Abstract
We report antiviral activity against human cytomegalovirus for certain dietary flavonoids and their likely biochemical mechanisms of action. Nine out of ten evaluated flavonoids blocked HCMV replication at concentrations that were significantly lower than those producing cytotoxicity against growing or stationary phase host cells. Baicalein was the most potent inhibitor in this series (IC50 = 0.4–1.2 μM), including positive control ganciclovir. Baicalein and genistein were chosen as model compounds to study the antiviral mechanism(s) of action for this series. Both flavonoids significantly reduced the levels of HCMV early and late proteins, as well as viral DNA synthesis. Baicalein reduced the levels of HCMV immediate-early proteins to nearly background levels while genistein did not. The antiviral effects of genistein, but not baicalein, were fully reversible in cell culture. Pre-incubation of concentrated virus stocks with either flavonoid did not inhibit HCMV replication, suggesting that baicalein did not directly inactivate virus particles. Baicalein functionally blocked epidermal growth factor receptor tyrosine kinase activity and HCMV nuclear translocation, while genistein did not. At 24 h post infection HCMV-infected cells treated with genistein continued to express immediate-early proteins and efficiently phosphorylate IE1-72. However, HCMV induction of NF-κB and increases in the levels of cell cycle regulatory proteins—events that are associated with immediate-early protein functioning – were absent. The data suggested that the primary mechanism of action for baicalein may be to block HCMV infection at entry while the primary mechanism of action for genistein may be to block HCMV immediate-early protein functioning.
Keywords: Baicalein, Genistein, Antiviral, Cytomegalovirus, Entry, Immediate-early function
1. Introduction
The majority of the population is infected with human cytomegalovirus (HCMV) yet only a small fraction of infected persons suffer from notable clinical manifestations (Alford and Britt, 1993). HCMV causes morbidities and mortalities in immunocompromised individuals such as transplant recipients and acquired immune deficiency syndrome patients (Landolfo et al., 2003, Davis et al., 1987). HCMV surface glycoprotein ligands interact with cellular receptors to produce multiple cell growth and inflammatory signals that are reactivated once HCMV immediate-early proteins are expressed (Evers et al., 2004). Many of the pathways that HCMV employs to activate cell growth and inflammatory events are required for viral replication and may be useful targets for antiviral drug development.
Currently approved drugs for the treatment of HCMV infections include viral DNA polymerase inhibitors: foscarnet, cidofovir, and ganciclovir (and pro-drug valganciclovir) (Griffiths, 2002). These compounds are virostatic, block late stages of HCMV replication, and do not prevent viral induction of multiple cell activation events. Thus, it may be useful to investigate potential new drug treatments for HCMV infections.
It was with this goal in mind that we investigated the potential anti-HCMV activities of flavonoids. Over 5000 naturally occurring flavonoids have been found in dietary or other botanical sources and many are proposed to be responsible for the health benefits of certain folk remedies and dietary supplements (Beecher, 2003). A number of biological properties have been described (or proposed) for various flavonoids. These include antioxidative, antiinflammatory, antiproliferative, and vasculoprotective effects (Gabor, 1986, Formica and Regelson, 1995), and specific modulation of the activities of a number of cellular enzymes, including inhibition of protein kinases (Middleton et al., 2000).
Two properties of flavonoids predicted possible antiviral activity against HCMV. First, the replication of multiple viruses is inhibited by various flavonoids. Genistein was active against bovine herpesvirus type 1 (Akula et al., 2002). 5,6,7-Trimethoxyflavone inhibited herpes simplex virus (HSV), HCMV, and poliovirus (Hayashi et al., 1997). Quercetin was active against herpes simplex, adeno-, respiratory syncytial (RSV), Rous sarcoma, Sindbis, pseudorabies, and parainfluenza viruses (Chiang et al., 2003, Formica and Regelson, 1995). Luteolin and quercetin inhibited the SARS coronavirus (Yi et al., 2004). Kaempferol and its derivatives inhibited HSV, HCMV, and poliovirus (Amoros et al., 1992, Mitrocotsa et al., 2000, Robin et al., 2001). Antiviral activity against several herpesviruses and respiratory viruses was reported for a series of biflavonoids (Lin et al., 1999). Several glycosylated flavonoids were active against RSV or influenza A H1N1 (Wei et al., 2004). Second, flavonoids affect signal transduction pathways that are required for HCMV replication. The effects of various flavonoids upon receptor tyrosine kinases, PI3-K, Akt, MAPKs, transcription factors, and prostaglandins vary according to the compound, compound concentration, stimulus, and cell type (Formica and Regelson, 1995, Middleton et al., 2000).
We obtained 10 commercially available dietary flavonoids to test as inhibitors of HCMV replication. This report describes selective inhibition of HCMV for nine of these flavonoids and our efforts to characterize the anti-HCMV mode of action for two model compounds: baicalein and genistein.
2. Materials and methods
2.1. Compounds, reagents, and antibodies
Flavonoids, ganciclovir (GCV), and ortho-nitrophenyl β-d-galactopyranoside (ONPG) were purchased from Sigma–Aldrich (St. Louis, MO). AG1478 was purchased from Calbiochem (La Jolla, CA). Stock solutions of drug compounds (either 1000× or 10 mg/ml) were prepared in dimethylsulfoxide (DMSO) and stored at −80 °C. Oligonucleotides were synthesized at the Nucleic Acids Core Facility of the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill.
Antibodies specific for HCMV IE1-72, IE2-86, UL84, and UL94 proteins were prepared in our laboratory as described previously (Kowalik et al., 1994, He et al., 1992, Wing et al., 1996). Antibody to β-actin (CP01) was purchased from Oncogene (San Diego, CA). Antibodies to EGFR (528), cdk2 (M2), cyclin E (HE-12), p53 (FL-393), and PCNA (PC-10; a gift from Dr. Yue Xiong) were from Santa Cruz (Santa Cruz, CA). Antibody to pp65 (8770) was purchased from Chemicon (Temecula, CA) and the corresponding fluorescein-conjugated secondary antibody (2078) was from Santa Cruz. Horseradish peroxidase (HRP)-conjugated antibody to phosphotyrosine (RC20H) was purchased from BD Biosciences. Appropriate secondary HRP-conjugated antibodies were purchased from Calbiochem, Sigma–Aldrich, or Oncogene.
