Abstract
Antimicrobial peptides (AMPs) are gaining importance as effective therapeutic alternatives to conventional antibiotics. Recently we have shown that a set of nine synthetic antimicrobial peptides, four originating from thrombin-induced human platelet-derived antimicrobial proteins named PD1–PD4 and five synthetic repeats of arginine-tryptophan (RW) repeats (RW1-5) demonstrate antibacterial activity in plasma and platelets. Using WR strain of vaccinia virus (VV) as a model virus for enveloped virus in the present study, we tested the same nine synthetic peptides for their antiviral activity. A cell culture-based standard plaque reduction assay was utilized to estimate antiviral effectiveness of the peptides. Our analysis revealed that peptides PD3, PD4, and RW3 were virucidal against VV with PD3 demonstrating the highest antiviral activity of 100-fold reduction in viral titers, whereas, PD4 and RW3 peptide treatments resulted in 10–30-fold reduction. The EC50 values of PD3, PD4 and RW3 were found to be 40 μg/ml, 50 μg/ml and 6.5 μM, respectively. In VV-spiked plasma samples, the virucidal activity of PD3, PD4 and RW3 was close to 100% (90–100-fold reduction). Overall, the present study constitutes a new proof-of-concept in developing peptide therapeutics for vaccinia virus infections in biothreat scenarios and as in vitro viral reduction agents.
Keywords: AMPs, RW peptides, Platelet-derived peptides, Microbicidal, pfu
1. Introduction
Ever since their discovery, antimicrobial peptides (AMPs) have been gaining attention as an important therapeutic intervention alternative in the field of disease prevention and care against a number of microbes (Brogden, 2005, Hancock and Sahl, 2006, Oyston et al., 2009, Zaiou, 2007). This can be mainly attributed to the rising microbial drug resistance, associated toxicity and higher production costs involved with conventional antimicrobial drugs. As a result of intense research in this field over the last decade, approximately 20,000 AMPs have so far been listed in the AMPs database (Brogden, 2005, Hancock and Sahl, 2006, Oyston et al., 2009, Zaiou, 2007). These peptides are either derived from nature (from microbes, vertebrates including humans) or are synthetic forms (Brogden, 2005, Hancock and Sahl, 2006, Zaiou, 2007). Each AMP could demonstrate their antimicrobial property either on a single class of organisms i.e., anti-bacterial, anti-viral, anti-parasitic, anti-fungal or on multiple classes of pathogens. The mechanism of action of many AMPs include either direct microbicidal activity or indirect action by blocking or inhibiting an important pathway in the microbial life cycle (Brogden, 2005, Chan et al., 2006, Hancock and Sahl, 2006, Oyston et al., 2009, Zaiou, 2007).
Though most of the currently reported AMPs are known to be either anti-bacterial or anti-fungal peptides, the number of antiviral peptides is slowly going up. Some of the AMPs that have been shown to be effective antivirals have been against viruses such as influenza A virus, severe-acute respiratory syndrome coronavirus (SARS-CoV), West Nile Virus (WNV), and other viruses (Bai et al., 2007, Basu et al., 2009, Chu et al., 2008, Daher et al., 1986, Guo et al., 2009, Oyston et al., 2009, Zaiou, 2007). Interestingly these antiviral peptides were originally either elicited due to an immune response by the host to the viral infection or virus-encoded (Bai et al., 2007, Basu et al., 2009, Chu et al., 2008, Daher et al., 1986, Guo et al., 2009, Oyston et al., 2009, Zaiou, 2007). Besides α-defensins two other classes of AMPs are produced by mammalian cells: β-defensins and cathelicidins (Gallo et al., 2002, Harder et al., 1997). Between the two, cathelicidin represented by LL-37 is the most famous antimicrobial peptide that has demonstrated potent anti-bacterial, anti-viral and anti-fungal properties (Dorschner et al., 2001, Howell et al., 2004). The α-defensins are known to inhibit replication of herpes simplex virus (HSV), cytomegalovirus (CMV), vesicular stomatitis virus (VSV) and influenza A virus (Daher et al., 1986). The mechanism of action of these peptides has been suggested to involve disruption of the microbial membrane and/or penetration of the microbial membranes to interfere with intracellular functions (Brogden, 2005, Harrison and Ramshaw, 2006, Howell et al., 2004, Liu et al., 2007, Tang et al., 2002). So far, the β-defensins, cathelicidins (LL-37), caragenins (synthetic AMPs), peptide mimetics of γ-interferon, the broad spectrum antiviral EB and peptide aptamers have been shown to possess antiviral activity against vaccinia virus (VV) (Ahmed et al., 2005, Altmann et al., 2009, Harrison and Ramshaw, 2006, Howell et al., 2004, Howell et al., 2007, Howell et al., 2009, Saccucci et al., 2009).
