Abstract
A fundamentally new strategy for the treatment of infectious disease is the modulation of host immune responses to enhance clearance of infectious agents and reduce tissue damage due to inflammation. Antimicrobial host defense peptides have been investigated for their potential as a new class of antimicrobial drugs. Recently their immunomodulatory activities have begun to be appreciated. Modulation of innate immunity by synthetic variants of host defense peptides, called innate defense regulators (IDRs), is protective without direct antimicrobial action. We discuss the potential and current limitations in exploiting the immunomodulatory activity of IDRs as a novel anti-infective pathway. IDRs show significant promise and current research is uncovering mechanistic information that will aid in the future development of IDRs for clinical use.
Infectious diseases and immunomodulatory therapy
Infectious diseases remain a leading cause of death and a major burden on healthcare systems worldwide [1]. For example, the HIV pandemic continues to claim two million lives per year, with an estimated 33 million people living with HIV/AIDS worldwide [2] and novel human pathogens, such as the severe acute respiratory syndrome (SARS) associated coronavirus and H5N1 avian influenza, have recently emerged [3]. Other infectious diseases, including tuberculosis and diarrhoreal disease, remain among the leading causes of death [2]. Dangers are also posed by the spread of infectious agents to new geographic locations, as exemplified by the recent cases of West Nile virus infection in North America [3]. In addition, the rapid evolution of antibiotic resistance in many bacterial pathogens creates an urgent need for new antimicrobial drugs and strategies for the treatment of infectious diseases [4].
Our rapidly expanding knowledge of the immune system is creating new opportunities for the development of immunomodulatory therapies 5, 6. Unlike conventional antibiotics that are designed to target a pathogen, such therapies exert their protective effects by acting on the host immune system. Many therapies designed to target the immune system have entered clinical use in recent decades and many more are progressing through clinical trials. These include immunosuppressive therapeutics for use in tissue transplantation, autoimmune and inflammatory conditions and compounds designed to stimulate immune responses, for example vaccine adjuvants. Although a wide variety of compounds are under investigation, most therapeutics approved to date belong to one of the following classes: monoclonal antibodies, Toll-like receptor (TLR) ligands and their derivatives, cytokines and chemokines, and small molecules.
Monoclonal antibodies are used clinically to stimulate and block cell-surface receptors, inactivate cytokines and other molecules in circulation and deliver antigens to dendritic cells or direct toxins towards tumor cells [5]. TLR ligands are used for their immunostimulatory activities 7, 8. For example, the TLR4 ligand monophosphoryl lipid-A (MPL) is used as an adjuvant in vaccines against hepatitis B virus (HBV) and human papillomavirus (HPV) 9, 10. In addition, the TLR7 agonist imiquimod is approved as a topical treatment for HPV-induced genital warts and for basal cell carcinoma. Various compounds designed to stimulate TLR3, TLR5, TLR7/8 and TLR9 are also undergoing clinical trials, primarily as adjuvants in vaccines against chronic viral infections or as adjuncts in cancer chemotherapy 7, 8.
Immunotherapeutic use of cytokines, chemokines and hormones is exemplified by type-I interferons for the treatment of chronic HBV infections, granulocyte monocyte-colony stimulating factor (GM-CSF) for the treatment of neutropenia and glucocorticoids for the treatment of asthma and other chronic inflammatory diseases [11]. A common application of small molecules is to target intracellular signal transduction pathways [12]. For example, organ transplant rejection can be limited by inhibiting the mammalian target of rapamycin (mTOR) with rapamycin or by inhibiting calcineurin with cyclosporine and FK506, thus reducing T-cell signal transduction. Many other small molecules that target components of pathways, such as those involving nuclear factor κB (NFκB), p38 mitogen-activated protein kinase (MAPK), and Janus kinase-signal transducers and activators of transcription (JAK-STAT), are also undergoing clinical trials [12]. Adoptive transfers of dendritic cells [13] and autologous cytotoxic T-cells [14], which are effective therapies for some forms of cancer, can also be considered immunotherapies.
Although many immunotherapies have been approved for use in the clinic and many others have shown efficacy in clinical trials, there are a number of obstacles that prevent their widespread use. The use of immunosuppressive therapies is often associated with increased risk of infections and might also predispose individuals to cancer 15, 16. Immunostimulatory treatments can result in inflammatory tissue damage, autoimmunity or potentially fatal cytokine storms 17, 18. These risks limit the application of most immunotherapies to life-threatening conditions for which better-tolerated treatments are not available. It is increasingly recognized that most conditions will not be treatable through generic stimulation or suppression of the immune system, as demonstrated by the failures of immunosuppressive therapies for sepsis [19]. Hence, we need to develop tools that precisely control, modulate and/or polarize the immune response. To be commercially viable, ideal therapies also need to be broad-spectrum and target a class of related conditions or pathogens. These challenges highlight the importance of studying the immune system from a systems biology perspective to predict the outcome and efficacy of immunomodulatory therapies 20, 21. This is discussed further in Box 1 .
Box 1. Systems biology approaches to studying immunomodulatory activity.
Drug development and complex systems
The innate immune system is a very complex, non-linear network for which there is great potential for subtle manipulations of signaling pathways and subsequent changes in cytokine production with far-reaching downstream effects that might be beneficial but are not completely predictable, desirable or controllable [21]. As recently discussed by Schadt et al. [20], progress in drug discovery and development requires an integrated approach that takes into account the non-linear causal relationships inherent in complex systems. This concept applies particularly to immunomodulation because of the significant cross-talk between pathways in the immune system. It is not possible to model the entire innate immune system either in vitro or computationally; however, systems biology approaches can aid in the understanding of complex interactions and signaling pathways. Attempts to understand the likely effects of immunmodulatory drugs require an appreciation of associated interactions within the immune system as a whole and their effects on other major systems, such as metabolism and hormonal and neuronal pathways.
