Abstract
Livestock production is an important source of animal protein worldwide. In the developed world meat consumption will remain steady but demand is forecast to grow enormously in developing countries. The use of genomics will speed genetic improvement and increase levels of production quickly in the developed world but might face problems in the developing world, including scientific, economic and political challenges. Considerable increases in public and private research funding will be required to develop and utilize novel tools and collections of detailed trait information on appropriate animals. The development of policies protecting the environment and managing all genetic resources will also be needed. Advances in livestock genomics have major implications for increasing food output as well as improving human health.
Introduction
Livestock production started ∼8000–10 000 years ago when pigs, chickens, cattle, goats and sheep were first domesticated. Following domestication, herdsmen made gradual genetic improvement of livestock, and later the ‘master breeders’ developed many breeds in Europe and Asia [1] leading to the definition of production characteristics and improvement in these species. The advent of modern genetics (see Glossary) and the rediscovery of Mendel's laws [2] led to increased rates of improvement and the use of the genetic tools of selection and crossbreeding. Pigs now grow considerably faster, are 25% leaner and use much less feed than 20 years ago, moreover, litter size has increased considerably. Commercially raised chickens and other poultry grow at incredible rates on far less feed than was required just ten years ago thanks to the implementation of modern genetics [3]. The overall rates of genetic improvement in these and other species have been considerable and have led to changes in livestock production in both developed and developing countries. Advances in reproductive technologies, including artificial insemination, embryo transfer and now the possibility of cloning, in combination with modern applications of nutrition and muscle physiology, have greatly improved the quality and efficiency of livestock production to provide healthy sources of animal protein.
The molecular genetics revolution, beginning in the late 1980s, led to the development of the field of genomics (see Glossary), the study of the entire genome of animals and the understanding of what individual genes do and how they interact to control biological processes. Genetic maps were developed in the mid 1990s for all species leading to early discoveries of individual genes controlling a small number of production traits [4]. Nowhere has that been more evident than in the swine industry, where several gene tests are being used in marker-assisted selection (MAS; see Glossary) programs to improve growth, meat quality and reproduction in over 60% of the pigs produced in the USA [5].
International efforts to sequence the human genome began in the 1990s, and initial drafts of the human genome were first published in 2001 6, 7. Using the infrastructure created by the billions of dollars of research investment, scientists working with food-producing species began efforts to capitalize on the human genome sequencing [8]. The first drafts of the sequencing of the chicken and cattle genomes were completed in 2005 9, 10 (http://www.genome.gov/12512874). In 2006, the sequencing of the horse and the pig genomes were also begun, with the first draft of the horse sequence completed in 2007, and the pig sequence expected to be completed in late 2008 or early 2009 [11]. Already, the first sequence information is revealing interesting results that will eventually impact poultry production. For example, the chicken sequence is elucidating a variety of aspects including gene diversity, role of non coding DNA, similarity of chickens to mammals and information on over 3 million single nucleotide polymorphisms (SNPs) 12, 13. In cattle, for instance, SNP gene chips with over 50 000 SNPs are being used to detect associations of genetic markers with traits to improve reproductive and production performance. Similar large-scale SNP gene chips for association analyses (see Glossary) are under development in chickens, pigs and other species. These developments have helped to create the opportunity for genomics to have enormous impacts on livestock. In addition, demands for fish and other aquaculture species have brought about increased dependence on farm-raised aquaculture programs that employ advanced genetics. Sequencing efforts, marker discovery and map developments are well underway and will likely lead to the use of MAS in many aquaculture species [14]. However, more than just the genomics tools need to be put into place. In order for the potential of genomics to be fulfilled, there needs to be parallel efforts on the collection of detailed trait information. This should also include the use of these technologies to help describe the environments where the animals are reared. For example, to describe the microbial environment that interacts with the animal, whether it be benign, as in the case of the rumen, or harmful, as in the case of pathogens. These aspects will require a new mindset for industry stakeholders, particularly those who provide research funding at federal and regional levels. This deepening of the phenotypic trait data are increasingly referred to as ‘the phenomic gap’ (see Glossary).
