Abstract
Respiratory viral infections (RVIs) can be associated with a wide range of clinical manifestations ranging from self-limited upper respiratory tract infections to more devastating conditions, such as pneumonia. RVIs constitute the most frequent reason for medical consultations in the world and they have a considerable impact on quality of life and productivity. Therefore, the prevention and control of RVIs remain major clinical goals. Currently, there are approximately 200 known respiratory viruses that can be grouped into one family of DNA viruses (Adenoviridae) and four families of RNA viruses (Orthomyxoviridae, Paramyxoviridae, Picornaviridae and Coronaviridae). In this paper, we review the major respiratory viruses that cause disesases in humans, with an emphasis on current treatment options.
Keywords: Viral infections, Epidemic influenza, Respiratory viruses, Antivirals
0. Introduction
Respiratory viral infections (RVIs) are the most frequent reason for medical consultations in the world (Anzueto and Niederman, 2003). They can be associated with a wide range of clinical manifestations from self-limited infections of the upper respiratory tract to more devastating conditions affecting the lower respiratory tract, such as pneumonia. In addition, RVIs have a considerable impact on quality of life and productivity of the society. For example, in the United States alone, these infections are responsible for 20 million absences from work and 22 million absences from school every year (Brooks et al., 2004). The annual burden of epidemic influenza alone averages millions of physicians visits, 150,000 hospitalizations, and 36,000 deaths (CDC, 2005). In the United States, the costs of non-influenza-related RVIs may reach 40 billion dollars annually (Fenderick et al., 2003). Each year, consumers spend 2–3 billion dollars on a myriad of products marketed to treat cold symptoms, such as antihistamine, analgesic, antitussive and anti-inflammatory agents (Temte, 2000).
Following the considerable research progress on respiratory viruses in the 60 s, species of rhinoviruses, coronaviruses, enteroviruses, adenoviruses, parainfluenza viruses and respiratory syncycial viruses were added to influenza viruses as causes of respiratory tract infections. Recently identified respiratory viruses such as human metapneumovirus (Van den Hoogen et al., 2001, Boivin et al., 2002a); human parechovirus-3 (Ito et al., 2004, Boivin et al., 2005), and new human coronaviruses (HCoVs), such as the one associated with severe acute respiratory syndrome (SARS) (Ksiazek et al., 2003) as well as HCoV-NL (Fouchier et al., 2004) and HCoV-HKU1 (Woo et al., 2005a, Woo et al., 2005b) are important additions to this list. Currently, there are approximately 200 known viruses that typically produce respiratory syndromes. They are grouped into five viral families including one family of DNA viruses (Adenoviridae) and four families of RNA viruses (Orthomyxoviridae, Paramyxoviridae, Picornaviridae and Coronaviridae) (Table 1 ). The purpose of this paper is to review the major respiratory viruses that cause diseases in humans, with an emphasis on current therapeutic options.
Table 1.
Classification of viruses that cause respiratory tract infections in humans
| DNA viruses | RNA viruses |
|---|---|
| Adenoviridae | Orthomyxoviridae |
| Adenovirus 1–51 | Influenza A, B and C viruses |
| Paramyxoviridae | |
| Respiratory syncytial virus | |
| Parainfluenza viruses 1–4 | |
| Metapneumovirus | |
| Picornaviridae | |
| Rhinoviruses 1–102 | |
| Enteroviruses (echovirus 1–34, coxsackievirus A1–A24, B1–B6) | |
| Parechoviruses (1–3) | |
| Coronaviridae | |
| 229E, OC43, SARS-CoV, NL, HK-U1 |
1. Orthomyxoviridae
Orthomyxoviruses are enveloped negative-sense RNA viruses containing 7 or 8 RNA segments which code for 9 or 11 proteins (Hayden and Palese, 2002). The RNA is protected by closely associated nucleoprotein forming a helical structure called the nucleocapsid (NC). Based on the NC and matrix (M1) antigens, influenza viruses are classified into three genera: influenza A, B, and C viruses. Influenza viruses contain two important surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), which are responsible for virus attachment to host-cell receptors and virion release from infected cells, respectively. Antigenic differences in the HA and NA proteins provide further classification of influenza A viruses into 16 HA and 9 NA subtypes. All of these subtypes have been identified in birds whereas some are known to infect pigs, horses and whales (Hayden and Palese, 2002). During the past century, only 3 HA (H1, H2, H3) and 2 NA (N1, N2) subtypes have efficiently infected humans.
Influenza viruses are spread by respiratory droplets resulting in a spectrum of clinical responses ranging from mildly symptomatic infection to a primary viral pneumonia rapidly progressing to death (Lamb and Webster, 2001). Influenza A viruses are usually associated with a more severe outcome than B or C viruses. A typical influenza A syndrome consists of a tracheobronchitis with the additional involvement of small airways (Lamb and Webster, 2001). The incubation period averages 2 days, whereas viral shedding in respiratory tract secretions generally lasts 3–6 days in immunocompetent adults. The onset of illness is usually abrupt with the occurrence of headache, chills, and dry cough followed by high fever, significant myalgias, malaise, and anorexia. More usual and benign complications of influenza include sinusitis, otitis media and bronchitis. More severe complications such as viral and/or bacterial pneumonia, as well as exacerbation of underlying illnesses, may also occur particularly in young children, the elderly, and subjects with cardiopulmonary diseases or immunosuppression (Wright and Webster, 2001).
Although annual immunization programs using the inactivated trivalent vaccine remain the most important means of reducing influenza-related morbidity and mortality, there are certain conditions where antiviral agents are warranted: (1) for the treatment and/or prevention of disease in individuals who are not vaccinated or do not mount an adequate immune response to the vaccine; (2) in years where there is a mismatch between the circulating and the vaccine viral strains; (3) in the advent of a new pandemic strain pending availability of an adequate vaccine.
1.1. Treatment of influenza infections
1.1.1. Adamantanes
1.1.1.1. Mechanism of action
Amantadine (1-adamantamine hydrochloride; Symmetrel) and its analogue, rimantadine (α-methyl-1-adamantanemethylamine; Flumadine), constitute the first class of antivirals licensed for influenza infections. These agents inhibit an early step in the influenza A virus replication cycle by interfering with the function of the viral M2 protein (Fig. 1 ). The latter acts as an ion channel through which the hydrogen ions pass into the interstices of the viral particle, resulting in the dissociation of the matrix protein (M1) from the ribonucleoprotein (RNP) complex. The RNP can then enter the cell nucleus and initiate replication (Martin and Helenius, 1991). Both amantadine and rimantadine are effective at low doses against various subtypes of influenza A viruses whereas they have no activity against influenza B viruses. In cell culture experiments, the concentration of amantadine that inhibits influenza A virus plaque formation by 50% (IC50) varies from 0.1 to 0.4 μg/ml (Hayden and Palese, 2002).
Fig. 1.
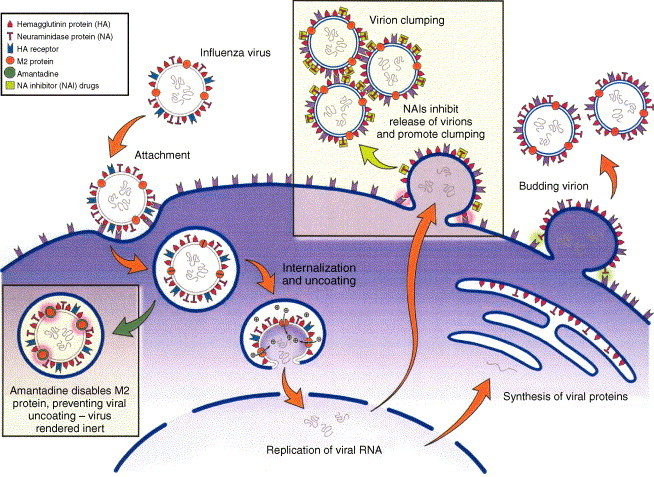
Mechanisms of action of anti-influenza compounds. The replicative cycle of influenza virus (attachment, internalization, replication and exit from the host respiratory cell) is illustrated. Amantadine blocks viral internalization and uncoating. Neuraminidase inhibitors prevent the neuraminidase from releasing budding viruses and dispersing virions (adapted from Stiver (2003) with permission).
1.1.1.2. Pharmacokinetics
Amantadine and rimantadine are administered orally as tablets or syrup at doses of 5 mg/kg of body weight for children and up to 200 mg/day for adults (Moscona, 2005). These drugs are absorbed rapidly and nearly completely with peak plasma concentrations reached within 2 h for amantadine and 4 h for rimantadine (Couch, 2000). Amantadine and rimantadine are mostly metabolized and highly excreted in the urine, with amantadine half-life of about 15 and 30 h in young adults and elderly subjects, respectively, whereas rimantadine half-life averages 30 h in both groups (Couch, 2000).
1.1.1.3. Clinical efficacy
Both amantadine and rimantadine are effective for the prevention of influenza A infection and illness (Hayden and Palese, 2002). When used prophylactically, either drug can prevent about 50% of infections and 70–90% of illnesses (Couch, 2000). Demicheli et al. (2000) have estimated that the average effectiveness of amantadine and rimantadine for the prevention of confirmed influenza illness was 61% and 72%, respectively. The therapeutic use of these drugs has also been associated with a reduction in the duration of symptoms by approximately 1 day when administered within 48 h of onset of symptoms (Couch, 2000). However, these drugs have not been shown to prevent complications associated with influenza infections (Monto, 2003, Bridges et al., 2003).
1.1.1.4. Side effects
Amantadine causes central nervous system (CNS) symptoms such as anxiety, depression, and insomnia in about 10% of subjects, whereas CNS symptoms have been reported in only 2% of individuals treated with rimantadine (Couch, 2000). Besides CNS symptoms, both amantadine and rimantadine can cause transient gastrointestinal symptoms, including nausea and vomiting (Couch, 2000).
1.1.1.5. Resistance to adamantanes
Despite the fact that naturally occurring amantadine-resistant influenza A viruses are rarely found, rates of resistance exceeding 30% and up to 80% have been reported after only a few days of therapy (Klimov et al., 1995, Shiraishi et al., 2003, Hayden et al., 1991). Molecular characterization of adamantane-resistant influenza A variants has revealed that a single substitution at one of five codons (26, 27, 30, 31, and 34) in the transmembrane region of the M2 protein may confer drug resistance (Klimov et al., 1995, Masuda et al., 2000, Boivin et al., 2002b, Saito et al., 2003). A recent report showed that H3N2-infected individuals shed more amantadine-resistant variants than their H1N1-infected counterparts (Saito et al., 2003). Of note, the current avian influenza H5N1 virus (Z strain), which emerged in Southeast Asia, is resistant to amantadine (Li et al., 2004). This concern is further enhanced by the fact that adamantane-resistant mutant viruses were found to maintain good replicative capacities in vitro and were at least as virulent as wild-type (sensitive) viruses in animal models (Sweet et al., 1991, Abed et al., 2005). Furthermore, adamantane-resistant influenza A viruses are transmissible in humans (Hayden et al., 1989).
1.1.2. Neuraminidase inhibitors (NAIs)
1.1.2.1. Mechanism of action
The NAIs target the active site of the NA enzyme, which is highly conserved in all influenza A and B viruses. NAIs inhibit the cleavage of the terminal sialic acid residues attached to viral glycoproteins and cellular glycolipids (Fig. 1), a process necessary for release of influenza virions from host cells and for spread of the virus throughout respiratory mucus (Palese and Compans, 1976). Zanamivir and oseltamir have excellent in vitro activity against all nine NA subtypes of influenza A and against the NA of influenza B viruses (Mendel and Roberts, 1998). Notably, these drugs are effective against avian viruses of the H5N1 subtype, although higher doses and prolonged treatment with oseltamivir were required to achieve inhibition of such viruses in mice (Yen et al., 2005). The zanamivir IC50 values for clinical isolates vary widely using a plaque reduction assay (PRA) in Madin Darby canine kidney (MDCK) cells (2–16,000 nM), but fall in a much narrower range (0.64–7.9 nM) by directly testing the inhibition of NA activity using an enzymatic assay (Whetherall et al., 2003). Zanamivir exhibits better in vitro antiviral activity against influenza B viruses, whereas oseltamivir has lower IC50 values against influenza A isolates (Boivin and Goyette, 2002, Whetherall et al., 2003).
