Highlights
►Houttuynia cordata Thunb. exhibits strong activity against herpes simplex virus. ►H. cordata blocks HSV-2 infection through inhibition of NF-κB, not MAPK. ► Flavonoids like quercetin, quercitrin and isoquercitrin as major active components.
Keywords: Houttuynia cordata, Herpes simplex virus, NF-κB, Flavonoid, Antiviral
Abbreviations: HCWE, Houttuynia cordata water extract; ACV, acyclovir
Abstract
Houttuynia cordata Thunb. is a medicinal plant widely used in folk medicine in several Asian countries. It has been reported that a water extract of H. cordata exhibits activity against herpes simplex virus (HSV) and the virus of severe acute respiratory syndrome (SARS), although the mechanisms are not fully understood yet. Previous studies have demonstrated absolute requirement of NF-κB activation for efficient replication of HSV-1 and HSV-2 and inhibition of NF-κB activation has been shown to suppress HSV infection. Here we show that a hot water extract of H. cordata (HCWE) inhibits HSV-2 infection through inhibition of NF-κB activation. The IC50 was estimated at 50 μg/ml of lyophilized HCWE powder. At 150 and 450 μg/ml, HCWE blocked infectious HSV-2 production by more than 3 and 4 logs, respectively. The inhibitory activity was concomitant with an inhibition of NF-κB activation by HSV-2 infection. Although activation of NF-κB and Erk MAPK has been implicated for HSV replication and growth, HCWE showed no effect on HSV-2-induced Erk activation. Furthermore, we show that treatment with quercetin, quercitrin or isoquercitrin, major water extractable flavonoids from H. cordata, significantly blocked HSV-2 infection. These results together demonstrated that H. cordata blocks HSV-2 infection through inhibition of NF-κB activation.
1. Introduction
Houttuynia cordata, a flowering plant native to southeastern and northeastern Asian countries, is an herbal garnish in Vietnamese cuisine and southern China. The plant is also used in folk medicine in several countries. Water extracts from this plant have been reported to have inhibitory activity against herpes simplex virus (HSV), influenza A virus, human immunodeficiency virus (HIV) (Hayashi et al., 1995, Chiang et al., 2003) and the virus of severe acute respiratory syndrome (SARS) (Lau et al., 2008). Although those viruses belong to diverse families and may utilize different mechanisms for cell entry and infection, a common feature of infection by those viruses is to exploit the NF-κB signaling pathway for replication (Gregory et al., 2004, Amici et al., 2006, Kumar et al., 2008, Bielinska et al., 1989). The transcriptional regulator of NF-κB family regulates host gene expression of cell survival, cell differentiation, inflammation as well as anti-viral responses. NF-κB activity is also essential in preventing virus-induced apoptosis, a critical event for virus replication (Hiscott et al., 2001, Goodkin et al., 2003).
In this report, we attempted to address mechanisms associated with H. cordata antiviral activity using HSV-2 infection as a model system. HSV-1 and -2, also known as human herpes virus 1 and 2 (HHV1 and HHV2), are enveloped DNA viruses of herpes virus family that infect humans. After cell entry, HSV-1 and HSV-2 infection promotes persistent NF-κB activation (Patel et al., 1998, Gregory et al., 2004, Yedowitz and Blaho, 2005) as well as MAPK activation (Hargett et al., 2005, Zhang et al., 2010, Karaca et al., 2004), since the activity is required for cell survival and virus growth (Smith et al., 2000, Perkins et al., 2002). The importance of NF-κB and MAPK activation in HSV-1 and HSV-2 infection has been demonstrated with pharmacological inhibitors and genetic approaches. IKK inhibition by cyclopentenone prostaglandin A blocks HSV-1 gene expression and reduces virus yield by more than 3000-fold (Amici et al., 2001). Inhibition of NF-κB mediated gene expression by resveratrol blocks both HSV-1 and HSV-2 infection (Faith et al., 2006), while curcumin a known inhibitor of NF-κB activation also has potential activity against HSV-1 and HSV-2 infections at relatively low concentrations (Kutluay et al., 2008). Zhang et al. (2010) recently demonstrated that inhibition or depletion of MEK1, an immediate upstream kinase of Erk1/2 MAPK, significantly blocks HSV replication.