Possible political, social and environmental impacts
Crystal ball gazing is often a dangerous business. However, some issues are clear given past developments in animal and crop production systems. The world is undergoing what has been called a ‘livestock revolution’ [15], driven by continued population growth, rising incomes and urbanization in developing countries. These social changes are driving a growing demand for meat and other animal protein sources (Table 1 ). In the developed world, animal production has generally increased at the expense of loss of the number of independent livestock producers, increased environmental challenges and growing animal welfare concerns. This is most obvious for pork and poultry production, where the largest units now produce most of the animals for consumption. In Europe, consumer demands for ‘farm raised’ instead of ‘factory farmed’ animals have slowed that process but the trend is well defined. In many developed countries or regions, environmental concerns are ever increasing. Pollution from large concentrated animal facilities is of major concern, not only because of large amounts of waste that must be disposed of, but also because of occasional waste spills, which can have disastrous outcomes in nearby rivers and streams. Many countries are regulating the amount of organic material that can be spread on the land and, in some regions, large-scale livestock production expansion is now limited. Finally, the developed world is particularly concerned about food safety and animal-borne diseases as illustrated by BSE (bovine spongiform encephalopathy), SARS (severe acute respiratory syndrome) and new strains of avian flu, which, it is feared, might lead to a human influenza pandemic.
Table 1.
Evidence of increasing demand for meat
| Annual growth of total meat consumption (%) |
Total meat consumption (Mt) |
||||
|---|---|---|---|---|---|
| Region | 1982–1994 | 1993–2020 | 1983 | 1993 | 2020 |
| China | 8.6 | 3.0 | 16 | 38 | 85 |
| India | 3.6 | 2.9 | 3 | 4 | 8 |
| Southeast Asia | 5.6 | 3.0 | 4 | 7 | 16 |
| Latin America | 3.3 | 2.3 | 15 | 21 | 39 |
| West Asia/North Africa | 2.4 | 2.8 | 5 | 6 | 15 |
| Sub-Saharan Africa | 2.2 | 3.5 | 4 | 5 | 12 |
| Developing world | 5.4 | 2.8 | 50 | 88 | 188 |
| Developed world | 1.0 | 0.6 | 88 | 97 | 115 |
| Total world | 2.9 | 1.8 | 139 | 184 | 303 |
Sources: FAO annual data. Total meat consumption for 1983 and 1993 are three-year moving averages. 2020 projections come from IFPRI's global model, IMPACT; Delgado C. et al. (1999) Livestock to 2020: The Next Food Revolution. Food Agriculture and Environment. Discussion Paper 28, International Food Policy Research Institute.
Abbreviation: Mt, million metric tonne.
In the developing world, animals have often meant an important source of food, fiber, fertilizer and power. In particular, animals are an important source of protein and offer the opportunity to survive economically and to provide sources of income for struggling families. Increased livestock production in some of these countries means competition for grain to feed humans and, in some cases where markets are growing rapidly, this displaces the smaller producers. In addition, native breeds, which are adapted to local environments and feed sources and might have had some disease resistance, are being replaced, often by Western breeds and lines that might be less appropriate for the systems where they are introduced. The resulting underperformance and losses resulting from native diseases can cause major losses to subsistence farmers and disruptions in the local economy.
Further clues on impacts can be derived from experiences relative to the use of genomics in crop production and the responses from the public. At present, genetically modified (GM) transgenic corn and soybeans are routinely used in many food products in the USA without labeling or restriction. However, in Europe, their use is being challenged. This situation has been transferred to the developing world where, despite advances brought about by the ‘green revolution’, there are individuals and countries now advocating the banning of improved GM varieties to be used in Africa. The condescending and paternalistic attitudes of some countries that seem to wish to prevent advanced crop stocks to be used in countries that need all the help they can get to feed their populations is alarming and points to a real fear, on the part of some societies, of the use of genomics in food production. However, there might need to be a distinction between the use of genomics technologies as an aid for animal breeding (as in MAS or potentially marker-assisted management) and the use of the technology to identify genes that can deliver benefits through genetic modification (see Glossary). Currently, transgenic (GM) animals have not been approved for food production although the use of cloned animals for food has recently moved closer to approval in the USA. The use of modern genomics does not require genetic modification or genetic engineering to be successful.
