Abstract
A prospective study was carried out on 92 randomly selected beef herds in the Midi-Pyrénées region in France. The objective was to determine factors associated with time to neonatal gastroenteritis. By taking into account the “intra-herd” correlation in failure time (in the semiparametric Cox model), we identified 12 management risk factors associated with hazard of diarrhoea. Some previously have been identified, but “new” risk factors were feeding of corn silage and the incidence of diarrhoea in the last season. We used the two main approaches which are often reviewed: marginal and frailty Cox models. Our results show that these two models give different parameter estimates, so the choice of the model remains crucial.
Keywords: Epidemiology, Risk factors, Beef calf, Diarrhoea, Correlation, Cox model
1. Introduction
We studied diarrhoea in newborn calves (the major cause of neonatal mortality) Sivula et al., 1996a, Wells et al., 1996, Bendali et al., 1999b. Calves are at the greatest risk of diarrhoea during the first week of life, and this risk decreases with age Vallet et al., 1985, Waltner-Toews et al., 1986. The incidence risks of diarrhoea in calves <30 days old reported by several studies varies between 15 and 20%. The mortality risk is 1.5–8% Clement et al., 1995, Quigley et al., 1995.
In the Midi-Pyrénées region (France), 80% of herds and 20% of neonatal calves are affected by diarrhoea; the case-fatality risk is 50% (Bichet, 1995).
Because of the large number of etiological agents (Escherichia coli, rotavirus, coronavirus and cryptosporidium), the prevention of neonatal diarrhoea in beef calves is difficult—but should be centred around management factors.
Two groups of factors involved in gastrointestinal disease are included in the statistical analysis: factors at the herd level (e.g. herd size, calf housing) and factors at the calf level (e.g. calving conditions, colostrum-feeding management, navel treatment). Our study is a continuation of a previous investigation conducted by Bendali et al. (1999b) on a large randomly selected sample of beef calves.
There has been considerable recent interest in the regression modelling of survival data, which has led to two broad classes of models: marginal and frailty models. With the marginal approach (Wei et al., 1989), a survival model is specified for each subject of the cluster along with assumptions about the dependence of survival times within clusters. Alternatively, a frailty model Vaupel et al., 1979, Andersen et al., 1999 postulates that the hazard within a particular cluster is the product of a function of the covariates and a cluster-specific parameter known as the “frailty”. The frailty commonly is used to indicate unobserved (i.e. “latent”) cluster-specific variables. Furthermore, independence among observed data items is assumed conditional on the frailty.
In interpretation of the results, the main difference between these two approaches is the meaning of the regression coefficients. In the marginal approach, the regression coefficients have a population-averaged interpretation—whereas in the frailty model, a cluster-specific response is modeled.
In addition to the previous study, based on a marginal approach, we used a frailty Cox model to determine factors associated with time to neonatal diarrhoea in French beef calves.
2. Statistical models and epidemiological data
2.1. The Cox model for survival data
Descriptive and analytical statistics were performed using S-PLUS© (MathSoft et al., 1999). The analysis was based on Cox’s proportional-hazards model (Cox, 1972).
In the semi-parametric Cox’s regression model, the hazard function related to the j th subject () of the i th herd () was:
| (1) |
where t is the observed age (days) when diarrhoea occurred (or censoring time), an unknown non-parametric baseline hazard function (which acts like a mean hazard for the whole population), the vector of observed covariates and the corresponding transposed vector of the regression coefficients. Then, was the estimated vector of relative risks (RRs), sometimes called “hazard risks” (HRs). This type of model permitted us to use the information even for an animal that was censored. The estimation is done by maximizing the Cox’s partial likelihood.
Although observations generally are correlated within the same unit, the estimator is consistent for (Wei et al., 1989). Unfortunately, the usual variance–covariance estimator is not valid.
We paid attention to the two types of Cox’s models, which were able to take into account a possible dependence among the components.
First, we used a marginal Cox’s model (MCM). To take correlation into account, it is possible to correct the variance estimate through the estimation function (Wei et al., 1989), as in the case of generalized estimating equations (Liang et al., 1992). The new variance estimator is similar to the sandwich estimator (Binder, 1983). The regression coefficient, which remains the same as in the naive (i.e. without correlation taken into account) model, is interpreted as a “population-average” coefficient. Interpretation of the parameters is obtained by considering the risk factors associated with the sample herds; consequences of a possible intra-herd correlation are taken into account. However, we may not extend the conclusions from this analysis to another herd.
