Short abstract
Objective
To investigate the safety and efficacy of chimeric antigen receptor T (CAR-T) cell infusion in patients with refractory multiple myeloma (MM).
Methods
Sixteen patients diagnosed with refractory MM were included in this study. Patients received initial infusions of T-derived CD19/B-cell maturation antigen (BCMA) CAR-T cells with 100% CD19, followed by second infusions with 40% BCMA and third infusions with 60% BCMA. The total doses were 0.5–1 × 107/kg CD19 and 1.2 − 6.2 × 107/kg BCMA. Patients were monitored after infusion. Levels of interleukin (IL)-2, IL-6, IL-10, tumor necrosis factor-α, and C-reactive protein were determined by enzyme-linked immunosorbent assay.
Results
Cytokine release syndrome (CRS) was observed in all 16 patients. Thirteen patients with CRS stage II−IV had persistent hyperthermia from 5−14 days after infusion, while most patients developed hyperthermia from 1 day after infusion and their temperatures returned to normal within 2−10 days. Levels of all factors were significantly elevated 2 days after infusion, peaked at 5 days, and then gradually decreased to normal levels. All inflammatory factors showed normal levels by 10 days after infusion.
Conclusion
Body temperature and levels of inflammatory factors all increased dramatically after infusion of CD19/BCMA CAR-T cells, but recovered to normal levels after appropriate treatment and nursing.
Keywords: Chimeric antigen receptor T cell, CD19, B-cell maturation antigen, BCMA-refractory multiple myeloma, temperature, inflammatory factor
Introduction
Multiple myeloma (MM) is characterized by the proliferation of a single clone of plasma cells in the bone marrow. MM accounts for 10% of all hematologic malignancies and about 1% of all cancers worldwide;1,2 >20,000 new cases of MM are diagnosed annually in the USA, with an incidence of 4/100,000.3,4 Despite the development of therapeutic approaches and a better understanding of its pathology, the prognosis of MM remains poor.5,6
Tumor immunotherapy has recently been increasingly applied in the clinic, involving monoclonal antibodies,7,8 cytokine-induced killer cells,9 tumor-infiltrating lymphocytes,10 chimeric antigen receptor T (CAR-T) cells,11 and B-cell maturation antigen (BCMA).12 CARs are genetically engineered molecules combining a single-chain variable fragment domain of a targeting antibody.13 Targeting CD19 or BCMA with CAR-T cells can recognize specific tumor antigens, leading to activation of antitumor functions.11,14 CAR-T cells have demonstrated potential use for the treatment of lymphocytic leukemia13 and MM.15 However, despite their promising effects in cancer treatment, CAR-T cells also have adverse effects, including cytokine release syndrome (CRS), which occurs due to the too-rapid clearance of tumor cells and the release of numerous cytokines.16
Few studies have reported on the application of CAR-T cells for the treatment of refractory MM, and none have focused on the use of CAR-T cells against the dual targets CD19 and BCMA in patients with refractory MM. In the present study, we examined the effects of CAR-T cell infusion on body temperature and inflammatory factors, and assessed its safety in patients with refractory MM. The results of this study might provide additional clinical evidence for the application of CAR-T cells for the treatment of MM.
Materials and methods
Patients and treatment
This observational study included patients diagnosed with refractory MM at The First Affiliated Hospital of Soochow University, Suzhou, China, between March 2017 and February 2018. All patients were older than 18 years. Refractory MM was defined according to the National Comprehensive Cancer Network criteria.17 Patients who received at least two standard therapies without complete recovery or who showed recurrence after recovery were considered as refractory. Patients with severe renal or liver dysfunction and patients who were pregnant were excluded. The patients’ basic clinical characteristics including age, sex, disease course, and International Staging System stage were recorded. Written informed consent was obtained from all patients. The present study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University.
After admission, T cells were collected from all patients and cultured for 1−2 weeks to obtain CAR-T cells. Cell culturing and T-derived CD19/BCMA CAR-T cell construction and generation were performed by Unicar Therapy Bio-Medicine Technology Co., Ltd. (Shanghai, China). Vector construction was carried by Unicar out using a primer designed by Unicar Therapy Bio-Medicine Technology Co., Ltd., and the sequences were amplified and inserted into the pWPT-GFP vector (Unicar). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Gaithersburg, MD, USA) at 37°C with 5% CO2 and then digested and transfected with the above vectors using Lipofectamine 3000 (Invitrogen, Waltham, MA, USA). After 48 hours, the vectors were collected and suspended in serum-free DMEM and stored at −80°C.
