papillomavirus; BRAP2, BRCA1-associated protein 2; CAV, chicken anaemia virus; CBP, CREB binding protein; Cdk, cyclin dependent kinase; CK1, protein kinase CK1; CK2, protein kinase CK2; Crm1, chromosome region maintenance protein 1; CTD, C-terminal domain; DLC, dynein light chain; DLC-AS, DLC-association sequence; dsDNA-PK, double stranded DNA-dependent protein kinase; EBV, Epstein–Barr virus; EXP, exportin; FG, phenylalanine–glycine; GSK3, glycogen synthase kinase 3; HCMV, human cytomegalovirus; HPV, human papilloma virus; HTLV, human T-cell leukaemia virus; IFN, interferon; IMP, importin; KSHV, Kaposi's sarcoma-associated herpes virus; MT, microtubule; MT-AS, MT-association sequence; LANA2, latency associated nuclear antigen 2; NE, nuclear envelope; NES, nuclear export sequence; NLS, nuclear localisation sequence; NPC, nuclear pore complex; Nup, nucleoporin; PKA, protein kinase A PKC, protein kinase C; PKC, protein kinase C; PML, promyelocytic leukaemia protein; Rb, retinoblastoma; RbBS, retinoblastoma binding site; RPP, Rabies virus phospho-protein; RV, Rabies virus; SARS, severe acute respiratory syndrome; STAT, signal transducer and activator of transcription; SV40, simian virus 40; T-ag, large tumour antigen; VZV, varicella zoster virus
Keywords: Simian virus 40 T-ag, Human cytomegalovirus ppUL44, Human papillomavirus E1, Rabies virus P, Phosphorylation, Nuclear import
Research highlights
► Nucleocytoplasmic trafficking of viral proteins is central to viral infection. ► Posttranslational modification is a key means to regulate viral protein trafficking. ► Nuclear trafficking of viral proteins can be a target for development of anti-virals.
1. Introduction
The mammalian cell is a highly organised, dynamic structure that compartmentalises its many functions into organelles such as the nucleus, Golgi, and endoplasmic reticulum. The nucleus retains the genetic material for cell maintenance and replication, whereby efficient signal dependent targeting of cellular proteins into or out of the nucleus, mediated by the importin (IMP) superfamily of transporters (see Fig. 2; Section 2) is required for the cell to function. During infection by various viruses, specific viral-encoded gene products exploit the host cell nucleocytoplasmic trafficking machinery to enter and exit the nucleus as part of the strategy of the virus to evade the host immune response and replicate productively. Many of these viral proteins appear not only to possess targeting signals mediating high affinity interaction with the cellular nuclear transport factors, but also show precise regulation thereof by phosphorylation of these interactions by cellular/virally encoded kinases or other enzymes (see Section 3).
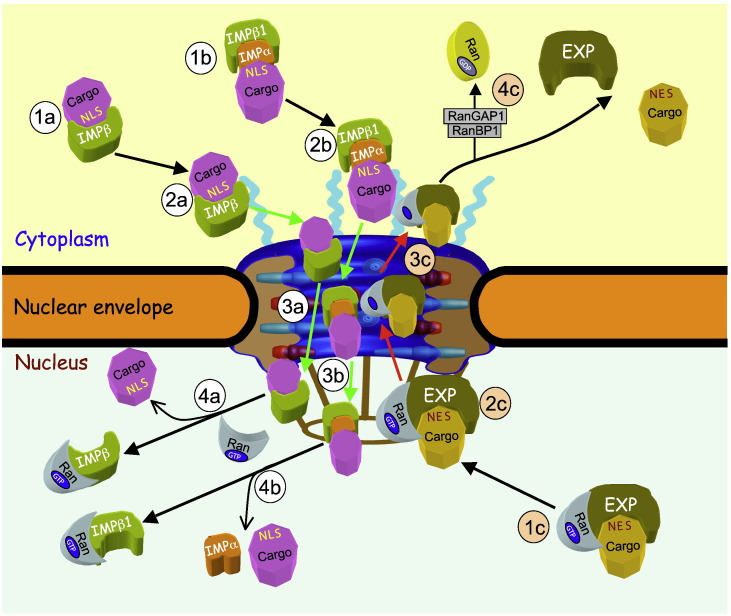
Fig. 2.
Schematic representation of IMP/EXP dependent nucleocytoplasmic transport. Transport of NLS-containing cargo proteins from the cytoplasm to the nucleus is either mediated by IMPβs alone (1a), or the IMPα/β1 heterodimer (1b), where the IMPα adaptor links the cargo protein to IMPβ1. The IMP/cargo complexes then dock onto the cytoplasmic side of the NPC (2a and 2b), followed by passage to the nuclear side of the NPC, through sequential, transient interactions of the IMPβ with the FG-Nups that make up the NPC (3a and 3b). Once within the nucleus, RanGTP binding to IMPβ disassociates the complex (4a and 4b) to release the NLS-containing cargo into the nucleus to perform its function. In analogous fashion to nuclear import, transport of NES-containing cargo proteins (1c) from the nucleus to the cytoplasm is mediated by EXPs which recognise the NES, dependent on RanGTP binding to the EXP. The EXP/RanGTP/cargo complex docks at the nuclear side of the NPC (2c), before passing to the cytoplasmic side of NPC through sequential, transient interactions of the EXP with the FG-Nups (3c). Once within the cytoplasm, RanGAP1 (RanGTPase-activating protein 1) and RanBP1 facilitate hydrolysis of GTP to GDP by Ran (4c), thereby dissociating cargo from the EXP.
This review will focus in detail on viral proteins for which there is evidence of regulated nucleocytoplasmic trafficking in infected cells, including gene products from DNA viruses such as simian virus 40 (SV40) large tumour antigen (T-ag), human cytomegalovirus (HCMV) processivity factor ppUL44, and the human papilloma virus (HPV) E1 protein, as well as the phospho “P” protein from the negative stranded RNA Rabies virus (RV). The regulatory mechanisms and evidence for a physiologically important role in the viral infectious cycle will be discussed (Section 4), with the implication being that the regulation of viral protein nuclear import is crucial for many diverse viruses, thereby representing a potential target for the future development of anti-viral agents.
2. Nucleocytoplasmic transport
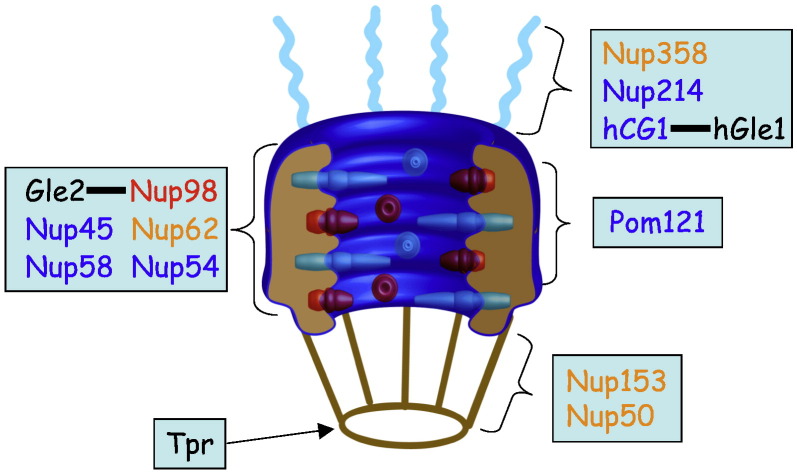
All transport into and out of the nucleus occurs through the nuclear pore complexes (NPCs), macromolecular structures (> 60 MDa) that span the double lipid bilayer of the nuclear envelope (NE) [1], [2], [3], [4]. There are approximately 2000 NPCs per “typical” vertebrate cell, depending on the stage of the cell cycle and the cell type. NPC structure is typified by 8-fold symmetry, being made up of multiple proteins called nucleoporins (Nups) [5], [6], [7], [8] which occur in multiples of eight [9]. With the exception of certain peripheral, asymmetric Nups, most Nups localise on both sides of a symmetry axis in the plane of the NE [2], [9], and can be grouped into several classes based on homology and functional similarity [10], including (i) transmembrane Nups (i.e. POM121 and Gp210 in vertebrates), which anchor the NPC within the NE and are bound by (ii) structural Nups (c. 50% of all Nups), which contribute to the overall architecture of the NPC and represent the scaffold linking the transmembrane Nups and (iii) FG-Nups (c. 33% of all Nups/50% of the NPC mass), which are distinguished by the fact that they contain multiple FxFG (single letter amino acid code, where x is any amino acid) or GLFG motifs separated by varying numbers of charged or polar amino acids [2], [11]. Fig. 1 shows the distribution of specific FG-Nups within the NPC, highlighting their position throughout the NPC. A number of studies indicate that FG-Nups are integral to bidirectional active transport through the NPC because of their ability to interact transiently with IMPs [9], [12], [13], [14], [15], [16].
Fig. 1.
Schematic representation of the NPC highlighting vertebrate FG-Nup subcomplexes (modified from [9]). Each box denotes a biochemically or functionally defined subcomplex, where “FG-Nups” containing predominantly FG, GLFG, and FXFG repeats are highlighted in blue, red and orange texts respectively, with selected structural, non-FG-Nups in black.
Translocation through the NPC of proteins > 45 kDa is generally mediated by members of the IMP superfamily of nuclear transporters, which includes 6 α and c. 20 β forms in humans. IMPαs are adaptors that function as heterodimers with IMPβ1 [1], [17], [18], [19] in nuclear import, whilst IMPβs can mediate transport in either direction through the NPC, with those mediating nuclear export called exportins (EXPs). IMPs/EXPs recognise specific sequences, nuclear localisation sequences (NLSs) or nuclear export signals (NESs) respectively within the cargo protein with which they interact, with the monomeric guanine nucleotide binding protein/GTPase Ran a key additional factor (see below) modulating cargo binding [17], [20].
Monopartite basic NLSs, such as that from SV40 T-ag (PKKKRKV132) [21], [22] and HCMV ppUL44 (PNTKKQK 431) [23] as well as bipartite NLSs, which comprise two clusters of basic residues such as the HPV E1 NLS (KRK 85-/-KKVKRR 125) [24], are generally recognised by the IMPα/IMPβ1 heterodimer. All IMPβs including IMPβ1, in contrast, are able to mediate import or export of their cargoes without the need for IMPα or other adaptors, although the NLS/NES sequences have not been defined in many cases. NESs recognised by EXP-1 (Crm1) [25], [26], [27] comprise 3–4 hydrophobic residues interspersed with 1 to 3 non-hydrophobic residues (L-x2-3-(L,I,M,F,M)-x2-3-L-x-(L,I,V) [17], [20]), the classic example being the NES from HIV-1 Rev (LPPLERLTL 83) [28].
As indicated above, IMP-dependent passage through the NPC is effected by transient interactions of the IMPβs with FG-Nups; Nup358 is proposed to play a key role in assembly of the IMP–cargo complex [29], [30], with a gradient of increasing affinity postulated to facilitate the passage of IMP–cargo complexes from cytoplasmic to nucleoplasmic side of the NPC (see [31]). In the case of nuclear import, release at the nuclear face requires Ran in its activated GTP-bound form to bind to the IMPβ to dissociate the import complex (Fig. 2 left). Nuclear export is analogous, where the EXP, only when in complex with RanGTP, recognises a NES within a cargo and forms a trimeric export complex (EXP/RanGTP/NES-cargo) that is able to translocate through the NPC through transient interactions with FG-Nups such as Nup98 [32] and the non-FG-Nup Tpr ([33]) on the nuclear side, and Nup214 [34] on the cytoplasmic side (see Fig. 1), where the complex is dissociated via GTP hydrolysis by Ran of GTP to GDP, facilitated by RanGTPase-activating protein (RanGAP) (Fig. 2 right) and Ran binding protein 1 (RanBP1) and/or the RanBP1-like domains of Nup358 [30].
Many viral proteins utilise the host cell nucleocytoplasmic trafficking machinery (Fig. 2) to achieve efficient nuclear import and/or export in order to carry out particular roles in viral replication and pathogenesis, and/or modulate the host cell cycle or innate immune response (see below and Table 1 ). The next sections examine a number of different viral proteins by way of illustrating the diverse mechanisms regulating viral protein nuclear import/export.
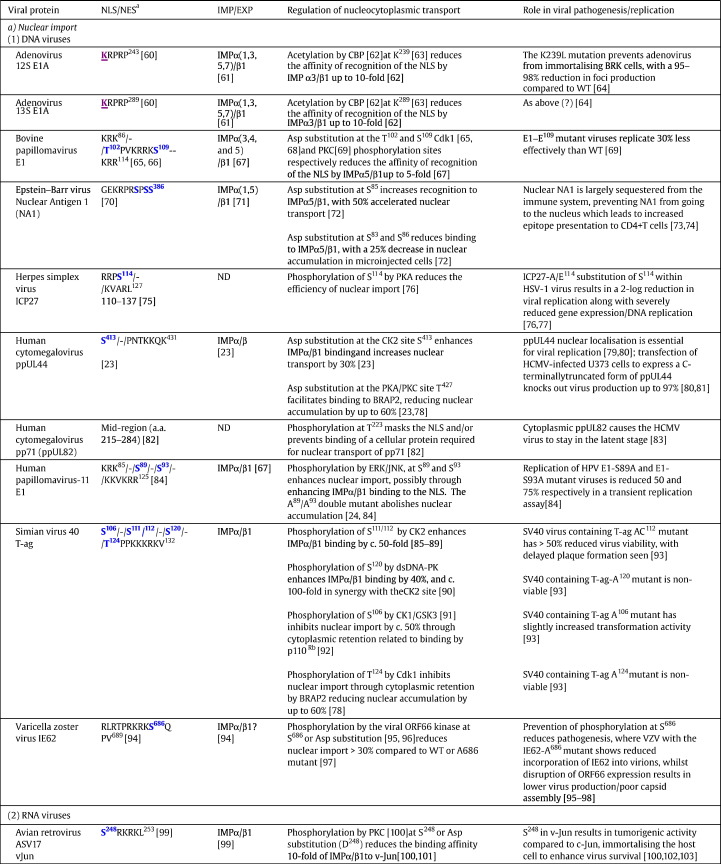
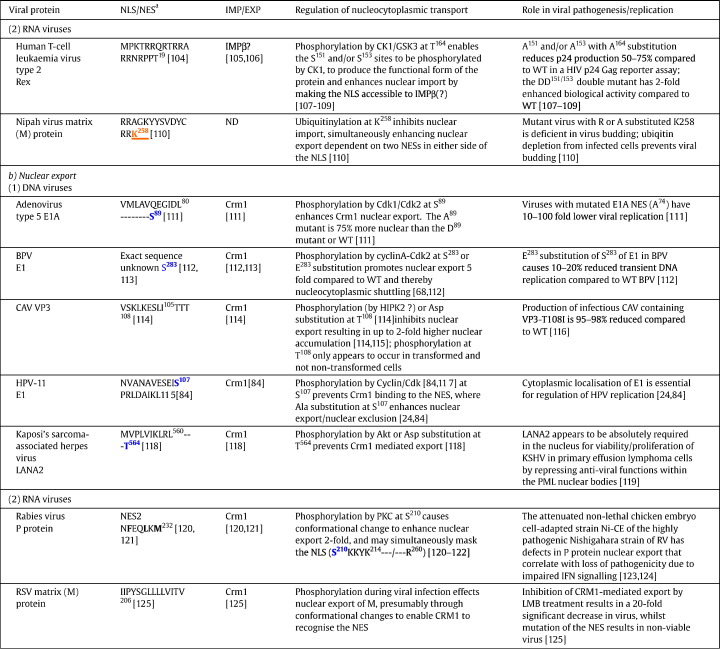
Table 1.