Secondly, we used a frailty Cox’s model (FCM) to formulate the instantaneous risk as (Vaupel et al., 1979):
| (2) |
where is a random variable corresponding to the i th herd effect, which increases failure risk (and is consequently named “frailty”). is said to be “cluster-specific”, i.e. its interpretation is conditional on the fact that a subject belongs to a particular herd. The distribution of usually is chosen to be either gamma, normal or positive stable distributions Hougaard, 1986, Hougaard, 1995, because of the positivity of these distributions. Furthermore, the family of frailty distributions is constrained to have a mean equal to 1 for identifiability (Elbers and Ridder, 1982).
The frailty term allows both modelling of the intra-cluster correlation and generalization of the results from the sampled beef herds to the population of beef herds.
2.2. Computation
All the fits were performed with the S-PLUS coxph procedure: the MCM required the cluster option, whereas for the FCM, we specified the frailty option. The risk-factor analysis was done using a backward-elimination method in four steps. Initially, all variables taken individually with the incidence of diarrhoea were offered in a naive univariable Cox’s regression model. Then, any variable with was eligible for the next step in a multivariable model. In the second step, previously qualified variables were grouped into the same model within categories: feeding system, management, calving, housing and so on. This grouping procedure took into account potential confounding factors and the inter-relationships between variables of the same class. In the third step, two final multivariable models were fitted with all the variables that had remained significant during the two previous steps: the first one was a MCM and the second one was a gamma FCM. The fourth step consisted into a backward-elimination method for each of these two models.
2.3. Data collection and target population
A prospective study on a random sample of 92 herds in the Midi-Pyrénées region was conducted in 8 departments of the southwest of France (Bendali et al., 1999a). A simple random stratified sampling design was used to select herds for the study. The stratification was based on the department and adjusted for the number of farms in each department using a proportional sampling method. A random-number sampling algorithm was used to identify the herd to be enrolled in the study. The enumeration rule was that if a farm was selected, then all the farm’s calves were enrolled in the study.
The sampling frame was the exhaustive listing of farms in that region. The inclusion criteria consisted of herds with >20 adult beef cows, at least three-quarters of calvings during the survey and the five most-important breeds of the region (Charolaise, Limousine, Gasconne, Aubrac, Blonde d’Aquitaine). A total of 7000 herds was eligible and represented 30% of the total herds of that region.
From 95 randomly selected beef herds in the Midi-Pyrénées, 92 were involved in this study and followed from December 1995 to April 1996. During this period, 3157 calves were born in the 92 selected herds. Complete data were obtained from 96.5% (3047) of the calves in the study. The global incidence of diarrhoea was 14.4% and the mortality was 4.2%, but these incidences included marked variations between the herds, age of calves and month of birth (Bendali et al., 1999a).
2.4. Data management
The cows were grouped according to their primiparous or multiparous status. Some initially continuous variables were transformed into categorical data, in accordance with a supposed cut-off point or minimum level of information given in the literature. The following cut-off values were used. The time of first colostrum (recorded in hours after birth) was grouped together for calves fed within the first 6 h of life and calves fed after 6 h Fourichon et al., 1996, Hall et al., 1992. Scale for the cleanliness of animals varied from 0 (cleanest) to 4 (most dirty) (Faye and Barnouin, 1985). Calf stocking density (the number of calves per available area) was considered sufficient when a calf had at least 1.6 or 1 in the tie stall or free stall, respectively (Vallet et al., 1985).
To assess passive immunity and the effectiveness of colostrum feeding, calves were sampled for total protein in the serum. The minimum “satisfactory” level of this total protein concentration was 50 g/L Perino et al., 1993, Rea et al., 1996.
The ambient-weather variables (temperature, dampness) were recorded at the visit closest to the date of birth of each calf. We calculated a parameter which took into account external and internal temperatures and dampness simultaneously (Andrieu et al., 1985); this method allows a single efficient measurement of atmospheric conditions to be used for each calf in accordance with birth.
3. Results
3.1. Marginal versus frailty fits for the hazard risks
Characteristics of the study population and management practices are shown in Table 1, Table 2, Table 3 .
Table 1.
