Highlights
-
•
Prospective cohort study on bovine respiratory disease (BRD) in a rosé veal farm.
-
•
Calves with immunoglobulins < 7.5g/L at arrival have a higher BRD hazard.
-
•
Higher BRD hazard when no bovine coronavirus antibodies at arrival.
-
•
Higher BRD hazard when no bovine respiratory syncytial virus antibodies at arrival.
-
•
These parameters can aid in selection of calves with a reduced BRD risk.
Keywords: Bovine respiratory disease, Failure of passive transfer, Immunoglobulin, Neonatal diarrhea, Average daily gain, Veal calves
Abstract
Failure of passive transfer is a common problem in calves destined for veal production. At present it is unknown whether the risk for respiratory disease (BRD) or neonatal calf diarrhea (NCD) in the veal herd is associated with total immunoglobulin (Ig) and/or on the serostatus for respiratory pathogens measured at arrival. Therefore, the first objective of this prospective longitudinal cohort study was to determine associations between serum protein fractions as determined by routine electrophoresis (total protein, albumin, alpha-1 and -2 globulins, beta-globulins and Ig's) at arrival and BRD and NCD in the first 3 weeks of the production cycle. The second objective was to determine whether the serostatus (seropositive/seronegative) of seven respiratory pathogens (bovine respiratory syncytial virus (BRSV), parainfluenzavirus-3, bovine coronavirus (BCV), bovine herpesvirus-1, bovine viral diarrhea virus, Mannheimia haemolytica and Mycoplasma bovis) of these arrival serum samples could be associated with the risk of having BRD. The third objective was to determine which of the electrophoresis proteins and respiratory serostatuses were associated with average daily gain (ADG) in the study period. The study population consisted of 150 rosé veal calves housed in a single air–space. The study period ended at day 18 post arrival, when BRD incidence was judged to be too high to further postpone a group treatment. A Cox regression model was used to determine the effect of the studied protein fractions and antibodies on the time to BRD and NCD occurrence. The effect of the studied predictors on ADG was determined by linear regression. Calves with Ig levels under 7.5 g/L had an increased BRD hazard (hazard ratio (HR) = 1.9 (95% confidence interval (CI) = 1.2–3.0)). NCD was only positively associated with the alpha-2 globulin concentration. Calves with a negative serostatus for BCV (HR = 1.7 (95% CI = 1.0–2.8)) or BRSV (HR = 2.0 (95% CI = 1.0–3.9)) had an increased BRD hazard. Average daily gain (ADG) was 0.242 kg/day (SD = 0.142) and was not related to the occurrence of BRD or NCD. Calves with Ig's below 7.5 g/L and with increased levels of alpha-2 globulins showed a decrease in ADG. This study showed the importance of providing sufficient colostrum to veal calves and the potential benefit of the presence of BCV and BRSV antibodies at arrival to reduce the BRD hazard in the first 3 weeks.
1. Introduction
In recent years, high levels of antimicrobial (multi)resistance have been detected in pathogenic, commensal indicator and zoonotic bacteria in both the European and North American veal sector (Catry et al., 2005; Di Labio et al., 2007; Cook et al., 2011, MARAN-2012, 2012). The emergence of livestock associated methicillin resistant Staphylococcus aureus and extended beta-lactamases (ESBL's) in enterobacteriaceae from food animals has initiated a public discussion in Western Europe, which severely increased pressure on veterinary antimicrobial use (Graveland et al., 2010; Vandendriessche et al., 2013). Antimicrobial resistance selection is predominantly driven by antimicrobial use (Bosman et al., 2013). Therefore, the intensive antimicrobial use in the veal sector urgently needs to be reduced, both for public health and political and economic reasons (MARAN, 2009, 2011; Pardon et al., 2012a).
Selection of calves based on disease risk and subsequently targetting metaphylactic antimicrobial treatment toward the high risk batches, is one potential strategy to rationally reduce antimicrobial consumption. Calf selection parameters include age at arrival, body weight, infection status for bovine viral diarrhea virus (BVDV), presence of disease (umbilical infection, bovine respiratory disease (BRD) or neonatal calf diarrhea (NCD)) (Wilson et al., 2000) and sufficient uptake of colostrum (Gulliksen et al., 2009, Brscic et al., 2012, Pardon et al., 2013). Failure of passive transfer (FPT) shows a prevalence ranging between 20 and 80% in the United States (McDonough et al., 1994, Wilson et al., 2000, Stilwell and Carvalho, 2011). Also in Belgium on average 40% of veal calves had FPT, assuming that immunoglobulin G (IgG) levels lower than 10 g/L upon arrival indicate colostral deficiency (Pardon, 2012). The crucial role of sufficient IgG uptake in protecting calves from BRD, NCD and mortality has been evidenced on multiple occasions (Postema and Mol, 1984, Furman-Fratczak et al., 2011, Stilwell and Carvalho, 2011). These effects have been shown to last up to 3 months for BRD (Virtala et al., 1996). To evaluate colostrum uptake, several tests have been validated for healthy animals in the age category of 2–7 days (Weaver et al., 2000). Although that cheap indirect screening tests, such as determination of total protein (TP) with a refractometer, perform reasonably well, direct determination of immunoglobulins (Ig) or IgG is generally more specific (Weaver et al., 2000). In calves sampled within a week after birth a cut off value for IgG of 10 g/L is generally accepted to determine FPT, and has been linked with disease occurrence, although some studies reported higher cut-offs in particular situations (Waldner and Rosengren, 2009, Furman-Fratczak et al., 2011, Stilwell and Carvalho, 2011). In contrast, it is currently unknown whether Ig levels measured at the age of arrival in veal calves (2–4 weeks old) can be used as predictors of BRD or NCD. For the veal sector it would be interesting to identify, through testing of calves upon arrival, individual calves requiring an adapted treatment and herds of origin with an insufficient colostrum management.
Therefore, the first objective of the present study was to determine whether Ig's or any other protein determined by routine electrophoresis measured at arrival, are associated with occurrence of BRD or NCD in veal calves in the first weeks after arrival, and are therefore potentially useful for risk classification of calves at arrival. The hypothesis was that calves with low TP or Ig would have a substantial higher risk acquiring BRD or NCD.
To gain further insight in which antibodies might play a protective role for BRD, the second objective was to determine the association between the serostatus for 7 respiratory pathogens, measured at arrival, and BRD occurrence.
Since earlier studies demonstrated a direct effect of FPT on average daily gain (ADG) in the first 3 months of life (Robison et al., 1988, Virtala et al., 1996), the third objective was to determine which of the studied serum parameters (electrophoresis protein fractions and respiratory antibodies) and diseases were associated with ADG in the study period.