Computational aids to systems biology approaches
There are many freely available and commercial bioinformatics tools and databases for data-mining and analysis of many aspects of biological systems. Of particular interest to the development of immunomodulatory drugs are those that include the following:
-
•
Interactome data specific to innate immunity, such as InnateDB (www.innatedb.com), a manually curated database and analysis platform for the genes, proteins, interactions, pathways and signaling responses in human and mouse innate immune responses;
-
•
Immunology-specific information, especially for immune-relevant transcriptome analysis, such as the Innate Immunity Database (http://db.systemsbiology.net/IIDB);
-
•
Pathway interaction data, such as the NCI-Nature Pathway Interaction Database (http://pid.nci.nih.gov); and
-
•
Tools for specific protein interaction analysis, such as IntAct (http://www.ebi.ac.uk/intact/).
The development and use of InnateDB [51], which integrates data from many other freely available databases and has manually curated interaction information, analysis tools and visualization capability, has provided an insight into the signaling pathways and potential downstream effects of peptide treatments. For example, we recently reported the application of such an approach to study LL-37, with experimental verification of bioinformatics predictions [52]. Conceivably, such a system could be used in conjunction with gene array or proteomics data to aid in the iterative rational design and testing of immunomodulatory peptides by analyzing how individual peptides influence different pathways. Pathway and ontology analyses can also implicate specific biological processes in the activity of these peptides.
Host defense peptides: multifunctional innate immune mediators
Host defense peptides (HDPs) (also known as antimicrobial peptides) are being investigated as potential immunotherapeutic agents because of to their unique combination of immunostimulatory and anti-inflammatory properties 6, 22. HDPs are produced by the immune systems of all multicellular organisms and are extremely diverse from the perspectives of both sequence and structure. Despite this diversity, most peptides are amphipathic molecules, with an overall net positive charge, and a high content of cationic and hydrophobic amino acids. Classes of peptides such as cathelicidins [23], defensins [24] and histatins [25] are distinguished by their sequence, structure or mechanism of production. The general characteristics of cathelicidins and defensins are described in Box 2 . In mammals these peptides are primarily produced by leukocytes, mucosal epithelial cells and keratinocytes.
Box 2. Mammalian host defense peptides.
Cathelicidins are cationic amphipathic peptides of diverse linear, α-helical or β-hairpin structure and are produced by proteolysis of the C-terminus of cathelin-domain-containing protein precursors [23]. In humans only one cathelicidin precursor, hCAP18, is produced, primarily in leukocytes and epithelial cells. It is cleaved to form peptide LL-37 and in some tissues a range of shorter peptides with altered properties [35]. Mice also produce one cathelicidin, CRAMP, whereas in cattle and pigs the cathelicidin peptide family is highly diverse.Defensins are cationic amphipathic peptides with an approximate length of 30 amino acids and a triple-stranded β-sheet structure containing three disulfide bonds [24]. Based on their pattern of disulfide bonding, defensins are classified into the α-, β-, and the less common θ-defensin families. In humans, α-defensins are produced in neutrophil azurophilic granules as part of their antimicrobial arsenal and by Paneth cells of the intestinal crypts, as well as by other leukocytes and epithelial cells, whereas β-defensins are produced by mucosal epithelia, skin and some leukocytes. θ-Defensins are circular peptides with anti-HIV activity that are not produced in humans and so far only found in old world monkeys [53].
Many HDPs show broad-spectrum microbicidal activity either due to interaction with and disruption of microbial membranes or translocation into bacteria to act on internal targets [26]. This undoubtedly plays a key role in immune defenses within some locations, such as phagolysosomes of leukocytes and the crypts of the small intestine. However, the microbicidal activity of the peptides is highly sensitive to antagonism by divalent cations, serum and anionic macromolecules such as glycosaminoglycans [27], and thus their immunomodulatory activity is probably more significant in many physiological environments. The immunomodulatory activities of HDPs include diverse effects on cell migration, survival and proliferation and the induction of many antimicrobial and immune mediators. The targets of peptide activity include leukocytes, mucosal epithelial cells, keratinocytes and vascular endothelial cells (Table 1 ). High sequence diversity and the multifunctional nature of HDPs provide many opportunities for the design of artificial peptides and derivatives with therapeutic applications 6, 22.
Table 1.
