Abstract
A novel binding site for angiotensins II and III that is unmasked by parachloromercuribenzoate has been reported in rat, mouse and human brains. Initial studies of this binding site indicate that it is not expressed in the adrenal, liver or kidney of the rat and mouse. To determine if this binding site occurs in other mouse tissues, 8 tissues were assayed for expression of this binding site by radioligand binding assay and compared with the expression of this binding site in the forebrain. Particulate fractions of homogenates of testis, epididymis, seminal vesicles, heart, spleen, pancreas, lung, skeletal muscle, and forebrain were incubated with 125I-sarcosine1, isoleucine8 angiotensin II in the presence or absence of 0.3 mM parachloromercuribenzoate plus 10 µM losartan and 10 µM PD123319 (to saturate AT1 and AT2 receptors). Specific (3 µM angiotensin II displaceable) high affinity binding occurred in the testis > forebrain > epididymis > spleen > pancreas > lung when parachloromercuribenzoate was present. Binding could not be reliably observed in heart, skeletal muscle and seminal vesicles. High affinity binding of 125I-sarcosine1, isoleucine8 angiotensin II was observed in the absence of parachloromercuribenzoate in the pancreas on occasion. This suggests that this novel angiotensin binding site may have a functional role in these tissues.
Keywords: Testis, Brain, Epididymis, Pancreas, Spleen, Lung, Parachloromercuribenzoate
1. Introduction
It is well established that the renin–angiotensin system (RAS) is an essential multi-organ endocrine system necessary for homeostatic regulation of blood volume and electrolyte balance, efforts which ultimately contribute to cardiovascular maintenance at tissue and organism levels [1]. Dysregulation of the renin–angiotensin system (RAS) causes several pathophysiological states of which hypertension is most prevalent. The octapeptide angiotensin II (Ang II) exerts its classical effects on mean arterial blood pressure primarily through the AT1 receptor [2]. These include direct vasoconstriction, release of aldosterone from the adrenal cortex, and centrally-mediated blood-pressure regulation [3], [4]. Additional effects of Ang II and the des Asp1 heptapeptide Ang III mediated through activation of AT1 receptors in the central nervous system (CNS) include dipsogenesis, secretion of vasopressin, and changes in reproductive hormone secretion [5], [6]. The brain RAS plays a major role in blood pressure regulation, but there are many aspects of this system that are not yet fully understood [7], [8].
Investigations of the brain RAS using radioligand binding assays have been hampered by significant metabolic degradation of the radiolabeled angiotensin peptides. This makes it impossible to attain steady-state conditions needed to accurately determine binding kinetics. Binding of angiotensin fragments as well as intact parent radioligand can also occur, and the concentration of free, intact radioligand is also continually decreasing [9]. Solutions to this problem include the use of peptidase-resistant analogs of angiotensin and the addition of protease inhibitors to the incubation medium [7], [8], [10].
In an effort to establish binding parameters for brain AT1 receptors that would insure stability of angiotensin peptides, specifically 125I-Ang II, work in this laboratory demonstrated that parachloromercuribenzoate (PCMB), an angiotensinase inhibitor [11], [12], unmasked a novel, high affinity, non-AT1, non-AT2 binding site for angiotensins in the rat brain hypothalamus [13]. The binding was considerably greater than that observed for AT1 and AT2 receptors in the rat brain, and was abundant throughout the cerebral cortex, a region where there is little AT1 or AT2 binding. Subsequently, autoradiographic analysis of this binding in the rat brain revealed that this novel binding site, which shows high affinity for Ang III as well as Ang II, but considerably lower affinity for other angiotensin fragments, is widespread [14].
To determine if this novel Ang II/Ang III binding site occurs in species other than the rat, its expression was assessed in the mouse [15]. The novel angiotensin binding site was identified both in the mouse cerebral cortex and hypothalamus. In addition, the binding site was absent in the mouse liver, adrenals, and kidney, a distribution pattern that was also reported in the rat [13]. A subsequent study reported the presence of this novel Ang II/Ang III binding site in the human brain [16].
While this novel Ang II/Ang III binding site is not present in tissues known to express high levels of AT1 receptors, e.g., liver, kidney and adrenal, a systematic study of other tissues in the body has not been undertaken. In this study we report the presence of this novel Ang II/Ang III binding site in a variety of tissues in the mouse, of which, several are considered to have minor involvement with the RAS.
2. Materials and methods
2.1. Animals
Adult ND4 male mice (Harlan) 30–35 g maintained in 12 h light/dark cycle, fed ad libitum were used. Some mice were initially used for behavioral studies (cannabinoid tetrad) with natural cannabinoid agonists, of which vehicle-treated (saline) and drug-tested animals were used at least 2 and 3 days after testing, respectively. Presented data are the summary of results from all animals as there was no apparent difference in observed effects from intact and vehicle/drug-treated mice. The protocol for these studies was approved by the University of Mississippi IACUC.