2. Materials and methods
The study design was a prospective longitudinal cohort study. The study protocol was reviewed and approved by the ethical committee of the Faculty of veterinary medicine (Ghent University) under license EC 2013/184.
2.1. Study population and study period
The study was carried out in a typical rosé starter veal farm located in the South of the Netherlands, in January 2014. This type of operation is specialized in raising calves for 8 weeks, after which they are transported to a finisher herd. The sole criterion for farm selection was willingness to cooperate. Study calves were housed on slatted floors in the same air–space. During the first 6 weeks calves were housed individually in metal framed pens, with 1.4 m2 floor surface and an air volume of 9.0 m3 per calf. After this period the metal framework was removed and the animals were kept in groups of 6 animals for the remaining of the raising period. The study compartment was physically isolated from the other calf units present at the farm by full walls and a separate ventilation system. The stable was mechanically ventilated at a 20% refreshing rate and temperature was maintained at 17 °C. Calves were fed a 21.2% crude protein (CP), 17.7% crude fat (CF) milk replacer (MR) diet at dry mater base. The amount of MR was gradually increased from 1.5 L (90 g powder/L) morning and evening at day 1 to twice daily 3 L at 110 g/L at the end of the study (day 18). Milk was individually provided in drinking buckets. In addition to MR calves received chopped straw (from 20 to 50 g/day over the 18 day study period) and starter concentrates (from 10 to 350 g/day; CP = 16%; CF = 2.8%). The study group was not vaccinated and did not receive any antimicrobial group treatment at arrival.
2.2. Sample size calculations
Sample size was calculated to detect a 25% difference in BRD incidence between calves with more or less than 10 g Ig/L (25% vs. 50%), with 95% confidence and 80% power (Winepiscope 2.0., Thrusfield et al., 2001). For a two-tailed test 56 animals within each group were needed. For practical reasons and to account for mortality, the study group consisted of all animals present in the same airspace, being 150 animals.
2.3. Measurements
For ethical and economic reasons it was agreed beforehand that the study would finish when the responsible veterinarian deemed it necessary to initiate a group treatment. Although no objective criteria were set, this usually is done when 10% of the animals is ill on the same day, which occurs with high predictability between week 2 and 3 (Pardon et al., 2011, Pardon et al., 2012a). In casu, the study period lasted from arrival to day 18.
2.3.1. Health monitoring, case definitions and antimicrobial treatment
At arrival, all calves were clinically examined. Health status was monitored twice daily (morning and evening) for the duration of the study by the same veterinarian. The case definition for NCD was presence of diarrhea (partial) anorexia and depression. For BRD the case definition was based on a scoring system using the following symptoms: depression, cough, rectal temperature and nasal discharge (Table 1 ). Animals with a score of ≥5 were considered a case. Omphalitis was defined as the presence of a painful umbilical swelling at arrival.
Table 1.
Score system used for detection and case definition of bovine respiratory disease in rosé veal calves.
| Symptom/score | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Fever | <39 °C | – | 39.1–39.5 | >39.5 |
| Cough | - Absent - Negative trachea reflex |
- Not spontaneous - Positive trachea reflex |
- Spontaneous (single occasion) - Positive trachea reflex |
- Spontaneous (continuous) - Positive trachea reflex |
| Depression | - Standing - Normal ear position |
- Standing - Drooping ears |
- Recumbency - Drooping ears - Rises when approached |
- Recumbency - Drooping ears - Remains recumbent upon approach |
| Nasal discharge | - Absent - Mildly serous |
- Bilateral serous discharge |
- Unilateral (muco)purulent discharge | - Bilateral (muco)purulent discharge |
Animals diagnosed with omphalitis were treated with 10,000 IU of benzylpenicillin (Procpen30®, Dopharma, Raamsdonksveer, The Netherlands) IM daily, for 5 days. NCD was treated with sulphadoxin–trimethoprim (Dofatrim-ject, Dopharma, Raamdonksveer, the Netherlands) (16 mg/kg IM, sid, 5 days) and meloxicam (Novem, Boehringer Ingelheim Vetmedica GmbH, Ingelheim/Rhein, Germany) (0.5 mg/kg IM, sid, 1 day). In case there was no response to treatment, NCD cases were treated with 2 mg/kg gentamicin IM (Genta-ject® 10%, Dopharma, Raamsdonksveer, Nederland). BRD cases were treated with a single injection of tildipirosin (4 mg/kg subcutaneously) (Zuprevo 18%, Merck, USA) and meloxicam. The drug use during the study period was quantified by standard daily dose methodology as described previously (Pardon et al., 2012a). The animal defined daily doses (ADD's) for each antimicrobial drug were as mentioned above (derived from the Belgian veterinary repertory (BCFI, 2013)). For combined products (e.g. sulphonamides with trimethoprim), each administration was counted as two doses. For long acting products, a long acting factor was used. For tildipirosin this was 9.25, as recommended by the manufacturer.
2.3.2. Sampling and storage
At arrival and at the end of the study period (day 23) all calves were weighed with an electronic balance (Rinstrum R323) at 0.2 kg precise. Blood was taken from the jugular vein with a 20 G needle vacutainer system (Venoject, Terumo, Belgium) at arrival and at the end of the study period. Blood was centrifuged (970 × g for 10 min at approximately 20 °C) and stored at −18 °C until laboratory analyses.
2.3.3. Laboratory analysis
2.3.3.1. Determination of the etiology of NCD
An antigen ELISA to detect Cyptosporidium parvum, bovine coronavirus (BCV), bovine rotavirus and enterotoxic Escherichia coli (Tetrastrips, BIO K 156, Bio-X, Jemelle, Belgium) was conducted in fresh fecal samples from NCD cases.
2.3.3.2. Determination of circulating BRD pathogens and serostatus at arrival
To identify circulating BRD pathogens in the study period, an antibody ELISA for bovine respiratory syncytial virus (BRSV), parainfluenzavirus (PI-3), bovine herpesvirus 1 (BHV-1), BVDV, Mycoplasma bovis (ELISA Respiratory kit, Bio-K 284, Bio-X, Jemelle, Belgium), BCV (Bio-K 127, Bio-X, Jemelle, Belgium) and Mannheimia haemolytica (Bio-K 139, Bio-X, Jemelle, Belgium) was performed on paired samples to determine seroconversion. Samples of the same animal were tested on the same plate. Determination of seroconversion and serostatus at arrival (positive or negative) were according to the manufacturer's guidelines.
2.3.3.3. Electrophoresis
To determine the amount of albumin, alpha-1 globulins (A1g), alpha-2 globulins (A2g), beta-globulins (Bg) and gamma-globulins (immunoglobulins, Ig's) in the arrival serum samples, electrophoresis was conducted by solid phase extraction (Capillarys, Sebia, France) at an accredited laboratory (Zoolyx, Aalst, Belgium) using optimized settings for animal samples. TP was measured colorimetrically on an automated biochemistry analyzer (Cobas 6000, Roche, Belgium). Quality controls previously run were within quality assurance specifications.