Immunomodulatory properties of mammalian host defense peptides
| Cell or tissue type | Peptide production and activity | References |
|---|---|---|
| Hematopoietic cells | ||
| Monocytes and macrophages | LL-37 and β-defensins are monocyte chemoattractants in vitro and in vivo. LL-37 has anti-endotoxic activity, induces chemokine production, promotes IL-1β secretion, but inhibits inflammatory responses to certain TLR ligands | 32, 61, 62 |
| Neutrophils | LL-37 and defensins are produced by neutrophils, stored within neutrophil granules and play an important microbicidal role in phagolysosomes. When released extracellularly, LL-37 acts as a neutrophil chemoattractant, inhibits neutrophil apoptosis, reduces pro-inflammatory cytokines and promotes both chemokine induction and the antimicrobial functions of neutrophils | 63, 64 |
| Mast cells | Mast cells are important producers of LL-37 in the skin. LL-37 and β-defensins are mast cell chemoattractants and promote mast cell degranulation | 65, 66 |
| Conventional dendritic cells | Defensins and cathelicidins are dendritic cell (DC) chemoattractants. LL-37 promotes differentiation of monocyte-derived DCs, but inhibits DC maturation and activation by TLR-ligands. β-Defensin 2 might promote DC activation as an endogenous TLR4 ligand. The adjuvant activities of defensins and cathelicidins in vivo might be mediated in part through their activity on DCs | 67, 68, 69 |
| Plasmacytoid dendritic cells | LL-37 in complex with DNA oligonucleotides strongly induces IFNα production by plasmacytoid DCs. This activity might contribute to the pathology of psoriasis | [70] |
| Epithelial cells | ||
| Keratinocytes | LL-37 promotes keratinocyte migration and production of IL-8, inhibits keratinocyte apoptosis, modulates responses to TLR ligands, and might have wound healing activities in the skin. Altered proteolytic processing of hCAP18 and LL-37 has been implicated in the pathology of rosacea | 54, 71, 72 |
| Bronchial epithelium | LL-37 acts on bronchial epithelial cells to stimulate cytokine and chemokine production and promote apoptosis | 73, 74 |
| Intestinal epithelium | α-Defensins are produced by Paneth cells and their microbiocidal activity plays an important role in the immune defenses of the gut. Reduced α-defensin production might contribute to Crohn's disease. LL-37 promotes mucin production and survival of intestinal epithelial cells | 75, 76 |
| Other cells | ||
| Vascular endothelium | LL-37 induces activation and proliferation of vascular endothelium, promoting angiogenesis | [77] |
| Mesenchymal stromal cells | LL-37 acts as a chemokine for mesenchymal stromal cells and promotes the production of various cytokines, as well as VEGF and MMP2; this can contribute to angiogenesis and tumor progression | [57] |
| Cancer cells | LL-37 promotes migration and proliferation of lung, ovarian and breast cancer cells and LL-37 production by cancer cells in vivo promotes tumor growth. However, LL-37 also augments the anti-cancer therapeutic activity of CpG oligonucleotides | 78, 79 |
Strategies and challenges in the design and screening of IDRs
The therapeutic potential of peptides with direct antimicrobial activity has been well studied and several of these peptides have entered clinical trials; however, the immunomodulatory activity of HDPs has not been explored to the same extent. One of the first synthetic immunomodulatory peptides based on the HDP template (i.e. that of the small bovine HDP bactenecin [28]), innate defense regulator 1 (IDR-1), can mediate in vivo protection against bacterial challenge in the absence of direct antimicrobial activity [29]. It has been proposed that its mechanism of action involves enhanced chemokine production accompanied by moderation of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and is mediated through modulation of specific intracellular signaling pathways. Such activities provide a powerful rationale for using HDPs to design synthetic peptides with optimized immunomodulatory activity. A diagrammatic overview of the current approach to IDR design is presented in Figure 1 .
Figure 1.
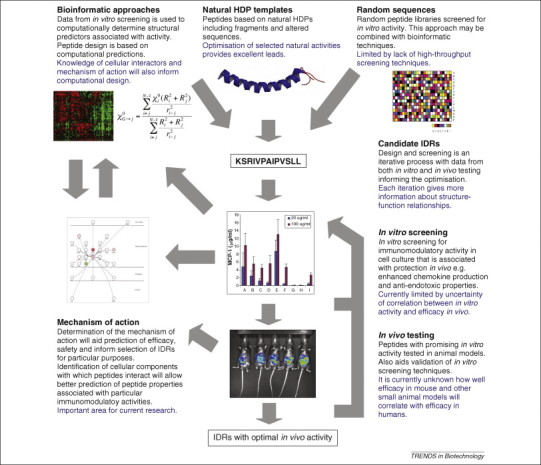
Design strategies for IDRs. Design and testing of IDRs are iterative processes. Initial candidate peptides are based on naturally occurring HDPs, designed using computational tools or randomly generated. These candidate peptides are tested in vitro and in vivo and computational analysis is used to select characteristics associated with function. New candidate peptides can then be designed using this structure–function relationship information and further testing reveals further information. Data from in vitro and in vivo testing also inform studies of the mechanism of action of IDRs and this information can feed back in to the bioinformatic and computational design approach. The structural diagram is LL-37 taken from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank.
An important aspect for the design of immunomodulatory peptides is the development and validation of screening techniques. High-throughput in vitro screening of antibacterial activity has been used for validation and iterative optimization of rationally designed antimicrobial peptides 30, 31. Through the use of large data sets, made possible by high-throughput screening of peptide libraries, it has been possible to identify particular physical characteristics that correlate well with direct antimicrobial activity. By contrast, owing to the complexity of the interactions between multiple cell types and effector molecules, it is not possible to screen for subtle immunomodulatory activities in vitro and surrogate markers of this activity must be used. Instead, it is necessary to use screens that correlate with enhanced protection. For example, IDR-1 was selected based on its in vitro immunomodulatory activities, namely enhanced release of chemokines from peptide-stimulated human peripheral blood mononuclear cells (PBMCs) [29] and its ability to protect mice against bacterial infections. Another possible screen would be ability to suppress TNF-α induction in response to treatment with TLR agonists [32]. Even with the use of such markers, candidate peptides must ultimately be tested in vivo because isolated cell systems cannot capture the complexity of the innate immune response. Thus, compared to testing of antimicrobial activity, for which there is often a strong correlation between in vitro and in vivo activity, it is much more labor-intensive and costly to generate the large data sets necessary for the application of computational methods to immunomodulatory peptide design. Despite this problem, we now have a few precedents. For example, synthetic endotoxin-binding peptides were optimized for protection in vivo [33] and a fragment of the human HDP LL-37 was optimized for anti-endotoxin activity through appropriate amino acid substitutions [34]. Gallo and colleagues [35] subsequently separated the weak antimicrobial and immunomodulatory properties of LL-37 in individual truncated peptides derived from LL-37. In certain studies the activities of smaller bactenecin- and indolicidin-like synthetic peptides were optimized as components of adjuvant formulations (because innate immunity instructs adaptive immunity) by screening for chemokine-inducing activity and then subjected to in vivo analysis 36, 37, 38.
