Short abstract
Objectives
This study assessed the protective effects of saturated hydrogen against CCl4-induced acute kidney injury (AKI) in mice, and investigated signaling pathways activated by exposure to saturated hydrogen.
Methods
A mouse model of CCl4-induced AKI was established; some mice were treated with saturated hydrogen. Levels of cystatin C and kidney injury molecule 1 were determined using enzyme-linked immunosorbent assays. Blood urea nitrogen and serum creatinine were measured on a fully automated biochemical analyzer. Interleukin-8, tumor necrosis factor-α, and interferon-γ in serum and kidney tissues were measured using enzyme-linked immunosorbent assays. Malondialdehyde, glutathione peroxidase, and superoxide dismutase in kidney tissues were measured using biochemical kits. Oxidative stress in kidney tissues was analyzed using nitrotyrosine staining. Expression levels of p-JAK2, p-STAT3, and p-p65 signal protein were assayed by immunohistochemistry and western blotting.
Results
Compared with untreated mice with CCl4-induced AKI, mice that were treated with saturated hydrogen exhibited improved renal function and reduced oxidative stress. Moreover, expression levels of p-JAK2, p-STAT3, and p-p65 were significantly reduced in mice treated with saturated hydrogen, compared with expression levels in untreated mice.
Conclusions
Treatment with saturated hydrogen can reduce oxidative stress and inflammatory cytokine activation, potentially through inhibition of JAK2/STAT3/p65 signaling, thereby protecting against AKI.
Keywords: Saturated hydrogen, acute kidney injury, oxidative stress, JAK2/STAT3/p65, carbon tetrachloride, glutathione peroxidase, cystatin C, malondialdehyde, kidney injury molecule 1, superoxide dismutase
Introduction
Acute kidney injury (AKI) is defined as acute and rapid deterioration of kidney function as a result of various etiologies. Acute tubular necrosis is the most common cause of AKI (>90%). Low blood volume, major surgery, crush syndrome, cardiogenic shock, and septic shock are main risk factors for AKI. Oxidative stress and inflammation of renal tissue cells are commonly observed in the presence of these risk factors.1 AKI is a critical and severe disease frequently encountered in clinical practice.1,2 Its incidence is rising yearly; notably, it is an important cause of death among affected patients.
In recent years, hydrogen molecules, which exhibit selective antioxidant action, have been shown to effectively inhibit hydroxyl radicals without influencing other oxygen radicals that have important physiological regulatory functions.3 Multiple studies have shown that hydrogen molecules can significantly ameliorate oxidative injury in the brain, liver, small intestine, heat, and lung, due to ischemia-reperfusion.4–7 Currently, hydrogen is widely used in clinical practice. The greatest advantage of hydrogen is its considerable biological safety. No clear adverse reactions have been observed in association with various hydrogen treatment methods. The application of hydrogen is also gradually enriched through methods such as inhalation, oral intake, and intravenous injection.4 This study was performed to assess the protective effect of a 0.9% sodium chloride solution containing saturated hydrogen against carbon tetrachloride (CCl4)-induced AKI in mice, and to investigate signaling pathways activated by exposure to saturated hydrogen.
Materials and methods
Preparation of saturated hydrogen-containing solution
High-pressure hydrogen (output pressure: 0.4 MPa) was added to 0.9% sodium chloride solution for 6 hours to achieve saturation. Following sterilization, the solution was stored at 4°C. Gas chromatography showed that the hydrogen concentration in the solution was 0.75 M, exceeding the effective therapeutic concentration (0.6 M).
