Abstract
More than thirty years have passed since canine parvovirus (CPV) emerged as a significant pathogen and it continues to pose a severe threat to world canine populations. Published information suggests that flies (Diptera) may play a role in spreading this virus; however, they have not been studied extensively and the degree of their involvement is not known. This investigation was directed toward evaluating the vector capacity of such flies and determining their potential role in the transmission and ecology of CPV. Molecular diagnostic methods were used in this cross-sectional study to detect the presence of CPV in flies trapped at thirty-eight canine facilities. The flies involved were identified as belonging to the house fly (Mucidae), flesh fly (Sarcophagidae) and blow/bottle fly (Calliphoridae) families.
A primary surveillance location (PSL) was established at a canine facility in south-central South Carolina, USA, to identify fly–virus interaction within the canine facility environment. Flies trapped at this location were pooled monthly and assayed for CPV using polymerase chain reaction (PCR) methods. These insects were found to be positive for CPV every month from February through the end of November 2011. Fly vector behavior and seasonality were documented and potential environmental risk factors were evaluated. Statistical analyses were conducted to compare the mean numbers of each of the three fly families captured, and after determining fly CPV status (positive or negative), it was determined whether there were significant relationships between numbers of flies captured, seasonal numbers of CPV cases, temperature and rainfall.
Flies were also sampled at thirty-seven additional canine facility surveillance locations (ASL) and at four non-canine animal industry locations serving as negative field controls. Canine facility risk factors were identified and evaluated. Statistical analyses were conducted on the number of CPV cases reported within the past year to determine the correlation of fly CPV status (positive or negative) for each facility, facility design (open or closed), mean number of dogs present monthly and number of flies captured. Significant differences occurred between fly CPV positive vs. negative sites with regard to their CPV case numbers, fly numbers captured, and number of dogs present. At the ASL, a statistically significant relationship was found between PCR-determined fly CPV status (positive or negative) and facility design (open vs. closed). Open-facility designs were likely to have more CPV outbreaks and more likely to have flies testing positive for CPV DNA.
Keywords: Canine parvovirus (CPV), Diptera, Canine facility, Real-time PCR assay, Vector capacity, Open vs. closed facility
1. Introduction
Canine parvovirus disease is extremely contagious, causing considerable morbidity and mortality in young canines (Prittie, 2004, Greene and Decaro, 2012) with reported mortality rates exceeding 90% for young non-immune animals (Bragg et al., 2012). An estimated one million dogs are infected annually in the United States alone, despite the widespread availability and use of vaccines (Otto et al., 2001). The virus first emerged in the United States during a nationwide outbreak in the fall of 1978 (Appel et al., 1980) and within two years the disease became established worldwide (Prittie, 2004, Greene and Decaro, 2012). The disease is presumed to be spread by oronasal exposure or fecal ingestion and is understood to occur as a result of direct contact with contaminated feces or indirect contact with virus-contaminated objects in the environment (Greene and Decaro, 2012). Enteric and myocardial forms of the disease exist but the enteric form is the most common form found in veterinary clinics, shelters, and kennels (MacLachlan and Dubovi, 2011, Greene and Decaro, 2012).
The virus is a small, non-enveloped, single-stranded DNA virus (Appel and Barr, 2009) with a tendency to form new types similar to the human influenza virus (Shackelton et al., 2005). Young, growing dogs 9–20 weeks of age are most susceptible because the virus has an affinity for rapidly dividing cells (MacLachlan and Dubovi, 2011). Factors thought to influence the severity of infection include: dog breed, age, gastrointestinal parasitism, overcrowded stressful environmental conditions, and co-infection with canine coronavirus (Smith-Carr et al., 1997, Sakulwira et al., 2003). CPV is extremely resilient and is able to survive desiccation and extreme temperature fluctuations, allowing it to persist in the environment for months and possibly years (Greene and Decaro, 2012). Two canine parvovirus types (CPV-2a and CPV-2b) were identified in 1980 and 1984 respectively (Greene and Decaro, 2012) and more recently a CPV-2c variant has evolved (Hong et al., 2007). In the United States CPV-2b is currently the most common isolate from diseased dogs, whereas in Europe CPV-2a is more prevalent (Appel and Barr, 2009).
Because no effective practical treatment exists for CPV disease except supportive therapy (Bragg et al., 2012), and because vaccination protocols do not completely protect young growing dogs (Appel and Barr, 2009), it is essential to understand how the virus survives in nature and spreads in the regional environment. In his veterinary practice, located in the southeastern United States, the senior author has noted seasonal fluctuations in the number of CPV cases, with the highest incidence rates occurring during the spring and fall. Such fluctuations could be explained by variations in the seasonality of an insect vector. Flies have been implicated as CPV vectors (Greene, 1984), which may help to explain how CPV reached pandemic proportions in only a few years. If flies are involved, it is important to know the particular fly family or fly family complex, and the vector capacity. The flies of interest are indigenous to most of the world and are referred to collectively as filth flies (White et al., 2011). They are all attracted to food, feces, animal odors, and carcasses, and have been implicated in the spread of many enteric disease agents (Greenberg, 1971); historically, they have impacted military field arenas, disaster relief efforts and refugee support operations (White et al., 2011).