2.2. Cell culture and viral infection
Primary human embryonic lung fibroblasts (HEL 299) were purchased from the American Tissue Culture Collection (ATCC; Manassas, VA) and were cultured in Dulbecco's modified Eagle's media (DMEM; Gibco BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin. Experiments with HEL cells were performed between passages 13 and 21. Stocks of the Towne strain of HCMV (passages 34–40) were prepared as described previously (Huang et al., 1973). Unless otherwise specified, cells were grown to confluence, then serum starved for 48 h in DMEM prior to HCMV infection at a multiplicity of infection (MOI) of 2–5 plaque-forming units (pfu)/cell that had been purified through a sucrose cushion to eliminate cytokines and growth factor contamination. For plaque reduction studies, virus from cell free infected culture supernatant was used as indicated. For colorimetric antiviral assays, the RC256 variant of HCMV (Spaete and Mocarski, 1987) was purchased from the ATCC and the supernatant from infected cell culture, 3 days after 100% cytopathic effect, was used to directly infect cell cultures.
2.2.1. Antiviral titer reduction assay
Various concentrations of drugs were added to confluent, serum-starved HEL fibroblasts in 24-well plates for 1 h at 37 °C in an atmosphere of 6% CO2. Cells were then infected with HCMV (Towne strain) at an MOI of approximately 1–2 pfu/cell in media with the indicated drug concentrations, to a total volume of 1 ml/well. Seven days post infection, 10 μl of the infected culture supernatant or the appropriate dilution from each well of each plate was used to inoculate the corresponding culture plate. Following a 1-h adsorption, the inoculant was removed, and a 1% methylcellulose (methocell) overlay containing DMEM with 4% FBS was added. Seven to ten days post infection, cells were fixed with 4% paraformaldehyde in PBS and stained with crystal violet. Plaques were counted under inverted light microscopy. Data were quantified as the percentage of plaques in the wells lacking drug (positive controls). In these experiments, some 100–200 plaques were present in each positive control well. For pre-incubation and reversibility studies, serial dilutions of supernatants were prepared in 96-well plates to quantitate titers by the procedure described elsewhere (Prichard et al., 1990). We modified this procedure to employ only one freeze-thaw cycle prior to titering because additional freeze-thaw cycles resulted in significantly lower virus yields.
2.2.2. Antiviral colorimetric assay
Antiviral colorimetric assay was modified from a procedure described elsewhere (Hippenmeyer and Dilworth, 1996). This assay used a recombinant virus, HCMV RC256, that has the Escherichia coli β-galactosidase gene under the control of HCMV major early β gene promoter integrated into the viral genome by homologous recombination (Spaete and Mocarski, 1987). Viral gene expression and replication in RC256-infected cells can then be measured by β-gal colorimetric assay. Various concentrations of drugs (2×) in DMEM were added to confluent, serum-starved HEL fibroblasts in 96-well plates. Plates were incubated for 1 h at 37 °C in an atmosphere of 6% CO2. Cells were then infected with an equal volume of DMEM containing HCMV (RC256; 1–2 PFU/cell). Seventy-two hours later, supernatants were removed and cell monolayers were rinsed with phosphate-buffered saline (PBS). To each well was added 50 μl of a lysis solution (10% glycerol, 1% Triton X-100, 2 mM DTT, 2 mM EDTA, and 25 mM Tris pH 7.8) and plates were incubated at 37 °C for 30 min. To each well was then added 50 μl of a freshly prepared and gravity filtered β-galactosidase substrate solution (33 mg ONPG added to 25 ml of a buffer consisting of 120 mM Na2HPO4, 80 mM NaH2PO4, 2 mM MgCl2, and 100 mM β-mercaptoethanol) and plates were incubated at room temperature (RT) for 30 min. Reactions were stopped by adding 100 μl/well 1 M Na2CO3. Optical densities were determined at 415 nm using a microplate reader. Following the subtraction of absorbance values determined for negative controls (uninfected cells, 16 wells/plate), data were quantified as percentages of the average absorbance determined for positive controls (infected cells, 16 wells/plate). The absorbances of positive controls were typically 1.2–1.8 AU and the absorbances of negative controls were ≤0.1 AU.
2.2.3. Cytotoxicity assays
To determine drug cytotoxicity in static HEL monolayers, cells were grown to confluence in 96-well plates and serum-starved for 48 h prior to the addition of various concentrations of compounds, as described for plaque reduction assays, except that virus inoculum and methylcellulose were omitted. Cytotoxicity against growing HEL monolayers was determined similarly, except that cells were not serum-starved and were approximately 10% confluent when drug was added. Seventy-two hour post treatment with selected drug concentrations, cell viability was assessed with a tetrazolium colorimetric dye assay kit (G1780), according to the manufacturer's instructions (Promega; Madison, WI). At each concentration of drug, the percentage of absorbance at 470 nm was determined as compared to negative control wells where an equivalent amount of DMSO had been added (Weislow et al., 1989).
2.2.4. Drug inhibition data analysis
Data were plotted as percent inhibition versus drug concentration, and each concentration curve was linearly regressed to determine 50% inhibitory concentration values (IC50). IC50 are presented as the mean ± standard deviation of multiple independent experiments, as indicated in the legend to Table 1 .
Table 1.
Antiviral activity and cytotoxicity of flavonoids
| Class | Compound | HCMV antiviral IC50a (μM) |
Cellular cytotoxic CC50a (μM) |
||
|---|---|---|---|---|---|
| Colorimetric (β-gal) assay | Titer reduction assay | Growing HEL fibroblasts | Static HEL fibroblasts | ||
| Flavones | Apigenin | 22 ± 3 | 6.4 | >100 | >100 |
| Baicalein | 0.4 ± 0.04 | 1.2 ± 0.8 | >100 | >100 | |
| Baicalin | 3.0 ± 1.0 | 15 ± 0.5 | >400 | >400 | |
| Luteolin | 6 ± 1 | 3.6 ± 0.7 | 62 ± 10 | >200 | |
| Isoflavones | Biochanin A | 28 ± 4 | 15 | 200 ± 50 | >400 |
| Daidzein | >40 | 7.2 | >200 | >200 | |
| Genistein | 38 ± 5 | 3.2 ± 0.1 | 124 ± 52 | >200 | |
| Flavonone | Naringenin | 32 ± 5 | >40 | >100 | >100 |
| Flavonols | Galangin | >40 | >40 | >400 | >400 |
| Quercetin | 13 ± 2 | 3.2 ± 0.8 | >400 | >400 | |
| Control | Ganciclovir | ≥100 | 2.0 ± 0.3 | ndb | nd |
IC50 and CC50; drug concentration producing 50% inhibition. Data are presented as the mean ± standard deviation of quadruplicate experiments, except titer reduction assays, where experiments were performed in duplicate for baicalin and genistein; triplicate for baicalein, luteolin, quercetin, and ganciclovir, or once.
nd, Not determined.