2.2. Reagents
Sarcosine1, isoleucine8 Ang II (SI Ang II) was obtained from Phoenix Pharmaceuticals. Losartan was a gift from Dr. Ron Smith of Dupont Merck. Parachloromercuribenzoic acid (PCMB) sodium salt was purchased from MP Biomedicals. [125I] SI Ang II was prepared in house by the chloramine T procedure and purified by reverse-phase HPLC as described previously [17]. All other reagents were purchased from major commercial suppliers.
2.3. Receptor binding assays
The measurement of 125I-SI Ang II binding in 9 mouse tissues (forebrain, heart, liver, pancreas, spleen, skeletal muscle, testis, seminal vesicles and epididymis) was carried out using established procedures [13], [18]. After mechanical homogenization (Tekmar Tissuemizer) of tissue in ice-cold hypotonic buffer, 20 mM NaPO4 (pH 7.2) the homogenates were centrifuged at 40,000 ×g for 20 min at 4 °C. The supernatant was removed and the pellet (total particulate including total cellular membrane fractions) was re-suspended by homogenization in assay buffer (150 mM NaCl, 5 mM EDTA, 0.1 mM bacitracin, 50 mM NaPO4, pH 7.1–7.2). The homogenates were re-centrifuged at high speed as before and the pellets re-suspended by homogenization in the assay buffer at a concentration of 50 mg initial wet weight/ml. Losartan and PD123319 (final concentration 10 μM from a 10 mM stock in de-ionized water) were added to saturate AT1 and AT2 receptors, respectively. PCMB (final concentration 0.3 mM from a 100 mM stock solution in 50 mM NaOH) was also added into an aliquot of the tissue homogenates 10–15 min before incubation to unmask this novel Ang II/Ang III binding site.
A similar protocol was used to determine the effect of a high ionic strength (400 mM NaCl) concentration on the occurrence of the novel Ang II/III binding site. For this experiment an aliquot of the brain tissue following the second centrifugation was re-suspended by homogenization either standard assay buffer or assay buffer containing 400 mM NaCl instead of 150 mM NaCl. The high ionic strength buffer comparison was performed to assess whether the novel binding protein is a membrane-associated protein that can be dissociated from brain membranes by a high ionic strength buffer, or if it is a membrane-integral protein that is not dissociable from membranes by a high ionic strength assay buffer.
The protein concentration of the homogenates was determined by the Coomassie blue dye method [19] using bovine γ-globulin as standard. Determination of B max (fmole of radioligand bound per mg protein) and K D values were carried out using the one-site saturation binding model of Prism software (Graphpad Software) on specific (3 μM Ang II displaceable) 125I-SI Ang II binding at 6 concentrations ranging from 0.3 to 6 nM. Values reported are mean ± SEM for assays in which the values obtained for K D and B max were significantly different from zero. Assays in which the K D value was greater than 20 nM were also excluded because this is more than 3 times the highest concentration of radioligand used and B max cannot be accurately determined with such a small occupancy (< 25%) of the binding site.
3. Results
3.1. Tissue distribution
Specific binding of 125I-SI Ang II in the presence of 0.3 mM PCMB, 10 µM losartan, and 10 µM PD123319 to the novel Ang II/Ang III binding site was observed in the following order of abundance: testis, forebrain, spleen, epididymis, pancreas, and lung membranes (Table 1 , Fig. 1 ). However, in 3 of the 9 assays of the lung, binding was not statistically different from zero. So, the true density of the novel Ang II/Ang III binding site in the lung is likely to be considerably less than that reported in Table 1 as it was not possible to include the undetectable values in the average.
Table 1.
Tissue distribution of the novel Ang II/III binding site.