2.4. Statistical analysis
Data were analyzed with SAS 9.4 (SAS Institute Inc., Cary, NC). The unit of analysis was the individual calf.
2.4.1. Ig and other routine serum electrophoresis proteins as predictors of BRD and NCD
The working hypothesis was that both total protein and Ig are predictors for BRD and NCD in veal calves, whereas the other protein fractions determined by routine electrophoresis (albumin, Ag and Bg) are not. Survival analysis was used to assess the association between the 6 serum protein fractions (TP, albumin, A1g, A2g, Bg and Ig) and the time until BRD or NCD diagnosis. Two Cox proportional hazards models (PROC PHREG) were built, with respectively NCD and BRD as outcome variable using Breslow ties. In each model, 7 predictors, being the 6 protein fractions (TP, albumin, A1g, A2g, Bg and Ig) and body weight at arrival as a potential confounder were evaluated. Calves arrived in three batches and in order to correct for clustering, arrival batch was added as fixed effect. Predictors were tested univariably both continuously as categorically, based on quartiles. If a significant effect was noted in one of the quartiles, a binary variable was constructed. The time between arrival and occurrence of NCD or BRD was defined as survival time and disease (either BRD or NCD) as the event. Censoring occurred at day 18, when the study ended, for each animal remaining undiseased. The model building procedure was as follows. First all predictors were tested univariably, and those with P < 0.20 were selected for multivariable modeling. The final multivariable model was built stepwise backwards, excluding non-significant variables. Next, all biologically relevant two-way interactions of significant fixed effects were tested. Significance was set at P < 0.05 and P < 0.10 was considered a trend. Wald's test was used to assess parameter estimate significance. Visual inspection of the log-cumulative hazard plots and/or Schoenfeld residuals (Schoenfeld, 1982) and construction of time-varying covariates were used to evaluate the proportional hazard assumption.
For significant protein fractions, their relationship with the studied disease was visualized by means of Kaplan–Meier survival curves (PROC LIFETEST) and a Log-rank test was performed. In practice, a recommendation regarding the threshold to identify future problematic animals based on a significant parameter is desired. Therefore, for significant parameters of primary interest, such as Ig, a receiver operating characteristics (ROC) curve was created to determine the optimal Ig cut-off value for BRD or NCD. Subsequently, a categorical variable was created for these variables based upon this cut-off, replacing the original continuous variable.
2.4.2. Serostatus for respiratory pathogens in arrival serum samples as predictors of BRD
Hypothesis was that animals, seronegative for a respiratory pathogen, would experience an increased BRD hazard in the study period. A Cox proportional hazard model was built with BRD as the event variable and time between arrival and occurrence of BRD as the survival time. All cases which did not experience BRD before day 18 were right censored. Predictors tested (n = 8) were the serostatus for BRSV, PI-3, BCV, BHV-1, BVDV, M. haemolytica and M. bovis and the body weight at arrival (potential confounder). Arrival batch was added as a fixed effect to adjust for clustering. The model building procedure, evaluation and visualization were as mentioned under Section 2.4.1.
2.4.3. Relation of serum protein fractions and respiratory pathogen serostatus with ADG
Working hypothesis was that low TP and Ig were associated with reduced ADG. Similarly, a reduced ADG in seronegative animals for any of the 7 respiratory pathogens was expected. Normal distribution of ADG was checked by Q–Q plot and by the Kolmogorov–Smirnov test. A linear mixed model (PROC MIXED) with arrival batch as a random effect to account for clustering within a batch was used. The covariance structure was specified as variance components. The effect of 15 predictors was evaluated (6 serum protein fractions (TP, albumin, A1g, A2g, Bg and Ig), 7 serostatuses for respiratory pathogens (BRSV, PI-3, BVDV, BHV-1, BCV, BHV-1, M. haemolytica and M. bovis) and 2 diseases (NCD and BRD). First all predictors were tested univariably for their association with ADG. Continuous predictors were added continuously and in quartiles.
All predictors with P < 0.2 were maintained for the multivariable model, which was built stepwise backward, excluding non-significant variables. Pearson and Spearman's rho correlations were calculated between significant predictors and when correlation was higher than 0.60, only the most significant variable was retained in the multivariable models. Relationships between significant categorical predictors were explored by univariable logistic regression (PROC LOGISTIC). In the final model, pairwise comparisons for categorical predictors were made, with Bonferroni adjustment for multiple comparisons. All biologically relevant two-way interactions of significant fixed effects were tested. Significance was set at P < 0.05 and P < 0.10 was considered a trend. Model fit and assumptions were evaluated by checking the normal distribution of the residuals.
3. Results
3.1. Disease occurrence, circulating pathogens, antimicrobial use and daily growth
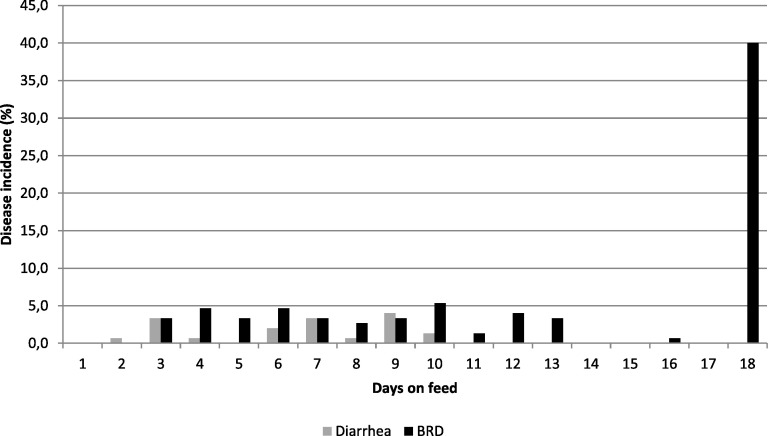
Calves arrived at the same day in three batches, of 27, 26 and 97 calves. The largest batch originated from Germany, the other two from the Netherlands. All calves were male, mean arrival age was 17.4 days (standard deviation (SD) = 4.7; range (R) = 12–41 days) and mean arrival weight 50.7 kg (SD = 3.5; R = 39.6–69.4). At arrival 4, calves (2.7%) had omphalitis and one (0.7%) diarrhea. In Fig. 1 the number of new cases of NCD and BRD over the study period is presented. Diarrhea only occurred in the first 10 days, with a cumulative incidence (CI) of 15.3% (23/150). Daily BRD incidence remained below 5% in the first 17 days, but at day 18 CI had accumulated to 40% (60/150). At day 18 a BRD outbreak occurred involving 40% (60/150) of the calves and the study was stopped. At this day the animals received an oral group treatment with 12 mg/kg tilmicosin phosphate (Pulmotil AC, Elanco, Indianapolis, USA) which blurred any further disease detection. In total, 61% (92/150) of the calves developed BRD in the study period of which 30.4% (28/92) relapsed. The mean score of BRD cases was 5.7 (SD = 0.8; R = 5–8) and 87.5% had a rectal temperature over 39.5 °C at treatment.