CCl4-induced AKI mouse model
Forty specific pathogen free male mice (weight: 20–30 g) were obtained from the Experimental Animal Center of Soochow University; a mouse model of CCl4-induced AKI was prepared in accordance with the experimental method described by Suzuki et al.8 The protocol was approved by the Institutional Animal Care and Use Committees of Soochow University (Permit Number: 3rSZ-2018443). CCl4 (Chongqing Chuandong Chemical (Group) Co., Ltd., Chongqing, China) and olive oil were mixed at a volume ratio of 1:9 to produce a 10% CCl4 solution. The mice were randomized into four groups: control (mice received a single dose of 300 µL physiological saline via intraperitoneal injection); hydrogen control (mice received a daily intragastric dose of 0.5 mL of H2, 6 hours after intraperitoneal injection of an equal volume of physiological saline); CCl4 model (mice received a single dose of 10% CCl4 solution [20 mL/kg] via intraperitoneal injection); and hydrogen treatment (mice received a daily intragastric dose of 0.5 mL of H2, 6 hours after intraperitoneal injection of 10% CCl4 solution [20 mL/kg]). Urine was collected by the tail-lifting method 48 hours following administration of CCl4; mice were then anesthetized by intraperitoneal injection and euthanized by cervical dislocation. Whole blood was collected via cardiac puncture, then incubated at room temperature for 2 hours without anticoagulant; it was then centrifuged (500 × g for 15 minutes at 4°C) and serum was collected. The abdomens of the mice were opened and kidney specimens were collected; the specimens were fixed in paraformaldehyde and embedded in paraffin, or cryopreserved (flash frozen) in liquid nitrogen.
Measurement of kidney function indicators in serum
Cystatin C (Cys C) was assayed using enzyme-linked immunosorbent assay (ELISA) kits purchased from Crystal Chem Inc. (Elk Grove Village, IL, USA); mouse kidney injury molecule 1 (KIM-1) was assayed using ELISA kits purchased from Shanghai Walan Biotechnology Co., Ltd. (Shanghai, China). Blood urea nitrogen (BUN) and serum creatinine (Scr) were measured on a 7600 fully automated biochemical analyzer (Hitachi, Beijing, China).
Measurement of serum and kidney tissue levels of interleukin-8, tumor necrosis factor-α, and interferon-γ
Expression levels of interleukin (IL)-8, tumor necrosis factor (TNF)-α and interferon (IFN)-γ in serum were measured in accordance with the instructions of the respective ELISA kits (Abcam, Cambridge, MA, USA). Approximately 100 mg of kidney tissue were homogenized in 1 mL of 0.05 M pre-cooled phosphate-buffered saline (pH 7.4), then centrifuged at 12,000 g for 10 minutes at 4°C. The levels of IL-8, TNF-α, and IFN-γ in homogenized kidney tissue were measured in accordance with the instructions of the respective ELISA kits.
Assessment of malondialdehyde, glutathione peroxidase, superoxide dismutase, and oxidative stress in kidney tissue
A 5% kidney homogenate was prepared by shearing 0.75 g of kidney tissue using scissors and homogenizing the tissue in a homogenizer with 0.05 M pre-cooled phosphate-buffered saline (pH 7.4) (solution volume 20-fold greater than that of dry kidney tissue). Levels of malondialdehyde (MDA) and glutathione peroxidase (GSH), as well as superoxide dismutase (SOD) activity, were measured in accordance with the instructions of MDA, GSH, and SOD assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The level of oxidative stress in tissue was detected by nitrotyrosine staining.
Hematoxylin-eosin staining and immunohistochemistry of kidney tissue
Paraffin blocks of kidney tissue were prepared as follows: complete left kidney tissue from each mouse was fixed in 10% neutral formaldehyde solution for 48 hours; this was followed by dehydration, clearing, and embedding. Paraffin-embedded sections (5-µm thick) were prepared and stained with hematoxylin–eosin, then observed via light microscopy for pathological changes. According to the degrees of renal tubular epithelial cell necrosis, renal tubular dilatation, and brush border degeneration, as well as the tubular type, the sections were graded as follows: 0, normal; 1, < 25%; 2, 25% to 50%; 3, 50% to 75%; 4, > 75%. At least 10 visual fields (× 400) at the junction of renal cortex and medulla in each section were randomly selected for analysis.
For immunocytochemistry, the slides were incubated with 0.3% hydrogen peroxide for 10 minutes, blocked with 0.5% bovine serum albumin (Boster Biological, Wuhan, China) for 30 minutes, and incubated overnight at room temperature with p-JAK2, p-STAT3, and p-p65 rabbit anti-mouse antibodies. The slides were washed three times and incubated with streptavidin-peroxidase complex (Boster Biological) at room temperature for 30 minutes, then rinsed twice with phosphate-buffered saline. An appropriate amount of diaminobenzidine was added and slides were rinsed twice with phosphate-buffered saline; they were counterstained with hematoxylin for 3 minutes, then observed via light microscopy.