| Brain | Testis | Epididymis | |
|---|---|---|---|
| Bmax | 139 ± 11.5 | 384 ± 70.7 | 80.8 ± 15.5 |
| KD | 1.85 ± 0.15 | 3.69 ± 0.77 | 5.89 ± 1.23 |
| n = 32 (32) | n = 11 (12) | n = 7 (7) | |
| Spleen | Pancreas | Lung | |
| Bmax | 72.9 ± 16.2 | 63.1 ± 9.9 | 56.9 ± 7.6 |
| KD | 2.87 ± 0.82 | 3.03 ± 0.75 | 2.55 ± 0.62 |
| n = 8 (10) | n = 10 (11) | n = 6 (9) | |
| Heart | Skeletal muscle | Seminal vesicles | |
| Bmax | 11.9 ± 4.8 | 13.3 ± 3.2 | 265 ± 36.5 |
| KD | 0.86 ± 0.44 | 3.15 ± 1.70 | 3.81 ± 1.63 |
| n = 3 (11) | n = 5 (10) | n = 3 (8) | |
Saturation binding analyses of 125I-SI Ang II binding to the novel Ang II/Ang III binding site in mouse forebrain, testis, spleen, pancreas, skeletal muscle, heart, lung, epididymis, and seminal vesicles. Bmax (fmol/mg protein) and KD (nM) (± SEM) values reported do not include samples for which Bmax or KD was not significantly different from zero (95% confidence interval from the saturation isotherm was zero or less from the non-linear regression analysis to the equation B = Bmax[D]/(KD + [D]) where [D] = concentration of 125I SI Ang II) [n = samples used (total samples)]. Values shown in italics are for tissues and conditions in which binding was not reliably detectable in half or more of the assays.
Fig. 1.
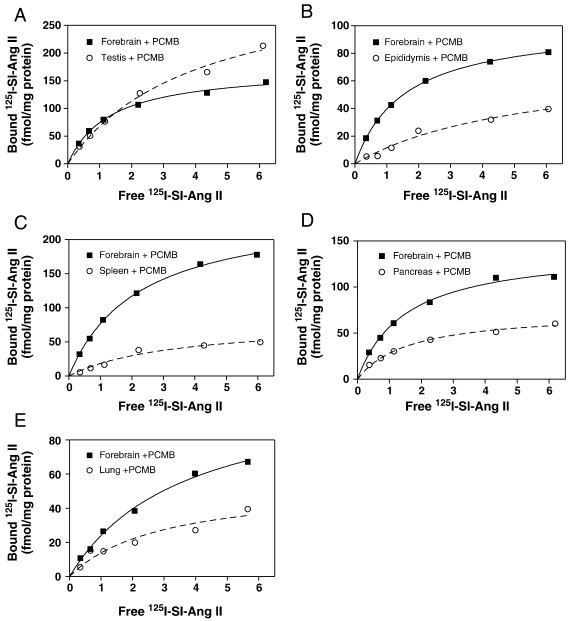
Representative saturation binding analyses of specific 125I-SI Ang II binding to the novel AngII/AngIII binding site in mouse brain (Panels A–E), testis (Panel A), epididymis (Panel B), spleen (Panel C), pancreas (Panel D), and lung (Panel E). Values for each panel are: Panel A, Brain (Bmax = 173.4 fmol/mg protein; KD = 1.32 nM) and Testis (Bmax = 342.2 fmol/mg protein; KD = 4.04 nM). Panel B, Brain (Bmax = 101.6 fmol/mg protein; KD = 1.56 nM) and Epididymis (Bmax = 75.3 fmol/mg protein; KD = 5.46 nM). Panel C, Brain (Bmax = 247.1 fmol/mg protein; KD = 2.21 nM) and Spleen (Bmax = 79.7 fmol/mg protein; KD = 3.34 nM). Panel D, Brain (Bmax = 141.9 fmol/mg protein; KD = 1.50 nM) and Pancreas (Bmax = 73.5 fmol/mg protein; KD = 1.60 nM). Panel E, Brain (Bmax = 122.0 fmol/mg protein; KD = 3.65 nM) and Lung (Bmax = 54.3 fmol/mg protein; KD = 2.93 nM).
Specific binding of 125I-SI Ang II in the presence of 0.3 mM PCMB, 10 µM losartan, and 10 µM PD123319 in skeletal muscle, heart, and seminal vesicles, though seen on occasion, was not significantly different from zero in half or more of the assays. Therefore the binding of 125I-SI Ang II to the novel Ang II/Ang III binding site in these tissues cannot be reliably observed and the values shown in Table 1 may not be representative of the expression of this binding site in these tissues.
Measurable specific binding in the absence of 0.3 mM PCMB, but in the presence of losartan (10 µM) and PD123319 (10 µM) was observed on more than two occasions in two tissues: the testis and pancreas. However, in the majority of the assays of the testis (8 of 12 assays) in the absence of 0.3 mM PCMB, binding did not differ from zero. And, when measurable binding was detectable (579 ± 163 fmol/mg protein 4 of 12 assays), the binding affinity was considerably lower (K D = 14.8 ± 1.8 nM) than in the presence of PCMB. In the pancreas 6 of the 11 assays were significantly greater than zero (106 ± 49.9 fmol/mg protein) in the absence of PCMB and showed high affinity (K D = 4.06 ± 0.30 nM) similar to that seen with PCMB, although the SEM, for the B max was nearly 50%. This indicates that under the in vitro conditions of this assay, the binding site in the pancreas may occur in a conformation capable of binding Ang II and Ang III.