As an herbal medicine, H. cordata has traditionally been used as remedy for inflammatory diseases and for antibacterial and antiviral purposes. During the screening of herbal medicines against sexually transmitted diseases, we found that a hot water extract from H. cordata (HCWE) exhibited strong anti-HSV-2 activity. The IC50 for HSV-2 infection was estimated at approximately 50 μg/ml of lyophilized powder. Although HSV-2 infection has been reported to induce Erk MAPK and NF-κB activation, we found HCWE blocked HSV-2 infection through selective inhibition of NF-κB activation. HCWE showed no effect on HSV-2 induced Erk MAPK activation. The HCWE mainly contains chlorogenic acid, flavonoids and the corresponding glycosides (Xu et al., 2006, Chiang et al., 2003). We found the flavonoids were responsible for the antiviral activity.
2. Materials and methods
2.1. Cells and virus
HeLa 229 cells were purchased from ATCC (Manassas, VA). African green monkey kidney epithelial Vero cells were purchased from Cell Bank of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in high glucose DMEM (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), 2 mM l-glutamine, nonessential amino acids and sodium pyruvate (Invitrogen), and maintained at 37 °C in a humidified incubator with 5% CO2.
Herpes simplex virus type 2 strain G was purchased from ATCC (VR-734). The virus was propagated in Vero cells and virus titer was determined as reported (Ejercito et al., 1968).
2.2. Herbal material
Dried leaves and stems of H. cordata Thunb. were purchased from Xuzhou Pharmaceutical Corporation (Xuzhou, China) and further identified by Ms. Yunxia Xu at Nanjing University. The air-dried material was extracted with water (100 mg material per milliliter distilled H2O) for 4 h in a water bath of 45 °C. The water extract was filtered through a 0.2 μm filter and then lyophilized into dry powder. The powder was stored at −80 °C and reconstituted with sterile water before use.
2.3. Reagents
Antibodies against NF-κB/p65 (sc-109), lamin B (sc-6216), p-Erk1/2 (sc-101760), Erk2 (sc-154) and JNK1/2 (sc-571) were purchased from Santa Cruz Biotechnologies (San Cruz, CA). Antibodies against p-p38 (9211), p-JNK1/2 (4671) and IκBα (9242S) were purchased from Cell Signaling (Beverly, MA). Anti-GAPDH monoclonal antibody (MB001) was purchased from Bioworld Technology (Minneapolis, MN). Rabbit antiserum against HSV-2 ICP0 protein was generated by immunizing rabbits with a synthetic peptide prepared by Abmart (Shanghai, China). Anti-p38 antibody was kindly provided by Dr. Jiahuai Han, Xiamen University (Xiamen, China). Recombinant human TNFα was purchased from Sino Biological Inc. (Beijing, China). HRP-conjugated secondary antibodies and chemical reagents including curcumin, quercetin, quercitrin, isoquercitrin, naringenin and acyclovir (ACV) were purchased from Sigma–Aldrich. Sodium houttuyfonate of Pharmacopeia grade was purchased from Hubei Hengshuo Corporation (Wuhan, China) and ECL reagent kit was from Pierce/Thermo Fisher.
2.4. Infection and inhibition assays
HSV-2 infection was assayed initially by measuring cytolytic effect using MTT assay for convenience. Briefly, Vero cells were seeded in 96-well plates at 5 × 103 cells per well in complete medium. Vero cells were infected in triplicate with HSV-2 at an MOI of 2. Cytolytic effect due to infection was monitored under an inverted microscope. Culture supernatants (10 μl) from infected and uninfected controls were sampled from those wells at 48 h PI for determination of infectious virion release using a secondary infection assay. At the end of an experiment (48 h), MTT (Sigma–Aldrich) was added to each well to a final concentration of 0.5 mg/ml for the measurement of formazan formation as previously described (Mosmann, 1983). The absorbance was measured at 570 nm in a Versa Max microtiter plate reader (Molecular Devices, Sunnyvale, CA). Data are presented as mean ± standard deviation of triplicate samples.