Genomics as part of the solution
Clearly genomics offers some real advantages and opportunities to improve livestock production in the developed world and advance the ’livestock revolution’ in the developing world. In the developed world, the use of genomic tools will rapidly increase as the sequence information is translated into tools that can be easily used. The first generation of tools are single-gene tests, which are rapidly being incorporated into selection programs 4, 5. More advanced outcomes include the millions of SNPs that are likely to be found in each species. These then can be used to create SNP chips for advanced genotyping and for association studies. Such efforts are well underway in chickens, cattle and pigs. The earliest discoveries will continue to improve the efficiency of production and will include increased reproductive performance, growth, increased lean content and meat quality. These increases in rates of improvement will range from 20% for growth and lean content to over 60% for reproduction and meat quality [16]. Major discoveries affecting disease resistance and food safety might offer rates of improvement in healthiness never seen before, because these traits are nearly impossible or very difficult to improve by traditional means (at least in terrestrial animals). Improved feed efficiency will be of increasing importance given the existing competition for grain between livestock feed, biofuels and human consumption and an increased focus on the contribution of livestock agriculture on greenhouse-gas production (methane, a major output from cattle and sheep has 21 times the impact of CO2).
Genomics can also help address environmental issues on several levels. Increases in feed efficiency [17] will lead to less waste output and lower production of methane. Already a transgenic phytase pig, which expresses phytase in its saliva and, hence, produces less phosphorus in the manure, has been developed in Canada [18]. This could have special benefit in areas where pig production is heavy and manure with large amounts of phosphorus has been polluting the environment. Although this pig model is excellent, no approval for the consumption of such pigs is allowed to date in Canada, where it was developed, or in the USA. Also, there might be more consumer-acceptable methods of achieving the same outcome, without needing to ‘interfere’ with the integrity of the animal. For example, researchers have been looking for SNPs associated with fast growth and no ill effects under lower feeding of phosphorus in the diet, which might be useful in standard selection schemes and, hence, might preclude the need for a transgenic pig expressing phytase. One novel transgenic pig is one that is rich in omega-3 fatty acids [19], which might have real health advantages for those who consume pork, but this awaits approval for consumption. Given the public's concerns over food safety, it is likely that such approval is years off in the USA, Canada and Europe. Again, it is possible to enrich pork for omega-3 fatty acids through changes to the feeding regime. Such an approach is more likely to deliver enriched ‘healthy pork’ in the short term. It is likely that animals will vary in their ability to incorporate the dietary omega-3 into meat and fat, so that genomics tools might also play a role in producing these new healthier foods.
Solutions involving genomics and not genetic modification are more likely to be adopted. The large, expected outputs from the SNP-association trials offer new challenges and opportunities. Chicken-, cattle- and pig-breeding companies might have literally hundreds or thousands of markers to select on. How will these be employed? The use of so-called ‘genomic selection’ is likely to be used, but new theories and developments are needed to use the markers effectively instead of the traditional use of estimated breeding values (EBV; see Glossary) [20]. More importantly, SNP associations can be devoted to traits previously overlooked, such as behavior, disease resistance, structural and environmental soundness and other traits, as long as efforts are made to collect the necessary data. This is not a trivial exercise and new paradigms might be required to get such work funded. Examination of genetic causes for differences related to behavior and stress might make it possible to breed healthier and more adaptable animals, which will lessen welfare concerns. Also, these genomic approaches will also look to measure more effectively genotype by environment interactions and to develop ways to select specific genotypes or ‘designer genes’ for specialized niche markets and products. Animals that increase the amount of omega-3 in their products would be an example. This is likely to raise incomes, at least for those producing specialized products.