The objectives of this study were to investigate the prevalence of CPV in fly populations, to identify the fly families involved, to document fly vector behavior and seasonality and to evaluate environmental and canine facility-related risk factors influencing fly activity and affecting fly numbers. Clarification and documentation of these insects’ vector capacity and potential role in transmission of CPV focused on the canine facility environment where disease incidence is traditionally high. Enzootic factors such as potential vectors, vector natural history, disease transmission, and ecology should be the same inside the canine facility environment as outside, only amplified.
2. Materials and methods
In a preliminary study that occurred in the early fall of 2010, the senior author first determined the filth fly families of interest by trapping, identifying and testing flies for CPV that were attracted to open bowls of infectious CPV positive canine feces. Three families of flies were found to test positive for CPV by real-time PCR assay. The three families of flies captured were the house fly (Mucidae), the blow/bottle fly (Calliphoridae) and the flesh fly (Sarcophagidae). To determine if the same flies could be carrying CPV in the canine facility environment, different canine facility surveillance locations were then selected. Fly trapping methods, fly identification and testing for CPV will be addressed later in this section.
2.1. Selection of primary surveillance location
From December 2010 through November 2011 fly activity was monitored at a private canine rescue/shelter facility in Aiken County, South Carolina, USA, where CPV had been known to occur frequently in the past. This facility was chosen as the primary surveillance location (PSL) because of its close proximity to a public animal control facility located only a few hundred meters away. Both facilities had a steady influx of large numbers of young susceptible dogs and open runs where flies could gain entry. At the PSL, monthly and seasonal fly population trends were recorded and fly-virus interaction was identified within the shelter environment.
The monthly and seasonal mean numbers of all flies captured were determined. In addition, fly families were identified1 and their mean numbers by month and season were determined. From December 2010 through November 2011 ambient temperature (°C) and rainfall (cm) were obtained daily from a US National Weather Service Station2 located 7.56 km north-west of the PSL to evaluate environmental risk factors. Possible relationships between the variables: number of flies captured, number of CPV cases, ambient temperature, and rainfall were determined.
2.2. Selection of additional surveillance locations
Data were also collected from thirty-seven additional canine facility surveillance locations (ASL) located in the eastern USA. Perhaps because of the stigma associated with CPV, not all canine facilities approached were willing to take part in the study. Facilities were therefore mostly selected based on physical proximity and the willingness of the facility to participate. Those facilities that were not within the state of South Carolina were selected in order to obtain some idea of the geographic extent of potential fly-borne CPV. Long-distance participants received their fly capture kits via the mail and returned captured, frozen flies using small Styrofoam coolers and refrigerant gel packs (PROPAK, Orlando, FL, USA).
ASL were chosen to determine the extent to which flies at other locations were carrying CPV and to gauge CPV disease frequency based on whether the facility was considered to be an open-air building or a completely closed structure. The mean number of dogs present monthly at each facility was also used to compare and assess differences in animal population numbers between facilities to determine if more populated facilities had more CPV outbreaks.
The majority (23) of the participating locations were from the state of South Carolina. Other facility locations included three from Florida, two each from Georgia, New York, and Rhode Island, and one each from Alabama, Connecticut, North Carolina, Virginia, and West Virginia. Additionally, there were four non-canine negative control facilities from South Carolina and Georgia, where high-density fly populations were common: one dairy and two poultry farms and a cattle-processing plant.
All facilities were evaluated for conditions that could affect the frequency of CPV disease. These conditions included: degree of fly access to areas where dogs were kept, numbers of flies captured, fly family composition, and the mean number of dogs present monthly. The number of CPV cases reported by each location within the past year was also recorded. Information on the mean monthly number of dogs and CPV case numbers were obtained from facility managers and by accessing facility records. Participants were asked to specify whether the dogs in runs and pens were exposed to the outside (open) or were kept completely indoors (closed). In this paper, the term canine facility is used to refer to a kennel or shelter where a number of dogs are housed and maintained. To respect privacy, the identities of participating facilities will not be disclosed.
2.3. Trapping methods
At the PSL, eight commercial fly traps (Black Flag Brands LLC, Joliet, IL, USA) were modified to capture flies alive, since dead flies in varying states of decomposition would make identification difficult. Two vials were placed in each trap, one containing a commercial natural fly bait (Rockwell Labs Ltd., North Kansas City, MO, USA) and the other a fly pheromone attractant (Black Flag Brands LLC, Joliet, IL, USA). The vials were covered with gauze and fastened with elastic bands to prevent fly entry. Fly traps were strategically placed at a suitable height (∼2 m). Traps were placed near drains where fly activity occurred at the outside end of each of eight inside-outside dog runs. All traps were retrieved at the end of each month and replaced with new traps. In late November 2011 a soil sample was collected by raking a 15 by 30 m fenced enclosure at the PSL where only healthy appearing dogs were occasionally kept yet flies were present and active. The soil sample obtained from this material and one obtained in the same manner from another area of the PSL where no dogs had been kept were tested for CPV by real-time PCR assay to determine if environmental contamination by CPV was present at this location.