Selected examples of viral proteins where regulation of nucleocytoplasmic trafficking is implicated in viral pathogenesis.
ND, not determined.
Abbreviations: CBP, CREB-binding protein; Cdk, cyclin dependent kinase; CK2, protein kinase CK2; dsDNA-PK, double stranded DNA-dependent protein kinase; GSK3, glycogen synthase kinase 3; HIPK2, homeodomain-interacting protein kinase 2; LANA2, latency associated nuclear antigen 2; LMB, leptomycin B; PKA, protein kinase A; PKC, protein kinase C; PML, promyelocytic leukaemia protein; WT, wild type.
aSingle letter code used for sequences; known/potential kinase sites (in bold blue), acetylation sites (in bold purple and underlined) or ubiquitination sites (in bold orange and underlined) are highlighted, with amino acid position in the protein of interest shown in the superscript.
3. Regulation of nuclear transport
General mechanisms by which nucleocytoplasmic trafficking can be regulated include modulation of the levels and distribution of IMPs/EXPs [35], [36] as well as the number and/or composition of NPCs [2], [11]. Fine-tuning of the localisation/transport of a single protein or group of proteins, however, requires more specific modification, generally of the protein cargo itself rather than of the transport machinery. The best understood mechanism of regulating nuclear transport is through phosphorylation near the NLS/NES modifying recognition by IMP/EXP [37], [38], but modifications such as acetylation, ubiquitinylation and sumoylation have also been described [39], [40], [41] to regulate nucleocytoplasmic trafficking of cellular proteins such as the tumour suppressors p110Rb and p53, Survivin, nuclear factor NF-κB, the phosphatase PTEN and the NF-κB essential modulator NEMO [42], [43], [44], [45], [46], [47], [48]. It is significant in this context that viral proteins are often highly posttranslationally modified (see Table 1), including through the action of cyclin-dependent kinases (Cdks), which can serve to effect cell cycle-dependent modulation of nucleocytoplasmic trafficking. A specific example is HPV E1, which will be examined in more detail in Section 4.2.
3.1. The cellular nuclear transport machinery as a viral target
The NPC and the Nups that constitute it are thought to be passive in nucleocytoplasmic transport in most situations. However, NPC composition and Nup conformation can have an influence on the transport of IMPs/EXPs as well as cargoes. Since IMPs/EXPs appear to have different affinities for the FG-Nups (see Section 2), the presence or absence of certain FG-Nups may favour one set of transport factors/cargo over another [13], [14], [15], [16].
Certain viral proteins are known to act directly or indirectly on the NPC and IMPs/EXPs [49] in order to act to alter host cell functions. An example with respect to the NPC is the 3C protease from the picornavirus Rhinovirus [50], which is thought to target Nups153, 214 and 358 for specific degradation in order to impair host cell nucleocytoplasmic transport (see Section 2 and Fig. 1), and thereby dampen anti-viral responses [50]; altered NPC structures have also been visualised in cells infected by the closely related poliovirus [51]. 2A protease from both Rhinovirus and poliovirus appears to act similarly to 3C in this respect [52], [53], [54], implying that the NPC is a key target of picornaviruses to disrupt host cell transport processes, and lead to “host cell shut down” to enable viral replication to proceed unchecked in the cytoplasm.
IMPs/EXPs can also be targets of viral proteins. Ebola virus VP24, for example, binds to and sequesters IMPα1 [55], [56], [57] in the cytoplasm, whilst IMPα2 is similarly sequestered by severe acute respiratory syndrome (SARS) coronavirus ORF6 [58]. In both cases, the IMPα is prevented from playing its normal role in mediating nuclear import of the STAT (signal transducer and activator of transcription) proteins in response to interferon (IFN), as part of the innate immune response (see [59]). Thus, it seems that various cytoplasmically replicating RNA viruses disrupt the cellular nuclear transport machinery in order to subvert the host cell transport processes necessary for the anti-viral response.
In the case of DNA viruses that replicate in the nucleus, however, efficient nuclear entry of many viral components is crucial for replication, so that disrupting the host cell nuclear import apparatus would not be a viable strategy to ensure efficient replication. The next section discusses the ways in which IMPs and cellular kinases can be subverted to enable efficient nuclear transport of gene products from DNA viruses that are required in the nucleus for replication.
3.2. Specific switches regulating IMP/EXP mediated trafficking
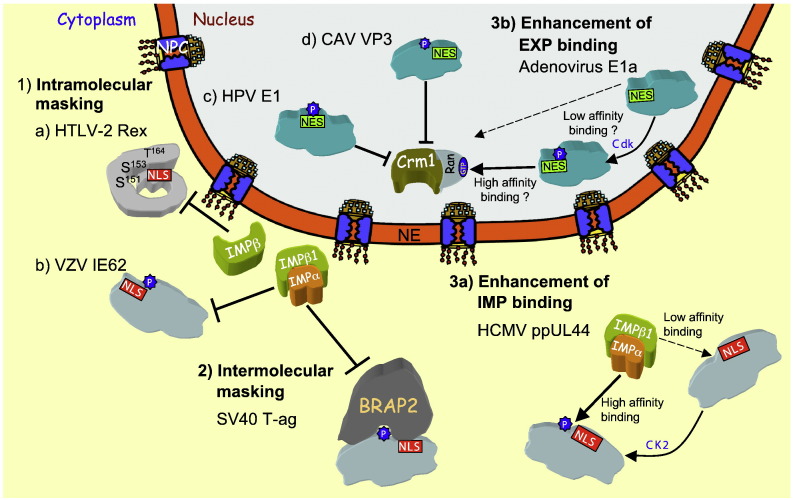
As indicated, the most common posttranslational modification known to regulate nuclear transport is phosphorylation. A number of viral proteins are known to require specific phosphorylation in different ways for efficient nuclear accumulation, including T-ag (see Section 4.1), HCMV ppUL44, chicken anaemia virus (CAV) VP3 and many others [23], [37], [38], [49]. Phosphorylation can regulate nuclear transport (see Fig. 3 ) by 1) directly modulating the affinity of an NLS/NES for its IMP/EXP; 2) facilitating masking or unmasking (intramolecular masking) of an NLS/NES within the protein carrying it; or 3) effecting the binding or release of an NLS/NES binding factor that is not an IMP/EXP (intermolecular masking) [4], [38].
Fig. 3.
Schematic representation of the mechanisms of regulation of IMP/EXP-dependent nuclear transport, as illustrated by examples of viral proteins. In intramolecular masking (1) IMPs/EXPs are prevented from binding the NLS/NES of the cargo by masking of the NLS/NES by sequences within the same protein. This is exemplified by (a) the human T-cell leukaemia virus type 2 (HTLV-2) Rex protein in its inactive p24 form, where specific phosphorylation by CK1/GSK3 at T164 and subsequent phosphorylation at S151/153 are required for IMPβ recognition of the NLS, and (b) the VZV IE62, where phosphorylation at S686 by the VZV kinase ORF66 inhibits nuclear import by impairing recognition by IMPα/β1 [97]. Examples of intramolecular masking in nuclear export are shown for (c) HPV E1, where Cdk mediated phosphorylation of S107 prevents Crm1 binding, resulting in nuclear retention [24], [84], and (d) CAV VP3, where phosphorylation of T108 specifically in cancer cells prevents nuclear export [114], [115]. In intermolecular masking (2), NLSs/NESs are masked from IMPs/EXPs binding by a heterologous protein/molecule. An example in the case of nuclear import is the cytoplasmic protein BRAP2 which, dependent on Cdk phosphorylation of T124, prevents recognition by IMPα/β1 of the SV40 T-ag NLS, a similar mechanism dependent on protein kinase C phosphorylation of T427 applies to HCMV ppUL44 protein (not shown) [78]. Enhanced nuclear import/export can occur through posttranslational modification enhancing NLS/NES recognition by IMP/EXP. An example (3a) is the increase in the affinity of binding of IMPα/β1 to the NLSs of HCMV ppUL44 and SV40 T-ag (not shown) by CK2 phosphorylation (of S413 and S111/112 respectively) leading to enhanced nuclear import [23], [85], [87]. In the case of nuclear export (3b), Cdk1/Cdk2 mediated phosphorylation of S89 enhances recognition of the Adenovirus type 5 E1a NES by Crm1, leading to more efficient nuclear export [111].
Table 1 summarises the mechanisms of regulation of nuclear import/export for a number of viral proteins for which nucleocytoplasmic trafficking is known to be important for the infectious cycle, with Fig. 3 illustrating several specific examples. As can be seen from Table 1, phosphorylation is a key modulator of nuclear transport of viral proteins, but other modifications, such as acetylation and ubiquitinylation, can also modulate nuclear transport.
Phosphorylation-mediated modulation of NLS/NES access, resulting in either inhibition (intramolecular masking) or enhancement of transport (see Fig. 3 and Table 1), is the most common means to regulate nuclear transport efficiency. The human T-cell leukaemia virus type 2 (HTLV-2) Rex protein (see Fig. 3) is an example; in its pre-mature (p24) form, the N-terminal IMPβ-recognised NLS is masked [107], [108], [109], but upon phosphorylation of T164 by protein kinase CK1 (CK1)/glycogen synthase kinase 3 (GSK3) [107], S151/153 is subsequently phosphorylated by CK1 to produce the active p26 form of the protein with an accessible NLS [107], [108], [109]. In the case of Kaposi's sarcoma-associated herpes virus LANA2 (latency-associated nuclear antigen 2), phosphorylation at T564 by Akt is believed to promote a conformational change that inhibits Crm1 binding to the NES [118]. A similar mechanism appears to apply to CAV VP3 (see Fig. 3) through the T108 phosphorylation site [114], [115], although phosphorylation in this case appears to only occur in transformed and not normal cells, making the nuclear targeting module of VP3 an exciting possibility for tumour-cell specific nuclear targeting. In the case of the SV40 T-ag protein, protein kinase CK2 (CK2) phosphorylation at S111/112 increases the affinity of recognition of the NLS by IMPα/β1, thereby accelerating the nuclear import rate c. 50-fold; this can be further enhanced by phosphorylation of the double-stranded DNA-dependent protein kinase (dsDNA-PK) site S120, which facilitates phosphorylation at the CK2 site, as well as IMPα/β1 recognition/nuclear import [85], [86], [87], [88], [89], [90]. In analogous fashion, HCMV ppUL44 is phosphorylated at S413 by CK2 to enable higher affinity recognition of the NLS by IMPα/β1 and increased nuclear import (see Fig. 3; [23]), and a similar mechanism appears to apply to the Adenovirus E1a protein (see Fig. 3), where phosphorylation by Cdk1 at S89 enhances Crm1-mediated nuclear export [111].
Intermolecular masking occurs when a heterologous protein prevents IMP/EXP recognition of normally accessible NLS/NES sequences in a cargo protein. Inhibitor protein I-κB is an example of a very specific cytoplasmic retention factor which binds to the NLS of the transcription factor NF-κB p65 to prevent IMPα/β1 interaction and thereby inhibit nuclear import. Upon activation of signal transduction, e.g. cytokine production during an immune response, I-κB is phosphorylated and degraded to unmask the p65 NLS and enable nuclear import [126], [127]. An example of a more general cytoplasmic retention factor that affects nuclear import of a number of different NLS-containing proteins, including SV40 T-ag and HCMV ppUL44 [78] is BRCA1 associated protein 2 (BRAP2). Intermolecular masking of the T-ag NLS by BRAP2 is dependent on phosphorylation of T124 by Cdk1 adjacent to the NLS (see Fig. 3 and Table 1), whilst PKA/PKC mediated phosphorylation of T427 within the ppUL44 NLS similarly facilitates interaction with BRAP2 and cytoplasmic retention [78]. Cellular proteins such as p53 and p21cip [78], [128], [129], [130], [131], [132], [133] which possess NLSs and adjacent phosphorylation sites resembling those of SV40 T-ag and HCMV ppUL44 also appear to be able to be recognised by BRAP2 and inhibited in terms of nuclear import.
The subcellular distribution of viral proteins is able to be precisely regulated by specific cellular mechanisms; this can be seen as representing part of the host cell anti-viral response, but is also able to be exploited by the various viruses to enhance replication. For example, although the inhibition of nuclear import of SV40 T-ag or HCMV ppUL44, by BRAP2 leads to slowing/prevention of viral replication, this may also contribute to viral replication by delaying it until the optimal stage of the cell cycle or cellular signal transduction state, which is achieved by the phosphorylation control of BRAP2 interaction with SV40 T-ag/HCMV ppUL44. The following section describes several specific examples where a physiological role of regulated nucleocytoplasmic trafficking is implicated in viral pathogenesis and/or the viral replication cycle.