In developing countries, the use of genomics will be implemented more slowly. As production systems are modified to be more capitalized, advanced genetic stock from developed countries will be used more frequently and increased production can be expected from the genomics-led improvements. Traditionally, such actions have not always been successful because ‘Western breeds’ are not well adapted to the environments of developing countries, which have different feed stuffs, weather and disease conditions. The use of genomics can be particularly powerful given that genomic research can be used to discover genes associated with ’native’ resistance. The ability to make these discoveries will possibly allow for the development of new lines and breeds that combine increased performance from Western breeds and increased disease resistance from the native stocks. Such new lines or breeds of animals could improve efficiencies by over 50% extremely rapidly. Production schemes that combine local and Western breeds of pigs have been successfully developed in Asia (J.P. Gibson, unpublished* ). Such schemes can lead to the conservation of local breeds, which otherwise are threatened by the introduction of Western genetics. Genomics can make important contributions here, including the characterization of genetic diversity as well as the identification of novel genetic variants explaining disease resistance or adaptation. Efforts will need to be made that will promote the use of genomics for the benefit of all producers, careful use of the environment, and safe and welfare conscious livestock production [15].
Animal genomics advance human health
There has been a continued interest in the pig as a biological model for human biology. A recent survey of U.S. government grants found over 400 active grants using pigs as models have received funding from the National Institute of Health. Research using pigs as animal models of human conditions has covered a vast array of disciplines, such as nutrition, digestive physiology, kidney function, heart function, diabetes, skin formation and healing, and obesity. With the growing evidence of the close genetic relation of the pig to the human, evidenced by the completed sequence analysis of the Sino-Danish project [21] and new sequence information as it becomes available, the extent of biomedical projects using the pig can be expected to grow in the future. For example, the pig is a very good model for human obesity, with very large amounts of data available on obesity-related traits in commercial herds of pigs. The phenomic gap has already been closed in this case so that there is a new opportunity to study genes that are involved in obesity [22]. Another possible application: the development of diabetes therapies that utilize the discovery of a diabetes gene in pigs [23], is illustrated in Figure 1 .
Figure 1.
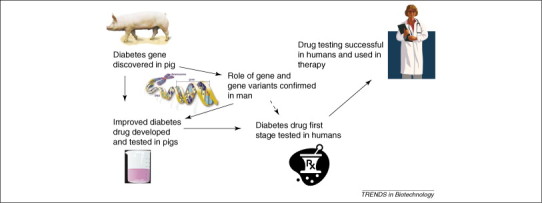
Possible application of pig genomics to human medicine. Shown is the potential development of diabetes therapies in humans, which utilize the discovery of a diabetes gene in pigs [23].
Shortages of human tissues and organs available for transplantation have fostered an interest in xenotransplantation (see Glossary), and the pig is the preferred donor owing to its size and comparative physiology. Recent concerns about retroviruses and difficulties in producing transgenic pigs meeting the standards required for safe transplantation have slowed the progress in the use of the pig for xenotransplantation and have caused some companies to scale back active research in this area. However it is possible that some portion of animal agriculture could be devoted to organ transplantation in the future.
Other species, particularly the chicken, have played an important role in early immunology research and work with developmental biology. More recently studies have involved virology and oncology. Such work has even been recognized with Nobel prizes to those researchers using the chicken for the basis of their discoveries. Opportunities to continue such work are immense given that the chicken genome is sequenced. Clearly, the involvement of NIH in paying for the entire sequencing effort suggests that the chicken has real relevance for human health. Certainly, given the sequencing efforts, this offers the opportunity to better understand the genetic basis for evolution/selection on adaptation.
