Abstract
The 3C-like protease (3CLpro) of SARS-coronavirus mediates the proteolytic processing of replicase polypeptides 1a and 1ab into functional proteins, becoming an important target for the drug development. In this study, Isatis indigotica root extract, five major compounds of I. indigotica root, and seven plant-derived phenolic compounds were tested for anti-SARS-CoV 3CLpro effects using cell-free and cell-based cleavage assays. Cleavage assays with the 3CLpro demonstrated that IC50 values were in micromolar ranges for I. indigotica root extract, indigo, sinigrin, aloe emodin and hesperetin. Sinigrin (IC50: 217 μM) was more efficient in blocking the cleavage processing of the 3CLpro than indigo (IC50: 752 μM) and beta-sitosterol (IC50: 1210 μM) in the cell-based assay. Only two phenolic compounds aloe emodin and hesperetin dose-dependently inhibited cleavage activity of the 3CLpro, in which the IC50 was 366 μM for aloe emodin and 8.3 μM for hesperetin in the cell-based assay.
Keywords: SARS-coronavirus, 3C-like protease, Isatis indigotica root, Phenolic compounds
Severe acute respiratory syndrome (SARS) was reported in 8447 cases with 811 deaths worldwide from February to June 2003 (Poutanen et al., 2003, Peiris et al., 2003, Drosten et al., 2003). A novel coronavirus, SARS-coronavirus (SARS-CoV) was identified as the etiological agent of the disease (Ksiazek et al., 2003, Lee et al., 2003, Tsang et al., 2003, Hsueh et al., 2003). SARS-CoV particle contains a single positive-stranded RNA genome encoding for replicase, spike, envelope, membrane, and nucleocapsid (Lai, 2003, Enjuanes et al., 2001, Holmes, 2003). The SARS-CoV 3CLpro mediates the proteolytic processing of replicase polypeptides into functional proteins, playing an important role in viral replication. Therefore, the SARS-CoV 3CLpro can be considered an attractive target for developing effective drugs against SARS. Several potential 3CLpro inhibitors with a 50% inhibitory concentration (IC50) below 10 μM were identified from the large number of the structurally diverse small molecules (Kao et al., 2004, Hsu et al., 2004).
Isatis indigotica root and phenolic Chinese herbs were frequently used for the prevention of SARS during the SARS outbreaks in China, Hong Kong, and Taiwan. I. indigotica root (Radix isatidis), belonging to the family Cruciferae, is native to China. Antiviral effects of I. indigotica root were found against influenza, hepatitis A and Japanese encephalitis (Qin and Xu, 1998, Wu et al., 1997). I. indigotica root contains indigo, indirubin, indican (indoxyl-β-d-glucoside), β-sitosterol, γ-sitosterol, sinigrin, etc. (Gilbert et al., 2004). Indigo and indirubin were identified as the promiscuous chymotrypsin inhibitors (McGovern and Shoichet, 2003). Recently, an anti-influenza virus effect of indirubin has been demonstrated (Mak et al., 2004). In addition, several herb-derived phenolics aloeemodin, hesperetin, quercetin, and naringenin have been accredited with antiviral effects against poliovirus, vesicular stomatitis virus, Sindbis virus, herpes simplex virus types 1 and 2, parainfluenza virus, and vaccinia virus (Semple et al., 2001, Andersen et al., 1991, Paredes et al., 2003, Kim et al., 2001).
In this study, we characterized the anti-SARS-CoV 3CLpro effect of the water extract of I. indigotica root, I. indigotica root-derived compounds, and herb-derived phenolics using a cell-free cleavage and cell-based cleavage assay.