Western blotting assessment of p-JAK2, p-STAT3, and p-p65 signal protein in kidney tissue
Kidney tissues were homogenized with RIPA lysis buffer (Dingguo, Beijing, China) on ice for 30 minutes; the supernatant was collected after centrifugation at 1200 × g for 15 minutes at 4°C. Proteins were quantified using the bicinchoninic acid method and then denatured; proteins were then separated via 12% sodium dodecylsulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with skim milk for 1 hour and then incubated with primary rabbit anti-mouse p-JAK2 (1:200, Cat. No. A00027), p-STAT3 (1:250, Cat. No. M00007) and p-p65 (1:100, Cat. No. M00284) antibodies overnight at 4°C; subsequently, the membrane was incubated with a horseradish peroxidase-labeled anti-rabbit secondary antibody (Cat. No. BA1054) at 37°C for 1 hour. All antibodies were purchased from Boster Biological. Enhanced chemiluminescence was used to visualize the protein bands (Edo Biotech, Shanghai, China), and the signals were captured via X-ray film. Relative densities were analyzed by a gel image system (Bio-Rad, Hercules, CA, USA) using glyceraldehyde 3-phosphate dehydrogenase as an internal control.9
Statistical methods
Data were analyzed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Measurement data are presented as mean ± standard deviation. Inter-group comparisons were performed by one-way analysis of variance. Multiple comparisons were performed by Student–Newman–Keuls test. A p value of < 0.05 was considered statistically significant.
Results
Treatment with saturated hydrogen improves kidney function
Compared with levels in the control group, levels of BUN, Scr, Cys C, and KIM-1 were increased at 48 hours after injection of CCl4 in the CCl4 model group (p < 0.01 for all). Compared with levels in the model group, levels of BUN, Scr, Cys C, and KIM-1 were reduced in the hydrogen treatment group (p < 0.05 for all) (Figure 1). These results indicated that treatment with saturated hydrogen can reduce kidney impairment.
Figure 1.
Treatment with saturated hydrogen improves kidney function. Levels of BUN and Scr were determined using an automated biochemical analyzer; Cys C and KIM-1 were assayed using ELISA. **p < 0.01 vs. Control; #p < 0.05 vs. CCl4.
Abbreviations: BUN, blood urea nitrogen; Scr, serum creatinine; Cys C, cystatin C; KIM-1, kidney injury molecule 1; H2, saturated hydrogen; CCl4, carbon tetrachloride.
Treatment with saturated hydrogen inhibits production of inflammatory factors, lowers MDA content, and reduces oxidative stress
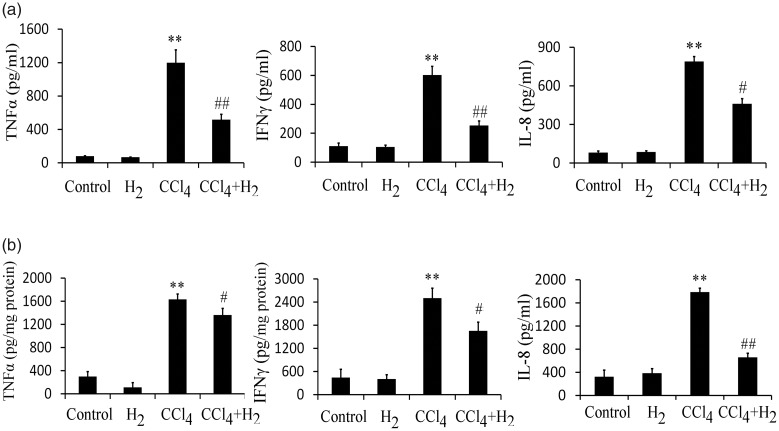
The expression levels of inflammatory cytokines TNF-α, IFN-γ, and IL-8 in serum significantly increased in the model group (p < 0.01 for all); compared with levels in the model group, the expression levels of inflammatory cytokines TNF-α, IFN-γ, and IL-8 significantly decreased in the hydrogen treatment group (p < 0.01 for TNF-α and IFN-γ; p < 0.05 for IL-8) (Figure 2a). Assessment of kidney tissue revealed similar results (Figure 2b).