4. Selected examples of regulation of subcellular trafficking of viral proteins
4.1. SV40 T-ag and HCMV ppUL44: multiple mechanisms of regulation of nuclear import through protein–protein interactions
SV40 virus replication uniquely is dependent on a single protein – T-ag – whose roles include as an initiation factor for viral DNA replication, dysregulation of the cell cycle and blocking apoptosis [134], [135]. T-ag's three main functional domains are the J domain (a.a. 1–82) that binds to hsc70, the constitutively expressed homologue of heat shock protein hsp70 [136], [137], the LxCxE motif (residues 103–107) that confers binding to the retinoblastoma (Rb) family of proteins p110Rb, p107Rb and p130Rb2 [138], [139], and a bipartite carboxyl-terminal domain (a.a. 351–450 and 533–626) that binds to the tumour suppressor p53 [137], [138], as well as the CREB binding protein (CBP) and the functional homologues, p300 and p400, all of which have roles in cell growth and transformation [140], [141]. T-ag's functions in replication are nuclear, as are the functions of the various host cell target proteins of T-ag; consistent with this, T-ag possesses a highly efficient NLS [21], [22], [142]. Early work showed that T-ag was a phosphoprotein [143], [144], with several clusters of phosphorylation sites [145], [146], [147] shown to be phosphorylated in SV40 infected cells and critical for T-ag function/virus replication (see Table 1) [148]. These include the CK2 site (S111/112) [85], [87], [88], the Cdk1 (cdc2) site (T124) [86] and the less well characterised CK1/GSK3 site (S106) [91], all of which affect virus replication [93], [148] as shown in Table 1, which summarises the effect of mutations at these sites on SV40 T-ag nuclear transport as well as SV40 pathogenesis/replication.
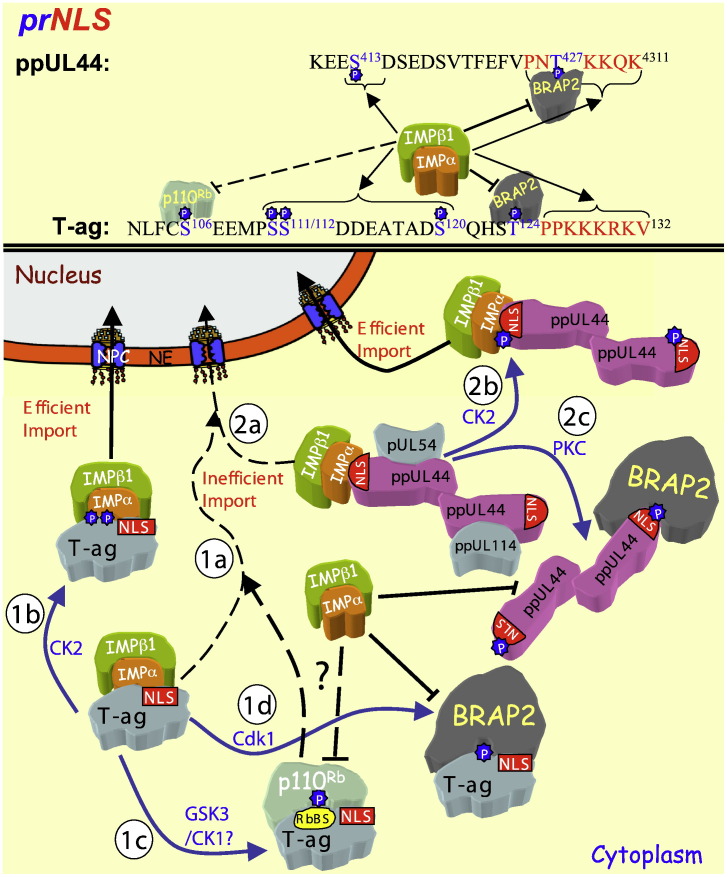
HCMV DNA replication occurs within the nucleus of the infected cell, through a “rolling circle” mechanism [149] that requires at least 6 essential virally encoded gene products [150], [151] which include the DNA holoenzyme complex, which is made up of a catalytic subunit (pUL54), and the phosphoprotein and processivity factor ppUL44 [79]. The ppUL44 N-terminal region possesses the ability to bind dsDNA in the absence of ATP and clamp loaders, and through its ability to bind to pUL54, can link pUL54 to DNA and stimulate DNA polymerase activity [79]. The N-terminal region also possesses dimerisation activity [152], [153]. Early in infection, ppUL44 localises to the nucleus through a C-terminally localised NLS (PNTKKQK431) [23], that also appears to be responsible, for “piggy-back” nuclear import of other viral replication fork proteins such as pUL54 and the uracil DNA glycosylase pUL114 [154], [155], whereby the proteins may assemble in the cytoplasm on the ppUL44 dimer before nuclear import ([156], [157]; Fig. 4 ). Importantly, ppUL44 is a target for cellular and viral kinases during infection [23], [158], [159], with several phosphorylation sites, including CK2 (S413) and PKC (T427) sites, N-terminal (a.a. 410–424) to the NLS [23], [78] (see Table 1 and Fig. 3, Fig. 4). This constellation of phosphorylation sites N-terminally proximal to the NLS is closely comparable to that of SV40 T-ag (see above) [23].
Fig. 4.
The regulation of viral protein nuclear import through phosphorylation near the NLS. The phosphorylation regulated NLSs (prNLSs) of SV40 T-ag and ppUL44 are shown (top — single letter amino acid code) with the regulatory phosphorylation sites (blue) and the NLS (red) highlighted, as well as the binding partners recognising them when phosphorylated (“P”). The T-ag NLS alone mediates IMPα/β1-mediated nuclear import, relatively inefficiently (black dotted arrow — 1a), but upon phosphorylation at serine111/112 by CK2 (blue arrow), IMPα/β1 is able to bind the T-ag NLS c. 50-fold better, to facilitate subsequent efficient nuclear import (1b). Phosphorylation at serine106 by GSK3/CK1 (blue arrow) allows p110Rb to bind T-ag at the RbBS, which leads to cytoplasmic retention and decreased nuclear import (1c). Phosphorylation at threonine124 by Cdk1 (blue arrow) allows BRAP2 to bind the T-ag NLS to prevent IMPα/β1 binding through intermolecular masking, and sequester T-ag in the cytoplasm (1d). (2a) HCMV ppUL44 IMPα/β1-mediated NLS-dependent nuclear import is inefficient (black dotted arrow) but upon phosphorylation at serine413 by CK2 (blue arrow), IMPα/β1 is able to bind the ppUL44 NLS with greater affinity to facilitate efficient nuclear import (2b). Phosphorylation at Thr427 by PK-C (blue arrow), enhances binding of ppUL44 to BRAP2 to prevent IMPα/β1 binding through intermolecular masking, and sequester ppUL44 in the cytoplasm and prevent nuclear import. Since ppUL44 may play a role in piggy-backing the HCMV proteins pUL54 and ppUL114 proteins into the nucleus early in infection, the various regulating mechanisms may apply to nuclear import of multiple HCMV proteins.
4.1.1. Positive and negative regulation of nuclear import through specific phosphorylation
Detailed analysis of the transport kinetics of bacterially expressed proteins microinjected into hepatoma cells indicated that the T-ag NLS alone (residues 126–132) conferred a much slower rate of import than the NLS together with the N-terminal flanking residues (a.a. 111–132) which contains the various phosphorylation sites mentioned above [88], [89]. Deletion/mutation of the CK2 site S111/112 to prevent phosphorylation decreased the import rate [88], whilst D112 substitution enhanced nuclear import [87], [160]; although S111 can function in its absence as a CK2 site, S112 is the main site of CK2 phosphorylation [87]. The mechanism of enhanced nuclear import through the CK2 site is through phosphorylation increasing the affinity of T-ag NLS recognition by the IMPα/β1 heterodimer [85]. Negative charge at S120, the dsDNA-PK site, apart from facilitating CK2 phosphorylation at S111/112, also enhances IMPα/β1 binding to the NLS [90]. That phosphorylation of S111/112 to enhance nuclear accumulation of T-ag is physiologically important in SV40 replication is indicated by the fact that viruses with mutations in the CK2 site (S112 and/or both S111/112) have markedly slower kinetics of DNA replication, and reduced viability (> 50%) [93], [148].
Significantly, HCMV ppUL44 processivity factor has an NLS comparable to that of SV40 T-ag, together with an adjacent CK2 site (see Table 1) that acts to increase the affinity of recognition by IMPα/β1 and nuclear transport efficiency [23]. Since ppUL44 contributes to nuclear accumulation of other HCMV gene products such as pUL54 and pUL114 involved in virus replication, the enhancement of ppUL44 nuclear import by CK2 would appear to be crucial to HCMV, with inhibition of CK2 potentially a viable future anti-viral approach to inhibit HCMV replication [23], [156], [157], [161].
That CK2 is exploited by SV40 and HCMV and possibly other viruses, to enhance nuclear localisation of proteins involved in their DNA replication can be understood in terms of CK2 being ubiquitously expressed and constitutively active [162]. Intriguingly, certain viruses have been shown to directly control CK2 localisation as well as up regulate its expression. During HSV-1 infection, for example, the ICP27 protein is known to recruit CK2 from the nucleus to the cytoplasm, resulting in a 3.5-fold increased CK2 activity by 6 h post infection that enhances cytoplasmic localisation of phosphorylated ICP27 and thereby facilitates its role in shuttling HSV mRNAs from the nucleus [163]. Analogously, CK2 appears to be recruited from subnuclear structures to regulate intranuclear transport of ribosomal RNA during Adenovirus infection [164]. The implication is that CK2 activity is integral to infection in the case of a number of viruses, with more examples of viruses using CK2 to modulate subcellular localisation likely to be identified in the near future.
In contrast to the effects of phosphorylation at S111/112/120, Cdk-phosphorylation or Asp substitution of T124 adjacent to the NLS inhibits T-ag nuclear import [78], [86]. The mechanism of inhibition of nuclear import is not through preventing IMPα/β1 recognition of the NLS, but rather through negative charge enhancing binding of the cytoplasmic retention factor BRAP2, first identified as a binding partner of BRCA1 in a yeast-2-hybrid screen [165]; negative charge at T124 appears to enhance specific binding of BRAP2 to SV40 T-ag, thereby inhibiting nuclear import [78].
Analogously, BRAP2 has also been shown to bind the HCMV processivity factor ppUL44, dependent on negative charge at T427 within the NLS (see Table 1 and Fig. 4) [78], making BRAP2 the first example of a cellular negative regulator of nuclear import (NRNI) that inhibits nuclear bound viral cargo in a phosphorylation-dependent manner. Although this has only been shown thus far for gene products from dsDNA viruses, it seems likely that this may apply to other viruses/viral gene products. The fact that BRAP2 may represent a general cellular defence mechanism to stem viral replication is an intriguing idea that warrants further investigation to examine its full potential as an anti-viral agent. It should not be ignored, however, that, as alluded to above, cytoplasmic retention of viral proteins until an optimal cell cycle/signal transduction state of the cell is attained is a strategy utilised by many viruses to facilitate rather than prevent virus production/infectivity etc. That virus replication is optimal at particular stages of the cell cycle has been shown for Hepatitis C, Epstein–Barr Virus (EBV), varicella zoster virus (VZV), Kaposi's sarcoma-associated herpes virus (KSHV), as well as HPV [166], [167], [168], [169], [170].
4.1.2. Inhibition of nuclear import through p110Rb
Unlike HCMV ppUL44, SV40 T-ag, as indicated above, is able to bind Rb family members through the Rb binding site (RbBS) [134], [135]. The CK1/GSK3 site (S106) within the RbBS has been shown to be critical for transformation and viral replication (see Table 1; [93], [148]), correlating with the fact that negative charge at this site inhibits nuclear transport [92] through modulation of binding of p110Rb, but not other Rb family members. Deletion or mutation of critical residues in the RbBS relieves inhibition of nuclear import for T-ag proteins carrying the RbBS, whilst cancer cells lacking functional p110Rb show no reduction in nuclear transport due to the RbBS. Based on fluorescence recovery after photobleaching (FRAP) experiments, the mechanism of inhibition appears to be through cytoplasmic retention of the Rb–T-ag complex [92].
Significantly, other DNA tumour viruses gene products such as adenovirus E1a [171], [172], [173], JC and Bk virus T-ag proteins [174], [175], [176], and pUL97 from HCMV [177] all possess RbBS's analogous to that of SV40 T-ag. Although the conventional view is that viral proteins target p110Rb to impair its role in the cell cycle [178], [179], it does not seem unreasonable to speculate that p110Rb in turn may act on a number of transforming viruses by modulating the nuclear import of diverse viral proteins.
4.2. HPV E1 protein: cell cycle phosphorylation controls levels of nuclear protein
HPV has a particular tropism for squamous mucosal or cutaneous epithelia [180], where infection can trigger hyperproliferation of epithelial keratinocytes and benign warts in the case of certain “lower risk” HPV genotypes (e.g. 6 and 11) [181], [182], or can lead to malignant cancer [183], [184] in the case of certain “high risk” HPV genotypes (e.g. 16 and 18) [181], [182], [184]. Most infections are latent, however, where the viral DNA persists in the host as low copy number extrachromosomal plasmids in the basal germinal stratum as a result of low-level expression of the viral genes [180].