Fig. 1.
Incidence of BRD and diarrhea in the first weeks after arrival in a rosé veal operation.
Regarding NCD cases, only C. parvum was detected in 30% of the fecal samples (n = 10). The most frequent respiratory pathogens circulating during the study period were M. bovis, BCV and BVDV (Table 2 ). One calf died during the study and necropsy demonstrated enteritis, dehydration and low body condition. During the study period a calf received on average 6.2 ADD's, of which 3.9 ADD's for respiratory disease, 1.9 ADD's for NCD and 0.4 ADD's for umbilical infection. ADG in the study period was 0.242 kg/day (SD = 0.142; R = −0.139 to 0.670).
Table 2.
Serostatus at arrival and seroconversion rate to 7 respiratory pathogens of rosé veal calves in the first three weeks after arrival at the finishing unit.
| Pathogen | Percentage of seropositives at arrival (number) | Number of seroconversions (%) |
|---|---|---|
| Bovine respiratory syncytial virus | 90.0 (133/148) | 0.8 (1/133) |
| Parainfluenzavirus type 3 | 92.6 (137/148) | 0.8 (1/133) |
| Bovine viral diarrhea virus | 40.5 (60/148) | 8.3 (11/133) |
| Bovine herpesvirus 1 | 48.6 (72/148) | 0.8 (1/133) |
| Bovine coronavirus | 75.0 (111/148) | 16.5 (22/133) |
| Mannheimia haemolytica | 21.0 (31/148) | 0 (0/133) |
| Mycoplasma bovis | 16.2 (24/148) | 12.0 (16/133) |
3.2. Ig and other routine serum electrophoresis proteins as predictors of BRD and NCD
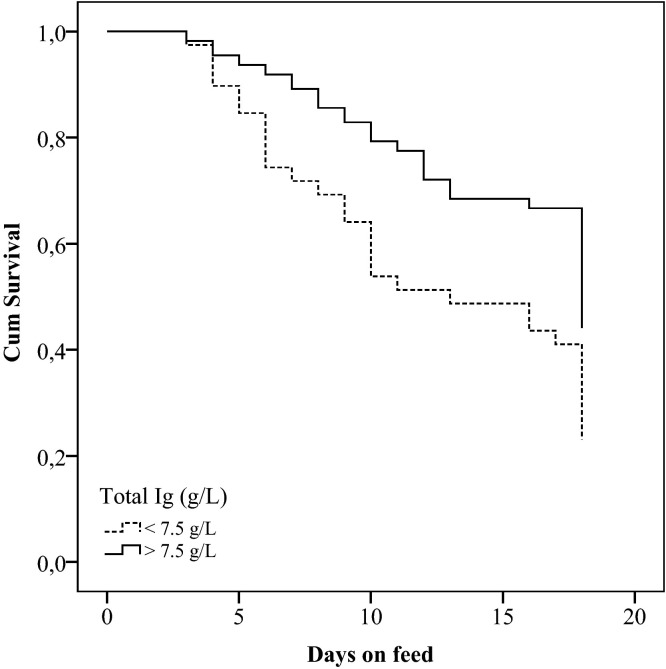
In the study population mean total Ig concentration was 10 g/L (SD = 4.2; R = 1–25) and 41.3% (62/150) of the calves had Ig levels below 10 g/L. In Table 3 mean values of the serum protein fractions in calves with or without NCD and BRD are displayed. ROC analysis for Ig demonstrated that the optimal cut-off (area under the curve = 0.60; sensitivity = 85%; specificity = 33%) to predict whether a calf would not develop BRD was 7.5 g/L. Subsequently, Ig was added to the model as a binary variable based on this cut off. Factors univariably associated (P < 0.20) with BRD hazard were body weight at arrival (P = 0.14) and Ig (>7.5/<7.5) (P < 0.05). In the final model only Ig remained significantly associated with the time until occurrence of BRD (Table 4 , Fig. 2 ).
Table 3.
Routine serum electrophoresis protein fractions (in g/L) at arrival and average daily gain (ADG) of rosé veal calves stratified according to bovine respiratory disease (BRD) and neonatal calf diarrhea (NCD) status.a
| Disease | Level | Calves (n) | ADG (kg/day) | Total protein | Albumin | Alfa1-globulins | Alfa2-globulins | Beta-globulins | Gamma-globulins |
|---|---|---|---|---|---|---|---|---|---|
| BRD | No | 57 | 0.255 ± 0.141 | 58.5 ± 6.1 | 25.9 ± 2.9 | 0.3 ± 0.5 | 11.8 ± 0.2 | 9.6 ± 1.4 | 10.7 ± 3.8 |
| Yes | 92 | 0.233 ± 0.143 | 57.4 ± 6.9 | 26.5 ± 2.6 | 0.3 ± 0.4 | 12.0 ± 2.1 | 9.3 ± 1.5 | 9.3 ± 4.4 | |
| NCD | No | 127 | 0.245 ± 0.143 | 57.7 ± 6.5 | 26.4 ± 2.6 | 0.2 ± 0.4 | 11.8 ± 1.9 | 9.3 ± 1.4 | 9.9 ± 4.3 |
| Yes | 22 | 0.221 ± 0.142 | 58.5 ± 7.1 | 25.6 ± 3.0 | 0.5 ± 0.5 | 12.6 ± 2.0 | 10.0 ± 1.6 | 9.8 ± 3.7 |
Values are displayed as mean ± standard deviation.
Table 4.
Cox regression model output on the relation between serum protein fractions determined by routine electrophoresis at arrival on time to diagnosis of bovine respiratory disease in a cohort of 150 rosé veal calves originating from 3 different batches.
| Variable | Category | Calves (n) | BRD positive (%) | HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Arrival batch | NL1 | 26 | 61.5 | ref. | ||
| NL2 | 27 | 74.1 | 0.9 | 0.5–1.6 | 0.75 | |
| GER | 97 | 57.7 | 1.3 | 0.7–2.5 | 0.44 | |
| Immunoglobulin (Ig) (g/L) | Positive | 111 | 55.9 | ref. | ||
| Negative | 39 | 76.9 | 1.9 | 1.2–3.0 | <0.05 |
HR = hazard ratio; ref. = referent category; NL = the Netherlands; GER = Germany; BRD = bovine respiratory disease.
Fig. 2.