We have recently demonstrated the antibacterial activity of two types of AMPs, the thrombin-induced human platelet-derived antimicrobial peptides (PD) and the arginine-tryptophan (RW) repeat peptides, against aerobic bacterial contaminants encountered in blood products (Mohan et al., 2009a). The structure and mechanism of action of PD and RW peptides has been elucidated previously in detail and these peptides are also known to be non-cytotoxic and non-hemolytic (Chan et al., 2006, Liu et al., 2007, Yeaman et al., 2007, Yeaman and Bayer, 1999). Because vaccinia virus has been used as a model to test methods of decontaminating blood products, and it has been found to be relatively resistant to solvent/detergent inactivation methods, we selected this virus to evaluate the antiviral activity of PD and RW peptides (Lin et al., 2005, Remington et al., 2004, Roberts, 2000, Wu and Snyder, 2003). In the present study we evaluated the same PD and RW peptides that were previously evaluated against bacteria (Mohan et al., 2009a) for their antiviral activity against VV as a model virus for enveloped viruses and observed that PD and RW peptides do demonstrate antiviral activity on VV in cell culture as well as in spiked plasma.
2. Materials and methods
2.1. Cells, virus and reagents
Simian kidney epithelial cell lines Vero 76 and B-SC-1 cells (ATCC, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Herndon, VA) containing 10% fetal bovine serum (FBS), 2 mM l-glutamine (Mediatech, Herndon, VA) and penicillin–streptomycin (Invitrogen, Gaithersberg, MD). Vaccinia virus (WR strain, kind gift from B. Moss) was propagated in Vero 76 cells as described previously (Jones-Trower et al., 2005, Kotwal et al., 1989, Mohan et al., 2009b).
2.2. Virus stock preparation and infection
VV seed stocks were prepared by infecting three T752 flasks of Vero 76 cells (Jones-Trower et al., 2005, Kotwal et al., 1989). Infected cells were freeze-thawed three times at 72 h post-infection (h.p.i.), followed by low speed centrifugation and the supernatant was collected, estimated for viral titers by plaque assay, aliquoted and stored at −80 °C, until used (Jones-Trower et al., 2005, Kotwal et al., 1989, Mohan et al., 2009b). VV infection of B-SC-1 (simian kidney epithelial) cells for antiviral analysis was performed in 12-well plates.
2.3. Peptide synthesis and reconstitution
A total of nine antimicrobial peptides were synthesized at CBER Core Facility. Four were tPMP-1 consensus sequence derived (PD) peptides of 15 amino acids in length and other 5 microbicidal peptides were 1–5 repeats of Arg-Trp (RW1-5) amino acids (Table 1 ) (Liu et al., 2007, Mohan et al., 2009a, Tang et al., 2002, Yeaman and Bayer, 1999). Reconstitution of the peptides was essentially as per a published protocol except for the solvent used (Mohan et al., 2009a). Both the PD and RW peptides were reconstituted in phosphate buffered saline (PBS), pH 7.2. Since PD peptides were of uniform length (15-mers), the stock solutions were made at 100 μg/μl by reconstituting the lyophilized powder in PBS and stored at 4–8 °C until used. Since the RW peptides were of variable length (2-, 4-, 6-, 8-, and 10-mer), the stocks were made at 10 mM concentration for these peptides by dissolving in PBS, pH 7.2.
Table 1.