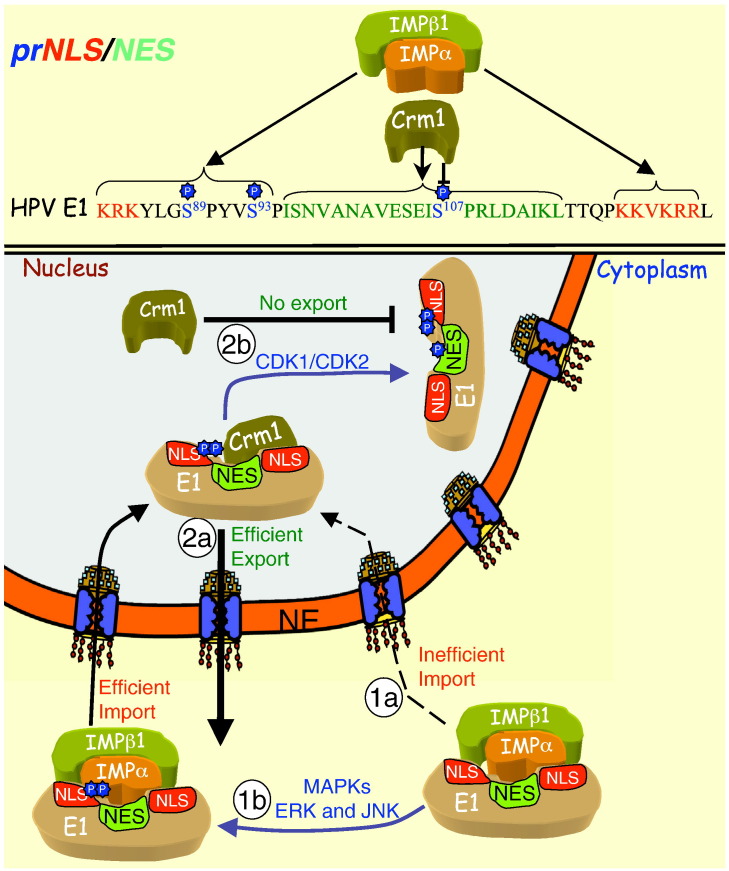
E1 is a 70 kDa site-specific ATP-dependent DNA helicase essential for virus replication, which is highly conserved amongst all HPV types, and is essential for viral replication and amplification [185], [186], [187]. Together with HPV E2 protein, which increases its affinity for DNA [188], [189], [190], [191], [192], [193], [194], E1 is able to bind to a specific binding element in the viral origin to act to facilitate origin DNA unwinding, recruit the host cell DNA polymerase α-primase complex, and thereby initiate viral DNA synthesis [195], [196], [197], [198], [199], [200], [201]. E1 performs its role in the nuclear compartment, which it accesses through a bipartite NLS (HPV-11 E1 83KRK/-/S89/-/S93/-/KKVKRR125 [24]). Between the basic amino acids of the bipartite NLS is a potent NES (HPV-11 E1 97NVANAVESEIS 107PRLDAIKL115 [84]) that confers rapid export out of the nucleus through Crm1 [24], [84]. Bovine papillomavirus (BPV) E1 is functionally homologous to HPV E1, being able to substitute for HPV E1 in replicating the HPV genome, and vice versa [185]. Although phosphorylation by Cdk2 at S-phase of the cell cycle promotes BPV E1 nuclear export via Crm1, where phosphorylation/dephosphorylation at S283 would appear to enable rapid nucleocytoplasmic shuttling [112], [113], Cdk phosphorylation appears to promote nuclear retention in the case of HPV-11 E1 [24], [84]. The presence of a NES in E1 presumably relates to the need for the virus to slow viral replication to establish a persistent infection in the basal keratinocytes and maintain low copy number by keeping the nuclear concentration of E1 low. Only once the basal cells differentiate and start to rise to the skin surface does E1 accumulate in the nucleus to enable HPV enter the vegetative stage of its life cycle, when the viral promoters are significantly up-regulated and late gene products produced to enable HPV to have the best chance to reinfect another host [170], [201], [202], [203], [204].
HPV E1 nuclear localisation is modulated by phosphorylation by MAPKs present in the cytoplasm, and Cdks in the nucleus, at specific stages of the cell cycle [24]. E1 has been shown to interact with several cyclin/Cdk complexes in vitro [68], [117], as well as directly with cyclin E [117], [205], an interaction that is essential for viral replication [117]. The N-terminal domain of E1 possesses a cyclin binding motif (RxL126) [117] as well as several Cdk phosphorylation sites, of which S89, S93 and S107 have been shown through mutational analysis to inhibit transient replication of viral origin-containing plasmids in transfected cells [117]. Phosphorylation of all three serines (see Fig. 5 ) appears to be required for efficient nuclear localisation that is dependent on active cyclin E/Cdk2 and/or cyclin A/Cdk2 at S107 [24], [84], [117], and MAPKs (ERK/JNK) at S89/93 [24]. S106 phosphorylation prevents Crm1 recognition of E1's NES [24], [84], whilst phosphorylation probably by MAPKs of S89 and S93 seems likely to facilitate recognition by IMPα/β1 [24], probably in a manner similar to the effect of the CK2 sites near/within the NLSs of T-ag and ppUL44 (see Section 4.1.1; Fig. 4, Fig. 5).
Fig. 5.
Cell cycle-dependent regulation of nuclear localisation for HPV E1 by cellular kinases. The prNLS of HPV E1 is shown (top), with the regulatory phosphorylation sites (blue), NLS (red) and NES (green), highlighted, as well as the binding partners that recognise them according to phosphorylation state (“P” indicates phosphorylation). The E1 NLS mediates IMPα/β1-mediated nuclear import inefficiently (black dotted arrow) (1a) but upon phosphorylation of S89 and S93 by ERK (and/or JNK for S89), IMPα/β1 is able to bind the NLS more strongly to facilitate efficient nuclear import (1b). Once in the nucleus E1 is quickly exported back to the cytoplasm, through Crm1 (2a). Nuclear export is prevented by the nuclear kinases Cdk1/Cdk2 (2b), present during S and G2 phases of cell cycle, which phosphorylate S107 to prevent Crm1 binding to the NES, leading to strong nuclear accumulation/nuclear retention.
Phosphorylation of HPV E1 by Cdk has been shown to be crucial for viral replication; mutation of all four Cdk sites within the protein (including S107) impairs HPV replication in vitro and in vivo, without affecting association with HPV E2 or cyclin E [84], [117]. It would thus appear that precise regulation of nucleocytoplasmic shuttling of HPV E1 is necessary in order to modulate the levels of E1 nuclear activity according to the differentiation state of the host cell; premature nuclear entry of E1 leading to virus production before the cell reaches the skin surface would be counterproductive, and so cell cycle-dependent phosphorylation appears to be exploited to HPV's advantage to fine tune the levels of E1 in the nucleus in order to optimise its chance of infecting a new host.
4.3. Rabies virus P protein
Although RNA viruses replicate in the cytoplasm, specific gene products from many of them are known to enter the nucleus (see Table 1) and/or alter nuclear transport of key cellular factors directly or indirectly (see Section 3). Nuclear localising proteins from RNA viruses generally interfere with transcription factors involved in signalling related to the innate immune system, though direct binding, or indirect effects [59]. STATs are the key factors involved in the innate immune response targeted by many RNA viruses, including Nipah, Sendai, measles and RV [59], [206], [207].
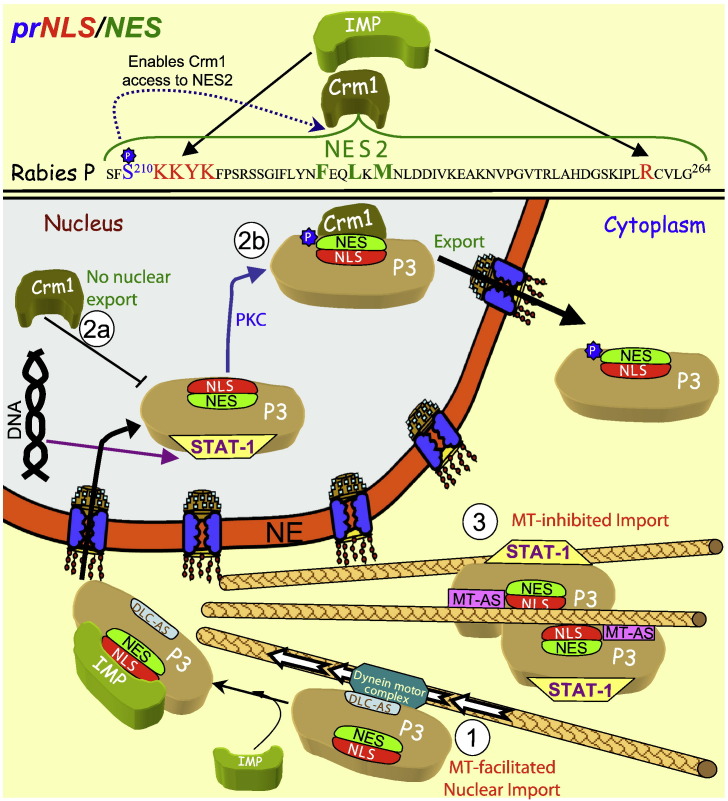
RV, genus lyssavirus, family Rhabdoviridea is a neurotropical virus, possessing a small 12 kb, negative stranded RNA genome comprising only five genes [208], [209]. Through a leaky scanning translation mechanism, the gene encoding the RV phospho (P)-protein (RPP) produces 5 forms (P1–5, where P1 is the full length protein), which have been implicated in various important functions in the viral life cycle [209], [210]. These include as a cofactor in viral genome replication through binding of its N-terminal 19 amino acids (only present in the P1) to the RV polymerase (L), and as a chaperone for nucleoprotein (N) either through direct binding (through a.a. 1–177) or indirectly bound to viral RNA genome (N-RNA) through the C-terminal domain (CTD, a.a. 174–297, present in all forms of RPP). Importantly, however, RPP also plays a key role as an antagonist of the host anti-viral response in part though binding to nuclear factors such as the transcription factor STAT-1 and promyelocytic leukaemia tumour suppressor protein (PML) [211], [212], [213], [214], [215] also through the CTD. RPP is also able to interact in two distinct modes with the host cell microtubule (MT) system, either through a dynein light chain (DLC) associated sequence (DLC-AS; a.a. 139–151, present in all forms of RPP) which confers interaction with DLC8 to enable dynein-facilitated nuclear import of RPP, or through a second distinct MT-association sequence (MT-AS, absent from P1 and P2), in combination with the RPP self-association domain (a.a. 54–139), which mediates dimerisation and causes the retention of associated STAT-1 on MTs, independent of DLC8, thereby preventing STAT-1 nuclear import and dampening the host cell response to IFNs [121], [210], [216].
4.3.1. Distinct roles of RPP P1 and P3 forms in the cytoplasm and nucleus
The key forms of RPP appear to be P1 (1–297) and P3 (53–297), which function predominantly in the cytoplasm and nucleus respectively, with the strong N-terminal NES (NES1) the main basis of predominantly cytoplasmic localisation of P1, in contrast to P3 that lacks it and is predominantly nuclear [120]. P1's key role is in the cytoplasm, both as a cofactor for replication, and as a binding partner of STAT-1 to prevent its role in IFN signalling, P3, however, can exist in either the nucleus, where its predominant role is to inhibit STAT-1 DNA binding activity, or in the cytoplasm, where, in a dimerised form, it prevents STAT-1 nuclear access by binding it and associating with MTs (Fig. 6 ). Multiple PKC sites [217] throughout RPP provide additional levels of control, the best understood of which is the PKC site at S210, which appears to function as a switch to inhibit the NLS (a.a. KKYK214 -/- R260) within the CTD [120], [122] and expose a second NES (NES2: NFEQLKM 232) normally buried within the CTD (see Fig. 6). Whether other PKC sites (e.g. S63, S64 and S162) within RPP modulate MT interaction and/or regulate dimerisation of the RPP to enable the MT-dependent retention of associated STAT-1, is unknown at this stage. What is clear, however, is that RPP dimerisation is critical for STAT-1 cytoplasmic retention, since deletion of the self-association domain abolishes P3 MT association, instead facilitating MT-enhanced nuclear import via the DLC-AS [210], [216]; a heterologous dimerisation domain functionally can substitute for the RPP self-association domain to restore STAT-1 cytoplasmic retention [210].
Fig. 6.
Regulation of nucleocytoplasmic shuttling of RV P3 protein to inhibit activity of the STAT-1 transcription factor in cytoplasmic and nuclear compartments. The prNLS/NES of RPP is shown (top), with the regulatory phosphorylation site (blue), NLS (red) and NES (green), highlighted, as well as the binding partners recognising them according to phosphorylation state (“P” indicating phosphorylation). In the absence of phosphorylation, P3 localises efficiently in the nucleus (1) through its NLS and the dynein light chain association sequence (DLC-AS), which confers binding to the microtubule (MT) motor dynein that acts to enhance IMP facilitated transport to the nucleus, where its role is to prevent DNA binding activity by the STAT-1 signalling molecule. P3 remains in the nucleus because NES2 is inaccessible (2a) until PKC phosphorylation of S210 induces conformation changes to render NES2 accessible to Crm1 (and mask the NLS) to permit Crm1 recognition and nuclear export (2b). Upon oligomerisation and binding to MTs through a MT-associating sequence (MT-AS) distinct from the DLC-AS, P3 remains cytoplasmic, acting to retain STAT-1 in the cytoplasm bound to MTs (3) and prevent its action in the anti-viral response.
4.3.2. Nucleocytoplasmic trafficking of forms of P protein contributes to pathogenicity through targeting STAT-1
That nucleocytoplasmic trafficking of RPP is critical to RV infection is implied by analysis of an attenuated non-lethal chicken embryo (CE) cell-adapted strain (Ni-CE) of the highly pathogenic Nishigahara (Ni) strain of RV. A chimeric CE(NiP) virus containing the Ni-P gene in the Ni-CE genetic background, is highly pathogenic, implying that RPP is a key virulence factor, and that mutations in the RPP are likely to be responsible for reduced pathogenicity of Ni-CE compared to Ni [123]. Intriguingly, 4 of a total of 7 amino acid substitutions in the Ni-CE strain, compared to that of Ni, are located within/near NES1 (see Section 4.3.1), correlating with the fact that the Ni-CE RPP P1 is more nuclear in infected cells than the Ni RPP, and thereby less able to prevent STAT-1 nuclear translocation in response to IFNα treatment. The implication is that the RPP NES1 plays a critical role in infection by specifically antagonising STAT-1 nuclear translocation to activate IFN-stimulated genes [124], [210], [218].
Similar mechanisms of regulating viral replication and immune evasion appear to be employed by Nipah virus which possesses 3 forms of the P protein, P (709 a.a.), V, and W proteins (456 and 450 a.a., respectively); all share the same N-terminal domain but vary in the C-terminal domain through frame shifting during translation [219]. The cytoplasmic and nuclear localisation of the gene products appears to be crucial to inhibit STAT-1 activities by directly interacting with STAT-1 and preventing its activation [207], [220]; W protein is found in the nucleus and V and P found in the cytoplasm [207], [221]. Nipah produces V and W in addition to the full length P protein to target STAT-1, with the varying forms acting in the cytoplasm and nucleus combining to effect inhibition of the innate immune response pathway. RV and Nipah and presumably other viruses thus utilise multiple forms of the same gene product to target STAT-1 in either the cytoplasm or nucleus to prevent the up-regulation of IFN-stimulated genes and thereby dampen the innate immune response. That STAT-1 is a target for many different viruses is known e.g. the V protein of measles [222], [223], [224] and rinderpest [225] viruses also binds STAT-1 to inhibit anti-viral responses. Clearly, perturbing the nucleocytoplasmic shuttling ability of the key viral proteins that sequester STAT-1 in nucleus/cytoplasm would represent an important step towards preventing viral evasion of the innate immune system, as would preventing interaction of STAT-1 with the viral proteins themselves.