Survival graph for occurrence of BRD in rosé veal calves in the first weeks after arrival, according to the immunoglobulin (Ig (g/L)) concentration measured upon arrival (Log Rank test: χ2 = 9.3, df = 1; P < 0.01).
For NCD, a significant association with the level of alpha-2 globulin was present. For every increase in alpha-2 globulin by 1 g/L the NCD hazard increased by 17% (Table 5 ). The arrival batch effect was significant, with calves from the smallest batch from the Netherlands experiencing a significantly higher NCD risk compared to the other batches.
Table 5.
Cox regression model output on the relation between serum protein fractions determined by routine electrophoresis at arrival on time to diagnosis of neonatal diarrhea in a cohort of 150 rosé veal calves originating from 3 different batches.
| Variable | Category | Calves (n) | NCD positive (%) | HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Arrival batch | NL1 | 26 | 34.6 | ref. | ||
| NL2 | 27 | 11.1 | 0.3 | 0.1–0.7 | <0.01 | |
| GER | 97 | 11.3 | 0.3 | 0.1–1.1 | 0.07 | |
| Alpha-2 globulin (g/L) | 1.2 | 1.0–1.4 | <0.05 |
HR = hazard ratio; ref. = referent category; NL = the Netherlands; GER = Germany; NCD = neonatal calf diarrhea.
3.3. Serostatus for respiratory pathogens in arrival serum samples as predictors of BRD
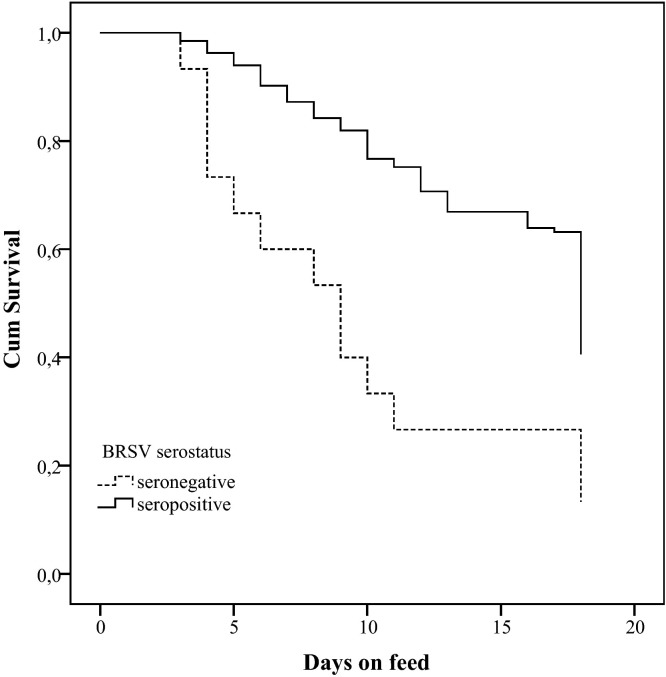
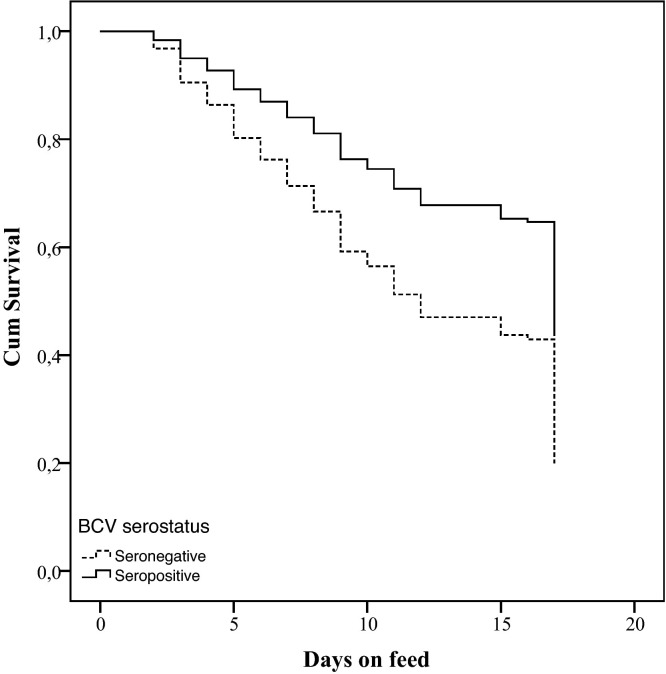
The antibody ELISA to determine the serostatus for the respiratory pathogens studied was not interpretable in 2 calves, leaving 148 calves for analysis. Table 6 shows the BRD incidence according to the serostatus for the 7 respiratory pathogens at arrival. Univariably associated predictors of the time until BRD occurrence, which were withheld for the multivariable model, were BRSV (P < 0.001), BCV (P < 0.01), PI-3 (P = 0.12) and M. bovis (P = 0.12). The serostatus for BCV and BRSV remained significant in the multivariable model (P < 0.05) (Table 7 , Fig. 3, Fig. 4 ).
Table 6.
Bovine respiratory disease (BRD) incidence stratified to the serostatus for 7 respiratory pathogens at arrival in 148 rosé veal calves.
| Pathogen | Serostatus | Number | BRD (%) |
|---|---|---|---|
| Bovine respiratory syncytial virus | Negative | 15 | 86.7 |
| Positive | 133 | 59.4 | |
| Parainfluenzavirus 3 | Negative | 11 | 72.7 |
| Positive | 137 | 61.3 | |
| Bovine viral diarrhea virus | Negative | 88 | 63.8 |
| Positive | 60 | 60.0 | |
| Bovine herpesvirus-1 | Negative | 76 | 68.4 |
| Positive | 72 | 55.6 | |
| Bovine coronavirus | Negative | 37 | 81.1 |
| Positive | 111 | 55.9 | |
| Mannheimia haemolytica | Negative | 117 | 64.1 |
| Positive | 31 | 54.8 | |
| Mycoplasma bovis | Negative | 124 | 65.3 |
| Positive | 24 | 45.8 |
Table 7.
Cox regression model output of the relation between the serostatus for respiratory pathogens at arrival on time to diagnosis of bovine respiratory disease in a cohort of 148 rosé veal calves originating from 3 different batches.
| Variable | Level | Calves (n) | BRD positive (%) | HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Arrival batch | NL1 | 24 | 62.5 | Ref. | ||
| NL2 | 27 | 74.1 | 1.0 | 0.5–1.9 | 0.96 | |
| GER | 97 | 57.7 | 0.8 | 0.4–1.4 | 0.38 | |
| BRSV-serostatus | Positive | 133 | 59.4 | Ref. | ||
| Negative | 15 | 86.7 | 2.0 | 1.0–3.9 | <0.05 | |
| BCV-serostatus | Positive | 111 | 55.9 | Ref. | ||
| Negative | 37 | 81.1 | 1.7 | 1.0–2.8 | <0.05 |
HR = hazard ratio; ref. = referent category; NL = the Netherlands; GER = Germany; BRSV = bovine respiratory syncytial virus; BCV = bovine coronavirus; BRD = bovine respiratory disease.
