Highlights
-
•
ER α-glucosidases are essential host factors for the morphogenesis of many enveloped viruses.
-
•
Imino sugars are competitive inhibitors of the ER α-glucosidases I and II.
-
•
Broad-spectrum antiviral efficacies of imino sugars have been demonstrated in vitro, and in vivo.
-
•
Strategies for development of potent and specific ER α-glucosidase inhibitors have been proposed.
-
•
Targeting glucosidase is promising for viral hemorrhagic fever and respiratory infections.
Keywords: ER α-glucosidases, imino sugars, antiviral therapy, flavivirus, filovirus, viral hemorrhagic fever
Abstract
Endoplasmic reticulum (ER)-resident α-glucosidases I and II sequentially trim the three terminal glucose moieties on N-linked glycans attached to nascent glycoproteins. These reactions are the first steps of N-linked glycan processing and are essential for proper folding and function of many glycoproteins. Because most viral envelope glycoproteins contain N-linked glycans, inhibition of ER α-glucosidases with derivatives of 1-deoxynojirimycin (DNJ) or castanospermine (CAST), two well-studied pharmacophores of α-glucosidase inhibitors, efficiently disrupts the morphogenesis of a broad spectrum of enveloped viruses. Moreover, both DNJ and CAST derivatives have been demonstrated to prevent the death of mice infected with several distinct flaviviruses and filoviruses and suppress the multiplication of several other species of viruses in infected animals. N-Butyl derivative of DNJ (NB-DNJ) and 6 O-bytanoyl prodrug of CAST (Bu-CAST) have been evaluated in human clinical trials for their antiviral activities against human immunodeficiency virus and hepatitis C virus, and there is an ongoing trial of treating dengue patients with Bu-CAST. This article summarizes the current status of ER α-glucosidase-targeted antiviral therapy and proposes strategies for development of more efficacious and specific ER α-glucosidase inhibitors as broad-spectrum, drug resistance-refractory antiviral therapeutics. These host function-targeted, broad-spectrum antiviral agents do not rely on time-consuming etiologic diagnosis, and should therefore be particularly promising in the management of viral hemorrhagic fever and respiratory tract viral infections, medical conditions that can be caused by many different enveloped RNA viruses, with a short window for medical intervention.
1. Introduction
The vast majority of antiviral drugs approved thus far by the United States Food and Drug Administration for the management of viral diseases inhibit virus-encoded enzymes, such as DNA/RNA polymerases, integrase, proteases and neuraminidase. The antiviral activity of these direct-acting antiviral agents is usually virus-specific, but there are numerous examples of treatment failure due to the emergence of drug-resistant viruses. In contrast, targeting host functions essential for viral replication has been considered as a potential broad-spectrum and resistance-refractory therapeutic approach. Accordingly, many host cellular proteins involved in nucleoside metabolism, RNA capping, protein folding and glycan processing have been empirically explored as broad-spectrum antiviral targets (De Clercq, 2004, Geller et al., 2012). Recently, the advent of genome-wide technologies has allowed for systematic identification and validation of potential host cellular targets for antiviral drug development (de Chassey et al., 2012, Hong-Geller and Micheva-Viteva, 2010, Meliopoulos et al., 2012, Watanabe et al., 2010).
Although many inhibitors of host functions have been demonstrated to inhibit a specific virus or a broad spectrum of viruses in cultured cells, the in vivo antiviral efficacy has so far only been demonstrated for inhibitors of a few host enzymes, including inosine-5′-monophosphate dehydrogenase (IMPDH) (Furuta et al., 2009), S-Adenosyl-L-homocysteine (SAH) hydrolase (Bray et al., 2000, Bray et al., 2002), cyclophilin (Inoue et al., 2007) and endoplasmic reticulum (ER) α-glucosidases (Noble et al., 2010). For ER α-glucosidase inhibitors in particular, 1-deoxynojirimycin (DNJ) and castanopermine (CAST) derivatives have been demonstrated in the last three decades by many independent research groups to inhibit the morphogenesis of many enveloped viruses (reviewed in Dwek et al., 2002). Several DNJ and CAST derivatives have been demonstrated to reduce viremia of dengue virus (DENV), and/or protect mice from lethal dose of DENV (Chang et al., 2011a, Chang et al., 2011b, Miller et al., 2012, Perry et al., 2013, Rathore et al., 2011, Schul et al., 2007, Watanabe et al., 2012, Whitby et al., 2005), Japanese encephalitis virus (JEV) (Wu et al., 2002), Ebola virus (EBOV) and Marburg virus (MARV) challenge in mice (Chang et al., 2013). Reduction in viremia by a DNJ derivative, N-nonyl-DNJ (NN-DNJ), was also shown in vivo in woodchucks chronically infected with woodchuck hepatitis virus (WHV), a model virus for human hepatitis B virus (HBV) (Block et al., 1994). Furthermore, human clinical trials of NB-DNJ and Bu-CAST have been performed to test their antiviral efficacy against human immunodeficiency virus (HIV) and hepatitis C virus (HCV), respectively. In both cases, modest reduction in virus titer in the serum of some of the treated patients was observed (Durantel, 2009, Fischl et al., 1994).
These studies have thus far validated that the host cellular ER α-glucosidases are viable drug targets for the treatment of a broad spectrum of enveloped viruses. Such broad-spectrum antiviral agents would be particularly promising in the management of viral hemorrhagic fever and respiratory tract viral infections. Both medical conditions are caused by many different viruses and have a short treatment window for medical intervention (Hussell et al., 2012, Jartti et al., 2012, Paessler and Walker, 2013). Development of drugs with broad-spectrum antiviral activity for these acute infections obviously holds clear clinical advantages in comparison with virus-specific antiviral agents, which rely on time-consuming etiologic diagnosis.
2. ER α-glucosidase I and II are key enzymes in N-linked glycan processing of nascent glycoproteins
Most proteins synthesized in the ER are glycoproteins. N-linked glycosylation is the most common type of glycosylation process, that occurs in the ER lumen in eukaryotes, and is initiated by the addition of a preformed oligosaccharide precursor Glc3Man9GlcNAc2 to an asparagines motif on the nascent polypeptide, by oligosaccharyltransferase. The N-linked oligosaccharide moieties serve highly diverse functions that are essential for the folding, sorting, secretion and function of the glycoproteins (Helenius and Aebi, 2004). As illustrated in Fig. 1 , the initial steps of N-linked oligosaccharide processing on a glycoprotein in the ER involve the sequential trimming of the three glucose residues on an oligosaccharide by the two ER glycoside cleaving enzymes, α-glucosidases I and II. α-glucosidase I removes the outer α-1,2-linked glucose residue and α-glucosidase II removes the two inner α-1,3-linked glucose residues. These processes are required for the proper folding and function of many glycoproteins by allowing their interaction with ER chaperones, calnexin and calreticulin (Hebert et al., 1995). ER contains a unique calnexin-dependent folding and quality control machinery in which calnexin/calreticuline act as chaperones and bind proteins with monoglucosidated glycan side chains. Incompletely folded proteins are re-glucosylated by UDP-glucosyltransferase 1, which serves as a sensor for correct protein folding. The properly folded proteins are eventually released from the re- and de-glucosylation cycle and moved to the Golgi complexes for further processing (Hammond et al., 1994). Abrogation of normal glycoprotein trafficking and processing by inhibition of ER α-glucosidases I and II, results in terminal glucose retention on N-linked glycans and subsequent generation of mis-folded glycoproteins, which may be retained in the ER and ultimately be directed to undergo ER-associated degradation (ERAD) (Alonzi et al., 2013, Simsek et al., 2005). Some of the mis-folded glycoproteins manage to escape the ER quality control, and end up expressed on the cell surface in the presence of glucosidase inhibition, but may present with compromised functionality (Burke et al., 1984, McLaughlin and Vandenbroeck, 2011).
Fig. 1.
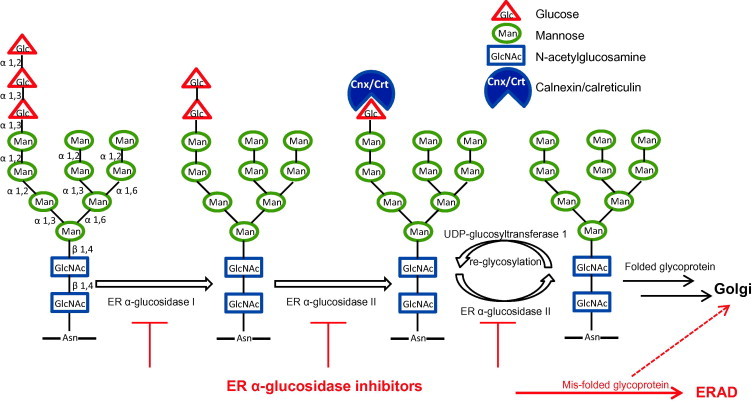
Illustration of ER α-glucosidases I and II in glycoprotein folding.