Figure 2.
Treatment with saturated hydrogen inhibits the production of inflammatory factors in serum and kidney tissue. a) Expression levels of inflammatory cytokines TNF-α, IFN-γ, and IL-8 in serum were assayed using ELISA. b) Expression levels of inflammatory cytokines TNF-α, IFN-γ, and IL-8 in kidney tissue were assayed using ELISA. **p < 0.01 vs. Control; ##p < 0.01 and #p < 0.05 vs. CCl4.
Abbreviations: TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; ELISA, enzyme-linked immunosorbent assay; H2, saturated hydrogen; CCl4, carbon tetrachloride.
The level of oxidative stress in kidney tissue was assessed by nitrotyrosine staining. As shown in Figure 3a, the model group had a large amount of nitrotyrosine staining. Conversely, the amount of nitrotyrosine staining tended to be lower after H2 treatment. These findings indicated that oxidative stress was reduced by H2 treatment.
Figure 3.
Treatment with saturated hydrogen reduces oxidative stress and MDA level in kidney tissue, while increasing GSH level and SOD activity. a) Oxidative stress was assayed using nitrotyrosine staining. b) Contents of MDA, GSH, and SOD in kidney tissue were assayed using biochemical analysis. **p < 0.01 vs. Control; ##p < 0.01 and #p < 0.05 vs. CCl4.
Abbreviations: MDA, malondialdehyde; GSH, glutathione peroxidase; SOD, superoxide dismutase; H2, saturated hydrogen; CCl4, carbon tetrachloride.
Compared with the level in the control group, MDA content in kidney tissue significantly increased in the model group (p < 0.01); this change was reversed by H2 treatment (p < 0.05 compared with the model group). In contrast, expression levels of GSH and SOD significantly decreased in the model group (p < 0.01 compared with the control group), whereas they significantly increased after H2 treatment (p < 0.05 compared with the model group) (Figure 3b).
Treatment with saturated hydrogen inhibits JAK2/STAT3 signaling
Hematoxylin–eosin staining revealed that glomerular and tubular structures in the control group were normal. Mice in the CCl4 model group exhibited the following structural features: renal tubular epithelial cell denaturation, renal tubular cyst contraction, evident vacuolar changes, cell swelling, necrosis, and exfoliation; they also exhibited massive congestion in glomeruli and microvessels, as well as infiltration in renal tubules, atrophy and degeneration of glomeruli, and infiltration of inflammatory cells in renal interstitium. Thus, the injury score of the model group was significantly greater than that of the control group (p < 0.01). The hydrogen treatment group exhibited vacuolar changes, congestion in glomeruli and microvessels, and tubular cell swelling, but these injuries were milder than those in the model group (p < 0.05 compared with the model group) (Figure 4a).
Figure 4.
Treatment with saturated hydrogen inhibits JAK2/STAT3 signaling. a) Hematoxylin-eosin staining. b) Expression levels of p-JAK2, p-STAT3, and p-p65 were assayed using immunohistochemistry. c, expression levels of p-JAK2, p-STAT3, and p-p65 were assayed using western blot. **p < 0.01 vs. Control; #p < 0.05 vs. CCl4.
Abbreviations: HE, hematoxylin-eosin; H2, saturated hydrogen; CCl4, carbon tetrachloride.
Compared with the control group, the expression levels of p-JAK2 and p-STAT3 significantly increased in the model group (p < 0.01); these changes were reversed after H2 treatment (p < 0.05 compared with the model group) (Figure 4b,c). These findings indicate that treatment with saturated hydrogen can inhibit JAK2/STAT3 signaling. p65 signal protein is a downstream molecule in the JAK2/STAT3 signaling pathway. Specifically, p65 enters the nucleus after phosphorylation, thereby serving as an indicator of NF-kB activation. Thus, we measured the expression level of p-p65; it was significantly higher in the model group (p < 0.01 compared with the control group), but significantly lower in the hydrogen treatment group (p < 0.05 compared with the model group) (Figure 4b,c).