3.2. High ionic strength (400 mM NaCl) effect
In the assay buffer containing 400 mM NaCl, no significant difference in 125I-SI Ang II binding, either B max (Fig. 2A) or K D (Fig. 2B) was observed compared to that in the 150 mM NaCl assay buffer.
Fig. 2.
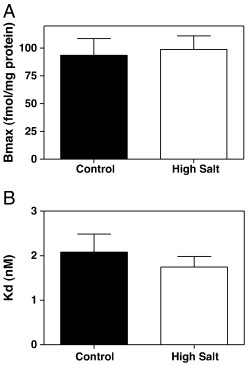
125I-SI Ang II binding to the novel Ang II/Ang III binding site. Control (150 mM NaCl) Bmax = 93.6 ± 15.1 fmol/mg protein and high ionic strength (400 mM NaCl) group Bmax = 98.9 ± 12.1 fmol/mg protein (Panel A). Control KD = 2.08 ± 0.41 nM and high salt treatment group KD = 1.74 ± 0.24 nM (Panel B) n = 6.
4. Discussion
4.1. Tissue distribution
Previous studies showing that the novel Ang II/Ang III binding site is present in rat and mouse brains, also show that it is not present in rat and mouse liver, adrenal glands, and kidney. Although previously described as brain-specific, binding assays in tissues not generally considered to be part of the classical RAS had not been done. Herein we report a broader examination of the tissue distribution of this novel binding site in 8 mouse tissues. This novel Ang II/Ang III binding site, which is unmasked by 0.3 mM PCMB in the brain, is also present in the testis, spleen, pancreas, epididymis, and lung, but was not reliably detectable in the heart, seminal vesicles and skeletal muscle. The presence of this novel Ang II/Ang III binding protein in tissues other than the brain suggests that it may mediate or regulate the effects of these angiotensins in these tissues. While the tissues in which this novel Ang II/Ang III binding site are not generally associated with the RAS, there is data to indicate that these tissues have local RAS's [1], [20], [21] and may also be responsive to systemically generated circulating angiotensins.
The highest level of 125I-SI Ang II binding to this novel Ang II/Ang III binding site was observed in the testis. Active angiotensins are produced in the testis [22]. Renin and angiotensinogen mRNA are present in mouse testis [23]. In rat however, renin mRNA is readily detectable but angiotensinogen mRNA could only be detected with a high sensitivity assay [23], [24], [25]. Within the rat and mouse testes, renin and renin-like-ir is present in Leydig cells [22], [26], [27], [28]. The testicular angiotensin-converting enzyme (ACE) variant [22], [29], [30] and Ang II receptors (predominantly AT1) are also found in Leydig cells [31], [32], [33], [34]. It has been proposed that Ang II functions in a negative feedback loop wherein LH stimulated Ang II production [35], [36] leads to inhibition of LH stimulated testosterone production [32]. Angiotensin II is not the only RAS component ascribed with functionality in the testicular RAS. Recent studies have established that Ang (1–7) activation of the Mas receptor is necessary for spermatogenesis in mice and rats [37] and that testicular ACE is necessary for capacitation and subsequent fertilization [38], [39], [40], [41], [42]. Thus, this novel Ang II/Ang III binding site could participate in Ang II-regulated male reproductive functions. See also reviews [1], [43], [44].
The next highest expression of the novel Ang II/Ang III binding site is in the brain. The binding site is present in high abundance relative to the expression of the AT1 and AT2 subtypes and is more widely distributed than either subtype in the rat brain which may indicate novel, heretofore undescribed actions of Ang II and Ang III in the brain [8], [14]. The B max values for mouse forebrain in this study are approximately 3 to 4 times greater than previously reported for rat and mouse hypothalamus and rat, mouse and human cerebral cortex [13], [15], [16] although the K D values are similar. It is not readily apparent why there is such a large difference in forebrain versus cerebral cortex and hypothalamus B max values.
There was an abundance of 125I-SI Ang II binding to the novel Ang II/Ang III binding site in the spleen. While expression of renin and angiotensinogen in the spleen is low [24], [45], [46], the spleen is reported to have a complete RAS, see reviews [1], [20]. High levels of Ang II receptor binding have been found in the red pulp of the spleen [47]. These receptors are of the AT1 subtype [48]. Splenic AT1 receptors might participate in regulation of splenic volume and blood flow [47], [49] or in regulation of lymphocyte function via AT1 receptor activation of cytotoxic T lymphocytes [50], [51]. Ang II stimulates proliferation of lymphocytes in the mouse spleen reportedly via stimulation of both AT1 and AT2 receptors [52]. See also reviews [1], [20]. The density of AT1 receptors in the rat spleen reported by Castren et al., [47] 81.6 fmol/mg protein, is very similar to the density of the novel Ang II/Ang III binding site, 72.9 fmol/mg protein. It remains to be determined if the novel Ang II/Ang III binding site is expressed on red or white pulp of the spleen. Thus its potential functional significance in the spleen remains uncertain.