The root of I. indigotica was purchased from Sun Ten Pharmaceutical Corporation (Taiwan). The plant root of Isatidis indigotica was extracted twice with 10 volumes of distilled boiling water for 1 h. The aqueous extract was concentrated under the reduced pressure at 50 °C, passed through 0.22-μm filters for sterilization, and diluted in culture medium to make a stock concentration of 10 mg/ml. Indigo and indirubin were kindly provided by Dr. Yuan-Shiun Chang, professor for Institute of Chinese Pharmaceutical Sciences, China Medical University. Indican (indoxyl-β-d-glucoside), β-sitosterol, sinigrin, aloe emodin, hesperetin, quercetin, naringenin, daidzein, emodin, and chrysophanol were purchased from Sigma Chemical.
To examine the trans-cleavage of SARS-CoV 3CLpro in the cell-free assay, recombinant 3CLpro was expressed in E. coli and purified using the HisTrap Kit (Amersham) as described in our previous report (Lin et al., 2004). Coomassie Blue-staining revealed that recombinant 3CLpro contained a major 34-kDa band for the monomer and a minor 68-kDa band for the dimer (Fig. 1A, lane 2). The cleavage substrate (TVRLQAGNATE) was generated as the substrate fusion protein with the N-terminal S-Tag and the C-terminal HSV-Tag. In the cell-free cleavage assay, the substrate fusion protein that was captured by anti-HSV-Tag antibodies in wells incubated with soluble 3CLpro for 3 h at 37 °C. The non-cleavage substrate protein was detected by an Enzyme Linked Immunosorbent Assay (ELISA) using peroxidase-conjugated S protein. ELISA showed that cell-free proteolytic activity correlated, in concentration-dependent manner, with the serial twofold dilution of recombinant 3CLpro protein in the range from 15 μg/ml to 240 μg/ml (Fig. 1B). Subsequently, the anti-3CLpro effect by the extract of I. indigotica root was evaluated using the cell-free cleavage assay. The cell-free cleavage assay indicated that the extract of I. indigotica root had a dose-dependent anti-3CLpro effect with an IC50 of 53.8 ± 4.2 μg/ml (Fig. 2 ; Table 1 ).
Fig. 1.
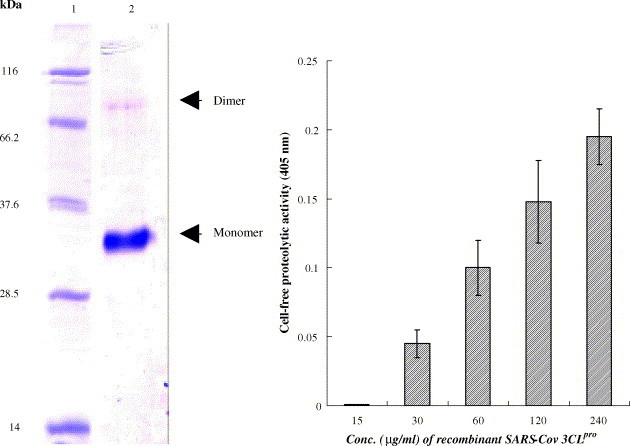
Cell-free cleavage activity of recombinant SARS-CoV 3CLpro. (A) The purified 3CLpro recombinant protein at the 1 mg/ml was analyzed by 10% SDS–PAGE with Coomassie blue staining (lane 2). (B) The trans-cleavage of the 3CLpro with a substrate fusion protein was determined using the ELISA. The substrate fusion protein was captured with anti-HSV mAb, followed by incubation with the serial dilution of the 3CLpro. The non-cleavage of the fusion protein was detected using the S protein-HRP conjugate and ABTS/H2O2 substrates. The ELISA product was measured at A405 nm. The relative cell-free cleavage activity was calculated as .
Fig. 2.
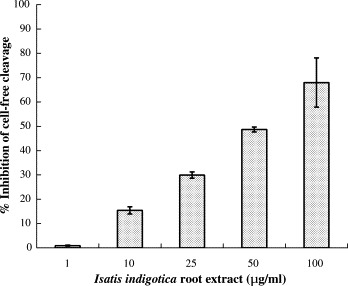
Inhibition of the cell-free cleavage of the 3CLpro by the Isatis indigotica root extract. The extract of the I. indigotica root was added into the mixture of the substrate fusion protein and the 3CLpro, and then incubated at room temperature for 3 h. The non-cleavage of substrate fusion protein was detected using the S protein-HRP conjugate and ABTS/H2O2 substrates. The ELISA product was measured at A405 nm. The relative inhibition of cell-free cleavage activity was calculated as .