For inhibition assays, monolayers of Vero cells were treated with HCWE or single compounds at doses and times as indicated. The reagents were left throughout the infection process.
2.5. Plaque forming assay
The antiviral activity was reassessed using a plaque forming assay as previously described (Ejercito et al., 1968). Briefly, Vero cells were seeded in 24-well plates 24 h prior to infection at a density of 5 × 104 cells per well in complete medium. Culture supernatants collected from infection assays were series-diluted and used for titration of infectious virus. Plaque formation was assessed 48 h PI after fixation with 3% formaldehyde for 30 min followed by the addition of crystal violet. After rinsing off crystal violet with PBS, the number of plaques was counted manually. The infection was performed in triplicate samples.
The residual HCWE in the primary cultures of an infection may interfere with secondary assays. We considered the effect negligible due to significant dilutions for secondary assays (at least 1:100 or higher).
2.6. Western blot analysis
Cells were lysed in a buffer containing 150 mM NaCl, 50 mM Tris–HCl (pH 7.4), 1 mM sodium vanadate, 1% NP-40, and a cocktail of protease inhibitors (Roche). The cell lysates were cleaned by centrifugation at 10,000g and soluble proteins were separated by SDS–PAGE. After being transferred to a polyvinylidene difluoride membrane (Millipore), the proteins were detected by incubation with a primary antibody, followed by horseradish peroxidase-conjugated secondary antibody and the ECL reagent (Pierce, Rockford, IL). The images were collected using Alpha Innotech Flour ChemHD2 imaging system (San Leandro, CA) and densities of corresponding bands were quantified using the pre-installed software.
To measure protein translocation biochemically, a nuclear protein extract kit (Beyotime Biotechnology) was used to separate nuclear proteins from cytosolic fractions.
To test the effect of curcumin and HCWE on TNFα induced IκBα degradation, HeLa 229 cells were treated with curcumin (10 μM) or HCWE (450 μg/ml) for 2 h, then stimulated with 1 ng/ml TNFα for 20 min. The cell lysates were subjected to western blot analysis.
3. Results
3.1. HCWE treatment blocks HSV-2 infection
To assay the antiviral effect of H. cordata, monolayers of Vero cells remained untreated or were preincubated with HCWE at varying concentrations for 2 h. Acyclovir (ACV) at 5 μM was included as a control. The cells were then infected with HSV-2 at an MOI = 2 for 2 days. HSV-2 infection caused a visible cytopathic effect 24 h PI in the infected but untreated controls. The effect became more obvious at 48 h PI since more cells were either partially detached or started to break up. Similar to ACV control, we found that preincubation with HCWE significantly blocked cell round up and cell detachment, suggesting that HCWE treatment potentially blocked HSV-2 infection. To semiquantitatively assess the effect, 10 μl culture supernatants were collected at 48 h PI for titration of virus release while cell viability was determined by an MTT assay (Mosmann, 1983). As shown in Fig. 1 A, HCWE treatment blocked cell death associated with HSV-2 infection, indicating that HCWE possesses antiviral activity. At 45, 150 and 450 μg/ml HCWE treatment significantly blocked HSV-2 infection. The IC50 was estimated at approximately 50 μg/ml.
Fig. 1.