1.1.2.2. Pharmacokinetics
Zanamivir (Relenza) is commercially available as a drug powder for inhaled oral administration (Diskhaler). About 80% of a dose of zanamivir is deposited in the oropharynx, whereas 10–20% reaches the lungs (Cass et al., 1999a). Ten to 20% of the inhaled dose is bioavailable; however, the concentration of the drug in the respiratory tract is estimated to be >1000 times that of the IC50 value of influenza A and B viruses (Peng et al., 2000). Absorbed drug is excreted in the urine without metabolic changes (Cass et al., 1999b). Oseltamivir (Tamiflu) is available for oral administration (capsules or liquid suspension) as an ethyl ester prodrug which is converted to its active form (oseltamivir carboxylate) after ester hydrolysis in the gut (Kim et al., 1997). About 75% of the dose is widely distributed in the body, allowing the drug to be active outside the respiratory tract (Moscona, 2005). Oseltamivir carboxylate is excreted mainly through the kidneys; therefore, dosing must be reduced in patients with advanced renal insufficiency (creatinine clearance <30 ml/min) (Moscona, 2005).
1.1.2.3. Clinical efficacy
Both zanamivir and oseltamivir are effective for the prevention of influenza infections (Table 2 ). Zanamivir provided a reduction in incidence of laboratory-confirmed symptomatic influenza by 67% during seasonal prophylaxis in the community (Monto et al., 1999a) and by 79% during post-exposure prophylaxis in households (Hayden et al., 2000). Oseltamivir reduced the incidence of laboratory-confirmed influenza by 76% during seasonal prophylaxis in the community (Hayden et al., 1999) and by 92% in institutionalized elderly subjects (Peters et al., 2001). When used as post-exposure prophylaxis in households, oseltamivir was associated with a reduction in incidence of laboratory-confirmed influenza by 89% and 84% in individuals ≥12 years old and in number of households, respectively (Welliver et al., 2001). Effectiveness of oseltamivir for post-exposure prophylaxis in children ≥1 year old has also been demonstrated, although the drug is currently only approved for prophylaxis in children older than 13 years (Hayden et al., 2004).
Table 2.
Selected trials of neuraminidase inhibitors for prevention of influenza infections
| Drug | Study population/setting | Reduction in incidence of laboratory-confirmed influenza | Reference |
|---|---|---|---|
| Zanamivir | Healthy adults/seasonal prophylaxis in the community | 67%, 84% (flu with fever) | Monto et al. (1999a) |
| Zanamivir | Healthy adults/post-exposure prophylaxis (PEP) in households | 79% | Hayden et al. (2000) |
| Zanamivir | Healthy adults/seasonal prophylaxis (meta-analysis) | 69% | Cooper et al. (2003) |
| Zanamivir | Healthy adults/PEP in households (meta-analysis) | 81% | Cooper et al. (2003) |
| Oseltamivir | Healthy adults/seasonal prophylaxis in the community | 76%, 90% (flu with fever) | Hayden et al. (1999) |
| Oseltamivir | Elderly subjects (>80% vaccinated)/seasonal prophylaxis in institutional setting | 92% | Peters et al. (2001) |
| Oseltamivir | Individuals ≥12 years old/PEP in households | 89% for individuals, 84% for households | Welliver et al. (2001) |
| Oseltamivir | Adults and children ≥1 year/PEP in households | 68% for individuals (81% when excluding patients positive at baseline), 58.5% for households (79% when excluding patients positive at baseline) | Hayden et al. (2004) |
| Oseltamivir | Healthy adults/seasonal prophylaxis (meta-analysis) | 74% | Cooper et al. (2003) |
| Oseltamivir | Adults/PEP in households (meta-analysis) | 90% | Cooper et al. (2003) |
Zanamivir and oseltamivir are also effective for the treatment of influenza infections (Table 3 ). Zanamivir at a dose of 10 mg twice daily for 5 days started within 48 h of onset of symptoms reduced the duration of symptoms of naturally occurring influenza infections by 1–2.5 days in adults (Monto et al., 1999b, Hayden et al., 1997, The MIST Study Group, 1998, Makela et al., 2000, Boivin et al., 2000). In children 5–12 years old, zanamivir reduced duration of symptoms by 1.25 days (Hedrick et al., 2000). A meta-analysis of randomized, placebo-controlled trials has also demonstrated that zanamivir conferred reduction in duration of symptoms by 2.5 days with a 43% reduction in complications in high-risk patients with confirmed influenza infection (Lalezari et al., 2001). Similarly, oseltamivir is effective in the treatment of naturally occurring influenza infections in adults (Nicholson et al., 2000, Treanor et al., 2000, Aoki et al., 2003). In patients treated with either 75 mg (recommended dose) or 150 mg of oseltamivir b.i.d. for 5 days, the median time to alleviation of symptoms was reduced by 1.2 and 1.45 days, respectively (Nicholson et al., 2000). In adults and adolescents with a proven influenza illness, oseltamivir treatment reduced the incidence of influenza-related lower respiratory tract complications and antibiotic use by 55% and 26.7%, respectively (Kaiser et al., 2003). In children (1–12 years old), oseltamivir treatment reduced the median duration of illness by 1.5 days and afforded reductions in cough, coryza and fever (Whitley et al., 2001). The incidence of antibiotic prescriptions was also significantly reduced in the oseltamivir group (31%) compared to the placebo group (41%). For maximal benefits, antiviral treatment must start as early as possible and within 48 h of the onset of symptoms. Indeed, for every 6 h reduction of oseltamivir treatment initiation time compared to symptom onset, median illness duration could be shortened by ∼10 h (Aoki et al., 2003). In United States of America, zanamivir is approved for treatment of individuals ≥7 years (10 mg twice daily for 5 days) whereas oseltamivir is licensed for treatment of persons >1 year (30–75 mg twice daily for 5 days) (Moscona, 2005).
Table 3.
Selected trials of neuraminidase inhibitors for treatment of influenza infections
| Drug | Study population | Reduction in the median time to alleviation of symptoms (days) | Reference |
|---|---|---|---|
| Zanamivir | Healthy adults with laboratory-confirmed influenza | 1 | Monto et al. (1999b) |
| Zanamivir | Healthy adults with laboratory-confirmed influenza | 1 | Hayden et al. (1997) |
| Zanamivir | Healthy adults with laboratory-confirmed influenza | 1.5 | The MIST Study Group (1998) |
| Zanamivir | Healthy adults with laboratory-confirmed influenza | 2.5 | Makela et al. (2000) |
| Zanamivir | Children (5–12 years) with laboratory-confirmed influenza | 1.25 | Hedrick et al. (2000) |
| Zanamivir | Healthy adults with laboratory-confirmed influenza (meta-analysis) | 1.25 | Cooper et al. (2003) |
| Zanamivir | Children with laboratory-confirmed influenza (meta-analysis) | 1 | Cooper et al. (2003) |
| Zanamivir | High-risk patients with laboratory-confirmed influenza (meta-analysis) | 2.0 | Cooper et al. (2003) |
| Zanamivir | High-risk patients with laboratory-confirmed influenza (meta-analysis) | 2.5 | Lalezari et al. (2001) |
| Oseltamivir | Healthy adults with laboratory-confirmed influenza | 1.3 | Treanor et al. (2000) |
| Oseltamivir | Healthy adults with laboratory-confirmed influenza | 1.2 (75 mg), 1.45 (150 mg) | Nicholson et al. (2000) |
| Oseltamivir | Children (1–12 years) with laboratory-confirmed influenza | 1.5 | Whitley et al. (2001) |
| Oseltamivir | Healthy adults with laboratory-confirmed influenza (meta-analysis) | 1.4 | Cooper et al. (2003) |
| Oseltamivir | Children with laboratory-confirmed influenza (meta-analysis) | 1.5 | Cooper et al. (2003) |
| Oseltamivir | High-risk patients with laboratory-confirmed influenza (meta-analysis) | 0.4 | Cooper et al. (2003) |
Note: Treatment was initiated within 48 h of the onset of symptoms.
1.1.2.4. Side effects
Minor side effects have been reported in less than 5% of persons who were given zanamivir in clinical trials. They include transient upper respiratory and gastrointestinal symptoms (Moscona, 2005). Nevertheless, zanamivir should be used with caution in patients with chronic respiratory diseases, since it can cause bronchospasm and reductions in air flow (Couch, 2000). Oseltamivir has also a few side effects, such as transient nausea, vomiting and abdominal pain, which occur in about 10% of treated persons (Couch, 2000). Such side effects are significantly reduced when the drug is administered with food.
1.1.2.5. Resistance to NAIs
In vitro studies have shown that numerous (12–20) viral passages in presence of the drug are required to select resistant variants. Resistance to NAIs can result from mutations in the active site of the NA enzyme, associated or not with aa changes in or near the receptor binding site of the HA protein. The latter changes are thought to reduce viral dependency on NA activity. However, the clinical consequences of HA mutations on development of NAI resistance have not been demonstrated, in contrast to NA mutations (Abed et al., 2002, Gubareva et al., 2001). NA mutations associated with NAI resistance are subtype-specific and drug-specific and involve both catalytic and framework residues (Table 4 ). Mutations at codon 292 (Arg292Lys) and 119 (Glu119Val) predominate in H3N2 viruses, whereas a His274Tyr mutation (N2 numbering) is the most frequent in H1N1 viruses (Abed et al., 2004, Whitley et al., 2001, Wang et al., 2002, Weinstock et al., 2003). Of note, the last mutation has been reported in H5N1 viruses isolated in Asia (Le et al., 2005; de Jong et al., 2005). Unlike amantadine-resistant variants, NAI-resistant influenza viruses were infrequent in clinical trials with estimated rates of resistance varying from 0.4% to 1% in the adult population and from 4% to 8% in children (Roberts, 2001). However, higher rates (18%) of oseltamivir resistance were reported in a recent treatment study of Japanese children (Kiso et al., 2004). Most, but probably not all, NAI-resistant variants seem to be compromised with regard to infectivity and transmissibility in mice (Ives et al., 2002) and ferrets (Herlocher et al., 2002). So far, resistance to zanamivir has been extremely infrequent, with one case reported from an immunocompromised child infected with influenza B (Gubareva et al., 1998). Whether this lower rate of resistance is due to intrinsic drug properties or lower use of this compound compared to oseltamivir should be clarified.
Table 4.
Selected influenza A and B neuraminidase mutations conferring resistance to neuraminidase inhibitors (adapted from Mishin et al., 2005)
| Virus | NAI used for selection | NA mutationa | Phenotype in NA inhibition assays using |
|||
|---|---|---|---|---|---|---|
| Zanamivir | Oseltamivir | Peramivir | A-315675 | |||
| A/Texas/36/91, A/New York/02/2001 (H1N1) | Oseltamivir/in humans | H274Y | S | R | R/I | S |
| A/Hanoi/30408/2005 (H5N1) | Oseltamivir/in humans | H274Y | S | R | ? | ? |
| A/Japan/305/57 (H2N2) | Oseltamivir/in mice | R292K | I | R | R | I |
| A/Turkey/Minnesota/833/80 (H4N2) | Zanamivir/in vitro | R292K | R | R | R | I |
| A/Texas/131/2002, A/Charlottesville/03/2004 (H3N2) | Oseltamivir/in humans | E119V | S | R | S | S |
| A/Turkey/Minnesota/833/80 (H4N2) | Zanamivir/in vitro | E119G | R | S | S | S |
| E119A | R | I | S | S | ||
| E119D | R | S | R | R | ||
| B/Memphis/20/1996 | Zanamivir/in humans | R152K | R | R | R | R |
| B/Rochester/02/2001 | Oseltamivir/in humans | D198N | R | R | S | S |
S, susceptible; R, resistant; I, intermediate.