Because the envelope proteins of many different viruses are glycoproteins containing N-linked glycans, and mammalian viruses do not encode their own carbohydrate-modifying enzymes, it is anticipated that the glycan processing and proper folding of those viral glycoproteins require host cellular ER α-glucosidases. Moreover, due to the highly dynamic nature of viral replication, it is conceivable that inhibition of ER α-glucosidases might preferentially disturb the maturation and function of viral envelope glycoproteins, which may consequentially reduce virion particle assembly, secretion and/or infectivity. Indeed, the essential role of cellular ER α-glucosidase II in the DENV life cycle was demonstrated in a genome-wide siRNA knockdown study (Sessions et al., 2009). In addition, suppression of ER α-glucosidase I and/or II expression with RNA interference technology efficiently reduced the yields of enveloped RNA viruses, such as DENV and HCV (Qu et al., 2011, Yu et al., 2012).
2. Inhibition of ER α-glucosidases compromises the replication of a broad spectrum of enveloped viruses via distinct mechanisms
Many natural products contain inhibitors of various types of glucosidases, and they have been extensively explored for application as medicines for carbohydrate-mediated diseases, such as cancer, diabetes, infections and lysosomal storage diseases (Kajimoto and Node, 2009, Moorthy et al., 2012, Tundis et al., 2010). Among these natural products, DNJ and CAST have been well documented for their broad-spectrum antiviral activities through inhibition of ER α-glucosidases I and II. Both DNJ and CAST are generally referred as imino sugars. As shown in Fig. 2 , DNJ is a 6-membered-ring polyhydroxylated secondary amine in which the molecule resembles a monosaccharide (glucose) with the ring oxygen replaced by nitrogen. CAST is indolizidine imino sugar, in which an ethylene links the endocyclic nitrogen to create a fused 5,6-ring system. Because imino sugars are similar in structure to glucose, the substrate of ER α-glucosidases, they competitively inhibit ER α-glucosidases I and II as transition-state substrate analogues (Hempel et al., 1993).
Fig. 2.
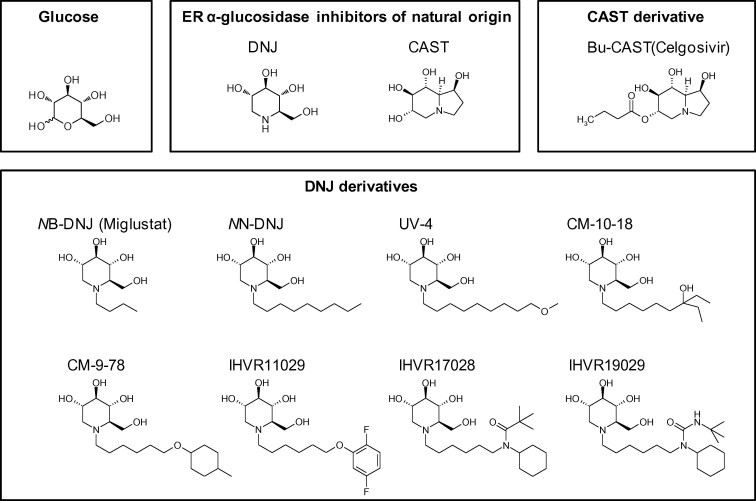
Structures of glucosidase inhibitors of natural origin and their derivatives in preclinical development.
DNJ and CAST, as well as their derivatives have been demonstrated to inhibit the replication of enveloped viruses from at least 12 different families, in vitro (Table 1 ). This includes DNA viruses, such as herpes simplex virus-2 (HSV-2), cytomegalovirus (CMV) and HBV, as well as RNA viruses, such as HIV, sindbis virus, HCV, JEV, West Nile virus (WNV), murine hepatitis virus, measles virus, vesicular stomatitis virus (VSV), and influenza A virus. Interestingly, multiple hemorrhagic fever viruses from four viral families, such as DENV, EBOV, Lassa fever, Junin, and Rift Valley fever virus are all sensitive to glucosidase inhibitor treatment (Chang et al., 2013, Noble et al., 2010, Silber et al., 1993). Although these viruses each have distinct genome structures and replication strategies, they are all enveloped with glycosylated viral proteins and therefore share a similar morphogenesis pathway that depends on the host glycoprotein processing machinery in the ER. However, it seems that not all viral glycoproteins are equally dependent on this mechanism to achieve functional conformations. For example, WNV was relatively less sensitive to imino sugar α-glucosidase inhibitors than other flaviviruses, such as DENV (Chang et al., 2009, Whitby et al., 2005). In addition, certain strains of VSV (Orsay) and influenza A virus (PR-8) were also reported to be insensitive to ER α-glucosidase inhibitors (Datema et al., 1984, Schlesinger et al., 1984).
Table 1.
Broad-spectrum antiviral activity of imino sugars against multiple families of enveloped viruses in vitro.
| Virus family | Virus genome | Viruses sensitive to α-glucosidase inhibitor | References |
|---|---|---|---|
| DNA viruses | |||
| Herpesviridae | Double strand linear DNA | Herpes simplex virus-2 Cytomegalovirus | Ahmed et al. (1995) |
| Taylor et al. (1988) | |||
| Hepadnaviridae | Partial double-stranded circular DNA | Hepatitis B virus | Block et al., 1994, Lu et al., 2001, Mehta et al., 2001 |
| RNA viruses | |||
| Retroviridae | Single strand RNA | Human immunodeficiency virus | Fischer et al., 1995, Fischer et al., 1996b, Taylor et al., 1994, Taylor et al., 1991 |
| Moloney murine leukemia virus | Taylor et al. (1991) | ||
| Rauscher murine leukemia virus | Ruprechat et al. (1989) | ||
| Togaviridae | Positive strand RNA | Sindbis viru | Datema et al. (1984) |
| Semliki forest virus | Kaluza et al. (1990) | ||
| Flaviviridae | Positive strand RNA | Bovine viral diarrhea virus | Jordan et al., 2002, Mehta et al., 2002, Zitzmann et al., 1999 |
| Hepatitis C virus | Qu et al., 2011, Steinmann et al., 2007 | ||
| Dengue virus | Courageot et al., 2000, Gu et al., 2007, Whitby et al., 2005, Wu et al., 2002 | ||
| Japanese encephalitis virus | Whitby et al., 2005, Wu et al., 2002 | ||
| West Nile virus | Chang et al., 2009, Gu et al., 2007 | ||
| Coronaviridae | Positive strand RNA | Severe acute respiratory syndrome coronavirus | Fukushi et al. (2012) |
| Murine hepatitis virus | Repp et al. (1985) | ||
| Paramyxoviridae | Negative strand RNA | Measles virus | Bolt et al. (1999) |
| Newcastle disease virus | Lee et al. (2011) | ||
| Rhabdoviridae | Negative strand RNA | Vesicular stomatitis virus | Schlesinger et al. (1984) |
| Filoviridae | Negative strand RNA | Ebola virus | Chang et al. (2013) |
| Arenaviridae | Bipartite segmented, negative-sense to ambisense, RNA | Tacaribe virus | Chang et al. (2013) |
| Lassa fever virus | Chang et al. (2013) | ||
| Junin virus | Silber et al. (1993) | ||
| Bunyaviridae | Tripartite segmented, negative-sense to ambisense, RNA | Rift Valley fever virus | Chang et al. (2013) |
| Orthomyxoviridae | 6–8 Segmented, negative-sense RNA | Influenza A virus | Datema et al., 1984, Saito and Yamaguchi, 2000 |
Concerning the antiviral mechanism of imino sugars, one of the anticipated biochemical consequences of suppressing the ER α-glucosidases is the production of viral glycoproteins with hyper-glycosylated glycan side chains. This has been demonstrated by the mobility shift of the intracellular viral glycoproteins in electrophoresis (Chang et al., 2009, Hammond et al., 1994, Taylor et al., 1994) and directly confirmed by analyzing glycan structures attached to the specific viral glycoproteins (Ritchie et al., 2010). As a consequence of the incomplete glucose trimming of N-linked oligosaccharide, the productive folding pathway of the viral glycoprotein is affected. This usually results in the reduced efficiency of virion assembly and secretion through degradation of viral glycoproteins bearing unprocessed N-linked oligosaccharide. In fact, it was well documented that imino sugars dose-dependently induced the mobility shift as well as degradation of the envelope glycoproteins of viruses from several families (Chang et al., 2009, Fukushi et al., 2012, Qu et al., 2011). Occasionally, the incomplete glucose trimming also affects virion secretion through prevention of the further processing and trafficking of viral glycoproteins. For instance, inhibition of the ER α-glucosidases delayed the formation and reduced the stability of DENV prM/E heterodimer (Courageot et al., 2000) or blocked the proteolytic cleavage of Sindbis virus precursor envelope glycoprotein (Datema et al., 1984).