Variables related to birth and prophylaxis categories associated with diarrhoea in the univariable model of diarrhoea in 3047 French beef calves in 92 herds (December 1995–April 1996)
| Variable | Levels | Sample |
Diarrhoea |
|||
|---|---|---|---|---|---|---|
| % | Cases | % | ||||
| Number of calves born | Single | 96.6 | 429 | 97.5 | 0.36 | |
| More | 103 | 3.4 | 11 | 2.5 | ||
| Calves born prematurely | No | 99.3 | 437 | 99.3 | 0.51 | |
| Yes | 20 | 0.7 | 3 | 0.7 | ||
| Calves born with malformation | No | 99.4 | 437 | 99.3 | 0.39 | |
| Yes | 17 | 0.6 | 3 | 0.7 | ||
| Respiration of calves at birth | Normal | 92.9 | 401 | 91.1 | 0.001 | |
| Dyspnea | 220 | 7.1 | 39 | 8.9 | ||
| Curative use of stimulants (orexigenic or other) at birth | No | 98.9 | 437 | 99.3 | 0.34 | |
| Yes | 35 | 1.1 | 3 | 0.7 | ||
| Preventive use of stimulants (orexigenic or other) at birth | No | 98.2 | 433 | 98.4 | 0.76 | |
| Yes | 56 | 1.8 | 7 | 1.6 | ||
| Navel dipping | Yes | 61.0 | 271 | 61.6 | 0.78 | |
| No | 39.0 | 169 | 38.4 | |||
| Navel dipping product | No | 40.0 | 169 | 38.4 | 0.05 | |
| Iodine | 880 | 29.0 | 146 | 33.2 | ||
| Other | 960 | 31.0 | 125 | 28.4 | ||
| First colostrum feeding assisted by the farmer | Yes | 74.0 | 335 | 76.1 | 0.53 | |
| No | 797 | 26.0 | 105 | 23.9 | ||
| Time of first feeding colostrum | >6 h | 88.0 | 392 | 89.1 | 0.47 | |
| <6 h | 363 | 12.0 | 48 | 10.9 | ||
| Frequency of feeding on the first day of life | 84.0 | 353 | 80.2 | 0.06 | ||
| 498 | 16.0 | 87 | 19.8 | |||
| Month of birth | December 1995 | 434 | 14.3 | 76 | 17.2 | >0.001 |
| January 1996 | 754 | 24.7 | 67 | 15.2 | ||
| February 1996 | 963 | 31.6 | 131 | 37.9 | ||
| March 1996 | 896 | 29.4 | 166 | 29.7 | ||
| Ease of calving | No assistance | 58.6 | 226 | 51.3 | 0.002 | |
| Easy pull | 795 | 26.1 | 138 | 31.3 | ||
| Hard pull | 467 | 15.3 | 76 | 17.4 | ||
| Frequency of feeding calf on second to third days of life | 84.0 | 361 | 82.0 | 0.36 | ||
| 494 | 16.0 | 79 | 18.0 | |||
| Sex of newborn | Male | 50.0 | 235 | 53.4 | 0.08 | |
| Female | 50.0 | 202 | 46.6 | |||
| Cleanliness of calves () | Clean | 78 | 84.7 | 374 | 85.0 | 0.02 |
| Dirty | 14 | 15.3 | 72 | 15.0 | ||
Table 2.
