Abstract
Porcine deltacoronavirus (PDCoV) was identified in multiple states across the United States (US) in 2014. In this study, we investigate the presence of PDCoV in diagnostic samples, which were further categorized by case identification (ID), and the association between occurrence, age, specimen and location between March and September 2014. Approximately, 7% of the case IDs submitted from the US were positive for PDCoV. Specimens were categorized into eight groups, and the univariate analysis indicated that oral fluids had 1.89 times higher odds of detecting PDCoV compared to feces. While the 43–56 day age group had the highest percentage of PDCoV positives (8.4%), the univariate analysis indicated no significant differences between age groups. However, multivariable analysis for age adjusted by specimen indicated the >147 day age group had 59% lower odds than suckling pigs of being positive for PDCoV. The percentage of PDCoV in diagnostic samples decreased to <1% in September 2014. In addition, 19 complete PDCoV genomes were sequenced, and Bayesian analysis was conducted to estimate the emergence of the US clade. The evolutionary rate of the PDCoV genome is estimated to be 3.8 × 10−4 substitutions/site/year (2.3 × 10−4–5.4 × 10−4, 95% HPD). Our results indicate that oral fluids continue to be a valuable specimen to monitor swineherd health, and PDCoV has been circulating in the US prior to 2014.
Keywords: Porcine deltacoronavirus, Bayesian analysis, Swine oral fluid
1. Introduction
Coronaviruses (CoVs) cause major diseases in a variety of animals, including humans. Belonging to the order Nidovirales, the family Coronaviridae consists of two subfamilies, Coronavirinae and Torovirinae (Masters and Perlman, 2013). The Coronavirinae subfamily consists of four genuses, Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and the recently identified Deltacoronavirus (Masters and Perlman, 2013). Coronaviruses are enveloped viruses that contain single-stranded, positive sense RNA with a genome size of approximately 25.4–31.7 kb (Masters and Perlman, 2013). Five CoVs have been identified in pigs: transmissible gastroenteritis virus (TGEV), porcine respiratory coronavirus (PRCV), porcine epidemic diarrhea virus (PEDV), hemagglutinating encephalomyelitis virus (HEV), and porcine deltacoronavirus (PDCoV). Within the past two years, PEDV and PDCoV were detected in the United States (US) domesticated pig population (Stevenson et al., 2013, Marthaler et al., 2013a, Marthaler et al., 2014b, Marthaler et al., 2014c, Li et al., 2014).
Initially, the species Deltacoronavirus was discovered during an investigation for new CoVs in a variety of mammalian and avian species (Woo et al., 2012). In swine, 10% of the samples were positive for PDCoV and 2 whole genomes were generated. In February 2014, the Ohio Department of Agriculture officially announced the identification of PDCoV in the US. Veterinary diagnostic laboratories quickly developed real time RT-PCR assays to detect the newly identified virus, which had been detected in 9 states (Wang et al., 2014, Marthaler et al., 2014c). However, the viral pathogenesis and virulence was unknown since clinical disease was not associated with the initial discovery of PDCoV. Nevertheless, pathogenesis and virulence was determined in gnotobiotic and conventionally raised pigs, and severe, watery diarrhea, and vomiting appeared at 48–72 h post infection (Ma et al., 2015, Jung et al., 2015).
During the initial outbreak of PDCoV, 30% of the diagnostic samples were positive (Marthaler et al., 2014c). We report the association between the occurrence, age, and specimen of PDCoV in diagnostic samples between March and September 2014, and sequence 19 PDCoV strains to estimate the emergence and the evolutionary rate of PDCoV.
2. Material and methods
Between March and September 2014, samples, including various specimen types, were submitted to the University of Minnesota Veterinary Diagnostic Laboratory (UMVDL) to determine the etiological agent of disease. Samples are assigned a case identification (ID), which encompasses several samples and specimens obtained from the same farm, and the submission may contain demographic information. The samples were processed, extracted, and tested for PDCoV per the clients’ request, using previously described methodology (Marthaler et al., 2013b, Marthaler et al., 2014a, Marthaler et al., 2014c). The results were analyzed by sample and case ID. One positive sample corresponded to a positive case ID. If a case ID contained more than one age group, the different ages within the same case ID were considered different epidemiological unit of interest. Samples were further categorized by age consisting of suckling pigs (<21 days), nursery pigs (21–42 days), growing pigs (43–56 days), finishing pigs (57–147 days) and mature pigs (>147 days). In addition, samples were categorized into 8 groups: environmental, feces, feedback, intestinal homogenate, oral fluid, rectal swab, semen, and miscellaneous, which included bacterial isolates, dried bovine plasma, feed, fluid, FTA card, plasma, RNA, serum, viral isolates, and vomitus.