However, inhibition of glycan processing by imino sugars does not always result in the degradation and/or disruption of the intracellular trafficking of certain viral glycoproteins. For instance, it has been demonstrated that although the oligosaccharides of VSV G protein or influenza A virus hemagglutinin were greatly altered in the presence of glucosidase inhibitors, their surface expression was not inhibited (Burke et al., 1984). Other studies showed that, while antiviral effect and changes in N-linked glycan structures can become obvious at low concentrations, degradation of viral glycoproteins usually occurs in the cells treated with much higher concentrations of glucosidase inhibitors (Chang et al., 2009, Du et al., 2013, Fukushi et al., 2012, Yu et al., 2012). In addition to suppressing virion assembly and secretion, the antiviral activity of glucosidase inhibitors from these examples might be explained by other mechanisms.
Indeed, inhibition of the N-linked glycan processing of viral glycoproteins could also result in secretion of virions with altered molecular composition, binding properties and/or functionalities, which prevent the infection of susceptible cells. In one extreme case, CMV infected cells continued to shed virions into the extracellular medium in the presence of CAST, as determined by electron microscopy, and the antiviral effect was mainly due to the reduction in infectivity as a result of the alteration of components on the virion envelope (Taylor et al., 1988). Another example is that imino sugars inhibited HIV infectivity at a post-CD4 binding step, by preventing the conformational change of glycoprotein gp-120 (Fischer et al., 1995, Fischer et al., 1996a, Fischer et al., 1996b). Nevertheless, for most enveloped viruses, it is more likely that reductions in both virion secretion and the infectivity are responsible for overall antiviral effects (Chapel et al., 2006, Fischer et al., 1996b, Lazar et al., 2007).
In addition to the above well-studied mechanisms, it is also possible that inhibition of ER α-glucosidases compromises the expression and/or function of viral receptors, since many of them are also glycoproteins with N-linked glycans. A study has been reported in HIV infection, where pre-treatment of HIV-permissive CD4+ cells with Bu-CAST substantially reduced their capacity to bind with cells chronically infected with HIV-1 (Bridges et al., 1994). To further test this hypothesis, we recently tested the effects of several DNJ-derivatives on the infection of retroviruses pseudotyped with envelope proteins from eight different families of viruses and demonstrated that the compounds efficiently inhibited the severe acute respiratory syndrome coronavirus (SARS-CoV) and human coronavirus NL63 spike protein-mediated entry (Zhao and Guo, unpublished observations).
Taken together, the studies have thus far illustrated that imino sugars inhibit a broad spectrum of enveloped viruses by disrupting virion assembly/secretion, and reducing infectivity of progeny virions by altering virion and/or cellular receptor components, in absence of effect on cell viability. These distinct antiviral mechanisms are all consistent with on-target inhibition of the ER α-glucosidases and subsequent disruption of viral and host cellular glycoprotein processing.
3. Structure–activity relationship (SAR) of imino sugars
Although demonstrated to be broadly active antiviral agents, the antiviral activity of DNJ and CAST, the two lead α-glucosidase inhibitors initially discovered from plants (Horne et al., 2011), was less than optimal and therefore limited their use. For example, it usually requires millimolar concentrations of DNJ to inhibit viruses. Thus, extensive lead optimization efforts have been made to obtain DNJ derivatives with improved antiviral efficacy (reviewed in Moorthy et al., 2012, Nash et al., 2011, Sayce et al., 2010). However, due to the lack of structural information of human ER α-glucosidases, all the lead optimization efforts have thus far been empirical. As mentioned above, DNJ is a substrate (glucose) analogue which competitively inhibits the ER α-glucosidases I and II. So far, limited attempts to alter the DNJ core structure have either not yielded derivatives with significantly improved antiviral activity, or resulted in loss of enzymatic inhibitory activity (Dwek et al., 2002, Horne et al., 2011, Zitzmann et al., 1999). The only exception is that a few five-membered iminocyclitol glucosidase inhibitors were demonstrated to have antiviral activity (Asano et al., 1995, Chapman et al., 2005, Liang et al., 2006). In contrast, the nitrogen atom in the DNJ ring structure provides a unique opportunity for generating a class of derivatives with DNJ as head group and various N-linked alkyl side chains. During the last two decades, more than 200 DNJ derivatives distinct in the length and composition of the N-linked side chains have been synthesized. Several strategies have been used to diversify the side-chain structures, which include heteroatom substitution, addition of terminal ring structure and conformation restriction. Through extensive comparison of antiviral activities, we have now obtained DNJ derivatives with submicromolar antiviral activity against several different viruses (Chang et al., 2009, Chang et al., 2013). Interestingly, it appears that compounds with more potent antiviral activity usually have a similar length of side chains, such as an approximately 9-carbon linear alkyl side chain or a 6-carbon linear chain linked with a terminal ring structure (Du et al., 2013, Howe et al., 2013, Yu et al., 2012) (Fig. 2). It is possible that those hydrophobic side chains anchor the DNJ derivatives on the ER membrane and present the DNJ head toward the active center of α-glucosidases (Kajimoto and Node, 2009, Moorthy et al., 2012). Hence, it is conceivable that proper length of the side chain is critical for optimal inhibition of the ER α-glucosidases, which are also integrated ER membrane proteins.
In addition, we also noticed that among the DNJ derivatives, the improvement of antiviral activity is not always correlated with an increased potency of inhibiting the ER α-glucosidases in cell-free biochemical assays (Chang et al., 2011a, Chang et al., 2013, Howe et al., 2013) (Chang et al. unpublished observations). One explanation for this phenomenon is that changes on N-linked side chain may not always enhance the potency of inhibition of the target enzymes, but may also improve cell penetration and/or distribution into the ER membrane (Du et al., 2013, Fleet et al., 1988, Karpas et al., 1988, Moorthy et al., 2012, Yu et al., 2012) and thus enhance antiviral activity.
Recently, the crystal structure of yeast ER α-glucosidase I has been determined together with modeled binding with substrate and inhibitors (Barker and Rose, 2013). Yeast and human α-glucosidase I share high identity in their catalytically active domains, similar substrate specificity, pH optimum, and inhibitor sensitivity (Bause et al., 1986, Schweden et al., 1986). Therefore, the structural information obtained from yeast ER α-glucosidase I may thus facilitate the rational design of more potent and specific imino sugar- or new pharmacophore of ER α-glucosidase inhibitors (Moorthy et al., 2012). While most SAR work has been focused on DNJ derivatives, very limited modification has been performed for CAST, partly due to the more complicated chemical nature of this pharmacophore. With progress in the understanding of the structural features of ER α-glucosidases, it is also possible, that rationally designed modifications of CAST can be made in the future.
4. Antiviral efficacies of imino sugars have been demonstrated in multiple animal models
The lead optimization efforts have successfully identified imino sugar derivatives with not only superior antiviral activity, but also favorable pharmacokinetic and toxicologic properties for in vivo antiviral efficacy studies. Fig. 2 lists all the DNJ and CAST derivatives that have been tested in animal models of virus infection. While several pioneer works proved the concept that imino sugar derivatives were efficacious in reducing viremia in WHV chronically infected woodchucks (Block et al., 1998), tissue virus load in murine zosteriform model of HSV-1 infection (Bridges et al., 1995) and inhibited Rauscher murine leukemia virus infection in mice (Ruprechat et al., 1989), the development of imino sugars for treatment of chronic human viral diseases, such as chronic hepatitis B, hepatitis C and AIDS, had been discouraging. This is because long term imino sugar treatment only resulted in mild reduction in viremia in a small proportion of treated individuals and brought limited clinical benefits in clinical trials of treating HIV and HCV-infected patients (Durantel, 2009, Fischl et al., 1994). Although these failures could be due to the use of early generations of imino sugar derivatives with low in vitro antiviral potency, another consideration is the different treatment goal for chronic and acute infection.
In contrast to the requirement of eradication or significant reduction in viremia for chronic viral infections, for many acute viral infections, such as viral hemorrhagic fever that can be caused by viruses from four different families, reduction of viremia by 1–2 logs in the early phase of infection could be life-saving (Noble et al., 2010, Vaughn et al., 2000). In addition, as mentioned in the previous section, antivirals broadly active against all four families of hemorrhagic fever viruses have a clear clinical advantage over direct-acting antiviral drugs that selectively inhibit specific viruses. It appears that imino sugars perfectly meet these criteria. Not surprisingly, as summarized in Table 2 , various imino sugar derivatives have thus far been tested in several mouse models of hemorrhagic fever virus infection, including DENV, EBOV and MARV, and demonstrated significant antiviral potency.
Table 2.