List of peptides used in the study. Peptides PD1–PD4 are platelet microbicidal protein (PMP)-derived peptides and superscript numbers on each peptide sequence indicate amino acid position on the PMP sequence.
| Peptides | Sequence | |
|---|---|---|
| PMP-derived peptides | PD1 | 1SDDPKESEGDLHCVC15 |
| PD2 | 13CVCVKTTSLVRPRHI27 | |
| PD3 | 49KNGRKLCLDLQAALY63 | |
| PD4 | 60AALYKKKIIKKLLES74 | |
| RW series peptides | RW1 | RW |
| RW2 | RWRW | |
| RW3 | RWRWRW | |
| RW4 | RWRWRWRW | |
| RW5 | RWRWRWRWRW | |
2.4. Antiviral assays
All the nine AMPs were evaluated for their antiviral potential. AMPs were tested at 3 different stages of virus infection: pre-, during- and post-infection. VV-infection without the peptides was taken as control and all experiments were performed in triplicate for statistical analyses.
2.4.1. Pre-infection
To analyze whether the AMPs act on the virus and/or cells both the VV and B-SC-1 cells were treated individually with the nine AMPs prior to infection and assessed for viral titers. Effect of AMPs on VV was performed by taking 100 μl of 104 pfu/ml concentration of WR virus, mixed individually with PD1–PD4 or RW1–RW5 peptides made up to a final volume of 1 ml with DMEM and incubated at room temperature for 2 h. Final concentration of the PD peptides was at 100 μg/ml whereas RW peptides were diluted to a 10 μM final concentration. The inoculum was then added to B-SC-1 cells maintained in 12-well plates. Following virus binding to the cells at 37 °C for 1 h, virus inoculum was aspirated and agar overlay containing DMEM was added to the cells and incubated for 72 h (Jones-Trower et al., 2005, Kotwal et al., 1989, Mohan et al., 2009b). At the end of incubation period the agar overlay was removed and cell monolayer was stained with crystal violet for enumeration of virus plaques. Results were expressed in plaque-forming units (pfu)/ml. Similarly, effect of PD and RW peptides on B-SC-1 cells was analyzed by individually treating cells with each of these peptides for 2 h at the same concentrations as above. Following incubation the peptide mixture was aspirated and cells washed once with fresh DMEM. Virus infectivity was assessed by inoculating a 1 ml mixture of VV (103 pfu/ml) onto these treated-cells and incubated for 1 h at 37 °C. Viral titers were measured by plaque assay as described above.
2.4.2. Virus-binding stage
100 μl of 104 pfu/ml concentration of WR virus was mixed with individual PD and RW peptides (final concentration 100 μg/ml and 10 μM, respectively) and made up to 1 ml with fresh DMEM and the mixture was added directly to B-SC-1 cells. Cells were incubated at 37 °C for 1 h. Following virus binding, the inoculum was aspirated and an agar overlay containing DMEM was added to the cell culture plates and incubated at 37 °C for 72 h (Jones-Trower et al., 2005, Kotwal et al., 1989). Cell monolayer was then stained with crystal violet for viral plaques as described above.
2.4.3. Post-infection stage
B-SC-1 cells maintained in 12-well culture plates were infected with 1 ml of VV inoculum (103 pfu/ml) and incubated at 37 °C for 1 h. Following virus binding, the inoculum was aspirated and fresh DMEM containing individual PD peptides at a final concentration of 100 μg/ml and RW peptides at 10 μM were added to each well and cell culture plates were incubated at 37 °C. Following 48 h of virus infection supernatant was aspirated and cell monolayer was freeze-thawed thrice to extract virus particles as described previously (Jones-Trower et al., 2005, Kotwal et al., 1989). Virus particles extracted from each well was then quantified by the plaque assay as described above.
2.5. Dose–response and EC50 value estimation
To evaluate whether the PD and RW peptides were microbicidal at concentrations lower than 100 μg/ml and 10 μM, respectively, a serial doubling dilution analysis was performed with PD3, PD4 and RW3 peptides. Since PD peptides were of uniform length (15 aa) they were constituted in μg/ml concentration. RW peptides though were of varying length (2, 4, 6, 8 and 10 aa) and hence expressed in μM concentrations. We maintained these two different concentration expression units to be consistent with our previously published work (Mohan et al., 2009a). The final concentration of the PD peptides for the assay were 100, 50, 25 and 12.5 μg/ml whereas the RW peptides concentration was 10, 5, 2.5 and 1.25 μM. 103 pfu/ml of WR virus was mixed individually with each of these peptides and the antiviral assay was performed as per the pre-infection protocol described above.