At the ASL, traps were also modified to capture flies in the same manner as at the PSL. Participants were instructed as to the placement of traps. Between two and four traps were hung at these surveillance locations for a minimum of two to four weeks before being evaluated. At least one fly had to be trapped at each location in order for the location to be included in the study.
2.4. Sampling methods
In order to evaluate flies at all surveillance locations, fly traps were first frozen to kill live flies. At the PSL, flies were sampled by the month in which they were captured, therefore, trapped flies from eight traps were collected at the end of each month, frozen and pooled together, to constitute a single sample.
At the ASL, location was the primary basis for sampling. All flies from each ASL were collected from multiple traps. Flies frozen and pooled by location were designated as a separate sample. Flies from the PSL and ASL were counted by month (PSL) or location (ASL) and identified by fly family (Evans, 2008). Sterile forceps were used for each sample to count and identify flies and care was taken to prevent cross contamination between samples. Flies were shipped frozen to a reference laboratory3 where they were assayed by real-time PCR and evaluated for the presence of CPV DNA. PCR was used throughout this study to evaluate flies for CPV, similar to methods that have been used extensively to assay insect vectors for arboviruses of livestock and man (Johnson et al., 2008).
2.5. Molecular detection
Template DNA was extracted from 10% (w/v) suspensions of flies that were ground with a mortar and pestle in sterile water, using a commercial DNA extraction kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA). All samples of flies pooled monthly at the PSL and flies pooled from each of the ASL were tested for CPV DNA using a previously-described CPV-2 group specific TaqMan-based real-time PCR assay (Decaro et al., 2005). An automated thermocycler (Cepheid, Sunnyvale, CA, USA) was used for real-time amplification and detection of CPV-DNA target sequences. CPV DNA, to be used as positive controls in all PCR assays, was derived from cell culture and known positive fecal samples. Sterile water was extracted using the same extraction process, which then served as both a negative control and an extraction process control. These controls were used for all assays to verify test accuracy and to determine if the extraction process affected test results. Soil samples taken from the PSL were prepared and assayed using the same method described above. The molecular detection methods used in this study are rapid, sensitive, pathogen-specific and cost-effective and are easily adapted to epidemiologic studies and virus surveillance systems (Johnson et al., 2008).
2.6. Statistical analyses
All statistical analyses were performed with a software package (SPSS, 2010). Some data sets were transformed as indicated below before their analysis could be performed because they satisfied neither the equal variance assumption (Levene's test) nor the normality assumption (Wilkes-Shapiro test, normal probability plot, and box plot) involved in ANOVA procedures. In those cases, transformed data were used for the analyses, but non-transformed values of means and their corresponding confidence intervals are presented in text and tables.
2.7. Evaluation of primary surveillance location
Rank transformation was found to normalize the data (P = 0.073) as well as make error variances equal or homogeneous (P = 0.12). A two-way analysis of variance (ANOVA) procedure was conducted on the fly count data to determine if there was a significant difference (P < 0.05) in the mean counts of the three fly families, after controlling for fly CPV status (positive vs. negative).
Correlation analysis was then conducted on monthly and seasonal fly data to determine if any significant correlations occurred between number of CPV cases, number of flies trapped, ambient temperature, and rainfall. P Values of all six pairs of Pearson correlation coefficients were calculated and compared with a Bonferroni-corrected level of significance (0.05/6 ≈ 0.01) where the significance level was averaged by the number of pairs being tested. Correlation coefficients with P-values < 0.01 were considered to be significant.
2.8. Evaluation of additional surveillance locations
A two-way ANCOVA was conducted to compare the means of the number of CPV cases at fly CPV positive sites vs. negative sites and at open facility design vs. closed, controlling for the number of flies captured. The same ANCOVA procedure was also used to compare the means of the number of flies captured at fly CPV positive sites vs. negative sites and at open vs. closed facility design, controlling for dog numbers. A two-way ANOVA procedure was then used to compare the means of the number of dogs at fly CPV positive sites vs. negative sites and at open vs. closed facility designs. Transformations were conducted on data for each of the three outcome variables so as to normalize errors and homogenize error variances. The natural-log transformation normalized the errors as well as homogenized the error variances for all three outcome variables.
A two-way ANOVA procedure was also performed on the transformed data for each of the three outcome variables: number of CPV cases, number of flies captured, and number of dogs, to compare fly CPV positive vs. negative sites and open vs. closed facility designs. The means of the natural-log-transformed counts of the three fly families were compared using the two-way ANOVA procedure, after controlling for fly CPV status (positive or negative).
A two-way MANOVA procedure was then conducted to determine if there was a difference between the PSL and ASL in their mean counts of the three fly families, after controlling for fly CPV status (positive or negative). Transformations were conducted on the numbers of each of the three fly families so as to normalize errors and satisfy compound symmetry (equality) of covariance matrices for fly numbers across surveillance locations and for fly CPV status (positive or negative). Rank transformation was the only transform found to normalize errors as well as satisfy equality of the covariance matrices for fly numbers (P-value = 0.468). Chi-squared test and Fisher's exact test were conducted to check for associations between fly CPV status (positive vs. negative) and facility design (open vs. closed).