Table 1.
The inhibitory effect on cell-free and cell-based cleavage activity of the SARS-CoV 3CLpro
| Compound | Structure | IC50a of cell-free cleavage (μg/ml) | IC50a of cell-based cleavage (μg/ml) | CC50b of cell death (μg/ml) |
|---|---|---|---|---|
| Isatis indigotica root | 53.8 ± 4.2 | 191.6 ± 8.2 | >5000 | |
| Indigo |  |
37.3 ± 8.1 (300 μM) | 190 ± 2.6 (752 μM) | 917 ± 18 (7375 μM) |
| Indirubin |  |
81.3 ± 5.2 (293 μM) | NSc | |
| Indican | 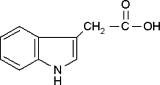 |
33.1 ± 1.2 (112 μM) | NSc | |
| Sinigrin | 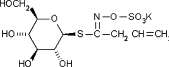 |
50.3 ± 1.5 (121 μM) | 90.1 ± 4.2 (217 μM) | >5000 (>10,000 μM) |
| Beta-sitosterol | 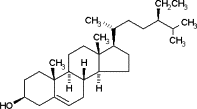 |
47.8 ± 8.6 (115 μM) | 502.1 ± 2.9 (1210 μM) | 613 ± 9 (1475 μM) |
| Aloeemodin | 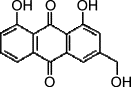 |
35.7 ± 1.5 (132 μM) | 99.1 ± 2.1 (366 μM) | 3135 ± 9 (11,592 μM) |
| Hesperetin | 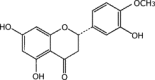 |
18.1 ± 0.6 (60 μM) | 2.5 ± 0.8 (8.3 μM) | 820 ± 15 (2718 μM) |
| Daidzein | 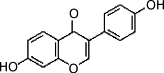 |
26.8 ± 1.2 (105 μM) | NSc |
IC50 (50% inhibitory concentration) was the concentration requiring for 50% inhibition on the cis-cleavage activity of 3CLpro.
CC50 (50% cytotoxic concentration) was the concentration giving half the OD570–630 of mock cells in MTT assay. IC50 and CC50 were determined using a computer program based on Fisher's statistical model.
Not significant.
The cell-based cleavage assay of 3CLpro for screening inhibitors does not require purification of the active 3CLpro, and represents closely the natural physiological state. Therefore, we used the cell-based cleavage assay for examining the inhibitory efficacy of the 3CLpro inhibitors. For the cell-based cleavage assay, the in-frame construction of the 3CLpro, the substrate, and the luciferase, designed as the plasmid pcDNA3.1-3CLpro-S-Luc, was co-transfected with the indicated vector pEGFP-N1 into Vero cells. The stable cell clone for the expression of the 3CLpro–substrate–luciferase fusion protein was selected by Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and 800 μg/ml of neomycin G418 (Fig. 3A). Since a more than 30 kDa protein fused at the N-terminus of the luciferase resulted in a dramatic decrease of luciferase activity (Joubert et al., 2000), the detection of luciferase activity could be considered as a measure for the cis-cleavage by the SARS-CoV 3CLpro. Western blotting with the anti-luciferase monoclonal antibody showed a 94-kDa band for the fusion protein 3CLpro-S-Luc and a 60-kDa band for the luciferase in Vero cells transfected with the plasmid pcDNA3.1-3CLpro-S-Luc (data not shown). The relative luciferase activity in the transfected cells was subsequently measured using the dual Luciferase Reporter Assay System (Fig. 3B). In the cell-based cleavage assay, the extract of I. indigotica root significantly inhibited the cis-cleavage activity of the SARS-CoV 3CLpro with an IC50 of 191.6 ± 8.2 μg/ml (Fig. 4 ; Table 1). The IC50 value from cell-based assay by the I. indigotica root extract was twofold higher than the IC50 value from the cell-free assay. The reason may be that the I. indigotica root extract could contain some inhibitory compounds that cannot permeate cellular membranes to reach intracellular SARS-CoV 3CLpro. The results of cell-free and cell-based cleavage assays demonstrated that the I. indigotica root extract might contain potent anti-SARS-CoV 3CLpro compounds.
