Abstract
In puppies weaning is a high risk period. Fecal changes are frequent and can be signs of infection by digestive pathogens (bacteria, viruses, parasites) and indicators of nutritional and environmental stress. The aim of this study was to define a pathological fecal score for weaning puppies, and to study the impact on that score of two intestinal viruses (canine parvovirus type 2 and canine coronavirus). For this, the quality of stools was evaluated on 154 puppies between 4 and 8 weeks of age (100 from small breeds and 54 from large breeds). The scoring was performed immediately after a spontaneous defecation based on a 13-point scale (from 1; liquid to 13; dry and hard feces). Fecal samples were frozen for further viral analysis. Each puppy was weighed once a week during the study period. The fecal score regarded as pathological was the highest score associated with a significant reduction in average daily gain (ADG). Fecal samples were checked by semi-quantitative PCR or RT-PCR for canine parvovirus type 2 and canine coronavirus identification, respectively. The quality of feces was affected by both age and breed size. In small breeds, the ADG was significantly reduced under a fecal score of 6 and 7 for puppies at 4–5 and 6–8 weeks of age, respectively. In large breeds, the ADG was significantly reduced under a fecal score of 5 whatever the age of the puppy. Whereas a high viral load of canine parvovirus type 2 significantly impacted feces quality, no effect was recorded for canine coronavirus. This study provides an objective threshold for evaluation of fecal quality in weaning puppies. It also emphasizes the importance to be given to age and breed size in that evaluation.
Keywords: Dog, Parvovirus, Coronavirus, Fecal score, Weaning, Breed size
1. Introduction
In dogs, feces quality can be influenced by own characteristics of the dog (breed size and age), digestive pathogens (bacteria, viruses, and parasites), diet (change without transition, type and quality of food), lifestyle and environment (stress) (Weber et al., 2002, Weber et al., 2003, Sokolow et al., 2005, Hernot et al., 2006, Stavisky et al., 2011). Various fecal scales have been proposed to evaluate feces quality in adult dogs (Meyer et al., 1999, Rolfe et al., 2002a, Rolfe et al., 2002b, Propst et al., 2003, Hernot et al., 2006, Allenspach et al., 2007). These scales are divided in 4–10 points, with lower grades corresponding to either dry feces or diarrhea, and with an optimal fecal score varying from 2 to 7.5 (Rolfe et al., 2002a, Rolfe et al., 2002b, Hernot et al., 2005, Allenspach et al., 2007). Digestive health is particularly challenged in puppies around the weaning period. Weaning is associated with major changes in food intake, together with profound environmental and social modifications. Besides these major alterations in digestive balance, the weaning period is associated with a high incidence of some gastro-intestinal pathogens. All these factors can affect feces quality and reduce the average daily gain (ADG). Canine parvovirus type 2 (CPV2) is frequently isolated in puppies from shelters (Marshall et al., 1984, Vanparijs et al., 1991, Naylor et al., 2001) and was reported to have a major impact on feces quality at weaning (Schulz et al., 2008). Canine coronavirus (CCV) is also frequently identified in the feces of puppies but its role in the etiology of acute diarrhea remains controversial (Tennant et al., 1993, Yesilbag et al., 2004).
Since weaning is a high risk period for puppies, a tool for precise follow-up of digestive health would be most useful. Scoring systems used in adult dogs cannot be applied to young puppies because of a physiological lower fecal quality prior to the weaning period (Giffard et al., 2004). No study has defined an abnormal fecal score during the weaning period. The objectives of this study were thus to determine in puppies a threshold in fecal score that could be regarded as pathological, based on its correlation with a drop average daily gain, and to evaluate the association between pathological scores and digestive infection by CPV2 or CCV.
2. Material and methods
2.1. Animals
The study was conducted from December 2009 to June 2010 in the North of France in one large breeding kennel housing 330 purebred dogs (280 females, 50 males) of 16 different breeds.