Fig. 3.
Survival graph for occurrence of BRD in rosé veal calves in the first weeks after arrival, according to the serostatus for bovine respiratory syncytial virus (BRSV) measured upon arrival (Log Rank test: χ2 = 13.8, df = 1; P < 0.001)
Fig. 4.
Survival graph for occurrence of BRD in rosé veal calves in the first weeks after arrival, according to the serostatus for bovine coronavirus (BCV) measured upon arrival (Log Rank test: χ2 = 10.7, df = 1; P < 0.01).
3.4. Influence of serum protein fractions and respiratory pathogen serostatus on ADG
Descriptives on ADG according to disease status are provided in Table 3. Factors univariably associated with ADG were albumin (P = 0.15), A1g (P = 0.13), A2g (P < 0.05), BCV (P = 0.10), BHV-1 (P < 0.05), BRSV (P < 0.05) and PI-3 (P = 0.08). The final multivariable model consisted of Ig and alpha-2 globulin (Table 8 ). Calves with Ig levels <7.5 g/L at arrival grew on average 72 g/day (95% CI = 29–129) less, and for every increase of alpha-2 globulin by 1 g/L, ADG decreased by 12 g/day (95% CI = 0–24).
Table 8.
Results of a linear mixed model on the relationship of serum protein fractions determined by routine electrophoresis and the serostatus for respiratory pathogens measured at arrival with average daily gain (kg/day) in 147 rosé veal calves.
| Variable | Level | Reference | β | 95% CI | P-value |
|---|---|---|---|---|---|
| Intercept | 0.391 | 0.079–0.703 | <0.001 | ||
| Alpha-2 globulin (g/L) | −0.012 | −0.024 to −0.0 | <0.05 | ||
| Immunoglobulin (Ig) (g/L) | <7.5 g/L | >7.5 g/L | −0.079 | −0.129 to −0.029 | <0.01 |
CI = confidence interval.
Arrival batch was added as a random effect (1% of total variation in average daily gain was situated at the arrival batch level).
4. Discussion
In contrast to previous studies on disease occurrence in veal calves (Postema and Mol, 1984, Postema et al., 1987, Brscic et al., 2012, Pardon, 2012), disease detection in the present study was not blurred by the routine administration of antimicrobials at arrival. Given the intensive daily monitoring it was no surprise to find a much higher BRD incidence (60% in the first three weeks) compared to previously reported figures in white veal calves over the complete production cycle (Brscic et al., 2012, Pardon et al., 2012b). Also for NCD the cumulative incidence in the present study was three times higher than previously reported (Pardon et al., 2012b). These observations confirm that disease incidence is masked in practice due to the administration of antimicrobial group treatments. Given the very similar prevalence of FPT in the present study compared to previous work in Europe and the US, and because of the systematic presence of all respiratory pathogens in veal herds (Wilson et al., 2000, Pardon et al., 2011, Pardon, 2012), the study most likely has a good external validity for the veal sector.
In previous studies on maternal Ig in calves, radial immunodiffusion is mentioned as the gold standard assay for IgG determination. This test is expensive in contrast to electrophoresis or refractometry to be used at large scale in the veal industry. Therefore the authors opted to use serum electrophoresis to determine the concentration of Ig's, a test which is used routinely in many veterinary diagnostic laboratories all over Europe. At the age of 3 weeks, the Ig fraction consists for the vast majority of IgG (95%), and therefore results obtained by electrophoresis are highly similar to those from tests which only determine IgG (Burton et al., 1989).
The main finding of the present study is a clear relation between Ig's measured at arrival and the time of BRD occurrence at the veal farm. This observation is in contrast with previous studies which could not evidence such an association (Postema and Mol, 1984, Postema et al., 1987). The most likely explanation for this difference is suboptimal disease expression and thus detection by the administration of antimicrobial group treatments in previous studies. Whereas in the present study the optimal cut-off to predict BRD in veal calves is 7.5 g/L, previous studies used 10 g/L as cut off. Maternal antibodies quickly decline after birth and normal IgG range for healthy calves, which have received enough colostrum, is reported to be between 10 and 15 g/L at the age of 2–3 weeks (Hassig et al., 2007). Therefore, it is not illogical that calves with FPT have levels below 10 g/L at the age of 2–3 weeks. Calves with Ig < 7.5 g/L had a substantial higher BRD hazard and according to Berge et al. (2005) animal welfare might be compromised when no antimicrobials are administered to this group at arrival. However, since only 26% of the calves were involved, group treatment does not comply with prudent use of antimicrobials. Also, for example in the Netherlands and Belgium, prophylactic administration of antimicrobials is forbidden or strongly discouraged, respectively.
At present the most practical application of Ig determination for the veal sector will be a regular screening in calves after purchase in order to identify the herds of origin with an insufficient colostrum management. The observed association between Ig levels, measured at the age of 2–3 weeks, and BRD is also interesting for conventional dairy and beef farming. It enlarges the window in which calves can be tested for FPT up to the third week of life, facilitating routine checking of FPT in a herd. In this context it is important to emphasize that measurement of TP, the cheapest option, is no longer useful at the age of 2–3 weeks in contrast to in the first days of life. In calves aged 2–3 weeks albumin concentration has increased, decreasing the proportion of Ig's in TP, and this might explain absence of a significant association of TP and disease (Mohri et al., 2007). Also the well-known influence of hydration status and protein loss by various causes (e.g. diarrhea) on TP levels (Weaver et al., 2000) might have played a role as some animals were already diseased at testing or shortly thereafter.
We did not find a similar relationship between Ig's and NCD, as observed for BRD. The most likely reason is that only C. parvum was identified, for which colostral antibodies do not confer protection. Also in previous studies C. parvum was the most frequently isolated pathogen in veal calf diarrhea (McDonough et al., 1994, Hoet et al., 2003). There was however a significant association of NCD with increasing levels of alpha-2 globulin. NCD already was present at arrival or developed shortly thereafter, and therefore several animals might already have had inflamed intestines. Since the alpha-2 globulin fraction contains the acute phase proteins haptoglobulin and ceruloplasmin, this already ongoing inflammation most likely explains the observed association.