Descriptive statistics of positive samples and case IDs were summarized as percentages. A relationship between veterinarian and swine company were visualized in terms of social networking using the computer package software UCINET 6.5, and the network was visualized with package software Netdraw (Borgatti et al., 2002, Borgatti, 2002). The distribution of specimens was summarized as percentages and visualized with R packages; maps (Becker et al., 2014), maptools (Bivand and Lewin-Koh, 2014), RColorBrewer (Neuwirth, 2014), and classInt (Bivand, 2013).
The independent variables, age with 5 categories and specimen with 8 categories, were first analyzed using univariate analysis of grouped data with binary dependent outcome of positive status of PDCoV (positive or negative)(Agresti, 2013). In multivariable analyses of grouped data, environment and miscellaneous samples were excluded from the analysis since they are not associated with an age of pigs. Independent variables, age with 5 categories and specimen with 6 categories, were modeled using binary dependent outcome of positive PDCoV. Then, the covariate, state, was introduced into the previous model. The suckling pigs (<21 days) and fecal samples were the predefined-baseline reference. At first preliminary analysis, a warning signal of Quasi-separation was reported. Therefore, Firth’s Penalized likelihood logistic regression models were employed to reduce the bias in maximum likelihood estimator for both univariate and multivariable logistic model (Firth, 1993, Heinze et al., 2013, Agresti, 2013). Comparing model and model selection was based on Penalized likelihood and Akaike information criterion (AIC), and lower values indicate a better fit of the analysis to the results. Finally, relative odds (OR) of PDCoV detection were estimated.
The total of submission and number of positive counts was aggregated monthly from March to September 2014. Percentage of positive samples was summarized, assuming that number of positive samples each month was identically-independent distributed with binomial distribution with probability p; n ∼ bin(p,N); where n is number of positive samples each month, N is total number of samples summited each month, and p is probability of being positive (prevalence). The number of positive PDCoV samples for each month was estimated using smoothing function. Since the observed number of positive samples in the last three months was near zero and the expected number cannot be lower than zero, a Quasi-binomial likelihood method was used to estimate mean-variance relationship and its confidence interval (Agresti, 2013). Graphics were produced with the R package ggplot2 (Hadley, 2009). Computational analysis was performed in (R version 3.2) with different packages as aforementioned (R, 2015). Statistical differences were considered significant when the p-value was lower than 0.05.
Positive PDCoV samples with Ct values <30 (n = 19, GenBank numbers KR265847–KR265865) were selected for sequencing using the Illumina Miseq (Marthaler et al., 2013a, Marthaler et al., 2014b). PDCoV sequences from GenBank (n = 23) and the sequences from this study were aligned using MAFFT and the Bayesian Markov chain Monte Carlo (MCMC) methods were used to infer a time scale phylogeny (Drummond and Strimmer, 2001, Drummond et al., 2002, Drummond et al., 2005, Drummond et al., 2006, Drummond et al., 2012, Drummond and Rambaut, 2007, Drummond and Suchard, 2010). A general-time reversible nucleotide substitution model, with gamma distributed among-site rate variation was applied, using a relaxed molecular clock and Bayesian skyline population prior as previously described (Marthaler et al., 2014d).