Discussion
The incidence of AKI is high and its prognosis remains poor. Treatment of renal ischemia-reperfusion, which is a common cause of AKI, has become a major focus of therapeutic efforts. Previous studies have shown that pathogeneses of ischemia-reperfusion injury include calcium overload, inflammation, apoptosis, and increased oxygen free radicals.10
CCl4 is a hepatophilic toxicant with a strong toxic effect on renal function.11 Free radicals (e.g., CCl3- and CCl3O2-) are generated after CCl4 interacts with the liver; these free radicals will enter kidneys via blood circulation and attack phospholipid molecules on the membranes of renal tubules and glomeruli, causing lipid peroxidation reactions and resulting in AKI.12 Therefore, CCl4 models are frequently used in experimental studies to explore the pathogenesis of drug-induced renal injury and evaluate the efficacies of anti-renal injury drugs. A previous experiment in rats showed that disordered reactive oxygen species reactions occurred in kidneys, and that phospholipid hydroperoxide and MDA levels were elevated in serum and kidney tissue, following induction of CCl4-induced AKI.8 In the present study, following intraperitoneal injection of CCl4, the model mice developed symptoms such as mental depression and bradykinesia. Moreover, levels of BUN and Scr increased; atrophy and deterioration of glomeruli, as well as degeneration and necrosis of proximal tubular epithelial cells, were identified via light microscopy; and the KIM-1 content in urine increased, suggesting that exposure to CCl4 causes severe renal damage in mice, similar to typical AKI in human patients. Moreover, the results showed that treatment with saturated hydrogen could significantly reduce renal function injury and inhibit the expression of inflammatory factors (i.e., TNF-α, IL-8, and IFN-γ), as well as generation of the oxidative stress product, MDA; concurrently, this treatment could promote the expression of the anti-oxidative stress enzyme, SOD.
AKI can be regulated via multiple signaling pathways, such as JAK2/STAT3 and PI3K/AKT.13,14 A prior study showed that activation of JAK2/STAT3 signaling is important for cell proliferation, inflammation, apoptosis, oxidative stress, and survival.15 Moreover, Li et al.16 reported that exogenous H2S contributed to recovery of ischemic post-conditioning-induced cardioprotection by reducing the reactive oxygen species level through downregulation of NF-κB and JAK2-STAT3 signaling pathways in aging cardiomyocytes; this indicated that hydrogen therapy may improve AKI via JAK2/STAT3 signaling. Our results in the present study showed that treatment with saturated hydrogen partially inhibited the activation of JAK2 and STAT3. In addition, p65 signal protein is a downstream molecule of the JAK2/STATA3 signaling pathway;17 this protein can regulate oxidative stress and inflammation, as well as modulate the levels of MDA, SOD, IL-6, TNF-α, and various chemokines.18 Treatment with saturated hydrogen significantly reduced the activation of p65 signal protein, which is consistent with the findings of a prior report where treatment with H2S reduced the expression levels of TNF-α, IL-6, IL-10, and monocyte chemoattractant protein-1, as well as the protein levels of p50, p65, and p-p65 in the kidney of rats with chronic renal failure via NF-κB signaling.19 To the best of our knowledge, the present study is the first to show that treatment with saturated hydrogen can reduce oxidative stress and inflammatory cytokine production through the JAK2/STAT3/p65 signaling pathway, thereby exhibiting protective effects against AKI. However, the specific mechanisms of hydrogen-related signaling pathways are very sophisticated in animals. Changes in the JAK2/STAT3/p65 signaling pathway after kidney injury and the exact mechanism by which this pathway affects AKI require further investigation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Shujun Zhou https://orcid.org/0000-0002-1570-1382
References