Broad-spectrum antiviral activity of imino sugars against multiple hemorrhagic fever viruses of Flaviviridae and Filoviridae families, in small animal models.
| Compound | Virus/family (virus strain) | Animal model | End point | Administration route | Efficacious dosea | Initiation, durationb | Reference |
|---|---|---|---|---|---|---|---|
| DNJ derivatives | |||||||
| IHVR17028 IHVR19029 |
Marburg Filoviridae (Mouse adapted RAVV) | BALB/c mouse | Survival | i.p. | 50 mg/kg, bid 75 mg/kg, bid |
d−1, 10 days +4 h, 10 days |
Chang et al. (2013) |
| IHVR11029 IHVR17028 IHVR19029 |
Ebola Filoviridae (Mouse adapted ebolavirus) | C57Bl/6 mouse | Survival | i.p. | 25 mg/kg, bid 25 mg/kg, bid 75 mg/kg, bid |
+4 h, 10 days | Chang et al. (2013) |
| UV-4 | Dengue-2 Flaviviridae (S221) | AG129 mouse | Survival viremia | i.g. | 5–100 mg/kg, tid | d0 to +2, 7 days | Perry et al. (2013) |
| NB-DNJ | Dengue-2 Flaviviridae (D2S10) | AG129 mouse | Survival | i.p. | 500 mg/kg, bid 0.088 mg/kg, qd, formulated in liposome |
d0, 7 days | Miller et al. (2012) |
| CM-10-18 | Dengue-2 Flaviviridae (D2S10 and D2Y98P-rc) | AG129 mouse | Survival | Oral | 10–150 mg/kg, bid | d0, 3 days | Chang et al. (2011b) |
| CM-10-18 CM-9-78 |
Dengue-2 Flaviviridae (TSV01) | AG129 mouse | Viremia | Oral | 75 mg/kg, bid | d0, 3 days | Chang et al. (2011a) |
| NN-DNJ | Dengue-2 Flaviviridae (TSV01) | AG129 mouse | Viremia | oral | 75 mg/kg, bid | d0, 3 days | Schul et al. (2007) |
| NN-DNJ | Japanese Encephalitis Flaviviridae (RP-9) | ICR mouse | Survival | Oral | 200 mg/kg, qd | d−1 | Wu et al. (2002) |
| Castanospermine derivative | |||||||
| Bu-CAST | Dengue-2 Flaviviridae (S221) | AG129 mouse | survival viremia | i.p. | 25–50 mg/kg, bid | d0 to +2, 5 days | Rathore et al., 2011, Watanabe et al., 2012 |
| CAST | Dengue-2 Flaviviridae (S221) | AG129 mouse | Survival | i.p. | 50 mg/kg, bid | d0, 5 days | Watanabe et al. (2012) |
| Bu-CAST | Dengue-2 Flaviviridae (TSV01) | AG129 mouse | Viremia | Oral | 75 mg/kg, bid | d0, 3 days | Schul et al. (2007) |
| CAST | Dengue-2 Flaviviridae (mouse-adapted DEN-2) | A/J mouse | Survival | i.p. | 50–250 mg/kg, qd | d0, 10 days | Whitby et al. (2005) |
Doses protected at least 50% of lethal infection.
d − 1 = 1 day prior to infection; d0 = at the time of infection; d + 1 = 1 day post infection.
Dengue hemorrhagic fever is the most common viral hemorrhagic fever and threatens more than one-third of world population without an approved antiviral therapy. As shown in Table 2, several imino sugar derivatives have been demonstrated by multiple research groups to be efficacious in both reducing DENV viremia and preventing lethality in mouse infection models. Particularly, significant protection against lethal dengue virus infection could be observed with both DNJ derivatives and Bu-CAST, when treatment was initiated up to 2 days after infection, suggesting their therapeutic value as post exposure treatment. Furthermore, we recently demonstrated that imino sugars IHVR17028 and IHVR19029 could efficiently protect mice from lethal dose challenge of EBOV and MARV, the two most dreaded hemorrhagic fever viruses.
From these studies, it becomes clear that in majority of the cases, in order to achieve close to 100% protection against lethal infection and/or significant reduction in viremia, greater than 50 mg/kg, twice-daily dosing of imino sugars is required. While as low as 10 mg/kg, three times a day also resulted in significant protection, 100 mg/kg once daily did not provide any benefit. These results are consistent with the relative short half-life of these imino sugar compounds (Chang et al., 2011a, Chang et al., 2013, Perry et al., 2013, Watanabe et al., 2012).
In an effort to confirm the therapeutic mechanism of imino sugars in vivo, we demonstrated that the antiviral efficacy of imino sugars was correlated with their inhibitory effect on the ER α-glucosidases, as suggested by analyzing protein-free oligosaccharides (FOS) in the serum of treated animals (Block et al., 1998, Chang et al., 2011a, Chang et al., 2013). Specifically, as a result of ER α-glucosidases inhibition, hyperglucosylated FOS with terminal glucose retention (such as Glc3Man7GlcNac2 or Glc1Man4GlcNac1) were observed to accumulate in treated animals, and thus supports the notion that the antiviral effect is likely through the proposed antiviral mechanism in vivo.
However, while the imino sugar derivatives efficiently protected against lethal DENV infection, the compounds only reduced the peak viremia in the infected mice by less than 1-log, except that, in one study, greater than 2-log reduction in viremia at a post peak time point, and at least 1-log reduction in tissue virus load were recorded (Chang et al., 2011a, Perry et al., 2013, Schul et al., 2007, Watanabe et al., 2012). These observations raise the possibility that imino sugar derivatives prevent the DENV-induced death of mice not only by inhibiting virus propagation, but also through suppression of viral pathogenesis. This later possibility is particularly consistent with the critical role of the interaction between DENV envelope glycans and the host cellular pattern-recognition receptor, C-type lectin, in DENV pathogenesis in mice (Chen et al., 2008).
It is generally acknowledged that massive production of inflammatory cytokines, secondary to macrophage and dendritic cell infection by the hemorrhagic fever viruses, plays an important role in the pathogenesis of viral hemorrhagic fever (Geisbert and Jahrling, 2004). However, the molecular mechanisms of the virus-induced “cytokine storm” and the cytokin mediated blood vessel leakage and other tissue damage remain elusive for most of the hemorrhagic fever viruses. In the case of DENV infection, it has been demonstrated with mouse embryonic fibroblasts derived from RIG-I- and MDA5-knockout mice that DENV infection is sequentially recognized by two cytoplasmic RNA helicase receptors, RIG-I- and MDA5. Sensing of DENV infection by these two receptors results in activation of both type I IFN and proinflammatory cytokine expression via their adaptor protein IPS-1 (or MAVS, Cardif and VISA)-dependent activation of IRF3 and NF-kB signaling pathways (Loo et al., 2008). However, DENV infection of macrophages induces a strong type I IFN and proinflammatory cytokine response by simultaneous activation of the plasma membrane C-type lectin receptor CLEC5A that recognizes oligosaccharides on the viral envelope glycoproteins and endosomal TLR7 that presumably recognizes viral genomic RNA (Chen et al., 2008). As illustrated in Fig. 3 , engagement of the two pattern-recognition receptors by the viral components activates signaling cascades by recruiting their distinct adaptor molecules DAP12 and MyD88 to induce proinflammatory cytokines and type I interferons in macrophages. The critical role of CLEC5A and proinflammatory cytokines in DENV pathogenesis was elegantly demonstrated by blockade of CLEC5A–DENV interaction with anti-CLEC5A monoclonal antibodies, which not only inhibited the production of proinflammatory cytokines, but also alleviated subcutaneous and vital-organ hemorrhagic symptoms and reduced the mortality of infected mice (Chen et al., 2008). Hence, it is entirely possible that by reducing DENV secretion and/or altering the glycan structure of the viral envelope glycoprotein, imino sugar derivatives might inhibit CLEC5A-mediated inflammatory cytokine production and consequentially alleviate viral pathogenesis. Indeed, several studies have demonstrated significant reduction in inflammatory cytokine levels upon treatment of DENV infected animals with imino sugars (Perry et al., 2013, Schul et al., 2007). Therefore, the potential anti-inflammatory activity of imino sugar ER α-glucosidase inhibitors should be further investigated in DENV and other hemorrhagic fever virus infections.
Fig. 3.
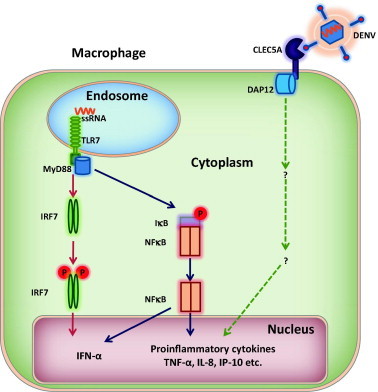
Illustration of DENV infection activated innate immune response in macrophages.
5. Antiviral efficacy and safety profiles of Bu-CAST and NB-DNJ in clinical trials
Imino sugar derivatives, such as Miglitol (Glyset®) and Miglustat (Zavesca®, NB-DNJ), have been approved by regulatory authorities for the treatment of non-insulin-dependent diabetes and lysosomal storage diseases (Gaucher’s disease), respectively. For antiviral applications, Bu-CAST (Celgosivir) was evaluated in clinical trials against HIV (NCT00002150 and NCT00002151) and HCV (NCT00157534) infections. In both cases, only modest antiviral effects were reported. While the trial for HIV was discontinued, Bu-CAST was further evaluated in a Phase IIb trial (NCT00332176) and demonstrated a synergistic effect in combination with pegylated IFN-α2b plus ribavirin in patients with HCV infection (Durantel, 2009). However, it was unclear where in the development path the agent is currently positioned, given the availability of much more potent drugs for this condition. Recently, a randomized, double-blind, placebo-controlled, Phase Ib clinical study to evaluate the activity, pharmacokinetics, safety and tolerability of Bu-CAST in adults with confirmed dengue fever (NCT01619969) was initiated in Singapore, based on efficacy in DEVN infected mouse models (Rathore et al., 2011, Schul et al., 2007, Watanabe et al., 2012).