We also observed that calves which were seronegative for BCV or BRSV had an increased BRD hazard. It might signify that among all viruses, BCV and BRSV contributed the most to BRD in the first weeks after arrival. On the other hand, the observed relationship might just be due to the fact that these viruses were the most prevalent pathogen in the study group, clarifying the benefit of the presence of BCV and BRSV antibodies at arrival. It is not unlikely, that in cohorts in which another virus predominates, a seronegative status for that particular virus will be a risk factor. Nevertheless, based upon the present observations providing sufficient specific immunity against respiratory viruses before transport to the veal farm, either by vaccination or by colostrum uptake might be an interesting strategy to reduce the risk of BRD in veal calves.
ADG is one of the primary economic outcomes in the veal sector. Average ADG was 0.242 kg/day, which is in line with the expected growth for that diet (3.1 Mcal at day 18 at an ambient temperature of 15 °C (NRC, 2001)). The well-known negative effects of BRD and NCD on ADG (Wittum et al., 1994, Rerat et al., 2012, Pardon et al., 2013) were not observed in the present study. Possibly intensive monitoring and early treatment alleviated inflammation or BRD was only of viral origin leading to mild inflammation. The association between low Ig and ADG, observed by other authors in dairy calves, was seen in veal calves at a later age (Robison et al., 1988, Virtala et al., 1996, Berge et al., 2009). The lower ADG in calves with higher alpha-2 globulin concentrations, most likely reflects the increased protein and caloric cost of an ongoing inflammation (Barnes et al., 2002).
In conclusion, a clear effect of Ig concentration at arrival on BRD and ADG in the first 3 weeks was demonstrated in veal calves. Possible applications of screening veal calves at arrival might be the identification of herds of origin with a poor colostrum management or classification of calves according to BRD risk to target antimicrobial treatment. For practical use, an Ig cut-off of 7.5 g/L is recommended. The other protein fractions determined by routine electrophoresis, including TP, did not display any association of direct practical use. Secondly, calves seronegative for BCV and BRSV at arrival had an increased BRD risk in the first 3 weeks of the production cycle. Therefore, assuring a seropositive status for these viruses either by vaccination on the farm of origin or by provision of colostrum from a vaccinated cow might be an appropriate approach to reduce the BRD risk and subsequently prudent antimicrobial use in the veal calf sector.
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organizations which could inappropriately influence or bias the content of the paper. The study was supported by funding of the first authors institute.
Acknowledgements
The authors are grateful to the veal farmer and integrator for their collaboration. Veterinary students are acknowledged for their help during the trial. S. Stuyvaert is acknowledged for performing the antibody ELISAs.
Contributor Information
Bart Pardon, Email: Bart.Pardon@UGent.be.
Jeroen Alliët, Email: Jeroen.Alliet@UGent.be.
Randy Boone, Email: Dapdeboskant@Gmail.com.
Sophie Roelandt, Email: Sophie.Roelandt@coda-cerva.be.
Bonnie Valgaeren, Email: Bonnie.Valgaeren@UGent.be.
Piet Deprez, Email: Piet.Deprez@UGent.be.
References
- Barnes D.M., Song Z., Klasing K.C., Bottje W. Protein metabolism during an acute phase response in chickens. Amino Acids. 2002;22:15–26. doi: 10.1007/s726-002-8198-6. [DOI] [PubMed] [Google Scholar]
- BCFI . 2013. Commented Drug Repertorium for Veterinary Use. Ed.: Belgian Center for Pharmacotherapeutic Information, P. Gustin, Ghent, Belgium. www.bcfi-vet.be (accessed 03.06.14) [Google Scholar]
- Berge A.C., Lindeque P., Moore D.A., Sischo W.M. A clinical trail evaluating prophylactic and therapeutic antibiotic use on health and performance of preweaned calves. J. Dairy Sci. 2005;88:2166–2177. doi: 10.3168/jds.S0022-0302(05)72892-7. [DOI] [PubMed] [Google Scholar]
- Berge A.C.B., Besser T.E., Moore D.A., Sischo W.M. Evaluation of the effects of oral colostrum supplementation during the first fourteen days on the health and performance of preweaned calves. J. Dairy Sci. 2009;92:286–295. doi: 10.3168/jds.2008-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman A.B., Wagenaar J.A., Stegeman J.A., Vernooij J.C., Mevius D.J. Antimicrobial resistance in commensal Escherichia coli in veal calves is associated with antimicrobial drug use. Epidemiol. Infect. 2013:1–12. doi: 10.1017/S0950268813002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brscic M., Leruste H., Heutinck L.F., Bokkers E.A., Wolthuis-Fillerup M., Stockhofe N., Gottardo F., Lensink B.J., Cozzi G., Van Reenen C.G. Prevalence of respiratory disorders in veal calves and potential risk factors. J. Dairy Sci. 2012;95:2753–2764. doi: 10.3168/jds.2011-4699. [DOI] [PubMed] [Google Scholar]
- Burton J.L., Kennedy B.W., Burnside E.B., Wilkie B.N., Burton J.H. Variation in serum concentrations of immunoglobulins G, A and M in Canadian Holstein–Friesian calves. J. Dairy Sci. 1989;72:135–149. doi: 10.3168/jds.S0022-0302(89)79089-5. [DOI] [PubMed] [Google Scholar]
- Catry B., Haesebrouck F., Vliegher S.D. Variability in acquired resistance of Pasteurella and Mannheimia isolates from the nasopharynx of calves, with particular reference to different herd types. Microb. Drug Resist. 2005;11:387–394. doi: 10.1089/mdr.2005.11.387. [DOI] [PubMed] [Google Scholar]
- Cook A., Reid-Smith R.J., Irwin R.J. Antimicrobial resistance in Escherichia coli isolated from retail milk-fed veal meat from Southern Ontario, Canada. J. Food Prot. 2011;74:1328–1333. doi: 10.4315/0362-028X.JFP-10-495. [DOI] [PubMed] [Google Scholar]
- Di Labio E., Regula G., Steiner A. Antimicrobial resistance in bacteria from Swiss veal calves at slaughter. Zoonoses Public Health. 2007;54:344–352. doi: 10.1111/j.1863-2378.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- Furman-Fratczak K., Rzasa A., Stefaniak T. The influence of colostral immunoglobulin concentration in heifer calves’ serum on their health and growth. J. Dairy Sci. 2011;94:5536–5543. doi: 10.3168/jds.2010-3253. [DOI] [PubMed] [Google Scholar]
