Abstract
Graphene is the basic building block of 0D fullerene, 1D carbon nanotubes, and 3D graphite. Graphene has a unique planar structure, as well as novel electronic properties, which have attracted great interests from scientists. This review selectively analyzes current advances in the field of graphene bioapplications. In particular, the biofunctionalization of graphene for biological applications, fluorescence-resonance-energy-transfer-based biosensor development by using graphene or graphene-based nanomaterials, and the investigation of graphene or graphene-based nanomaterials for living cell studies are summarized in more detail. Future perspectives and possible challenges in this rapidly developing area are also discussed.
Introduction
‘Where nature finishes producing its own species, man begins, using natural things and with the help of this nature, to create an infinity of species.’ Just as the scientist/artist Leonardo da Vinci said, nanoscience is always exciting the imagination of biologists and biotechnologists [1]. New-found nanomaterials are especially providing fascinating opportunities for biotechnological development because of their unique structures, components and properties [2]. Hence, the advancement of nanomaterial research plays an essential role in the exploration of the biochemical and nanotechnological fields [3]. Today, impressive achievements have been made at the cross-section of nanotechnology and biotechnology by employing modern nanomaterials as elements to generate mechanical, optical, and electronic devices and biosensors 4, 5, 6.
Graphene, a free-standing 2D crystal with one-atom thickness, has become one of the hottest topics in the fields of materials science, physics, chemistry, and nanotechnology [7]. This allotrope of carbon comprises layers of six-atom rings in a honeycombed network and can be conceptually viewed as a true planar aromatic macromolecule [8]. As a basic building block of other carbon allotropes, graphene can be wrapped to generate 0D fullerenes, rolled up to form 1D carbon nanotubes, and stacked to produce 3D graphite [9] (Figure 1a). Although graphene is the fundamental basis of different carbon forms, it was first prepared unambiguously in 2004, 440 years after the invention of graphite, by peeling a single layer of graphene using sticky tape and a pencil [10]. During the past several years, various methods for producing graphene have been developed, such as micromechanical exfoliation, chemical vapour deposition, epitaxial growth, and chemically synthesis (see Box 1 for more details). Graphene samples produced by chemical method are also called chemically modified graphene (CMG). Here, we use graphene and graphene-based nanomaterial to define the graphene materials referred to in this review.
Figure 1.
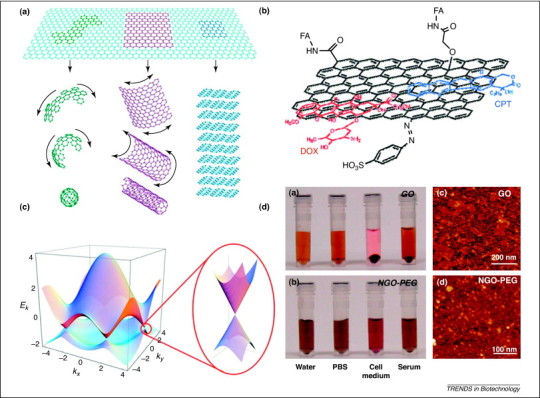
(a) The epitome of graphite forms. Graphene is a 2D building material for carbon materials of all other dimensionalities. It can be wrapped up into 0D buckyballs, rolled into 1D nanotubes, or stacked into 3D graphite [12]. (b) Schematic image representing the loading of doxorubicin (DOX) and camptothecin (CPT) onto FA-modified NGO. The NGO is functionalized with sulfonic acid groups to form NGO–SO3H, which render it stable in physiological solution. NGO–FA was prepared through formation of an amide bond by the reaction between the NH2 groups of FA and COOH groups of NGO–SO3H. Finally, two anticancer drugs, DOX and CPT were conjugated onto the FA–NGO via π–π stacking and hydrophobic interactions [68]. (c) Electronic dispersion in graphene. Left: energy spectrum (in units of t) for finite values of t and t′, with t=2.7 eV and t′= –0.2t. Right: magnified image of energy bands close to one of the Dirac points [11]. (d) PEG modification of NGO: photos of (i) GO and (ii) NGO–PEG in different solutions recorded after centrifugation at 10 000 times gravity for 5 min. GO crashed out slightly in PBS and completely in cell medium and serum (top panel). NGO–PEG was stable in all solutions; Atomic force microscopy (AFM) images of (iii) GO and (iv) NGO–PEG [66]. Reproduced with permission from 11, 12, 66, 68.
Box 1. Methods for production of graphene and graphene-based nanomaterials.
Graphene was first prepared in 2004 by peeling a single layer of graphene using sticky tape and a pencil [10]. During the past several years, various methods for producing graphene have been developed, such as micromechanical exfoliation, epitaxial growth or chemical vapour deposition (CVD) epitaxial growth, and chemical synthesis from graphite.
The sticky tape and pencil strategy is also considered as the micromechanical exfoliation or peel-off method, which can be used successfully against the strong exfoliation energy of the π-stacked layers in graphite, to produce pure and single-layered graphene sheets with a honeycomb lattice. However, this process has a disadvantage in terms of yielding small samples. A method of liquid phase exfoliation of graphite in surfactant/water solutions has been demonstrated recently. This method produces graphene that consists of 40% of sheets thinner than five layers and about 3% monolayers [70].
Graphene synthesis by epitaxy on transition metals has been reported. For example, epitaxial growth of single- and multi-layered graphene epitaxy on Ru (0001) has been observed by in situ surface microscopy, with characterization by electron scattering and microscopy [71]. CVD epitaxial growth is another approach for production of graphene, by dissolving carbon into a nickel substrate, and then forcing it to precipitate out by cooling the nickel. The thickness and crystalline ordering of the precipitated carbon is controlled by the cooling rate and concentration of carbon dissolved in the nickel. After chemical etching of the nickel, the graphene membrane detaches and can be transferred to another substrate [72].