One challenge for the rational design of IDRs is the identification of their specific interacting partners. To date, several receptors have been identified for peptides [39]. However, these are extracellular receptors and HDP are known to have the features of cell-penetrating peptides [40]. Indeed, it was shown that IDR-1 is also taken up into cells, a feature required for its chemokine-inducing activities [29]. We propose that all IDR-like peptides enter cells and interact with particular cellular components to modulate the intracellular signaling pathways of immune cells and have recently identified two such intracellular receptors (unpublished data and Ref. [41]) and we suspect that there are multiple receptors for each HDP and IDR. As these interactors are identified, peptides with the optimal shape, hydrophobicity and/or charge characteristics might be designed in docking studies, although the requirement for uptake might place certain restraints on peptide design. In the absence of such information, iterative optimization via amino acid substitution and screening would seem to be the most effective strategy.
Animal models also present challenges for the testing of immunomodulatory agents. Significant variations exist within the immune systems of different mammals and therefore particular peptides that are highly protective in mice might not be similarly effective in humans. To address this challenge, it might be necessary to use primary human cells, e.g. PBMCs, for in vitro studies and to demonstrate parallel data in animal infection studies. However, as is always the case for new drugs, efficacy in humans cannot be confirmed until these drugs enter clinical trials.
Safety has already been demonstrated for three immunomodulatory cationic peptides in human clinical trials. Two of these, MX-226 and hLF1-11, were originally developed as antimicrobial peptides. However, the former was found to be safe when delivered topically, with efficacy in phase II clinical trials targeting both the inflammatory sequellae of severe acne and a non-infectious inflammatory skin disease, rosacea. Systemic safety was demonstrated for hLF1-11 in immunocompromised hematopoetic stem cell transplant recipients. Another interesting peptide, RDP58, which was derived from the heavy chain of HLA class I molecules, was also safe in clinical trials (although much of the information regarding this peptide is not publicly available) [42]. According to Genzyme Corporation (http://www.genzyme.com/corp/licensing/genz_p_rdp58_login.asp), RDP58 enhances heme oxygenase activity and inhibits inflammatory responses to stimuli such as lipopolysaccharide (LPS), phytohemagglutinin (PHA), double-stranded RNA and cytokines such as TNF-α and interleukin (IL)-1β. RDP58 reduced the production of pro-inflammatory cytokines such as TNF-α, interferon (IFN)-γ and IL-12, but did not affect the production of several other cytokines, including IL-4, IL-6, IL-8 and IL-10. The proposed mechanism of action involves interference with intracellular signaling pathways, although the specific details and interactors are currently unknown [42].
Whereas IDR-1 and RDP58 are both cationic peptides, they might modulate the innate immune response to inflammatory stimuli in different ways. Both peptides suppress the induction of certain pro-inflammatory cytokines, such as TNF-α, but their effects on other cytokines vary. For example, RDP58 does not affect IL-6 or IL-10 production, whereas IDR-1 enhances their production. The distinct responses to these peptides indicate the value of different design strategies, because RDP58 was based on HLA class I molecules whereas IDR-1 design was based on a bovine cathelicidin. Importantly, this range of potential activities indicates that if we can understand the intricacies of immunomodulatory peptide interactions with host cell components and pathways, it might be possible to design peptides with customized immunomodulatory effects to target different diseases (Box 3 ).
Box 3. Potential applications of IDRs.
Broad-spectrum anti-infectives
Exploitation of HDPs has tended to focus on their potential use as direct antibacterials. However, immunomodulatory peptides have finally come of age as clinical candidates. Both approaches have potential applications in targeting other infectious agents, such as viruses, fungi and parasites, through direct action or, more likely, the modulation of appropriate signaling pathways.
Anti-inflammatory agents
Regardless of their anti-infective activities, immunomodulatory peptides could also act as anti-inflammatory drugs because many seem to moderate pro-inflammatory cytokines such as TNF-α. Some of the peptides listed in Table 2 are being considered for the treatment of sepsis and chronic inflammatory conditions such as inflammatory bowel disease. In addition, HDPs such as LL-37 and IDRs have a strong ability to reverse the pro-inflammatory activities of LPS, suggesting potential as anti-endotoxic agents [50].
Wound healing and anti-tumor activity
LL-37 promotes wound healing, independent of its direct antimicrobial properties, which could potentially be exploited for peptides that mimic this activity 54, 55. Another important activity in this context is the ability to stimulate angiogenesis. Although LL-37 can enhance the potential for tumor metastasis 56, 57, there is potential for peptide-based drugs as anti-tumor agents, as demonstrated by current trials of glutoxim/NOV-002 (Table 2).
Novel vaccine adjuvants
Strong data support the use of synthetic IDRs as components of vaccine formulations. The inclusion of poly-L-lysine in a vaccine formulation with CpG-oligodinucleotide (ODN) moderated induction of the potentially dangerous pro-inflammatory cytokine response [58]. In addition, both IC31 [comprising a synthetic peptide (KLKL(5)KLK) attached to a synthetic ODN] and a formulation of indolicidin, CpG ODN and polyphosphazene induced antigen-specific cellular and humoral responses 37, 38, 59. Garlapati et al. [36] recently demonstrated adjuvant activity for IDRs in neonatal pigs in combination with other molecules such as CpG ODNs and polyphosphazenes. The authors proposed that such formulations could be used as adjuvants that are effective in neonates, a population that is notoriously difficult to successfully vaccinate and that is highly susceptible to many potentially fatal infectious diseases. It is also conceivable that peptides can be developed as adjuvants for mucosal vaccines because they could be optimized for enhanced induction of pro-inflammatory cytokines (e.g. IL-6, IL-12, IL-15 and IL-18) and chemokines, such as MCP-1, which show adjuvant activity when administered with mucosal vaccinations [60].