Binding of 125I-SI Ang II was also observed in the epididymis, a tissue which is contiguous with the testis and completes or complements many of its functions. Components of the RAS present in the epididymis include renin-like activity [53], angiotensinogen, the testicular ACE variant [29], [54] and Ang II receptors [55]. Ang II and both AT1 and AT2 receptors have been identified in epididymis with a proposed functionality in the maturation of spermatozoa, specifically by regulating fluid and electrolyte balance and stimulating spermatozoal transport, see review [43]. Thus this novel Ang II/Ang III binding site may also participate in this functionality.
The novel Ang II/Ang III binding site was also observed in the mouse pancreas. Components of the RAS that have been observed in the pancreas of various species include renin mRNA [56], angiotensinogen mRNA, Ang II and Ang II receptors [57]. Ang II-like immunoreactivity was reported to be present in endothelial cells of pancreatic blood vessels and the epithelial cells of pancreatic ducts, with less pronounced immunoreactivity for Ang II in the acinar cells and in the smooth muscle layers overlying the pancreatic ducts as well as the blood vessels but with no Ang II-like immunoreactivity in islet cells [58]. Ang II-like immunoreactivity was not detected in islet cells. The density of Ang II receptors in the canine pancreas was relatively low, 15.8 fmol/mg protein [57] and is predominantly made up of the AT2 subtype [59]. In the rat pancreas Ang II receptors were also reported to have a low (15 fmol/mg protein) density, but were observed on islet cell membranes as well as on the exocrine pancreas [60]. This is about ¼ the density of the novel Ang II/Ang III binding site in the mouse pancreas. AT1 receptor immunoreactivity was observed in rat pancreatic acinar cells [61] and was tied to the induction of apoptosis of acinar cells during experimental pancreatic fibrosis.
Clinical evidence has clearly established that RAS blockade is protective against type 2 diabetes mellitus. There is evidence supporting a role for both systemic and pancreatic islet RASs in regulation of islet blood flow and insulin biosynthesis [62]. The use of angiotensin receptor blockers (ARBs) and ACE inhibitors appears to attenuate or prevent RAS-mediated inflammatory responses, apoptosis, fibrosis, and superoxide anion production in islet cells [63]. A less classical role of the RAS has also been noted: acute diabetes seen in some SARS patients is mediated by ACE2 which functions as a receptor for the SARS coronavirus [64]. See also reviews: [1], [56], [65]. In view of the continuing ambiguities regarding the functionality of the pancreatic RAS and the effects of systemically generated circulating angiotensins on the pancreas, it remains to be determined how the novel Ang II/Ang III binding protein would affect the actions of angiotensins on the pancreas.
The pancreas was unique inasmuch as it was the only tissue in which nearly similar amounts and affinity of binding was seen with and without PCMB, although binding was not always observed in the absence of PCMB (5 of 11 assays). This suggests that the novel Ang II/Ang III binding site may be more stably expressed in a conformation capable of binding Ang II and Ang III so as to be observable in vitro without PCMB. Further studies of this unique characteristic of the pancreas are needed to verify this possibility.
Binding of 125I-SI Ang II to the novel Ang II/Ang III binding site was observed in the lung, but only in 6 of the 9 assays carried out. The vasculature of the lung contains a rich supply of ACE [66], [67] making the lung the most integral tissue for the systemic production of Ang II. Renin and angiotensinogen mRNA have been observed in low levels in the rat lung [24], [46], [68]. The predominant Ang II receptor subtype in the lung is AT1 and was initially reported to have a density of 704 ± 60 fmol/mg protein and K D of 2.8 ± 0.9 nM [69]. However, a subsequent study showed a much lower density of 128.4 ± 15.7 fmol/mg protein and a K D of 0.63 ± 0.05 nM [70]. The novel Ang II/Ang III binding site shows a density below both of those reported for the AT1, (B max = 56.9 ± 7.6 fmole/mg protein). Ang II has several functions in the lung including induction of microvascular permeability and subsequent pulmonary edema [71], pulmonary vasoconstriction [72], fibroblast proliferation mediated by AT1 receptors [73], and AT1 mediated apoptosis of alveolar epithelial cells in vitro [74]. A recent study showed that renin released by mast cell degranulation, possibly in concert with concurrent chymase release [75], leads to local generation of Ang II which then promotes bronchoconstriction via the AT1 receptor on bronchial smooth muscle cells [76]. This study highlights the interplay between different tissue types in the functional use of angiotensin II as a paracrine hormone. The novel Ang II/Ang III binding site may mediate more typical actions of angiotensins II and III, or it may participate in novel and complex regulatory pathways in the lung, see also reviews [77], [78].