2.9. Significant canine facility and vector-related risk factor associations
The risk factors for CPV disease frequency were number of flies, canine facility design, and fly CPV status, while the risk factors for a fly positive CPV status were canine facility design and dog-numbers present in the facility. The degree of association between an outcome variable and its corresponding risk factors is referred to as the effect size. The measure of effect size for the association between the number of CPV cases and its risk factors are Eta-squared values and they were obtained from the ANOVA procedure. The measure of effect size for the association between fly CPV positive result and its risk factors are odds ratios and they were obtained by conducting a logistic regression procedure.
3. Results
At the PSL, laboratory analyses of flies captured monthly in traps from December 2010 through November 2011 demonstrated that these insects were positive for canine parvoviral DNA beginning in February 2011 and for each subsequent month for the remainder of the year. Increased disease incidence appeared to occur mainly in spring and early summer when these flies were most numerous and active, and again in fall when flies tended to congregate in large numbers (Fig. 1 ). Although flies were positive for virus for most of the year and were more active in spring, early summer and late fall, the monthly and seasonal fly captures did not correlate with monthly and seasonal disease incidence (P = 0.232 for monthly and P = 0.988 for seasonal incidence). Ambient temperature (r = 0.696, P = 0.012), but not rainfall (r = 0.035, P = 0.913), was found to be correlated with the number of flies captured monthly. As ambient temperatures exceeded (27 °C) in mid-summer, fly captures declined dramatically and this downward trend continued into fall as temperatures steadily decreased. The soil sample collected from an area where only healthy appearing dogs were occasionally kept and flies were active, tested positive for virus. The sample taken from an area where no fly activity occurred and no dogs were kept, tested negative.
Fig. 1.
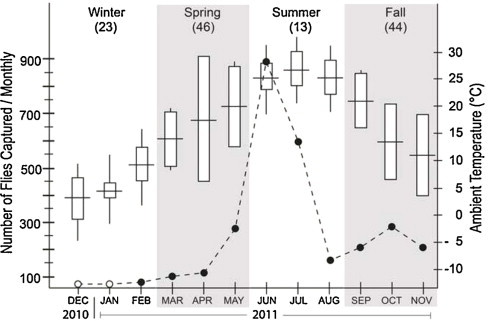
Fly vector surveillance at the primary study location. Fly capture rates (number of Flies Captured/Monthly, 2010–2011) are compared with ambient temperature. Values in parentheses represent the number of CPV cases by season. Spring and fall months, when disease incidence is typically high, are shaded. Fly CPV-negative (PCR) results are represented by open circles and fly CPV-positive (PCR) results are represented by solid circles. Horizontal lines represent mean monthly ambient temperatures; rectangles represent two standard errors above and below the means; vertical lines represent the range. Data were taken from Edgefield Veterinary Clinic medical records, Edgefield, SC, 1996–2011 (126 cases).
The prevalence of CPV in different canine facility fly populations, by state, (Fig. 2 ) is expressed as the positive site rate (PSR): PSR = ∑(PS)/∑(LS) × 100 where the sum of the positive sites is ∑(PS) and the sum of the locations sampled is ∑(LS). The PSR for different states was found to be: SC = 17/22 (77%), GA = 3/3 (100%), AL = 1/1 (100%), CT = 1/1 (100%), NC and RI = 2/2 (100%), and FL = 1/3 (33%). Flies at two locations in NY and one location in VA and WV were found to be negative. None of the flies from any of the four non-canine, negative control locations tested positive for virus. Three additional closed-design facilities in SC, PA, and MA did not meet the study inclusion criteria as there were no flies inside the facilities and no flies were caught in traps.
Fig. 2.
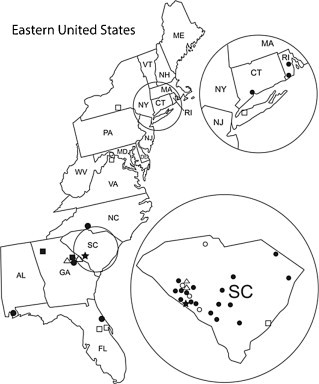
Geographic location of the primary study location (star) and of 26 positive and 11 additional negative canine facility surveillance locations. Positive sites are black. Negative sites are open. Circles represent open canine facility designs. Squares represent closed canine facility designs. Open triangles represent 4 non-canine, livestock and poultry facility negative field controls.
At the ASL, 70% (n = 26) of the 37 facilities not including the four negative field controls, were positive for CPV. Ninety-two percent of all positive sites were open-design facilities, but only 22% of nine closed-design facilities were positive (Table 1 ). Statistically significant associations (Chi-square and Fisher's exact test; P-values < 0.001 and 0.002 respectively) were found between fly CPV status (positive vs. negative) and facility design (open vs. closed). The odds ratio of a positive fly CPV status for open-facility designs vs. closed-facility designs was estimated to be 21 with a 95% asymptotic confidence interval of (3.16–139.67). Thus the probability of flies testing positive in open-facility designs were estimated to be 21 times higher than at closed-facility designs.
Table 1.