Variables related to feeding and management categories associated with diarrhoea in the univariable model of diarrhoea in 3047 French beef calves in 92 herds (December 1995–April 1996)
| Variable | Levels | Sample |
Diarhoea |
|||
|---|---|---|---|---|---|---|
| N | % | Cases | % | |||
| Feeding concentrate | Yes | 31 | 33.7 | 123 | 27.9 | >0.001 |
| No | 61 | 66.3 | 323 | 72.1 | ||
| Feeding corn silage | Yes | 41 | 44.5 | 189 | 42.9 | 0.003 |
| No | 51 | 55.5 | 257 | 57.1 | ||
| Feeding grass silage | Yes | 57 | 61.9 | 270 | 61.3 | 0.001 |
| No | 35 | 38.1 | 176 | 38.7 | ||
| Quantities of feed | Free | 22 | 23.9 | 107 | 24.3 | 0.33 |
| Restricted | 70 | 76.1 | 339 | 75.7 | ||
| Different diet for pregnant and lactating cows | Yes | 42 | 45.6 | 178 | 40.4 | 0.02 |
| No | 50 | 54.4 | 268 | 59.6 | ||
| Different diet between primiparous and multiparous | Yes | 29 | 31.5 | 153 | 34.7 | 0.80 |
| No | 63 | 68.5 | 293 | 65.3 | ||
| Flushing used | Yes | 16 | 17.4 | 76 | 17.3 | 0.26 |
| No | 76 | 82.6 | 370 | 82.7 | ||
| Type of housing | Free-loose | 47 | 51.1 | 193 | 43.8 | 0.49 |
| Tie stall-tethered | 45 | 48.9 | 253 | 56.2 | ||
| Adequate ventilation of building | Yes | 49 | 53.2 | 206 | 46.8 | 0.74 |
| No | 43 | 46.8 | 240 | 53.2 | ||
| Presence of ammonia detected by technician | No | 86 | 93.4 | 394 | 89.5 | 0.002 |
| Yes | 6 | 6.6 | 52 | 10.5 | ||
| Building dampness estimated by technician | No | 86 | 93.4 | 396 | 90.0 | 0.003 |
| Yes | 6 | 6.6 | 50 | 10.0 | ||
| Nursing (quarantine) location for adults | Yes | 25 | 27.1 | 80 | 18.2 | 0.03 |
| No | 67 | 72.9 | 366 | 81.8 | ||
| Nursing (quarantine) location for calves | Yes | 31 | 33.7 | 115 | 26.1 | 0.006 |
| No | 61 | 66.3 | 331 | 73.9 | ||
| Calf stocking | Sufficient | 61 | 66.3 | 258 | 58.6 | >0.001 |
| Insufficient | 31 | 33.7 | 188 | 41.4 | ||
| Animals grouped on lot | Yes | 74 | 80.4 | 375 | 85.2 | 0.02 |
| No | 18 | 19.6 | 71 | 14.8 | ||
| Calf location | Hutches-group pen | 10 | 10.8 | 54 | 12.3 | 0.10 |
| Individual tied | 67 | 72.8 | 319 | 72.5 | ||
| Free | 15 | 16.4 | 73 | 15.2 | ||
| Calving grouped in winter | Yes | 42 | 45.6 | 255 | 57.9 | 0.83 |
| No | 50 | 54.4 | 191 | 42.1 | ||
| Incidence of diarrhoea in the last season | >5% | 28 | 30.4 | 78 | 17.7 | <0.001 |
| 5 % | 64 | 69.6 | 368 | 82.3 | ||
Table 3.
Variables related to prophylaxis and miscellaneous categories associated with diarrhoea in the univariable model of diarrhoea in 3047 French beef calves in 92 herds (December 1995–April 1996)
| Variable | Levels | Sample |
Diarhoea |
|||
|---|---|---|---|---|---|---|
| N | % | Cases | % | |||
| Straw for caw barn (regular 1/day, sufficient 2–5 kg/cow) | Yes | 39 | 42.4 | 148 | 33.6 | 0.30 |
| No | 53 | 57.6 | 298 | 66.4 | ||
| Straw for calves barn (regular 1/day, sufficient 1.5 kg/calf) | Yes | 31 | 33.7 | 191 | 43.4 | 0.70 |
| No | 61 | 66.3 | 255 | 56.6 | ||
| Cleaning before calving season in calf barn | Yes | 24 | 26.1 | 162 | 36.8 | 0.001 |
| No | 68 | 73.9 | 284 | 63.2 | ||
| Cleaning after calving season in calf barn | Yes | 20 | 21.7 | 71 | 16.1 | 0.003 |
| No | 72 | 78.3 | 375 | 83.9 | ||
| Cleaning after each diarrhoea episode in calf barn | Yes | 4 | 4.3 | 30 | 6.8 | 0.64 |
| No | 88 | 95.7 | 416 | 93.2 | ||
| Disinfecting calf area (frequently) | Yes | 39 | 42.4 | 189 | 42.9 | 0.65 |
| No | 53 | 57.6 | 257 | 57.1 | ||
| Cleanliness of cows () | Clean | 52 | 56.5 | 212 | 48.2 | 0.003 |
| Dirty | 40 | 43.5 | 234 | 51.8 | ||