Numbers indicate the position of the substituted residue in the NA amino acid sequence (N2 numbering).
1.1.3. Other NAIs
Besides zanamivir and oseltamivir, other compounds that target the NA enzyme of influenza viruses have been developed, although they are not commercially available. Peramivir, an orally bioavailable cyclopentane compound, is a potent NA inhibitor in vitro and in vivo (Sidwell and Smee, 2002). A-315675, a pyrrolidone-based compound, also demonstrated in vitro activity against influenza A (N1, N2, and N9 subtypes) and influenza B viruses which was similar to that of zanamivir and oseltamivir (Hanessian et al., 2002). Zanamivir derivative compounds containing different side-chains could also be considered as possible anti-influenza agents (De Clerq, 2002). The efficacy of the new NAIs against influenza A and B mutants is summarized in Table 4. Finally, ribavirin (discussed below) has in vitro activity against influenza viruses (Couch, 2000). In addition, it demonstrated high efficacy in preventing death and reducing lung virus titers in mice infected with influenza A or B viruses (Sidwell et al., 2005).
2. Paramyxoviridae
This viral family contains some of the most important respiratory viruses which cause epidemics of major medical interest. Paramyxoviruses are single-stranded negative-sense RNA viruses that are divided into two subfamilies (Fig. 2 A). Paramyxoviruses include important viruses associated with upper and lower respiratory tract infections in humans i.e. human respiratory syncycial virus (HRSV), human parainfluenza viruses (HPIV) and human metapneumovirus (HMPV). Unlike orthomyxoviruses, the RNA genome of paramyxoviruses is not segmented and encodes several subgenomic messenger RNA (mRNAs). For HRSV, there are 10 subgenomic mRNAs which are translated into 11 proteins, including 4 nucleocapsid proteins (N, P, L and M2-1), 3 transmembrane glycoproteins (F, G and SH), 2 non-structural proteins (NS1 and NS2), a matrix M protein and a RNA regulatory factor M2-2. Compared to HRSV, HMPV has no NS proteins whereas HPIVs possess a hemagglutinin-neuraminidase (HN) protein instead of the G protein with no NS, M2 and SH proteins (Fig. 2B).
Fig. 2.
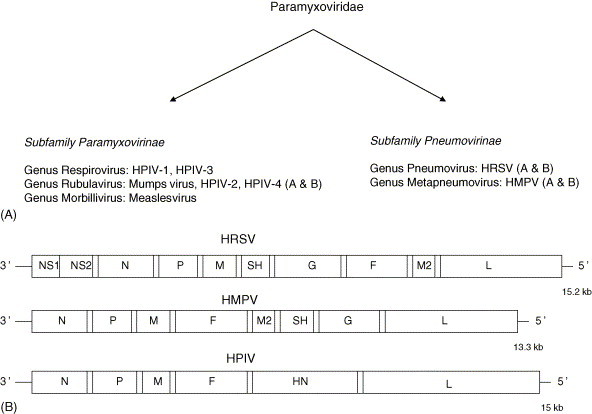
Major members of the Paramyxoviridae family that cause respiratory tract infections in humans: (A) classification; (B) schematic representation showing genomic organizations and gene orders.
2.1. Human respiratory syncycial virus (HRSV)
HRSV is a major cause of lower respiratory tract disease in premature babies (≤35 months of gestation), infants less than 6 months old, and elderly institutionalized subjects (Welliver, 2003, Greenough, 2002). The outcome of HRSV infection usually involves mild upper respiratory tract infections; however, more severe conditions, such as pneumonia and bronchiolitis occur in 25–40% of children (Meissner, 2003). Approximately 1% of HRSV-infected infants require hospitalization (Greenough, 2002). Each year, in the US, pediatric HRSV infections are associated with 50–90% of hospitalizations attributable to bronchiolitis and 20–50% of those attributable to pneumonia (Ogra, 2004). In the Northern Hemisphere, and in particular in the US, HRSV circulates predominantly during winter months and accounts for approximately 90,000 hospitalizations and 4000–17,000 deaths annually (Ogra, 2004, Thompson et al., 2003). There are currently no approved vaccines for HRSV despite considerable work in this area.
2.1.1. Treatment of HRSV infections
2.1.1.1. Ribavirin
Ribavirin (Virazole: 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) is a nucleoside analogue which is currently licensed for treatment of severe HRSV disease in high-risk infants (Brooks et al., 2004). This drug has a broad spectrum of activity against DNA and RNA viruses (Balfour, 1999). Ribavirin inhibits viral replication by several mechanisms, including inhibition of viral polymerase, inhibition of 5′ cap formation of mRNA, and inhibition of IMP dehydrogenase leading to a decrease of intracellular GTP concentrations (Balfour, 1999). Despite initial promising clinical studies, ribavirin was not found to confer clinical benefits in a large non-randomized cohort study of 223 previously healthy infants with respiratory failure due to HRSV infection (Moler et al., 1996). The clinical impact of ribavirin for treatment of HRSV lower respiratory tract infections in infants and children was also evaluated in a meta-analysis of 10 controlled trials (Vuvojic and Mills, 2001). The drug provided significant reduction in the duration of assisted ventilation whereas it had no significant impact on mortality rates, length of hospitalization and pulmonary functions. In 1996, the American Academy of Pediatrics revised its recommendation by replacing the statement ‘ribavirin should be used’ by ‘ribavirin may be considered’ in infants and young children at risk of severe HRSV disease. Nevertheless, ribavirin therapy remains recommended in high-risk patients for whom HRSV infection is associated with significant rates of mortality, such as bone marrow transplant recipients (Moscona, 2000, Englund et al., 1988). Ribavirin is highly toxic for bone marrow progenitors and is administered in an aerosolized form for treatment of respiratory tract infections.
2.1.1.2. Fusion inhibitors
The HRSV F protein is constituted of two subunits (F1 and F2). The F1 subunit contains two heptad repeat regions (HR1 and HR2) which are highly conserved among paramyxoviruses. A conformational change of the F protein leading to the formation of a stable HR1/HR2 six-helix bundle is responsible for the fusion of viral and cellular membranes (Zhao et al., 2000). Therefore the HR1/HR2 region seems to be a suitable target for the development of anti-HRSV agents. Recombinant HR1 and HR2 proteins expressed in E. coli showed a strong inhibitory effect on HRSV fusion with IC50 values of 1.68 and 2.93 μM, respectively (Wang et al., 2003). Recently, several small molecules with potent inhibitory effects on HRSV fusion have been identified. The triphenyl compound VP-14637 (ViroPharma) and the benzimidazole analogue JNJ2408068 (Johnson and Johnson) are small molecules that bind into a hydrophobic cavity in the inner core of the F protein, interacting simultaneously with both HR1 and HR2 (Douglas et al., 2005). These agents efficiently inhibited HRSV infection in vitro with IC50 values of 1.4 and 2.1 nM, respectively (Douglas et al., 2005). In a cell fusion assay, the IC50 values of the same agents were 5.4 and 0.9 nM, respectively. The characterization of drug-resistant HRSV variants selected in vitro in presence of such inhibitors revealed amino acid changes in the HR2 (Asp486Asn, Glu487Asp, Phe488Tyr) and in the intervening domain between HR1 and HR2 (Lys399Ile and Thr400Ala) (Douglas et al., 2005). Further in vitro studies showed that substitutions at residues 486–488, which are involved in direct contact with bound inhibitor, could be responsible for the resistance phenotype (Douglas et al., 2005).
The polynuclear aromatic compound RFI-641 (Wyeth) has also potent anti-HRSV activity by blocking two viral F glycoprotein-mediated fusion events, including fusion of the virion envelope with the cellular plasma membrane and syncytium formation (Huntley et al., 2002). In vitro, RFI-641 inhibited HRSV A and B replication with average IC50 values ranging from 0.018 to 0.055 μg/ml (Huntley et al., 2002). In addition, in an African green monkey model, prophylactic administration of RFI-641 resulted in a reduction in virus lung titers by ≥1.5 log during days 3–10 post HRSV challenge.
2.1.1.3. Antisense oligodeoxynucleotides
Antisense oligodeoxynucleotides (ODNs) are short (18–25 nucleotides) single-stranded molecules designed to inhibit RNA expression by annealing to complementary regions of the targeted mRNA. The newly formed RNA–DNA hybrid attracts endogenous ribonuclease H (RNase H) which cleaves the RNA portion of the hybrid (Crooke, 1992). This results in transcript degradation and/or prevents translation into proteins. A phosphorothioate ODN targeting HRSV repetitive intergenic sites has shown potent in vitro activity (Jairath et al., 1997).
2.1.1.4. Immunoprophylactic agents
Two immunoprophylactic agents have been approved for the prevention of HRSV disease. RespiGam is an intravenous polyclonal immune globulin enriched in neutralizing antibodies against HRSV which was recommended for the prevention of serious HRSV disease in high-risk infants. RespiGam reduced hospitalizations, hospital stay and total HRSV-related intensive care unit days by 41%, 53%, and 44%, respectively (Maggon and Barik, 2004). However, this product requires large volume infusion and its availability is currently limited.
Palivizumab (Synagis) is a monoclonal antibody composed of human (95%) and murine (5%) antibody sequences that is directed against the HRSV fusion (F) glycoprotein (Meissner, 2003, Johnson et al., 1997). Palivizumab is 50–100 times more potent than RespiGam and was approved by the US food and drug administration (FDA) in 1998 for prevention of serious lower respiratory tract infections caused by HRSV. Palivizumab is recommended during the HRSV season (monthly intramuscular injection) for premature babies (≤32 weeks of gestation) and infants younger than 2 years of age with chronic lung disease or congenital heart disease. In a phase III clinical study involving 1502 high-risk infants, palivizumab reduced the incidence of hospitalization by 55% (The IMpact-RSV Study Group, 1998). Another double-blind, randomized, placebo-controlled study in 1287 children with congenital heart disease showed a 45% reduction in hospitalization in the treated group (Feltes et al., 2003). On the other hand, palivizumab did not seem to provide direct cost savings related to hospitalization or ambulatory care in infants who were born between 32 and 35 weeks of estimated gestational age (Wegner et al., 2004).
2.1.1.5. Steroids
A possible effect of glucocorticoid therapy for improving pulmonary inflammatory response after HRSV infection has been suggested (Vuvojic and Mills, 2001). A meta-analysis of six randomized controlled trials of systemic corticosteroid use in bronchiolitis showed that the duration of symptoms and length of hospital stay were significantly reduced by corticosteroid treatment (Garrison et al., 2000). However, a subsequent randomized, double-blind, placebo-controlled trial, demonstrated that dexamethasone impaired the decline of HRSV in the lower respiratory tract of infants with severe HRSV disease and was not associated with improvement of clinical outcomes (Buckingham et al., 2002).
2.2. Parainfluenza viruses (HPIVs)
Four distinct serotypes of human parainfluenza viruses have been described (Henrickson, 2003). These viruses can cause upper respiratory tract diseases in individuals of all age groups, although young children between 6 months and 3 years present more severe diseases (Henrickson, 2003). In the US, HPIVs account for approximately 33% of lower respiratory tract infections in children younger than 5 years (Denny and Clyde, 1986, Glezen et al., 1984). HPIV-1 and -2 have been reported to cause disease in the fall months of alternate years (Knott et al., 1994, Reed et al., 1997) whereas HPIV-3 appears to be endemic during most months of the year. HPIVs have been associated with laryngotracheobronchitis or croup (mainly HPIV-1), bronchiolitis and pneumonia (Henrickson, 2003).