Chemical synthesis of graphene from graphite is becoming a promising method with some advantages such as scalable, high-volume production and ease of chemical modification. However, the chemical approach does introduce some defects in graphene during the oxidation or reduction steps, and results in structural damage of the graphene. Hence, graphene produced by chemical methods is also called CMG in some reports and reviews [73]. Here, we use the terms graphene and graphene-based nanomaterial. Briefly, the chemical method for graphene production involves initial oxidation of graphite to graphite oxide, followed by mechanical or thermal exfoliation of graphite oxide to GO sheets. GO sheets are hydrophilic, oxygenated graphene sheets and graphite oxide is a layered material that consists of GO sheets, which bear oxygen functional groups on their basal planes and edges. To derive graphene from GO, chemical reduction is the final step to remove the functional groups, which makes the product strongly hydrophilic. Reduction of GO to graphene can be carried out by using reducing chemical agents such as hydroquinone, dimethylhydrazine and hydrazine hydrate, electrochemical reduction [74], thermal reduction [75], and photocatalytic reduction [76].
Although it is considered as the basic block of carbon allotropes, graphene exhibits distinctly different properties, such as its unusual structural characteristics and electronic flexibility 11, 12, 13. Other extraordinary properties include high planar surface (calculated value, 2630 m2/g) [14], superlative mechanical strength (Young's modulus, ∼1100 GPa) [15], unparalleled thermal conductivity (∼5000 W/m/K) [16], and remarkable electronic properties. Typically, the notable electronic characteristics of graphene are a high integer quantum Hall effect at room temperature [17]; a Dirac fermion system with linear energy dispersion [18]; an ambipolar electric field effect, along with ballistic conduction of charge carriers [19]; and electron-hole symmetry and freedom internal degree [20]. For example, electronic dispersion in the graphene lattice is shown in Figure 1c. According to the linear dispersion of the electron energy (E) with respect to the wave vector (k), the Dirac equation that describes the linear E-k relation is , where the Fermi velocity v F is about 106 m/s. It is expected that quasiparticles in graphene behave differently from those in conventional metals and semiconductors, which exhibit a parabolic dispersion relation [21]. Specific information on graphene synthesis, as well as its chemical and physical properties, has been discussed in several reviews 6, 7, 9, 13, 22, 23, 24.
As a result of the unique chemical and physical properties mentioned above, graphene and graphene-based nanomaterials have attracted strong interest in biological studies 25, 26, 27, 28. Various graphene-based nanomaterials 29, 30, 31, 32, 33, 34 have been used to fabricate functionalized biosystems integrated with nucleic acids (NAs), peptides, proteins and even cells. Moreover, the discovery of the adsorption of single-stranded DNA (ssDNA) onto graphene sheets, the ability of graphene to quench electron donors, the ability of graphene to protect biomolecules from enzymatic cleavage, as well as transportation capability in living cells and in vivo systems, have revealed the potential for graphene application in biological studies and biotechnology.
Graphene, graphene oxide (GO), CMG and graphene derivates are promising candidates in biotechnology development, therefore, this review selectively summarizes approaches that are currently emerging at the intersection of graphene-based nanomaterials and biotechnological investigations. We first describe the achievements of graphene-based functional biosystems because the combination of graphene and biomolecules introduces graphene into biotechnology. Fluorescence resonance energy transfer (FRET) biosensors based on graphene and its derivates are then discussed, with the sensing applications ranging from small molecules and DNA to proteins and cells. Moreover, recent achievements using graphene as an advanced transporter for drug delivery, as well as bioimaging in living cells, are summarized at the end of this article.
Biofunctionalization of graphene and graphene-based nanomaterials
Parallel with the advancement of nanomaterial science and biotechnology, various nano/bio interfaces have been realized in the areas of biological device design, biomolecule detection, bioassays, and molecular medicine 35, 36, 37, 38, 39. Graphene has been employed as a substrate to be interfaced with various biomolecules and cells (Figure 2 ). Biological modification in turn benefits graphene by improving its biocompatibility, solubility and selectivity. Hence, several studies have focused on graphene modification and functionalization (reviewed in [22]). For the purpose of this review, biofunctionalization approaches for graphene are separated into two categories: functionalization with DNA and proteins. Tandem functionalization with DNA and protein, as well as functionalization with other biomolecules is also briefly mentioned.
Figure 2.

Graphene and its derivatives have been reported to be functionalized with avidin–biotin, peptides, NAs, proteins, aptamers, small molecules, bacteria, and cells through physical adsorption or chemical conjugation. The functionalized graphene biosystems with the unique properties have been used to build up biological platforms, biosensors, and biodevices.
Biofunctionalization with DNA
The large 2D aromatic surface of graphene makes it an ideal substrate for adsorption of certain biomolecules. Importantly, ssDNA can be strongly interfaced onto the graphene surface. In particular, the tethering process of ssDNA on graphene is preferential on thicker sheets (∼8 nm), and on wrinkled rather than flat surfaces of chemically modified graphene. Patterning ssDNA on graphene layers increases the hole-density in the graphene layer in the order of 5.61×1012/cm2. The resultant ssDNA–graphene biointerface has been used in a field-effect transistor (FET) for the label-free, reversible detection of complementary ssDNA [40]. Through a self-assembly process under strong ultrasonication, monolayers of ssDNA molecules have been adsorbed on both sides of graphene sheets by π–π stacking [41]. In another example, graphene adsorbed with ssDNA has been used directly for the study of surface-enhanced laser desorption ionization time-of-flight mass spectrometry (SELDI-TOF-MS) [42]. Graphene efficiently preconcentrated the ssDNA and served as a direct platform for SELDI-TOF-MS analysis. Another elegant approach of graphene tethering with DNA includes formation of nanoparticles (NPs) decorated with graphene biosystems. Thiolated ssDNA is first adsorbed onto GO sheets. The resultant thiol-DNA–GO sheet is used as a 2D bionanointerface for assembly of gold NPs. Furthermore, the gold NPs maintain optical properties after assembly [43].