For infusion of CAR-T cells, three sequential doses of CD19/BCMA CAR-T cells were infused into patients using intravenous infusion, with 100% CD19, 40% BCMA, and 60% BCMA, respectively. The total doses of CD19 and BCMA were 0.5–1 × 107/kg and 1.2−6.2 × 107/kg, respectively. Two patients were infused with 0.5 × 107/kg BCMA-CAR-T cells on the surface lesion under B-mode ultrasound guidance. Patients were then monitored closely for vital signs and basic clinical indices.
Monitoring and nursing
After infusion, patients were monitored strictly to detect CRS occurrence. CRS stage was defined according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events.18 Temperature and electrocardiograms were monitored constantly in all patients. Because most of the patients were older with abnormal cardiac and renal function indexes, sensitive indicators and cytokines associated with renal failure and heart failure were monitored intensively. Hormonal drugs were forbidden during CAR-T treatment, and nonsteroidal anti-inflammatory drugs were therefore prepared previously. In the event of hyperthermia, accompanied by heart rate acceleration, chills, elevated blood pressure, or shortness of breath, patients were administered physical cooling and fluid infusion, and low dose non-steroidal drugs were used to reduce the temperature. Liver and kidney functions, routine blood, urine, blood gas and blood electrolyte monitoring were also performed. Appropriate psychological intervention was provided to encourage the patients and reduce their anxiety. All monitoring and nursing management was performed by the same team according to the same protocols.
Levels of the inflammatory factors interleukin (IL)-2, IL-6, IL-10, tumor necrosis factor (TNF)-α, and C-reactive protein (CRP) were determined by enzyme-linked immunosorbent assay (ELISA) using the corresponding kits (LifeSpan BioSiences, Inc., WA, USA) according to the manufacturer’s instructions. In the event of abnormal levels of the above factors, the hemorrhage condition was intensively monitored and accidents such as falling down or falling out of bed should be avoided. The incidences of CRS and complications were also recorded.
Statistical analysis
Measured data were expressed as mean ± standard deviation or median (range). Comparisons between two groups were performed using Student’s t-test. A value of P<0.05 was considered significant. All calculations were made using SPSS Statistics for Windows, Version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients
Sixteen patients who met the inclusion criteria were included in this study. Eleven patients received fludarabine and cyclophosphamide before infusion and five were infused directly after hematopoietic reconstitution of autologous hematopoietic stem cell transplantation.
CRS incidence and complications
CRS occurred in all 16 patients, with an incidence rate of 100% (Table 1). Among all patients, the overall response rate was 87.5% (14/16), including 12 complete and two partial responses. The complications are listed in Table 1. The most common complications were hyperthermia, muscle pain, headache, nausea, and increase of inflammatory factors. One patient developed neurological impairment, but no patients died.
Table 1.
Basic clinical characteristics and incidences of cytokine release syndrome and complications in all patients.
| Variable | Value |
|---|---|
| Age, years | 55.1 ± 8.2 (50–72) |
| Sex, male:female | 14:2 |
| Course of disease, months | 5.5 (3–18) |
| Treg cell number | 1.41 ± 0.3 × 108 |
| ISS stage, n (%) | |
| I | 3 (18.8) |
| II | 7 (43.8) |
| II | 6 (37.5) |
| CRS stage, n (%) | |
| I | 3 (18.8) |
| II | 9 (56.3) |
| III | 2 (12.5) |
| IV | 2 (12.5) |
| Complication, n (%) | |
| Hyperthermia | 15 (93.8) |
| Increase of inflammatory factors | 13 (81.3) |
| Muscle pain | 11 (68.8) |
| Headache | 9 (56.3) |
| Nausea | 8 (50.0) |
| Vomiting | 5 (31.3) |
| Diarrhea | 3 (18.8) |
| Renal impairment | 3 (18.8) |
| Liver impairment | 1 (6.3) |
| Neurological impairment | 1 (6.3) |
| Drugs used before study, n (%) | |
| Lenalidomide | 9 (56.3) |
| Dexamethasone | 12 (75.0) |
| Bortezomib | 7 (43.8) |
| Adriamycin | 6 (37.5) |
ISS, International Staging System; CRS cytokine release syndrome.