| Disinfecting calving area | Yes | 15 | 16.3 | 95 | 21.6 | 0.65 |
| No | 77 | 83.7 | 351 | 78.4 | ||
| Cleaning calving location after each calving | Yes | 76 | 82.6 | 390 | 88.6 | >0.001 |
| No | 16 | 17.4 | 56 | 11.4 | ||
| Dam vaccinated against other agents | Yes | 751 | 25.0 | 81 | 18.4 | >0.001 |
| No | 75.0 | 359 | 81.6 | |||
| Dam parity | Primiparous | 620 | 20.0 | 93 | 21.1 | 0.44 |
| Multiparous | 80.0 | 347 | 78.9 | |||
| Dam vaccinated against coronavirus | Yes | 809 | 27.0 | 110 | 27.5 | 0.19 |
| No | 73.0 | 330 | 72.5 | |||
| Dam vaccinated against rotavirus | Yes | 807 | 26.0 | 110 | 27.5 | 0.20 |
| No | 74.0 | 330 | 72.5 | |||
| Dam vaccinated against E. coli | Yes | 777 | 26.0 | 114 | 25.9 | 0.68 |
| No | 74.0 | 326 | 74.1 | |||
| Majority of cows vaccinated against at least one infectious agent | Yes | 22 | 23.9 | 113 | 25.7 | 0.03 |
| No | 70 | 76.1 | 333 | 74.3 | ||
| Majority of calves vaccinated against at least one infectious agent | Yes | 21 | 22.8 | 113 | 25.7 | 0.90 |
| No | 71 | 77.2 | 333 | 74.3 | ||
| Additional vitamins and minerals to cows | Yes | 55 | 59.8 | 264 | 60.0 | >0.001 |
| No | 37 | 40.2 | 182 | 40.0 | ||
| Additional vitamins and minerals to calves | Yes | 40 | 43.4 | 255 | 57.9 | 0.18 |
| No | 52 | 56.6 | 191 | 42.1 | ||
No significant difference was observed between the parameters (HR and value) estimated at the first and second steps of the analysis. The univariable screening procedure identified more than 20 variables associated with diarrhoea () in the neonatal period.
Among the variables the most positively associated with hazard of diarrhoea (Table 4 ), were the dam vaccination against other agents, the presence of ammonia and the cleaning after calving season (1.54 and 1.45).
Table 4.
Marginal and frailty multivariable Cox models of diarrhoea in 3047 French beef calves in 92 herds (December 1995–April 1996)
| Variable | Marginal model |
Frailty model |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | S.E. | HR | 95% CI | S.E. | |||
| Month of birth | ||||||||
| January | 0.44 | 0.24, 0.82 | 0.31 | 0.01 | 0.44 | 0.30, 0.63 | 0.18 | >0.001 |
| February | 0.73 | 0.38, 1.39 | 0.32 | 0.34 | 0.79 | 0.56, 1.12 | 0.17 | 0.15 |
| March | 1.46 | 0.81, 2.65 | 0.30 | 0.21 | 1.88 | 1.32, 2.68 | 0.17 | >0.001 |
| Ease of calving | ||||||||
| Easy | 1.42 | 1.07, 1.89 | 0.14 | 0.02 | 1.24 | 0.98, 1.58 | 0.12 | 0.06 |
| Difficult | 1.36 | 1.01, 1.84 | 0.15 | 0.04 | 1.40 | 1.04, 1.88 | 0.15 | 0.03 |
| Vacc. others (no) | 1.58 | 1.18, 2.10 | 0.14 | 0.002 | 1.67 | 1.33, 2.10 | 0.11 | >0.001 |
| Concentrate (no) | 1.32 | 1.06, 1.65 | 0.11 | 0.01 | 1.43 | 1.10, 1.84 | 0.13 | 0.006 |
| Corn silage (no) | 0.73 | 0.57, 0.94 | 0.12 | 0.01 | 0.69 | 0.54, 0.89 | 0.12 | 0.004 |
| Ammonia (yes) | 1.62 | 1.20, 2.18 | 0.15 | 0.001 | 1.60 | 0.99, 2.60 | 0.24 | 0.09 |
| Stocking (sufficient) | 1.34 | 1.09, 1.64 | 0.10 | 0.005 | 1.36 | 1.04, 1.76 | 0.13 | 0.02 |
| Diar. incidence (high) | 1.41 | 1.07, 1.86 | 0.14 | 0.01 | 1.45 | 1.12, 1.88 | 0.13 | 0.004 |
| Cleaning aft. (no) | 1.54 | 1.20, 1.96 | 0.12 | >0.001 | 1.45 | 1.07, 1.95 | 0.15 | 0.02 |
| Diar. cleaning (no) | 0.56 | 1.09, 1.87 | 0.28 | 0.04 | 0.45 | 0.25, 0.81 | 0.29 | 0.007 |
| Cows clean. (low) | 1.27 | 1.02, 1.60 | 0.11 | 0.03 | 1.37 | 1.08, 1.73 | 0.12 | 0.007 |
The variables associated with the lowest hazard in our study were the cleaning after each diarrhoea episode in calf barn and the feeding corn silage.