A cross-tabulation of descriptive statistics and confidence intervals for dog numbers, fly numbers and CPV case numbers by fly CPV status and facility design for additional surveillance locations.
| No. canine facility surveillance locations = 37 | No. non-canine negative field control locations = 4 | ||||||
|---|---|---|---|---|---|---|---|
| (PCR) positive sites = 26; 70% | |||||||
| Facility design | N | Total no. dogs present monthly | Mean no. dogs present monthly (95% CI) | Total no. flies | Mean no. flies (95% CI) | Total no. CPV cases | Mean no. CPV cases (95% CI) |
| Open | 24 | 3953 | 165 (72.3–257.1) | 5396 | 225 (73.7–375.9) | 903 | 38 (9.2–66.1) |
| Closed | 2 | 550 | 275 (SD = 0) | 175 | 88 (SD = 0) | 140 | 70 (SD = 0) |
| (PCR) negative sites = 11; 30% | |||||||
| Open | 4 | 85 | 21 (4.9–37.7) | 247 | 62 (14.9–108.5) | 0 | 0 (0) |
| Closed | 7 | 810 | 116 (0–276.3) | 633 | 90 (0–242.7) | 44 | 6 (1.2–11.3) |
The mean number of dogs present monthly at the ASL was significantly greater (P = 0.008) at fly CPV positive sites ( 95% CI = 87.6–258.8) than at fly CPV negative sites ( 95% CI = 0–177.3). The number of CPV cases ( 95% CI = 13.8–66.4) at fly CPV positive sites was also significantly greater (P < 0.001) than at fly CPV negative sites ( 95% CI = 0.5–7.6), and the mean number of all flies was also found to be significantly greater (P = 0.043) at fly CPV positive sites ( 95% CI = 74.8–353.7) than at fly CPV negative sites ( 95% CI = 0–166.9). The mean number of CPV cases was significantly greater (P = 0.009) at open-design facilities ( 95% CI = 7.6–56.9) than at closed-design facilities ( 95% CI = 0–42.3).
At the PSL, there was no significant difference between the mean counts of the three fly families (P = 0.902), even after controlling for fly CPV status (positive or negative). However, at the ASL, a significant difference did occur among the mean counts of the three fly families (P = 0.018), even after controlling for fly CPV status (positive or negative). Therefore, post hoc tests were conducted on ASL data to check for pairwise differences in the mean counts of the three fly families. No significant difference was found between the mean number of blow/bottle flies (Calliphoridae) and flesh flies (Sarcophagidae) (P = 0.428 for Bonferroni and 0.434 for Tukey tests) or between flesh flies (Sarcophagidae) and house flies (Mucidae) (P = 0. 135 for Bonferroni and 0.139 for Tukey tests). The mean number of blow/bottle flies (Calliphoridae) ( 95% CI = 24.8–225.9), however, was significantly greater (P = 0.005 for Bonferroni and 0.006 for Tukey tests) than that of house flies (Mucidae) ( 95% CI = 19.4–44).
The two-way MANOVA procedure for comparing PSL and ASL revealed no significant differences between PSL and ASL in the mean numbers of the three fly families (P = 0.7 for both Wilk's lambda and Pillai's trace), even after controlling for fly CPV status (positive or negative). The mean number of CPV-positive flies was significantly greater (P < 0.001 for both Wilk's lambda and Pillai's trace) than that of CPV-negative flies regardless of the surveillance location. Thus, fly family numbers varied little across all locations, but fly numbers tended to be greater at CPV-positive facilities. The means, standard deviations, and 95% confidence intervals for each of the three fly families, averaging values across all surveillance locations, are presented in Table 2 .
Table 2.
Descriptive statistics and 95% confidence intervals for the mean numbers of each fly family captured at CPV positive and negative surveillance locations. Data was averaged across all surveillance locations.
| Fly family | Fly/CPV positive sites |
Fly/CPV negative sites |
||||
|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | |
| Blow/bottle fly (Calliphoridae) | 123.89 | 299.36 | (22.6–225.18) | 47.08 | 116.72 | (0–111.61) |
| House fly (Mucidae) | 31.78 | 33.27 | (20.52–43.03) | 5.54 | 6.13 | (1.83–9.24) |
| Flesh fly (Sarcophagidae) | 31.78 | 33.27 | (20.52–43.03) | 16.77 | 22.29 | (3.3–30.24) |
The effect sizes (Eta-squared values and odds ratios) for important canine facility and vector-related risk factor associations are shown in Table 3 .
Table 3.
Significant canine facility and vector-related risk factor associations determined from analysis of data from 37 canine facility surveillance locations.
| Outcome variable | Risk factor (predictor variable) | Eta-squareda | Odds ratiob | Confidence interval for odds ratio | Risk factor designation |
|---|---|---|---|---|---|
| No. of CPV cases (continuous data) | Fly numbers (continuous) | 0.021 | N/A | N/A | Vector |
| Canine facility design (binary) | 0.036 | Vector | |||
| Fly CPV status (binary) | 0.139 | Vector | |||
| Fly CPV status (binary) | Canine facility design (binary) | N/A | 19.7 | (2.7–136.01) | Canine facility |
| Fly numbers (continuous) | 1.004 | (0.996–1.012) | Canine facility | ||
The Eta-squared values are a measure of the association or effect size and are interpreted as follows: 2.1% of the variation in the number of CPV cases that can be explained by the variation in the number of flies captured, 3.6% of the variation in the number of CPV cases that can be explained by the variation in the canine facility design, and 13.9% of the variation in the number of CPV cases that can be explained by the variation in the fly CPV result.