2.3. Western blot analyses
Baicalein and genistein were added to make final concentrations of 20 and 50 μM, respectively, in 100 mm dishes of confluent, serum-starved HEL. These compounds were present from 1 h before, during, and throughout infection. Cells were infected with HCMV and incubated at 37 °C in an atmosphere of 6% CO2 until harvesting. Monolayers were harvested at the indicated times by scraping dishes in 2× sodium dodecyl sulfate (SDS) sample buffer. Samples were boiled and loaded onto 8–10% SDS-polyacrylamide gels. Proteins were separated by electrophoresis and transferred to Immobilon-P membranes (Millipore, Bedford, MA). Membranes were blocked for 1 h in 5% (w/v) non-fat dry milk (bovine serum albumin was used to block membranes when detecting phosphorylated proteins) in PBS with 0.1% Tween-20 (PBS-T). Membranes were probed with dilutions of primary antibodies overnight at 4 °C. Blots were washed and probed with horse radish peroxidase-conjugated secondary antibodies, then washed and developed by enhanced chemiluminescence (ECL), according to the manufacturer's instructions (Amersham Biosciences Corp., Piscataway, NJ). Relative protein levels were quantified from films scanned into TIFF files using ImageQuant (Amersham) software.
2.4. HCMV DNA assay (dot blot)
Quantification of HCMV DNA synthesis was performed as described previously (Johnson et al., 1999). HEL cells were grown to confluence in 24-well plates, serum-starved for 48 h, and infected with 2–5 pfu/cell HCMV in the presence or absence of compounds. At 72 hpi, individual wells were harvested by removing the cell culture medium and replacing it with 0.1 ml PBS and freezing at −80 °C. Plates were thawed and 0.4 ml of a denaturation buffer consisting of 1.5 M NaCl and 1 M NaOH was added to wells. After 10 min, 0.4 ml of a neutralization buffer consisting of 1 M NaCl and 1 M Tris:HCl pH 7.0 was added to wells. After 5 min samples were transferred to and immobilized on nitrocellulose membranes using a minifold apparatus (Schleicher & Schuell; Keene, NH) and baking at 80 °C for 2 h under vacuum. Non-specific binding was blocked by incubation in 5× Denhardt's, 5× SSC, 50% (v/v) formamide, 1% (w/v) SDS, with 75 μg/ml salmon sperm DNA at 42 °C for 3 h. Membranes were probed in blocking buffer for 14 h at 42 °C with heat denatured purified Towne genomic DNA, that had been labeled with [α-32P]dATP (MP Biomedicals; Irvine, CA) by nick translation according to the manufacturer's instructions (Invitrogen; Carlsbad, CA). Following washes, membranes were developed by autoradiography.
2.5. Immunoprecipitations (IP)
One hour prior to infection, drugs were added to 100 mm dishes of HEL cells that were subsequently infected or mock-infected with HCMV. In IE1-72 protein experiments the media was replaced with phosphate-free DMEM (Gibco BRL) spiked with 100 μCi 32PO4 (MP Biomedicals) for a 2 h labeling period prior to harvesting. At various times post infection, cells were harvested by scraping in ice-cold PBS and resuspended in 0.6 ml of IP buffer consisting of 120 mM NaCl, 100 mM NaF, 0.2 mM NaVO4, 0.5% Nonidet P-40 (NP-40), and 50 mM Tris pH 8.0, with protease inhibitor cocktail (Sigma) in microfuge vials. Samples were freeze-thawed and incubated on ice for 15 min with occasional vortexing. Debris was removed by centrifugation and equivalent protein content of supernatants was determined by colorimetric assays (Bio-Rad; Hercules, CA). Samples were incubated with 0.5 μg primary antibody with rocking at 4 °C for 2 h. Twenty microliters of a protein G-sepharose slurry (Amersham) was added and samples were incubated for 10–14 h at 4 °C with gentle agitation. Samples were centrifuged, supernatants were removed, and pellets were washed four times with 4 °C PBS-T. Beads were boiled in 2× SDS-PAGE loading buffer and the supernatant was loaded onto polyacrylamide gels, electrophoresed, and transferred to Immobilon-P membranes as described above. For phosphorylated EGFR experiments, membranes were analyzed first with antibody to phosphotyrosine then stripped and probed with antibody to EGFR. For phosphorylated IE1-72 experiments, membranes were autoradiographed then probed with antibody to IE1-72 to confirm the protein identity of reactive bands.
2.5.1. Cell nuclear extract isolations
Extracts were prepared as described previously (Yurochko et al., 1995, Yurochko et al., 1997a, Yurochko et al., 1997b). Briefly, mock-infected and infected HEL fibroblasts (with and without flavonoids) were collected by scraping the cell monolayers of 100 mm dishes in ice-cold PBS, washing, and centrifuging. Cells were resuspended and incubated for 10 min on ice in 50 μl of a cytoplasmic isolation buffer, consisting of 10 mM HEPES (pH 7.6), 60 mM KCl, 1 mM EDTA, 0.1% NP-40, 1 mM DTT, and 1× protease inhibitor cocktail (Sigma). Samples were centrifuged and the cytoplasmic supernatants were removed. The nuclear pellets were washed with cytoplasmic extraction buffer (minus NP-40) and then incubated for 10 min on ice in 25 μl of a nuclear isolation buffer, consisting of 20 mM Tris (pH 8.0), 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 25% (v/v) glycerol, and 1× protease inhibitor cocktail. Samples were centrifuged and the desired supernatant extracts were stored at −80 °C.
2.5.2. Electrophoretic mobility shift assays (EMSA)
EMSA were performed as described previously (Yurochko et al., 1995, Yurochko et al., 1997a, Yurochko et al., 1997b). Briefly, equal amounts of nuclear extracts (as determined by colorimetric protein content assays; Bio-Rad) were aliquoted and placed on ice. One to two micrograms poly(dI-dC), 32P-labeled double-stranded deoxy oligonucleotide NF-κB specific probe 5′-CCTTTTTTTTTGGGGATTCCCCA-3′ (Yurochko et al., 1995), and binding buffer (10 mM Tris:HCl, 50 mM NaCl, 7.5 mM MgCl2, 0.5 mM EDTA, 10% (v/v) glycerol, 1 mM DTT) were added to a final volume of 10 μl. Samples were incubated for 15 min at RT, electrophoresed on a 5% polyacrylamide gel, dried, and analyzed by autoradiography. Probes were labeled by annealing complimentary deoxy oligonucleotides and filling in overhanging ends with [α-32P]dATP.