Conclusions
Initial genomic discoveries in laboratories have quickly found their way to farms as commercial companies continue to employ these technological advances. It is envisioned that, in the future, instead of selection on EBVs for traditional traits, genomic selection based on thousands of genes markers is likely to be used for a wider range of traits of economic performance. Further discoveries and enhanced understanding of the complexity of livestock genomes will simply boost livestock's contribution in providing a sustainable source of protein worldwide, as well as playing a valuable role in biomedical research. Policy decisions that support smart and environmentally sound growth, access to technology by all levels of producers and continued governmental financial support for both short- and long-term genomics research will be required if the full impact of genomics on animal agriculture is to be realized 15, 24.
Acknowledgements
M.F.R. appreciates the support provided in part by PIC/Genus, Monsanto Choice Genetics, Hatch, Iowa Agricultural Experiment Station and State of Iowa funds. M.F.R. also wishes to thank financial support received from the USDA NRSP8, which supports the National Pig Genome Coordination project. G.S.P. is funded by the Alberta Livestock Industry Development Fund and the Alberta Agricultural Research Institute. Both authors are actively involved in animal genomics research, work with industry, academic groups and funding agencies around the world and have granted patents or pending patent applications on DNA markers for application in livestock species.
Glossary
- Association analysis
is the determination of whether an SNP can explain variation in a trait of interest, such as litter size in pigs or milk yield in dairy cows. One of the bases at the SNP is on average associated with a significantly different value for the trait being studied. For example, pigs with an adenosine (A) in codon 298 of MC4R on both of its chromosomes eat more and grow faster than pigs with a guanidine (G) at this position [18].
- Estimated breeding values (EBV)
is the value of an individual animal for a specific trait or combination of traits compared to its contemporaries. It is calculated from the animals own performance and/or the performance of its progeny and relatives. For example, a dairy bull's EBV for milk is calculated based on the production records of his daughters. Nowadays, it is possible to incorporate DNA marker information to increase the accuracy of the EBV.
- Genetic modification
is the science of altering the genome of an organism by inserting or deleting specific genes or modifying the sequence of the organism. Although genetically modified crop production is now common in some countries (USA, Canada, China) and genetically modified livestock were made as long ago as 1980s, none are approved for marketing at this time.
- Genetics
is the science of heredity and variation in living organisms. Long before G. Mendel first wrote about the laws of inheritance in 1866, farmers used their understanding of inheritance of characteristics to improving crop plants and animals through selective breeding.
- Genomics
is the science of an organism's entire genome resulting from its sequencing. Genomics includes the study of single genes, gene networks, gene interactions and expression.
- Marker-assisted selection (MAS)
is the use of the DNA genotype variation, for example SNPs, to calculate the breeding value of an animal. Marker-assisted management is the use of this information to predict the potential performance of an animal in order to help decide how to rear it in order to optimize product quality and commercial return.
- Phenomic gap
refers to the lack of data on the traits that can benefit most from the application of genomics and MAS. With the phenomenal increase in genomic resources (sequence, SNPs and low-cost genotyping), this gap has recently been recognized as the biggest hurdle for the exploitation of the technology in livestock and improving our understanding of biology.
- Single nucleotide polymorphisms (SNPs)
are differences in the genome sequence between chromosome pairs and between individuals at the finest level of the DNA sequence, the four bases that make up the DNA alphabet: adenosine (A), cytosine (C), guanidine (G) and thymine (T). Once identified, these differences can be routinely assayed (genotyped) in cells collected from an animal.
- Xenotransplantation
is the transplantation of cells, tissue or organs between species, for example between pigs and humans. Such an approach has been proposed as a means of overcoming the shortage in human donors for transplant surgery. However, the recipient mounts a powerful rejection response to such foreign organs; this response must be overcome if xenotransplantation is to be undertaken successfully in the future. Genetic modification is being used to remove some of these problems, however, concerns such as the potential for novel disease transmission remain.
Footnotes
Gibson, J.P. et al., Paper 06–12, 8th World Congress of Genetics Applied to Livestock Production, Belo Horizonte, MG., Brazil, August 2006.