Clinical applications of immunomodulatory peptides
Peptide based immunotherapies are still in their infancy and thus we have very little clinical experience to lean on and must look to the field of immunomodulators in general [6]. Clinical evaluation of immunotherapeutics must be approached in a manner that differs from traditional pharmacology, because the immune system self-amplifies its responses to rapidly overcome potential infectious agents that multiply exponentially. It is perhaps naïve to assume that immunomodulatory drugs will demonstrate typical dose–response pharmacodynamics. In addition, the potential for harm that can accompany inappropriate stimulation of immunity dictates a cautious approach. Immune responses are often delicately balanced and upsetting this balance too greatly can render the system unable to return to homeostasis. Minor perturbations of immune homeostasis observed in pre-clinical testing can translate into catastrophic effects in humans, as was the case for the near-lethal cytokine storm induced by the CD28 antagonist TGN1412 in phase I trials [18].
Therefore, clinical trials of immunomodulatory drugs often evaluate compounds in approaches that avoid excessive responses or systemic absorption. For example, RDP58, which is under investigation for the treatment of ulcerative colitis [42], was structurally modified to prevent both proteolytic degradation and systemic absorption [43]. Two phase II trials of RDP58 in a combined cohort of 127 patients revealed that the drug was well tolerated (adverse effects equivalent to placebo) and efficacious in improving sigmoidoscopy scores in colitis patients receiving medium or high doses of the drug [42]. The pathogenesis of ulcerative colitis is thought to be at least partially due to dysregulated host responses to intestinal microbiota [44] and although RDP58 is being investigated for its anti-inflammatory properties it might also alter host responses to microbiota in a manner similar to that of anti-infective peptides.
Several peptide-based drugs with both immunomodulatory and antimicrobial activity have entered clinical trials as topical antimicrobials and some of these have exhibited immunomodulatory (generally anti-inflammatory) activity (Table 2 ). An advantage of topical application is circumvention of the need to demonstrate systemic safety. Thus, a series of peptides have been used as topical antimicrobials, including pexiganan (MSI-78), omiganan (MX-226), iseganan (IB-367), hLF1-11, XOMA-629, and HB-1345 (Table 2). Subsequent investigations revealed that MX-226 and hLF1-11 have clinically useful immunomodulatory activity and that IB-367 reduces plasma TNF-α levels in animal sepsis models [45]. A phase III clinical trial of IB-367 was recently initiated for oral mucositis in patients receiving radiation therapy for neoplastic disease for which the drug is not explicitly referred to as an antimicrobial (http://clinicaltrials.gov/ct2/show/NCT00022373). Based on these observations we feel there is a strong possibility that immunomodulatory activity will play a substantial role in any clinical benefit demonstrated by cationic peptide drugs.
Table 2.
Immunomodulatory peptides in clinical trials
| Drug | Description | Intended use | Progress | Ref./Reg. no.a |
|---|---|---|---|---|
| Immunomodulatory anti-infective peptides lacking antimicrobial action | ||||
| EA-230 (Exponential Biotherapies) | Oligopeptide fragment from β-hCG (4-mer, LQGV) | Sepsis | Phase II | [80] |
| Glutoxim/NOV-002 (Pharma BAM/Novelos) | Hexapeptide with a stabilized disulfide bond [bis-(γ-L-glutamyl)-L-cysteinyl-bis-glycine disodium salt] | Tuberculosis, non small cell lung cancer | Market (Russia), phase III (N. America) | [46]NCT00347412 |
| IMX942 (Inimex) | Synthetic cationic host defense peptide, derivative of IDR-1 and indolicidin | Nosocomial infections, febrile neutropenia, | Phase IA | Websiteb |
| Immunomodulatory anti-infectives with antimicrobial action | ||||
| hLF1-11 (AM-Pharma) | Cationic peptide, human lactoferricin (amino acid fragment 1–11) | Bacteremia and fungal infections in immunocompromised hematopoetic stem cell transplant recipients | Phase 1/II | NCT00509938 |
| Omiganan [MX-226] (Migenix) | Synthetic cationic host defense peptide (12-mer), indolicidin derivative | Topical antiseptic, acne vulgaris, papulopustular rosacea | Phase III | NCT00000435, NCT00027248 |
| Opebacan (Xoma) | 21-amino-acid peptide derivative of bactericidal/permeability-increasing protein | Endotoxemia in hematopoetic stem cell transplant recipients | Phase I/II | NCT00454155 |
| XOMA-629 (Xoma) | 9-amino-acid peptide derivative of bactericidal/permeability-increasing protein | Impetigo | Phase IIA | Websitec,d |
| Immunomodulatory peptides | ||||
| DiaPep277 (DeveloGen) | HSP60 derivative (24-mer peptide) that induces T regulatory cells | Type 1 diabetes mellitus | Phase III | NCT00644501 |
| RDP58 (Genzyme) | Semisynthetic D-amino acid decapeptide derived from HLA class I B2702 | Inflammatory bowel disease | Post phase II | [42], websitee |
| Anti-infective peptides with unknown immunomodulatory activityf | ||||
| PAC-113 (Pacgen Biopharmaceuticals) | Synthetic cationic host defense peptide (12-mer), histatin derivative | Antifungal | Phase II | NCT00659971 |
| PMX-30063 (PolyMedix) | Defensin structural mimetic, non-peptide, small molecule/copolymer | Antibiotic | Phase IB | Websiteg |
| HB-1345 (Helix BioMedix) | Lipohexapeptide | Acne | Pre-phase I | Websiteh |
| Pexiganan acetate [MSI-78] (MacroChem) | Synthetic cationic host defense peptide (22-mer), magainin derivative | Topical antibiotic | Phase III | NCT00563433, NCT00563394 |
| Iseganan [IB-367] (Ardea Biosciences) | Synthetic protegrin-1 derivative (17 amino acids) | Oral mucositis in radiation therapy patients | Phase III | NCT00022373 |
Ref., reference; Reg. no., registration number from http://www.clinicaltrials.gov.