- Graveland H., Wagenaar J.A., Heesterbeek H. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS One. 2010;5:e10990. doi: 10.1371/journal.pone.0010990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliksen S.M., Jor E., Lie K.I., Loken T., Akerstedt J., Osteras O. Respiratory infections in Norwegian dairy calves. J. Dairy Sci. 2009;92:5139–5146. doi: 10.3168/jds.2009-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassig M., Stadler T., Lutz H. Transition from maternal to endogenous antibodies in newborn calves. Vet. Rec. 2007;160:234–235. doi: 10.1136/vr.160.7.234. [DOI] [PubMed] [Google Scholar]
- Hoet A.E., Nielsen P.R., Hasoksuz M., Thomas C., Wittum T.E., Saif L.J. Detection of bovine torovirus and other enteric pathogens in feces from diarrhea cases in cattle. J. Vet. Diagn. Invest. 2003;15:205–212. doi: 10.1177/104063870301500301. [DOI] [PubMed] [Google Scholar]
- MARAN-2009 . In: Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands. Mevius D.J., Koene M.G.J., Wit B., van Pelt W., Bondt N., editors. Lelystad; The Netherlands: 2011. http://www.wageningenur.nl/nl/Publicatie-details.htm?publicationId=publication-way-343035323134 (accessed 4 February 2014). [Google Scholar]
- MARAN-2012 . In: Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands. Mevius D.J., Koene M.G.J., Wit B., van Pelt W., Bondt N., editors. Lelystad; The Netherlands: 2012. http://www.wageningenur.nl/nl/Publicatie-details.htm?publicationId=publication-way-343330383332 (accessed 4 February 2014) [Google Scholar]
- McDonough S.P., Stull C.L., Osburn B.I. Enteric pathogens in intensively reared veal calves. Am. J. Vet. Res. 1994;55:1516–1520. [PubMed] [Google Scholar]
- Mohri M., Sharifi K., Eidi S. Hematology and serum biochemistry of Holstein dairy calves: age related changes and comparison with blood composition in adults. Res. Vet. Sci. 2007;83:30–39. doi: 10.1016/j.rvsc.2006.10.017. [DOI] [PubMed] [Google Scholar]
- NRC . 7th rev. ed. The National Academies Press; Washington, DC: 2001. Nutrient Requirements of Dairy Cattle. Ed. National Research Council; pp. 214–233. [Google Scholar]
- Pardon B. Ghent University; 2012. Morbidity, Mortality and Drug Use in White Veal Calves with Emphasis on Respiratory Disease; p. p316. (Ph.D. thesis) [Google Scholar]
- Pardon B., De Bleecker K., Dewulf J., Callens J., Boyen F., Catry B., Deprez P. Prevalence of respiratory pathogens in diseased, non-vaccinated, routinely medicated veal calves. Vet. Rec. 2011;169:278. doi: 10.1136/vr.d4406. [DOI] [PubMed] [Google Scholar]
- Pardon B., Catry B., Dewulf J., Persoons D., Hostens M., De Bleecker K., Deprez P. Prospective study on quantitative and qualitative antimicrobial and anti-inflammatory drug use in white veal calves. J. Antimicrob. Chemother. 2012;67:1027–1038. doi: 10.1093/jac/dkr570. [DOI] [PubMed] [Google Scholar]
- Pardon B., De Bleecker K., Hostens M., Callens J., Dewulf J., Deprez P. Longitudinal study on morbidity and mortality in white veal calves in Belgium. BMC Vet. Res. 2012;8:26. doi: 10.1186/1746-6148-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon B., Hostens M., Duchateau L., Dewulf J., De Bleecker K., Deprez P. Impact of respiratory disease, diarrhea, otitis and arthritis on mortality and carcass traits in white veal calves. BMC Vet. Res. 2013;9:79. doi: 10.1186/1746-6148-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postema H.J., Mol J. Risk of disease in veal calves: relationships between colostrum-management, serum immunoglobulin levels and risk of disease. Zentralbl. Veterinarmed. A. 1984;31:751–762. doi: 10.1111/j.1439-0442.1984.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Postema H.J., Franken P., van der Ven J.B. A study in veal calves for a possible correlation between serum immunoglobulin levels, nutrition levels and risk of disease in the first few weeks of the fattening period. Tijdschr. Diergeneeskd. 1987;112:665–671. [PubMed] [Google Scholar]
- Rerat M., Albini S., Jaquier V., Hussy D. Bovine respiratory disease: efficacy of different prophylactic treatments in veal calves and antimicrobial resistance of isolated Pasteurellaceae. Prev. Vet. Med. 2012;103:265–273. doi: 10.1016/j.prevetmed.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Robison J.D., Stott G.H., DeNise S.K. Effects of passive immunity on growth and survival in the dairy heifer. J. Dairy. Sci. 1988;71:1283–1287. doi: 10.3168/jds.S0022-0302(88)79684-8. [DOI] [PubMed] [Google Scholar]
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- Stilwell G., Carvalho R.C. Clinical outcome of calves with failure of passive transfer as diagnosed by a commercially available IgG quick test kit. Can. Vet. J. 2011;52:524–526. [PMC free article] [PubMed] [Google Scholar]
- Thrusfield M., Ortega C., de Blas I., Noordhuizen J.P., Frankena K. Win Episcope 2.0: improved epidemiological software for veterinary medicine. Vet. Rec. 2001;148:567–572. doi: 10.1136/vr.148.18.567. [DOI] [PubMed] [Google Scholar]
- Vandendriessche S., Vanderhaeghen W., Soares F.V. Prevalence, risk factors and genetic diversity of methicillin-resistant Staphylococcus aureus carried by humans and animals across livestock production sectors. J. Antimicrob. Chemother. 2013;68:1510–1516. doi: 10.1093/jac/dkt047. [DOI] [PubMed] [Google Scholar]
- Virtala A.M., Mechor G.D., Grohn Y.T., Erb H.N. The effect of calfhood diseases on growth of female dairy calves during the first 3 months of life in New York State. J. Dairy. Sci. 1996;79:1040–1049. doi: 10.3168/jds.S0022-0302(96)76457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldner C.L., Rosengren L.B. Factors associated with serum immunoglobulin levels in beef calves from Alberta and Saskatchewan and association between passive transfer and health outcomes. Can. Vet. J. 2009;50:275–281. [PMC free article] [PubMed] [Google Scholar]
- Weaver D.M., Tyler J.W., VanMetre D.C., Hostetler D.E., Barrington G.M. Passive transfer of colostral immunoglobulins in calves. J. Vet. Int. Med. 2000;14:569–577. doi: 10.1892/0891-6640(2000)014<0569:ptocii>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Wilson L.L., Smith J.L., Smith D.L., Swanson D.L., Drake T.R., Wolfgang D.R., Wheeler E.F. Characteristics of veal calves upon arrival, at 28 and 84 days, and at end of the production cycle. J. Dairy. Sci. 2000;83:843–854. doi: 10.3168/jds.S0022-0302(00)74948-4. [DOI] [PubMed] [Google Scholar]
- Wittum T.E., Salman M.D., King M.E., Mortimer R.G., Odde K.G., Morris D.L. The influence of neonatal health on weaning weight of Colorado, USA beef-calves. Prev. Vet. Med. 1994;19:15–25. [Google Scholar]