Among the DNJ derivatives, NB-DNJ (Zavesca, Miglustat) was evaluated in Phase II trials (NCT00001993, NCT00002079) for HIV infection with limited efficacy, mainly due to its low potency and the difficulty in achieving steady-state therapeutic concentrations in vivo (Fischl et al., 1994). None of the new generation of DNJ derivatives, with demonstrated significantly improved antiviral potency, has reached the point of clinical trials. Considering the antiviral potency of imino sugar and the treatment goal for different viral infections, the most likely therapeutic applications of the DNJ derivatives should be acute viral infections, particularly viral hemorrhagic fevers and respiratory tract viral infections. The promising antiviral efficacy against DENV and filoviruses in mice warrants their further development as broad-spectrum antiviral agents for hemorrhagic fever viruses. In addition, the potent protective effect of a DNJ derivative UV-4 (Fig. 2) against H1N1 influenza A virus-infected mice (Ramstedt et al. patent number 20110065752. http://www.faqs.org/patents/app/20110065752) and potent antiviral activity and unique antiviral mechanism of other DNJ derivatives against SARS-CoV (Fukushi et al., 2012) also make the imino sugar derivatives potential broad-spectrum antiviral agents against respiratory enveloped RNA virus infections.
In addition to therapeutic efficacy, important safety information has been obtained from these clinical trials. In general, Bu-Cast was well tolerated in the 12–24 week treatment, at 400–600 mg/day dose, without serious adverse events. Mild to moderate, reversible gastrointestinal symptoms, including flatulence and diarrhea, were reported by about half of the treated individuals, which could be controlled with anti-diarrhea agents and with a low-sucrose/starch and high-glucose diet (Durantel, 2009) (Roth et al., 1996 Int. Conf. AIDS). A similar degree of gastrointestinal side effects was also observed in the NB-DNJ trial (Tierney et al., 1995).
6. Approaches toward improved ER-alpha glucosidase-targeted antiviral therapies
Both DNJ- and CAST-derivatives, the two currently available pharmacophores of ER α-glucosidase inhibitors, are glucose mimetics that not only inhibit ER α-glucosidases, but also other host glucosidases using glucose as substrate. These include intestinal disaccharidases, such as maltase, lactase and sucrose, the enzymes that hydrolyze ingested carbohydrates from disaccharides to monosaccharides (Reuser and Wisselaar, 1994). Not surprisingly, one of the common side-effects of these antiviral compounds is intestinal stress and osmotic diarrhea. Because intestinal disaccharidases localize on the brush border of the small intestine with their active sites facing the lumen, this side effect is largely associated with oral administration of imino sugars, and therefore, could be avoided by the parenteral route of administration. Alternatively, orally available prodrugs of imino sugars can be explored. For example, Bu-CAST is an oral prodrug of CAST which is rapidly converted into CAST in the body. Another approach is ester prodrugs of DNJ that are hydrolyzed in the circulation and intracellular compartments by ubiquitous esterases, thus avoiding inhibition of gut glucosidases (Liederer and Borchardt, 2006). A perbutyrylated ester prodrug of NB-DNJ has been demonstrated to improve the safety profile of the parent compound, however, its bioavailability was compromised in some species of animals, due to the less efficient conversion rate (Cook et al., 1995). In addition, the prodrug approach could increase the exposure of the drug in plasma to achieve an efficacious dose, and improve the metabolic stability of drug to increase the half-life. In the future, other approaches should be explored to optimize the safety profile and the bioconversion of imino sugar prodrugs.
In principle, the antiviral efficacy and safety profiles of imino sugars can be further improved by computer-assisted design of novel imino sugar derivatives that more potently and selectively inhibit human ER α-glucosidases. Achievement of this goal should be facilitated by the recent determination of the crystal structure of a yeast ER α-glucosidase (Barker and Rose, 2013). In addition, ER-targeted delivery and design of imino sugar derivatives with increased hydrophobicity and ER distribution (Du et al., 2013) are also viable approaches to improving the antiviral efficacy of imino sugars. Indeed, it has been demonstrated that targeted delivery of imino sugars with liposomes to the ER membrane immensely improved their antiviral activity both in cultured cells and in vivo in mice against DENV (Miller et al., 2012, Pollock et al., 2008).
Moreover, in order to improve ER α-glucosidase targeted antiviral therapy, imino sugars should not be the only chemical space to be explored. The most selective inhibitors of ER α-glucosidases may not be the substrate analogues targeting the catalytic center that may be similar between ER glucosidases and intestinal glucosidases, but compounds that target allosteric sites unique to ER α-glucosidases. In fact, this approach has been explored by others for the identification of gut maltase-specific inhibitors for the treatment of diabetes (Hakamata et al., 2012). Development of a convenient fluorescent substrate-based in vitro enzymatic assay for ER α-glucosidases will be the first step towards discovery of novel allosteric inhibitors, which may ultimately improve ER α-glucosidase-targeted antiviral therapy (Hakamata et al., 2009).
In summary, the cellular ER α-glucosidases are among a few host enzymes that have been demonstrated to be validated targets for a broad spectrum of enveloped viruses, particularly those that cause hemorrhagic fever. However, the currently available imino sugar derivatives are still not highly efficacious, producing only a modest reduction in viremia, while requiring high and frequent doses, and have mild side effects due to the non-selective inhibition of gut glucosidases. In the future, a combined effort of rationally designed chemical modification, ER-targeted drug delivery and prodrug design should further improve the efficacy and safety profiles of imino sugars, and ultimately pave the way to the clinic. In addition, the discovery of novel allosteric ER α-glucosidase inhibitors should be a new direction towards improving ER α-glucosidase-targeted antiviral therapy.
Acknowledgements
Preparation of this manuscript was supported in part by NIH grant (AI232758), The Hepatitis B Foundation and The Commonwealth of Pennsylvania.
References
- Ahmed S.P., Nash R.J., Bridges C.G., Taylor D.L., Kang M.S., Porter E.A., Tyms A.S. Antiviral activity and metabolism of the castanospermine derivative MDL 28,574, in cells infected with herpes simplex virus type 2. Biochem. Biophys. Res. Commun. 1995;208:267–273. doi: 10.1006/bbrc.1995.1333. [DOI] [PubMed] [Google Scholar]
- Alonzi, D.S., Kukushkin, N.V., Allman, S.A., Hakki, Z., Williams, S.J., Pierce, L., Dwek, R.A., Butters, T.D., 2013. Glycoprotein misfolding in the endoplasmic reticulum:identification of released oligosaccharides reveals a second ER-associated degradation pathway for Golgi-retrieved proteins. Cell. Mol. Life Sci. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Asano N., Kizu H., Oseki K., Tomioka E., Matsui K., Okamoto M., Baba M. N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J. Med. Chem. 1995;38:2349–2356. doi: 10.1021/jm00013a012. [DOI] [PubMed] [Google Scholar]
- Barker M.K., Rose D.R. Specificity of processing alpha-glucosidase I is guided by the substrate conformation: crystallographic and in silico studies. J. Biol. Chem. 2013;288:13563–13574. doi: 10.1074/jbc.M113.460436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bause E., Gunther R., Schweden J., Tillmann U. In vitro studies on the subcellular location of glucosidase I and glucosidase II in dog pancreas. Biosci. Rep. 1986;6:827–834. doi: 10.1007/BF01117106. [DOI] [PubMed] [Google Scholar]
- Block T.M., Lu X., Platt F.M., Foster G.R., Gerlich W.H., Blumberg B.S., Dwek R.A. Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin. Proc. Natl. Acad. Sci. USA. 1994;91:2235–2239. doi: 10.1073/pnas.91.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block T.M., Lu X., Mehta A.S., Blumberg B.S., Tennant B., Ebling M., Korba B., Lansky D.M., Jacob G.S., Dwek R.A. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nat. Med. 1998;4:610–614. doi: 10.1038/nm0598-610. [DOI] [PubMed] [Google Scholar]