3. Results
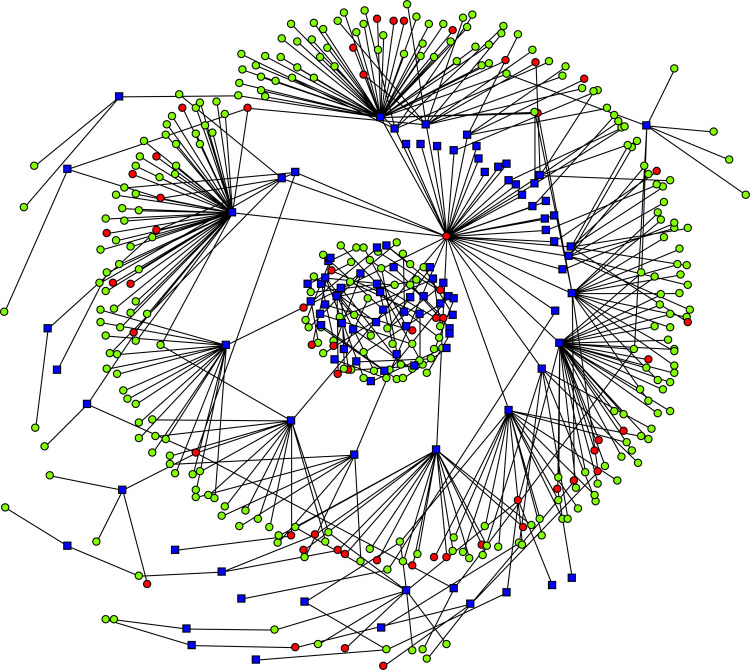
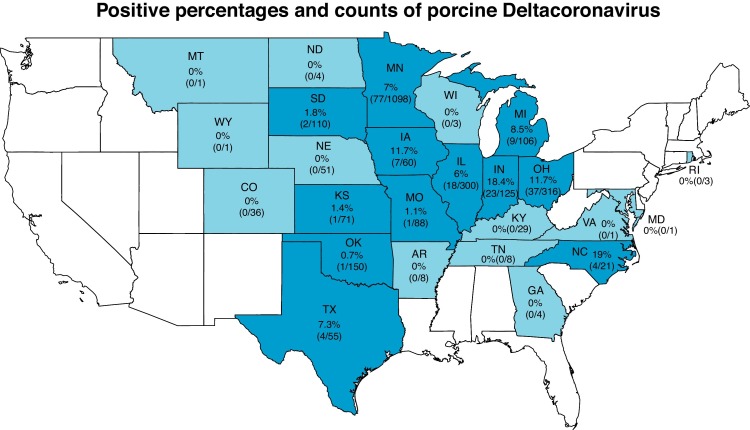
Samples are routinely submitted to the UMVDL to detect the causative agents of clinical disease. Between March and September 2014, 102 veterinarians submitted 10,127 individual samples, which corresponded to 2723 case IDs from 382 different swine companies. Out of the 102 submitting veterinarians, 31 veterinarians submitted case IDs that were positive for PDCoV. Veterinarians submitted case IDs from both negative and positive swine companies (Fig. 1 ). The samples were submitted from Canada, Chile, Mexico, and US (Table 1 ). While all case IDs from Canada and Chile were negative for PDCoV, only one case ID submitted from Mexico was positive for PDCoV. The 6.9% of case IDs submitted from the US were positive for PDCoV. Twelve of the 25 states represented in this sample set were positive for PDCoV while North Carolina, Indiana, Ohio, and Iowa had the highest percentage of positive case IDs (>10%) (Fig 2 ).
Fig. 1.
Network connectivity of veterinarians and swine companies. The blue squares represent the submitting clients while the circles represent swine companies. Green circles were negative for PDCoV while red circles were positive for PDCoV. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Distribution of PDCoV by Country.
| Country | Positive sample results | Positive case ID results |
|---|---|---|
| Canada | 0.0% (0/198) | 0.0% (0/44) |
| Chile | 0.0% (0/32) | 0.0% (0/4) |
| Mexico | 4.4% (3/68) | 4.0% (1/25) |
| United States | 5.2% (513/9829) | 6.9% (184/2650) |
| Total | 5.1% (516/10127) | 6.8% (185/2723) |
Fig. 2.
Geographical distribution of PDCoV positive case IDs in the US. Case IDs were submitted from blue shaded states while dark blue indicates states with at least one positive case ID.
The majority specimens were intestines (n = 854), feces (n = 619), fecal swabs (n = 358), and oral fluids (n = 602). To estimate the relationship between the positive case IDs and specimen, specimens were grouped by case ID into 8 different categories and the odds were estimated using feces as a reference (Tables 2 ). Oral fluids contained the highest percentage of positive case IDs (12.1%) and had 1.89 times higher odds (95% CI: 1.28–2.82) to test positive for PDCoV than feces. Interestingly, the odds of PDCoV detection in fecal swabs and intestines were lower compared to feces, OR = 0.45 (95% CI: 0.22–0.85) and OR = 0.62 (95% CI:0.40–0.98), respectively.
Table 2.