The odds ratio values are interpreted as the odds of a fly CPV positive result at an open facility design. The odds of a fly testing positive for CPV at an open facility design is 19.17 times greater than a fly testing positive at a closed facility design. For every additional fly captured, the odds of a fly CPV positive result at an open facility design increases by 0.004 or 0.4%.
4. Discussion
To our knowledge, this represents the first epidemiological field investigation using molecular diagnostic methods to identify the presence of CPV in flies and to evaluate these insects as potential arthropod vectors of CPV disease. These insects could potentially play a significant role in the natural history and ecology of this disease, helping to explain at least some of its ubiquitous and persistent presence in many canine facilities. The animal odors and abundant food supply found in these environments make them especially attractive to these insects. Dissemination of CPV by flies would certainly increase opportunities for both dispersal and survival of the virus, since flies have been shown to fly 8 km/h (Murvosh and Thaggard, 1966) and up to 20 km in 24 h (Hogsette and Farkas, 2000). In the canine shelter environment, mere physical separation of infected and healthy canines would not prevent this disease from spreading if flies can move between infected and non-infected animal quarters. Thus, if flies can be shown to actually transmit CPV, young susceptible dogs housed outside in the presence of flies would have an increased risk of contracting this disease.
The stability and ability of CPV to persist in the environment is an important factor responsible for both its success as a pathogen and for its survival in nature (MacLachlan and Dubovi, 2011). As with other fly-borne diseases, flies feeding on infectious fecal material may carry virus in their saliva, feces and on body surfaces (White et al., 2011). These insects could thus be very effective at moving significant amounts of virus in the course of their diurnal activities. Their feeding and elimination behavior, whereby they regurgitate and defecate each time they land (Jacobs, 2007), would make them ideal transmitters of this disease. Flies could also transport and indirectly transfer virus to other flies congregating and feeding on the same substrate. In this way, they could potentially carry this virus a considerable distance in search of food and ovipositional sites (Jacobs, 2007).
One explanation for the lack of correlation between disease frequency and fly numbers is that disease transmission is probably not direct. Most likely, host exposure is indirect via viral contamination of the host's environment by these insects. This indirect mode of transmission, combined with the virus's ability to persist in the environment, would make it difficult to determine how a particular host exposure occurred. A soil sample testing positive for virus was collected in early winter at the primary location, from an area where flies were still active but only healthy appearing dogs were kept. Thus shelter contamination by flies could occur and flies could contaminate the environment by spreading virus at this time of year, resulting in a source of infection that could link fall outbreaks to those of early spring. A statistically significant positive correlation between ambient temperature and fly numbers suggests that warm weather conditions could be a precursor to larger fly populations which could possibly indirectly influence the incidence of CPV disease. CPV disease has been reported by Houston et al. (1996) to show a distinct seasonality with a higher incidence occurring between July and September. Disease frequency itself probably does not have a distinct seasonal pattern but may be affected by fly numbers which, in turn, could be influenced by variations in climatic conditions. Thus, environmental factors have been shown (MacLachlan and Dubovi, 2011) to affect disease occurrence, especially in those diseases transmitted by arthropods. Therefore, in order to understand the epidemiology of this disease it, may be helpful to understand the ecology of these potential insect vectors.
At the current study's PSL, flies were present all year, but numbers decreased substantially in the winter, probably due to the cold. The dramatic decreases in fly capture rates in mid-summer and the tendency of these insects to congregate in late fall were probably due to senescent, end of lifecycle-related behaviors, which were most likely influenced by cooling environmental temperatures and shortening photoperiods. In the spring and early summer, it was evident that flies were more active and more numerous. Corresponding seasonal disease incidence was also found to be greatest at this time of year. Disease frequency was noted to increase again in the fall when these insects were observed to congregate or swarm in large numbers at canine and other animal facilities. Regional differences in fly family distribution do occur and are probably due to a multitude of factors including, but not limited to, environmental temperature, preferred food availability, and ovipositional site preference (Jacobs, 2007).
CPV disease appears to be very common in the shelter/kennel environment and some spillover into other local canine populations is likely to occur. Greater than a billion viral particles can potentially occur in a single gram of infectious canine feces (Sherding, 2000). Considering this and knowing the behavior of these insects, it is easy to understand how a large-scale shelter or kennel could serve as a significant reservoir of infection. Random, isolated outbreaks at smaller shelters and kennels could possibly be attributable to fly food resources attracting flies carrying virus which then expose susceptible dogs through regurgitation of virus during feeding and transfer of virus to objects in the host's immediate environment. Flies are also known to be an important vector of coccidia (Cystoisospora spp.) and to spread other enteric pathogens (Lappin and Spindel, 2009, White et al., 2011). Crowded, stressed environments especially favor synergistic infections of CPV and canine coronavirus along with other endoparasites (Appel and Barr, 2009). Infections with these enteric organisms may also affect individual immunity and could predispose young dogs to CPV disease (Smith-Carr et al., 1997, Sakulwira et al., 2003).