Of the eight mouse tissues analyzed, significant specific binding of 125I-SI Ang II was not reliably observed in the heart, seminal vesicles, and skeletal muscle. The undetectability of the novel Ang II/Ang III binding site in heart was surprising given the preponderance of evidence which attributes a significant direct role of Ang II on cardiac function and pathophysiology, see review [20]. Cardiac tissue has been shown to express ACE, as well as AT1 and AT2 receptors, however expression of angiotensinogen, and renin are still uncertain [20], [44]. In contrast, increased expression of ACE2 has been noted following myocardial infarction, heart failure, and treatment with ACE inhibitors or ARBs [44]. It has been proposed that locally produced Ang II is not responsible for ventricular hypertrophy but may have a significant role in fibrosis induction [21].
The low level of binding to the novel Ang II/Ang III binding site in skeletal muscle and seminal vesicles is in accord with the minimal evidence available describing a functional RAS in both of these tissues in adulthood.
4.2. Membrane localization
Although we have previously established that the binding site is present in plasma membrane-enriched fraction of brain homogenates [13], [15], [16], those studies did not distinguish between a membrane-associated protein and a membrane bound protein. It should be further noted that in the current binding study the cellular fraction used included total cellular membranes, not solely plasma membrane. To address the possibility that this novel Ang II/Ang III binding protein is membrane associated rather than an integral membrane protein, the concentration of NaCl in the assay buffer was increased to 400 mM so as to dissociate membrane-associated proteins from the membranes. Specific binding of 125I-SI Ang II remained constant between control and high-salt treated membranes, suggesting that the novel Ang II/Ang III binding site protein is not a membrane-associated protein, and is instead embedded within the plasma membrane. The cellular localization of this novel binding site as an integral protein of the plasma membrane ascribes it several possible roles: in signaling (as a receptor), as a transporter (to internalize these angiotensins into cells), or as a peptidase that could function as a highly specific angiotensinase that modulates the availability of Ang II and Ang III [13], [15].
4.3. Protein identification inferred from the tissue distribution patterns in the mouse
At present the identity of this novel binding protein is unknown. However, one available method for identifying its structure is to find complementary data on protein expression patterns in the mouse. A search of global tissue expression patterns using Mouse Genome Informatics MGI database (www.informatics.jax.org) revealed several candidate proteins, which were chosen based on similarities with the novel Ang II/Ang III binding site. The criteria for inclusion included: tissue distribution in adult mice (present in testis and brain, the tissues with the highest observed binding; see Table 1), cellular localization (plasma membrane integral protein), protein size (approx. 80 kDa) and absence of glycosylation sites [79]. The search returned eight proteins of interest: Cldn 17, Emp1, Fbox2, Maged1, Mmp24, Rxfp2, Septin 3, and St8sia3. These candidate proteins were then arbitrarily scored to determine which best fit the criteria set described above. Three of the eight proteins, Maged1, Mmp24, and Rxfp2, showed good concordance with characteristics of the novel Ang II/Ang III binding protein.
MAGE-D1 (also known as NRAGE or Dlxin-1) is a plasma membrane protein which functions as an adapter protein mediating various pathways including neuronal growth factor receptor (NGFR, p75NTR) and UNCD5H1-induced apoptosis and Dlx/Msx-mediated transcription [80]. It has been detected in most adult tissues, and is predominantly expressed in the brain, but has not been observed in liver and lung (www.informatics.jax.org) [80].
Rxfp2 is a cell surface receptor for the peptide relaxin and may also serve as a receptor for Leydig insulin-like peptide (INSL3). Its expression pattern extends from fetus to adult in male and female gonads and the brain, but has not been detected in the kidney, spleen, or heart (www.informatics.jax.org; MGI – uniprot.org).
The metalloproteinase Mmp24 (MT5-MMP) most closely parallels reported biochemical characteristics of the novel Ang II/Ang III binding site [15], [79]. According to data from MGI, Mmp24 has been detected in the plasma membranes of adult mouse brain and testis, but not in heart, skeletal muscle, liver, kidney, and lung (www.informatics.jax.org). One striking difference between the novel Ang II/Ang III binding site and Mmp24 is that expression of Mmp24 in the brain is predominantly located in the cerebellum [81], whereas autoradiographic analysis of the novel Ang II/Ang III binding site in the rat brain shows low levels of binding in the cerebellum and highest density in the forebrain, specifically the olfactory bulb [14].