2.6. Fluorescence microscopy
HEL fibroblasts were seeded onto glass chamber slides (Falcon), grown to confluence and serum starved overnight. Thirty minutes prior to infection, slides were placed on ice. Ten minutes prior to infection, the media was changed to ice-cold DMEM containing 20 μM baicalein, 50 μM genistein, or none. Media was removed and replaced with ice-cold DMEM containing 2–5 pfu/cell HCMV in the presence and absence of drugs. Slides were incubated at 4 °C for 1 h, then at 37 °C in an atmosphere of 5% CO2 for 1 h. Wells were washed (4× 1 min) with either PBS or PBS containing the above drug concentrations. Media with or without drugs was added to all wells and slides were incubated for 5 h at 37 °C. Slides were washed with ice-cold PBS (3× 1 min) then simultaneously fixed and permeabilized in 100% methanol at −20 °C for 10 min. Slides were washed with PBS (2× 1 min), then with blocking buffer (BB) consisting of PBS with 5% FBS and 0.1% Tween-20 (2× 2 min). Wells were incubated with antibody to pp65 (1:1000 in BB) for 90 min, then washed with PBS (3× 5 min) and BB (1× 5 min). Wells were incubated with fluorescein-conjugated secondary antibody (1:500 in BB) for 60 min, then washed with PBS (1× 1 min), 0.1 μg/ml 4,6-diamidino-2-phenylindole (DAPI: Santa Cruz) in PBS (2× 5 min), and PBS (2× 5 min). Slides were mounted in Molwiol and analyzed with a Zeiss Axioskop using filters for DAPI and fluorescein.
3. Results
3.1. Antiviral activity and cytotoxicity of flavonoids
We examined the abilities of a panel of flavonoids (Fig. 1 ) to inhibit the replication of HCMV. Two complementary antiviral endpoint measurements were employed: titer reduction and colorimetric assays. The colorimetric assay was based upon the β-galactosidase activity of a transgenic version of the AD169 strain expressing this enzyme from the HCMV major early (β-)promoter (Spaete and Mocarski, 1987). Nine out of ten compounds showed antiviral activity at concentrations below 40 μM in at least one of the antiviral assays (Table 1). Baicalein was the most potent inhibitor that we examined (including positive control ganciclovir), displaying an IC50 of 0.4 ± 0.04 μM by colorimetric assay and 1.2 ± 0.8 μM by titer reduction assay.
Fig. 1.
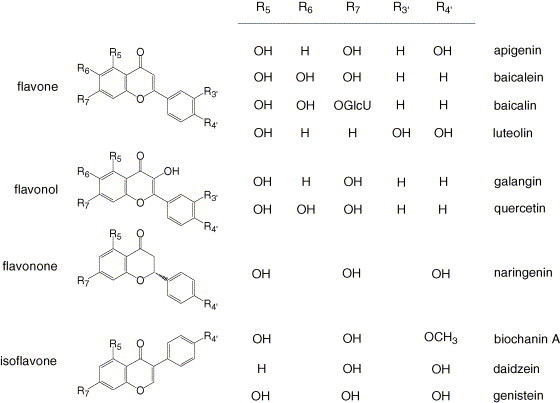
The chemical structures of compounds evaluated in this study. GlcU represents β-d-glucuronic acid.
To rule out the possibility that the antiviral activities of flavonoids were artifacts of cytotoxicity, we employed a commercial tetrazolium colorimetric dye assay to measure drug effects on cell viability. Significantly higher concentrations of HCMV-inhibitory flavonoids were necessary to produce cellular cytotoxicity in both static and growing HEL cells (Table 1). We chose two structurally different model compounds to study the antiviral mode of action for this series. Subsequent experiments were performed at 20 μM (5.4 μg/ml) baicalein and 50 μM genistein (13.5 μg/ml), because these concentrations potently inhibited viral replication but did not display obvious cytotoxicity in cell culture.
3.2. Effects of flavonoids upon HCMV proteins
In order to identify the stage(s) of HCMV replication that were specifically inhibited by flavonoids, we determined their effects upon viral proteins that were representative of immediate-early, early, and late viral gene expression. Western blot analyses revealed that all three kinetic classes of HCMV proteins were reduced to nearly background levels by baicalein (Fig. 2 ). Fifty micromoles genistein reproducibly resulted in slightly lower levels of IE1-72 at 24 h post infection (hpi). IE2-86 levels were more sensitive to genistein, but were never reduced by more than 65%. Since IE1-72 is present prior to IE2-86 (Cherrington and Mocarski, 1989), we reasoned that the mechanism of action for genistein might be direct inhibition of IE1-72. IE1-72 is a protein kinase that autophosphorylates (Pajovic et al., 1997). We reproducibly detected substantially the same amounts phosphorylated relative to total IE1-72 in the presence and absence of genistein (Fig. 2). In contrast to its effects upon immediate-early proteins, genistein treatment resulted in an unambiguous block to HCMV early and late proteins. This flavonoid reduced UL84 and UL94 proteins to nearly background levels at 48 and 72 hpi, respectively (Fig. 2).
Fig. 2.
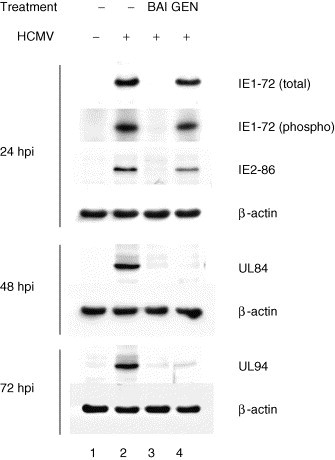
Effects of flavonoids upon HCMV proteins. IE1-72 and IE2-86 (immediate-early), UL84 (early), and UL94 (true late) protein levels were measured by immunoblotting at 24, 48, and 72 h post infection, respectively. To determine phosphorylated IE1-72, cells were labeled with 32PO4; IE1-72 was isolated by immunoprecipitation, subjected to SDS-PAGE, and detected by autoradiography. BAI indicates treatment with 20 μM baicalein and GEN indicates treatment with 50 μM genistein. Control β-actin levels indicate that similar levels of protein were loaded in each lane. These experiments were performed independently at least six times with similar results.
3.3. Effects of flavonoids upon HCMV DNA
To confirm the results obtained for drug effects upon HCMV proteins we measured viral DNA levels in HCMV-infected cells treated with flavonoids by dot (Southern) blotting. We employed viral DNA synthesis inhibitor ganciclovir as a positive control (Mar et al., 1983). HCMV DNA was reduced to nearly background levels in infected cells treated with baicalein (Fig. 3a), similar to the effects of ganciclovir. Genistein reproducibly inhibited HCMV DNA replication by approximately 95% at 72 hpi.
Fig. 3.
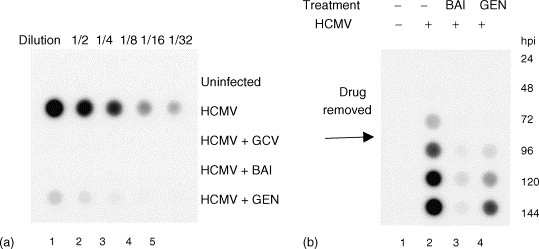
Effects of flavonoids upon HCMV DNA levels (a) and reversibility of flavonoids (b). (a) HCMV DNA replication was determined by dot (Southern) blotting hybridization at 72 h post infection. Controls were performed for uninfected cells, HCMV-infected cells, and HCMV-infected cells treated with 50 μM ganciclovir (GCV). BAI indicates treatment with 20 μM baicalein and GEN indicates treatment with 50 μM genistein. Fractions indicate the dilution factor of samples applied to membranes. This experiment was performed independently four times with substantially the same results. (b) Reversibility of flavonoids. HCMV DNA replication was blocked for 72 h with 20 μM baicalein (BAI) or 50 μM genistein (GEN) then released from drug pressure and allowed to replicate for an additional 72 h. Viral DNA levels were determined at 24 h intervals by dot blotting. This experiment was reproduced twice.