Biofunctionalization with proteins
As a result of their varied surface functional groups and secondary structure, proteins can exfoliate and modify graphene through physical adsorption or chemical bonding. Graphene and GO have been tethered with various proteins, which results in biosystems with unique properties. For example, an enzyme immobilization matrix based on GO has been reported [24]. It has been found that horseradish peroxidase (HRP) and lysozyme can be spontaneously immobilized on GO because the individual GO sheet is enriched with oxygen-containing groups, which makes it possible to immobilize enzymes without any surface modifications or coupling reagents [44]. To monitor protein adsorption on graphene further, an electrolyte-gated graphene FET has been built using single-layer graphene as a channel [45].
Exfoliation and functionalization of graphene can be accomplished with interfacial engineering by hydrophobin; a microbial adhesion protein [46]. Exfoliation might also be carried out in future studies by using proteins with specified functionality, such as antibody/antigen or biotin/avidin. NPs have been introduced into graphene that has been previously modified with proteins. An adhesive-protein-functionalized graphene nanosheet with the electrostatic assembly of various metallic NPs (e.g. Au, Pt, Ag, Pd) or latex NPs has been reported. As a result of the tunable amphiphilic feature of bovine serum albumin (BSA) according to the solution pH, the hydrophobic and hydrophilic patches on the BSA surface can be switched easily. At a suitable pH and reaction temperature, various positive- or negative-charged NPs can be decorated on the BSA–graphene biointerface based on the electrostatic adherence [47].
Tandem DNA and protein biofunctionalization
DNA and protein can be incorporated in tandem with graphene biofunctionalization. As an example, an ssDNA–protein–graphene composite has been fabricated [41]. ssDNA was first co-assembled with graphene sheets. Then, an interlayer of the ssDNA-stabilized graphene sheet was expanded by positively charged cytochrome c (a redox protein). As a result, a multi-layered protein–DNA–graphene nanocomposite was obtained. In a recent study, HRP has been immobilized on ssDNA–graphene sheets to create an HRP–ssDNA–graphene-coated electrode [48]. Such an electrochemical biosensor has been demonstrated with a wider linear relationship of hydrogen peroxide sensing (115.5–9.25 mM), which could be used to study the mechanism of enzyme direct electrochemistry. However, more effort is desired in this field because it is still a problem how to realize a practical electrochemical biosensor based on the DNA–graphene–enzyme biosystem, how to maintain enzyme activity on the modified electrode, and how to carry out real sample detection.
Biofunctionalization with other biomolecules
Other biomolecules, such as peptides and cellulose, have also been used to functionalize graphene. A synthetic approach for producing a diphenylalanine-modified graphene core/shell by single-step solution processing has been proposed [49]. The reduced monolayer graphene sheets decorated with chemical functionalities such as epoxide, hydroxyl and carboxylic acid groups were dispersed in aqueous medium. Then, peptide/graphene core/shell nanowires were immediately created as soon as an organic peptide solution (100 mg/ml solution of diphenylalanine in 1,1,1,3,3,3-hexafluoro-2-propanol) was diluted in aqueous graphene dispersion under mild mechanical shaking. The diameter and graphene-shell thickness of the prepared nanowire were 400 nm and 10 nm, respectively. Moreover, biologically compatible and biodegradable natural polymeric dispersants, such as lignin and cellulose derivatives, have been employed to fabricate high-concentration and stable aqueous suspensions of graphene nanosheets, which exhibit advanced priorities compared with some general chemical agents. The resultant suspensions of graphene nanosheets are stable in water at high concentrations (0.6–2 mg/ml) [50]. Such nanosheets can be used as the loading platform for enzymes or biomarkers in biosensors, or the carriers of NAs or drugs in therapy studies.
Graphene-based FRET biosensors
There is increasing interest in the use of graphene for the development of FRET biosensors (Figure 3 ). FRET involves the transfer of energy from a donor fluorophore to an acceptor fluorophore, and is one of the advanced tools available for measuring nanometer-scale distance and changes, both in vivo and in vitro [51]. Recent theoretical and experimental studies have shown that graphene can be a highly efficient quencher for various organic dyes and quantum dots (QDs) [13]. Compared with organic quenchers, graphene has shown superior quenching efficiency for a variety of fluorophores, with low background and high signal-to-noise ratio, as well as other advantages derived from the nanomaterial itself, such as protection from enzymatic cleavage 52, 53. Graphene and GO have been reported to interact strongly with NAs through π– π stacking interactions between the ring structures in the NA bases and the hexagonal cells of graphene and GO; whereas double-stranded DNA (dsDNA) cannot be stably adsorbed onto the surface because of efficient shielding of nucleobases within the negatively charged dsDNA phosphate backbone 54, 55. The development of graphene-based FRET biosensors has been motivated greatly by reliance on this particular principle and integrating it with the advantages of graphene. Here, we selectively summarize recent progress in biosensors that integrate the super quenching property of graphene and the recognition properties of NAs (Table 1 ).
Figure 3.