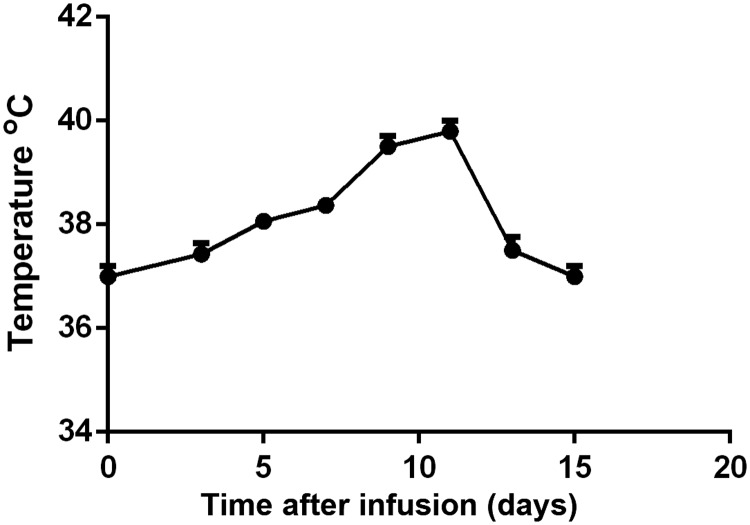
Temperature changes
Temperature changes in all patients within the first 2 weeks after infusion are shown in Figure 1. Thirteen (81.3%) patients with CRS stage II–IV had persistent hyperthermia from 5–14 days after infusion. Most patients developed hyperthermia from 1 day after infusion and their temperature returned to normal within 2–10 days after appropriate treatment and nursing management. Temperature returned to normal in all patients by 14 days after infusion.
Figure 1.
Mean temperature in all patients.
Changes in serum levels of inflammatory factors
We analyzed the changes in levels of the inflammatory factors IL-2, IL-6, IL-10, TNF-α, and CRP within 10 days after infusion. Mean levels of all factors were significantly elevated at 2 days after infusion (P < 0.05) (Table 2). Levels peaked at 3–5 days and then gradually decreased to normal levels after appropriate treatment and nursing management. Levels of all the measured inflammatory factors had returned to normal by 10 days after infusion.
Table 2.
Changes in serum levels of inflammatory factors within 10 days after CAR-T cell infusion.
| Before infusion | 2 days | 3 days | 5 days | 7 days | 10 days | |
|---|---|---|---|---|---|---|
| IL-2, pg/mL | 34.8 ± 10.7 | 35.4 ± 10.9 | 72.8 ± 23.4* | 148.5 ± 23.6* | 95.6 ± 19.9* | 35.9 ± 11.4 |
| IL-6, pg/mL | 58.3 ± 12.5 | 64.5 ± 16.4 | 214.9 ± 20.3* | 458.2 ± 334.7* | 103.8 ± 26.6* | 59.6 ± 13.3 |
| IL-10, pg/mL | 54.0 ± 11.7 | 63.5 ± 18.9 | 103.8 ± 22.4* | 161.3 ± 33.5* | 121.6 ± 25.7* | 58.9 ± 14.6 |
| TNF-α, pg/mL | 47.9 ± 12.4 | 58.2 ± 13.1 | 113.4 ± 21.8* | 163.4 ± 36.8* | 118.7 ± 25.0* | 47.8 ± 10.3 |
| CRP, mg/L | 16.8 ± 4.9 | 17.4 ± 4.2 | 34.7 ± 8.3* | 44.7 ± 11.6* | 31.5 ± 7.9* | 17.2 ± 5.3 |
*P < 0.05, compared with the value before infusion.
Discussion
Along with the development of cancer immunotherapy, the application of CAR-T cells has attracted much attention in recent years. CAR-T cells are considered to provide a potentially novel method for the treatment of hematological tumors. However, information on the clinical application of CAR-T cells for the treatment of MM is currently lacking, and more clinical evidence for the efficacy and safety of CAR-T cells in cancer therapy is urgently needed. In the present observational study, we examined the effects of CAR-T cell infusion on the CRS situation, and on body temperature and levels of inflammatory factors in patients with refractory MM.