Each pregnant bitch was housed singly for the 2 weeks preceding the expected delivery date. All puppies stayed with their dam in heated whelping boxes (surface: 2 m2) from birth to approximately 8 weeks of age, when they were sold. They were fed a dry expanded complete diet balanced for growing dogs (food composition: moisture 8%, protein 30%, crude fat 22%, crude fiber 1.8%, ash 6.9%, and metabolizable energy 4251 kcal/kg). The daily ration was calculated based on the number of puppies in the litter and on breed size. Half the quantity was distributed early in the day and half in the end of the day. For young puppies 4–6 weeks of age, the dry diet was rehydrated with water just before distribution.
Each puppy was treated with a single dose of diclazuril (Vecoxan®; Janssen Animal Health; Issy-Les-Moulineaux, France, 2.5 mg/kg, per os) at 4 and 7 weeks of age and with fenbendazole (Panacur®, Intervet; Beaucouzé, France, 50 mg/kg, per os, q 24 h) for 3 consecutive days at 2, 4, 6 and 8 weeks of age. Puppies were vaccinated at 5, 6 and 7 weeks of age with a non-adjuvant, modified-live vaccine containing parvovirus Cornell 780916-115 strain with a viral titer of 105.5 TCID50 (Primodog®, Merial, Lyon, France).
154 puppies (75 males and 79 females, from 46 litters) between 4 and 8 weeks of age were included in the study. Depending on the mean adult body weight of their respective breed, two groups of dogs were considered: small breed dogs including breeds with a mean adult body weight <10 kg (West Highland White Terrier, Bichon Frise, Poodle, Shih Tzu, Lhasa apso) and large breed dogs including breeds with a mean adult body weight >25 kg (German Shepherd, Golden retriever, Labrador retriever). 100 small breed puppies and 54 large breed puppies were enrolled. Within one litter, puppies were identified by wool collars of various colors.
2.2. Average daily gain
Puppies between 4 and 8 weeks of age were weighed every 7 days using calibrated electronic scales. The average daily gain (ADG) was calculated for each puppy every week between 4 and 8 weeks of age as: (weight of week (n) − weight of week (n − 1))/7).
2.3. Feces sampling and scoring
All stool samples were scored by a single operator (AG) using a 13-point scale, based on the texture and shape of the feces (from liquid to hard and dry) (Table 1 ). A rectal swab was performed immediately after sampling for CPV2 and CCV detection. The swab was stored at −20 °C until qPCR or qRT-PCR.
Table 1.
Fecal scale for the evaluation of the quality of feces in weaning puppies.
| Fecal score | Visual aspect of the feces | Description of the feces |
|---|---|---|
| 1 |  |
Feces completely liquid |
| 2 |  |
Liquid feces associated with soft feces (soft feces do not represent the main quantity of feces) |
| 3 |  |
Liquid feces associated with soft feces (soft feces represent the main quantity of feces) |
| 4 |  |
Pasty feces with no shape |
| 5 |  |
Pasty unformed feces visualization of the apparition of a cylindrical shaped (1) which has not kept its shape due to the high humidity |
| 6 |  |
Feces mainly unformed (1) but with a part which is formed (2) |
| 7 |  |
Formed stools but very soft. Cylindrical shaped feces without any stria observed. |
| 8 |  |
Formed stools but very soft. Cylindrical shaped feces with presence of stria (1) |
| 9 |  |
Formed stools but very soft. Cylindrical shaped feces separated in pellets |
| 10 |  |
Formed, drier but not hard feces. Cylindrical shaped feces, slightly tacky to the touch, separated in pellets |
| 11 |  |
Formed, drier but not hard feces. Cylindrical shaped feces, dry appearance, separated in pellets, can be easily crushed out of shape |
| 12 |  |
Formed, drier but not hard feces. Cylindrical shaped feces, dry appearance, separated in pellets, can be crushed out of shape with a moderate difficulty |
| 13 |  |
Formed, dry and hard feces |
2.4. CCV and CPV2 detection