- Bolt G., Pedersen I.R., Blixenkrone-Moller M. Processing of N-linked oligosaccharides on the measles virus glycoproteins: importance for antigenicity and for production of infectious virus particles. Virus Res. 1999;61:43–51. doi: 10.1016/s0168-1702(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Bray M., Driscoll J., Huggins J.W. Treatment of lethal Ebola virus infection in mice with a single dose of an S-adenosyl-L-homocysteine hydrolase inhibitor. Antiviral Res. 2000;45:135–147. doi: 10.1016/s0166-3542(00)00066-8. [DOI] [PubMed] [Google Scholar]
- Bray M., Raymond J.L., Geisbert T., Baker R.O. 3-Deazaneplanocin A induces massively increased interferon-alpha production in Ebola virus-infected mice. Antiviral Res. 2002;55:151–159. doi: 10.1016/s0166-3542(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Bridges C.G., Brennan T.M., Taylor D.L., McPherson M., Tyms A.S. The prevention of cell adhesion and the cell-to-cell spread of HIV-1 in vitro by the alpha-glucosidase 1 inhibitor, 6-O-butanoyl castanospermine (MDL 28574) Antiviral Res. 1994;25:169–175. doi: 10.1016/0166-3542(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Bridges C.G., Ahmed S.P., Kang M.S., Nash R.J., Porter E.A., Tyms A.S. The effect of oral treatment with 6-O-butanoyl castanospermine (MDL 28,574) in the murine zosteriform model of HSV-1 infection. Glycobiology. 1995;5:249–253. doi: 10.1093/glycob/5.2.249. [DOI] [PubMed] [Google Scholar]
- Burke B., Matlin K., Bause E., Legler G., Peyrieras N., Ploegh H. Inhibition of N-linked oligosaccharide trimming does not interfere with surface expression of certain integral membrane proteins. EMBO J. 1984;3:551–556. doi: 10.1002/j.1460-2075.1984.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Wang L., Ma D., Qu X., Guo H., Xu X., Mason P.M., Bourne N., Moriarty R., Gu B., Guo J.T., Block T.M. Novel imino sugar derivatives demonstrate potent antiviral activity against flaviviruses. Antimicrob. Agents Chemother. 2009;53:1501–1508. doi: 10.1128/AAC.01457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Schul W., Butters T.D., Yip A., Liu B., Goh A., Lakshminarayana S.B., Alonzi D., Reinkensmeier G., Pan X., Qu X., Weidner J.M., Wang L., Yu W., Borune N., Kinch M.A., Rayahin J.E., Moriarty R., Xu X., Shi P.Y., Guo J.T., Block T.M. Combination of alpha-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antiviral Res. 2011;89:26–34. doi: 10.1016/j.antiviral.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Schul W., Yip A., Xu X., Guo J.T., Block T.M. Competitive inhibitor of cellular alpha-glucosidases protects mice from lethal dengue virus infection. Antiviral Res. 2011;92:369–371. doi: 10.1016/j.antiviral.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Warren T.K., Zhao X., Gill T., Guo F., Wang L., Comunale M.A., Du Y., Alonzi D.S., Yu W., Ye H., Liu F., Guo J.T., Mehta A., Cuconati A., Butters T.D., Bavari S., Xu X., Block T.M. Small molecule inhibitors of ER alpha-glucosidases are active against multiple hemorrhagic fever viruses. Antiviral Res. 2013;98:432–440. doi: 10.1016/j.antiviral.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapel C., Garcia C., Roingeard P., Zitzmann N., Dubuisson J., Dwek R.A., Trepo C., Zoulim F., Durantel D. Antiviral effect of alpha-glucosidase inhibitors on viral morphogenesis and binding properties of hepatitis C virus-like particles. J. Gen. Virol. 2006;87:861–871. doi: 10.1099/vir.0.81503-0. [DOI] [PubMed] [Google Scholar]
- Chapman T.M., Davies I.G., Gu B., Block T.M., Scopes D.I., Hay P.A., Courtney S.M., McNeill L.A., Schofield C.J., Davis B.G. Glyco- and peptidomimetics from three-component Joullie-Ugi coupling show selective antiviral activity. J. Am. Chem. Soc. 2005;127:506–507. doi: 10.1021/ja043924l. [DOI] [PubMed] [Google Scholar]
- Chen S.T., Lin Y.L., Huang M.T., Wu M.F., Cheng S.C., Lei H.Y., Lee C.K., Chiou T.W., Wong C.H., Hsieh S.L. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453:672–676. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- Cook C.S., Karabatsos P.J., Schoenhard G.L., Karim A. Species dependent esterase activities for hydrolysis of an anti-HIV prodrug glycovir and bioavailability of active SC-48334. Pharm. Res. 1995;12:1158–1164. doi: 10.1023/a:1016259826037. [DOI] [PubMed] [Google Scholar]
- Courageot M.P., Frenkiel M.P., Dos Santos C.D., Deubel V., Despres P. Alpha-glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum. J. Virol. 2000;74:564–572. doi: 10.1128/jvi.74.1.564-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R., Romero P.A., Rott R., Schwarz R.T. On the role of oligosaccharide trimming in the maturation of Sindbis and influenza virus. Arch. Virol. 1984;81:25–39. doi: 10.1007/BF01309294. [DOI] [PubMed] [Google Scholar]
- de Chassey B., Meyniel-Schicklin L., Aublin-Gex A., Andre P., Lotteau V. New horizons for antiviral drug discovery from virus–host protein interaction networks. Curr. Opin. Virol. 2012;2:606–613. doi: 10.1016/j.coviro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antivirals and antiviral strategies. Nat. Rev. Microbiol. 2004;2:704–720. doi: 10.1038/nrmicro975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Ye H., Gill T., Wang L., Guo F., Cuconati A., Guo J.T., Block T.M., Chang J., Xu X. N-Alkyldeoxynojirimycin derivatives with novel terminal tertiary amide substitution for treatment of bovine viral diarrhea virus (BVDV), Dengue, and Tacaribe virus infections. Bioorg. Med. Chem. Lett. 2013;23:4258–4262. doi: 10.1016/j.bmcl.2013.01.108. [DOI] [PubMed] [Google Scholar]
- Durantel D. Celgosivir, an alpha-glucosidase I inhibitor for the potential treatment of HCV infection. Curr. Opin. Investig. Drugs. 2009;10:860–870. [PubMed] [Google Scholar]
- Dwek R.A., Butters T.D., Platt F.M., Zitzmann N. Targeting glycosylation as a therapeutic approach. Nat. Rev. Drug Discovery. 2002;1:65–75. doi: 10.1038/nrd708. [DOI] [PubMed] [Google Scholar]
- Fischer P.B., Collin M., Karlsson G.B., James W., Butters T.D., Davis S.J., Gordon S., Dwek R.A., Platt F.M. The alpha-glucosidase inhibitor N-butyldeoxynojirimycin inhibits human immunodeficiency virus entry at the level of post-CD4 binding. J. Virol. 1995;69:5791–5797. doi: 10.1128/jvi.69.9.5791-5797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P.B., Karlsson G.B., Butters T.D., Dwek R.A., Platt F.M. N-butyldeoxynojirimycin-mediated inhibition of human immunodeficiency virus entry correlates with changes in antibody recognition of the V1/V2 region of gp120. J. Virol. 1996;70:7143–7152. doi: 10.1128/jvi.70.10.7143-7152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P.B., Karlsson G.B., Dwek R.A., Platt F.M. N-butyldeoxynojirimycin-mediated inhibition of human immunodeficiency virus entry correlates with impaired gp120 shedding and gp41 exposure. J. Virol. 1996;70:7153–7160. doi: 10.1128/jvi.70.10.7153-7160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl M.A., Resnick L., Coombs R., Kremer A.B., Pottage J.C., Jr., Fass R.J., Fife K.H., Powderly W.G., Collier A.C., Aspinall R.L. The safety and efficacy of combination N-butyl-deoxynojirimycin (SC-48334) and zidovudine in patients with HIV-1 infection and 200–500 CD4 cells/mm3. J. Acquir. Immune Defic. Syndr. 1994;7:139–147. [PubMed] [Google Scholar]
- Fleet G.W., Karpas A., Dwek R.A., Fellows L.E., Tyms A.S., Petursson S., Namgoong S.K., Ramsden N.G., Smith P.W., Son J.C. Inhibition of HIV replication by amino-sugar derivatives. FEBS Lett. 1988;237:128–132. doi: 10.1016/0014-5793(88)80185-6. [DOI] [PubMed] [Google Scholar]