The concordance of the novel Ang II/Ang III binding site to Mmp24 is not entirely surprising given the ability of PCMB to inhibit metalloendopeptidases EC 3.4.24.15, thimet oligopeptidase and EC 3.4.24.16, neurolysin [82], [83] and the abundance of EC 3.4.24.15 in brain and testis [13]. Additional correlates between angiotensins and metalloproteinases include ACE/ACE2 and IRAP (AT4 receptor; EC 3.4.11.3) [84], see also reviews [3], [85]. The functions of several brain metalloproteinases have been correlated to pathological sequelae in neuroinflammatory events and to beneficial roles in angio- and neurogenesis [86]. These pathological consequences of metalloproteinase activation are most directly a result of increased blood-brain barrier permeability but also include extracellular matrix (ECM) regulation in Alzheimer's and Parkinson's disease. If the novel Ang II/III binding site is interacting with a metalloproteinase it suggests that there could be other substrates besides Ang II and Ang III whose functionality is affected by this binding site. Future studies, directed at sequence identification of the novel Ang II/Ang III binding protein and characterization of its physiological functions and possible involvement in pathophysiological processes will be needed.
Acknowledgments
This work was supported by The Peptide Radioiodination Service Center of the University of Mississippi. The authors thank Dr. Abir El-Alfy for providing the mice used in this study as well as for reviewing the work.
References
- 1.Phillips M.I., Speakman E.A., Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul Pept. 1993;43:1–20. doi: 10.1016/0167-0115(93)90403-u. [DOI] [PubMed] [Google Scholar]
- 2.de Gasparo M., Catt K.J., Inagami T., Wright J.W., Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 3.Thomas W.G., Mendelsohn F.A. Angiotensin receptors: form and function and distribution. Int J Biochem Cell Biol. 2003;35:774–779. doi: 10.1016/s1357-2725(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 4.Bourassa E.A., Sved A.F., Speth R.C. Angiotensin modulation of rostral ventrolateral medulla (RVLM) in cardiovascular regulation. Mol Cell Endocrinol. 2009;302:167–175. doi: 10.1016/j.mce.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips M.I., Sumners C. Angiotensin II in central nervous system physiology. Regul Pept. 1998;78:1–11. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 6.Kaschina E., Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 7.Speth R.C., Karamyan V.T. The significance of brain aminopeptidases in the regulation of the actions of angiotensin peptides in the brain. Heart Fail Rev. 2008;13:299–309. doi: 10.1007/s10741-007-9078-2. [DOI] [PubMed] [Google Scholar]
- 8.Speth R., Karamyan V.T. Brain angiotensin receptors and binding proteins. Naun Schmied Arch Pharmacol. 2008;377:283–293. doi: 10.1007/s00210-007-0238-7. [DOI] [PubMed] [Google Scholar]
- 9.Karamyan V.T., Gadepalli R., Rimoldi J., Speth R. Brain AT1 angiotensin receptor subtype binding: Importance of peptidase inhibition for identification of angiotensin II as an endogenous ligand. J Pharmacol Exp Ther. 2009;331:170–177. doi: 10.1124/jpet.109.157461. [DOI] [PubMed] [Google Scholar]
- 10.Karamyan V., Speth R. Enzymatic pathways of the brain renin-angiotensin system: unsolved problems and continuing challenges. Regul Pept. 2007;143:15–27. doi: 10.1016/j.regpep.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujioka H., Okabe T., Yamaguchi H. Purification and characterization of angiotensin II degradation factor from porcine endothelial cells. Tohoku J Exp Med. 1995;177:183–192. doi: 10.1620/tjem.177.183. [DOI] [PubMed] [Google Scholar]
- 12.Kohara K., Tabuchi Y., Senanayake P., Brosnihan K.B., Ferrario C.M. Reassessment of plasma angiotensins measurement: effects of protease inhibitors and sample handling procedures. Peptides. 1991;12:1135–1141. doi: 10.1016/0196-9781(91)90070-6. [DOI] [PubMed] [Google Scholar]