5. Conclusions and future research
Precise regulation of the function of specific viral proteins is central to viral replication and pathogenesis, and as discussed here, nucleocytoplasmic trafficking plays a critical role in the case of many DNA and even RNA viruses (see Table 1). Phosphorylation appears to be the main mechanism by which viral protein nucleocytoplasmic trafficking is regulated during the virus life cycle, involving various cellular kinases as well as virally encoded kinases. The ubiquitously expressed CK2 enhances nuclear localisation of specific proteins involved in replication in the case of DNA tumour viruses such as SV40 and HCMV, whilst Cdks have been shown to play a crucial role in maintaining a persistent HPV infection in the skin through modulating E1 localisation; viruses that encode their own kinases such as VZV (ORF66, see Table 1) are less reliant on cellular kinases for control over subcellular localisation. Clearly, targeting the activity of kinases, such as CK2 and Cdk in order to perturb viral protein subcellular trafficking using specific inhibitors, represents a potential anti-viral strategy, although hampered by the obvious problem of effects on normal cellular functions of conventional kinase inhibitors. Screening for and/or developing compounds that block the nuclear transport of specific viral proteins though disrupting their interaction with IMP/EXPs seems an intriguing alternative, whereby a counter screening approach could be used to discard inhibitors of general host protein–IMP interaction, in order to identify inhibitors specific to IMP–viral protein interaction without affecting cellular proteins (Wagstaff et al., manuscript in preparation). Along similar lines, a unique approach would be to screen for compounds that stabilise or enhance the interaction with negative regulators of nuclear import such as BRAP2 with the SV40 T-ag or HCMV ppUL44 proteins or p110Rb with SV40 T-ag etc., as a means to inhibit viral protein nuclear import and thereby virus production.
In conclusion, based on the results summarised here (e.g. Table 1) and elsewhere, viral protein nucleocytoplasmic trafficking is central to viral infection/pathogenesis in many cases. Developing reagents directed specifically towards nuclear import/export of viral proteins rather than inhibitors of general transport looms as a fruitful avenue of research. In the face of the growing need for therapeutics to combat the consistently emerging lethal zoonotic viral threats to human health, such as SARS, Ebola and Nipah, as well as more familiar lethal pathogens, such as HIV and influenza, this avenue should probably be exploited in the near future with some urgency.
Footnotes
This article was originally intended for the special issue “Regulation of Signaling and Cellular Fate through Modulation of Nuclear Protein Import”. The Publisher apologizes for any inconvenience caused.
References
- 1.Mosammaparast N., Pemberton L.F. Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol. 2004;14:547–556. doi: 10.1016/j.tcb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Suntharalingam M., Wente S.R. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev. Cell. 2003;4:775–789. doi: 10.1016/s1534-5807(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 3.Gorlich D., Kutay U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 4.Jans D.A., Xiao C.Y., Lam M.H. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays. 2000;22:532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Brohawn S.G., Partridge J.R., Whittle J.R., Schwartz T.U. The nuclear pore complex has entered the atomic age. Structure. 2009;17:1156–1168. doi: 10.1016/j.str.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Angelo M.A., Hetzer M.W. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–466. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorlich D., Mattaj I.W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 8.Hinshaw J.E., Carragher B.O., Milligan R.A. Architecture and design of the nuclear pore complex. Cell. 1992;69:1133–1141. doi: 10.1016/0092-8674(92)90635-p. [DOI] [PubMed] [Google Scholar]
- 9.Terry L.J., Wente S.R. Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport. Eukaryot. Cell. 2009;8:1814–1827. doi: 10.1128/EC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran E.J., Wente S.R. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–1053. doi: 10.1016/j.cell.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Rout M.P., Aitchison J.D. Pore relations: nuclear pore complexes and nucleocytoplasmic exchange. Essays Biochem. 2000;36:75–88. doi: 10.1042/bse0360075. [DOI] [PubMed] [Google Scholar]
- 12.Walde S., Kehlenbach R.H. The Part and the Whole: functions of nucleoporins in nucleocytoplasmic transport. Trends Cell Biol. 2010;20:461–469. doi: 10.1016/j.tcb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Frey S., Richter R.P., Gorlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314:815–817. doi: 10.1126/science.1132516. [DOI] [PubMed] [Google Scholar]
- 14.Lim R.Y., Huang N.P., Koser J., Deng J., Lau K.H., Schwarz-Herion K., Fahrenkrog B., Aebi U. Flexible phenylalanine–glycine nucleoporins as entropic barriers to nucleocytoplasmic transport. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9512–9517. doi: 10.1073/pnas.0603521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters R. Translocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionality. Traffic. 2005;6:421–427. doi: 10.1111/j.1600-0854.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 16.Rout M.P., Aitchison J.D., Suprapto A., Hjertaas K., Zhao Y., Chait B.T. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J. Cell Biol. 2000;148:635–651. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried H., Kutay U. Nucleocytoplasmic transport: taking an inventory. Cell. Mol. Life Sci. 2003;60:1659–1688. doi: 10.1007/s00018-003-3070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldfarb D.S., Corbett A.H., Mason D.A., Harreman M.T., Adam S.A. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Pemberton L.F., Paschal B.M. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187–198. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 20.Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–451. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 21.Kalderon D., Richardson W.D., Markham A.F., Smith A.E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984;311:33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- 22.Kalderon D., Roberts B.L., Richardson W.D., Smith A.E. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 23.Alvisi G., Jans D.A., Guo J., Pinna L.A., Ripalti A. A protein kinase CK2 site flanking the nuclear targeting signal enhances nuclear transport of human cytomegalovirus ppUL44. Traffic. 2005;6:1002–1013. doi: 10.1111/j.1600-0854.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu J.H., Lin B.Y., Deng W., Broker T.R., Chow L.T. Mitogen-activated protein kinases activate the nuclear localization sequence of human papillomavirus type 11 E1 DNA helicase to promote efficient nuclear import. J. Virol. 2007;81:5066–5078. doi: 10.1128/JVI.02480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson A.W., Lund E., Dahlberg J. Nuclear export of ribosomal subunits. Trends Biochem. Sci. 2002;27:580–585. doi: 10.1016/s0968-0004(02)02208-9. [DOI] [PubMed] [Google Scholar]
- 26.Ohno M., Segref A., Bachi A., Wilm M., Mattaj I.W. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell. 2000;101:187–198. doi: 10.1016/S0092-8674(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 27.Paraskeva E., Izaurralde E., Bischoff F.R., Huber J., Kutay U., Hartmann E., Luhrmann R., Gorlich D. CRM1-mediated recycling of snurportin 1 to the cytoplasm. J. Cell Biol. 1999;145:255–264. doi: 10.1083/jcb.145.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer U., Huber J., Boelens W.C., Mattaj I.W., Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 29.Hutten S., Walde S., Spillner C., Hauber J., Kehlenbach R.H. The nuclear pore component Nup358 promotes transportin-dependent nuclear import. J. Cell Sci. 2009;122:1100–1110. doi: 10.1242/jcs.040154. [DOI] [PubMed] [Google Scholar]
- 30.Hutten S., Flotho A., Melchior F., Kehlenbach R.H. The Nup358–RanGAP complex is required for efficient importin alpha/beta-dependent nuclear import. Mol. Biol. Cell. 2008;19:2300–2310. doi: 10.1091/mbc.E07-12-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pyhtila B., Rexach M. A gradient of affinity for the karyopherin Kap95p along the yeast nuclear pore complex. J. Biol. Chem. 2003;278:42699–42709. doi: 10.1074/jbc.M307135200. [DOI] [PubMed] [Google Scholar]
- 32.Oka M., Asally M., Yasuda Y., Ogawa Y., Tachibana T., Yoneda Y. The mobile FG nucleoporin Nup98 is a cofactor for Crm1-dependent protein export. Mol. Biol. Cell. 2010;21:1885–1896. doi: 10.1091/mbc.E09-12-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Efraim I., Frosst P.D., Gerace L. Karyopherin binding interactions and nuclear import mechanism of nuclear pore complex protein Tpr. BMC Cell Biol. 2009;10:74. doi: 10.1186/1471-2121-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutten S., Kehlenbach R.H. Nup214 is required for CRM1-dependent nuclear protein export in vivo. Mol. Cell. Biol. 2006;26:6772–6785. doi: 10.1128/MCB.00342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogarth C.A., Calanni S., Jans D.A., Loveland K.L. Importin alpha mRNAs have distinct expression profiles during spermatogenesis. Dev. Dyn. 2006;235:253–262. doi: 10.1002/dvdy.20569. [DOI] [PubMed] [Google Scholar]
- 36.Loveland K.L., Hogarth C., Szczepny A., Prabhu S.M., Jans D.A. Expression of nuclear transport importins beta 1 and beta 3 is regulated during rodent spermatogenesis. Biol. Reprod. 2006;74:67–74. doi: 10.1095/biolreprod.105.042341. [DOI] [PubMed] [Google Scholar]
- 37.Jans D.A., Hubner S. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol. Rev. 1996;76:651–685. doi: 10.1152/physrev.1996.76.3.651. [DOI] [PubMed] [Google Scholar]
- 38.Poon I.K., Jans D.A. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 39.Durairaj G., Garg P., Bhaumik S.R. Nuclear export of mRNA and its regulation by ubiquitylation. RNA Biol. 2009;6:531–535. doi: 10.4161/rna.6.5.10078. [DOI] [PubMed] [Google Scholar]
- 40.Palancade B., Doye V. Sumoylating and desumoylating enzymes at nuclear pores: underpinning their unexpected duties? Trends Cell Biol. 2008;18:174–183. doi: 10.1016/j.tcb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Sim H., Argentaro A., Harley V.R. Boys, girls and shuttling of SRY and SOX9. Trends Endocrinol. Metab. 2008;19:213–222. doi: 10.1016/j.tem.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Pickard A., Wong P.P., McCance D.J. Acetylation of Rb by PCAF is required for nuclear localization and keratinocyte differentiation. J. Cell Sci. 2010;123:3718–3726. doi: 10.1242/jcs.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H., Holloway M.P., Ma L., Cooper Z.A., Riolo M., Samkari A., Elenitoba-Johnson K.S., Chin Y.E., Altura R.A. Acetylation directs survivin nuclear localization to repress STAT3 oncogenic activity. J. Biol. Chem. 2010;285:36129–36137. doi: 10.1074/jbc.M110.152777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lohrum M.A., Woods D.B., Ludwig R.L., Balint E., Vousden K.H. C-terminal ubiquitination of p53 contributes to nuclear export. Mol. Cell. Biol. 2001;21:8521–8532. doi: 10.1128/MCB.21.24.8521-8532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M., Brooks C.L., Wu-Baer F., Chen D., Baer R., Gu W. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003;302:1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- 46.Trotman L.C., Wang X., Alimonti A., Chen Z., Teruya-Feldstein J., Yang H., Pavletich N.P., Carver B.S., Cordon-Cardo C., Erdjument-Bromage H., Tempst P., Chi S.G., Kim H.J., Misteli T., Jiang X., Pandolfi P.P. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shcherbik N., Haines D.S. Ub on the move. J. Cell. Biochem. 2004;93:11–19. doi: 10.1002/jcb.20130. [DOI] [PubMed] [Google Scholar]
- 48.Huang T.T., Wuerzberger-Davis S.M., Wu Z.H., Miyamoto S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell. 2003;115:565–576. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 49.Terry L.J., Shows E.B., Wente S.R. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 50.Ghildyal R., Jordan B., Li D., Dagher H., Bardin P.G., Gern J.E., Jans D.A. Rhinovirus 3C protease can localize in the nucleus and alter active and passive nucleocytoplasmic transport. J. Virol. 2009;83:7349–7352. doi: 10.1128/JVI.01748-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belov G.A., Lidsky P.V., Mikitas O.V., Egger D., Lukyanov K.A., Bienz K., Agol V.I. Bidirectional increase in permeability of nuclear envelope upon poliovirus infection and accompanying alterations of nuclear pores. J. Virol. 2004;78:10166–10177. doi: 10.1128/JVI.78.18.10166-10177.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gustin K.E., Sarnow P. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J. 2001;20:240–249. doi: 10.1093/emboj/20.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gustin K.E., Sarnow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 2002;76:8787–8796. doi: 10.1128/JVI.76.17.8787-8796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park N., Katikaneni P., Skern T., Gustin K.E. Differential targeting of nuclear pore complex proteins in poliovirus-infected cells. J. Virol. 2008;82:1647–1655. doi: 10.1128/JVI.01670-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basler C.F., Amarasinghe G.K. Evasion of interferon responses by Ebola and Marburg viruses. J. Interferon Cytokine Res. 2009;29:511–520. doi: 10.1089/jir.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mateo M., Reid S.P., Leung L.W., Basler C.F., Volchkov V.E. Ebolavirus VP24 binding to karyopherins is required for inhibition of interferon signaling. J. Virol. 2010;84:1169–1175. doi: 10.1128/JVI.01372-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reid S.P., Leung L.W., Hartman A.L., Martinez O., Shaw M.L., Carbonnelle C., Volchkov V.E., Nichol S.T., Basler C.F. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J. Virol. 2006;80:5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frieman M., Yount B., Heise M., Kopecky-Bromberg S.A., Palese P., Baric R.S. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 2007;81:9812–9824. doi: 10.1128/JVI.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Randall R.E., Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 60.Lyons R.H., Ferguson B.Q., Rosenberg M. Pentapeptide nuclear localization signal in adenovirus E1a. Mol. Cell. Biol. 1987;7:2451–2456. doi: 10.1128/mcb.7.7.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kohler M., Gorlich D., Hartmann E., Franke J. Adenoviral E1A protein nuclear import is preferentially mediated by importin alpha3 in vitro. Virology. 2001;289:186–191. doi: 10.1006/viro.2001.1151. [DOI] [PubMed] [Google Scholar]