At the present time, there exists no licensed HPIV vaccine, although recombinant and live-attenuated HPIV-3 vaccines, combined or not with HRSV glycoproteins, can induce satisfactory immune responses (Belshe et al., 2004).
2.2.1. Treatment of HPIV infections
Currently, there are no antivirals approved for the treatment of HPIV infections, although several compounds have demonstrated in vitro and/or in vivo activity against these viruses (Henrickson, 2003).
2.2.1.1. Ribavirin
Ribavirin exhibits in vitro antiviral activity against HPIVs (Browne, 1981). In plaque inhibition experiments, the drug reduced HPIV-1 and -2 plaque formation by 50% at a concentration of approximately 10 and 12 μg/ml, respectively (Browne, 1981). Aerosolized and oral ribavirin were also associated with reduction in HPIV shedding and clinical improvement in infected immunocompromised patients (Chakrabarti et al., 2001, Malinowski and Hostoffer, 2001).
2.2.1.2. Other antivirals and immunomodulators
The HN of HPIV is responsible for virus binding to the host cell and for promoting F protein-mediated fusion. In addition, it facilitates the spread of infection through its NA activity which cleaves the receptors and thus prevents virion aggregation (Henrickson, 2003). Zanamivir inhibits the NA of HPIV-3 with an IC50 value of 250 μM in NA assays (Greengard et al., 2000). Of note, the drug is approximately (100,000 times) less active against HPIV-3 compared to influenza viruses.
Two novel HPIV inhibitors (BCX2798 and BCX2855), which target the HN protein, have demonstrated potent in vitro activity against HPIV-1, -2, and -3 in LLC-MK2 cells with IC50 values of 0.1–0.6 μM in HA inhibition assays and 0.02–20 μM in NA inhibition assays (Alymova et al., 2004). In addition, these agents were associated with significant reduction in viral lung titers and protection from death in a mouse model of infection with a recombinant HPIV-1 virus. Also, some peptides have shown a potent inhibitory effect on HPIV-induced syncytium formation by targeting a specific domain of the F protein, which may provide a useful antiviral approach for severe HPIV infections (Gosh and Shay, 1998, Lambert et al., 1996).
Oral or parenteral steroids also appear to improve croup symptoms associated with moderate to severe HPIV infections (Somani and Evans, 2001). High-titer pooled immunoglobulins may also reduce lower respiratory tract infections due to HPIV (The PREVENT Study Group, 1997). Finally, combined immunotherapy and steroids was also shown to reduce pulmonary virus titers and inflammation in a cotton rat model (Prince and Porter, 1996). This strategy could constitute a therapeutic option for patients with severe HPIV disease until effective vaccines and antivirals are available.
2.3. Human metapneumovirus (HMPV)
Human metapneumvirus (HMPV) was first identified in 2001 from respiratory tract secretions of Dutch children with bronchiolitis (Van den Hoogen et al., 2001). HMPV can cause severe respiratory diseases, most particularly in young children, elderly subjects and immunocompromised hosts (Boivin et al., 2002a). The spectrum of disease and the epidemiology of HMPV resemble that of HRSV, including upper respiratory tract and lower respiratory tract illnesses i.e. cold and influenza-like illnesses, bronchiolitis, croup, pneumonia, and exacerbation of asthma and chronic obstructive pulmonary disease (Hamelin et al., 2004, Hamelin et al., 2005). In several pediatric studies, HMPV was found to be the second or third cause of hospitalizations in young children after HRSV and possibly influenza (Hamelin et al., 2004).
There are currently no approved vaccines for HMPV, although recombinant live-attenuated vaccines generated by reverse genetics have shown promise in animal studies (Biacchesi et al., 2005).
2.3.1. Treatment of HMPV infections
Ribavirin has similar in vitro activity against HMPV and HRSV with IC50 values of approximately 88 and 74 μM, respectively (Wyde et al., 2003). This drug also decreased HMPV lung titers when administered by the intra-peritoneal route in mice (Hamelin et al., 2006). Viral inhibition was also shown with heparin and the sulfated sialyl lipid NMSO3, two drugs that seem to alter viral fusion when assayed in cell culture (Wyde et al., 2004). The humanized monoclonal antibody against HRSV (palizumab) has no in vitro activity against HMPV whereas most conventional immunoglobulin preparations seem to contain sufficient amounts of neutralizing titers (Wyde et al., 2003). There has been no clinical study of antivirals or immunoprophylactic agents in the treatment of HMPV infections.
3. Picornaviridae
Picornaviruses constitute a diverse family of non-enveloped single-stranded positive-sense RNA viruses whose genome encodes a single polyprotein of 2100–2400 aa (Mackie, 2003). Three genera within this family (Rhinovirus, Enterovirus, and Parechovirus) can cause respiratory tract infections in humans. Human rhinoviruses (HRVs) are the most frequent cause of mild upper respiratory tract infections i.e. common colds (Makela et al., 1998, Savolainen et al., 2003). This genus includes more than 100 serotypes which are divided into two groups on the basis of their cellular receptors. The majority of rhinoviruses (91 serotypes) use the intracellular adhesion molecule 1 (ICAM-1) as their cell receptor for virus attachment, whereas the remaining serotypes use the low density lipoprotein (LDL) receptor (Mackie, 2003). In temperate climates, HRV infections can be observed throughout the year although there are peaks of activity during the fall and spring seasons (Makela et al., 1998). Besides the common cold, HRVs can cause acute otitis media, lower respiratory tract infections including pneumonia, wheezing in children and exacerbations of asthma and chronic obstructive pulmonary disease (COPD) in adults (Savolainen et al., 2003). Enteroviruses (echo and coxsackieviruses) and parechoviruses have been associated with numerous clinical syndromes including respiratory tract infections. There are no vaccines or specific immunoglobulins against the respiratory picornaviruses.
3.1. Treatment of picornavirus infections
3.1.1. Interferons
Interferons are glycoproteins that induce a number of antiviral, antiproliferative and immunological effects which, collectively, affect host-cell susceptibility to picornavirus infections (Rotbart et al., 1998). In conjunction with double-stranded RNA, interferons induce the expression of proteins, some of which mediate an antiviral activity. Interferons may also contribute with humoral antibodies and macrophages to the elimination of picornavirus infections (Balfour, 1999). Commercial preparations of interferon alpha are not orally bioavailable and need to be given by intramuscular or subcutaneous injections. Intranasal interferon α-2b was found to be active during clinical prophylactic studies of HRV infections, but has not demonstrated benefits in the treatment of established infection (McKinlay, 2001). Furthermore, nasal irritations and bleeding are major side effects that limit its use (Rotbart, 2000).
3.1.2. Pleconaril
Pleconaril ({3-[3,5-dimethyl-4-[(3-methyl-5-isoxasolyl)-propyl]-phenyl]-5-(trifluoromethyl)-1,2,4-oxadiazole}, ViroPharma Inc.), is an orally bioavailable anti-picornaviral agent (Pevear et al., 1999). It acts by integrating into the cavity of the viral capsid to inhibit its uncoating, thus blocking attachment to host-cell receptors and inhibiting viral replication (Pevear et al., 1999). Pleconaril demonstrated a potent broad spectrum activity against 93 of the 101 HRV serotypes and 45 of 55 culturable enterovirus serotypes (McKinlay, 2001). Pleconaril exhibited a median IC50 value of 0.07 μg/ml when tested against 5 selected HRV serotypes and 46 clinical HRV isolates (Kaiser et al., 2000). Pharmacokinetic studies in adults and children have shown that plasma concentrations were above the drug concentration that inhibits >90% of HRVs in vitro after oral administration (Pevear et al., 1999). In clinical trials, pleconaril conferred reductions in symptom severity and duration in individuals with naturally occurring colds (Ison et al., 2002). A recent study has shown that the clinical benefit correlated with drug susceptibility of the baseline virus isolate (Pevear et al., 2005). Pleconaril-treated subjects infected with highly susceptible viruses (IC50 ≤ 0.38 μg/ml) experienced a median reduction in symptom duration of 1.9–3.9 days compared with that for the placebo-treated subjects. By contrast, subjects whose baseline virus isolate susceptibility was >0.38 μg/ml did not benefit from pleconaril treatment. During a 6-week prophylactic study on picornavirus respiratory infections, pleconaril was associated with an increase in menstrual irregularities. Subsequently, concerns have been raised that this drug might increase the metabolism and reduce the efficacy of some hormonal contraceptives and drugs used to treat HIV (Webster, 2005). As a result, pleconaril was not licensed by the FDA. An intranasal formulation of the drug is under investigation.
3.1.3. BTA188
BTA188 (Biota Inc.), another capsid-function inhibitor, has shown potent antiviral activity against 87 selected HRV serotypes, with a median IC50 value of 0.01 μg/ml (Hayden et al., 2001). BTA188 was also effective against all tested clinical HRV isolates (n = 40) with a median IC50 value of 0.004 μg/ml. This agent had a high bioavailability when evaluated in rodents and dogs, with accumulation in nasal tissue (Hayden et al., 2001). In dogs, mean concentrations of BTA188 in the nasal epithelium 24 h after dosing were 25 times higher than plasma concentrations and significantly higher than HRV IC50 values.
3.1.4. Soluble ICAM
Recombinant soluble ICAM-1 (Tremacamra, Boehringer Mannheim) binds competitively to the viral receptor of HRV in vitro, thus preventing attachment and subsequent replication (Shigeta, 1998). Moreover, in a randomized clinical trial of experimentally infected volunteers, this agent had a significant impact on the total symptom score (reduced by 45%), the proportion of subjects with clinical colds (reduced by 23%) and the total nasal mucus weight (reduced by 56%) (Turner et al., 1999). In addition, this antiviral appeared to be well tolerated.
3.1.5. 3C protease inhibitors
The 3C protein of picornaviruses is a protease which cleaves viral precursor polypeptide into structural proteins and enzymes which are essential for viral replication (Rotbart, 2000). AG7088 (Ruprintrivir, Agouron Pharmaceuticals, Inc.) is a potent inhibitor of HRV 3C protease. In vitro, it efficiently inhibited all 48 HRV serotypes tested with a mean IC50 value of 0.023 μM (range: 0.003–0.081 μM) (Patick et al., 1999). In prophylactic studies, AG7088 reduced the incidence of colds, total symptom scores, respiratory symptoms and nasal discharge (Kaiser et al., 2000). Moreover, ruprintrivir provided reduction in total and respiratory symptoms by 2–3 days in subjects who started treatment within 24 h or less of onset of respiratory symptoms (Schmidt et al., 2001). Mild nausea and taste disturbance are common adverse effects (Munoz et al., 2000). On the other hand, the intranasal spray of ruprintrivir seemed to be well tolerated, with blood-tinged mucus and nasal irritation being the most common side effects (Hayden et al., 2003).
4. Coronaviridae
Coronaviruses were first isolated 40 years ago in organ cultures of human embryonic trachea and nasal epithelium and in primary human kidney cell cultures (Tyrrell and Bynoe, 1965). These viruses have a single-stranded positive-sense RNA genome of approximately 30 kb, which is considered to be the largest known autonomously replicating RNA (Thiel et al., 2001). About two-thirds of the genome encodes proteins involved in the replication and transcription of the viral RNA. There are three groups of coronaviruses that include several animal viruses and now five human viruses (Fig. 3 ). The human coronaviruses (HCoVs) OC43 and 229E are considered to be the second cause of the common cold after HRVs accounting for approximately 35% of mild upper respiratory infections in adults (Falsey et al., 2002). The virus responsible for the severe acute respiratory syndrome (SARS) outbreak between 2002 and 2003 was identified as a new coronavirus (SARS-CoV), which led to more than 8000 infections and 800 deaths in 26 countries (Ksiazek et al., 2003, Peiris et al., 2003). Clinically, SARS is characterized by systemic symptoms such as fever and myalgia followed by respiratory symptoms, including non-productive cough and dyspnea. Approximately 15% of cases deteriorate and require mechanical ventilation due to development of acute respiratory distress syndrome (ARDS) (Tsui et al., 2003). More recently, HCoV-NL and HCoV-HKU1 have been associated with small numbers of cases of bronchiolitis and pneumonitis in humans (Fouchier et al., 2004, Woo et al., 2005a, Woo et al., 2005b). There are currently no approved vaccines or specific immunoglobulins for the prevention of human coronaviruses although several groups are working on the development of effective vaccines using different approaches (Zhu, 2004).