Fig. 3.
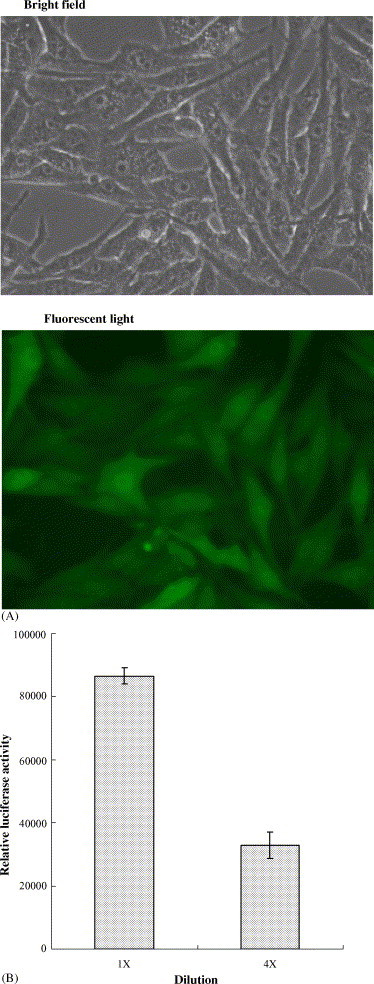
Cell-based cleavage assay of the 3CLpro in Vero cells. (A) Vero cells were transfected with the plasmid containing the 3CLpro–substrate–luciferase in-frame gene plus the indicated vector pEGFP-N1. (B) Relative Luc activity in the dilution of transfected cell lysates was determined using the dual Luciferase Reporter Assay System and the Luminometer TROPIX TR-717.
Fig. 4.
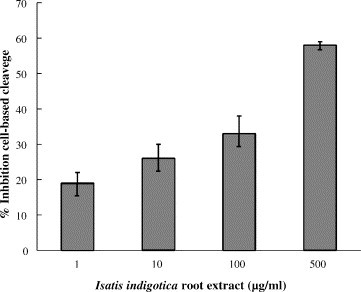
Inhibition of the cell-based cleavage of the 3CLpro by the Isatis indigotica root extract. Vero cells generating the 3CLpro–substrate–luciferase fusion protein were treated with the indicated concentration of the I. indigotica root extract. Equal amounts (100 μg) of cell lysates were used to determine the luciferase activity (LUC) using the dual Luciferase Reporter Assay System. The relative inhibition of cell-based cleavage activity was calculated as 1 − (LUCwith inhibitor)/(LUCwithout inhibitor).
The in vitro cytotoxicity profile of the I. indigotica root extract was examined using Vero cells. Vero cells in MEM medium with 10% FBS were plated in 96-well plates (5 × 104 cells/well) and then treated with the indicated compounds. After the treatment for 20 h, 25 μl of a MTT solution at 5 mg/ml was added to each well and incubated at 37 °C in 5% CO2 for 3 h. After a three-time washing of phosphate buffer saline, 100 μl DMSO was then added into the plates for dissolving the formazan crystals. OD570–630 in each well was then measured with a micro-ELISA reader. The result indicated that the 50% cytotoxic concentration (CC50) was greater than 5000 μg/ml.