Total nucleic acids were extracted from the rectal swabs using a commercial kit (Nucleospin, Macherey Nagel; Hoerdt, France), as recommended by the supplier and eluted in 400 μL. The analyses were performed by real-time polymerase chain reaction (PCR) or reverse-transcriptase PCR, for CPV2 and CCV detection, respectively, in a 7900 HT sequence detection system (Applied Biosystems, Villebon sur Yvette, France). Five microliters of eluate was used for PCR and absence of PCR inhibitors was checked for each eluate. The detection threshold of the CPV2 real-time PCR (i.e. the lower number of genomic copies detected in 95% of runs) is 4 copies of genomic DNA VP2 template. The quantification threshold is 200 copies. These were determined by probit analysis as recommended by European Pharmacopeia for PCR assays assessment. The detection and quantification thresholds for canine coronavirus detection are, respectively, 36 and 200 copies of genomic RNA. Results from duplicate analysis (mean of the two results) were expressed semi-quantitatively as 5 viral load levels (Table 2 ). For CPV2, viral load levels have been determined in asymptomatic non-vaccinated, asymptomatic vaccinated or diseased dogs with confirmed parvovirosis (histolopathologic exam and/or positive testing using antigen assays). High and very high viral loads as defined in Table 2 are typical loads in dogs with parvovirosis and correlated to positive antigen testing and detection of wild-type strains of virus, i.e. CPV2a, b or c (unpublished results). In vaccinated dogs the CPV2 viral loads are mostly low or very low but sometimes may be medium especially when the dog has been vaccinated a few days before sampling with high-titer vaccines. Whatever the vaccine used we neither obtained high viral loads of vaccine strain even in the days after vaccination (unpublished results). As far as CCV is concerned, the viral load levels have been arbitrary defined on the basis of several hundreds of tests in asymptomatic or diarrheic puppies from different breeds.
Table 2.
Viral load in rectal swab for canine parvovirus type 2 (CPV2) and canine coronavirus (CCV).
| Viral load level | CPV2 | CCV |
|---|---|---|
| 1 | <104.2 copies | <104.2 copies |
| 2 | <105.7 copies | <105.3 copies |
| 3 | <107.7 copies | <107.3 copies |
| 4 | <1010.3 copies | <109.3 copies |
| 5 | >1010.3 copies | >109.3 copies |
2.5. Statistical analysis
The effects of age, size of the breed and interaction between them were evaluated with the Catmod procedure of SAS (SAS Institute, Cary, NC, USA). Two ages (4–5 weeks and 6–8 weeks) were combined with two sizes (small and large breeds) in 2 × 2 factorial designs.
For determination of a threshold value for pathological fecal scores, a comparison of weight gain above and below of each fecal score was performed with a variance analysis (PROC GLM, SAS).
The effect of age, and breed size on the average daily gain was evaluated by variance analysis (PROC GLM, SAS).
The effects of CPV2 and CCV on the pathological feces and the interaction between them were evaluated using the Catmod procedure of SAS. Two levels of CPV2 (levels 1–3 vs 4–5) were combined with two levels of CCV (levels 1–3 vs 4–5) in 2 × 2 factorial designs. A p value < 0.05 was considered statistically significant.
3. Results
A spontaneous defecation was not observed from each puppy during each observation session. 74 feces samples (27 from small breeds and 47 from large breeds) and 204 feces samples (133 from small breeds and 71 from large breeds) were collected.
3.1. Effect of age and breed size on feces quality
Over the whole study, a median fecal score of 8 was obtained (ranging from 1 to 11). The proportion of feces with a score ≥ 8 was affected by age (p = 0.023) and breed size (p = 0.006). A significant interaction (p = 0.047) between age and breed size was recorded. Fecal scores of small breed puppies improved with age whereas no such effect was observed in large breed puppies (Fig. 1, Fig. 2A ). In view of this difference in effect of breed size on fecal score, further analyses were conducted separately for the two groups of puppies (small and large breeds).
Fig. 1.
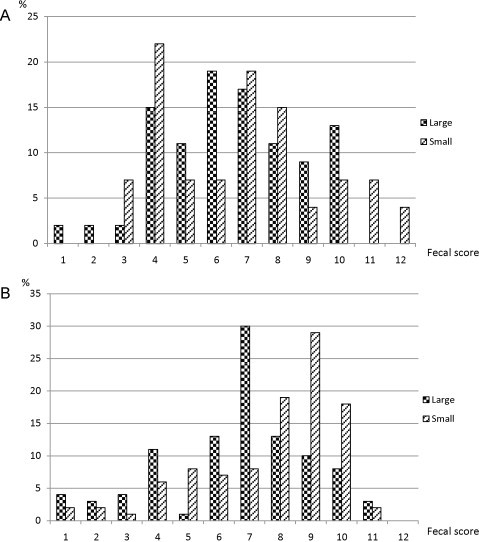
Feces quality in puppies of 4–5 (Fig. 2A) and 6–8 (Fig. 2B) weeks of age depending on breed size (n = 278 feces).