Fig. 3.
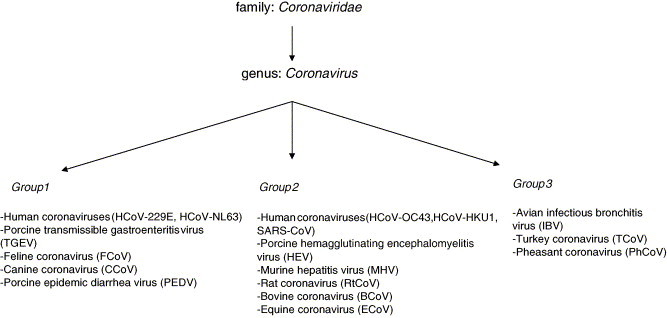
Classification of human and animal coronaviruses.
4.1. Treatment of SARS-CoV infections
At the time of the SARS outbreak, no specific treatment was shown to prevent disease progression. However, research in that field has been intensified resulting in certain promising therapeutic strategies that still need to be validated during subsequent outbreaks.
4.1.1. Ribavirin
Several small clinical studies have assessed the effectiveness of ribavirin against SARS-CoV. However, none of them clearly demonstrated its clinical efficacy (Groneberg et al., 2005). Furthermore, the drug is not active against SARS-CoV in vitro (Chen et al., 2004). In addition, Knowles et al. (2003) reported common adverse effects in individuals treated with high-dose ribavirin therapy for suspected or probable SARS, i.e. haemolytic anemia, hypocalcemia and hypomagnesemia reported in 61%, 58% and 46% of cases, respectively.
4.1.2. Protease inhibitors
The combination of HIV protease inhibitors such as lopinavir and ritonavir (Kaletra, Abbott Laboratories) could be potentially useful for the treatment of SARS because these agents have shown antiviral activity against SARS-CoV in vitro (Chan et al., 2003, Chu et al., 2004). In a small group of patients who received lopinavir/ritonavir as initial treatment, there was a significant reduction in the intubation and mortality rates (Chan et al., 2003). Chu et al. (2004) also evaluated lopinavir/ritonavir treatment compared with historic controls; all patients were also treated with ribavirin and steroids. Development of ARDS or death within 21 days were significantly lower in the lopinavir/ritonavir group compared to historic controls. Nosocomial infections were also reduced in the active group. Other protease inhibitors may be considered for the treatment of SARS because of their in vitro activity against SARS-CoV. These include nelfinavir, Calpain inhibitor III (Z-Val-Phe-Ala-CHO) and Calpain inhibitor IV (Val-Leu-CHO) (Yamamoto et al., 2004).
4.1.3. Fusion inhibitors
Fusion inhibitors are promising candidates for the treatment of SARS. It was postulated that the heptad region in the spike protein of SARS-CoV is involved in the mechanism mediating fusion between the virus and host-cell membranes (Liu et al., 2004). By analogy with the heptad regions 1 and 2 of the HIV-1 gp41 protein which provided the basis for anti-HIV fusion inhibitors, one peptide (CP1) within the heptad region 1 of the spike protein of SARS-CoV specifically inhibited viral replication in vitro (Liu et al., 2004).
4.1.4. Steroids
In addition to standard antiviral agents, some studies reported that high-dose steroids were associated with improvements of chest X-rays, oxygenation rates and fever, although their clinical value is still uncertain in the context of SARS. In addition, their use was associated with joint pain caused by avascular necrosis (Groneberg et al., 2005).
4.1.5. siRNA
RNA interference therapy is a process by which small interfering RNAs (siRNA) mediate the specific degradation of mRNAs with identical sequences. Specific siRNAs targeting the replicase 1A region of SARS-CoV demonstrated a strong inhibitory effect on replication of different strains of SARS-CoV in vitro (He et al., 2003). However, their clinical benefit awaits evaluation.
5. Adenoviridae
Adenoviruses (Ads) are non-enveloped double-stranded DNA viruses that were first observed in human adenoid tissues in 1953. Fifty-one serotypes of human Ads have been classified into six groups (A–F) based on their biological characteristics, tumorigenicity, and DNA homology. Despite their multiorgan tropism, some types of Ads have a predilection for the respiratory tract and can cause a wide range of respiratory symptoms, including coryza, pharyngitis, tonsillitis, bronchitis, and pneumonia. Ads belonging to subgroups B (Ad3, 7, 14, 16, 21, 34, 35), C (Ad1, 2, 5, 6), and E (Ad4) account for up to 8% of respiratory tract infections in young children (Ruuskanen et al., 1985). Ad4 and Ad7 are also significant causes of acute respiratory diseases in military recruits (Wadell, 1984, Erdman et al., 2002) and these serotypes were part of a live adenovirus vaccine. Ads were not found to be associated with the seasonality observed with most other respiratory viruses although they can cause sporadic outbreaks of disease. In general, Ad infections are mild or self-limited and resolve within 2 weeks without long-term complications. However, Ads constitute an important cause of mortality and morbidity in immunocompromised children and in neonates (Munoz et al., 1997, Azbug and Levin, 1991). Adenovirus pneumonia is associated with mortality rates as high as 60% in bone marrow transplant recipients (Hierhozler, 1992). Despite the fact that live, enteric-coated oral vaccines developed for Ad4 and Ad7 significantly lowered Ad morbidity by 95–99%, their production was discontinued in 1996. However, the U.S. Department of Defense recently awarded a contract to Barr Laboratories, Inc. to resume production of such vaccine (Blasiole et al., 2004).
5.1. Treatment of Ad infections
There are no approved therapeutic agents against Ad infections. However, some broad spectrum antivirals have been used in the treatment of severe Ad infections in immunocompromised hosts.
5.1.1. Ribavirin
Intravenous (IV) ribavirin has shown some apparent success in the treatment of Ad disease in children including stem cell transplant (SCT) recipients with pneumonia (Howard et al., 1999), bone marrow transplant (BMT) recipients with gastroenteritis (Kapelushnik et al., 1995) and leukemic children with disseminated disease (McCarthy et al., 1995) although most reports are anecdotal or small series of cases. The most common adverse effect of IV ribavirin is a reversible mild anemia which is rarely symptomatic.
5.1.2. Cidofovir
Cidofovir, a broad spectrum cytosine nucleotide analogue, has shown some efficacy in treating symptomatic Ad-associated disease in immunocompromised children and adults (Ribaud et al., 1999, Bordigoni et al., 2001). In a study on 22 SCT patients (aged from 1 to 9 years), 2 of 3 patients treated with cidofovir recovered compared to 3 of 13 patients treated with ribavirin (Bordigoni et al., 2001). However, the clinical use of IV cidofovir has been limited by its nephrotoxicity.
6. Conclusion
The prevention and control of respiratory viral infections remain major clinical goals because of their impact on the society in terms of health, quality of life and economy. With the notable exception of influenza viruses, there are no approved vaccines for the prevention of most respiratory viral infections despite continual efforts in this field. However, the immunoprophylactic agents RespiGam and Synagis constitute an effective strategy for the prevention of severe HRSV infections in premature and at-risk infants. Similarly, with the exception of anti-influenza agents, including adamantanes (amantadine and rimantadine) and neuraminidase inhibitors (zanamivir and oseltamivir), there are no other licensed antivirals against the large variety of clinically important respiratory viruses such as HPIV, HRV, and Ads. Although ribavirin has been approved for the treatment of HRSV infection, its clinical use has been limited by its side effects combined to its minor clinical efficacy. Because most respiratory viral infections are self-limited and due to the lack of rapid point-of-care diagnostic methods, the commercialization of antiviral agents against respiratory viruses represents an important challenge. Nevertheless, numerous antiviral agents have shown potent in vitro and in vivo activities against different families of respiratory viruses and merit additional studies.
References
- Abed Y., Bourgault A.M., Fenton R.J., Morley P.J., Tisdale M., Boivin G. Characterization of two influenza A: H3N2 viruses with reduced susceptibility to neuraminidase inhibitors from untreated patients due to mutations in the hemagglutinin gene. J. Infect. Dis. 2002;186:1074–1080. doi: 10.1086/344237. [DOI] [PubMed] [Google Scholar]
- Abed Y., Goyette N., Boivin G. A reverse genetics study of resistance to neuraminidase inhibitors in an influenza A/H1N1 virus. Antivir. Ther. 2004;9:577–581. [PubMed] [Google Scholar]
- Abed Y., Goyette N., Boivin G. Generation and characterization of recombinant influenza A/H1N1 viruses harboring amantadine resistance mutations. Antimicrob. Agents Chemother. 2005;49:556–559. doi: 10.1128/AAC.49.2.556-559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alymova I.V., Taylor G., Takimoto T., Lin T.H., Chand P., Babu Y.S., Li C., Xiong X., Portner A. Efficacy of novel hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 against human parainfluenza viruses in vitro and in vivo. Antimicrob. Agents Chemother. 2004;48:1495–1502. doi: 10.1128/AAC.48.5.1495-1502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzueto A., Niederman M.S. Diagnosis and treatment of rhinovirus respiratory infections. Chest. 2003;123:1664–1672. doi: 10.1378/chest.123.5.1664. [DOI] [PubMed] [Google Scholar]
- Aoki F.Y., Macleod M.D., Paggiaro P., Carewisz O., Elsawi A., Wat C., Griffiths M., Waalberg E., Ward P., The IMPACT Study Group Early administration of oral oseltamivir increases the benefits of influenza treatment. J. Antimicrob. Chemother. 2003;51:123–129. doi: 10.1093/jac/dkg007. [DOI] [PubMed] [Google Scholar]
- Azbug M.J., Levin M.J. Neonatal adenovirus infection: four patients and review of the literature. Pediatrics. 1991;87:890–896. [PubMed] [Google Scholar]
- Balfour H.H. Antiviral drugs. New Engl. J. Med. 1999;340:1255–1268. doi: 10.1056/NEJM199904223401608. [DOI] [PubMed] [Google Scholar]
- Belshe R.B., Newman F.K., Anderson E.L., Wright P.F., Karron R.A., Tollefson S. Evaluation of combined live attenuated respiratory syncycial virus and parainfluenza 3 virus vaccines in infants and young children. J. Infect. Dis. 2004;190:2013–2096. doi: 10.1086/425981. [DOI] [PubMed] [Google Scholar]
- Biacchesi S., Pam Q.N., Skiadopoulos M.H., Murphy B.R., Collins B.L., Buchholz U.J. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J. Virol. 2005;79:12608–12613. doi: 10.1128/JVI.79.19.12608-12613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiole D.A., Metzgar D., Daum L.T., Ryan M.A.K., Wu J., Wills C., Le C.T., Freed N.E., Hansen C.J., Gray G.C., Russell K.L. Molecular analysis of adenovirus isolates from vaccinated and unvaccinated young adults. J. Clin. Microbiol. 2004;42:1686–1693. doi: 10.1128/JCM.42.4.1686-1693.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G., Goyette N., Hardy I., Aoki F., Wagner A., Trottier S. Rapid antiviral effect of inhaled zanamivir in the treatment of naturally occurring influenza in otherwise healthy adults. J. Infect. Dis. 2000;181:1471–1474. doi: 10.1086/315392. [DOI] [PubMed] [Google Scholar]