The ratio of the robust (marginal or frailty model) to the naive variances varied from 1.14 to 6.25.
Among the 11 variables which were significantly associated with time to diarrhoea in the MCM, only 1 became nonsignificant in the FCM: the presence of ammonia ( updated to 0.09).
The hazard of diarrhoea was higher in calves born in March than those born in December. Moreover, a high incidence of diarrhoea in the previous season (1994–1995) and dystocia increased the hazard.
We noted that hygiene and cleanliness seemed to have an impact on the time to diarrhoea. Newborn calves had a higher hazard of diarrhoea when stalls were not cleaned after the calving season, and when dams were not clean (as was observed in 43.5% of the farms).
The stocking of calves was considered insufficient in 33.7% of the herds and was associated with an increased hazard of diarrhoea—as was dam vaccination against other agents than pathogens associated with diarrhoea.
3.2. Frailty as herd effect
Standard S-PLUS output provides a significance test for the frailty: its effect was very significant (). We also studied prediction of the frailty (Fig. 1 ). Herd with a predicted frailty >1 corresponded to a weakened herd (i.e. to herd with a detrimental effect on its baseline hazard). As expected, we noted that among herds with the lowest predicted frailties, number of diarrhoea cases and number of censorings were deeply unbalanced: the censoring rate was always >90%.
Fig. 1.
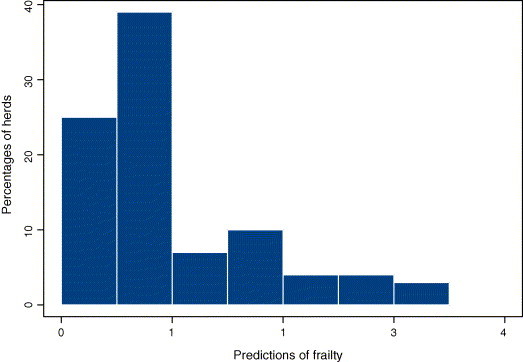
Histogram of the frailty predictions .
4. Discussion
4.1. Factors related to birth
Several factors were associated with diarrhoea hazard (including practices related to calving and the care of the calf). Difficult calving causes stress for the newborn calf, which decreases resistance to pathogens owing to a combination of reduced calf vigour and delayed ingestion of colostrum. In addition, newborn calves which require assistance during parturition can be weakened for long periods after birth, and thus become exposed to more faecal pathogens than calves which stand up shortly after birth.
In contrast to previous studies Roy, 1990, Schumman et al., 1990, Sivula et al., 1996b, dyspnea was not associated with the hazard of diarrhoea. One explanation might be that the most calves (92.9%) had adequate respiration at birth.
Colostrum factors were not associated with the hazard of gastroenteritis. One explanation may be that the most calves (76%) has a sufficient level of total serum protein (>50 g/L).
No effect of the navel treatment on the days to diarrhoea was established—in agreement with Waltner-Toews et al. (1986).
Because testing the effect of birth season needs a follow-up longer than 1 or 2 years and more calendar months, we estimated the effect of the month rather than the season. The higher hazard was observed in March (as in the initial study; Bendali et al. 1999b). This could be explained by calf overcrowding in addition to climate and weather conditions, and to a high burden of infection in the late calving season, which could be the result of the accumulation of manure and contaminated bedding.
4.2. Management and ambient conditions
Herd size or type of housing did not have a significant influence on time to morbidity in our study.