Fig. 2.
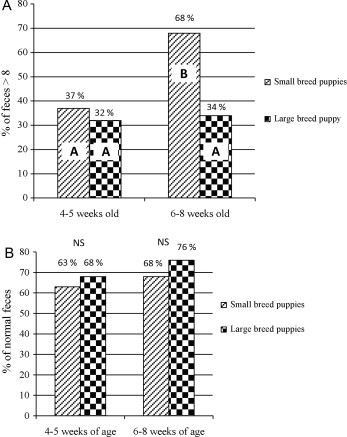
Percentage of puppies with a fecal score > 8 (A) and with normal feces (using the new scale) (B) depending on breed size and age of puppies (n = 278 feces). NS: non significant; and A, B: categories with different letters were significantly different (P < 0.05).
3.2. Determination of a threshold value for pathological fecal score
The ADG was affected by breed size but not by age. As expected, significantly lower ADGs were observed in small breeds compared to large breeds, whatever the period (P < 0.001). No significant difference in ADG was observed between the two periods either in small (P = 0.842) or in large (P = 0.494) breed puppies (Fig. 1, Fig. 2).
Small breed puppies with a fecal score ≤ 6 at 4–5 weeks of age and ≤7 at 6–8 weeks of age had a significantly lower ADG. Large breeds with a fecal score ≤ 5 had a significantly lower ADG (Table 3 ). The pathological fecal score for threshold was defined as the highest score associated with a significant reduction in ADG. With these threshold values, 29.9% (83/278) of the feces samples were classified as abnormal. The proportion of normal feces was affected neither by age (P = 0.128) nor by breed size (P = 0.113) (Fig. 2B).
Table 3.
Cut-off points for pathological fecal score from 154 puppies (n = 278 feces).
| Threshold (fecal score) | Small breed dogs |
Large breed dogs |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4–5 weeks old (n = 27) |
6–8 weeks old (n = 133) |
4–5 weeks old (n = 47) |
6–8 weeks old (n = 71) |
|||||||||
| Mean daily weight gain (g day−1) |
Mean daily weight gain (g day−1) |
Mean daily weight gain (g day−1) |
Mean daily weight gain (g day−1) |
|||||||||
| At or below threshold | Above threshold | P-value | At or below threshold | Above threshold | P-value | At or below threshold | Above threshold | P-value | At or below threshold | Above threshold | P-value | |
| 4 | 28 | 34.4 | 0.087 | 15.4 | 33.5 | 0.001 | 38.6 | 79.3 | 0.002 | 54.8 | 82.8 | 0.043 |
| 5 | 27.4 | 35.5 | 0.018 | 20.2 | 34.1 | 0.002 | 51.2 | 79.8 | 0.016 | 54.4 | 83.4 | 0.032 |
| 6 | 28.8 | 35.4 | 0.049 | 22.9 | 34.5 | 0.003 | 61.5 | 80.2 | 0.098 | 62.8 | 84.4 | 0.073 |
| 7 | 30.8 | 35.2 | 0.219 | 25.4 | 34.7 | 0.009 | 70.2 | 71.6 | 0.907 | 69.3 | 90.5 | 0.083 |
| 8 | – | – | – | 31.1 | 32.3 | 0.713 | – | – | – | 73.4 | 87.9 | 0.313 |
| 9 | – | – | – | – | – | – | – | – | – | 74.1 | 95.5 | 0.246 |
n, Number of data collected (ADG associated with a fecal score.
Bold value indicates highest fecal score associated with a significant reduction in average daily gain.
3.3. Association between fecal score and viral load in intestinal viruses
A total of 264 rectal swabs were performed immediately after feces sampling on the 154 puppies. 14 swabs could not be carried out because of the exceedingly small size of some 4–5 week puppies. Both CPV2 and CCV were isolated in 100% of the collected feces. High viral loads of CPV2 and CCV (levels 4–5) were detected in 12.5% (33/264) and 67% (177/264) of the samples, respectively, with 8.7% (23/264) containing high loads of both viruses (Fig. 3 ).