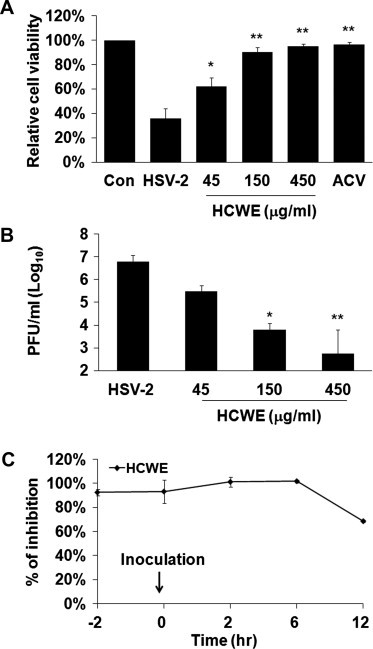
Inhibition of herpes simplex virus-2 infection by Houttuynia cordata water extract. Dose response of HCWE on HSV-2 infection. (A) Monolayers of Vero cells in 96-well plates were infected with HSV-2 at an MOI of 2 in the presence or absence of HCWE at 45, 150, 450 μg/ml. HSV-2 infection caused cytolytic effect. At 48 h PI, cells that remained attached were quantitatively measured for their activity to reduce MTT to formazan. An increased reading from MTT assay indicates reduction of virus infection. Acyclovir (ACV) at 5 μM was used as a positive control against virus infection. Data are representative of 3 independent experiments of triplicate samples. (B) In parallel experiments, culture supernatants were collected and assayed for production of infectious virus by plaque forming assay. Data are presented as mean ± standard deviation of triplicate samples. ∗P < 0.05, ∗∗P < 0.01 compared with HSV-2 samples determined by ANOVA. (C) Time course of HCWE treatment on HSV-2 infection. HCWE at 450 μg/ml was added at 2 h prior to (-2 h), during (0 h), or at indicated times post virus inoculation (2, 6, and 12 h PI). Inhibition of virus infection was determined by using MTT assay. The data are presented as mean ± standard deviation of triplicate samples, and the results represent three independent experiments. No significant difference on HSV-2 infection was detected when HCWE was added 2 h prior to or within 6 h post inoculation.
Using a secondary infection assay, we determined the effect of HCWE treatment on the yield of virus production. HCWE at 45 μg/ml, 150 μg/ml and 450 μg/ml resulted in reduction of more than 1, 3 and 4 logs of infectious virus, respectively, in those samples (Fig. 1B). Those results indicated HCWE has anti-HSV-2 activity.
HSV infection is initiated by virus attachment followed by membrane fusion for cell entry and eventual replication and assembly in the cells. To preliminarily investigate the stages of HCWE action on HSV-2 infection, monolayers of Vero cells were treated with HCWE 2 h prior to, during or after virus inoculation. Virus infection was evaluated by measuring cell viability using MTT assay. The inhibitory effect was significant when HCWE was added at 2 h prior to, during or 2 h PI, early stages of virus cell entry and infection (Fig. 1C). The effect became less significant when HCWE was added at later stages of infection (12 h PI). HSV-2 cell entry is a relative rapid event (Nicola and Straus, 2004). We hence interpreted the data as HCWE acted at a stage(s) after the virus has entered host cells since addition of HCWE at 2 h PI showed a similar inhibitory effect as earlier addition.
3.2. HCWE treatment blocks HSV-2 induced NF-κB activation
NF-κB activation is required for efficient replication of HSV-1 and HSV-2 virus (Patel et al., 1998, Gregory et al., 2004). We next examined whether HCWE was capable to block NF-κB pathway by investigating NF-κB nuclear translocation. HeLa 229 cells were treated with HCWE at 450 μg/ml for 2 h or remained untreated. The cells were then infected with HSV-2 at an MOI of 5 for varying times. NF-κB nuclear translocation was determined by immunoblotting after fractionation. As shown in Fig. 2 A, p65 was detected at relatively low levels in uninfected cells; whereas, HSV-2 infection significantly induced p65 nuclear translocation at 6, 12 and 24 h PI. Treatment with HCWE abated HSV-2 induced p65 nuclear translocation, suggesting HCWE treatment blocked HSV-2 induced NF-κB activation.
Fig. 2.