3.4. Effect of pre-treating virus particles with flavonoids
To assess potential virucidal activity for baicalein, we determined the effects of pre-treatment of infectious virus stocks with flavonoids. Stocks of HCMV were pre-incubated with either media or drug-containing media and 1 h later used to infect cells that were pre-treated with media or the concentration of drug equal to the dilution of that used for pre-incubation of virus. The antiviral effects of pre-treatment of virions with baicalein, ganciclovir, or genistein were similar to pre-treatment of cells with the effective diluted concentration (data not shown).
3.5. Reversibility of flavonoids
We examined whether or not the antiviral effects of baicalein and genistein could be reversed following the “removal” of drug. Since both flavonoids blocked HCMV DNA synthesis, we employed dot (Southern) blots as an endpoint assay. We infected cells in the presence of baicalein and genistein, and 72 hpi the media was replaced with drug-free media. Samples were harvested on each of 6 days: 3 days in the presence of flavonoids, and 3 days of release from drug pressure (Fig. 3b). Input virus in these experiments was similar to that in Fig. 3a, however, films were under exposed in Fig. 3b so that the signal of virus controls would not be saturated at 5–6 days post infection. Upon release from genistein inhibition, HCMV DNA replication resumed and increased over time delayed approximately 48 h after virus controls, suggesting that the genistein block occurred at about 24 hpi. Low levels of rebound viral DNA were present upon release from baicalein inhibition, suggesting that the antiviral effects of baicalein were poorly reversible. Similar experiments employing virus titer as an endpoint were performed with substantially the same results.
3.6. Effects of flavonoids upon HCMV induction of cell cycle regulatory proteins
HCMV-infected fibroblasts were elsewhere shown to undergo cell cycle arrest (Jault et al., 1995, Dittmer and Mocarski, 1997). This was evidenced by increased levels of cyclin-dependent kinase 2 (cdk2), cyclin E, and proliferating cell nuclear antigen (PCNA), as well as cellular DNA levels consistent with a block at either G1/S or G2/M (Jault et al., 1995, Dittmer and Mocarski, 1997). Cell cycle arrest was reproduced in cells transfected with IE2-86 (Wiebusch and Hagemeier, 1999). IE1-72 and IE2-86 have roles in the disruption of a variety of cell cycle regulatory pathways (Castillo et al., 2000). HCMV immediate-early proteins induce the p53 tumor suppressor protein (Muganda et al., 1994, Speir et al., 1994), and p53 levels may provide some measure of immediate-early protein transactivation. We measured the ability of baicalein, genistein, and control viral DNA replication inhibitor ganciclovir to inhibit HCMV-mediated cell cycle regulatory protein induction at 24 hpi by immunoblotting (Fig. 4 ). Increases in the steady-state levels of cdk2, cyclin E, p53, and PCNA were not affected by treatment with ganciclovir. The activation of these proteins by HCMV was inhibited by both baicalein and genistein. Genistein treatment did not always result in full inhibition of HCMV induction of these proteins (Fig. 4), however, none were increased to levels reflecting those of immediate-early proteins as compared to HCMV positive controls in response to genistein (Fig. 2).
Fig. 4.
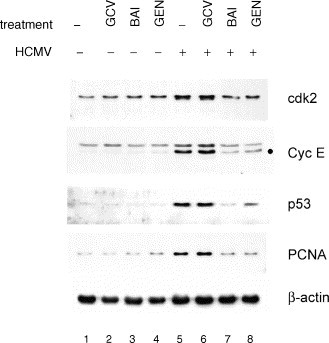
Effects of flavonoids upon HCMV-induced cell cycle regulatory proteins at 24 h post infection. Protein levels were determined by immunoblotting. GCV: 50 μM ganciclovir; BAI: 20 μM baicalein; GEN: 50 μM genistein; cdk2: cyclin-dependent kinase 2; Cyc E: cyclin E—the band consistent with cyclin E-specific binding is marked “●”; PCNA: proliferating cell nuclear antigen. Control β-actin levels indicate that similar levels of protein were loaded in each lane. This experiment was performed independently at least three times with substantially the same results.
3.7. Effects of flavonoids upon HCMV-mediated transcription factor activation
In order to further assess the possibility that genistein might act by inhibiting the transactivating functions of HCMV immediate-early proteins, we measured its effects upon NF-κB transcription factor activation. Our laboratory has previously described biphasic induction of NF-κB upon HCMV infection and that immediate-early proteins are likely to be the major contributors to this effect at 24 hpi (Yurochko et al., 1995). NF-κB was induced in HCMV-infected fibroblasts as compared to uninfected (Fig. 5 ). This induction was absent in HCMV-infected cells treated with baicalein or genistein.
Fig. 5.
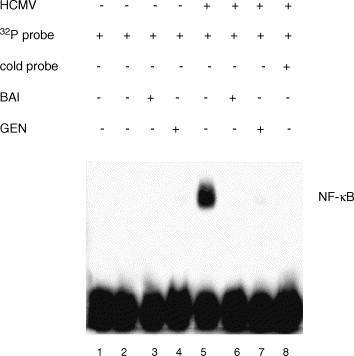
Effects of flavonoids upon HCMV-mediated NF-κB transcription factor activation at 24 h post infection. NF-κB binding to a 32P-labeled MHC class I double-stranded deoxyoligonucleotide probe was measured by EMSA. BAI indicates treatment with 20 μM baicalein and GEN indicates treatment with 50 μM genistein. Controls were also performed for probe alone (lane 1) and the nuclear extracts of HCMV-infected cells in the presence of 100-fold excess unlabeled probe (“cold probe,” lane 8). This experiment was independently performed three times with similar results.
3.8. Effects of flavonoids upon HCMV-mediated receptor tyrosine kinase activity
Since the antiviral properties of baicalein appeared to function prior to HCMV protein expression, we performed experiments to assess its effects upon initial events in the viral replication cycle. EGFR autophosphorylation is a receptor-ligand event that is required for virus replication and initiates HCMV-induced cellular signal transduction and virus entry (Wang et al., 2003). We examined the abilities of flavonoids to block HCMV-mediated phosphorylation of EGFR shortly following infection. Immunoprecipitation of EGFR followed by Western blotting for total and tyrosine phosphorylated forms revealed similar total EGFR in virus-infected and uninfected cells regardless of drug treatment (Fig. 6 ). HCMV-induced phosphorylation was reduced to background levels by baicalein (Fig. 6a), but not by genistein (Fig. 6b).