Principles of graphene-based FRET biosensors. ssDNA, aptamers and MBs can be adsorbed onto the surfaces of graphene or graphene derivates (which also possess a planar surface and 2D structure). Fluorophore labels on the ends of probes are quenched rapidly when adsorbed onto the graphene surface. When analytes (e.g. cDNA, thrombin and a designed complementary ssDNA or functional NAs like survivin mRNA [64]) are introduced into the systems and bind their probes (ssDNA, aptamer and MB, respectively), the probe fluorescence is recovered, thus allowing detection. Conversely, dsDNA remains fluorescent before an enzyme (e.g. helicase) is introduced; ssDNA is then released, and fluorophore on the ssDNA is quenched by graphene-based nanomaterials.
Table 1.
FRET biosensors fabricated with graphene-based nanomaterials
| Graphene category | Probe type | Probe sequence | Target | LOD (nM) | Refs |
|---|---|---|---|---|---|
| Graphene | ssDNA | 5′-FAM-AATCAACTG GGA GAA TGTAACTG-3′ | cDNA | N/A | [52] |
| Graphene oxide | ssDNA | 5′-FAM-AGTCAGTGTGGAAAATCTCTAGC-3′ | cDNA | N/A | [54] |
| Graphene oxide | Aptamer | 5′-FAM-TCTCTCAGTCCGTGGTAGGGCAGGTTGGGGTGACT-3′ | Human thrombin | 2.0 | [54] |
| Graphene oxide | ssDNA and dsDNA | 5′-FAM-GGAATTCTAATGTAGTATAGTAATCCGCTC-3′ 5′-(T)15GAGCGGATTACTATACTACATTAGAATTCC-3′ | SCV helicase | 0.625 | [55] |
| Graphene oxide | ssDNA | 5′-FAM- TCGTTGGAGTTTGTCTG-3′ 5′-Cy5-CCCTAATCCGCCCAC-3′ 5′-ROX-CCTGGTGCCGTAGAT-3′ | cDNA | 0.1 | [56] |
| Graphene oxide | Hairpin-structured DNA | 5′-Dabcyl-CGACGGAGAAAGGGCTGCCACGTCG-FAM-3′ | cDNA | 2.0 | [59] |
| Graphene oxide | Aptamer | 5′-FAM-CTCTCTTCTCTTCATTTTTCAACACAACACAC-3′ | Silver (I) ions | 5 | [60] |
| SDBS-graphene | Aptamer | 5′-FAM-GGTTGGTGTGGTTGG-3′ | Bovine thrombin | 0.0313 | [61] |
| Graphene oxide | MB | 5′-Dabcyl-CGACGGAGAAAGGGCTGCCACGTCG-Cy5-3′ | survivin mRNA | N/A | [63] |
| Graphene oxide | ssDNA | 5′-H2N-(T)6GCACACGCGCAC-3′ | Au NP-labeled cDNA | 200 | [77] |
Abbreviations: Cy5 a reactive water-soluble fluorescent dye of the cyanine dye family; ROX: reference dye from Invitrogen; SCV: severe acute respiratory syndrome coronavirus.
DNA detection
The first graphene-based FRET biosensor included a fluorescein amidite (FAM)-labeled ssDNA adsorbed onto GO [54]. As a result of the FRET effect between FAM and GO, fluorescence is quenched rapidly; however, binding between probe ssDNA and a complementary ssDNA alters the conformation, and consequently, releases FAM–ssDNA from the GO surface and results in fluorescence recovery. Detection of cDNA is therefore realized. Similarly, multicolor DNA analysis has been accomplished with graphene-based FRET biosensors [56]. The planar GO surface allows simultaneous quenching of multiple ssDNA probes labeled with different dyes, which leads to a multicolor sensor for the detection of multiple DNA targets. In this case, a graphene-based FRET biosensor is able to detect different DNA targets with various sequences by using ssDNA probes with different sequences. In another example, a graphene-based FRET platform has been developed for detection of helicase-mediated unwinding of duplex DNA [55]. By reliance on the preferential binding of GO to ssDNA over dsDNA, the dsDNA substrate that contains a fluorescent dye at the end of one strand is prepared first. As helicase unwinding of dsDNA proceeds, the fluorescence decreases and is quenched completely because of strong interaction of GO with unwound ssDNA. Helicase activity can thus be monitored in real time.
To enhance the sequence-specific detection of target DNA, molecular beacons (MBs) have been employed to fabricate graphene-based FRET biosensors [57]. Typically, the two ends of an MB are labeled with a fluorophore and a quencher. MBs do not fluoresce until they have hybridized with the target NAs. The unique thermodynamics and specificity of MBs have led to their broad application in biotechnology [58]. However, the dual labeling limits the preparation of MBs, and variable or residual fluorescence can limit detection sensitivity. In studies of MB-based FRET biosensors, graphene can serve as a nanoquencher for fluorophores as well as a nanoscaffold for MBs. The incorporation of graphene into a MB-based FRET biosensor not only provides a more convenient synthesis/purification protocol compared to conventional MBs, but also improves the sensitivity. As an example, GO has been employed as the ‘nanoquencher’, with increased sensitivity and single-base mismatch selectivity for target DNA [59]. An MB-based GO FRET biosensor has also been integrated with QDs as fluorophores owning to its broad absorption and narrow emission spectra [58]. The MB-QD GO-based FRET biosensor has displayed good selectivity and high sensitivity, with a detection limit for target cyclin DNA of 12 nM (Table 1), and could be used for quantification of NAs and for single nucleotide polymorphism analysis.