Distribution of PDCoV by specimen.
| Samples | Positive case ID results | OR (95% CI) | P(>χ2) |
|---|---|---|---|
| Feces | 6.8%(42/619) | – | – |
| Intestine | 4.3%(37/854) | 0.62* (0.40–0.98) | 0.0402 |
| Fecal swab | 3.1%(11/358) | 0.45** (0.22–0.85) | 0.0124 |
| Feedback | 3.4%(1/29) | 0.72 (0.08–2.83) | 0.6802 |
| Oral fluid | 12.1%(73/602) | 1.89*** (1.28–2.82) | 0.0013 |
| Semen | 0.0% (0/17) | 0.39 (0.00–2.94) | 0.4452 |
| Environmental | 7.6%(11/144) | 1.17 (0.56–2.23) | 0.6533 |
| Miscellaneous | 11.0%(11/100) | 1.73 (0.83–3.33) | 0.1365 |
P < 0.05.
P < 0.01.
P < 0.001.
Samples were further categorized into age groups to investigate the association between age and PDCoV detection. A majority of the case IDs were from suckling piglets (n = 397) while 1554 case IDs lacked age demographics (Table 3 ). Pigs in the 43–56 day age group had the highest percentage of PDCoV positive case IDs (8.4%) while pigs in the >147 age group had the lowest percentage of positive case IDs (3.6%). There were no statistical differences (p > 0.01) in the OR of being PDCoV positive between the different age groups.
Table 3.
Distribution of PDCoV by age.
| Age | Positive case ID results | Crude OR (95%CI) | P(>χ2) |
|---|---|---|---|
| <21 days | 5.5%(22/397) | – | |
| 21–42 days | 6.7%(19/285) | 1.22 (0.65–2.28) | 0.533 |
| 43–56 days | 8.4%(9/107) | 1.61 (0.70–3.45) | 0.252 |
| 57–147 days | 6.2%(16/256) | 1.14 (0.59–2.19) | 0.687 |
| >147 days | 3.6%(8/233) | 0.66 (0.28–1.42) | 0.297 |
| Missing age | 7.8%(121/1554) | 1.41 (0.91–2.30) | 0.13 |
| All | 6.9%(46/2822) |
While there was no statistical difference for detecting PDCoV by age group, we wanted to investigate the association between specimen and age group, which was possible since there was no interaction between age and specimen (Table 4 ). Environmental and miscellaneous categories were removed from the analysis since these specimens are not associated with an age group. In the >147 day age group, PDCoV detection had 59% lower odds than suckling pigs (OR = 0.41, 95% CI: 0.17–0.90). Oral fluids had 2.05 times higher odds (95% CI: 1.40–3.05) of PDCoV compared to feces while intestines and fecal swabs had lower odds of PDCoV detection compared to feces, OR = 0.51 (95% CI: 0.31–0.83) and OR = 0.44 (95% CI: 0.22–0.82), respectively. The age and specimen were further adjusted by state to investigate the association with state, and the odds were similar to Table 4 (not shown); however, the AIC value was lower, indicating state should be included in analysis with this data set (Table 5 ).
Table 4.
Multiple variable analysis.
| Age adjusted by specimen | ||
|---|---|---|
| Age | Crude OR (95%CI) | P(>χ2) |
| <21 days | – | |
| 21–42 days | 1.08 (0.57–2.04) | 0.819 |
| 43–56 days | 1.37 (0.59–2.99) | 0.448 |
| 57–147 days | 0.66 (0.33–1.29) | 0.226 |
| >147 days | 0.41*** (0.17–0.90) | 0.025 |
| Missing age | 0.69 (0.42–1.17) | 0.164 |
| Specimen adjusted by age | ||
|---|---|---|
| Specimen | Crude OR (95%CI) | P(>χ2) |
| Feces | – | |
| Feedback | 0.68 (0.07–2.69) | 0.633 |
| Intestine | 0.51** (0.31–0.83) | 0.007 |
| Oral fluid | 2.05*** (1.40–3.05) | <0.001 |
| Fecal swab | 0.44** (0.22–0.82) | 0.009 |
| Semen | 0.62 (0.00–5.06) | 0.722 |
P < 0.01.
P < 0.001.
Table 5.