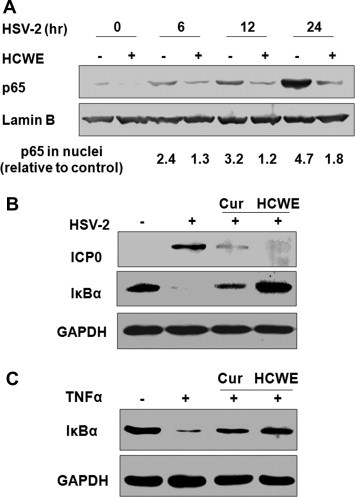
HSV-2 infection induces NF-κB activation and HCWE treatment blocks NF-κB activation. HeLa 229 cells were infected with HSV-2 (MOI = 5) in the presence or absence of 450 μg/ml HCWE for 6, 12 and 24 h. NF-κB/p65 nuclear translocation was determined by protein fractionation followed by immunoblotting with anti-p65 antibody. HSV-2 infection induced persistent NF-κB/p65 protein nuclear translocation, while treatment with HCWE blocked NF-κB/p65 activation induced by HSV-2 infection (A). The numbers represent relative intensity of a p65 protein band to Lamin B, a loading control, in each sample compared to untreated and uninfected control. In separate experiments, curcumin, a known inhibitor of NF-κB activation and HSV-2 immediate early gene expression, was included as a control. Treatment with curcumin (Cur) at 10 μM or HCWE at 450 g/ml significantly blocked HSV-2 ICP0 expression as detected at 24 h PI (B) as well as infection and TNFα induced IκBα degradation (B and C).
To substantiate this conclusion, we then repeated the experiment by including curcumin, a natural product that has been shown to block NF-κB activation as well as HSV-1 and HSV-2 replication (Shishodia et al., 2005, Kutluay et al., 2008). As shown in Fig. 2B and C, treatment with curcumin or HCWE significantly blocked HSV-2 ICP0 expression, indicating an inhibition of HSV-2 replication. In agreement with a role of NF-κB for HSV-2 replication, an inhibition of IκBα degradation induced by HSV-2 infection as well as by TNFα treatment was also detected in those samples. These results together indicated that HCWE blocked HSV-2 infection potentially through inhibition of NF-κB activation.
3.3. HCWE treatment does not inhibit Erk MAPK activation during HSV-2 infection
In addition to NF-κB activation, HSV-1 and HSV-2 infection also induces MAPK activation (Zachos et al., 1999, Hargett et al., 2005, Zhang et al., 2010, Smith et al., 2000). We also investigated whether HCWE had an effect on MAPK activation by detection of protein phosphorylation. Consistently with previous report (Zhang et al., 2010), HSV-2 infection selectively promoted Erk MAPK phosphorylation at 3 and 6 h PI. HCWE treatment however did not significantly block HSV-2 induced MAPK activation Fig. 3 . These results together suggest that HCWE block HSV-2 infection through NF-κB inhibition.
Fig. 3.
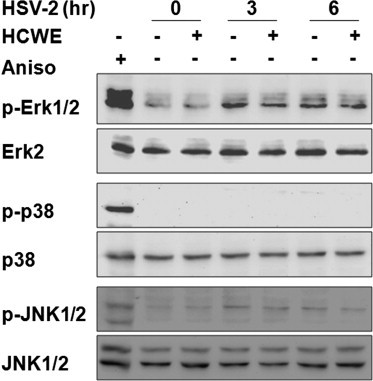
HSV-2 infection promotes Erk MAPK activation, HCWE treatment showed no effect on Erk activation. HeLa 229 cells were infected with HSV-2 (MOI = 5) in the presence or absence of 450 μg/ml HCWE. Cell lysates were harvested at 3 and 6 h PI, and the levels of phosphorylated forms of Erk1/2, p38 and JNK1/2 as well as total Erk2, p38 and JNK1/2 proteins were determined by immunoblotting analysis. Anisomycin (Aniso) at 5 μM was used as a positive control since it activates all three MAPK classes examined.