- 62.Madison D.L., Yaciuk P., Kwok R.P., Lundblad J.R. Acetylation of the adenovirus-transforming protein E1A determines nuclear localization by disrupting association with importin-alpha. J. Biol. Chem. 2002;277:38755–38763. doi: 10.1074/jbc.M207512200. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Q., Yao H., Vo N., Goodman R.H. Acetylation of adenovirus E1A regulates binding of the transcriptional corepressor CtBP. Proc. Natl. Acad. Sci. U.S.A. 2000;97:14323–14328. doi: 10.1073/pnas.011283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Douglas J.L., Quinlan M.P. Efficient nuclear localization of the Ad5 E1A 12S protein is necessary for immortalization but not cotransformation of primary epithelial cells. Cell Growth Differ. 1994;5:475–483. [PubMed] [Google Scholar]
- 65.Lentz M.R., Pak D., Mohr I., Botchan M.R. The E1 replication protein of bovine papillomavirus type 1 contains an extended nuclear localization signal that includes a p34cdc2 phosphorylation site. J. Virol. 1993;67:1414–1423. doi: 10.1128/jvi.67.3.1414-1423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leng X., Wilson V.G. Genetically defined nuclear localization signal sequence of bovine papillomavirus E1 protein is necessary and sufficient for the nuclear localization of E1-beta-galactosidase fusion proteins. J. Gen. Virol. 1994;75(Pt 9):2463–2467. doi: 10.1099/0022-1317-75-9-2463. [DOI] [PubMed] [Google Scholar]
- 67.Bian X.L., Rosas-Acosta G., Wu Y.C., Wilson V.G. Nuclear import of bovine papillomavirus type 1 E1 protein is mediated by multiple alpha importins and is negatively regulated by phosphorylation near a nuclear localization signal. J. Virol. 2007;81:2899–2908. doi: 10.1128/JVI.01850-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cueille N., Nougarede R., Mechali F., Philippe M., Bonne-Andrea C. Functional interaction between the bovine papillomavirus virus type 1 replicative helicase E1 and cyclin E-Cdk2. J. Virol. 1998;72:7255–7262. doi: 10.1128/jvi.72.9.7255-7262.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanardi T.A., Stanley C.M., Saville B.M., Spacek S.M., Lentz M.R. Modulation of bovine papillomavirus DNA replication by phosphorylation of the viral E1 protein. Virology. 1997;228:1–10. doi: 10.1006/viro.1996.8375. [DOI] [PubMed] [Google Scholar]
- 70.Ambinder R.F., Mullen M.A., Chang Y.N., Hayward G.S., Hayward S.D. Functional domains of Epstein–Barr virus nuclear antigen EBNA-1. J. Virol. 1991;65:1466–1478. doi: 10.1128/jvi.65.3.1466-1478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito S., Ikeda M., Kato N., Matsumoto A., Ishikawa Y., Kumakubo S., Yanagi K. Epstein–Barr virus nuclear antigen-1 binds to nuclear transporter karyopherin alpha1/NPI-1 in addition to karyopherin alpha2/Rch1. Virology. 2000;266:110–119. doi: 10.1006/viro.1999.0054. [DOI] [PubMed] [Google Scholar]
- 72.Kitamura R., Sekimoto T., Ito S., Harada S., Yamagata H., Masai H., Yoneda Y., Yanagi K. Nuclear import of Epstein–Barr virus nuclear antigen 1 mediated by NPI-1 (Importin alpha5) is up- and down-regulated by phosphorylation of the nuclear localization signal for which Lys379 and Arg380 are essential. J. Virol. 2006;80:1979–1991. doi: 10.1128/JVI.80.4.1979-1991.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leung C.S., Haigh T.A., Mackay L.K., Rickinson A.B., Taylor G.S. Nuclear location of an endogenously expressed antigen, EBNA1, restricts access to macroautophagy and the range of CD4 epitope display. Proc. Natl. Acad. Sci. U.S.A. 2010;107:2165–2170. doi: 10.1073/pnas.0909448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leung C.S., Taylor G.S. Nuclear shelter: the influence of subcellular location on the processing of antigens by macroautophagy. Autophagy. 2010;6 doi: 10.4161/auto.6.4.11814. [DOI] [PubMed] [Google Scholar]
- 75.Mears W.E., Lam V., Rice S.A. Identification of nuclear and nucleolar localization signals in the herpes simplex virus regulatory protein ICP27. J. Virol. 1995;69:935–947. doi: 10.1128/jvi.69.2.935-947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhi Y., Sandri-Goldin R.M. Analysis of the phosphorylation sites of herpes simplex virus type 1 regulatory protein ICP27. J. Virol. 1999;73:3246–3257. doi: 10.1128/jvi.73.4.3246-3257.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rojas S., Corbin-Lickfett K.A., Escudero-Paunetto L., Sandri-Goldin R.M. ICP27 phosphorylation site mutants are defective in herpes simplex virus 1 replication and gene expression. J. Virol. 2010;84:2200–2211. doi: 10.1128/JVI.00917-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fulcher A.J., Roth D.M., Fatima S., Alvisi G., Jans D.A. The BRCA-1 binding protein BRAP2 is a novel, negative regulator of nuclear import of viral proteins, dependent on phosphorylation flanking the nuclear localization signal. FASEB J. 2010;24:1454–1466. doi: 10.1096/fj.09-136564. [DOI] [PubMed] [Google Scholar]
- 79.Ertl P.F., Powell K.L. Physical and functional interaction of human cytomegalovirus DNA polymerase and its accessory protein (ICP36) expressed in insect cells. J. Virol. 1992;66:4126–4133. doi: 10.1128/jvi.66.7.4126-4133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ripalti A., Boccuni M.C., Campanini F., Landini M.P. Cytomegalovirus-mediated induction of antisense mRNA expression to UL44 inhibits virus replication in an astrocytoma cell line: identification of an essential gene. J. Virol. 1995;69:2047–2057. doi: 10.1128/jvi.69.4.2047-2057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pari G.S., Kacica M.A., Anders D.G. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J. Virol. 1993;67:2575–2582. doi: 10.1128/jvi.67.5.2575-2582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen W., Westgard E., Huang L., Ward M.D., Osborn J.L., Chau N.H., Collins L., Marcum B., Koach M.A., Bibbs J., Semmes O.J., Kerry J.A. Nuclear trafficking of the human cytomegalovirus pp 71 (ppUL82) tegument protein. Virology. 2008;376:42–52. doi: 10.1016/j.virol.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Penkert R.R., Kalejta R.F. Nuclear localization of tegument-delivered pp 71 in human cytomegalovirus-infected cells is facilitated by one or more factors present in terminally differentiated fibroblasts. J. Virol. 2010;84:9853–9863. doi: 10.1128/JVI.00500-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng W., Lin B.Y., Jin G., Wheeler C.G., Ma T., Harper J.W., Broker T.R., Chow L.T. Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase. J. Virol. 2004;78:13954–13965. doi: 10.1128/JVI.78.24.13954-13965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hubner S., Xiao C.Y., Jans D.A. The protein kinase CK2 site (Ser111/112) enhances recognition of the simian virus 40 large T-antigen nuclear localization sequence by importin. J. Biol. Chem. 1997;272:17191–17195. doi: 10.1074/jbc.272.27.17191. [DOI] [PubMed] [Google Scholar]
- 86.Jans D.A., Ackermann M.J., Bischoff J.R., Beach D.H., Peters R. p34cdc2-mediated phosphorylation at T124 inhibits nuclear import of SV-40 T antigen proteins. J. Cell Biol. 1991;115:1203–1212. doi: 10.1083/jcb.115.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jans D.A., Jans P. Negative charge at the casein kinase II site flanking the nuclear localization signal of the SV40 large T-antigen is mechanistically important for enhanced nuclear import. Oncogene. 1994;9:2961–2968. [PubMed] [Google Scholar]
- 88.Rihs H.P., Jans D.A., Fan H., Peters R. The rate of nuclear cytoplasmic protein transport is determined by the casein kinase II site flanking the nuclear localization sequence of the SV40 T-antigen. EMBO J. 1991;10:633–639. doi: 10.1002/j.1460-2075.1991.tb07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rihs H.P., Peters R. Nuclear transport kinetics depend on phosphorylation-site-containing sequences flanking the karyophilic signal of the Simian virus 40 T-antigen. EMBO J. 1989;8:1479–1484. doi: 10.1002/j.1460-2075.1989.tb03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiao C.Y., Hubner S., Jans D.A. SV40 large tumor antigen nuclear import is regulated by the double-stranded DNA-dependent protein kinase site (serine 120) flanking the nuclear localization sequence. J. Biol. Chem. 1997;272:22191–22198. doi: 10.1074/jbc.272.35.22191. [DOI] [PubMed] [Google Scholar]
- 91.Grasser F.A., Scheidtmann K.H., Tuazon P.T., Traugh J.A., Walter G. In vitro phosphorylation of SV40 large T antigen. Virology. 1988;165:13–22. doi: 10.1016/0042-6822(88)90653-8. [DOI] [PubMed] [Google Scholar]
- 92.Fulcher A.J., Dias M.M., Jans D.A. Binding of p110 retinoblastoma protein inhibits nuclear import of simian virus SV40 large tumor antigen. J. Biol. Chem. 2010;285:17744–17753. doi: 10.1074/jbc.M109.055491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneider J., Fanning E. Mutations in the phosphorylation sites of simian virus 40 (SV40) T antigen alter its origin DNA-binding specificity for sites I or II and affect SV40 DNA replication activity. J. Virol. 1988;62:1598–1605. doi: 10.1128/jvi.62.5.1598-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kinchington P.R., Turse S.E. Regulated nuclear localization of the varicella-zoster virus major regulatory protein, IE62. J. Infect. Dis. 1998;178(Suppl 1):S16–S21. doi: 10.1086/514263. [DOI] [PubMed] [Google Scholar]
- 95.Kinchington P.R., Fite K., Seman A., Turse S.E. Virion association of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, requires expression of the VZV open reading frame 66 protein kinase. J. Virol. 2001;75:9106–9113. doi: 10.1128/JVI.75.19.9106-9113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kinchington P.R., Fite K., Turse S.E. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J. Virol. 2000;74:2265–2277. doi: 10.1128/jvi.74.5.2265-2277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eisfeld A.J., Turse S.E., Jackson S.A., Lerner E.C., Kinchington P.R. Phosphorylation of the varicella-zoster virus (VZV) major transcriptional regulatory protein IE62 by the VZV open reading frame 66 protein kinase. J. Virol. 2006;80:1710–1723. doi: 10.1128/JVI.80.4.1710-1723.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schaap A., Fortin J.F., Sommer M., Zerboni L., Stamatis S., Ku C.C., Nolan G.P., Arvin A.M. T-cell tropism and the role of ORF66 protein in pathogenesis of varicella-zoster virus infection. J. Virol. 2005;79:12921–12933. doi: 10.1128/JVI.79.20.12921-12933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chida K., Vogt P.K. Nuclear translocation of viral Jun but not of cellular Jun is cell cycle dependent. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4290–4294. doi: 10.1073/pnas.89.10.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tagawa T., Kuroki T., Vogt P.K., Chida K. The cell cycle-dependent nuclear import of v-Jun is regulated by phosphorylation of a serine adjacent to the nuclear localization signal. J. Cell Biol. 1995;130:255–263. doi: 10.1083/jcb.130.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schlummer S., Vetter R., Kuder N., Henkel A., Chen Y.X., Li Y.M., Kuhlmann J., Waldmann H. Influence of serine O-glycosylation or O-phosphorylation close to the vJun nuclear localisation sequence on nuclear import. Chembiochem. 2006;7:88–97. doi: 10.1002/cbic.200500212. [DOI] [PubMed] [Google Scholar]
- 102.Wong W.Y., Havarstein L.S., Morgan I.M., Vogt P.K. c-Jun causes focus formation and anchorage-independent growth in culture but is non-tumorigenic. Oncogene. 1992;7:2077–2080. [PubMed] [Google Scholar]
- 103.Morgan I.M., Havarstein L.S., Wong W.Y., Luu P., Vogt P.K. Efficient induction of fibrosarcomas by v-jun requires mutations in the DNA binding region and the transactivation domain. Oncogene. 1994;9:2793–2797. [PubMed] [Google Scholar]
- 104.Siomi H., Shida H., Nam S.H., Nosaka T., Maki M., Hatanaka M. Sequence requirements for nucleolar localization of human T cell leukemia virus type I pX protein, which regulates viral RNA processing. Cell. 1988;55:197–209. doi: 10.1016/0092-8674(88)90043-8. [DOI] [PubMed] [Google Scholar]
- 105.Palmeri D., Malim M.H. Importin beta can mediate the nuclear import of an arginine-rich nuclear localization signal in the absence of importin alpha. Mol. Cell. Biol. 1999;19:1218–1225. doi: 10.1128/mcb.19.2.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Truant R., Cullen B.R. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol. Cell. Biol. 1999;19:1210–1217. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kesic M., Ward M., Semmes O.J., Green P.L. Site-specific phosphorylation regulates human T-cell leukemia virus type 2 Rex function in vivo. J. Virol. 2009;83:8859–8868. doi: 10.1128/JVI.00908-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Narayan M., Kusuhara K., Green P.L. Phosphorylation of two serine residues regulates human T-cell leukemia virus type 2 Rex function. J. Virol. 2001;75:8440–8448. doi: 10.1128/JVI.75.18.8440-8448.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Narayan M., Younis I., D'Agostino D.M., Green P.L. Functional domain structure of human T-cell leukemia virus type 2 rex. J. Virol. 2003;77:12829–12840. doi: 10.1128/JVI.77.23.12829-12840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y.E., Park A., Lake M., Pentecost M., Torres B., Yun T.E., Wolf M.C., Holbrook M.R., Freiberg A.N., Lee B. Ubiquitin-regulated nuclear-cytoplasmic trafficking of the nipah virus matrix protein is important for viral budding. PLoS Pathog. 2010;6:e1001186. doi: 10.1371/journal.ppat.1001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang H., Olson M.V., Medrano D.R., Lee O.H., Xu J., Piao Y., Alonso M.M., Gomez-Manzano C., Hung M.C., Yung W.K., Fueyo J. A novel CRM1-dependent nuclear export signal in adenoviral E1A protein regulated by phosphorylation. FASEB J. 2006;20:2603–2605. doi: 10.1096/fj.06-6433fje. [DOI] [PubMed] [Google Scholar]