2.6. Plasma sample spiking assay
To assess whether these three AMPs would maintain their antiviral activity in a biological material, human plasma samples were spiked with VV and the peptides were tested for their activity. Human plasma samples were acquired from the NIH blood bank, Bethesda, MD and were spiked with 102 pfu of VV-WR virus. PD3, PD4 and RW3 peptides were each incubated with the virus inoculum for 2 h at room temperature and tested for antiviral activity by performing the plaque assay as described in the pre-infection experiment.
2.7. Statistical analyses
All assays described here were performed a minimum of three times and mean values ± SD (Standard Deviation) were calculated using Microsoft Excel®. Statistical analyses were performed using a two-tailed Student's t-test and values were considered significant when p < 0.05. Dose–response curves (EC50 values) for PD3, PD4 and RW3 peptides were estimated using the GraphPad™ Prism 5.0 software.
3. Results
3.1. PD3, PD4 and RW3 inhibit vaccinia virus during the pre-infection stage
In order to evaluate at which stage of the virus infection are the PD and RW peptides demonstrate antiviral activity, we tested all nine AMPs in a pre-infection incubation step. Results from this experiment revealed that preincubation of AMPs with VV were able to inhibit viral infection (Fig. 1 ). Platelet-derived peptides PD3 and PD4 were able to bring down the VV titer by 10–100-fold. The RW3 peptide was able to elicit a 10-fold reduction in VV titers (Fig. 1). Remaining six peptides (PD1, PD2, RW1, RW2, RW4 and RW5) did not demonstrate any antiviral activity above what was observed with the control group.
Fig. 1.
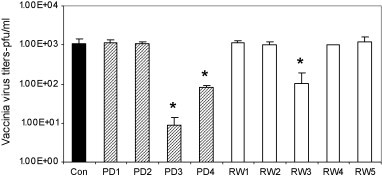
Antiviral activity of PD and RW peptides on VV during the pre-infection stage. WR strain of VV was incubated with PD and RW peptides at 100 μg/ml and 10 μM concentration, respectively, for 2 h at RT and titers were measured by counting plaques on B-SC-1 cells. Since PD peptides were of uniform length (15 aa) they were constituted in μg/ml whereas RW peptides though were of varying length (2, 4, 6, 8 and 10 aa) and hence expressed in μM concentrations. These two different concentration expression units were maintained to be consistent with our previously published work (Mohan et al., 2009a). VV infection without the peptides was included as control. PD3, PD4 and RW3 peptides demonstrate significant (p < 0.05, indicated by *) reduction in viral titers.
3.2. PD3, PD4 and RW3 peptides do not inhibit vaccinia virus after virus-binding and post-infection stage
Once we observed that the AMPs were able to inhibit VV, the next step was to address whether the AMPs would still inhibit VV post a virus-binding step or a post-infection stage. Analysis of the post virus-binding experiment revealed that none of the PD or RW peptides were able to inhibit virus infection as there was no significant difference in the viral titers between the test and control groups (Fig. 2 ). Similarly, the pre- and post-infection treatment of B-SC-1 cells with PD and RW peptides did not have any significant effect on virus titers between control and the peptide-treated groups (Fig. 2).
Fig. 2.
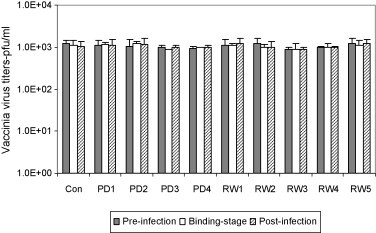
Antiviral activity of PD and RW peptides during pre-infection, VV-binding and post-infection stage. PD and RW peptides were added to B-SC-1 cells at 3 different stages: (a) prior to infection (filled bars), (b) during virus binding (open bars) or (c) post-infection stage (shaded bars) and resulting viral titers were compared to that of VV-infection lacking the peptides. In the pre-infection experiment B-SC-1 cells were incubated with PD and RW peptides for 2 h at 37 °C, followed by washing of the cells and infection with VV. For the virus-binding stage experiment peptides were mixed individually with VV and the mixture was added to cells. Following incubation for 1 h inoculum was aspirated and agar overlay with DMEM was added. The post-infection assay was performed by first infecting B-SC-1 cells with VV for 1 h and then adding fresh medium containing PD and RW peptides. Note that both PD and RW peptides do not exhibit significant antiviral activity (p > 0.05) during these three stages of treatment.