- Boivin G., Abed Y., Pelletier G., Ruel L., Moison D., Cote S., Peret T.C.T., Erdman D.D., Anderson L.J. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- Boivin G., Goyette N., Bernatchez H. Prolonged excretion of amantadine-resistant influenza A virus quasi species after cessation of antiviral therapy in an immunocompromised patient. Clin. Infect. Dis. 2002;34:E23–E25. doi: 10.1086/338870. [DOI] [PubMed] [Google Scholar]
- Boivin G., Goyette N. Susceptibility of recent Canadian influenza A and B virus isolates to different neuraminidase inhibitors. Antivir. Res. 2002;54:143–147. doi: 10.1016/s0166-3542(01)00219-4. [DOI] [PubMed] [Google Scholar]
- Boivin G., Abed Y., Boucher F.D. Human parechovirus-3 and neonatal infections. Emerg. Infect. Dis. 2005;11:103–105. doi: 10.3201/eid1101.040606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordigoni P., Carret A.S., Venard V., Witz F., Faou L.L. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem-cell transplantation. Clin. Infect. Dis. 2001;32:1290–1297. doi: 10.1086/319984. [DOI] [PubMed] [Google Scholar]
- Bridges C., Harper S., Fukuda K. Recommendations of the Advisory Committee of Immunisation Practices (ACIP) Centers for Disease Control; 2003. Prevention and control of influenza. pp. 1–34. [Google Scholar]
- Brooks M.J., Sasadeusz J.J., Tannock G.A. Antiviral chemotherapeutic agents against respiratory viruses: where are we now and what's in the pipeline? Curr. Opin. Pulm. Med. 2004;10:197–203. doi: 10.1097/00063198-200405000-00009. [DOI] [PubMed] [Google Scholar]
- Browne M.J. Comparative inhibition of influenza and parainfluenza virus replication by ribavirin. Antimicrob. Agents Chemother. 1981;19:712–715. doi: 10.1128/aac.19.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham S.C., Jafri H.S., Bush A.J., Carubelli C.M., Sheeran P., Hardy R.D., Ottolini M.G., Ramilo O., De Vincenzo J.P. A randomized, double-blind, placebo-controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: effects on RSV quantity and clinical outcome. J. Infect. Dis. 2002;185:1222–1229. doi: 10.1086/340024. [DOI] [PubMed] [Google Scholar]
- Cass L.M.R., Efthymiopoulos C., Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration in healthy volunteers. Clin. Pharmacokinet. 1999;36:1–11. doi: 10.2165/00003088-199936001-00001. [DOI] [PubMed] [Google Scholar]
- Cass L.M.R., Brown J., Pickford M. Pharmacoscintigraphic evaluation of lung deposition of inhaled zanamivir in healthy volunteers. Clin. Pharmacokinet. 1999;36:21–31. doi: 10.2165/00003088-199936001-00003. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb. Mortal. Wkly Rep. 2005;54:1–40. [Google Scholar]
- Chakrabarti S., Collingham K.E., Holder K., Fegan C.D., Osman H., Milligan D.W. Pre-emptive oral ribavirin therapy of paramyxovirus infections after hematopoietic stem-cell transplantation: a pilot study. Bone Marrow Transplant. 2001;28:759–763. doi: 10.1038/sj.bmt.1703216. [DOI] [PubMed] [Google Scholar]
- Chan K.S., Lai S.T., Chu C.M., Tsui E., Tam C.Y., Wong M.M. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med. J. 2003;9:399–406. [PubMed] [Google Scholar]
- Chen F., Chan K.H., Jiang Y., Kao R.Y.T., Lu H.T., Fan K.W., Cheng V.C.C., Tsui W.H.W., Hung I.F.N., Lee T.S.W., Guan Y., Peiris M.S., Yuen K.Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N.J., Sutton A.J., Abrams K.R., Wailou A., Turner D., Nicholson K.G. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomized controlled trials. BMJ. 2003;326:1235–1242. doi: 10.1136/bmj.326.7401.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch R.B. Prevention and treatment of influenza. New Engl. J. Med. 2000;343:1778–1787. doi: 10.1056/NEJM200012143432407. [DOI] [PubMed] [Google Scholar]
- Crooke S.T. Therapeutic application of oligonucleotides. Annu. Rev. Pharmacol. Toxicol. 1992;32:329–376. doi: 10.1146/annurev.pa.32.040192.001553. [DOI] [PubMed] [Google Scholar]
- De Clerq E. Highlights in the development of new antiviral agents. Mini Rev. Med. Chem. 2002;2:163–175. doi: 10.2174/1389557024605474. [DOI] [PubMed] [Google Scholar]
- de Jong M.D., Thanh T.T., Khanh T.H., Hien V.M., Smith G.J.D., Chau N.V., Van Cam B., Qui P.T., Ha D.Q., Guan Y., Peiris J.S.M., Hien T.T., Farrar J. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 2005;353:2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- Demicheli V., Jefferson T., Rivetti D., Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine. 2000;18:957–1030. doi: 10.1016/s0264-410x(99)00332-1. [DOI] [PubMed] [Google Scholar]
- Denny F.W., Clyde W.A., Jr. Acute lower respiratory infections in nonhospitalized children. J. Pediatr. 1986;108:635–646. doi: 10.1016/s0022-3476(86)81034-4. [DOI] [PubMed] [Google Scholar]
- Douglas J.L., Panis M.L., Ho E., Lin K.Y., Krawzyk S.H., Grant D.B., Cai R., Swaminathan S., Chen X., Cihlar T. Small molecules VP-14637 and JNJ-2408068 inhibit respiratory syncycial virus infection by similar mechanisms. Antimicrob. Agents Chemother. 2005;49:2460–2466. doi: 10.1128/AAC.49.6.2460-2466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund J.A., Sullivan C.J., Jordan M.C., Dehner L.P., Vercellotti J.M., Balfour H.H., Jr. Respiratory syncytial virus infection in immunocompromised adults. Ann. Intern. Med. 1988;109:203–208. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- Erdman D.D., Xu W., Gerber S.I., Gray G.C., Shnurr D., Kajon A.E., Anderson L.J. Molecular epidemiology of adenovirus type 7 in the United States, 1966–2000. Emerg. Infect. Dis. 2002;8:269–277. doi: 10.3201/eid0803.010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R., Walshe E.E., Hayden F.G. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J. Infect. Dis. 2002;185:1338–1341. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltes T.F., Cabalca A.K., Meissner H.C., Cardiac Synagis Study Group Palizumab prophylaxis reduces hospitalizations due to respiratory syncycial virus infection in high-risk infants. Pediatrics. 2003;102:531–537. [Google Scholar]
- Fenderick A.M., Monto A.S., Nightengale B., Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A., Hartwig N.G., Bestebroer T.M., Niemeyer B., de Jong J.C., Simon J.H., Osterhaus A.D. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison M.M., Christakis D.A., Harvey E., Cummings P., Davis R.L. Systemic corticosteroids in infant bronchiolitis: a meta-analysis. Pediatrics. 2000;105:E44. doi: 10.1542/peds.105.4.e44. [DOI] [PubMed] [Google Scholar]
- Glezen W.P., Frank A.L., Taber L.H., Kasel J.A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J. Infect. Dis. 1984;150:851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- Gosh J.K., Shay Y. A peptide derived from a conserved domain of sendai virus fusion protein inhibits virus-cell fusion. J. Biol. Chem. 1998;273:7252–7259. doi: 10.1074/jbc.273.13.7252. [DOI] [PubMed] [Google Scholar]
- Greenough A. Respiratory syncycial virus infections: clinical features, management and prophylaxis. Curr. Opin. Pulm. Med. 2002;8:214–217. doi: 10.1097/00063198-200205000-00011. [DOI] [PubMed] [Google Scholar]
- Greengard O., Poltoratskaia N., Leikina E., Zimmerberg J., Moscona A. The anti-influenza virus agent 4-GU-DANA (zanamivir) inhibits cell fusion mediated by human parainfluenza virus and influenza virus HA. J. Virol. 2000;74:11108–11114. doi: 10.1128/jvi.74.23.11108-11114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg D.A., Poutanen S.M., Low D.E., Lode H., Welte T., Zabel P. Treatment and vaccines for severe acute respiratory syndrome. Lancet. 2005;5:147–155. doi: 10.1016/S1473-3099(05)01307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva L.V., Matrosovich M.N., Brenner M.K., Bethell R.C., Webster R.G. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J. Infect. Dis. 1998;178:1257–1262. doi: 10.1086/314440. [DOI] [PubMed] [Google Scholar]
- Gubareva L.V., Kaiser L., Matrosovich M.N., Soo-Hoo Y., Hayden F.G. Selection of influenza virus mutants in experimentally infected volunteers treated with oseltamivir. J. Infect. Dis. 2001;183:523–531. doi: 10.1086/318537. [DOI] [PubMed] [Google Scholar]
- Hamelin M.E., Abed Y., Boivin G. The human metapneumovirus: a new player among respiratory viruses. Clin. Infect. Dis. 2004;38:983–990. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin M.E., Cote S., Laforge J., Lampron N., Bourbeau J., Weiss K., Gilca R., De Serres G., Boivin G. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin. Infect. Dis. 2005;41:498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- Hamelin M.E., Prince G.A., Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob. Agents Chemother. 2006;50:774–777. doi: 10.1128/AAC.50.2.774-777.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanessian S., Bayrakdaryan M., Luo X. Total synthesis of A-315675: a potent inhibitor of influenza neuraminidase. J. Am. Chem. Soc. 2002;124:4716–4721. doi: 10.1021/ja0126226. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Belshe R.B., Clover R.D., Hay A.J., Oakes M.G., Soo W. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N. Engl. J. Med. 1989;321:1696–1702. doi: 10.1056/NEJM198912213212502. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Sperber S.J., Belshe R.B., Clover R.D., Hay A.J., Pyke S. Recovery of drug-resistant influenza A virus during therapeutic use of rimantadine. Antimicrob. Agents Chemother. 1991;35:1741–1747. doi: 10.1128/aac.35.9.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G., Osterhaus A.D., Treanor J.J., Fleming D.M., Aoki F.Y., Nicholson K.G., Bohnen A.M., Hirst H.M., Keene O., Wightman K., GG167 Influenza Study Group Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N. Engl. J. Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Atmar R.L., Schilling M., Johnson C., Poretz D., Paar D., Huson L., Ward P., Mills R.G. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N. Engl. J. Med. 1999;341:1336–1343. doi: 10.1056/NEJM199910283411802. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Gubareva L.V., Monto A.S., Klein T.C., Elliott M.J., Hammond J.M., Sharp S.J., Ossi M.J., The Zanamivir Family Study Group Inhaled zanamivir for the prevention of influenza in families. N. Engl. J. Med. 2000;343:1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Crump C., Reece P.A., Watson K., Ryan J., Cameron-Smith R., Tucker S.P. In vitro anti-rhinovirus spectrum and potency of BTA188, a novel, oral picornavirus capsid-binder. Antivir. Res. 2001;50:A75. (Abstract) [Google Scholar]
- Hayden F., Palese P. Influenza Virus. In: Richman D.D., Whitley R.J., Hayden F.G., editors. Clinical Virology. 2nd ed. ASM Press; Washington, DC: 2002. pp. 891–920. [Google Scholar]
- Hayden F., Turner R.B., Gwaltney J.M., Chi-Burris K., Gersten M., Hsyu P., Patick A.K., Smith G.J., III, Zalman L.S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003;47:3907–3916. doi: 10.1128/AAC.47.12.3907-3916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G., Belshe R., Villanueva C., Lanno R., Hughes C., Small I., Dutkowski R., Ward P., Carr J. Management of influenza in households: a prospective, randomized comparison of oral treatment with or without post-exposure prophylaxis. J. Infect. Dis. 2004;189:440–449. doi: 10.1086/381128. [DOI] [PubMed] [Google Scholar]