- 112.Hsu C.Y., Mechali F., Bonne-Andrea C. Nucleocytoplasmic shuttling of bovine papillomavirus E1 helicase downregulates viral DNA replication in S phase. J. Virol. 2007;81:384–394. doi: 10.1128/JVI.01170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosas-Acosta G., Wilson V.G. Identification of a nuclear export signal sequence for bovine papillomavirus E1 protein. Virology. 2008;373:149–162. doi: 10.1016/j.virol.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poon I.K., Oro C., Dias M.M., Zhang J., Jans D.A. Apoptin nuclear accumulation is modulated by a CRM1-recognized nuclear export signal that is active in normal but not in tumor cells. Cancer Res. 2005;65:7059–7064. doi: 10.1158/0008-5472.CAN-05-1370. [DOI] [PubMed] [Google Scholar]
- 115.Kuusisto H.V., Wagstaff K.M., Alvisi G., Jans D.A. The C-terminus of apoptin represents a unique tumor cell-enhanced nuclear targeting module. Int. J. Cancer. 2008;123:2965–2969. doi: 10.1002/ijc.23884. [DOI] [PubMed] [Google Scholar]
- 116.Prasetyo A.A., Kamahora T., Kuroishi A., Murakami K., Hino S. Replication of chicken anemia virus (CAV) requires apoptin and is complemented by VP3 of human torque teno virus (TTV) Virology. 2009;385:85–92. doi: 10.1016/j.virol.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 117.Ma T., Zou N., Lin B.Y., Chow L.T., Harper J.W. Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication. Proc. Natl. Acad. Sci. U.S.A. 1999;96:382–387. doi: 10.1073/pnas.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Munoz-Fontela C., Collado M., Rodriguez E., Garcia M.A., Alvarez-Barrientos A., Arroyo J., Nombela C., Rivas C. Identification of a nuclear export signal in the KSHV latent protein LANA2 mediating its export from the nucleus. Exp. Cell Res. 2005;311:96–105. doi: 10.1016/j.yexcr.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 119.Marcos-Villar L., Lopitz-Otsoa F., Gallego P., Munoz-Fontela C., Gonzalez-Santamaria J., Campagna M., Shou-Jiang G., Rodriguez M.S., Rivas C. Kaposi's sarcoma-associated herpesvirus protein LANA2 disrupts PML oncogenic domains and inhibits PML-mediated transcriptional repression of the survivin gene. J. Virol. 2009;83:8849–8858. doi: 10.1128/JVI.00339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pasdeloup D., Poisson N., Raux H., Gaudin Y., Ruigrok R.W., Blondel D. Nucleocytoplasmic shuttling of the rabies virus P protein requires a nuclear localization signal and a CRM1-dependent nuclear export signal. Virology. 2005;334:284–293. doi: 10.1016/j.virol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 121.Moseley G.W., Filmer R.P., DeJesus M.A., Jans D.A. Nucleocytoplasmic distribution of rabies virus P-protein is regulated by phosphorylation adjacent to C-terminal nuclear import and export signals. Biochemistry. 2007;46:12053–12061. doi: 10.1021/bi700521m. [DOI] [PubMed] [Google Scholar]
- 122.Mavrakis M., McCarthy A.A., Roche S., Blondel D., Ruigrok R.W. Structure and function of the C-terminal domain of the polymerase cofactor of rabies virus. J. Mol. Biol. 2004;343:819–831. doi: 10.1016/j.jmb.2004.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shimizu K., Ito N., Mita T., Yamada K., Hosokawa-Muto J., Sugiyama M., Minamoto N. Involvement of nucleoprotein, phosphoprotein, and matrix protein genes of rabies virus in virulence for adult mice. Virus Res. 2007;123:154–160. doi: 10.1016/j.virusres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 124.Ito N., Moseley G.W., Blondel D., Shimizu K., Rowe C.L., Ito Y., Masatani T., Nakagawa K., Jans D.A., Sugiyama M. Role of interferon antagonist activity of rabies virus phosphoprotein in viral pathogenicity. J. Virol. 2010;84:6699–6710. doi: 10.1128/JVI.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghildyal R., Ho A., Dias M., Soegiyono L., Bardin P.G., Tran K.C., Teng M.N., Jans D.A. The respiratory syncytial virus matrix protein possesses a Crm1-mediated nuclear export mechanism. J. Virol. 2009;83:5353–5362. doi: 10.1128/JVI.02374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beg A.A., Ruben S.M., Scheinman R.I., Haskill S., Rosen C.A., Baldwin A.S., Jr. I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 1992;6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 127.Traenckner E.B., Wilk S., Baeuerle P.A. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bischoff J.R., Friedman P.N., Marshak D.R., Prives C., Beach D. Human p53 is phosphorylated by p60-cdc2 and cyclin B-cdc2. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4766–4770. doi: 10.1073/pnas.87.12.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liang S.H., Clarke M.F. Regulation of p53 localization. Eur. J. Biochem. 2001;268:2779–2783. doi: 10.1046/j.1432-1327.2001.02227.x. [DOI] [PubMed] [Google Scholar]
- 130.Kim I.S., Kim D.H., Han S.M., Chin M.U., Nam H.J., Cho H.P., Choi S.Y., Song B.J., Kim E.R., Bae Y.S., Moon Y.H. Truncated form of importin alpha identified in breast cancer cell inhibits nuclear import of p53. J. Biol. Chem. 2000;275:23139–23145. doi: 10.1074/jbc.M909256199. [DOI] [PubMed] [Google Scholar]
- 131.Child E.S., Mann D.J. The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle. 2006;5:1313–1319. doi: 10.4161/cc.5.12.2863. [DOI] [PubMed] [Google Scholar]
- 132.Rodriguez-Vilarrupla A., Diaz C., Canela N., Rahn H.P., Bachs O., Agell N. Identification of the nuclear localization signal of p21(cip1) and consequences of its mutation on cell proliferation. FEBS Lett. 2002;531:319–323. doi: 10.1016/s0014-5793(02)03549-4. [DOI] [PubMed] [Google Scholar]
- 133.Asada M., Ohmi K., Delia D., Enosawa S., Suzuki S., Yuo A., Suzuki H., Mizutani S. Brap2 functions as a cytoplasmic retention protein for p21 during monocyte differentiation. Mol. Cell. Biol. 2004;24:8236–8243. doi: 10.1128/MCB.24.18.8236-8243.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ali S.H., DeCaprio J.A. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- 135.Garcea R.L., Imperiale M.J. Simian virus 40 infection of humans. J. Virol. 2003;77:5039–5045. doi: 10.1128/JVI.77.9.5039-5045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kierstead T.D., Tevethia M.J. Association of p53 binding and immortalization of primary C57BL/6 mouse embryo fibroblasts by using simian virus 40 T-antigen mutants bearing internal overlapping deletion mutations. J. Virol. 1993;67:1817–1829. doi: 10.1128/jvi.67.4.1817-1829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu J., Rice P.W., Gorsch L., Abate M., Cole C.N. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J. Virol. 1992;66:2780–2791. doi: 10.1128/jvi.66.5.2780-2791.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peden K.W., Srinivasan A., Farber J.M., Pipas J.M. Mutants with changes within or near a hydrophobic region of simian virus 40 large tumor antigen are defective for binding cellular protein p53. Virology. 1989;168:13–21. doi: 10.1016/0042-6822(89)90398-x. [DOI] [PubMed] [Google Scholar]
- 139.Srinivasan A., Peden K.W., Pipas J.M. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J. Virol. 1989;63:5459–5463. doi: 10.1128/jvi.63.12.5459-5463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eckner R., Ludlow J.W., Lill N.L., Oldread E., Arany Z., Modjtahedi N., DeCaprio J.A., Livingston D.M., Morgan J.A. Association of p300 and CBP with simian virus 40 large T antigen. Mol. Cell. Biol. 1996;16:3454–3464. doi: 10.1128/mcb.16.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lill N.L., Tevethia M.J., Eckner R., Livingston D.M., Modjtahedi N. p300 family members associate with the carboxyl terminus of simian virus 40 large tumor antigen. J. Virol. 1997;71:129–137. doi: 10.1128/jvi.71.1.129-137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lanford R.E., Butel J.S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984;37:801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- 143.Rundell K., Collins J.K., Tegtmeyer P., Ozer H.L., Lai C.J., Nathans D. Identification of simian virus 40 protein A. J. Virol. 1977;21:636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tegtmeyer P., Rundell K., Collins J.K. Modification of simian virus 40 protein A. J. Virol. 1977;21:647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scheidtmann K.H., Kaiser A., Carbone A., Walter G. Phosphorylation of threonine in the proline-rich carboxy-terminal region of simian virus 40 large T antigen. J. Virol. 1981;38:59–69. doi: 10.1128/jvi.38.1.59-69.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scheidtmann K.H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J. Virol. 1982;44:116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scheidtmann K.H., Hardung M., Echle B., Walter G. DNA-binding activity of simian virus 40 large T antigen correlates with a distinct phosphorylation state. J. Virol. 1984;50:1–12. doi: 10.1128/jvi.50.1.1-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kalderon D., Smith A.E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984;139:109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- 149.Mocarski E.S., Jr., Kemble G.W. Recombinant cytomegaloviruses for study of replication and pathogenesis. Intervirology. 1996;39:320–330. doi: 10.1159/000150503. [DOI] [PubMed] [Google Scholar]
- 150.Anders D.G., McCue L.A. The human cytomegalovirus genes and proteins required for DNA synthesis. Intervirology. 1996;39:378–388. doi: 10.1159/000150508. [DOI] [PubMed] [Google Scholar]
- 151.Pari G.S., Anders D.G. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 1993;67:6979–6988. doi: 10.1128/jvi.67.12.6979-6988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weiland K.L., Oien N.L., Homa F., Wathen M.W. Functional analysis of human cytomegalovirus polymerase accessory protein. Virus Res. 1994;34:191–206. doi: 10.1016/0168-1702(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 153.Appleton B.A., Loregian A., Filman D.J., Coen D.M., Hogle J.M. The cytomegalovirus DNA polymerase subunit UL44 forms a C clamp-shaped dimer. Mol. Cell. 2004;15:233–244. doi: 10.1016/j.molcel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 154.Alvisi G., Ripalti A., Ngankeu A., Giannandrea M., Caraffi S.G., Dias M.M., Jans D.A. Human cytomegalovirus DNA polymerase catalytic subunit pUL54 possesses independently acting nuclear localization and ppUL44 binding motifs. Traffic. 2006;7:1322–1332. doi: 10.1111/j.1600-0854.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 155.Prichard M.N., Lawlor H., Duke G.M., Mo C., Wang Z., Dixon M., Kemble G., Kern E.R. Human cytomegalovirus uracil DNA glycosylase associates with ppUL44 and accelerates the accumulation of viral DNA. Virol. J. 2005;2:55. doi: 10.1186/1743-422X-2-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alvisi G., Jans D.A., Ripalti A. Human cytomegalovirus (HCMV) DNA polymerase processivity factor ppUL44 dimerizes in the cytosol before translocation to the nucleus. Biochemistry. 2006;45:6866–6872. doi: 10.1021/bi060086u. [DOI] [PubMed] [Google Scholar]
- 157.Alvisi G., Rawlinson S.M., Ghildyal R., Ripalti A., Jans D.A. Regulated nucleocytoplasmic trafficking of viral gene products: a therapeutic target? Biochim. Biophys. Acta. 2008;1784:213–227. doi: 10.1016/j.bbapap.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 158.Krosky P.M., Baek M.C., Jahng W.J., Barrera I., Harvey R.J., Biron K.K., Coen D.M., Sethna P.B. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J. Virol. 2003;77:7720–7727. doi: 10.1128/JVI.77.14.7720-7727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marschall M., Freitag M., Suchy P., Romaker D., Kupfer R., Hanke M., Stamminger T. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology. 2003;311:60–71. doi: 10.1016/s0042-6822(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 160.Xiao C.Y., Jans P., Jans D.A. Negative charge at the protein kinase CK2 site enhances recognition of the SV40 large T-antigen NLS by importin: effect of conformation. FEBS Lett. 1998;440:297–301. doi: 10.1016/s0014-5793(98)01478-1. [DOI] [PubMed] [Google Scholar]
- 161.Sinigalia E., Alvisi G., Mercorelli B., Coen D.M., Pari G.S., Jans D.A., Ripalti A., Palu G., Loregian A. Role of homodimerization of human cytomegalovirus DNA polymerase accessory protein UL44 in origin-dependent DNA replication in cells. J. Virol. 2008;82:12574–12579. doi: 10.1128/JVI.01193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.St-Denis N.A., Litchfield D.W. Protein kinase CK2 in health and disease: from birth to death: the role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell. Mol. Life Sci. 2009;66:1817–1829. doi: 10.1007/s00018-009-9150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koffa M.D., Kean J., Zachos G., Rice S.A., Clements J.B. CK2 protein kinase is stimulated and redistributed by functional herpes simplex virus ICP27 protein. J. Virol. 2003;77:4315–4325. doi: 10.1128/JVI.77.7.4315-4325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Souquere-Besse S., Pichard E., Filhol O., Legrand V., Rosa-Calatrava M., Hovanessian A.G., Cochet C., Puvion-Dutilleul F. Adenovirus infection targets the cellular protein kinase CK2 and RNA-activated protein kinase (PKR) into viral inclusions of the cell nucleus. Microsc. Res. Tech. 2002;56:465–478. doi: 10.1002/jemt.10060. [DOI] [PubMed] [Google Scholar]
- 165.Li S., Ku C.Y., Farmer A.A., Cong Y.S., Chen C.F., Lee W.H. Identification of a novel cytoplasmic protein that specifically binds to nuclear localization signal motifs. J. Biol. Chem. 1998;273:6183–6189. doi: 10.1074/jbc.273.11.6183. [DOI] [PubMed] [Google Scholar]