Ion, small molecule, and protein detection
Aptamers are selected single-strand oligonucleotides isolated from random-sequence DNA or RNA libraries by an in vitro selection process. Aptamers boast high specificity to a wide range of targets, which is advantageous for many bio-analytical applications (reviewed in [53]). By using aptamers as probes, we can extend the targeting field of graphene-based FRET biosensors from DNA to ions, small molecules, and proteins. Ag+ ion detection has been realized by using GO and an Ag+-specific aptamer [60]. The target-induced conformational change of the aptamer leads to the recovery of FAM fluorescence. Ag+ ions can be easily differentiated when some other metal ions are present with a 10-fold higher concentration, which demonstrates a sensitive responding ability of the proposed graphene FRET biosensor. Another highly specific FRET sensor has been developed for detection of bovine thrombin, based on a dye-labeled aptamer probe and graphene 54, 61. To obtain a good water-dispersion graphene sample, sodium dodecyl benzene sulfonate (SDBS) has been used for the chemical reduction of graphite oxide to produce SDBS-graphene [61]. Thrombin aptamer labeled with FAM was first incubated with SDBS–graphene. When thrombin was introduced into the aptamer/graphene solution, obvious fluorescence recovery was observed. This is mainly due to the quadruplex structure formed by thrombin and its aptamer, which owns the weak affinity to graphene. The graphene-based FRET aptasensor has a sensitive detection ability for thrombin in buffer (down to 31.3 pM) and in serum solutions (Table 1). The graphene-based FRET aptasensor has exhibited an extraordinary response in buffer and serum solutions.
Graphene-based biotechnology for living cell studies
As outlined above, the unique ability of DNA adsorption, super quenching capacity, and protection from enzyme cleavage have resulted in graphene being a robust artificial nanomaterial in biotechnology, and a novel candidate to be utilized in biomedical investigations, including in vivo targeting of cellular ATP and in situ localization of mRNA, real-time monitoring, drug delivery, and cell imaging. In this section, we selectively emphasize such applications of graphene and graphene-based nanomaterials in living cells.
Cellular probing and real-time monitoring
An aptamer–FAM/GO nanosheet (aptamer–FAM/GO-nS) complex has been designed for in situ molecular probing of ATP in JB6 Cl 41-5a mouse epithelial cells [62]. The aptamer–FAM/GO-nS complex, coupled with a wide-field fluorescence microscope, serves as a real-time sensing platform (Figure 4 ). ATP recognition by the ATP aptamer has been used as a model system to elucidate certain properties and advantages of the GO nanosheet: (i) GO-nS can serve as a transporter of DNA aptamers into living cells; (ii) GO-nS shows efficient protection of oligonucleotides from enzymatic cleavage during delivery to inter- or intracellular spaces; and (iii) GO-nS can act as a real-time sensing platform in living cells with high fluorescence quenching efficiency [62]. The capability of graphene for DNA protection from cleavage during cellular delivery has indeed been demonstrated: MBs can be used as oligonucleotide probes in conjunction with GO-nS to deliver DNA to HeLa cells [63].
Figure 4.
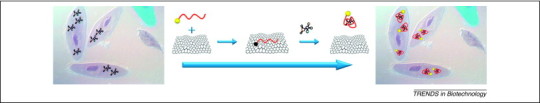
Design of aptamer-carboxyfluorescein/GO-nS (aptamer-FAM/GO-nS) used for preliminarily investigation of the ability to probe living cells at the molecular level. A FAM-labeled ATP aptamer was first incubated with GO-nS to produce the aptamer-FAM/GO-nS nanocomplex. Then, the nanocomplex was cultured together with JB6 cells and observed with a wide-field microscope. Significant FAM fluorescence could be observed for JB6 cells incubated with aptamer-FAM/GO-nS, which indicated successful intracellular aptamer delivery and ATP probing in living cells. As controls, JB6 cells were also cultured with or without random DNA-FAM/GO-nS as well as with ATP aptamer–FAM or GO alone, and almost no fluorescence signals were observed in the control samples. These results demonstrate that GO-nS is an efficient cargo for DNA transport through cell membranes. Reproduced with permission from [62].
Graphene FET for living cell detection
Nanomaterial-based FETs have been proven to be powerful building elements for nanoscale bioelectronic interfaces with cells and tissues, owing to their ability to form coupled interfaces with cell membranes. Graphene-based FETs have been reported in recent studies as promising chemical and biological sensors in living cells 64, 65. For example, a graphene-based FET has been used to investigate electrogenic cells [64]. The FET conductance signals that are recorded from beating chicken embryonic cardiomyocytes yields well-defined extracellular signals with a signal-to-noise ratio routinely above 4, which exceeds typical values for other planar devices. A graphene-based FET (with active channel of 20.8 × 9.8 μm) has also been used as a biosensor to detect hormonal catecholamine molecules in neuroendocrine PC12 rat adrenal medulla cells [65]. This patterned GO film-based FET has realized the label-free and real-time monitoring of catecholamine secretion from living cells.