Fig. 3.
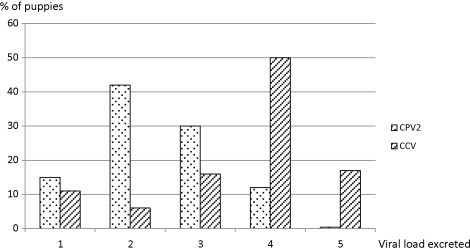
Parvovirus type 2 and coronavirus loads excreted by puppies between 4 and 8 weeks of age (n = 264 feces).
Puppies with a high load of parvovirus had significantly more pathological feces than puppies with a low load of parvovirus (P < 0.001). On the contrary, high loads of coronavirus were not significantly associated with pathological feces (P = 0.261). No interaction between high load of coronavirus and parvovirus and abnormal feces was observed (P = 0.227) (Fig. 4 ).
Fig. 4.
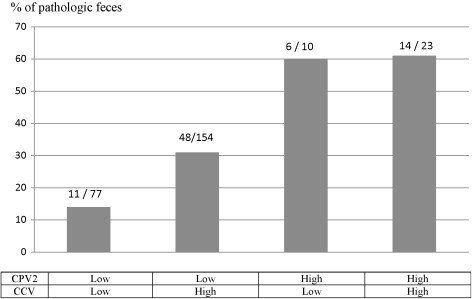
Relationship between viral load (CCV and CPV2) and abnormal feces on puppies between 4 and 8 weeks of age (n = 264 feces). x/X; x = number of pathological feces; and X = total number of feces in the group.
4. Discussion
The weaning period is critical for puppies. The maternally derived antibodies (MDA), acquired by ingestion of immunoglobulin-rich colostrum, are essential for the protection of puppies against neonatal infection and death (Day, 2007). However during the weaning period, MDAs may interfere with an active immune response to vaccine administration, but the low titers do not prevent infection with a virulent virus (Chappuis, 1998). So puppies are sensitive to infection by intestinal pathogens such as parvoviruses. Most earlier studies considered only the aspects of feces (consistency, color…) to define “diarrhea” and to evaluate the impact of enteropathogens on feces quality (Finlaison, 1995, Del Amo et al., 1999, Sokolow et al., 2005). However, young puppies have a higher fecal moisture content (Weber et al., 2003) and a lower fecal quality (Giffard et al., 2004) which may be related to a relatively low total mucosal surface (Paulsen et al., 2003) or to faster gastric emptying (Miyabayashi and Morgan, 1991, Weber et al., 2002). So, digestive disorders may be overestimated in studies considering all soft feces as abnormal. An objective definition of abnormal feces in puppies appeared desirable. This study was designed to propose a fecal scoring scale for puppies in the weaning period, i.e. at 6–8 weeks of age and to provide an objective definition of an abnormal fecal score, based on a reduction in the average daily weight gain.
All puppies included in this study were born and grown in the same kennel. They were thus exposed to the same management, food distribution and environmental pathogens. This made it possible to study the effect of breed size on the fecal score. Large breed puppies had more frequently soft and unformed feces. Such an effect of breed size has already been reported in adult dogs. In large breed dogs, such as German shepherds or Great Danes, the fecal moisture content is higher, soft stools are more frequent and the number of defecations is higher than in small breeds (Weber et al., 2001, Weber et al., 2002, Weber et al., 2003, Hernot et al., 2006). This difference may be due to a lower mineral absorption and/or to higher fermentative activity reflecting higher intestinal colic permeability and a longer colic transit time, respectively (Herschel et al., 1981, Kirkwood, 1985, Meyer et al., 1993, Meyer et al., 1999, Rolfe et al., 2002a, Weber et al., 2004).
Distinct pathological thresholds were determined in our study for small and large breeds (Table 3). The proposed definition of abnormal fecal score, based on a reduction in ADG, makes it possible to balance the effects of age and breed size (Fig. 2). The relationship between infection by enteropathogens and abnormal fecal scores, whatever the age and breed size of puppies, can then be investigated.