This study underscores the importance of facility design as an important first line of defense in preventing potential fly-borne CPV disease and, hypothetically, protecting young, susceptible canines from CPV. A closed-design facility that eliminates, or at least significantly limits, fly-ingress and that allows for efficient disposal of animal waste would be an effective method of controlling and minimizing the spread of CPV disease if, as suggested by this study, these insects might play a role in disease transmission. It is only logical that open-design facilities would create more opportunities for fly-borne disease contagion than closed designs where flies have only limited or no access. A reasonable requirement would be to have at least a fully enclosed structure for housing young, particularly susceptible dogs. It is possible that closed designs, by having fewer flies and less fly-borne disease, could potentially reduce the expense of CPV-related medical treatments and also decrease the need for CPV disease-related euthanasia. Indoor quarantine facilities, separate from the main shelter area, would also be important and could prevent already-infected animals from introducing disease to healthy individuals. The implementation of aggressive fly control, vigilant disease surveillance, vaccination, and routine sanitation using parvocidal disinfectants are also important additional measures (Greene and Decaro, 2012). Common-sense approaches such as keeping doors and windows closed and employing screening and externally-directed fans at doors could also diminish fly entry. Covering trash receptacles, positioning them far away from entrances and areas where dogs are kenneled, and placing fly traps near waste drains could also help. The use of fly traps can be beneficial as they minimize insecticide use and decrease the development of insecticide resistance. Granular insecticide baits can be used outside at bait stations in locations where animals are denied access.4
Additional studies evaluating the risk factors discussed here are still needed, however, to confirm these findings. Of particular importance is the need to conduct a conclusive confirmatory disease transmission study in which flies are exposed to a CPV-positive bait source and then are introduced to non-immune young dogs under carefully controlled laboratory conditions.
To summarize, potential CPV-related risk factors include: inadequate fly control, fly accessible open facilities, warm weather, crowded and unsanitary conditions, increased fly vector numbers in the spring and early summer and the tendency of these insects to congregate at canine and other animal facilities in late fall. Fly-borne disease targets congregations of animals whether they be livestock, dogs or man. Specific guidelines and more effective methods of fly control need to be developed for controlling these insects in the canine facility environment.
5. Conclusion
Prior to this study, no information that defined factors influencing the spread of CPV existed, aside from dog-to-dog or indirect contact with virus-contaminated objects in the environment. This investigation has now shown that flies may play a significant role in CPV disease transmission. Hypothetically, this information can now be used to develop and implement more effective disease control measures that will benefit veterinarians, canine shelters, rescue organizations, and private kennels. One limitation of the present study was that, although the molecular data was obtained using quantitative techniques, the study itself was designed to be primarily qualitative. PCR testing of pooled fly samples by month (PSL) or location (ASL) resulted in limitations in that a lower sensitivity of CPV DNA detection probably existed when fly numbers were lower. To further understand the disease transmission capacity of these insects and to more completely understand the intricacies of fly-virus interaction as well as the dynamics of disease transmission, expanded quantitative studies are also needed to evaluate the CPV types involved, the number of viral particles a fly can carry, the length of time a fly is infective and where the virus resides in these insects.
Acknowledgments
This study was funded by the Edgefield Veterinary Clinic, Edgefield, SC, USA, and Allen Isdell, AE, Augusta, GA, USA. The authors thank Diane M. Peterson for invaluable technical assistance and Jerome A. Hogsette, Jr., of the USDA-ARS Center for Medical, Agricultural and Veterinary Entomology, Gainesville, FL, USA, for providing a critical reading of the manuscript. Manuscript preparation was supported, in part, by the U.S. Department of Energy under award number DE-FC09-07SR 22506 to the University of Georgia Research Foundation.
Footnotes
Allen E. Isdell, AE, Field Entomologist, 2605 Peach Orchard Road, Augusta, GA 30906, USA.
US National Weather Service Automated Weather Observation System (AWOS-3). Environmental data monitored at Aiken Municipal Airport, 129 Aviation Boulevard, Aiken, SC, USA (lat. 333906N; long. 08140.92W).
College of Veterinary Medicine, Athens Veterinary Diagnostic Laboratory, The University of Georgia, Athens, GA, USA.
Dr. Jerome A. Hogsette, Jr., USDA-ARS Center for Medical, Agricultural and Veterinary Entomology, Gainesville, FL, USA.