- Fukushi M., Yoshinaka Y., Matsuoka Y., Hatakeyama S., Ishizaka Y., Kirikae T., Sasazuki T., Miyoshi-Akiyama T. Monitoring of S protein maturation in the endoplasmic reticulum by calnexin is important for the infectivity of severe acute respiratory syndrome coronavirus. J. Virol. 2012;86:11745–11753. doi: 10.1128/JVI.01250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Shiraki K., Sakamoto K., Smee D.F., Barnard D.L., Gowen B.B., Julander J.G., Morrey J.D. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T.W., Jahrling P.B. Exotic emerging viral diseases: progress and challenges. Nat. Med. 2004;10:S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Geller R., Taguwa S., Frydman J. Broad action of Hsp90 as a host chaperone required for viral replication. Biochim. Biophys. Acta. 2012;1823:698–706. doi: 10.1016/j.bbamcr.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B., Mason P., Wang L., Norton P., Bourne N., Moriarty R., Mehta A., Despande M., Shah R., Block T. Antiviral profiles of novel iminocyclitol compounds against bovine viral diarrhea virus, West Nile virus, dengue virus and hepatitis B virus. Antiviral Chem. Chemother. 2007;18:49–59. doi: 10.1177/095632020701800105. [DOI] [PubMed] [Google Scholar]
- Hakamata W., Kurihara M., Okuda H., Nishio T., Oku T. Design and screening strategies for alpha-glucosidase inhibitors based on enzymological information. Curr. Top. Med. Chem. 2009;9:3–12. doi: 10.2174/156802609787354306. [DOI] [PubMed] [Google Scholar]
- Hakamata W., Ishikawa R., Ushijima Y., Tsukagoshi T., Tamura S., Hirano T., Nishio T. Virtual ligand screening of alpha-glucosidase: identification of a novel potent noncarbohydrate mimetic inhibitor. Bioorg. Med. Chem. Lett. 2012;22:62–64. doi: 10.1016/j.bmcl.2011.11.084. [DOI] [PubMed] [Google Scholar]
- Hammond C., Braakman I., Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc. Natl. Acad. Sci. USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert D.N., Foellmer B., Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helenius A., Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Hempel A., Camerman N., Mastropaolo D., Camerman A. Glucosidase inhibitors: structures of deoxynojirimycin and castanospermine. J. Med. Chem. 1993;36:4082–4086. doi: 10.1021/jm00077a012. [DOI] [PubMed] [Google Scholar]
- Hong-Geller E., Micheva-Viteva S.N. Functional gene discovery using RNA interference-based genomic screens to combat pathogen infection. Curr. Drug Discov. Technol. 2010;7:86–94. doi: 10.2174/157016310793180657. [DOI] [PubMed] [Google Scholar]
- Horne G., Wilson F.X., Tinsley J., Williams D.H., Storer R. Iminosugars past, present and future: medicines for tomorrow. Drug Discovery Today. 2011;16:107–118. doi: 10.1016/j.drudis.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Howe, J.D., Smith, N., Lee, M.J., Ardes-Guisot, N., Vauzeilles, B., Desire, J., Baron, A., Bleriot, Y., Sollogoub, M., Alonzi, D.S., Butters, T.D., 2013. Novel imino sugar alpha-glucosidase inhibitors as antiviral compounds. Bioorg. Med. Chem. [Epub ahead of print]. [DOI] [PubMed]
- Hussell T., Godlee A., Salek-Ardakani S., Snelgrove R.J. Respiratory viral infections: knowledge based therapeutics. Curr. Opin. Immunol. 2012;24:438–443. doi: 10.1016/j.coi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Inoue K., Umehara T., Ruegg U.T., Yasui F., Watanabe T., Yasuda H., Dumont J.M., Scalfaro P., Yoshiba M., Kohara M. Evaluation of a cyclophilin inhibitor in hepatitis C virus-infected chimeric mice in vivo. Hepatology. 2007;45:921–928. doi: 10.1002/hep.21587. [DOI] [PubMed] [Google Scholar]
- Jartti T., Jartti L., Ruuskanen O., Soderlund-Venermo M. New respiratory viral infections. Curr. Opin. Pulm. Med. 2012;18:271–278. doi: 10.1097/MCP.0b013e328351f8d4. [DOI] [PubMed] [Google Scholar]
- Jordan R., Nikolaeva O.V., Wang L., Conyers B., Mehta A., Dwek R.A., Block T.M. Inhibition of host ER glucosidase activity prevents Golgi processing of virion-associated bovine viral diarrhea virus E2 glycoproteins and reduces infectivity of secreted virions. Virology. 2002;295:10–19. doi: 10.1006/viro.2002.1370. [DOI] [PubMed] [Google Scholar]
- Kajimoto T., Node M. Inhibitors against glycosidases as medicines. Curr. Top. Med. Chem. 2009;9:13–33. doi: 10.2174/156802609787354333. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Repges S., McDowell W. The significance of carbohydrate trimming for the antigenicity of the Semliki Forest virus glycoprotein E2. Virology. 1990;176:369–378. doi: 10.1016/0042-6822(90)90007-e. [DOI] [PubMed] [Google Scholar]
- Karpas A., Fleet G.W., Dwek R.A., Petursson S., Namgoong S.K., Ramsden N.G., Jacob G.S., Rademacher T.W. Aminosugar derivatives as potential anti-human immunodeficiency virus agents. Proc. Natl. Acad. Sci. USA. 1988;85:9229–9233. doi: 10.1073/pnas.85.23.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar C., Durantel D., Macovei A., Zitzmann N., Zoulim F., Dwek R.A., Branza-Nichita N. Treatment of hepatitis B virus-infected cells with alpha-glucosidase inhibitors results in production of virions with altered molecular composition and infectivity. Antiviral Res. 2007;76:30–37. doi: 10.1016/j.antiviral.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Lee D., Woo J.K., Kim D., Kim M., Cho S.K., Kim J.H., Park S.P., Lee H.Y., Riu K.Z., Lee D.S. Antiviral activity of methylelaiophylin, an alpha-glucosidase inhibitor. J. Microbiol. Biotechnol. 2011;21:263–266. [PubMed] [Google Scholar]
- Liang P.H., Cheng W.C., Lee Y.L., Yu H.P., Wu Y.T., Lin Y.L., Wong C.H. Novel five-membered iminocyclitol derivatives as selective and potent glycosidase inhibitors: new structures for antivirals and osteoarthritis. ChemBioChem. 2006;7:165–173. doi: 10.1002/cbic.200500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liederer B.M., Borchardt R.T. Enzymes involved in the bioconversion of ester-based prodrugs. J. Pharm. Sci. 2006;95:1177–1195. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]
- Loo Y.M., Fornek J., Crochet N., Bajwa G., Perwitasari O., Martinez-Sobrido L., Akira S., Gill M.A., Garcia-Sastre A., Katze M.G., Gale M., Jr. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Lu Y., Geschwindt R., Dwek R.A., Block T.M. Hepatitis B virus MHBs antigen is selectively sensitive to glucosidase-mediated processing in the endoplasmic reticulum. DNA Cell Biol. 2001;20:647–656. doi: 10.1089/104454901753340631. [DOI] [PubMed] [Google Scholar]
- McLaughlin M., Vandenbroeck K. The endoplasmic reticulum protein folding factory and its chaperones: new targets for drug discovery? Br. J. Pharmacol. 2011;162:328–345. doi: 10.1111/j.1476-5381.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., Carrouee S., Conyers B., Jordan R., Butters T., Dwek R.A., Block T.M. Inhibition of hepatitis B virus DNA replication by imino sugars without the inhibition of the DNA polymerase: therapeutic implications. Hepatology. 2001;33:1488–1495. doi: 10.1053/jhep.2001.25103. [DOI] [PubMed] [Google Scholar]
- Mehta A., Ouzounov S., Jordan R., Simsek E., Lu X., Moriarty R.M., Jacob G., Dwek R.A., Block T.M. Imino sugars that are less toxic but more potent as antivirals, in vitro, compared with N-n-nonyl DNJ. Antiviral Chem. Chemother. 2002;13:299–304. doi: 10.1177/095632020201300505. [DOI] [PubMed] [Google Scholar]
- Meliopoulos V.A., Andersen L.E., Birrer K.F., Simpson K.J., Lowenthal J.W., Bean A.G., Stambas J., Stewart C.R., Tompkins S.M., van Beusechem V.W., Fraser I., Mhlanga M., Barichievy S., Smith Q., Leake D., Karpilow J., Buck A., Jona G., Tripp R.A. Host gene targets for novel influenza therapies elucidated by high-throughput RNA interference screens. FASEB J. 2012;26:1372–1386. doi: 10.1096/fj.11-193466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.L., Lachica R., Sayce A.C., Williams J.P., Bapat M., Dwek R., Beatty P.R., Harris E., Zitzmann N. Liposome-mediated delivery of iminosugars enhances efficacy against dengue virus in vivo. Antimicrob. Agents Chemother. 2012;56:6379–6386. doi: 10.1128/AAC.01554-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy N.S., Ramos M.J., Fernandes P.A. Studies on alpha-glucosidase inhibitors development: magic molecules for the treatment of carbohydrate mediated diseases. Mini Rev. Med. Chem. 2012;12:713–720. doi: 10.2174/138955712801264837. [DOI] [PubMed] [Google Scholar]
- Nash R.J., Kato A., Yu C.Y., Fleet G.W. Iminosugars as therapeutic agents: recent advances and promising trends. Future Med. Chem. 2011;3:1513–1521. doi: 10.4155/fmc.11.117. [DOI] [PubMed] [Google Scholar]
- Noble C.G., Chen Y.L., Dong H., Gu F., Lim S.P., Schul W., Wang Q.Y., Shi P.Y. Strategies for development of dengue virus inhibitors. Antiviral Res. 2010;85:450–462. doi: 10.1016/j.antiviral.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Paessler S., Walker D.H. Pathogenesis of the viral hemorrhagic fevers. Annu. Rev. Pathol. 2013;8:411–440. doi: 10.1146/annurev-pathol-020712-164041. [DOI] [PubMed] [Google Scholar]