3.3. PD and RW peptides exhibit antiviral activity at low concentrations
In order to evaluate the minimal inhibitory concentration of the PD3, PD4 and RW3 peptides further analysis by serial doubling dilution of these peptides and their effect on virus inhibition was performed. Dose–response curves for PD3, PD4 and RW3 were generated using the GraphPad Prism 5.0 software and their EC50 values calculated. Analysis revealed that PD3 (Fig. 3A) and PD4 (Fig. 3B) exhibited an EC50 value of 40 μg/ml and concentration, respectively. RW3 on the other hand revealed an EC50 dose at 6.5 μM concentration (Fig. 3C).
Fig. 3.
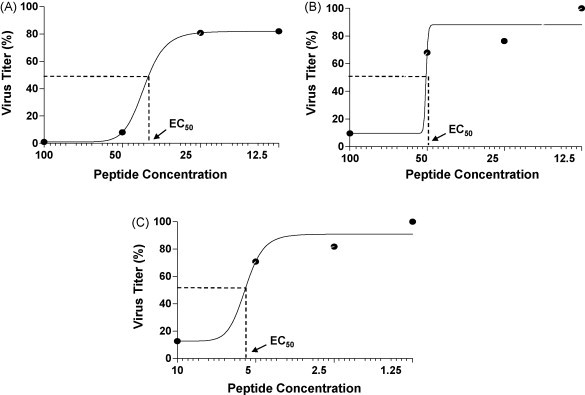
Dose–response curves or EC50 estimation of PD and RW peptides. Serial doubling dilution analysis was performed with PD3, PD4 and RW3 peptides. The final concentration of the PD peptides for the assay were 100, 50, 25 and 12.5 μg/ml whereas the RW peptides concentration was 10, 5, 2.5 and 1.25 μM. 1 ml of WR virus (103 pfu/ml) was mixed individually with each of these peptides at RT for 2 h, followed by infection of B-SC-1 cells for 1 h. Following virus binding agar overlay with DMEM was added to cells and incubated at 37 °C for 72 h. Viral titers were estimated by counting plaques at the four different concentrations of the peptides tested and EC50 values were deduced by using the GraphPad Prism 5.0 software. Peptide concentrations are represented on the x-axis (log scale) with PD3 and PD4 expressed in μg/ml and RW3 concentration expressed in μM. Analysis reveals that the EC50 values for PD3 (A), PD4 (B) and RW3 (C) were 40 μg/ml, 50 μg/ml and 6.5 μM, respectively.
3.4. PD and RW peptides inhibit vaccinia virus in spiked plasma samples
Since these peptides have already been known to act as potent antibacterial agents (Mohan et al., 2009a) in blood products, we analyzed whether these AMPs could inhibit VV in a VV-spiked plasma sample as well. Analysis of the effect of PD3, PD4 and RW3 peptides on VV in spiked plasma samples revealed that PD3 was the most potent peptide with 100% virus inhibition at 100 μg/ml concentration. PD4 peptide on the other hand demonstrated a 90-fold reduction in virus titer at 100 μg/ml concentration (Fig. 4 ). RW3 was able to inhibit VV by ∼90% at 10 μM concentration (Fig. 4).
Fig. 4.
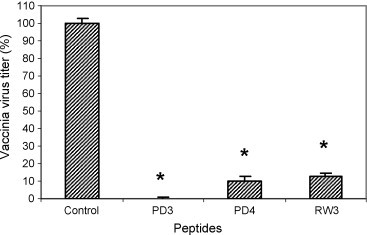
Antiviral activity of PD and RW peptides against VV in spiked-plasma samples. Human plasma samples spiked with 102 pfu of VV-WR virus were incubated with PD3, PD4 and RW3 peptides individually for 2 h and tested for antiviral activity by infecting B-SC-1 cells and measuring virus titer by performing the plaque assay as described in the pre-infection experiment. Note that PD3, PD4 and RW3 treatment results in 90–100-fold reduction of viral titers (p < 0.05, indicated by *).