Fecal score thresholds were based on in ADG, but in a second time, the scoring scale was validated in evaluating the association between fecal score and the load of two intestinal viruses, CPV2 and CCV. Both viruses were isolated from all feces samples collected. Earlier serological and virological studies had demonstrated that puppies and dogs from kennels and animal shelters are particularly exposed to these infections (Rimmelzwaan et al., 1991, Tennant et al., 1993, Bandai et al., 1999, Naylor et al., 2001, Yesilbag et al., 2004, Schulz et al., 2008). The high prevalence observed here reflects the high contagiosity of these two viruses. Whereas viruses identified in the feces may sign the excretion of a wild viral strain, the low level of CPV2 detected in 87.3% of the feces may also result from vaccination. Puppies in our study were vaccinated at 5, 6, and 7 weeks of age with a non-adjuvant, modified live-vaccine containing a type 2 parvovirus strain. The use of such vaccines has been associated with fecal excretion of the vaccinal virus, the excretion remaining nevertheless markedly lower than in the case of a natural infection (Carmichael et al., 1981). The PCR assay used in this study has been shown to detect viruses in the feces of low-grade shedding animals (Desario et al., 2005, Schmitz et al., 2009).
In this work, a deterioration of the fecal score was recorded in dogs with high amounts of fecal CPV2. This virus is well known to cause weaning diarrhea (Potgieter et al., 1981, Carman and Povey, 1982, Schulz et al., 2008). So the fecal score proposed in this study may be of use in epidemiological studies designed to evaluate the effect of various enteropathogens on the quality of feces.
Interestingly, none of the puppies suffered from bloody diarrhea. Our study thus demonstrated that puppies may excrete high viral loads and yet present no systemic signs (anorexia, prostration). These healthy looking animals may however become major sources of virus for other animals. Whereas we confirmed the impact of CPV2 on fecal quality, we did not evidence any influence of CCV infection on the fecal score, whatever the amount excreted (Fig. 4). This virus is a common pathogen in dogs, but its actual role in acute dog diarrhea remains controversial (Tennant et al., 1993, Yesilbag et al., 2004). An interaction between CCV and CPV2 (aggravation of clinical signs in dogs infected by both viruses) was observed in 8 week old puppies under experimental condition (Appel, 1988). This interaction was not observed in our study.
5. Conclusion
Based on this study, a new objective fecal score, specific for puppies during the weaning period, could be defined. Thresholds below which feces are to be considered as pathological vary, according to age and breed size. This tool for management of digestive health may be of major interest to evaluate the impact of various infectious and environmental risk factors of digestive alterations in puppies during the weaning period.
Acknowledgments
The authors would like to thank Drs Mickael Weber, Vincent Biourge, and Marc Chodkiewicz whose comments on the manuscript were highly appreciated.
References
- Allenspach K., Wieland B., Grone A., Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007;21:700–708. doi: 10.1892/0891-6640(2007)21[700:ceideo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Appel M.J.G. Does canine coronavirus augment the effects of subsequent parvovirus infection? Vet. Med. 1988:360–366. [Google Scholar]
- Bandai C., Ishiguro S., Masuya N., Hohdatsu T., Mochizuki M. Canine coronavirus infections in Japan: virological and epidemiological aspects. J. Vet. Med. Sci. 1999;61:731–736. doi: 10.1292/jvms.61.731. [DOI] [PubMed] [Google Scholar]
- Carman S., Povey C. Successful experimental challenge of dogs with canine parvovirus-2. Can. J. Comp. Med. 1982;46:33–38. [PMC free article] [PubMed] [Google Scholar]