An immunomodulatory peptide currently in use as an anti-infective agent is the drug glutoxim (NOV-002), although this peptide is quite different from the cationic IDRs described above. A tripeptide derivative of oxidized glutathione, glutoxim is currently prescribed in Russia as an adjunct to traditional therapies for the treatment of pulmonary and disseminated Mycobacterium tuberculosis infections. The addition of glutoxim to standard tuberculosis therapy regimes reduced the time needed to clear the bacterium, increased the time to resolution of pulmonary infiltrate and increased the rate of post infection weight gain [46]. Adverse effects were limited to transient febrile events that resolved on discontinuation of the drug. Pre-clinical studies in human neutrophils and whole blood demonstrated an immunomodulatory mode of action [47], suggesting that immunomodulatory peptide-based drugs can be safe and effective. Glutoxim is currently undergoing phase III clinical trials in the USA as a chemotherapy adjuvant (http://www.clinicaltrials.gov/ct2/show/NCT00347412) in non-small-cell lung cancer rather than as an anti-infective, possibly because of the relatively low incidence of tuberculosis in the USA and lesser potential for return on investment. Early results are promising and include reductions in chemotherapy-induced myelosuppression and modulation of lymphocyte subsets (www.clinicaltrials.gov/ct2/show/NCT00347412), suggesting that glutoxim might also have an immunomodulatory role in the treatment of neoplastic disease.
An exciting area in which the dual anti-infective and anti-inflammatory actions of peptide immunotherapies have potential is the treatment of bacteremia and septicemia. Although no clinical data are available yet, IMX-942, which is a derivative of IDR-1, is being used in phase I safety trials in chemotherapy patients with febrile neutropenia (http://www.inimexpharma.com). Thus, the future for immunomodulatory peptides is extremely promising.
Limitations and advantages of IDRs
For all drug candidates the issues of potential toxicity, in vivo stability, and appropriate delivery routes must be overcome. For anti-infectives the potential for resistance development is also important. Clearly it is possible to design IDRs without substantial in vitro or animal model toxicity [29] but issues still remain regarding potential in vivo toxicity (especially in humans and for multiple dosing) and appropriate formulations and routes of administration. The completion of clinical safety trials with peptide-based drugs, such as those listed in Table 2, shows that at least these peptides can be administered safely via topical application. However, whether such peptides are safe when delivered by other routes is still an open question.
In vivo stability and proteolytic processing of the peptides is also of potential concern. Although small peptides are likely to be degraded in minutes to hours inside the host, results for IDR-1 indicate that treatment up to 48 h prior to or 6 h after bacterial challenge is still protective in animal models [29]. This raises the question of how a peptide that is presumably degraded rapidly can have long-lasting effects and whether enhancement of peptide stability is desirable in this context. One possibility is that the peptides prime immune cells; indeed, their ability to translocate into immune cells [40] might protect them from proteolytic degradation. This is consistent with the observation that the peptides are active when delivered by many different routes, possibly indicating that these locally primed cells (or cells containing peptide) migrate to the infection site.
One way to enhance stability is to create D-amino acid forms of the peptides, exploiting the specificity of proteases for L-amino acid forms. Because the three-dimensional structure of the peptides is likely to be important for their interactions with specific receptors, this might render the peptides inactive. This problem can be circumvented using retro-inverso peptides comprising D-amino acids in the reversed sequence because this preserves the spatial positions of the side chains and maintains the protease resistance of the D-amino acid forms [48]. The cost of manufacturing synthetic peptides is very high, so strategies that enhance bioavailability (i.e. appropriate formulations) are also important in decreasing the cost of treatment.
One very important characteristic of IDRs is that there is a lower likelihood of microorganisms developing resistance to them than is the case for direct antimicrobials [49]. Because the innate immune system has evolved alongside microbes, subtly augmentation of this response should not enhance the selective pressure for bacterial immune evasion mechanisms. In addition, enhanced innate clearance of microbes mediated by IDRs should not result in the creation of bacterial debris that continues to promote inflammation in the absence of live bacteria [50], providing yet another advantage over traditional antibiotic therapies. Indeed, some of these peptides actually suppress such septic inflammatory responses.
Conclusions
Synthetic immunomodulatory IDR peptides have significant potential as anti-infective therapeutics and current research promises to reveal crucial mechanistic information that should greatly enhance the development of optimized IDRs. There is the potential to tailor the design of IDRs for different purposes, not all of which will necessarily be anti-infective. The ability to subtly alter the normal balance between protective innate immune responses, such as chemokine production and concomitant leukocyte recruitment (which they enhance), and the potentially harmful effects of excessive inflammation (which they can suppress) provides a unique opportunity to assist in innate immune protection against pathogens. This approach is unlikely to result in the development of microbial resistance to the IDRs and also avoids or suppresses the inflammation associated with bacterial debris that often accompanies conventional antibiotic therapy. Although much research is still required in this area, the potential of this novel approach to anti-infective therapy is vast.
Disclosure statement
R.E.W. Hancock is a founder of and scientific advisory board member for Inimex Pharmaceuticals Inc. and is a shareholder in Migenix Inc.