- He M.L., Zheng B., Peng Y. Inhibition of SARS-associated coronavirus infection and replication by RNA interference. JAMA. 2003;290:2665–2666. doi: 10.1001/jama.290.20.2665. [DOI] [PubMed] [Google Scholar]
- Hedrick J.A., Barzilai A., Behre U., Henderson F.W., Hammond J., Reilly R., Keene O. Zanamivir for treatment of symptomatic influenza A and B infection in children five to twelve years of age: a randomized controlled trial. Pediatr. Infect. Dis. J. 2000;19:410–417. doi: 10.1097/00006454-200005000-00005. [DOI] [PubMed] [Google Scholar]
- Henrickson K.J. Parainfluenza viruses. Clin. Microbiol. Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlocher M.L., Carr J., Ives J., Elias S., Truscon S., Roberts N., Monto A.S. Influenza virus carrying an R292K mutation in the neuraminidase gene is not transmitted in ferrets. Antivir. Res. 2002;54:99–111. doi: 10.1016/s0166-3542(01)00214-5. [DOI] [PubMed] [Google Scholar]
- Hierhozler J.C. Adenoviruses in the immunocompromised host. Clin. Microbiol. Rev. 1992;5:262–274. doi: 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D.S., Phillips G.L., Reece D.E. Adenovirus infections in hematopoietic stem-cell transplant recipients. Clin. Infect. Dis. 1999;29:1494–1501. doi: 10.1086/313514. [DOI] [PubMed] [Google Scholar]
- Huntley C.C., Weiss W.J., Gazumyan A., Buklan A., Feld B., Hu W., Jones T.R., Murphy T., Nikitenko A.A., O’Hara B., Prince G., Quartuccio S., Raifeld Y.E., Wude P., O’Connell J.F. RFI-641, a potent respiratory syncytial virus inhibitor. Antimicrob. Agents Chemother. 2002;46:841–847. doi: 10.1128/AAC.46.3.841-847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison M.G., Mills J., Openshaw P., Zambon M., Osterhaus A., Hayden F.G. Proceedings of the Fourth International Symposium on Current Research on Respiratory Viral InfectionsAntivir. Res. 2002;55:227–278. doi: 10.1016/S0166-3542(02)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Yamashita T., Tsuzuki H., Takeda N., Sakae K. Isolation and identification of a novel human parechovirus. J. Gen. Virol. 2004;85:391–398. doi: 10.1099/vir.0.19456-0. [DOI] [PubMed] [Google Scholar]
- Ives J.A., Carr J.A., Mendel D.B., Tai C.Y., Lambkin R., Kelly R., Oxford J.S., Hayden F.G., Roberts N.A. The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leaves virus severely compromised both in vitro and in vivo. Antivir. Res. 2002;55:307–317. doi: 10.1016/s0166-3542(02)00053-0. [DOI] [PubMed] [Google Scholar]
- Jairath S., Vargas P.B., Hamlin H.A., Field A.K., Kilkuskie R.E. Inhibition of respiratory syncytial virus replication by antisense oligodeoxyribonucleotides. Antivir. Res. 1997;33:201–213. doi: 10.1016/s0166-3542(96)01015-7. [DOI] [PubMed] [Google Scholar]
- Johnson S., Oliver C., Prince G.A. Development of humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- Kaiser L., Crump C.E., Hayden F.G. In vitro activity of pleconaril and AG7088 against selected serotypes and clinical isolates of human rhinovirus. Antivir. Res. 2000;47:215–220. doi: 10.1016/s0166-3542(00)00106-6. [DOI] [PubMed] [Google Scholar]
- Kaiser L., Wat C., Mills T., Mahoney P., Ward P., Hayden F.G. Impact of oseltamivir treatment of influenza-related lower respiratory tract complications and hospitalizations. Arch. Intern. Med. 2003;163:1667–1672. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- Kapelushnik J., Or R., Delukina M., Nagler A., Livni N., Engelhard D. Intravenous ribavirin therapy for adenovirus gastroenteritis after bone marrow transplantation. J. Pediatr. Gastroenterol. Nutr. 1995;21:110–112. doi: 10.1097/00005176-199507000-00021. [DOI] [PubMed] [Google Scholar]
- Kim C., Lew W., Williams M. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design synthesis and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- Kiso M., Mitamura K., Sakai-Tagawa Y., Shiraishi K., Kawakami C., Kimura K., Hayden F.G., Sugaya N., Kawaoka Y. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–765. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- Klimov A.I., Rocha E., Hayden F.G., Shult P.A., Roumillat L.F., Cox N.J. Prolonged shedding of amantadine-resistant influenza A viruses by immunodeficient patients: detection by polymerase chain reaction-restriction analysis. J. Infect. Dis. 1995;172:1352–1355. doi: 10.1093/infdis/172.5.1352. [DOI] [PubMed] [Google Scholar]
- Knott A.M., Long C.E., Hall C.B. Parainfluenza viral infections in pediatric outpatients: seasonal patterns and clinical characteristics. Pediatr. Infect. Dis. J. 1994;13:269–273. doi: 10.1097/00006454-199404000-00005. [DOI] [PubMed] [Google Scholar]
- Knowles S.R., Phylips E.J., Dresser L., Matukas L. Common adverse events associated with use of ribavirin for severe acute respiratory syndrome in Canada. Clin. Infect. Dis. 2003;37:1139–1142. doi: 10.1086/378304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lamb R., Webster R. Orthomyxoviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott Williams and Wilkins; Philadelphia, PA: 2001. pp. 1487–1519. [Google Scholar]
- Lambert D.M., Barney S., Lambert A.L., Guthrie K., Medinas R., Davis D.E. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2186–2191. doi: 10.1073/pnas.93.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalezari J., Campion K., Keene O., Silagy C. Zanamivir for treatment of influenza A and B in high-risk patients: a pooled analysis of randomized controlled trials. Arch. Intern. Med. 2001;161:212–217. doi: 10.1001/archinte.161.2.212. [DOI] [PubMed] [Google Scholar]
- Le Q.M., Kiso M., Someya K., Sakai Y.T., Nguyen T.H., Nguyen K.H.L., Pham N.D., Ngyen H.H., Yamada S., Muramoto Y., Horimoto T., Takada A., Goto H., Suzuki T., Suzuki Y., Kawaoka Y. Isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- Li K.S., Guan Y., Wang J., Smith G.J., Xu K.M., Duan L., Rahardjo A.P., Puthavathana P., Buranathai C., Nguyen T.D. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;8:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- Liu S., Xiao G., Chen Y., He Y., Niu J., Escalante C.R. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie P.L. The classification of viruses infecting the respiratory tract. Paediatr. Resp. Rev. 2003;4:84–90. doi: 10.1016/S1526-0542(03)00031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggon K., Barik S. New drugs and treatment for respiratory syncytial virus. Rev. Med. Virol. 2004;14:149–168. doi: 10.1002/rmv.423. [DOI] [PubMed] [Google Scholar]
- Makela M.J., Puhukka T., Ruskanen O., Leinonen M., Saikku P., Kimpimaki M. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela M.J., Pauksens K., Rostila T., Fleming D.M., Man C.Y., Keene O.N., Webster A. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J. Infect. 2000;40:42–48. doi: 10.1053/jinf.1999.0602. [DOI] [PubMed] [Google Scholar]
- Malinowski M.D., Hostoffer R.W. Home care based treatment with short course inhaled ribavirin of parainfluenzavirus in a patient with severe combined immunodeficiency. J. Allergy Clin. Immunol. 2001;107:663. [Google Scholar]
- Martin K., Helenius A. Nuclear transport of influenza virus ribonucleoproteins: the viral matrix protein (M1) promotes export and inhibits import. Cell. 1991;67:117–130. doi: 10.1016/0092-8674(91)90576-k. [DOI] [PubMed] [Google Scholar]
- Masuda H., Suzuki H., Oshitani H., Saito R., Kawasaki S., Nishikawa M., Satoh H. Incidence of amantadine-resistant influenza A viruses in sentinel surveillance sites and nursing homes in Niigata, Japan. Microbiol. Immunol. 2000;44:833–839. doi: 10.1111/j.1348-0421.2000.tb02571.x. [DOI] [PubMed] [Google Scholar]
- McCarthy A.J., Bergin M., Silva L.M.D., Stevens M. Intravenous ribavirin therapy for disseminated adenovirus infection. Pediatr. Infect. Dis. J. 1995;4:1003–1004. doi: 10.1097/00006454-199511000-00017. [DOI] [PubMed] [Google Scholar]
- McKinlay M.A. Recent advances in the treatment of rhinovirus infections. Curr. Opin. Pharmacol. 2001;5:477–481. doi: 10.1016/s1471-4892(01)00083-2. [DOI] [PubMed] [Google Scholar]
- Meissner H.C. Selected populations at increased risk for respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2003;22:S40–S45. doi: 10.1097/01.inf.0000053884.21238.13. [DOI] [PubMed] [Google Scholar]
- Mendel D., Roberts N. In vitro and in vivo efficacy of influenza neuraminidase inhibitors. Curr. Opin. Infect. Dis. 1998;11:727–732. doi: 10.1097/00001432-199812000-00013. [DOI] [PubMed] [Google Scholar]
- Mishin V.P., Hayden F.G., Gubareva L.V. Susceptibilities of antiviral-resistant influenza viruses to novel neuraminidase inhibitors. Antimicrob. Agents Chemother. 2005;49:4515–4520. doi: 10.1128/AAC.49.11.4515-4520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–1881. [PubMed] [Google Scholar]
- Moler F.W., Steinhart C.M., Ohmit S.E., Stidham G.L., Pediatric Critical Study Group Effectiveness of ribavirin in otherwise well infants with respiratory syncytial virus-associated respiratory failure. J. Pediatr. 1996;128:422–428. doi: 10.1016/s0022-3476(96)70294-9. [DOI] [PubMed] [Google Scholar]
- Monto A.S., Robinson D.P., Herlocher M.L., Hinson J.M., Jr., Elliott M.J., Crisp A. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA. 1999;282:31–35. doi: 10.1001/jama.282.1.31. [DOI] [PubMed] [Google Scholar]
- Monto A.S., Fleming D.M., Henry D., De Groot R., Makela M., Klein T., Elliott M., Keene O.N., Man C.Y. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J. Infect. Dis. 1999;180:254–261. doi: 10.1086/314904. [DOI] [PubMed] [Google Scholar]
- Monto A.S. The role of antivirals in the control of influenza. Vaccine. 2003;21:1796–1800. doi: 10.1016/s0264-410x(03)00075-6. [DOI] [PubMed] [Google Scholar]
- Moscona A. Management of respiratory syncytial virus infection in the immunocompromised child. Pediatr. Infect. Dis. J. 2000;19:253–254. doi: 10.1097/00006454-200003000-00017. [DOI] [PubMed] [Google Scholar]
- Moscona A. Neuraminidase inhibitors for influenza. N. Engl. J. Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- Munoz F.M., Piedra P.A., Demmler G.J. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin. Infect. Dis. 1997;27:1197–1200. doi: 10.1086/514978. [DOI] [PubMed] [Google Scholar]
- Munoz F.M., Galasso G.J., Gwaltney J.M., Jr., Hayden F.G., Murphy B., Webster R., Wright P., Couch R.B. Current research on influenza and other respiratory viruses. Antivir. Res. 2000;46:91–124. doi: 10.1016/S0166-3542(00)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K.G., Aoki F.Y., Osterhaus A.D., Trottier S., Carewicz O., Mercier C.H., Rode A., Kinnersley N., Ward P. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- Ogra P.L. Respiratory syncycial virus: the virus, the disease and the immune response. Paediatr. Respir. Rev. 2004;5:S119–S126. doi: 10.1016/S1526-0542(04)90023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Compans R.W. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J. Gen. Virol. 1976;33:159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- Patick A.K., Binfor S.L., Brothers M.A., Jackson R.L., Ford C.E., Diem M.D., Maldonado F., Dragovich P.S., Zhou R., Prins J.T., Fuhrman S.A., Meador J.W., Zalman L.S., Matthews D.A., Worland S.T. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 1999;43:2444–2450. doi: 10.1128/aac.43.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Yeun K.Y., Osterhaus A.D., Stohr K. The acute severe respiratory syndrome. N. Engl. J. Med. 2003;349:2341–2344. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- Peng A.W., Milleri S., Stein D. Direct measurement of the anti-influenza agent Zanamivir in the respiratory tract following inhalation. Antimicrob. Agents Chemother. 2000;44:1974–1976. doi: 10.1128/aac.44.7.1974-1976.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P.H., Jr., Gravenstein S., Norwood P., De Bock V., Van Couter A., Gibbens M., Von Planta P.A., Ward P. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J. Am. Geriatr. Soc. 2001;249:1025–1031. doi: 10.1046/j.1532-5415.2001.49204.x. [DOI] [PubMed] [Google Scholar]