- Carmichael L.E., Joubert J.C., Pollock R.V. A modified live canine parvovirus strain with novel plaque characteristics. I. Viral attenuation and dog response. Cornell Vet. 1981;71:408–427. [PubMed] [Google Scholar]
- Chappuis G. Neonatal immunity and immunisation in early age: lessons from veterinary medicine. Vaccine. 1998;16:1468–1472. doi: 10.1016/S0264-410X(98)00110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M.J. Immune system development in the dog and cat. J. Comp. Pathol. 2007;137(Suppl. 1):S10–S15. doi: 10.1016/j.jcpa.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Del Amo A.N., Aprea A.N., Petruccelli M.A. Detection of viral particles in feces of young dogs and their relationship with clinical signs. Rev. Microbiol. 1999;30:237–241. [Google Scholar]
- Desario C., Decaro N., Campolo M., Cavalli A., Cirone F., Elia G., Martella V., Lorusso E., Camero M., Buonavoglia C. Canine parvovirus infection: which diagnostic test for virus? J. Virol. Methods. 2005;126:179–185. doi: 10.1016/j.jviromet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Finlaison D.S. Faecal viruses of dogs – an electron microscopy study. Vet. Microbiol. 1995;46:295–305. doi: 10.1016/0378-1135(95)00094-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard C.J., Seino M.M., Markwell P.J., Bektash R.M. Benefits of bovine colostrum on fecal quality in recently weaned puppies. J. Nutr. 2004;134:2126S–2127S. doi: 10.1093/jn/134.8.2126S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernot D.C., Biourge V.C., Martin L.J., Dumon H.J., Nguyen P.G. Relationship between total transit time and faecal quality in adult dogs differing in body size. J. Anim. Physiol. Anim. Nutr. (Berl.) 2005;89:189–193. doi: 10.1111/j.1439-0396.2005.00544.x. [DOI] [PubMed] [Google Scholar]
- Hernot D.C., Dumon H.J., Biourge V.C., Martin L.J., Nguyen P.G. Evaluation of association between body size and large intestinal transit time in healthy dogs. Am. J. Vet. Res. 2006;67:342–347. doi: 10.2460/ajvr.67.2.342. [DOI] [PubMed] [Google Scholar]
- Herschel D.A., Argenzio R.A., Southworth M., Stevens C.E. Absorption of volatile fatty acid Na, and H2O by the colon of the dog. Am. J. Vet. Res. 1981;42:1118–1124. [PubMed] [Google Scholar]
- Kirkwood J. The influence of size on the biology of the dog. J. Small Anim. Pract. 1985;26:97–110. [Google Scholar]
- Marshall J.A., Healey D.S., Studdert M.J., Scott P.C., Kennett M.L., Ward B.K., Gust I.D. Viruses and virus-like particles in the faeces of dogs with and without diarrhoea. Aust. Vet. J. 1984;61:33–38. doi: 10.1111/j.1751-0813.1984.tb07186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Kienzle E., Zentek J. Body size and relative weights of gastrointestinal tract and liver in dogs. J. Vet. Nutr. 1993;2:31–35. [Google Scholar]
- Meyer H., Zentek J., Habernoll H., Maskell I. Digestibility and compatibility of mixed diets and faecal consistency in different breeds of dog. Zentralbl. Veterinarmed. A. 1999;46:155–165. doi: 10.1046/j.1439-0442.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- Miyabayashi T., Morgan J. Upper gastrointestinal examinations: a radiographic study of clinically normal beagle puppies. J. Small Anim. Pract. 1991;32:83–88. [Google Scholar]
- Naylor M.J., Monckton R.P., Lehrbach P.R., Deane E.M. Canine coronavirus in Australian dogs. Aust. Vet. J. 2001;79:116–119. doi: 10.1111/j.1751-0813.2001.tb10718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen D.B., Buddington K.K., Buddington R.K. Dimensions and histologic characteristics of the small intestine of dogs during postnatal development. Am. J. Vet. Res. 2003;64:618–626. doi: 10.2460/ajvr.2003.64.618. [DOI] [PubMed] [Google Scholar]
- Potgieter L.N., Jones J.B., Patton C.S., Webb-Martin T.A. Experimental parvovirus infection in dogs. Can. J. Comp. Med. 1981;45:212–216. [PMC free article] [PubMed] [Google Scholar]