3.4. Flavonoids in HCWE have anti-HSV activity
H. cordata contains volatile essential oil such as houttuynin, an aliphatic aldehyde, and water soluble components, mainly flavonoids like quercetin, quercitrin and isoquercitrin (Xu et al., 2006, Chou et al., 2009). To investigate whether the major chemical components of H. cordata also contained antiviral activity, Vero cells were treated with quercetin, quercitrin or isoquercitrin at 10 μM. We also included a more stable and water soluble derivative of houttuynin (sodium houttuyfonate), since houttuynin was identified as an antimicrobial component of H. cordata (Kosuge, 1952). As shown in Fig. 4 , quercetin, quercitrin or isoquercitrin at 10 μM showed significant activity against HSV-2 infection, while naringenin, a dihydroflavonoid from grape fruit showed no effect against HSV-2 infection, indicating that the flavonoids are responsible for anti-HSV-2 activity in HCWE.
Fig. 4.
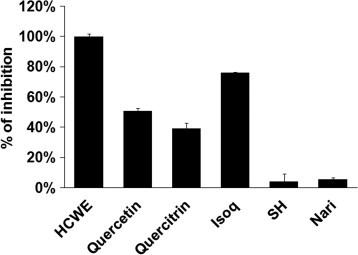
Major flavonoids in HCWE exhibit anti-HSV-2 activity. Vero cells were infected with HSV-2 (MOI = 2) in the presence or absence of 450 μg/ml HCWE or 10 μM of quercetin, quercitrin, isoquercitrin (Isoq), sodium houttuyfonate (SH) or naringenin (Nari). Their effect on HSV-2 infection was determined by measuring cell viability using MTT assay. The data are presented as mean ± standard deviation of triplicate samples. The results are representative of three independent experiments.
These results therefore demonstrated that H. cordata blocked HSV-2 infection through inhibition of NF-κB activation by the flavonoids.
4. Discussion
Many plants and their components have anti-herpetic activity (Khan et al., 2005). As a traditional Chinese herbal medicine, H. cordata is frequently used for its antibacterial and antiviral properties. The water extracts from H. cordata have been reported to inhibit viral infection even though the mechanisms were not elucidated. Both HSV-1 and HSV-2 infection promotes NF-κB as well as MAPK activation for efficient virus replication and growth (Smith et al., 2000, Perkins et al., 2002, Patel et al., 1998, Gregory et al., 2004) and pharmacological reagents that block NF-κB or MAPK activation have been reported to inhibit HSV infection. We found HCWE had anti-herpetic activity against HSV-2 and HSV-1 (data not shown) infection and the activity was associated with its effect on NF-κB pathway activation. Although HSV-2 infection promotes Erk MAPK activation and Erk activity is required for HSV-2 replication (Zhang et al., 2010), we found HCWE treatment had no effect on HSV-2-induced MAPK activation.
The transcriptional regulator NF-κB plays a critical role in cell proliferation and viral gene expression of some viruses. NF-κB activation also enables cells with anti-apoptotic activity, crucial for viral replication. Several medically important viral pathogens are known to require NF-κB activity for replication and infection. Compounds that block NF-κB activation have been reported to have antiviral activities. Cyclopentenone prostaglandins (cyPG) of the A and J type block TNFα-induced NF-κB activation by direct inhibition and modification of the IKKβ subunit of IKK. Amici et al. (2001) reported that cyPG were also powerful inhibitors of IKK and NF-κB activation induced by HSV-1 infection, an effect that was attributed to the potent antiviral activity in human cells. Curcumin, an inhibitor of constitutive NF-κB activation (Shishodia et al., 2005), has been shown to inhibit herpes simplex virus immediate-early gene expression and virus replication at relatively low concentrations (Kutluay et al., 2008). Resveratrol, a polyphenolic compound implicated with diverse biological activities, suppresses HSV induced activation of NF-κB within the nucleus, resulting in impaired expression of HSV genes and synthesis of viral DNA (Faith et al., 2006). Those and our results suggest that reagents that target signaling pathways may exert selective activity against a diverse group of viral pathogens as previously suggested (Ludwig, 2009, Baba, 2006).