Fig. 6.
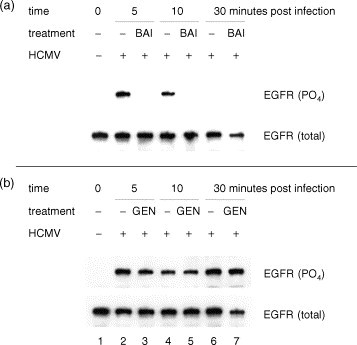
(a and b) Effects of flavonoids upon HCMV-mediated receptor tyrosine kinase activation. At the indicated times post infection, EGFR was isolated by immunoprecipitation and analyzed for phosphotyrosine. The same membranes were stripped and re-probed for total EGFR. BAI indicates treatment with 20 μM baicalein and GEN indicates treatment with 50 μM genistein. This experiment was independently performed three times with similar results.
3.9. Effects of flavonoids upon HCMV nuclear translocation
We examined the effects of baicalein and genistein upon the entry and nuclear translocation of HCMV particles. HEL fibroblasts were infected with HCMV and nuclear localization of tegument protein pp65 was examined at 6 hpi by fluorescence microscopy. In HCMV-positive controls, pp65 was observed in cell nuclei (Fig. 7 ). Baicalein but not genistein blocked efficient nuclear accumulation of pp65.
Fig. 7.
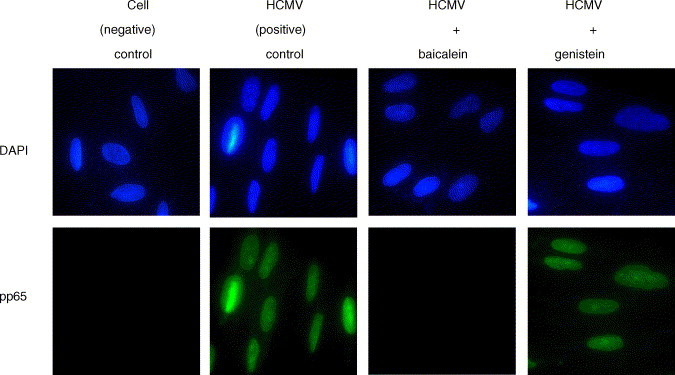
Effects of flavonoids upon HCMV nuclear translocation. HEL fibroblasts were infected with HCMV in the presence and absence of 20 μM baicalein and 50 μM genistein. Six hpi cells were fixed and stained for viral tegument protein pp65 (green). Nuclei were stained with DAPI (blue) and cells were examined by fluorescence microscopy.
4. Discussion
Our data indicated that a number of flavonoids possessed antiviral activity against HCMV at concentrations below those producing cytotoxicity (Table 1). This suggests that this series of natural products or synthetic derivatives may provide useful pharmacophore(s) for future proprietary antivirals. Others reported that genistein did not inhibit HCMV replication (Slobbe-van Drunen et al., 1997). However, their endpoint assay was limited to the effects of ≤12 μM genistein upon HCMV IE1-72 protein levels at 24 hpi.
There were discrepancies in the antiviral data (IC50 values) from the titer reduction assay in comparison to the colorimetric assay, particularly in the antiviral potency of positive control ganciclovir. This compound inhibits HCMV replication at the stage of viral DNA replication via the inhibition of HCMV-specific DNA polymerase, it does not inhibit immediate-early or early gene expression. Because the expression of reporter enzyme, β-galactosidase in RC256 HCMV-infected cells is under early (β-)promoter control, thus, the colorimetric assay can not faithfully measure the inhibitory effects of certain compounds that act on the DNA replication or L stage of virus replication. In contrast to its effects upon viral DNA (Fig. 3) and titer (Table 1), the colorimetric assay detected little antiviral activity for positive control ganciclovir. A reproducible IC50 of 9 μM was reported for ganciclovir in the original description of this assay (Hippenmeyer and Dilworth, 1996). In our adapted assay, we examined ganciclovir ten times and nine times obtained an IC50 of >100 μM (Table 1 and data not shown). It is possible that different infection conditions were responsible for this discrepancy. They infected fibroblasts by centrifuging virus (0.05 pfu/cell) onto trypsinized cell pellets, while we infected by adding supernatant virus (1.2 pfu/cell) to the media of adherent cells. Infecting cells with HCMV by centrifugation was elsewhere shown to accelerate (viral DNA replication was detectable at 16 instead of 56 hpi) and amplify (30–50 fold increased HCMV protein staining) HCMV replication (Ho et al., 1993, Gleaves et al., 1989). Others reported using an MOI of 1 pfu/cell in adapting their 72 hpi β-galactosidase assay to infection without centrifugation (van der Strate et al., 2003).
As shown in Table 1, this series of compounds suggests trends toward structure–activity relationships for HCMV inhibition. In general, flavones were the most active group of flavonoids examined. Among flavone and flavonol derivatives, those hydroxylated at the 6 position had greater anti-HCMV activities. Since naringenin was essentially inactive in both antiviral assays, it is possible that anti-HCMV activity requires aromatic conjugation or coplanar arrangement of the benzene rings. While baicalein was the most potent compound, it lost only 10-fold activity when conjugated to glucuronic acid at the 7 position (baicalin). Similarly, 3-glycosides of kaempferol were active against HCMV and less toxic than the parent compound (Mitrocotsa et al., 2000). The two most potent and selective compounds in our series, baicalein and quercetin, have the same arrangement of hydroxyl substituents on the A ring as well as unsubstituted B rings. The most toxic compound in the series (luteolin) was dihydroxylated on the B ring.
To investigate potential mechanism(s) of action for flavonoids against HCMV, we began by checking the effects of compounds upon viral protein expression and viral DNA synthesis. The results (Fig. 2, Fig. 3) suggested that baicalein operated prior to HCMV immediate-early protein expression while genistein operated between immediate-early and early protein expression. The antiviral effects of genistein and baicalein were fully and poorly reversible, respectively (Fig. 3b). However, pre-incubation of virus with baicalein (or genistein) did not result in marked inhibition of HCMV. This suggested that baicalein did not directly inactivate virus particles.
Our data suggest that the primary mechanism of action for baicalein's antiviral activity against HCMV is to prevent entry by targeting the kinase activity of EGFR. We have shown previously that this target is required for HCMV entry and cellular activation (Wang et al., 2003). It was surprising that genistein—a known inhibitor of tyrosine protein kinase activity – did not (Fig. 7). Genistein had an IC50 of 22 μM against the phosphorylation of histone H2B by purified EGFR in a test tube, and an IC50 of 111 μM against EGF-stimulated EGFR autophosphorylation in human epidermoid carcinoma cells (Akiyama et al., 1987). These differences from our experimental conditions may explain this discrepancy. Additionally, EGF and the HCMV gB glycoprotein specifically recognize different species of ErbB homo- and hetero-oligomers (Wang et al., 2003).