CAR-T cells have been reported as a novel therapeutic approach in many diseases, and basic clinical studies have demonstrated different kinds of specific CAR-T cells. Craddock et al.19 showed that GD2 CAR-T cells could enhance tumor trafficking via expression of the chemokine receptor CCR2b, while Kenderian et al.20 showed that CAR-T cells targeting CD33 could avoid long-term myelosuppression in patients with acute myeloid leukemia. Humanized anti-epidermal growth factor receptor variant III CAR-T cells could also be generated to treat glioblastoma.21
Despite the above studies, clinical evidence for the use of CAR-T cells is still lacking. CAR-T cells have been used to treat neuroblastoma.22 In addition, Garfall et al.15 recently demonstrated the application of CAR-T cells against CD19 for the treatment of MM, and found that infusion of CAR-T cells after high-dose melphalan and autologous transplantation could lead to a durable complete response. Sidaway et al.23 found that anti-BCMA CAR-T cells showed promising efficacy for the treatment of MM patients. However, the safety and adverse effects of CAR-T have rarely been reported in patients with MM. CRS is generally one of the main adverse effects of CAR-T cell treatment, and infusion of CAR-T cells has been reported to lead to varying degrees of CRS.24,25 In the present study, the incidence of CRS was as high as 100%, and both temperature and levels of inflammatory factors increased dramatically after the infusion of CAR-T cells; however, these all returned to normal levels after appropriate treatment and nursing. Further clinical studies are needed to confirm these results.
The present study had some limitations. First, the sample size was small, and second, the long-term effects of the treatment remain unclear. Further studies are therefore needed to address these issues.
In conclusion, we conducted an observational study to investigate the safety and efficacy of CD19/BCMA CAR-T cell infusion in patients with refractory MM. Both body temperature and levels of inflammatory factors increased dramatically after the infusion of CAR-T cells, but recovered to normal levels after treatment and nursing. This study provides more clinical evidence for the therapeutic application of CAR-T cells in patients with MM.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Xiaming Zhu https://orcid.org/0000-0001-7430-6238
References
- 1.Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet 2014; 385: 2197. [DOI] [PubMed] [Google Scholar]
- 2.Vincent RS. Multiple myeloma: 2014 Update on diagnosis, risk-stratification, and management. Am J Hematol 2014; 89: 998–1009. [DOI] [PubMed] [Google Scholar]
- 3.Harousseau JL, Dreyling M. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24: vi133–vi137. [DOI] [PubMed] [Google Scholar]
- 4.Kazandjian D. Multiple myeloma epidemiology and survival: a unique malignancy. Semin Oncol 2016; 43: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pour L, Sevcikova S, Greslikova Het al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica 2014; 99: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koskela K, Pelliniemi TT, Rajamäki Aet al. Serum oncostatin M in multiple myeloma: impact on disease severity and prognosis. Eur J Haematol 2015; 65: 52–56. [DOI] [PubMed] [Google Scholar]
- 7.Gubin MM, Artyomov MN, Mardis ERet al. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest 2015; 125: 3413–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sautto G, Mancini N, Gorini Get al. Possible future monoclonal antibody (mAb)-based therapy against arbovirus infections. Biomed Res Int 2013; 2013: 838491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J Transl Med 2013; 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smyrk TC, Watson P, Kaul Ket al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 2015; 91: 2417–2422. [PubMed] [Google Scholar]
- 11.Scholler J, Brady TL, Binderscholl Get al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012; 4: 132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams GB, Feng J, Ghogha Aet al. Abstract 4979: development of KITE-585: a fully human BCMA CAR T-cell therapy for the treatment of multiple myeloma. Cancer Res 2017; 77: 4979. [Google Scholar]
- 13.Porter DL, Hwang WT, Frey NVet al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7: 303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman KM, Garrett TE, Evans JWet al. Effective targeting of multiple BCMA-expressing hematological malignancies by anti-BCMA CAR T cells. Hum Gene Ther 2018; 29: 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garfall AL, Maus MV, Hwang WTet al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med 2015; 373: 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruella M, Kenderian SS, Shestova Oet al. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms. Leukemia 2016; 31: 246. [DOI] [PubMed] [Google Scholar]
- 17.Anderson KC, Alsina M, Atanackovic Det al. Multiple myeloma, version 2.2016: clinical practice guidelines in oncology. J Natl Compr Canc Netw 2015; 13: 1398–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong X, Lim EA, Hershman DLet al. ReCAP: identifying severe adverse event clusters using the National Cancer Institute’s common terminology criteria for adverse events. J Oncol Pract 2016; 12: e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craddock JA, An L, Bear Aet al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother 2010; 33: 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenderian SS, Ruella M, Shestova Oet al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia 2015; 29: 1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson LA, Scholler J, Ohkuri Tet al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 2015; 7: 275ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis CU, Savoldo B, Dotti Get al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011; 118: 6050–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidaway P. Haematological cancer: anti-BCMA CAR T cells show promise in MM. Nat Rev Clin Oncol 2016; 13: 530. [DOI] [PubMed] [Google Scholar]
- 24.Teachey DT, Lacey SF, Shaw PAet al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016; 6: 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Jie S, Zhao Wet al. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J Hematol Oncol 2016; 9: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]