- 166.Sarfraz S., Hamid S., Ali S., Jafri W., Siddiqui A.A. Modulations of cell cycle checkpoints during HCV associated disease. BMC Infect. Dis. 2009;9:125. doi: 10.1186/1471-2334-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guo Q., Qian L., Guo L., Shi M., Chen C., Lv X., Yu M., Hu M., Jiang G., Guo N. Transactivators Zta and Rta of Epstein–Barr virus promote G0/G1 to S transition in Raji cells: a novel relationship between lytic virus and cell cycle. Mol. Immunol. 2010;47:1783–1792. doi: 10.1016/j.molimm.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 168.Moffat J.F., Greenblatt R.J. Effects of varicella-zoster virus on cell cycle regulatory pathways. Curr. Top. Microbiol. Immunol. 2010;342:67–77. doi: 10.1007/82_2010_28. [DOI] [PubMed] [Google Scholar]
- 169.Schang L.M. The cell cycle, cyclin-dependent kinases, and viral infections: new horizons and unexpected connections. Prog. Cell Cycle Res. 2003;5:103–124. [PubMed] [Google Scholar]
- 170.Wang H.K., Duffy A.A., Broker T.R., Chow L.T. Robust production and passaging of infectious HPV in squamous epithelium of primary human keratinocytes. Genes Dev. 2009;23:181–194. doi: 10.1101/gad.1735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Whyte P., Buchkovich K.J., Horowitz J.M., Friend S.H., Raybuck M., Weinberg R.A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- 172.Whyte P., Williamson N.M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989;56:67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- 173.Herrmann C.H., Su L.K., Harlow E. Adenovirus E1A is associated with a serine/threonine protein kinase. J. Virol. 1991;65:5848–5859. doi: 10.1128/jvi.65.11.5848-5859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bollag B., Chuke W.F., Frisque R.J. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: identification of sequences important for efficient transformation. J. Virol. 1989;63:863–872. doi: 10.1128/jvi.63.2.863-872.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Haggerty S., Walker D.L., Frisque R.J. JC virus–simian virus 40 genomes containing heterologous regulatory signals and chimeric early regions: identification of regions restricting transformation by JC virus. J. Virol. 1989;63:2180–2190. doi: 10.1128/jvi.63.5.2180-2190.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Harris K.F., Christensen J.B., Imperiale M.J. BK virus large T antigen: interactions with the retinoblastoma family of tumor suppressor proteins and effects on cellular growth control. J. Virol. 1996;70:2378–2386. doi: 10.1128/jvi.70.4.2378-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Prichard M.N., Sztul E., Daily S.L., Perry A.L., Frederick S.L., Gill R.B., Hartline C.B., Streblow D.N., Varnum S.M., Smith R.D., Kern E.R. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J. Virol. 2008;82:5054–5067. doi: 10.1128/JVI.02174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.DeCaprio J.A. The role of the J domain of SV40 large T in cellular transformation. Biologicals. 1999;27:23–28. doi: 10.1006/biol.1998.0173. [DOI] [PubMed] [Google Scholar]
- 179.Zhang B., Chen W., Roman A. The E7 proteins of low- and high-risk human papillomaviruses share the ability to target the pRB family member p130 for degradation. Proc. Natl. Acad. Sci. U.S.A. 2006;103:437–442. doi: 10.1073/pnas.0510012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hebner C.M., Laimins L.A. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev. Med. Virol. 2006;16:83–97. doi: 10.1002/rmv.488. [DOI] [PubMed] [Google Scholar]
- 181.de Villiers E.M. Human pathogenic papillomavirus types: an update. Curr. Top. Microbiol. Immunol. 1994;186:1–12. doi: 10.1007/978-3-642-78487-3_1. [DOI] [PubMed] [Google Scholar]
- 182.zur Hausen H., de Villiers E.M. Human papillomaviruses. Annu. Rev. Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]
- 183.Monsonego J., Bosch F.X., Coursaget P., Cox J.T., Franco E., Frazer I., Sankaranarayanan R., Schiller J., Singer A., Wright T.C., Jr., Kinney W., Meijer C.J., Linder J., McGoogan E., Meijer C. Cervical cancer control, priorities and new directions. Int. J. Cancer. 2004;108:329–333. doi: 10.1002/ijc.11530. [DOI] [PubMed] [Google Scholar]
- 184.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 185.Chiang C.M., Ustav M., Stenlund A., Ho T.F., Broker T.R., Chow L.T. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5799–5803. doi: 10.1073/pnas.89.13.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sverdrup F., Khan S.A. Replication of human papillomavirus (HPV) DNAs supported by the HPV type 18 E1 and E2 proteins. J. Virol. 1994;68:505–509. doi: 10.1128/jvi.68.1.505-509.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ustav M., Stenlund A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 1991;10:449–457. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Frattini M.G., Laimins L.A. Binding of the human papillomavirus E1 origin-recognition protein is regulated through complex formation with the E2 enhancer-binding protein. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12398–12402. doi: 10.1073/pnas.91.26.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Frattini M.G., Laimins L.A. The role of the E1 and E2 proteins in the replication of human papillomavirus type 31b. Virology. 1994;204:799–804. doi: 10.1006/viro.1994.1596. [DOI] [PubMed] [Google Scholar]
- 190.Kuo S.R., Liu J.S., Broker T.R., Chow L.T. Cell-free replication of the human papillomavirus DNA with homologous viral E1 and E2 proteins and human cell extracts. J. Biol. Chem. 1994;269:24058–24065. [PubMed] [Google Scholar]
- 191.Lusky M., Hurwitz J., Seo Y.S. Cooperative assembly of the bovine papilloma virus E1 and E2 proteins on the replication origin requires an intact E2 binding site. J. Biol. Chem. 1993;268:15795–15803. [PubMed] [Google Scholar]
- 192.Mohr I.J., Clark R., Sun S., Androphy E.J., MacPherson P., Botchan M.R. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990;250:1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- 193.Sanders C.M., Stenlund A. Transcription factor-dependent loading of the E1 initiator reveals modular assembly of the papillomavirus origin melting complex. J. Biol. Chem. 2000;275:3522–3534. doi: 10.1074/jbc.275.5.3522. [DOI] [PubMed] [Google Scholar]
- 194.Sedman T., Sedman J., Stenlund A. Binding of the E1 and E2 proteins to the origin of replication of bovine papillomavirus. J. Virol. 1997;71:2887–2896. doi: 10.1128/jvi.71.4.2887-2896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Park P., Copeland W., Yang L., Wang T., Botchan M.R., Mohr I.J. The cellular DNA polymerase alpha-primase is required for papillomavirus DNA replication and associates with the viral E1 helicase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8700–8704. doi: 10.1073/pnas.91.18.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sedman J., Stenlund A. Co-operative interaction between the initiator E1 and the transcriptional activator E2 is required for replicator specific DNA replication of bovine papillomavirus in vivo and in vitro. EMBO J. 1995;14:6218–6228. doi: 10.1002/j.1460-2075.1995.tb00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang L., Mohr I., Fouts E., Lim D.A., Nohaile M., Botchan M. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5086–5090. doi: 10.1073/pnas.90.11.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Conger K.L., Liu J.S., Kuo S.R., Chow L.T., Wang T.S. Human papillomavirus DNA replication. Interactions between the viral E1 protein and two subunits of human DNA polymerase alpha/primase. J. Biol. Chem. 1999;274:2696–2705. doi: 10.1074/jbc.274.5.2696. [DOI] [PubMed] [Google Scholar]
- 199.Lin B.Y., Makhov A.M., Griffith J.D., Broker T.R., Chow L.T. Chaperone proteins abrogate inhibition of the human papillomavirus (HPV) E1 replicative helicase by the HPV E2 protein. Mol. Cell. Biol. 2002;22:6592–6604. doi: 10.1128/MCB.22.18.6592-6604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Masterson P.J., Stanley M.A., Lewis A.P., Romanos M.A. A C-terminal helicase domain of the human papillomavirus E1 protein binds E2 and the DNA polymerase alpha-primase p68 subunit. J. Virol. 1998;72:7407–7419. doi: 10.1128/jvi.72.9.7407-7419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stenlund A. Initiation of DNA replication: lessons from viral initiator proteins. Nat. Rev. Mol. Cell Biol. 2003;4:777–785. doi: 10.1038/nrm1226. [DOI] [PubMed] [Google Scholar]
- 202.Stubenrauch F., Laimins L.A. Human papillomavirus life cycle: active and latent phases. Semin. Cancer Biol. 1999;9:379–386. doi: 10.1006/scbi.1999.0141. [DOI] [PubMed] [Google Scholar]
- 203.Banerjee N.S., Chow L.T., Broker T.R. Retrovirus-mediated gene transfer to analyze HPV gene regulation and protein functions in organotypic “raft” cultures. Methods Mol. Med. 2005;119:187–202. doi: 10.1385/1-59259-982-6:187. [DOI] [PubMed] [Google Scholar]
- 204.Stoler M.H., Wolinsky S.M., Whitbeck A., Broker T.R., Chow L.T. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology. 1989;172:331–340. doi: 10.1016/0042-6822(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 205.Lin B.Y., Ma T., Liu J.S., Kuo S.R., Jin G., Broker T.R., Harper J.W., Chow L.T. HeLa cells are phenotypically limiting in cyclin E/CDK2 for efficient human papillomavirus DNA replication. J. Biol. Chem. 2000;275:6167–6174. doi: 10.1074/jbc.275.9.6167. [DOI] [PubMed] [Google Scholar]
- 206.Gerlier D., Valentin H. Measles virus interaction with host cells and impact on innate immunity. Curr. Top. Microbiol. Immunol. 2009;329:163–191. doi: 10.1007/978-3-540-70523-9_8. [DOI] [PubMed] [Google Scholar]
- 207.Shaw M.L., Garcia-Sastre A., Palese P., Basler C.F. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J. Virol. 2004;78:5633–5641. doi: 10.1128/JVI.78.11.5633-5641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tordo N., Poch O., Ermine A., Keith G., Rougeon F. Walking along the rabies genome: is the large G–L intergenic region a remnant gene? Proc. Natl. Acad. Sci. U.S.A. 1986;83:3914–3918. doi: 10.1073/pnas.83.11.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schnell M.J., McGettigan J.P., Wirblich C., Papaneri A. The cell biology of rabies virus: using stealth to reach the brain. Nat. Rev. Microbiol. 2010;8:51–61. doi: 10.1038/nrmicro2260. [DOI] [PubMed] [Google Scholar]
- 210.Moseley G.W., Lahaye X., Roth D.M., Oksayan S., Filmer R.P., Rowe C.L., Blondel D., Jans D.A. Dual modes of rabies P-protein association with microtubules: a novel strategy to suppress the antiviral response. J. Cell Sci. 2009;122:3652–3662. doi: 10.1242/jcs.045542. [DOI] [PubMed] [Google Scholar]
- 211.Chelbi-Alix M.K., Vidy A., El Bougrini J., Blondel D. Rabies viral mechanisms to escape the IFN system: the viral protein P interferes with IRF-3, Stat1, and PML nuclear bodies. J. Interferon Cytokine Res. 2006;26:271–280. doi: 10.1089/jir.2006.26.271. [DOI] [PubMed] [Google Scholar]
- 212.Blondel D., Regad T., Poisson N., Pavie B., Harper F., Pandolfi P.P., De The H., Chelbi-Alix M.K. Rabies virus P and small P products interact directly with PML and reorganize PML nuclear bodies. Oncogene. 2002;21:7957–7970. doi: 10.1038/sj.onc.1205931. [DOI] [PubMed] [Google Scholar]
- 213.Heilman D.W., Teodoro J.G., Green M.R. Apoptin nucleocytoplasmic shuttling is required for cell type-specific localization, apoptosis, and recruitment of the anaphase-promoting complex/cyclosome to PML bodies. J. Virol. 2006;80:7535–7545. doi: 10.1128/JVI.02741-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Toriumi H., Honda Y., Morimoto K., Tochikura T.S., Kawai A. Structural relationship between nucleocapsid-binding activity of the rabies virus phosphoprotein (P) and exposure of epitope 402–13 located at the C terminus. J. Gen. Virol. 2002;83:3035–3043. doi: 10.1099/0022-1317-83-12-3035. [DOI] [PubMed] [Google Scholar]
- 215.Vidy A., Chelbi-Alix M., Blondel D. Rabies virus P protein interacts with STAT1 and inhibits interferon signal transduction pathways. J. Virol. 2005;79:14411–14420. doi: 10.1128/JVI.79.22.14411-14420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Moseley G.W., Roth D.M., DeJesus M.A., Leyton D.L., Filmer R.P., Pouton C.W., Jans D.A. Dynein light chain association sequences can facilitate nuclear protein import. Mol. Biol. Cell. 2007;18:3204–3213. doi: 10.1091/mbc.E07-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gupta A.K., Blondel D., Choudhary S., Banerjee A.K. The phosphoprotein of rabies virus is phosphorylated by a unique cellular protein kinase and specific isomers of protein kinase C. J. Virol. 2000;74:91–98. doi: 10.1128/jvi.74.1.91-98.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shimizu K., Ito N., Sugiyama M., Minamoto N. Sensitivity of rabies virus to type I interferon is determined by the phosphoprotein gene. Microbiol. Immunol. 2006;50:975–978. doi: 10.1111/j.1348-0421.2006.tb03875.x. [DOI] [PubMed] [Google Scholar]
- 219.Kulkarni S., Volchkova V., Basler C.F., Palese P., Volchkov V.E., Shaw M.L. Nipah virus edits its P gene at high frequency to express the V and W proteins. J. Virol. 2009;83:3982–3987. doi: 10.1128/JVI.02599-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rodriguez J.J., Parisien J.P., Horvath C.M. Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J. Virol. 2002;76:11476–11483. doi: 10.1128/JVI.76.22.11476-11483.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ciancanelli M.J., Volchkova V.A., Shaw M.L., Volchkov V.E., Basler C.F. Nipah virus sequesters inactive STAT1 in the nucleus via a P gene-encoded mechanism. J. Virol. 2009;83:7828–7841. doi: 10.1128/JVI.02610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Caignard G., Guerbois M., Labernardiere J.L., Jacob Y., Jones L.M., Wild F., Tangy F., Vidalain P.O. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology. 2007;368:351–362. doi: 10.1016/j.virol.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 223.Ohno S., Ono N., Takeda M., Takeuchi K., Yanagi Y. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J. Gen. Virol. 2004;85:2991–2999. doi: 10.1099/vir.0.80308-0. [DOI] [PubMed] [Google Scholar]
- 224.Palosaari H., Parisien J.P., Rodriguez J.J., Ulane C.M., Horvath C.M. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 2003;77:7635–7644. doi: 10.1128/JVI.77.13.7635-7644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nanda S.K., Baron M.D. Rinderpest virus blocks type I and type II interferon action: role of structural and nonstructural proteins. J. Virol. 2006;80:7555–7568. doi: 10.1128/JVI.02720-05. [DOI] [PMC free article] [PubMed] [Google Scholar]