The ammonia concentration was associated with the hazard of gastroenteritis. This result might be a consequence of bad ventilation or an insufficient quantity of straw. The same value was reported by Schumman et al. (1990) when inadequate draining was observed.
Cleaning after each calving season probably leads to a reduction in the spread of the micro-organisms which are the origin of bovine diarrhoea. Two unexpected negative associations were found with the cleaning after each diarrhoea episode and the cleaning before each calving season. One possible explanation is that farmers with herds with a history of disease would be more likely to clean and disinfect locations. The second possible explanation is that the cleaning might have no substantial effect because the calf housing was already empty just before calving and therefore the number of pathogens already reduced.
4.3. Prophylaxis
Having dams vaccinated against agents such as bovine viral diarrhoea and Clostridium perfringens—but not against E. coli—rotavirus and coronavirus, seems to decrease the hazard of diarrhoea. This result could be explained by a general good management of the herd.
The initial study (Bendali et al., 1999b) found an unexpected negative association between dam vaccination with E. coli and calf diarrhoea. Although the hazard risk remained <1 in our study, the effect was no longer significant in these robust approaches.
Minerals and vitamins offered to cows during the dry period were associated with increased diarrhoea hazard.
4.4. Feed factors
Several feed factors were tested. Calves from herds with no concentrate feeding were at higher hazard of diarrhoea ( in the FCM).
Feeding corn silage has been associated with an increased hazard of diarrhoea. One explanation might be that feeding additional corn silage could induce a transient increase in blood triglyceride and blood urea nitrogen, which may contribute to the hazard of diarrhoea.
Herds with high incidences of diarrhoea in the previous calving season were more likely to show this high rate ( in the FCM). This result may be explained by the fact that herd management did not change markedly from one season to the next and the concentration of pathogens remained roughly constant. Another explanation may be the possibility of transmission from older to younger animals.
Our results showed that it is not easy to identify separately individual effects of several management practices, and very often, many factors are associated. The consequence is that providing advises to farmers should simultaneously takes several parameters into account.
4.5. Conclusion
Conclusions regarding factors influencing the hazard of diarrhoea in neonatal calves from the two different models (with correlation taken into account) were similar. Hazards were higher when cows were not vaccinated (against a variety of specific agents), calvings needed assistance, cows were not clean, the building smelled of ammonia, and calf-diarrhoea incidence was >5% the previous year. However, we recommend use of the FCM if statistical inference is an intention.
Furthermore, the study of frailty allows us to distinguish between herds according to the fact that this component is an indicator of fragility.
References
- Andersen P.K., Klein J.P., Zhang M.-J. Testing for centre effects in multi-center survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat. Med. 1999;18:1489–1500. doi: 10.1002/(sici)1097-0258(19990630)18:12<1489::aid-sim140>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Andrieu, S., Fostier, B., Tillie, M., Mathieu, P., 1985. L’ambiance dans les btiments d’élevages bovins: méthodes pour le diagnostic de l’ambiance dans les btiments d’élevages bovins. Doc. 87101, ITEB/EDE, Aisne.
- Bendali F., Bichet H., Schelcher F., Sanaa M. Pattern of diarrhoea in newborn beef calves in south-west France. Vet. Res. 1999;30:61–74. [PubMed] [Google Scholar]
- Bendali F., Sanaa M., Bichet H., Schelcher F. Risk factors associated with diarrhoea in newborn calves. Vet. Res. 1999;30:509–522. [PubMed] [Google Scholar]
- Bichet H. L’activité vétérinaire passée au peigne fin. Réseau VEGA. Rev. d’Epidémiosurveillance Anim. 1995;7:4–14. [Google Scholar]