The major chemical components of H. cordata consist of essential oils including houttuynin, an aliphatic aldehyde, and water soluble components, including flavonoids of quercetin, quercitrin and isoquercitrin. The content of quercitrin can exceed 0.1% in dried leaves, while others are minor components (Xu et al., 2006). Kosuge (1952) first isolated houttuynin from H. cordata and reported its antibacterial activity. Hence houttuynin as well as the sodium houttuyfonate (bisulphite adduct as a prodrug due to its stability) had been used in China to treat bacterial infection and other complications until its withdrawal from the market due to lack of defined activity. We found that the once approved sodium houttuyfonate had no activity against HSV-2 infection or signaling pathway activation. Instead, we found that the antiviral activity was associated with the flavonoids, since quercetin, quercitrin and isoquercitrin all displayed significant activity against HSV-2 infection. This is consistent with the reported activity of some flavonoids on virus infection as well as inflammatory response (Kaul et al., 1985, Lyu et al., 2005, Choi et al., 2009, Kim et al., 2010).
Acknowledgments
This work was partially supported by grants from the Natural Science Foundation of China (90813036) and Basic Research Funds for Central Universities (1114021404 and 1082021406).
References
- Amici C., Belardo G., Rossi A., Santoro M.G. Activation of IkappaB kinase by herpes simplex virus type 1: a novel target for anti-herpetic therapy. J. Biol. Chem. 2001;276:28759–28766. doi: 10.1074/jbc.M103408200. [DOI] [PubMed] [Google Scholar]
- Amici C., Rossi A., Costanzo A., Ciafre S., Marinari B., Balsamo M., Levrero M., Santoro M.G. Herpes simplex virus disrupts NF-kappaB regulation by blocking its recruitment on the IkappaBalpha promoter and directing the factor on viral genes. J. Biol. Chem. 2006;281:7110–7117. doi: 10.1074/jbc.M512366200. [DOI] [PubMed] [Google Scholar]
- Baba M. Recent status of HIV-1 gene expression inhibitors. Antiviral Res. 2006;71:301–306. doi: 10.1016/j.antiviral.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Bielinska A., Krasnow S., Nabel G.J. NF-kappaB-mediated activation of the human immunodeficiency virus enhancer: site of transcriptional initiation is independent of the TATA box. J. Virol. 1989;63:4097–4100. doi: 10.1128/jvi.63.9.4097-4100.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang L.C., Chang J.S., Chen C.C., Ng L.T., Lin C.C. Anti-Herpes simplex virus activity of Bidens pilosa and Houttuynia cordata. Am. J. Chin. Med. 2003;31:355–362. doi: 10.1142/S0192415X03001090. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Song J.H., Park K.S., Kwon D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci. 2009;37:329–333. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Chou S.C., Su C.R., Ku Y.C., Wu T.S. The constituents and their bioactivities of Houttuynia cordata. Chem. Pharm. Bull. (Tokyo) 2009;57:1227–1230. doi: 10.1248/cpb.57.1227. [DOI] [PubMed] [Google Scholar]
- Ejercito P.M., Kieff E.D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Faith S.A., Sweet T.J., Bailey E., Booth T., Docherty J.J. Resveratrol suppresses nuclear factor-kappaB in herpes simplex virus infected cells. Antiviral Res. 2006;72:242–251. doi: 10.1016/j.antiviral.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Goodkin M.L., Ting A.T., Blaho J.A. NF-kappaB is required for apoptosis prevention during herpes simplex virus type 1 infection. J. Virol. 2003;77:7261–7280. doi: 10.1128/JVI.77.13.7261-7280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory D., Hargett D., Holmes D., Money E., Bachenheimer S.L. Efficient replication by herpes simplex virus type 1 involves activation of the IkappaB kinase-IkappaB-p65 pathway. J. Virol. 2004;78:13582–13590. doi: 10.1128/JVI.78.24.13582-13590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargett D., McLean T., Bachenheimer S.L. Herpes simplex virus ICP27 activation of stress kinases JNK and p38. J. Virol. 2005;79:8348–8360. doi: 10.1128/JVI.79.13.8348-8360.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Kamiya M., Hayashi T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med. 1995;61:237–241. doi: 10.1055/s-2006-958063. [DOI] [PubMed] [Google Scholar]