Baicalein was described elsewhere as a specific inhibitor of 12/15-lipoxygenase with IC50 values ranging from 15 to 120 nM (Sekiya and Okuda, 1982, Cho et al., 1991). We cannot summarily discard the possibility that 12/15-lipoxygenase is required for HCMV entry and could operate prior to HCMV activation of EGFR. However, direct virion interactions have been detected between HCMV and EGFR (Wang et al., 2003), and receptor tyrosine kinases are capable of acting upstream of lipoxygenases (Seth et al., 2001).
Our data suggest that the primary mechanism of action for genistein's antiviral activity against HCMV is to block immediate-early protein function. Genistein appeared to prevent early and late HCMV gene expression (Fig. 2, Fig. 3). While immediate-early proteins were more or less present, two downstream effector systems (cell cycle perturbation and NF-κB transcription factor induction) were more or less absent upon treatment with genistein (Fig. 4, Fig. 5). Genistein reduced the levels of IE2-86 to a greater extent than those of IE1-72 (Fig. 2). IE1-72 is expressed prior to and transactivates the expression of IE2-86 (Cherrington and Mocarski, 1989, Sambucetti et al., 1989). This implicated IE1-72 as a possible target, but genistein did not affect the phosphorylation of IE1-72 (Fig. 2).
Flavonoids are clearly abundant in many common dietary sources and commercial dietary supplements. While it has been estimated that persons on a high soy diet have steady-state serum levels of genistein of 1–5 μM (Adlercreutz et al., 1993), it is not clear that flavonoids are generally bioavailable from dietary sources or maintain steady-state levels sufficient to produce human benefits or maladies. The issue of potential toxicity should not be overlooked by those seeking justification for anecdotal or documented health benefits from flavonoid consumption. This study supports further inquiries into the possibility that flavonoid(s) may assist in clearing HCMV infections as well as other possible health benefits. If safety and efficacy can be demonstrated and standardization can be achieved (Barnes, 2003a, Barnes, 2003b), then flavonoid-rich diets or natural products may provide – or may continue to provide – widely accessible and inexpensive alternative antiviral medicines.
Acknowledgements
We thank Haobin Cai and Shunbin Ning for helpful discussions and useful advice. This study was funded by AI47678 and CA19014 grants from the National Institutes of Health, USA (to E.-S. Huang). D.L. Evers was partially supported by NIH training grant CA09156.
References
- Adlercreutz H., Markkanen H., Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Akula S.M., Hurley D.J., Wixon R.L., Wang C., Chase C.C. Effect of genistein on replication of bovine herpesvirus type 1. Am. J. Vet. Res. 2002;63:1124–1128. doi: 10.2460/ajvr.2002.63.1124. [DOI] [PubMed] [Google Scholar]
- Alford C.A., Britt W.J. In: The Human Herpesviruses. Roizman B., Whitley R.J., Lopez C., editors. Raven Press; New York: 1993. pp. 227–255. [Google Scholar]
- Amoros M., Simoes C.M., Girre L., Sauvager F., Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992;55:1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- Barnes J. Quality, efficacy and safety of complementary medicines: fashions, facts and the future. Part I. Regulation and quality. Br. J. Clin. Pharmacol. 2003;55:226–233. doi: 10.1046/j.1365-2125.2003.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J. Quality, efficacy and safety of complementary medicines: fashions, facts and the future. Part II: Efficacy and safety. Br. J. Clin. Pharmacol. 2003;55:331–340. doi: 10.1046/j.1365-2125.2003.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher G.R. Overview of dietary flavonoids: nomenclature, occurrence and intake. J. Nutr. 2003;133:3248S–3254S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- Castillo J.P., Yurochko A.D., Kowalik T.F. Role of human cytomegalovirus immediate-early proteins in cell growth control. J. Virol. 2000;74:8028–8037. doi: 10.1128/jvi.74.17.8028-8037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington J.M., Mocarski E.S. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J. Virol. 1989;63:1435–1440. doi: 10.1128/jvi.63.3.1435-1440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang L.C., Chiang W., Liu M.C., Lin C.C. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J. Antimicrob. Chemother. 2003;52:194–198. doi: 10.1093/jac/dkg291. [DOI] [PubMed] [Google Scholar]
- Cho H., Ueda M., Tamaoka M., Hamaguchi M., Aisaka K., Kiso Y., Inoue T., Ogino R., Tatsuoka T., Ishihara T. Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J. Med. Chem. 1991;34:1503–1505. doi: 10.1021/jm00108a039. [DOI] [PubMed] [Google Scholar]
- Davis M.G., Kenney S.C., Kamine J., Pagano J.S., Huang E.S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc. Natl. Acad. Sci. U.S.A. 1987;84:8642–8946. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer D., Mocarski E.S. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers D.L., Wang X., Huang E.S. Cellular stress and signal transduction responses to human cytomegalovirus infection. Microbes Infect. 2004;6:1084–1093. doi: 10.1016/j.micinf.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Formica J.V., Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Gabor M. Anti-inflammatory and anti-allergic properties of flavonoids. Prog. Clin. Biol. Res. 1986;213:471–480. [PubMed] [Google Scholar]
- Gleaves C.A., Hursh D.A., Rice D.H., Meyers J.D. Detection of cytomegalovirus from clinical specimens in centrifugation culture by in situ DNA hybridization and monoclonal antibody staining. J. Clin. Microbiol. 1989;27:21–23. doi: 10.1128/jcm.27.1.21-23.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P.D. The 2001 Garrod lecture. The treatment of cytomegalovirus infection. J. Antimicrob. Chemother. 2002;49:243–253. doi: 10.1093/jac/49.2.243. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Hayashi T., Otsuka H., Takeda Y. Antiviral activity of 5,6,7-trimethoxyflavone and its potentiation of the antiherpes activity of acyclovir. J. Antimicrob. Chemother. 1997;39:821–824. doi: 10.1093/jac/39.6.821. [DOI] [PubMed] [Google Scholar]
- He Y.S., Xu L., Huang E.S. Characterization of human cytomegalovirus UL84 early gene and identification of its putative protein product. J. Virol. 1992;66:1098–1108. doi: 10.1128/jvi.66.2.1098-1108.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer P.J., Dilworth V.M. A rapid assay for determination of antiviral activity against human cytomegalovirus. Antiviral Res. 1996;32:35–42. doi: 10.1016/0166-3542(95)00976-0. [DOI] [PubMed] [Google Scholar]