AIC value for various analysis.
| Dependent variable | AIC value |
|---|---|
| Age | 1402.626 |
| Specimen + age | 1203.842 |
| State + age + specimen | 1186.636 |
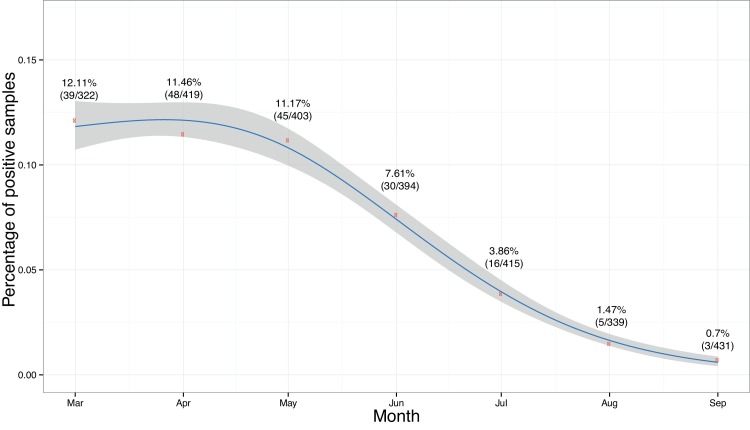
We further investigated the seasonal component of PDCoV detection in diagnostic samples. The percentage of positive PDCoV was <13% between March and September 2014 (Fig 3 ). In July 2014, the occurrence decreased to <4% while in September, the occurrence was <1%. While there was no evidence of a direct seasonal relationship within our data set, there was a strong trend, indicating a decrease of PDCoV in diagnostic samples during the summer.
Fig. 3.
The percentages of positive PDCoV case IDs between March and September 2014. Red dots represent the observed number of positives while the blue line represents the predicted value. The shaded area represents the CI.
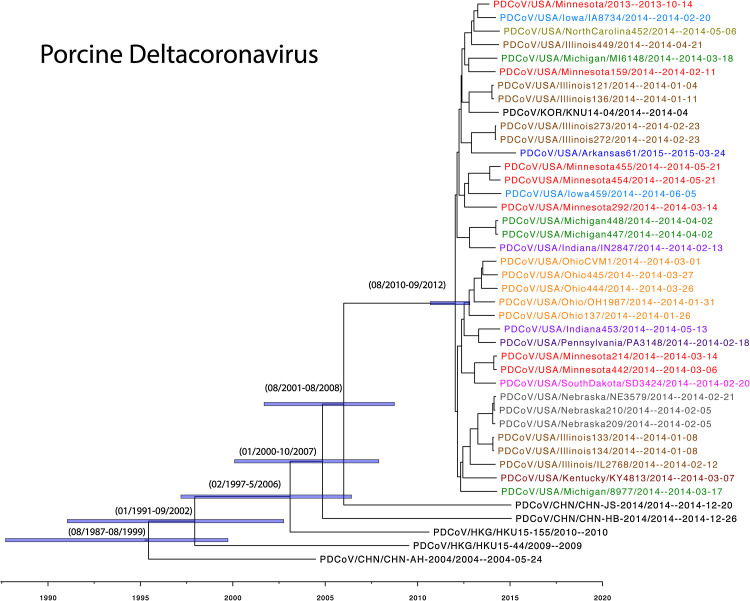
The 19 new PDCoV sequences were aligned with the 23 available PDCoV sequences from GenBank China (n = 3), Hong Kong (n = 2), South Korea (n = 1), and US (n = 17). The complete PDCoV genome sequences from the US and South Korea shared a 99.7–100% nucleotide identity while sharing a 98.9–99.3% nucleotide identity with the strains from China and Hong Kong. A time-scaled maximum clade credibility (MCC) phylogeny was inferred for the concatenated open reading frames (24,507 nt). In the phylogenetic tree, the 36 US and single Korean strains group together in a clade (posterior probability = 1.0) (Fig 4 ). Viruses from different states are mixed within the tree, evidencing of extensive spatial dispersal of the virus and multiple viral introductions into states such as Minnesota, Michigan, Indiana, and Illinois. The diversity of US PDCoV strains traces back to a common ancestor that is estimated to be between August 2010 and September 2012, indicating that PDCoV has recently emerged in the US swine population. The viruses from Asia are not closely related, and inferring the origin country of PDCoV is difficult. The phylogeny is consistent with dispersal of PDCoV from the US to South Korea, although the lack of PDCoV genetic data from other countries makes this inference tenuous. The evolutionary rate of the PDCoV genome is estimated to be 3.8 × 10−4 substitutions/site/year (2.3 × 10−4–5.4 × 10−4, 95% HPD). The evolutionary rate of the Spike gene (3483 nt) is estimated to be 2.0 × 10−3 substitutions/site/year (8.2 × 10−4–3.4 × 10−3, 95% HPD).