Drug delivery and cell imaging
Another exciting area of graphene research is drug delivery in living cells. For instance, modified GO has been investigated as a cargo for the delivery of water-soluble cancer drugs [66]. Nanoscale GO (NGO) was first functionalized with polyethylene glycol (PEG) to render high solubility in aqueous solutions, as well as stability in physiological solutions, such as serum. A water-insoluble aromatic molecule, SN38, has been attached to PEGylated NGO (NGO-PEG). Finally, the NGO-PEG–SN38 complex exhibits high potency with IC50 values of 6 nM for HCT-116 human colon cancer cells, which is 1000-fold more potent than camptothecin (CPT-11), and has similar potency to that of free SN38 [67]. To enhance the loading efficiency and targeting ability of anticancer drugs, NGO can be covalently modified with folic acid (FA) [68]. Controlled loading of two anticancer drugs, doxorubicin and CPT-11 onto the FA-conjugated NGO (FA-NGO) has been investigated. In this case, FA-NGO loaded with the two anticancer drugs shows specific targeting to MCF-7 human breast cancer cells and remarkably high cytotoxicity compared to unmodified NGO loaded with doxorubicin or irinotecan. In a final example, PEG-modified nanoscale graphene sheets (NGSs) have been prepared, and the strong optical absorbance of NGSs in the near-infrared region has been utilized to achieve ultra-high in vivo tumor uptake of anticancer drugs, which can be used for photothermal therapy of cancer [69]. The behavior of PEGylated NGSs in mice has been studied by fluorescence imaging, and surprisingly high tumor accumulation was observed. NGSs with a biocompatible coating might therefore constitute a novel type of 2D nanomaterial with great potential in cancer therapy. With the success of the above studies, it is likely that graphene-based nanocarriers will find widespread application in biomedicine in the future.
Concluding remarks
As a result of the fascinating properties of graphene, with respect to structures that can be oriented and surfaces that can be modified, we believe that it offers some important advantages for biotechnological applications, especially in the areas of bioelectronics, biosensors and medicine. However, the merging of graphene and biotechnology is in its infancy, with many challenges remaining. Control of the size and number of single layers of graphene separated from bulk graphite might be the foremost challenge for the application of graphene as the building block of functionalized biosystems. Pristine graphene possesses a super quenching ability for FRET applications, but it is highly hydrophobic. Likewise, GO has been widely used in the fields of FRET biosensor studies because of its good solubility, but it is lack of super fast electron transfer. Preparation of soluble, well-defined graphene or graphene derivates with high quenching efficiency is another challenge. Cytotoxicity, the cellular uptake mechanism, and the intracellular metabolic pathway of graphene and its derivates remain almost unknown, which are highly important research areas and are crucial if we are going to use graphene in living cell studies and for in vivo applications.
Furthermore, although non-covalent adsorption between ssDNA and graphene is considered as a prior principle, we think the novel graphene-based FRET biosensors based on covalent binding or other binding forms will be one probable direction for future research. Many promising results about graphene chemistry have been produced in the past several years, which might open a new stage for the modification and functionalization of graphene with biomolecules. In our opinion, more novel designs for graphene-based FRET are highly desired for future studies. Such exploration will benefit the FRET biosensor field, and provide better ideas for the applications of graphene in biotechnology in vitro and in vivo, such as graphene-based cellular probing, diagnostics, drug delivery, and therapy.
Acknowledgments
This work was supported partially by Grant U54 ES016015-010003 from the National Institute of Environmental Health Sciences, NIH, and Grant U01 NS058161-01 from the NIH CounterACT Program through the National Institute of Neurological Disorders and Stroke. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. Part of the research described in this paper was performed using EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory (PNNL). PNNL is operated for DOE by Battelle under Contract DE-AC05-76RL01830. This work was also financially supported by the National Natural Science Foundation of China (No. 20975060, No. 21005046, No. 11079002), National Basic Research Program of China (No. 2007CB310500, No. 2011CB935700).
Contributor Information
Jinghong Li, Email: jhli@mail.tsinghua.edu.cn.
Yuehe Lin, Email: Yuehe.lin@pnl.gov.
References
- 1.Niemeyer C.M. Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science. Angew. Chem. Int. Ed. 2001;40:4128–4158. doi: 10.1002/1521-3773(20011119)40:22<4128::AID-ANIE4128>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Martin C.R., Kohli P. The emerging field of nanotube biotechnology. Nat. Rev. Drug Discov. 2003;2:29–37. doi: 10.1038/nrd988. [DOI] [PubMed] [Google Scholar]
- 3.Bechet D. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26:612–621. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Ulijn R.V., Smith A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008;37:664–675. doi: 10.1039/b609047h. [DOI] [PubMed] [Google Scholar]
- 5.Kotov N.A. Nanomaterials for neural interfaces. Adv. Mater. 2009;21:3970–4004. [Google Scholar]
- 6.Yang W.R. Carbon nanomaterials in biosensors: should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010;49:2114–2138. doi: 10.1002/anie.200903463. [DOI] [PubMed] [Google Scholar]