Acknowledgements
Our own peptide research is funded by the Foundation for the National Institutes of Health, the Bill and Melinda Gates Foundation, the Canadian Institutes of Health Research (CIHR) through two separate Grand Challenges in Global Health Research Initiatives and an Additional research grant from CIHR, and by Genome BC and the advanced Foods and Materials Network. A.N. has a postdoctoral fellowship and MLM an MD/PhD studentship from CIHR with additional support from the Michael Smith Foundation for Medical Research. R.E.W.H. holds a Canada Research Chair.
References
- 1.Rappuoli R. From Pasteur to genomics: progress and challenges in infectious diseases. Nat. Med. 2004;10:1177–1185. doi: 10.1038/nm1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO (2008) World Health Statistics 2008, World Health Organisation Press
- 3.Weiss R.A., McMichael A.J. Social and environmental risk factors in the emergence of infectious diseases. Nat. Med. 2004;10:S70–76. doi: 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spellberg B. The epidemic of antibiotic resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann T.A. Immunotherapy: past, present and future. Nat. Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 6.Hamill P. Novel anti-infectives: is host defence the answer? Curr. Opin. Biotechnol. 2008;19:628–636. doi: 10.1016/j.copbio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Romagne F. Current and future drugs targeting one class of innate immunity receptors: the Toll-like receptors. Drug Discov. Today. 2007;12:80–87. doi: 10.1016/j.drudis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Kanzler H. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 9.Pashine A. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 2005;11:S63–68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 10.Harper D.M. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 11.Viola A., Luster A.D. Chemokines and their receptors: drug targets in immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill L.A. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat. Rev. Drug Discov. 2006;5:549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- 13.Figdor C.G. Dendritic cell immunotherapy: mapping the way. Nat. Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg S.A. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello C.A. Anti-cytokine therapeutics and infections. Vaccine. 2003;21(Suppl. 2):S24–34. doi: 10.1016/s0264-410x(03)00196-8. [DOI] [PubMed] [Google Scholar]
- 16.Askling J., Bongartz T. Malignancy and biologic therapy in rheumatoid arthritis. Curr. Opin. Rheumatol. 2008;20:334–339. doi: 10.1097/BOR.0b013e3282f7c706. [DOI] [PubMed] [Google Scholar]
- 17.Ponce R. Adverse consequences of immunostimulation. J. Immunotoxicol. 2008;5:33–41. doi: 10.1080/15476910801897920. [DOI] [PubMed] [Google Scholar]
- 18.Suntharalingam G. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 19.O’Callaghan A., Redmond H.P. Treatment of sepsis: current status of clinical immunotherapy. Surgeon. 2006;4:355–361. doi: 10.1016/s1479-666x(06)80111-3. [DOI] [PubMed] [Google Scholar]
- 20.Schadt E.E. A network view of disease and compound screening. Nat. Rev. Drug Discov. 2009;8:286–295. doi: 10.1038/nrd2826. [DOI] [PubMed] [Google Scholar]
- 21.Brown K.L. Complexities of targeting innate immunity to treat infection. Trends Immunol. 2007;28:260–266. doi: 10.1016/j.it.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Hancock R.E.W., Sahl H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 23.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 24.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 25.Kavanagh K., Dowd S. Histatins: antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol. 2004;56:285–289. doi: 10.1211/0022357022971. [DOI] [PubMed] [Google Scholar]
- 26.Powers J.P., Hancock R.E.W. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 27.Bowdish D.M. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 2005;77:451–459. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 28.Bowdish D.M. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother. 2005;49:1727–1732. doi: 10.1128/AAC.49.5.1727-1732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott M.G. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 2007;25:465–472. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
- 30.Cherkasov A. Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem. Biol. 2009;4:65–74. doi: 10.1021/cb800240j. [DOI] [PubMed] [Google Scholar]
- 31.Fjell C.D. Identification of novel antibacterial peptides by chemoinformatics and machine learning. J. Med. Chem. 2009;52:2006–2015. doi: 10.1021/jm8015365. [DOI] [PubMed] [Google Scholar]
- 32.Mookherjee N. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 33.Dankesreiter S. Synthetic endotoxin-binding peptides block endotoxin-triggered TNF-alpha production by macrophages in vitro and in vivo and prevent endotoxin-mediated toxic shock. J. Immunol. 2000;164:4804–4811. doi: 10.4049/jimmunol.164.9.4804. [DOI] [PubMed] [Google Scholar]
- 34.Nagaoka I. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin. Diagn. Lab. Immunol. 2002;9:972–982. doi: 10.1128/CDLI.9.5.972-982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braff M.H. Structure–function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J. Immunol. 2005;174:4271–4278. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 36.Garlapati S. Strategies to link innate and adaptive immunity when designing vaccine adjuvants. Vet. Immunol. Immunopathol. 2009;128:184–191. doi: 10.1016/j.vetimm.2008.10.298. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs-Nolan J. The novel adjuvant combination of CpG ODN, indolicidin and polyphosphazene induces potent antibody- and cell-mediated immune responses in mice. Vaccine. 2009;27:2055–2064. doi: 10.1016/j.vaccine.2009.01.118. [DOI] [PubMed] [Google Scholar]
- 38.Kindrachuk J. A novel vaccine adjuvant comprised of a synthetic innate defence regulator peptide and CpG oligonucleotide links innate and adaptive immunity. Vaccine. 2009;27:4662–4671. doi: 10.1016/j.vaccine.2009.05.094. [DOI] [PubMed] [Google Scholar]
- 39.Bowdish D.M. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Pept. Sci. 2005;6:35–51. doi: 10.2174/1389203053027494. [DOI] [PubMed] [Google Scholar]