4. Discussion
We had previously reported the proof-of-concept of usage of PD and RW peptides as bactericidal agents in experimentally contaminated blood products (Mohan et al., 2009a). Ideally, to be the most useful, any antimicrobial agent has to exhibit a broad-spectrum antimicrobial activity viz. anti-bacterial, anti-viral, anti-fungal and anti-parasitic. Hence, in the present study we evaluated the efficacy of the same AMPs against vaccinia virus reduction in experimentally infected plasma. Though current virus inactivation procedures are considered highly efficient and the transmission risks due to viruses is said to be minimal, the possibility still does exist. In order to address this hypothetical but possible occurrence we selected vaccinia virus as a model virus as it poses considerable challenge in being the most resistant virus to solvent/detergent inactivation methods (Lin et al., 2005, Remington et al., 2004, Roberts, 2000, Srinivasan et al., 2006, Wu and Snyder, 2003). Of the nine AMPs tested, three peptides (PD3, PD4 and RW3) exhibited virucidal activity against vaccinia virus. The same three peptides also exhibited potent bactericidal activity in plasma and platelets stored in plasma (Mohan et al., 2009a). The mechanism of action of these AMPs on bacteria is well known but their mechanism of action on viruses is not yet clearly understood (Liu et al., 2007, Yeaman and Bayer, 1999).
Antimicrobial peptides have been reported against a variety of viruses that include influenza A virus, SARS co-V, WNV, HSV and many others (Bai et al., 2007, Basu et al., 2009, Chu et al., 2008, Guo et al., 2009). The precise mechanism of action of the various AMPs is similar in some and different between other viruses. These AMPs could even have a stage-specific action on different viruses such as some may act on the extracellular virus, some may do so on the intracellular form, and some at the time of virus release (Bai et al., 2007, Basu et al., 2009, Chu et al., 2008, Guo et al., 2009). Our analysis of the PD and RW peptides indicate that all three peptides exhibit their antiviral activity on the virus during in vitro conditions. These peptides do not have any effect once the VV is bound to the cell membrane or during post-infection. More interestingly, pre-treatment of B-SC-1 cells with PD and RW peptides did not adversely affect virus titers suggesting that these peptides do not affect virus entry and may not be able to penetrate the cell as reported with some of the recently tested AMPs (Snyder and Dowdy, 2004).
Our analysis of the VV-spiked plasma samples further confirm that the PD3, PD4 and RW3 peptides are able to inhibit the virus and are able to retain their antiviral activity in biological fluids as well. While one would argue that a peptide that provides a 10-fold reduction in VV titers is not an effective therapeutic agent, it could make a difference in blood transfusion settings where blood donor deferral takes care of the high titer symptomatic individuals and inadvertently collected blood from an asymptomatic individual (i.e. with very low titers). In this scenario a reduction of ∼100 VV particles to less than 10 or, 10 particles to 0 would certainly make a difference, if the peptide can achieve a 10-fold reduction of the virus.
In the present study, the highest concentration of the PD and RW peptides tested was only 100 μg/ml and 10 μM, respectively, based on our previous experience with bacteria (Mohan et al., 2009a). Regardless of whether there is substantial risk of acquiring VV by blood products or not, the demonstration of anti-vaccinia properties of these AMPs in plasma samples is very promising. The antiviral potency of these AMPs may have potential applications, as observed with Caragenins, for topical therapy of poxviral infections (Howell et al., 2009). Interestingly, the Caragenins are synthetic antimicrobial compounds designed to mimic the structure and function of endogenous AMPs (Howell et al., 2009). Future application of these AMPs in other potentially challenging scenarios include treatment of various microbial infections, especially the drug-resistant ones, through systemic administration (Groneberg et al., 2004). More pertinently, small animal testing of these AMPs would provide further evidence of such an application for these peptides and additionally reveal peptide clearance or safety aspects of these AMPs in vivo. The distinct advantage of the PD and RW peptides is that these are non-immunogenic, non-cytotoxic and non-hemolytic and thus have very minimal safety concerns. Additionally, since these AMPs are synthetic they are easy to produce in large quantities with a good product consistency. A more comprehensive analysis using these AMPs on some of the more recently emerging viruses such as H1N1, West Nile virus (WNV), SARS Co-V, CMV could bring about the value of these AMPs in virus inactivation/intervention strategies in general.