References
- Appel L.D., Barr S.C. Infectious Disease Management in Animal Shelters. 1st ed. Wiley-Blackwell; Ames, IA: 2009. Canine parvovirus and coronavirus; pp. 197–208. [Google Scholar]
- Appel M.G., Meunier P.C., Pollock R.H., Greisen H. Canine viral enteritis. Canine Pract. 1980;7:22–36. [Google Scholar]
- Bragg R.F., Duffy A.L., DeCecco F.A., Chung D.K., Green M.T., Veir J.K., Dow S.W. Clinical evaluation of a single dose of immune plasma for treatment of canine parvovirus infection. J. Am. Vet. Med. Assoc. 2012;240:700–704. doi: 10.2460/javma.240.6.700. [DOI] [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Desario C., Campolo M., Trani L.D., Tarsitano E., Tempesta M., Buonavoglia C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 in the feces of dogs. Vet. Microbiol. 2005;105:19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Evans A.E. 1st ed. Sterling Publishing Co., Inc.; New York, NY: 2008. Flies: Order Diptera. Field Guide to Insects and Spiders of North America; pp. 250–252. [Google Scholar]
- Greenberg B. vol. I. Princeton Univ. Press; Princeton, NJ: 1971. p. 856. (Flies and Disease). [Google Scholar]
- Greene C.E. W.B. Saunders Co.; Philadelphia, PA: 1984. Canine Viral Enteritis. Clinical Microbiology and infectious Diseases of the Dog and Cat; p. 438. [Google Scholar]
- Greene C.E., Decaro N. 4th ed. Saunders Elsevier; St. Louis, MO: 2012. Canine Viral Enteritis. Infectious Diseases of the Dog and Cat; pp. 67–76. [Google Scholar]
- Hogsette J.A., Farkas R. Contributions to a Manual of Palearctic Diptera, General and Applied Dipterology. 2000. Secretophagous and hematophagous higher Diptera; pp. 769–792. [Google Scholar]
- Hong C., Decaro N., Desario C., Tanner P., Pardo M.C., Sanchez S., Buonavoglia C., Saliki J.T. Occurrence of canine parvovirus type 2c in the United States. J. Vet. Diag. Invest. 2007;19:535–539. doi: 10.1177/104063870701900512. [DOI] [PubMed] [Google Scholar]
- Houston D.M., Ribble C.S., Head L.L. Risk factors associated with parvovirus enteritis in dogs: 283 cases (1982–1991) J. Am. Vet. Med. Assoc. 1996;208:542–546. [PubMed] [Google Scholar]
- Jacobs S.B. Pennsylvania State University, Department of Entomology; University Park, PA: 2007. Entomological notes: House flies. Revised. Cooperative extension works. Available at: http://ento.psu.edu/extension/factsheets/house-flies (accessed 12.05.2012) [Google Scholar]
- Johnson N., Voller K., Phillips L.P., Mansfield K., Fooks A. Rapid molecular detection methods for arboviruses of livestock of importance to northern Europe. Prev. Vet. Med. 2008;87:4–20. doi: 10.1155/2012/719402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin M.R., Spindel M. Infectious Disease Management in Animal Shelters. 1st ed. Wiley-Blackwell; Ames, IA: 2009. Bacterial and protozoal gastrointestinal disease; pp. 223–240. [Google Scholar]
- MacLachlan N.J., Dubovi E.J. 4th ed. Elsevier Inc.; Boston, MA: 2011. Fenner's Veterinary Virology; pp. 230–232. [Google Scholar]
- Murvosh E.M., Thaggard C.W. vol. 59. Annals of the Entomological Society of America; Annapolis, MD: 1966. pp. 533–547. (Ecological Studies of the House Fly). [DOI] [PubMed] [Google Scholar]
- Otto C.M., Jackson C.B., Rogell E.J., Prior R.B., Ammons W.S. Recombinant bactericidal/permeability-increasing protein (rBPI21) for treatment of parvovirus enteritis: a randomized, double-blinded, placebo-controlled trial. J. Vet. Intern. Med. 2001;15:355–360. [PubMed] [Google Scholar]
- Prittie J. Canine parvoviral enteritis: a review of diagnosis, management, and prevention. J. Vet. Emerg. Crit. Care. 2004;14:167–176. [Google Scholar]
- Sakulwira K., Vanapongtipagorn P., Theamboonlers A., Oraveerakul K., Poovorawan Y. Prevalence of canine coronavirus and parvovirus infections in dogs with gastroenteritis in Thailand. Vet. Med.-Czech. 2003;48:163–167. [Google Scholar]
- Shackelton L., Parish C., Truyen V., Holmes E. High rate of viral evolution associated with the emergence of a carnivore parvovirus. Proc. Natl. Acad. Sci. U.S.A. 2005;102:379–384. doi: 10.1073/pnas.0406765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherding R.G. Saunders Manual of Small Animal Practice. 2nd ed. WB Saunders Co.; Philadelphia, PA: 2000. Intestinal viruses; pp. 110–116. [Google Scholar]
- Smith-Carr S., Macintire D.K., Swango L.J. Canine parvovirus. Part I. Pathogenesis and vaccination. Compendium. 1997;19:125–133. [Google Scholar]
- SPSS . IBM Corp.; Armonk, NY: 2010. Statistics Version 19.0. [Google Scholar]
- White G.B., Rosales A.L., Evans E.S., Hogsette J.A. Armed Forces Pest Management Board, Defense Pest Management Information Analysis Center, Forest Glen Section, Walter Reed Army Medical Center; Washington, DC: 2011. Filth Flies: Significance, Surveillance and Control in Contingency Operations. Technical Guide No. 30; pp. 1–54. [Google Scholar]