- Ho W.Z., Cherukuri R., Ge S.D., Cutilli J.R., Song L., Whitko S., Douglas S.D. Centrifugal enhancement of human immunodeficiency virus type 1 infection and human cytomegalovirus gene expression in human primary monocyte/macrophages in vitro. J. Leukoc. Biol. 1993;53:208–212. doi: 10.1002/jlb.53.2.208. [DOI] [PubMed] [Google Scholar]
- Huang E.S., Chen S.T., Pagano J.S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J. Virol. 1973;12:1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jault F.M., Jault J.M., Ruchti F., Fortunato E.A., Clark C., Corbeil J., Richman D.D., Spector D.H. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.A., Huong S.M., Huang E.S. Inhibitory effect of 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H—imidazole on HCMV DNA replication and permissive infection. Antiviral Res. 1999;41:101–111. doi: 10.1016/s0166-3542(99)00002-9. [DOI] [PubMed] [Google Scholar]
- Kowalik T.F., Yurochko A.D., Rinehart C.A., Lee C.Y., Huang E.S. Productive infection of human endometrial stromal cells by human cytomegalovirus. Virology. 1994;202:247–257. doi: 10.1006/viro.1994.1340. [DOI] [PubMed] [Google Scholar]
- Landolfo S., Gariglio M., Gribaudo G., Lembo D. The human cytomegalovirus. Pharmacol. Ther. 2003;98:269–297. doi: 10.1016/s0163-7258(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Lin Y.M., Flavin M.T., Schure R., Chen F.C., Sidwell R., Barnard D.L., Huffman J.H., Kern E.R. Antiviral activities of biflavonoids. Planta Med. 1999;65:120–125. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- Mar E.C., Cheng Y.C., Huang E.S. Effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine on human cytomegalovirus replication in vitro. Antimicrob. Agents Chemother. 1983;24:518–521. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E., Jr., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Mitrocotsa D., Mitaku S., Axarlis S., Harvala C., Malamas M. Evaluation of the antiviral activity of kaempferol and its glycosides against human cytomegalovirus. Planta Med. 2000;66:377–379. doi: 10.1055/s-2000-8550. [DOI] [PubMed] [Google Scholar]
- Muganda P., Mendoza O., Hernandez J., Qian Q. Human cytomegalovirus elevates levels of the cellular protein p53 in infected fibroblasts. J. Virol. 1994;68:8028–8034. doi: 10.1128/jvi.68.12.8028-8034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajovic S., Wong E.L., Black A.R., Azizkhan J.C. Identification of a viral kinase that phosphorylates specific E2Fs and pocket proteins. Mol. Cell Biol. 1997;17:6459–6464. doi: 10.1128/mcb.17.11.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard M.N., Turk S.R., Coleman L.A., Engelhardt S.L., Shipman C., Jr., Drach J.C. A microtiter virus yield reduction assay for the evaluation of antiviral compounds against human cytomegalovirus and herpes simplex virus. J. Virol. Methods. 1990;28:101–106. doi: 10.1016/0166-0934(90)90091-s. [DOI] [PubMed] [Google Scholar]
- Robin V., Irurzun A., Amoros M., Boustie J., Carrasco L. Antipoliovirus flavonoids from Psiadia dentata. Antivir. Chem. Chemother. 2001;12:283–291. doi: 10.1177/095632020101200503. [DOI] [PubMed] [Google Scholar]
- Sambucetti L.C., Cherrington J.M., Wilkinson G.W., Mocarski E.S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya K., Okuda H. Selective inhibition of platelet lipoxygenase by baicalein. Biochem. Biophys. Res. Commun. 1982;105:1090–1095. doi: 10.1016/0006-291x(82)91081-6. [DOI] [PubMed] [Google Scholar]
- Seth R.K., Haque M.S., Zelenka P.S. Regulation of c-fos induction in lens epithelial cells by 12(S)HETE-dependent activation of PKC. Invest Ophthalmol. Vis. Sci. 2001;42:3239–3246. [PubMed] [Google Scholar]
- Slobbe-van Drunen M.E., Vossen R.C., Couwenberg F.M., Hulsbosch M.M., Heemskerk J.W., van Dam-Mieras M.C., Bruggeman C.A. Activation of protein kinase C enhances the infection of endothelial cells by human cytomegalovirus. Virus Res. 1997;48:207–213. doi: 10.1016/s0168-1702(96)01441-4. [DOI] [PubMed] [Google Scholar]
- Spaete R.R., Mocarski E.S. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc. Natl. Acad. Sci. U.S.A. 1987;84:7213–7217. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir E., Modali R., Huang E.S., Leon M.B., Shawl F., Finkel T., Epstein S.E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994;265:391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- van der Strate B.W., De Boer F.M., Bakker H.I., Meijer D.K., Molema G., Harmsen M.C. Synergy of bovine lactoferrin with the anti-cytomegalovirus drug cidofovir in vitro. Antiviral Res. 2003;58:159–165. doi: 10.1016/s0166-3542(02)00211-5. [DOI] [PubMed] [Google Scholar]
- Wang X., Huong S.M., Chiu M.L., Raab-Traub N., Huang E.S. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- Wei F., Ma S.C., Ma L.Y., But P.P., Lin R.C., Khan I.A. Antiviral flavonoids from the seeds of Aesculus chinensis. J. Nat. Prod. 2004;67:650–653. doi: 10.1021/np030470h. [DOI] [PubMed] [Google Scholar]
- Weislow O.S., Kiser R., Fine D.L., Bader J., Shoemaker R.H., Boyd M.R. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 1989;81:577–586. doi: 10.1093/jnci/81.8.577. [DOI] [PubMed] [Google Scholar]
- Wiebusch L., Hagemeier C. Human cytomegalovirus 86-kilodalton IE2 protein blocks cell cycle progression in G(1) J. Virol. 1999;73:9274–9283. doi: 10.1128/jvi.73.11.9274-9283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing B.A., Lee G.C., Huang E.S. The human cytomegalovirus UL94 open reading frame encodes a conserved herpesvirus capsid/tegument-associated virion protein that is expressed with true late kinetics. J. Virol. 1996;70:3339–3345. doi: 10.1128/jvi.70.6.3339-3345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L., Shen Y., Luo M., Zuo G., Hu J., Duan D., Nie Y., Shi X., Wang W., Han Y., Li T., Liu Y., Ding M., Deng H., Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko A.D., Hwang E.S., Rasmussen L., Keay S., Pereira L., Huang E.S. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-kappaB during infection. J. Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko A.D., Kowalik T.F., Huong S.M., Huang E.S. Human cytomegalovirus upregulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 and p65 promoters. J. Virol. 1995;69:5391–5400. doi: 10.1128/jvi.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko A.D., Mayo M.W., Poma E.E., Baldwin A.S., Jr., Huang E.S. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J. Virol. 1997;71:4638–4648. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