Five major compounds of the I. indigotica root, including indigo, indirubin, indican, sinigrin, and beta-sitosterol were further tested for anti-SARS-CoV 3CLpro action (Fig. 5 , Table 1). Of the five compounds, sinigrin, beta-sitosterol and indigo dose-dependently inhibited cleavage activities of the 3CLpro in cell-free and cell-based assays (Fig. 5, Table 1). The IC50 in the cell-free assays was 115 μM for beta-sitosterol, 121 μM for sinigrin, and 300 μM for indigo. The cell-based assay indicated that sinigrin (IC50: 217 μM) was more efficient in blocking the cleavage processing of the 3CLpro than indigo (IC50: 752 μM) and beta-sitosterol (IC50: 1210 μM). Sinigrin showed a strong correlation between the effects on cell-free and cell-based cleavage of the SARS-CoV 3CLpro. Moreover, indigo (CC50: 7.4 mM) and sinigrin (CC50: >10 mM) were not toxic to Vero cells. Sinigrin, an antioxidant, has been reported to possess inhibitory effects on quinine reductase and glutathione S-transferase, antiproliferative effects against cancer cells, and antimicrobial activity against Bacillus subtilis and Saccharomyces cerevisiae (Brabban and Edwards, 1995, Munday and Munday, 2002, Smith et al., 2004). This study is the first report in that sinigrin significantly blocks the cleavage processing of a viral protease.
Fig. 5.
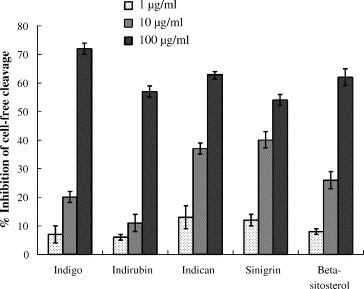
Inhibition of the cell-free cleavage of the 3CLpro by the compounds derived from Isatis indigotica root. The indicated compound was added into the mixture of the substrate fusion protein and the 3CLpro, and then the uncleaved substrate was detected using the S protein-HRP conjugate and ABTS/H2O2 substrates. The ELISA product was measured at A405 nm. The relative inhibition of cell-free cleavage activity was calculated as .
Seven phenolic compounds, aloeemodin, hesperetin, quercetin, naringenin, daidzein, emodin, and chrysophanol were also tested for their inhibitory effects on the SARS-CoV 3CLpro (Fig. 6 ; Table 1). Only two of the phenolic compounds, aloeemodin and hesperetin dose-dependently inhibited cleavage activity of the 3CLpro in cell-free and cell-based assays (Fig. 6; Table 1). In the cell-free assay, the IC50 values were 132 μM for aloe emodin and 60 μM for hesperetin. Quercetin has been reported to block the entry of SARS-CoV into host cells (Yi et al., 2004). However, no inhibitory effect on SARS-CoV 3CLpro was found for quercetin in the cell-free and cell-based cleavage assays. Interestingly, hesperetin (CC50: 2.7 mM) had an IC50 of 8.3 μM in the cell-based assay (Table 1). Hesperetin is poorly soluble in water; so, hesperetin was less inhibitory in the cell-free assay than in the cell-based assay. The finding of the anti-3CLpro effects of hesperetin at the micromolar range was consistent with a previous report indicating that hesperetin had an inhibitory activity on Sindbis virus infection with an IC50 of 20.5 μg/ml (about 68 μM) by plaque assay (Paredes et al., 2003). Of the compounds tested, hesperetin was the most potent inhibitor of SARS-CoV 3CLpro (Table 1).
Fig. 6.
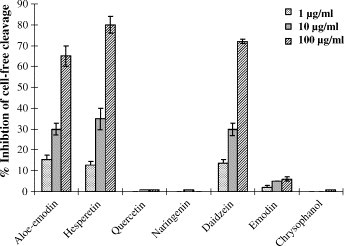
Inhibition of the cell-free cleavage of the 3CLpro by the phenolic compounds. The indicated compound was added into the mixture of the substrate fusion protein and the 3CLpro, and then the uncleaved substrate was detected. The relative inhibition of cell-free cleavage activity was calculated as .