- Hiscott J., Kwon H., Genin P. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J. Clin. Invest. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca G., Hargett D., McLean T.I., Aguilar J.S., Ghazal P., Wagner E.K., Bachenheimer S.L. Inhibition of the stress-activated kinase, p38, does not affect the virus transcriptional program of herpes simplex virus type 1. Virology. 2004;329:142–156. doi: 10.1016/j.virol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Kaul T.N., Middleton E., Jr., Ogra P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985;15:71–79. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- Khan M.T., Ather A., Thompson K.D., Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral Res. 2005;67:107–119. doi: 10.1016/j.antiviral.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Kim Y., Narayanan S., Chang K.O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Res. 2010;88:227–235. doi: 10.1016/j.antiviral.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Kosuge T. Structure of an antimicrobial substance isolated from Houttuynia cordata Thunb. Yakugaku Zasshi (J. Pharm. Soc. Jpn.) 1952;72:1227–1231. [Google Scholar]
- Kumar N., Xin Z.T., Liang Y., Ly H. NF-kappaB signaling differentially regulates influenza virus RNA synthesis. J. Virol. 2008;82:9880–9889. doi: 10.1128/JVI.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutluay S.B., Doroghazi J., Roemer M.E., Triezenberg S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology. 2008;373:239–247. doi: 10.1016/j.virol.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.M., Lee K.M., Koon C.M., Cheung C.S., Lau C.P., Ho H.M., Lee M.Y., Au S.W., Cheng C.H., Lau C.B., Tsui S.K., Wan D.C., Waye M.M., Wong K.B., Wong C.K., Lam C.W., Leung P.C., Fung K.P. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig S. Targeting cell signalling pathways to fight the flu: towards a paradigm change in anti-influenza therapy. J. Antimicrob. Chemother. 2009;64:1–4. doi: 10.1093/jac/dkp161. [DOI] [PubMed] [Google Scholar]
- Lyu S.Y., Rhim J.Y., Park W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res. 2005;28:1293–1301. doi: 10.1007/BF02978215. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nicola A.V., Straus S.E. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 2004;78:7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Hanson J., McLean T.I., Olgiate J., Hilton M., Miller W.E., Bachenheimer S.L. Herpes simplex type 1 induction of persistent NF-kappaB nuclear translocation increases the efficiency of virus replication. Virology. 1998;247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- Perkins D., Pereira E.F., Gober M., Yarowsky P.J., Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 2002;76:1435–1449. doi: 10.1128/JVI.76.3.1435-1449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S., Amin H.M., Lai R., Aggarwal B.B. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem. Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Smith C.C., Nelson J., Aurelian L., Gober M., Goswami B.B. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J. Virol. 2000;74:10417–10429. doi: 10.1128/jvi.74.22.10417-10429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Ye H., Wang W., Yu L., Chen G. Determination of flavonoids in Houttuynia cordata Thunb. and Saururus chinensis (Lour.) Bail. by capillary electrophoresis with electrochemical detection. Talanta. 2006;68:759–764. doi: 10.1016/j.talanta.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Yedowitz J.C., Blaho J.A. Herpes simplex virus 2 modulates apoptosis and stimulates NF-kappaB nuclear translocation during infection in human epithelial HEp-2 cells. Virology. 2005;342:297–310. doi: 10.1016/j.virol.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Zachos G., Clements B., Conner J. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 1999;274:5097–5103. doi: 10.1074/jbc.274.8.5097. [DOI] [PubMed] [Google Scholar]
- Zhang H., Feng H., Luo L., Zhou Q., Luo Z., Peng Y. Distinct effects of knocking down MEK1 and MEK2 on replication of herpes simplex virus type 2. Virus Res. 2010;150:22–27. doi: 10.1016/j.virusres.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