- Perry S.T., Buck M.D., Plummer E.M., Penmasta R.A., Batra H., Stavale E.J., Warfield K.L., Dwek R.A., Butters T.D., Alonzi D.S., Lada S.M., King K., Klose B., Ramstedt U., Shresta S. An iminosugar with potent inhibition of dengue virus infection in vivo. Antiviral Res. 2013;98:35–43. doi: 10.1016/j.antiviral.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Pollock S., Dwek R.A., Burton D.R., Zitzmann N. N-Butyldeoxynojirimycin is a broadly effective anti-HIV therapy significantly enhanced by targeted liposome delivery. AIDS. 2008;22:1961–1969. doi: 10.1097/QAD.0b013e32830efd96. [DOI] [PubMed] [Google Scholar]
- Qu X., Pan X., Weidner J., Yu W., Alonzi D., Xu X., Butters T., Block T., Guo J.T., Chang J. Inhibitors of endoplasmic reticulum {alpha}-glucosidases potently suppress hepatitis C virus virion assembly and release. Antimicrob. Agents Chemother. 2011;55:1036–1044. doi: 10.1128/AAC.01319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore A.P., Paradkar P.N., Watanabe S., Tan K.H., Sung C., Connolly J.E., Low J., Ooi E.E., Vasudevan S.G. Celgosivir treatment misfolds dengue virus NS1 protein, induces cellular pro-survival genes and protects against lethal challenge mouse model. Antiviral Res. 2011;92:453–460. doi: 10.1016/j.antiviral.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Repp R., Tamura T., Boschek C.B., Wege H., Schwarz R.T., Niemann H. The effects of processing inhibitors of N-linked oligosaccharides on the intracellular migration of glycoprotein E2 of mouse hepatitis virus and the maturation of coronavirus particles. J. Biol. Chem. 1985;260:15873–15879. doi: 10.1016/S0021-9258(17)36339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuser A.J., Wisselaar H.A. An evaluation of the potential side-effects of alpha-glucosidase inhibitors used for the management of diabetes mellitus. Eur. J. Clin. Invest. 1994;24(Suppl. 3):19–24. doi: 10.1111/j.1365-2362.1994.tb02251.x. [DOI] [PubMed] [Google Scholar]
- Ritchie G., Harvey D.J., Feldmann F., Stroeher U., Feldmann H., Royle L., Dwek R.A., Rudd P.M. Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology. 2010;399:257–269. doi: 10.1016/j.virol.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprechat R.M., Mullaney S., Andersen J., Bronson R. In vivo analysis of castanospermine, a candidate antiretroviral agent. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1989;2:149–157. [PubMed] [Google Scholar]
- Saito T., Yamaguchi I. Effect of glycosylation and glucose trimming inhibitors on the influenza A virus glycoproteins. J. Vet. Med. Sci. 2000;62:575–581. doi: 10.1292/jvms.62.575. [DOI] [PubMed] [Google Scholar]
- Sayce A.C., Miller J.L., Zitzmann N. Targeting a host process as an antiviral approach against dengue virus. Trends Microbiol. 2010;18:323–330. doi: 10.1016/j.tim.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Malfer C., Schlesinger M.J. The formation of vesicular stomatitis virus (San Juan strain) becomes temperature-sensitive when glucose residues are retained on the oligosaccharides of the glycoprotein. J. Biol. Chem. 1984;259:7597–7601. [PubMed] [Google Scholar]
- Schul W., Liu W., Xu H.Y., Flamand M., Vasudevan S.G. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J. Infect. Dis. 2007;195:665–674. doi: 10.1086/511310. [DOI] [PubMed] [Google Scholar]
- Schweden J., Borgmann C., Legler G., Bause E. Characterization of calf liver glucosidase I and its inhibition by basic sugar analogs. Arch. Biochem. Biophys. 1986;248:335–340. doi: 10.1016/0003-9861(86)90429-7. [DOI] [PubMed] [Google Scholar]
- Sessions O.M., Barrows N.J., Souza-Neto J.A., Robinson T.J., Hershey C.L., Rodgers M.A., Ramirez J.L., Dimopoulos G., Yang P.L., Pearson J.L., Garcia-Blanco M.A. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber A.M., Candurra N.A., Damonte E.B. The effects of oligosaccharide trimming inhibitors on glycoprotein expression and infectivity of Junin virus. FEMS Microbiol. Lett. 1993;109:39–43. doi: 10.1111/j.1574-6968.1993.tb06140.x. [DOI] [PubMed] [Google Scholar]
- Simsek E., Mehta A., Zhou T., Dwek R.A., Block T. Hepatitis B virus large and middle glycoproteins are degraded by a proteasome pathway in glucosidase-inhibited cells but not in cells with functional glucosidase enzyme. J. Virol. 2005;79:12914–12920. doi: 10.1128/JVI.79.20.12914-12920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann E., Whitfield T., Kallis S., Dwek R.A., Zitzmann N., Pietschmann T., Bartenschlager R. Antiviral effects of amantadine and iminosugar derivatives against hepatitis C virus. Hepatology. 2007;46:330–338. doi: 10.1002/hep.21686. [DOI] [PubMed] [Google Scholar]
- Taylor D.L., Fellows L.E., Farrar G.H., Nash R.J., Taylor-Robinson D., Mobberley M.A., Ryder T.A., Jeffries D.J., Tyms A.S. Loss of cytomegalovirus infectivity after treatment with castanospermine or related plant alkaloids correlates with aberrant glycoprotein synthesis. Antiviral Res. 1988;10:11–26. doi: 10.1016/0166-3542(88)90011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.L., Sunkara P.S., Liu P.S., Kang M.S., Bowlin T.L., Tyms A.S. 6-O-butanoylcastanospermine (MDL 28,574) inhibits glycoprotein processing and the growth of HIVs. AIDS. 1991;5:693–698. doi: 10.1097/00002030-199106000-00008. [DOI] [PubMed] [Google Scholar]
- Taylor D.L., Kang M.S., Brennan T.M., Bridges C.G., Sunkara P.S., Tyms A.S. Inhibition of alpha-glucosidase I of the glycoprotein-processing enzymes by 6-O-butanoyl castanospermine (MDL 28,574) and its consequences in human immunodeficiency virus-infected T cells. Antimicrob. Agents Chemother. 1994;38:1780–1787. doi: 10.1128/aac.38.8.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney M., Pottage J., Kessler H., Fischl M., Richman D., Merigan T., Powderly W., Smith S., Karim A., Sherman J. The tolerability and pharmacokinetics of N-butyl-deoxynojirimycin in patients with advanced HIV disease (ACTG 100). The AIDS clinical trials group (ACTG) of the national institute of allergy and infectious diseases. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995;10:549–553. [PubMed] [Google Scholar]
- Tundis R., Loizzo M.R., Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini Rev. Med. Chem. 2010;10:315–331. doi: 10.2174/138955710791331007. [DOI] [PubMed] [Google Scholar]
- Vaughn D.W., Green S., Kalayanarooj S., Innis B.L., Nimmannitya S., Suntayakorn S., Endy T.P., Raengsakulrach B., Rothman A.L., Ennis F.A., Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Watanabe S., Kawaoka Y. Cellular networks involved in the influenza virus life cycle. Cell Host Microbe. 2010;7:427–439. doi: 10.1016/j.chom.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Rathore A.P., Sung C., Lu F., Khoo Y.M., Connolly J., Low J., Ooi E.E., Lee H.S., Vasudevan S.G. Dose- and schedule-dependent protective efficacy of celgosivir in a lethal mouse model for dengue virus infection informs dosing regimen for a proof of concept clinical trial. Antiviral Res. 2012;96:32–35. doi: 10.1016/j.antiviral.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Whitby K., Pierson T.C., Geiss B., Lane K., Engle M., Zhou Y., Doms R.W., Diamond M.S. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J. Virol. 2005;79:8698–8706. doi: 10.1128/JVI.79.14.8698-8706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.F., Lee C.J., Liao C.L., Dwek R.A., Zitzmann N., Lin Y.L. Antiviral effects of an iminosugar derivative on flavivirus infections. J. Virol. 2002;76:3596–3604. doi: 10.1128/JVI.76.8.3596-3604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Gill T., Wang L., Du Y., Ye H., Qu X., Guo J.T., Cuconati A., Zhao K., Block T.M., Xu X., Chang J. Design, synthesis, and biological evaluation of N-alkylated deoxynojirimycin (DNJ) derivatives for the treatment of dengue virus infection. J. Med. Chem. 2012;55:6061–6075. doi: 10.1021/jm300171v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann N., Mehta A.S., Carrouee S., Butters T.D., Platt F.M., McCauley J., Blumberg B.S., Dwek R.A., Block T.M. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc. Natl. Acad. Sci. USA. 1999;96:11878–11882. doi: 10.1073/pnas.96.21.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]