Our results have demonstrated significantly inhibitory effects on SARS-CoV 3CLpro by I. indigotica root extract, indigo, sinigrin, aloeemodin and hesperetin in the micromolar range. Particularly, the cell-based assay demonstrated that hesperetin (IC50: 8.3 μM) and sinigrin (IC50: 217 μM) could be potential inhibitors of SARS-CoV 3CLpro. In addition, sinigrin and hesperetin with a CC50 of over 2 mM were considerably less cytotoxic to Vero cells (Table 1). Akin to other reported anti-SARS substances, such as glycyrrhizin (Cinatl et al., 2003a), nelfinavir (Yamamoto et al., 2004), aurintricarboxylic acid (He et al., 2004), and interferon (Cinatl et al., 2003b), the compounds reported here may be considered as potential leads in the development of inhibitors of SARS-CoV 3CLpro.
Acknowledgments
We would like to thank the National Science Council (Taiwan) and China Medical University for financial support (NSC93-2320-B-039-051, NSC 92-2314-B-039-030, NSC 92-2751-B-039-009-Y, and CMU92-MT-03).
References
- Andersen D.O., Weber N.D., Wood S.G., Hughes B.G., Murray B.K., North J.A. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antiviral Res. 1991;16:185–196. doi: 10.1016/0166-3542(91)90024-l. [DOI] [PubMed] [Google Scholar]
- Brabban A.D., Edwards C. The effects of glucosinolates and their hydrolysis products on microbial growth. J. Appl. Bacteriol. 1995;79:171–177. doi: 10.1111/j.1365-2672.1995.tb00931.x. [DOI] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Sola I., Almazan F., Izeta A., Gonzalez J.M., Alonso S. Coronavirus derived expression systems. Progress and problems. Adv. Exp. Med. Biol. 2001;494:309–321. doi: 10.1007/978-1-4615-1325-4_47. [DOI] [PubMed] [Google Scholar]
- Gilbert K.G., Maule H.G., Rudolph B., Lewis M., Vandenburg H., Sales E., Tozzi S., Cooke D.T. Quantitative analysis of indigo and indigo precursors in leaves of Isatis spp. and Polygonum tinctorium. Biotechnol. Prog. 2004;20:1289–1292. doi: 10.1021/bp0300624. [DOI] [PubMed] [Google Scholar]
- He R., Adonov A., Traykova-Adonova M., Cao J., Cutts T., Grudesky E., Deschambaul Y., Berry J., Drebot M., Li X. Potent and selective inhibition of SARS coronavirus replication by aurintricarboxylic acid. Biochem. Biophys. Res. Commun. 2004;320:1199–1203. doi: 10.1016/j.bbrc.2004.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K.V. SARS-associated coronavirus. N. Engl. J. Med. 2003;348:1948–1951. doi: 10.1056/NEJMp030078. [DOI] [PubMed] [Google Scholar]
- Hsu J.T., Kuo C.J., Hsieh H.P., Wang Y.C., Huang K.K., Lin C.P., Huang P.F., Chen X., Liang P.H. Evaluation of metal-conjugated compounds as inhibitors of 3CL protease of SARS-CoV. FEBS Lett. 2004;574:116–120. doi: 10.1016/j.febslet.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh P.R., Hsiao C.H., Yeh S.H., Wang W.K., Chen P.J., Wang J.T., Chang S.C., Kao C.L., Yang P.C. Microbiologic characteristics, serologic responses, and clinical manifestations in severe acute respiratory syndrome, Taiwan. Emerg. Infect. Dis. 2003;9:1163–1167. doi: 10.3201/eid0909.030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert P., Pautigny C., Madelaine M.F., Rasschaert D. Identification of a new cleavage site of the 3C-like protease of rabbit haemorrhagic disease virus. J. Gen. Virol. 2000;81:481–488. doi: 10.1099/0022-1317-81-2-481. [DOI] [PubMed] [Google Scholar]