- Binder D.A. On the variance of asymptotically normal estimators from complex surveys. Int. Stat. Rev. 1983;51:279–292. [Google Scholar]
- Clement J.C., King M.E., Salman M.D., Wittum T.E., Casper H.H., Odde K.G. Use of epidemiologic principles to identify risk factors associated with the development of diarrhoea in calves in five beef herds. J. Am. Vet. Med. Assoc. 1995;207:1334–1338. [PubMed] [Google Scholar]
- Cox D.R. Regression models and life tables (with discussion) J. Roy. Stat. Soc. B. 1972;74:187–220. [Google Scholar]
- Elbers C., Ridder G. True and spurious duration dependance: the identifiability of the proportional hazard model. Rev. Econ. Stud. 1982;49:403–409. [Google Scholar]
- Faye B., Barnouin J. Objectivation de la propreté des vaches laitires et des stabulations: l’indice de propreté. Bull. Tech. C. R. Z. V. Theix Inra. 1985;59:61–67. [Google Scholar]
- Fourichon C., Seegers H., Beaudeau F. Élevages des veaux et risque d emortalité et de troubles de santé en exploitations laitires. Rencontres Recherches Ruminants. 1996;3:143–148. [Google Scholar]
- Hall G.A., Jones P.W., Morgan J.H. vol. 12. Blackwell Scientific Publications; 1992. (Bovine Medicine: Calf Diarrhoea). pp. 154–180. [Google Scholar]
- Hougaard P. Survival models for heterogeneous populations derived from stable distributions. Biometrika. 1986;73:387–396. [Google Scholar]
- Hougaard P. Frailty models for survival data. Lifetime Data Anal. 1995;1:255–273. doi: 10.1007/BF00985760. [DOI] [PubMed] [Google Scholar]
- Liang K.-Y., Zeger S.L., Qaqish B. Multivariate regression analyses for categorical data. J. Roy. Stat. Soc. B. 1992;54:3–40. [Google Scholar]
- MathSoft Inc. S-PLUS 2000, 1999. Users’s Guide, USA Edition. Data Analysis Products Division, Cambridge.
- Perino L., Sutherland R.L., Woolen N.E. Serum gamma-glutamyl-transferase activity and protein concentration at birth and after suckling in calves with adequate and inadequate passive transfer of immunoglobulin. G. Am. J. Vet. Res. 1993;54:56–59. [PubMed] [Google Scholar]
- Quigley J.D., Martin K.R., Bemis D.A., Potgieter L.N.D., Reinemerger C.R., Rohrbach B.W., Dowlen H.H. Effects of housing and colostrum feeding on serum immunoglobulins, growth, and fecal scores of Jersey calves. J. Dairy Sci. 1995;78:893–901. doi: 10.3168/jds.S0022-0302(95)76703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea D.E., Tyler J.W., Hancock D.D., Basser T.E., Wilson L., Krytenberg D.S., Sanders B.A. Prediction of calf mortality by use of test for passive transfer of colostral immunoglobulin. J. Am. Vet. Med. Assoc. 1996;208:2047–2049. [PubMed] [Google Scholar]
- Roy, J.H.B., 1990. The Calf, 5th ed., vol. 1, Management of Health. British Library Cataloguing in Publication Data, pp. 1–117.
- Schumman F.J., Townsend H.G.G., Naylor J.M. Risk factors for mortality from diarrhoea in beef calves in Alberta. Can. J. Vet. Res. 1990;54:336–372. [PMC free article] [PubMed] [Google Scholar]
- Sivula N.J., Ames T.R., Marsh W.E., Werdin R.E. Descriptive epidemiology of morbidity and mortality in Minnesota dairy heifer calves. Prev. Vet. Med. 1996;27:155–171. [Google Scholar]
- Sivula N.J., Ames T.R., Marsh W.E. Management practices and risk factors for morbidity and mortality in Minnesota dairy calves. Prev. Vet. Med. 1996;27:173–182. [Google Scholar]
- Vallet A., Grenet N., Gauthier D. Influence des conditions d’élevage sur la fréquence des diarrhées de veau nouveau-nés et sur l’efficacité de leur traitement par voie orale. Ann. Rech. Vét. 1985;16:297–303. [PubMed] [Google Scholar]
- Vaupel J.W., Manton K.G., Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- Waltner-Toews D., Martin S.W., Meek A.H. Dairy calf management, morbidity and mortality in Ontario Holstein herds. Prev. Vet. Med. 1986;4:103–171. [Google Scholar]
- Wei L.J., Lin D.Y., Weissfeld L. Regression analysis of multivariate incomplete failure time data by modelling marginal distributions. J. Roy. Stat. Soc. B. 1989;84:1065–1073. [Google Scholar]
- Wells S.J., Garber L.P., Hill G.W. Health status of preweaned dairy heifers in the United States. Prev. Vet. Med. 1996;29:185–199. doi: 10.1016/s0167-5877(96)01078-1. [DOI] [PubMed] [Google Scholar]