- 7.Geim A.K. Graphene: status and prospects. Science. 2009;324:1530–1534. doi: 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- 8.Ruoff R. Calling all chemists. Nat. Nanotechnol. 2008;3:10–11. doi: 10.1038/nnano.2007.432. [DOI] [PubMed] [Google Scholar]
- 9.Rao C.N.R. Graphene: the new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009;48:7752–7777. doi: 10.1002/anie.200901678. [DOI] [PubMed] [Google Scholar]
- 10.Novoselov K.S. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 11.Castro Neto, A.H. et al. (2009) The electronic properties of graphene. Rev. Mod. Phys. 81, 109–162 (link to the on line abstract: http://rmp.aps.org/abstract/RMP/v81/i1/p109_1)
- 12.Geim A.K., Novoselov K.S. The rise of graphene. Nat. Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 13.Chen D. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010;39:3157–3180. doi: 10.1039/b923596e. [DOI] [PubMed] [Google Scholar]
- 14.Stoller M.D. Graphene-based ultracapacitors. Nano Lett. 2008;8:3498–3502. doi: 10.1021/nl802558y. [DOI] [PubMed] [Google Scholar]
- 15.Lee C. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321:385–388. doi: 10.1126/science.1157996. [DOI] [PubMed] [Google Scholar]
- 16.Balandin A.A. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008;8:902–907. doi: 10.1021/nl0731872. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y.B. Experimental observation of the quantum Hall effect and Berry's phase in graphene. Nature. 2005;438:201–204. doi: 10.1038/nature04235. [DOI] [PubMed] [Google Scholar]
- 18.Novoselov K.S. Two-dimensional gas of massless Dirac fermions in graphene. Nature. 2005;438:197–200. doi: 10.1038/nature04233. [DOI] [PubMed] [Google Scholar]
- 19.Novoselov K.S. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10451–10453. doi: 10.1073/pnas.0502848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morozov S.V. Strong suppression of weak localization in graphene. Phys. Rev. Lett. 2006;97:016081. doi: 10.1103/PhysRevLett.97.016801. [DOI] [PubMed] [Google Scholar]
- 21.Avouris P. Carbon-based electronics. Nat. Nanotechnol. 2007;2:605–615. doi: 10.1038/nnano.2007.300. [DOI] [PubMed] [Google Scholar]
- 22.Allen M.J. Honeycomb carbon: a review of graphene. Chem. Rev. 2010;110:132–145. doi: 10.1021/cr900070d. [DOI] [PubMed] [Google Scholar]
- 23.Compton O.C., Nguyen S.T. Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. Small. 2010;6:711–723. doi: 10.1002/smll.200901934. [DOI] [PubMed] [Google Scholar]
- 24.Shao Y.Y. Graphene based electrochemical sensors and biosensors: a review. Electroanalysis. 2010;22:1027–1036. [Google Scholar]
- 25.Wang Y. Graphene oxide amplified electrogenerated chemiluminescence of quantum dots and its selective sensing for glutathione from thiol-containing compounds. Anal. Chem. 2009;81:9710–9715. doi: 10.1021/ac901935a. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009;11:889–892. [Google Scholar]
- 27.Tang L.H. Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv. Funct. Mater. 2009;19:2782–2789. [Google Scholar]
- 28.Kang X. Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009;25:901–905. doi: 10.1016/j.bios.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Stankovich S. Graphene-based composite materials. Nature. 2006;442:282–286. doi: 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano. 2010;4:1790–1798. doi: 10.1021/nn100315s. [DOI] [PubMed] [Google Scholar]
- 31.Li X.L. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science. 2008;319:1229–1232. doi: 10.1126/science.1150878. [DOI] [PubMed] [Google Scholar]
- 32.Kong B.S. Layer-by-layer assembly of graphene and gold nanoparticles by vacuum filtration and spontaneous reduction of gold ions. Chem. Commun. 2009:2174–2176. doi: 10.1039/b821920f. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H. P25-graphene composite as a high performance photocatalyst. ACS Nano. 2010;4:380–386. doi: 10.1021/nn901221k. [DOI] [PubMed] [Google Scholar]
- 34.Ramanathan T. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008;3:327–331. doi: 10.1038/nnano.2008.96. [DOI] [PubMed] [Google Scholar]
- 35.Katz E., Willner I. Integrated nanoparticle-biomolecule hybrid systems: synthesis, properties, and applications. Angew Chem. Int. Ed. 2004;43:6042–6108. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 36.Kim J. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009;38:372–390. doi: 10.1039/b709883a. [DOI] [PubMed] [Google Scholar]
- 37.Nel A.E. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 38.Biju V. Delivering quantum dots to cells: bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chem. Soc. Rev. 2010;39:3031–3056. doi: 10.1039/b926512k. [DOI] [PubMed] [Google Scholar]
- 39.Park S. Biocompatible, robust free-standing paper composed of a TWEEN/graphene composite. Adv. Mater. 2010;22:1736–1740. doi: 10.1002/adma.200903611. [DOI] [PubMed] [Google Scholar]
- 40.Mohanty N., Berry V. Graphene-based single-bacterium resolution biodevice and DNA transistor: interfacing graphene derivatives with nanoscale and microscale biocomponents. Nano Lett. 2008;8:4469–4476. doi: 10.1021/nl802412n. [DOI] [PubMed] [Google Scholar]
- 41.Patil A.J. Aqueous stabilization and self-assembly of graphene sheets into layered bio-nanocomposites using DNA. Adv. Mater. 2009;21:3159–3164. [Google Scholar]
- 42.Tang L.A.L. Graphene-based SELDI probe with ultrahigh extraction and sensitivity for DNA oligomer. J. Am. Chem. Soc. 2010;132:10976–10977. doi: 10.1021/ja104017y. [DOI] [PubMed] [Google Scholar]
- 43.Liu J.B. Noncovalent DNA decorations of graphene oxide and reduced graphene oxide toward water-soluble metal-carbon hybrid nanostructures via self-assembly. J. Mater. Chem. 2010;20:900–906. [Google Scholar]
- 44.Zhang J.L. Graphene oxide as a matrix for enzyme immobilization. Langmuir. 2010;26:6083–6085. doi: 10.1021/la904014z. [DOI] [PubMed] [Google Scholar]