- 40.Lau, Y.E. et al. (2005) Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect. Immun. 73, 583-591 41 [DOI] [PMC free article] [PubMed]
- 41.Mookherjee, N. et al., Intracellular receptor for human host defence peptide LL-37 in monocytes. J. Immunol. (in press) DOI:10.4049/jimmunol.0802586, Epub ahead of print PMFD 19605696 [DOI] [PubMed]
- 42.Travis S. RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm. Bowel Dis. 2005;11:713–719. doi: 10.1097/01.mib.0000172807.26748.16. [DOI] [PubMed] [Google Scholar]
- 43.Holtmann M.M. RDP-58 (SangStat Medical) IDrugs. 2003;6:1188–1194. [PubMed] [Google Scholar]
- 44.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 45.Giacometti A. Antiendotoxin activity of protegrin analog IB-367 alone or in combination with piperacillin in different animal models of septic shock. Peptides. 2003;24:1747–1752. doi: 10.1016/j.peptides.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 46.Sokolova, G.B. et al. (2002) [Glutoxim in the complex treatment of tuberculosis]. Antibiot. Khimioter. 47, 20-23 (English translation available at http://www.glutoxim.ru/eng/index.php?id=9&issueId=2) [PubMed]
- 47.Fimiani V. Immunomodulatory effect of glutoxim on some activities of isolated human neutrophils and in whole blood. Immunopharmacol. Immunotoxicol. 2002;24:627–638. doi: 10.1081/iph-120016039. [DOI] [PubMed] [Google Scholar]
- 48.Fischer P.M. The design, synthesis and application of stereochemical and directional peptide isomers: a critical review. Curr. Protein Pept. Sci. 2003;4:339–356. doi: 10.2174/1389203033487054. [DOI] [PubMed] [Google Scholar]
- 49.Kraus D., Peschel A. Staphylococcus aureus evasion of innate antimicrobial defense. Future Microbiol. 2008;3:437–451. doi: 10.2217/17460913.3.4.437. [DOI] [PubMed] [Google Scholar]
- 50.Andra J. Mechanisms of endotoxin neutralization by synthetic cationic compounds. J. Endotoxin Res. 2006;12:261–277. doi: 10.1179/096805106X118852. [DOI] [PubMed] [Google Scholar]
- 51.Lynn D.J. InnateDB: facilitating systems-level analyses of the mammalian innate immune response. Mol. Syst. Biol. 2008;4:218. doi: 10.1038/msb.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mookherjee N. Systems biology evaluation of immune responses induced by human host defence peptide LL-37 in mononuclear cells. Mol. Biosyst. 2009;5:483–496. doi: 10.1039/b813787k. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen T.X. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides. 2003;24:1647–1654. doi: 10.1016/j.peptides.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Carretero M. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J. Invest. Dermatol. 2008;128:223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 55.Steinstraesser L. Host defense peptides in wound healing. Mol. Med. 2008;14:528–537. doi: 10.2119/2008-00002.Steinstraesser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber G. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res. 2009;11:R6. doi: 10.1186/bcr2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coffelt S.B. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lingnau K. Poly-L-arginine synergizes with oligodeoxynucleotides containing CpG-motifs (CpG-ODN) for enhanced and prolonged immune responses and prevents the CpG-ODN-induced systemic release of pro-inflammatory cytokines. Vaccine. 2002;20:3498–3508. doi: 10.1016/s0264-410x(02)00343-2. [DOI] [PubMed] [Google Scholar]
- 59.Schellack C. IC31, a novel adjuvant signaling via TLR9, induces potent cellular and humoral immune responses. Vaccine. 2006;24:5461–5472. doi: 10.1016/j.vaccine.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 60.Stevceva L., Ferrari M.G. Mucosal adjuvants. Curr. Pharm. Des. 2005;11:801–811. doi: 10.2174/1381612053381846. [DOI] [PubMed] [Google Scholar]
- 61.Soehnlein O. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elssner A. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1β processing and release. J. Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 63.Barlow P.G. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J. Leukoc. Biol. 2006;80:509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng Y. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human α-defensins from neutrophils. Br. J. Dermatol. 2007;157:1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 65.Di Nardo A. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 66.Niyonsaba F. Evaluation of the effects of peptide antibiotics human β-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur. J. Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 67.Davidson D.J. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004;172:1146–1156. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 68.Kandler K. The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int. Immunol. 2006;18:1729–1736. doi: 10.1093/intimm/dxl107. [DOI] [PubMed] [Google Scholar]
- 69.Biragyn A. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 70.Lande R. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 71.Yamasaki K. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 72.Tokumaru S. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 73.Tjabringa G.S. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 74.Pistolic J. Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-κB pathway. J. Innate Immun. 2008;1:254–267. doi: 10.1159/000171533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Otte J.M. Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity. Regul. Pept. 2009;156:104–117. doi: 10.1016/j.regpep.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Wehkamp J. Defensins and cathelicidins in gastrointestinal infections. Curr. Opin. Gastroenterol. 2007;23:32–38. doi: 10.1097/MOG.0b013e32801182c2. [DOI] [PubMed] [Google Scholar]
- 77.Koczulla R. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.von Haussen J. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 79.Chuang C.M. Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum. Gene Ther. 2009;20:303–313. doi: 10.1089/hum.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van den Berg H.R. Synthetic oligopeptides related to the β-subunit of human chorionic gonadotropin attenuate inflammation and liver damage after (trauma) hemorrhagic shock and resuscitation. Shock. 2009;31:285–291. doi: 10.1097/SHK.0b013e31817fd62a. [DOI] [PubMed] [Google Scholar]