- Propst E.L., Flickinger E.A., Bauer L.L., Merchen N.R., Fahey G.C., Jr. A dose-response experiment evaluating the effects of oligofructose and inulin on nutrient digestibility, stool quality, and fecal protein catabolites in healthy adult dogs. J. Anim. Sci. 2003;81:3057–3066. doi: 10.2527/2003.81123057x. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., Groen J., Egberink H., Borst G.H., UytdeHaag F.G., Osterhaus A.D. The use of enzyme-linked immunosorbent assay systems for serology and antigen detection in parvovirus, coronavirus and rotavirus infections in dogs in The Netherlands. Vet. Microbiol. 1991;26:25–40. doi: 10.1016/0378-1135(91)90039-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe V.E., Adams C.A., Butterwick R.E., Batt R.M. Relationships between fecal consistency and colonic microstructure and absorptive function in dogs with and without nonspecific dietary sensitivity. Am. J. Vet. Res. 2002;63:617–622. doi: 10.2460/ajvr.2002.63.617. [DOI] [PubMed] [Google Scholar]
- Rolfe V.E., Adams C.A., Butterwick R.F., Batt R.M. Relationship between faecal character and intestinal transit time in normal dogs and diet-sensitive dogs. J. Small Anim. Pract. 2002;43:290–294. doi: 10.1111/j.1748-5827.2002.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Schmitz S., Coenen C., Konig M., Thiel H.J., Neiger R. Comparison of three rapid commercial Canine parvovirus antigen detection tests with electron microscopy and polymerase chain reaction. J. Vet. Diagn. Invest. 2009;21:344–345. doi: 10.1177/104063870902100306. [DOI] [PubMed] [Google Scholar]
- Schulz B.S., Strauch C., Mueller R.S., Eichhorn W., Hartmann K. Comparison of the prevalence of enteric viruses in healthy dogs and those with acute haemorrhagic diarrhoea by electron microscopy. J. Small Anim. Pract. 2008;49:84–88. doi: 10.1111/j.1748-5827.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolow S.H., Rand C., Marks S.L., Drazenovich N.L., Kather E.J., Foley J.E. Epidemiologic evaluation of diarrhea in dogs in an animal shelter. Am. J. Vet. Res. 2005;66:1018–1024. doi: 10.2460/ajvr.2005.66.1018. [DOI] [PubMed] [Google Scholar]
- Stavisky J., Radford A.D., Gaskell R., Dawson S., German A., Parsons B., Clegg S., Newman J., Pinchbeck G. A case–control study of pathogen and lifestyle risk factors for diarrhoea in dogs. Prev. Vet. Med. 2011;99:185–192. doi: 10.1016/j.prevetmed.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant B.J., Gaskell R.M., Jones R.C., Gaskell C.J. Studies on the epizootiology of canine coronavirus. Vet. Rec. 1993;132:7–11. doi: 10.1136/vr.132.1.7. [DOI] [PubMed] [Google Scholar]
- Vanparijs O., Hermans L., van der Flaes L. Helminth and protozoan parasites in dogs and cats in Belgium. Vet. Parasitol. 1991;38:67–73. doi: 10.1016/0304-4017(91)90010-s. [DOI] [PubMed] [Google Scholar]
- Weber M., Martin L., Biourge V., Nguyen P., Dumon H. Influence of age and body size on the digestibility of a dry expanded diet in dogs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2003;87:21–31. doi: 10.1046/j.1439-0396.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- Weber M., Stambouli F., Martin L., Dumon H., Biourge V., Nguyen P. Gastrointestinal transit of solid radiopaque markers in large and giant breed growing dogs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2001;85:242–250. doi: 10.1046/j.1439-0396.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Weber M.P., Hernot D., Nguyen P.G., Biourge V.C., Dumon H.J. Effect of size on electrolyte apparent absorption rates and fermentative activity in dogs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2004;88:356–365. doi: 10.1111/j.1439-0396.2004.00494.x. [DOI] [PubMed] [Google Scholar]
- Weber M.P., Stambouli F., Martin L.J., Dumon H.J., Biourge V.C., Nguyen P.G. Influence of age and body size on gastrointestinal transit time of radiopaque markers in healthy dogs. Am. J. Vet. Res. 2002;63:677–682. doi: 10.2460/ajvr.2002.63.677. [DOI] [PubMed] [Google Scholar]
- Yesilbag K., Yilmaz Z., Torun S., Pratelli A. Canine coronavirus infection in Turkish dog population. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2004;51:353–355. doi: 10.1111/j.1439-0450.2004.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