References
- 1.Mason I.L., editor. A World Dictionary of Livestock Breeds, Types, and Varieties. 2nd ed. CABI; 1969. [Google Scholar]
- 2.Mendel, G.J., ed. (1866) Versuche über Pflanzenhybriden. Verhandlungen des Naturforschenden Vereins Brünn 4, 3–47
- 3.Muir W.M., Aggrey S.E., editors. Poultry Genetics, Breeding and Biotechnology. CABI; 2003. [Google Scholar]
- 4.Andersson L., Georges M. Domestic animal genomics: Deciphering the genetics of complex traits. Nat. Rev. Genet. 2004;5:202–212. doi: 10.1038/nrg1294. [DOI] [PubMed] [Google Scholar]
- 5.Rothschild M.F. Porcine genomics delivers new tools and results: this little piggy did more than just go to market. Genet. Res. 2004;83:1–6. doi: 10.1017/s0016672303006621. [DOI] [PubMed] [Google Scholar]
- 6.Lander E.S. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 7.Venter J.C. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 8.Womack J.E. Advances in livestock genomics: opening the barn door. Genome Res. 2005;15:1699–1705. doi: 10.1101/gr.3809105. [DOI] [PubMed] [Google Scholar]
- 9.International Chicken Genome Sequencing Consortium (2004) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432, 695–716 [DOI] [PubMed]
- 10.Dodgson J.B. Chicken genome sequence: A centennial gift to poultry genetics. Cytogenet. Genome Res. 2003;102:291–296. doi: 10.1159/000075765. [DOI] [PubMed] [Google Scholar]
- 11.Schook L.B. Swine genome sequencing consortium (SGSC): a strategic roadmap for sequencing the pig genome. Comp. Funct. Genom. 2005;6:251–255. doi: 10.1002/cfg.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourque G. Comparative architectures of mammalian and chicken genomes reveal highly variable rates of genomic rearrangements across different lineages. Genome Res. 2005;15:98–110. doi: 10.1101/gr.3002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Chicken Polymorphism Map Consortium (2004) A genetic variation map for chicken with 2.8 million single-nucleotide polymorphisms. Nature 432, 717–722 [DOI] [PMC free article] [PubMed]
- 14.Rothschild, M.F. and Ruvinsky, A. (2007). Marker assisted selection for aquaculture species. In: Aquaculture Genome Technologies (Liu, J.,ed.) Blackwell Press (in press)
- 15.Delgado C., editor. Livestock to 2020: The Next Food Revolution. International Food Policy Research Institute; 1999. [Google Scholar]
- 16.Meuwissen T.H.E., Goddard M.E. The use of marker haplotypes in animal breeding schemes. Genet. Sel. Evol. 1996;28:161–176. [Google Scholar]
- 17.Kim K.S. A missense variant of the melanocortin 4 receptor (MC4R) gene is associated with fatness, growth and feed intake traits. Mamm. Genome. 2000;11:131–135. doi: 10.1007/s003350010025. [DOI] [PubMed] [Google Scholar]
- 18.Golovan S.P. Pigs expressing salivary phytase produce low-phosphorus manure. Nat. Biotechnol. 2001;19:741–745. doi: 10.1038/90788. [DOI] [PubMed] [Google Scholar]
- 19.Lai L. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat. Biotechnol. 2006;24:435–436. doi: 10.1038/nbt1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meuwissen T.H.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 2001;157:1819–1829. doi: 10.1093/genetics/157.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wernersson R. Pigs in sequence space: a 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genomics. 2005;6:70. doi: 10.1186/1471-2164-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocha D., Plastow G. Commercial pigs: an untapped resource for human obesity research? Drug Discov. Today. 2006;11:475–477. doi: 10.1016/j.drudis.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Kim K.S. A comparative study of obesity QTL and candidate genes in the pig: a model organism for human obesity. Obes. Res. 2004;12:1981–1984. [Google Scholar]
- 24.Green R.D. Identifying the future needs for long-term USDA efforts in agricultural animal genomics. Int. J. Biol. Sci. 2007;3:185–191. doi: 10.7150/ijbs.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]