- 45.Ohno Y. Electrolyte-gated graphene field-effect transistors for detecting pH protein adsorption. Nano Lett. 2009;9:3318–3322. doi: 10.1021/nl901596m. [DOI] [PubMed] [Google Scholar]
- 46.Laaksonen P. Interfacial engineering by proteins: exfoliation and functionalization of graphene by hydrophobins. Angew. Chem. Int. Ed. 2010;49:4946–4949. doi: 10.1002/anie.201001806. [DOI] [PubMed] [Google Scholar]
- 47.Liu J.B. Toward a universal “adhesive nanosheet” for the assembly of multiple nanoparticles based on a protein-induced reduction/decoration of graphene oxide. J. Am. Chem. Soc. 2010;132:7279–7281. doi: 10.1021/ja100938r. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Q.A. Fabrication of a biocompatible and conductive platform based on a single-stranded DNA/graphene nanocomposite for direct electrochemistry and electrocatalysis. Chem. Eur. J. 2010;16:8133–8139. doi: 10.1002/chem.201000684. [DOI] [PubMed] [Google Scholar]
- 49.Han T.H. Peptide/graphene hybrid assembly into core/shell nanowires. Adv. Mater. 2010;22:2060–2064. doi: 10.1002/adma.200903221. [DOI] [PubMed] [Google Scholar]
- 50.Yang Q. Fabrication of high-concentration and stable aqueous suspensions of graphene nanosheets by noncovalent functionalization with lignin and cellulose derivatives. J. Phys. Chem. C. 2010;114:3811–3816. [Google Scholar]
- 51.Selvin P.R. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Biol. 2000;7:730–734. doi: 10.1038/78948. [DOI] [PubMed] [Google Scholar]
- 52.Tang Z.W. Constraint of DNA on functionalized graphene improves its biostability and specificity. Small. 2010;6:1205–1209. doi: 10.1002/smll.201000024. [DOI] [PubMed] [Google Scholar]
- 53.Lu Y., Liu J.W. Functional DNA nanotechnology: emerging applications of DNAzymes and aptamers. Curr. Opin. Biotechnol. 2006;17:580–588. doi: 10.1016/j.copbio.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Lu C.H. A graphene platform for sensing biomolecules. Angew. Chem. Int. Ed. 2009;48:4785–4787. doi: 10.1002/anie.200901479. [DOI] [PubMed] [Google Scholar]
- 55.Jang H. A graphene-based platform for the assay by helicase. Angew. Chem. Int. Ed. 2010;49:5703–5707. doi: 10.1002/anie.201001332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He S.J. A graphene nanoprobe for rapid, sensitive, and multicolor fluorescent DNA analysis. Adv. Funct. Mater. 2010;20:453–459. [Google Scholar]
- 57.Broude N.E. Stem-loop oligonucleotides: a robust tool for molecular biology and biotechnology. Trends Biotechnol. 2002;20:249–256. doi: 10.1016/s0167-7799(02)01942-x. [DOI] [PubMed] [Google Scholar]
- 58.Dong H.F. Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal. Chem. 2010;82:5511–5517. doi: 10.1021/ac100852z. [DOI] [PubMed] [Google Scholar]
- 59.Lu C.H. Increasing the sensitivity and single-base mismatch selectivity of the molecular beacon using graphene oxide as the “nanoquencher”. Chem. Eur. J. 2010;16:4889–4894. doi: 10.1002/chem.200903071. [DOI] [PubMed] [Google Scholar]
- 60.Wen Y.Q. A graphene-based fluorescent nanoprobe for silver(I) ions detection by using graphene oxide and a silver-specific oligonucleotide. Chem. Commun. 2010;46:2596–2598. doi: 10.1039/b924832c. [DOI] [PubMed] [Google Scholar]
- 61.Chang H.X. Graphene fluorescence resonance energy transfer aptasensor for the thrombin detection. Anal. Chem. 2010;82:2341–2346. doi: 10.1021/ac9025384. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J. Am. Chem. Soc. 2010;132:9274–9276. doi: 10.1021/ja103169v. [DOI] [PubMed] [Google Scholar]
- 63.Lu C.H. Using graphene to protect DNA from cleavage during cellular delivery. Chem. Commun. 2010;46:3116–3118. doi: 10.1039/b926893f. [DOI] [PubMed] [Google Scholar]
- 64.Cohen Karni T. Graphene and nanowire transistors for cellular interfaces and electrical recording. Nano Lett. 2010;10:1098–1102. doi: 10.1021/nl1002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He Q.Y. Centimeter-long and large-scale micropatterns of reduced graphene oxide films: fabrication and sensing applications. ACS Nano. 2010;4:3201–3208. doi: 10.1021/nn100780v. [DOI] [PubMed] [Google Scholar]
- 66.Liu Z. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008;130:10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun X.M. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1:203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L.M. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010;6:537–544. doi: 10.1002/smll.200901680. [DOI] [PubMed] [Google Scholar]
- 69.Yang K. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 70.Lotya M. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009;131:3611–3620. doi: 10.1021/ja807449u. [DOI] [PubMed] [Google Scholar]
- 71.Sutter P.W. Epitaxial graphene on ruthenium. Nat. Mater. 2008;7:406–411. doi: 10.1038/nmat2166. [DOI] [PubMed] [Google Scholar]
- 72.Obraztsov A.N. Chemical vapour deposition: making graphene on a large scale. Nat. Nanotechnol. 2009;4:212–213. doi: 10.1038/nnano.2009.67. [DOI] [PubMed] [Google Scholar]
- 73.Park S., Ruoff R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009;4:217–224. doi: 10.1038/nnano.2009.58. [DOI] [PubMed] [Google Scholar]
- 74.Shao Y.Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010;20:743–748. [Google Scholar]
- 75.Zhu Y.W. Exfoliation of graphite oxide in propylene carbonate and thermal reduction of the resulting graphene oxide platelets. ACS Nano. 2010;4:1227–1233. doi: 10.1021/nn901689k. [DOI] [PubMed] [Google Scholar]
- 76.Williams G. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano. 2008;2:1487–1491. doi: 10.1021/nn800251f. [DOI] [PubMed] [Google Scholar]
- 77.Liu F. Graphene oxide arrays for detecting specific DNA hybridization by fluorescence resonance energy transfer. Biosens. Bioelectron. 2010;25:2361–2365. doi: 10.1016/j.bios.2010.02.022. [DOI] [PubMed] [Google Scholar]