Acknowledgements
The authors wish to acknowledge Dr. Bernard Moss, NIH, Bethesda, for providing the WR virus strain. SSR is a recipient of a postdoctoral fellowship at the Center for Biologics Evaluation and Research (CBER) administered by the Oak Ridge Institute for Science and Education (ORISE) through an intra-agency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration. Review of the manuscript by Drs. Tahir Malik and Zhiping Ye, CBER is gratefully acknowledged.
Footnotes
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination policy.
References
- Ahmed C.M., Burkhart M.A., Subramaniam P.S., Mujtaba M.G., Johnson H.M. Peptide mimetics of gamma interferon possess antiviral properties against vaccinia virus and other viruses in the presence of poxvirus B8R protein. J. Virol. 2005;79:5632–5639. doi: 10.1128/JVI.79.9.5632-5639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann S.E., Jones J.C., Schultz-Cherry S., Brandt C.R. Inhibition of vaccinia virus entry by a broad spectrum antiviral peptide. Virology. 2009;388:248–259. doi: 10.1016/j.virol.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F., Town T., Pradhan D., Cox J., Ashish, Ledizet M., Anderson J.F., Flavell R.A., Krueger J.K., Koski R.A., Fikrig E. Antiviral peptides targeting the west nile virus envelope protein. J. Virol. 2007;81:2047–2055. doi: 10.1128/JVI.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D., Walkiewicz M.P., Frieman M., Baric R.S., Auble D.T., Engel D.A. Novel influenza virus NS1 antagonists block replication and restore innate immune function. J. Virol. 2009;83:1881–1891. doi: 10.1128/JVI.01805-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Chan D.I., Prenner E.J., Vogel H.J. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim. Biophys. Acta. 2006;1758:1184–1202. doi: 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Chu L.H., Chan S.H., Tsai S.N., Wang Y., Cheng C.H., Wong K.B., Waye M.M., Ngai S.M. Fusion core structure of the severe acute respiratory syndrome coronavirus (SARS-CoV): in search of potent SARS-CoV entry inhibitors. J. Cell Biochem. 2008;104:2335–2347. doi: 10.1002/jcb.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher K.A., Selsted M.E., Lehrer R.I. Direct inactivation of viruses by human granulocyte defensins. J. Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorschner R.A., Pestonjamasp V.K., Tamakuwala S., Ohtake T., Rudisill J., Nizet V., Agerberth B., Gudmundsson G.H., Gallo R.L. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Invest. Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- Gallo R.L., Murakami M., Ohtake T., Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- Groneberg D.A., Fischer A., Chung K.F., Daniel H. Molecular mechanisms of pulmonary peptidomimetic drug and peptide transport. Am. J. Respir. Cell Mol. Biol. 2004;30:251–260. doi: 10.1165/rcmb.2003-0315TR. [DOI] [PubMed] [Google Scholar]
- Guo Y., Tisoncik J., McReynolds S., Farzan M., Prabhakar B.S., Gallagher T., Rong L., Caffrey M. Identification of a new region of SARS-CoV S protein critical for viral entry. J. Mol. Biol. 2009;394:600–605. doi: 10.1016/j.jmb.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R.E., Sahl H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Harder J., Bartels J., Christophers E., Schroder J.M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Harrison J.M., Ramshaw I.A. Cytokines, skin, and smallpox-a new link to an antimicrobial peptide. Immunity. 2006;24:245–247. doi: 10.1016/j.immuni.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Howell M.D., Jones J.F., Kisich K.O., Streib J.E., Gallo R.L., Leung D.Y. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J. Immunol. 2004;172:1763–1767. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- Howell M.D., Streib J.E., Kim B.E., Lesley L.J., Dunlap A.P., Geng D., Feng Y., Savage P.B., Leung D.Y. Ceragenins: a class of antiviral compounds to treat orthopox infections. J. Invest. Dermatol. 2009;129:2668–2675. doi: 10.1038/jid.