- 1.Zhou HL, Zhang R, Anand Pet al. Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury. Nature 2019; 565: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han SJ, Li H, Kim Met al. Kidney proximal tubular TLR9 exacerbates ischemic acute kidney injury. J Immunol 2018; 201: 1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Yang G, Kim YJet al. Hydrogen-rich medium protects mouse embryonic fibroblasts from oxidative stress by activating LKB1-AMPK-FoxO1 signal pathway. Biochem Biophys Res Commun 2017; 491: 733–739. [DOI] [PubMed] [Google Scholar]
- 4.Cui J, Chen X, Zhai Xet al. Inhalation of water electrolysis-derived hydrogen ameliorates cerebral ischemia-reperfusion injury in rats - a possible new hydrogen resource for clinical use. Neuroscience 2016; 335: 232–241. [DOI] [PubMed] [Google Scholar]
- 5.Uto K, Sakamoto S, Que Wet al. Hydrogen-rich solution attenuates cold ischemia-reperfusion injury in rat liver transplantation. BMC Gastroenterol 2019; 19: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zálešák M, Kura B, Graban Jet al. Molecular hydrogen potentiates beneficial anti-infarct effect of hypoxic postconditioning in isolated rat hearts: a novel cardioprotective intervention. Can J Physiol Pharmacol 2017; 95: 888–893. [DOI] [PubMed] [Google Scholar]
- 7.Liu CX, Tan YR, Xiang Yet al. Hydrogen Sulfide protects against chemical hypoxia-induced injury via attenuation of ROS-mediated Ca2+ overload and mitochondrial dysfunction in human bronchial epithelial cells. Biomed Res Int 2018; 2018: 2070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki K, Nakagawa K, Yamamoto Tet al. Carbon tetrachloride-induced hepatic and renal damages in rat: inhibitory effects of cacao polyphenol. Biosci Biotechnol Biochem 2015; 79: 1669–1675. [DOI] [PubMed] [Google Scholar]
- 9.Qin L, Qiu K, Hu Cet al. Respiratory syncytial virus promoted the differentiation of Th17 cells in airway microenvironment through activation of Notch-1/Delta3. J Med Microbiol 2019; 68: 649–656. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Yao L, Liang Qet al. Propofol protects hippocampal neurons from hypoxia-reoxygenation injury by decreasing calcineurin-induced calcium overload and activating YAP signaling. Oxid Med Cell Longev 2018; 2018: 1725191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pegoraro CMR, Nai GA, Garcia LAet al. Protective effects of Bidens pilosa on hepatoxicity and nephrotoxicity induced by carbon tetrachloride in rats. Drug Chem Toxicol 2018. doi: 10.1080/01480545.2018.1526182. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Rjeibi I, Feriani A, Saad ABet al. Lycium europaeum Linn as a source of polysaccharide with in vitro antioxidant activities and in vivo anti-inflammatory and hepato-nephroprotective potentials. J Ethnopharmacol 2018; 225: 116–127. [DOI] [PubMed] [Google Scholar]
- 13.Zhu S, Zhang C, Weng Qet al. Curcumin protects against acute renal injury by suppressing JAK2/STAT3 pathway in severe acute pancreatitis in rats. Exp Ther Med 2017; 14: 1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Q, Zhang X, Li Qet al. TLR2 protects cisplatin-induced acute kidney injury associated with autophagy via PI3K/Akt signaling pathway. J Cell Biochem 2019; 120: 4366–4374. [DOI] [PubMed] [Google Scholar]
- 15.Shi X, Guan Y, Jiang Set al. Renin-angiotensin system inhibitor attenuates oxidative stress induced human coronary artery endothelial cell dysfunction via the PI3K/AKT/mTOR pathway. Arch Med Sci 2019; 15: 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Li M, Li Yet al. Exogenous H2S contributes to recovery of ischemic post-conditioning-induced cardioprotection by decrease of ROS level via down-regulation of NF-κB and JAK2-STAT3 pathways in the aging cardiomyocytes. Cell Biosci 2016; 6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JY, Jiang L, He Tet al. NETO2 promotes invasion and metastasis of gastric cancer cells via activation of PI3K/Akt/NF-κB/Snail axis and predicts outcome of the patients. Cell Death Dis 2019; 10: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng B, Su M, Chen Qet al. Protective effect of a polysaccharide from Anoectochilus roxburghii against carbon tetrachloride-induced acute liver injury in mice. J Ethnopharmacol 2017; 200: 124–135. [DOI] [PubMed] [Google Scholar]
- 19.Wu D, Luo N, Wang Let al. Hydrogen sulfide ameliorates chronic renal failure in rats by inhibiting apoptosis and inflammation through ROS/MAPK and NF-κB signaling pathways. Sci Rep 2017; 7: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]