- Kao R.Y., Tsui W.H., Lee T.S., Tanner J.A., Watt R.M., Huang J.D., Hu L., Chen G., Chen Z., Zhang L., He T., Chan K.H., Tse H., To A.P., Ng L.W., Wong B.C., Tsoi H.W., Yang D., Ho D.D., Yuen K.Y. Identification of novel small-molecule inhibitors of severe acute respiratory syndrome-associated coronavirus by chemical genetics. Chem. Biol. 2004;11:1293–1299. doi: 10.1016/j.chembiol.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.K., Jeon W.K., Ko B.S. Flavanone glycosides from Citrus junos and their anti-influenza virus activity. Planta Med. 2001;67:548–549. doi: 10.1055/s-2001-16484. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lai M.M.C. SARS virus: the beginning of the unraveling of a new coronavirus. J. Biomed. Sci. 2003;10:664–675. doi: 10.1007/BF02256318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Lin C.W., Tsai C.H., Tsai F.J., Chen P.J., Lai C.C., Wan L., Chiu H.H., Lin K.H. Characterization of trans- and cis-cleavage activity of the SARS coronavirus 3CLpro protease: basis for the in vitro screening of anti-SARS drugs. FEBS Lett. 2004;574:131–137. doi: 10.1016/j.febslet.2004.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak N.K., Leung C.Y., Wei X.Y., Shen X.L., Wong R.N., Leung K.N., Fung M.C. Inhibition of RANTES expression by indirubin in influenza virus-infected human bronchial epithelial cells. Biochem. Pharmacol. 2004;67:167–174. doi: 10.1016/j.bcp.2003.08.020. [DOI] [PubMed] [Google Scholar]
- McGovern S.L., Shoichet B.K. Information decay in molecular docking screens against holo, apo, and modeled conformations of enzymes. J. Med. Chem. 2003;46:2895–2907. doi: 10.1021/jm0300330. [DOI] [PubMed] [Google Scholar]
- Munday R., Munday C.M. Selective induction of phase II enzymes in the urinary bladder of rats by allyl isothiocyanate, a compound derived from Brassica vegetables. Nutr. Cancer. 2002;44:52–59. doi: 10.1207/S15327914NC441_7. [DOI] [PubMed] [Google Scholar]
- Paredes A., Alzuru M., Mendez J., Rodriguez-Ortega M. Anti-Sindbis activity of flavanones hesperetin and naringenin. Biol. Pharm. Bull. 2003;26:108–109. doi: 10.1248/bpb.26.108. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Qin G.W., Xu R.S. Recent advances on bioactive natural products from Chinese medicinal plants. Med. Res. Rev. 1998;18:375–382. doi: 10.1002/(sici)1098-1128(199811)18:6<375::aid-med2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Semple S.J., Pyke S.M., Reynolds G.D., Flower R.L. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antiviral Res. 2001;49:169–178. doi: 10.1016/s0166-3542(01)00125-5. [DOI] [PubMed] [Google Scholar]
- Smith T.K., Lund E.K., Parker M.L., Clarke R.G., Johnson I.T. Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis. 2004;25:1409–1415. doi: 10.1093/carcin/bgh149. [DOI] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Wu X.Y., Qin G.W., Cheung K.K., Cheng K.F. New alkaloids from Isatis indigotica. Tetrahedron. 1997;53:13323–13328. [Google Scholar]
- Yamamoto N., Yang R., Yoshinaka Y., Amari S., Nakano T., Cinatl J., Rabenau H., Doerr H.W., Hunsmann G., Otaka A., Tamamura H., Fujii N., Yamamoto N. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L., Shen Y., Luo M., Zuo G., Hu J., Duan D., Nie Y., Shi X., Wang W., Han Y., Li T., Liu Y., Ding M., Deng H., Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