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M.D., Streib J.E., Leung D.Y. Antiviral activity of human beta-defensin 3 against vaccinia virus. J. Allergy Clin. Immunol. 2007;119:1022–1025. doi: 10.1016/j.jaci.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Trower A., Garcia A., Meseda C.A., He Y., Weiss C., Kumar A., Weir J.P., Merchlinsky M. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology. 2005;343:128–140. doi: 10.1016/j.virol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kotwal G.J., Hugin A.W., Moss B. Mapping and insertional mutagenesis of a vaccinia virus gene encoding a 13,800-Da secreted protein. Virology. 1989;171:579–587. doi: 10.1016/0042-6822(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Lin L., Hanson C.V., Alter H.J., Jauvin V., Bernard K.A., Murthy K.K., Metzel P., Corash L. Inactivation of viruses in platelet concentrates by photochemical treatment with amotosalen and long-wavelength ultraviolet light. Transfusion. 2005;45:580–590. doi: 10.1111/j.0041-1132.2005.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Brady A., Young A., Rasimick B., Chen K., Zhou C., Kallenbach N.R. Length effects in antimicrobial peptides of the (RW)n series. Antimicrob. Agents Chemother. 2007;51:597–603. doi: 10.1128/AAC.00828-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan K.V., Rao S.S., Atreya C.D. Evaluation of antimicrobial peptides as novel bactericidal agents for room temperature-stored platelets. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- Mohan K.V., Zhang C.X., Atreya C.D. The proteoglycan bamacan is a host cellular ligand of vaccinia virus neurovirulence factor N1L. J. Neurovirol. 2009:1–9. doi: 10.1080/13550280902913636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyston P.C., Fox M.A., Richards S.J., Clark G.C. Novel peptide therapeutics for treatment of infections. J. Med. Microbiol. 2009;58:977–987. doi: 10.1099/jmm.0.011122-0. [DOI] [PubMed] [Google Scholar]
- Remington K.M., Trejo S.R., Buczynski G., Li H., Osheroff W.P., Brown J.P., Renfrow H., Reynolds R., Pifat D.Y. Inactivation of West Nile virus, vaccinia virus and viral surrogates for relevant and emergent viral pathogens in plasma-derived products. Vox Sang. 2004;87:10–18. doi: 10.1111/j.1423-0410.2004.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. Resistance of vaccinia virus to inactivation by solvent/detergent treatment of blood products. Biologicals. 2000;28:29–32. doi: 10.1006/biol.1999.0236. [DOI] [PubMed] [Google Scholar]
- Saccucci L., Crance J.M., Colas P., Bickle M., Garin D., Iseni F. Inhibition of vaccinia virus replication by peptide aptamers. Antiviral Res. 2009;82:134–140. doi: 10.1016/j.antiviral.2009.02.191. [DOI] [PubMed] [Google Scholar]
- Snyder E.L., Dowdy S.F. Cell penetrating peptides in drug delivery. Pharm. Res. 2004;21:389–393. doi: 10.1023/B:PHAM.0000019289.61978.f5. [DOI] [PubMed] [Google Scholar]
- Srinivasan K., Akolkar P.N., Taffs R.E., Hewlett I.K. Absence of detectable viremia in plasma and peripheral blood mononuclear cells from smallpox vaccinees: implications for blood safety. Transfusion. 2006;46:1589–1592. doi: 10.1111/j.1537-2995.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- Tang Y.Q., Yeaman M.R., Selsted M.E. Antimicrobial peptides from human platelets. Infect. Immun. 2002;70:6524–6533. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.Y., Snyder E.L. Safety of the blood supply: role of pathogen reduction. Blood Rev. 2003;17:111–122. doi: 10.1016/s0268-960x(02)00063-2. [DOI] [PubMed] [Google Scholar]
- Yeaman M.R., Bayer A.S. Antimicrobial peptides from platelets. Drug Resist. Updat. 1999;2:116–126. doi: 10.1054/drup.1999.0069. [DOI] [PubMed] [Google Scholar]
- Yeaman M.R., Yount N.Y., Waring A.J., Gank K.D., Kupferwasser D., Wiese R., Bayer A.S., Welch W.H. Modular determinants of antimicrobial activity in platelet factor-4 family kinocidins. Biochim. Biophys. Acta. 2007;1768:609–619. doi: 10.1016/j.bbamem.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiou M. Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. J. Mol. Med. 2007;85:317–329. doi: 10.1007/s00109-006-0143-4. [DOI] [PubMed] [Google Scholar]