Fig. 4.
A time scaled phylogenetic tree of PDCoV. The US strains are colored by state while the Asain strains are represented in black. At the major nodes, the purple boxes represent the posterior distribution and the 95% tMRCAs are reported.
4. Discussion
Porcine deltacoronavirus is a newly identified virus in the US, and its virulence has been recently described (Jung et al., 2015, Ma et al., 2015). Diagnostic results are a valuable commodity to understanding the occurrence and spread of pathogens in production animals. Data analysis by case ID further enhances the results of our study compared to analyzing each sample individually. Our results could be further enhanced if individual farm identification was included in the multivariable analysis. However, farm identification was not be included with each case ID. The results of the network connection between veterinarians and swine companies highlight the importance of biosecurity since veterinarians were listed on both positive and negative case IDs. While our results indicate that PDCoV has spread throughout the US, the occurrence of PDCoV decreased to <1% by September, which more accurately reflects the current situation compared to the previous study where the occurrence of positive PDCoV in diagnostic samples was 30% (Marthaler et al., 2014c). During the summer of 2014, the prevalence of PEDV also decreased, suggesting seasonality association with enteric coronavirus in swine (Swine Health Monitoring Program).
A variety of different specimens were submitted for PDCoV testing by real time RT-PCR. Real time RT-PCR is a valuable tool for detecting pathogens, and more recently, oral fluids have been used to screen for a variety of pathogens, including porcine reproductive and respiratory syndrome virus, influenza A virus, African swine fever, classical swine fever, and foot-and-mouth disease viruses (Detmer et al., 2011, Olsen et al., 2013, Grau et al., 2015). While the previous viruses are shed in oral fluids, PDCoV is an enteric pathogen. Oral fluids are contaminated with fecal material, and our results indicated that oral fluids had the highest OR associated with PDCoV detection. Pigs constantly chew on a variety of materials including feces. When the pigs chew on the ropes used to collect oral fluids, the feces, which can contain numerous pathogens, is transferred to the ropes. Our results indicate that oral fluids are extremely beneficial to determine if a swineherd is infected with PDCoV, and oral fluids continue to be a valuable tool to monitor the health of swine herds.
The detection of PDCoV in oral fluids may explain the higher ORs in growing pigs. Individual samples may be more likely to be taken from suckling piglets while group samples may be taken from older pigs. Piglets are weaned from their mother at approximately 21 days and comingled with a numerous piglets at nursery barns, possibly from multiple different sow farms. The comingling of piglets can contribute to the transmission of PDCoV. If one farm is positive for PDCoV while the other farms are negative, the virus will spread to the negative pigs due to fecal contamination from positive animals. In addition, the maternal immunity diminishes at weaning, which makes the piglets more susceptible to infections (Saif et al., 1994, Sestak et al., 1996). Numerous viral pathogens including rotavirus A, B, and C, have high co-infection rates in growing pigs (Marthaler et al., 2012, Marthaler et al., 2013b). Decreasing the number of enteric pathogens in growing pigs is vital to maximizing feed conversion and weight gain to reduce the number of days to market.
The lack of global PDCoV strains severely hinders our understanding of the origins of PDCoV in the US. At this time, it is impossible to infer that the PDCoV strains found in US swine came from China simply because the five Chinese sequences are basal to US diversity. Despite sampling from many geographically dispersed states the diversity of PDCoV found in US swine is restricted (≥99.7 nucleotide percent identity), which is consistent with the relatively recent introduction of the virus inferred using a time-scaled Bayesian phylogeny. The lack of spatial structure observed for PDCoV in the US is also consistent with rapid dissemination of the virus across US state lines, including multiple introductions into a given state. The presence of PDCoV in approximately 2010 is supported by recent serological data, which detected swine PDCoV IgG antibodies in 2010 (Thachil et al., 2014). The evolutionary rate for PDCoV was estimated at 3.8 × 10−4 substitutions/site/year and has a similar evolutionary rate (6.2 × 10−4 substitutions/site/year) to PEDV, suggesting porcine enteric coronavirus, including TGEV, may evolve the same within pigs.