- Pevear D.C., Tull T.M., Seipel M.E., Groarke J.M. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 1999;43:2109–2115. doi: 10.1128/aac.43.9.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D.C., Hayden F.G., Demenczuk T.M., Barone L.R., McKinlay M.A., Collett M.S. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob. Agents Chemother. 2005;49:4492–4499. doi: 10.1128/AAC.49.11.4492-4499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The PREVENT Study Group Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- Prince G.A., Porter D.D. Treatment of parainfluenza type 3 bronchiolitis and pneumonia in a cotton rat model using topical antibody and glucocorticosteroid. J. Infect. Dis. 1996;173:598–608. doi: 10.1093/infdis/173.3.598. [DOI] [PubMed] [Google Scholar]
- Ribaud P., Scieux C., Freymuth F., Morinet F., Gluckman E. Successful treatment of adenovirus disease with intravenous cidofovir in an unrelated stem-cell transplant recipient. Clin. Infect. Dis. 1999;28:690–691. doi: 10.1086/517222. [DOI] [PubMed] [Google Scholar]
- Reed G., Jewett P.H., Thompson J., Tollefson S., Wright P.F. Epidemiology and clinical impact of parainfluenza virus infection in otherwise healthy infants and young children <5 years old. J. Infect. Dis. 1997;175:807–813. doi: 10.1086/513975. [DOI] [PubMed] [Google Scholar]
- Roberts N.A. Treatment of influenza with neuraminidase inhibitors: virological implications. Phil. Trans. Biol. Sci. 2001;353:1895–1897. doi: 10.1098/rstb.2001.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H.A., O’Connell J.F., McKinlay M.A. Treatment of human enterovirus infections. Antivir. Res. 1998;38:1–14. doi: 10.1016/s0166-3542(97)00068-5. [DOI] [PubMed] [Google Scholar]
- Rotbart H.A. Review: antiviral therapy for enteroviruses and rhinoviruses. Antivir. Chem. Chemother. 2000;11:261–271. doi: 10.1177/095632020001100402. [DOI] [PubMed] [Google Scholar]
- Ruuskanen O., Meurman O., Sarkkinen H. Adenoviral diseases in children: a study of 105 cases. Pediatrics. 1985;76:79–83. [PubMed] [Google Scholar]
- Saito R., Sakai T., Sato I., Sano Y., Oshitani H., Sato M., Suzuki H. Frequency of amantadine-resistant influenza A viruses during two seasons featuring cocirculation of H1N1 and H3N2. J. Clin. Microbiol. 2003;41:2164–2165. doi: 10.1128/JCM.41.5.2164-2165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen C., Blomqvist S., Hori T. Human rhinoviruses. Pediatr. Respir. Rev. 2003;4:91–98. doi: 10.1016/s1526-0542(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Schmidt A.C., Couch R.B., Galasso G.J., Hayden F.G., Murphy B.R., Chanock R.N. Current research on respiratory viral infections. Antivir. Res. 2001;50:157–196. doi: 10.1016/S0166-3542(01)00136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta S. Approaches to antiviral chemotherapy for acute respiratory infections. Antivir. Chem. Chemother. 1998;9:93–107. doi: 10.1177/095632029800900201. [DOI] [PubMed] [Google Scholar]
- Shiraishi K., Mitamura K., Sakai-Tagawa Y., Goto H., Sugaya N., Kawaoka Y. High frequency of resistant viruses harboring different mutations in amantadine-treated children with influenza. J. Infect. Dis. 2003;188:57–61. doi: 10.1086/375799. [DOI] [PubMed] [Google Scholar]
- Sidwell R.W., Smee D.F. Peramivir (BCX-1812, RWJ-270201): potential new therapy for influenza. Expert Opin. Invest. Drugs. 2002;11:859–869. doi: 10.1517/13543784.11.6.859. [DOI] [PubMed] [Google Scholar]
- Sidwell R.W., Bailey K.W., Wong M.H., Barnard D.L., Smee D.F. In vitro and in vivo influenza virus-inhibitory effects of viramidine. Antivir. Res. 2005;68:10–17. doi: 10.1016/j.antiviral.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Somani R., Evans M.J. Role of glucocorticosteroids in treating croup. Can. Fam. Physician. 2001;47:733–735. [PMC free article] [PubMed] [Google Scholar]
- Stiver G. The treatment of influenza with antiviral drugs. Can. Med. Assoc. J. 2003;168:49–56. [PMC free article] [PubMed] [Google Scholar]
- Sweet C., Hayden F.G., Jakeman K.J., Grambas S., Hay A.J. Virulence of rimantadine-resistant human influenza A (H3N2) viruses in ferrets. J. Infect. Dis. 1991;164:169–172. doi: 10.1093/infdis/164.5.969. [DOI] [PubMed] [Google Scholar]
- Temte J.L. A family physician's perspective on picornavirus infections in primary care. Arch. Fam. Med. 2000;9:921–922. doi: 10.1001/archfami.9.9.921. [DOI] [PubMed] [Google Scholar]
- The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- Thiel V., Herold J., Schell B., Sidell S.G. Infectious RNA transcribed in vitro from cDNA copy of the human coronavirus genome cloned in vaccinia virus. J. Gen. Virol. 2001;82:1273–1281. doi: 10.1099/0022-1317-82-6-1273. [DOI] [PubMed] [Google Scholar]
- Thompson W.W., Shay D.K., Weintraub E., Brammer L., Cox N., Anderson L.J., Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Treanor J.J., Hayden F.G., Vrooman P.S., Barbarash R., Bettis R., Riff D., Singh S., Kinnersley N., Ward P., Mills R.G. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- Tsui P.T., Kwok M.L., Yuen H., Hai S.T. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg. Infect. Dis. 2003;9:1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R., Wecker M., Pohl G., Witek T.J., McNally E., St George R., Winther B., Hayden F.G. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA. 1999;281:1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- Tyrrell D.A.J., Bynoe M.L. Cultivation of a novel common cold virus in organ cultures. BMJ. 1965;1:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., De Groot R., Fouchier R.A., Osterhaus A.D.M.E. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuvojic O., Mills J. Preventive and therapeutic strategies for respiratory syncytial virus infection. Curr. Opin. Pharmacol. 2001;20:232–255. doi: 10.1016/s1471-4892(01)00087-x. [DOI] [PubMed] [Google Scholar]
- Wadell G. Molecular epidemiology of human adenoviruses. Curr. Top. Microbiol. Immunol. 1984;110:191–220. doi: 10.1007/978-3-642-46494-2_7. [DOI] [PubMed] [Google Scholar]
- Wang E., Sun X., Qian Y., Zhao L., Tien P., Gao G.F. Both heptad repeats of human respiratory syncycial virus fusion protein are potent inhibitors of viral fusion. Biochem. Biophys. Res. Commun. 2003;302:469–475. doi: 10.1016/s0006-291x(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Wang M.Z., Tai C.Y., Mendel D.B. Mechanism by which mutations at His274 alter sensitivity of influenza A virus N1 neuraminidase to oseltamivir carboxylate and zanamivir. Antimicrob. Agents Chemother. 2002;46:3809–3816. doi: 10.1128/AAC.46.12.3809-3816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A.D.B. Pleconaril—an advance in the treatment of enteroviral infection in immunocompromised patients. J. Clin. Virol. 2005;32:1–6. doi: 10.1016/j.jcv.2004.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver R., Monto A.S., Carewicz O., Schatteman E., Hassman M., Hedrick J., Jackson H.C., Huson L., Ward P., Oford J.S. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285:748–754. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- Welliver R.C. Respiratory syncycial virus and other respiratory viruses. Pediatr. Infect. Dis. J. 2003;22:S6–S12. doi: 10.1097/01.inf.0000053880.92496.db. [DOI] [PubMed] [Google Scholar]
- Whetherall N.T., Trivedi T., Zeller J., Hodges-Savola C., McKimm-Breshkin J.L., Zambon M., Hayden F.G. Evaluation of neuraminidase enzyme assays using different substrates to measure susceptibility of influenza virus clinical isolates to neuraminidase inhibitors: report of the neuraminidase inhibitor susceptibility network. J. Clin. Microbiol. 2003;41:742–750. doi: 10.1128/JCM.41.2.742-750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner S., Van J.J., Liu G., Byrns P., Cypra C., Campbell W., Stiles A. Direct cost analyses of palivizumab treatment in a cohort of at-risk children: evidence from the North Carolina Medicaid Program. Pediatrics. 2004;114:1612–1619. doi: 10.1542/peds.2004-0959. [DOI] [PubMed] [Google Scholar]
- Weinstock D.M., Gubareva L.V., Zucotti G. Prolonged shedding of multidrug-resistant influenza A virus in an immunocompromised patient. N. Engl. J. Med. 2003;348:867–868. doi: 10.1056/NEJM200302273480923. [DOI] [PubMed] [Google Scholar]
- Whitley R.J., Hayden F.G., Reisinger K.S., Young N., Dutkowski R., Ipe D., Mills R.G., Ward P. Oral oseltamivir treatment of influenza in children. Pediatr. Infect. Dis. J. 2001;20:127–133. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y., Wang B.H., Poon R.W., Cai J.J., Luk W.K., Poon L.L., Wong S.S., Guan Y., Peiris J.S., Yuen K.Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Tsoi H.W., Huang Y., Poon R.W., Chu C.M., Lee R.A., Luk W.K., Wong G.K., Wong B.H., Cheng V.C., Tang B.S., Wu A.K., Yung R.W., Chen H., Guan Y., Chan K.H., Yuen K.Y. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J. Infect. Dis. 2005;192:1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P., Webster R. Orthomyxoviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott Williams and Wilkins; Philadelphia, PA: 2001. pp. 1533–1562. [Google Scholar]
- Wyde P.R., Chetty S.N., Jewell A.M., Boivin G., Piedra P.A. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antivir. Res. 2003;60:51–59. doi: 10.1016/s0166-3542(03)00153-0. [DOI] [PubMed] [Google Scholar]
- Wyde P.R., Moylett E.H., Chetty S.N., Jewell A.M., Bowlin T.L., Piedra P.A. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by NMSO3 in tissue culture assays. Antivir. Res. 2004;63:51–59. doi: 10.1016/j.antiviral.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Yang R., Yoshinaka Y., Amari S., Nakano T., Cinatl J., Rabenau H., Doerr H.W., Hunsmann G., Otaka A., Tamamura H., Fujii N., Yamamoto N. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H.L., Monto A.S., Webster R.G., Govorkova E.A. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J. Infect. Dis. 2005;192:665–672. doi: 10.1086/432008. [DOI] [PubMed] [Google Scholar]
- Zhao X., Singh M., Malashkevich V.N., Kim P.S. Structural characterization of the HRSV fusion protein core. Proc. Natl. Acad. Sci. U.S.A. 2000;97:14172–14177. doi: 10.1073/pnas.260499197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M. SARS immunity and vaccination. Cell. Mol. Immunol. 2004;1:193–198. [PubMed] [Google Scholar]