5. Conclusion
In conclusion, the percentage of PDCoV case IDs severely decreased and was <1% in September 2014. While oral fluids are a valuable specimen type to detect respiratory pathogens, oral fluids can also be used to detect enteric pathogens was well; implying oral fluids are an excellent sample type to monitor the health of swineherds. Bayesian analysis estimates the evolutionary rate of PDCoV at 3.8 × 10−4 substitutions/site/year.
Acknowledgements
This study was supported by the Rapid Agricultural Response Fund, established by the Minnesota legislature and administered by the UM Agricultural Experiment Station. We would like to thank the faculty and personnel, especially Mary Thurn and Katerina Herzberg, at the UMVDL for their technical services and diagnostic testing.
References
- Agresti A. Introduction to Generalized Linear Model. In: Anonymous, editor. John Wiley & Sons Inc.; Hoboken, New Jersey: 2013. pp. 113–162. [Google Scholar]
- Becker, R.A., Wilks, A.R., Brownrigg, R., Minka, T.P., 2014. Maps: draw geographical maps. R package version 2. 3–9.
- Bivand, R., Lewin-Koh, N., 2014. Maptools: Tools for reading and handling spatial objects. R package version 0. 8–30.
- Bivand, R., 2013. classInt: Choose univariate class intervals. R package version 0. 1–21.
- Borgatti S.P., Everett M.G., Freeman L.C. Analytic Technologies; Harvard, MA: 2002. Ucinet 6 for Windows: Software for Social Network Analysis. [Google Scholar]
- Borgatti S.P. Analytic Technologies; Lexington, KY: 2002. NetDraw Software for Network Visualization. [Google Scholar]
- Detmer S.E., Patnayak D.P., Jiang Y., Gramer M.R., Goyal S.M. Detection of influenza A virus in porcine oral fluid samples. J. Vet. Diagn. Invest. 2011;23:241–247. doi: 10.1177/104063871102300207. [DOI] [PubMed] [Google Scholar]
- Drummond A.J., Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A., Strimmer K. PAL: an object-oriented programming library for molecular evolution and phylogenetics. Bioinformatics. 2001;17:662–663. doi: 10.1093/bioinformatics/17.7.662. [DOI] [PubMed] [Google Scholar]
- Drummond A.J., Suchard M.A. Bayesian random local clocks, or one rate to rule them all. BMC Biol. 2010;8:114. doi: 10.1186/1741-7007-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A.J., Nicholls G.K., Rodrigo A.G., Solomon W. Estimating mutation parameters, population history and genealogy simultaneously from temporally spaced sequence data. Genetics. 2002;161:1307–1320. doi: 10.1093/genetics/161.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A.J., Rambaut A., Shapiro B., Pybus O.G. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol. Biol. Evol. 2005;22:1185–1192. doi: 10.1093/molbev/msi103. [DOI] [PubMed] [Google Scholar]
- Drummond A.J., Ho S.Y., Phillips M.J., Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A.J., Suchard M.A., Xie D., Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- Grau F.R., Schroeder M.E., Mulhern E.L., McIntosh M.T., Bounpheng M.A. Detection of african swine fever, classical swine fever, and foot-and-mouth disease viruses in swine oral fluids by multiplex reverse transcription real-time polymerase chain reaction. J. Vet. Diagn. Invest. 2015;27:140–149. doi: 10.1177/1040638715574768. [DOI] [PubMed] [Google Scholar]
- Hadley, W., 2009. Ggplot2: elegant graphics for data analysis.
- Heinze, G., Ploner, M., Dunkler, D., Southworth, H., 2013. Logistf: Firth’s bias reduced logistic regression. R package version 1.21.
- Jung K., Hu H., Eyerly B., Lu Z., Chepngeno J., Saif L.J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerging Infect. Dis. J. 2015;21:650. doi: 10.3201/eid2104.141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Chen Q., Harmon K.M., Yoon K.J., Schwartz K.J., Hoogland M.J., Gauger P.C., Main R.G., Zhang J. Full-length genome sequence of porcine deltacoronavirus strain USA/IA/2014/8734. Genome Announc. 2014;2:00278–314. doi: 10.1128/genomeA.00278-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang Y., Liang X., Lou F., Oglesbee M., Krakowka S., Li J. Origin, evolution, and virulence of porcine deltacoronaviruses in the united states. MBio. 2015;6 doi: 10.1128/mBio.00064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Rossow K., Gramer M., Collins J., Goyal S., Tsunemitsu H., Kuga K., Suzuki T., Ciarlet M., Matthijnssens J. Detection of substantial porcine group B rotavirus genetic diversity in the united states, resulting in a modified classification proposal for G genotypes. Virology. 2012;433:85–96. doi: 10.1016/j.virol.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Jiang Y., Otterson T., Goyal S., Rossow K., Collins J. Complete genome sequence of porcine epidemic diarrhea virus strain USA/colorado/2013 from the united states. Genome Announc. 2013;1:00555–613. doi: 10.1128/genomeA.00555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Rossow K., Culhane M., Collins J., Goyal S., Ciarlet M., Matthijnssens J. Identification, phylogenetic analysis and classification of porcine group C rotavirus VP7 sequences from the united states and Canada. Virology. 2013;446:189–198. doi: 10.1016/j.virol.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Marthaler D., Homwong N., Rossow K., Culhane M., Goyal S., Collins J., Matthijnssens J., Ciarlet M. Rapid detection and high occurrence of porcine rotavirus A, B, and C by RT-qPCR in diagnostic samples. J. Virol. Methods. 2014;209:30–34. doi: 10.1016/j.jviromet.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Marthaler D., Jiang Y., Collins J., Rossow K. Complete genome sequence of strain SDCV/USA/Illinois121/2014, a porcine deltacoronavirus from the united states. Genome Announc. 2014;2:00218–314. doi: 10.1128/genomeA.00218-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Raymond L., Jiang Y., Collins J., Rossow K., Rovira A. Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerg. Infect. Dis. 2014;20:1347–1350. doi: 10.3201/eid2008.140526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Rossow K., Culhane M., Goyal S., Collins J., Matthijnssens J., Nelson M., Ciarlet M. Widespread rotavirus H in commercially raised pigs, united states. Emerg. Infect. Dis. 2014;20:1203–1206. doi: 10.3201/eid2007.140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P., Perlman S. Coronaviridae. In: Knipe D., Howley P., editors. Fields Virology. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 825–858. [Google Scholar]
- Neuwirth, E., 2014. RColorBrewer: ColorBrewer palettes. R package version 1. 1–2.
- Olsen C., Wang C., Christopher-Hennings J., Doolittle K., Harmon K.M., Abate S., Kittawornrat A., Lizano S., Main R., Nelson E.A., Otterson T., Panyasing Y., Rademacher C., Rauh R., Shah R., Zimmerman J. Probability of detecting porcine reproductive and respiratory syndrome virus infection using pen-based swine oral fluid specimens as a function of within-pen prevalence. J. Vet. Diagn. Invest. 2013;25:328–335. doi: 10.1177/1040638713481471. [DOI] [PubMed] [Google Scholar]
- R, C.T., 2015. R: A language and environment for statistical computing.
- Saif L.J., van Cott J.L., Brim T.A. Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine. Vet. Immunol. Immunopathol. 1994;43:89–97. doi: 10.1016/0165-2427(94)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K., Lanza I., Park S.K., Weilnau P.A., Saif L.J. Contribution of passive immunity to porcine respiratory coronavirus to protection against transmissible gastroenteritis virus challenge exposure in suckling pigs. Am. J. Vet. Res. 1996;57:664–671. [PubMed] [Google Scholar]
- Stevenson G.W., Hoang H., Schwartz K.J., Burrough E.R., Sun D., Madson D., Cooper V.L., Pillatzki A., Gauger P., Schmitt B.J., Koster L.G., Killian M.L., Yoon K.J. Emergence of porcine epidemic diarrhea virus in the united states: clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- Thachil, A., Gerber, P., Xio, C., Huan, Y., Opriessnig, T., Halbur, P., 2014. A porcine deltacoronavirus serological survey using an indirect PDCoV anti-IgG ELISA confirms that PDCoV infection in US pigs is low and has been present since 2010. AASV.
- Wang L., Byrum B., Zhang Y. Porcine coronavirus HKU15 detected in 9 US states. Emerg. Infect. Dis. 2014;20:1594–1595. doi: 10.3201/eid2009.140756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]