- 13.Karamyan V.T., Speth R.C. Identification of a novel non-AT1, non-AT2 angiotensin binding site in the rat brain. Brain Res. 2007;1143:83–91. doi: 10.1016/j.brainres.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Karamyan V.T., Speth R.C. Distribution of the non-AT1, non-AT2 angiotensin-binding site in the rat brain: preliminary characterization. Neuroendocrinology. 2008;88:256–265. doi: 10.1159/000140635. [DOI] [PubMed] [Google Scholar]
- 15.Karamyan V.T., Gembardt F., Rabey F.M., Walther T., Speth R.C. Characterization of the brain-specific non-AT(1), non-AT(2) angiotensin binding site in the mouse. Eur J Pharmacol. 2008;590:87–92. doi: 10.1016/j.ejphar.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Karamyan V.T., Stockmeier C.A., Speth R.C. Human brain contains a novel non-AT1, non-AT2 binding site for active angiotensin peptides. Life Sci. 2008;83:421–425. doi: 10.1016/j.lfs.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speth RC, Harding JW, Radiolabeling of angiotensin peptides. In: Wang D, editor. Angiotensin Protocols, Methods in Molecular Biology, Humana Press, Totowa, NJ, 2001;275–295. [DOI] [PubMed]
- 18.Speth R.C. Sarcosine1, glycine8 angiotensin II is an AT1 angiotensin II receptor subtype selective antagonist. Regul Pept. 2003;115:203–209. doi: 10.1016/s0167-0115(03)00172-1. [DOI] [PubMed] [Google Scholar]
- 19.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Paul M., Poyan M.A., Kreutz R. Physiology of local renin–angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 21.Bader M., Ganten D. Update on tissue renin–angiotensin systems. J Mol Med. 2008;86:615–621. doi: 10.1007/s00109-008-0336-0. [DOI] [PubMed] [Google Scholar]
- 22.Pandey K.N., Misono K.S., Inagami T. Evidence for intracellular formation of angiotensins: coexistence of renin and angiotensin-converting enzyme in leydig cells of rat testis. Biochem Biophys Res Commun. 1984;122:1337–1343. doi: 10.1016/0006-291x(84)91238-5. [DOI] [PubMed] [Google Scholar]
- 23.Dzau V.J., Ellison K.E., Brody T., Ingelfinger J., Pratt R.E. A comparative study of the distributions of renin and angiotensinogen messenger ribonucleic acids in rat and mouse tissues. Endocrinology. 1987;120:2334–2338. doi: 10.1210/endo-120-6-2334. [DOI] [PubMed] [Google Scholar]
- 24.Campbell D.J., Habener J.F. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest. 1986;78:31–39. doi: 10.1172/JCI112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellmann W., Suzuki F., Ohkubo H., Nakanishi S., Ludwig G., Ganten D. Angiotensinogen gene expression in extrahepatic rat tissues: application of a solution hybridization assay. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:327–331. doi: 10.1007/BF00173408. [DOI] [PubMed] [Google Scholar]
- 26.Sigmund C.D., Gross K.W. Structure, expression, and regulation of the murine renin genes. Hypertension. 1991;18:446–457. doi: 10.1161/01.hyp.18.4.446. [DOI] [PubMed] [Google Scholar]
- 27.Pandey K.N., Inagami T. Detection of renin mRNA in mouse testis by hybridization with renin cDNA probe. Biochem Biophys Res Commun. 1984;125:662–667. doi: 10.1016/0006-291x(84)90590-4. [DOI] [PubMed] [Google Scholar]
- 28.Deschepper C.F., Mellon S.H., Cumin F., Baxter J.D. Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal and pituitary of the rat. Proc Natl Acad Sci U S A. 1986;83:7552–7556. doi: 10.1073/pnas.83.19.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar R.S., Kusari J., Roy S.N., Soffer R.L., Sen G.C. Structure of testicular angiotensin-converting enzyme. A segmental mosaic isozyme. J Biol Chem. 1989;264:16754–16758. [PubMed] [Google Scholar]
- 30.Hohlbrugger G., Schweisfurth H., Dahlheim H. Angiotensin I converting enzyme in rat testis, epididymis, and vas deferens under different conditions. J Reprod Fertil. 1982;65:97–103. doi: 10.1530/jrf.0.0650097. [DOI] [PubMed] [Google Scholar]
- 31.Millan M.A., Aguilera G. Angiotensin II receptors in testes. Endocrinology. 1988;122:1984–1990. doi: 10.1210/endo-122-5-1984. [DOI] [PubMed] [Google Scholar]
- 32.Khanum A., Dufau M.L. Angiotensin II receptors and inhibitory actions in Leydig cells. J Biol Chem. 1988;263:5070–5074. [PubMed] [Google Scholar]
- 33.Kitami Y., Okura T., Marumoto K., Wakamiya R., Hiwada K. Differential gene expression and regulation of type-1 angiotensin II receptor subtypes in the rat. Biochem Biophys Res Commun. 1992;188:446–452. doi: 10.1016/0006-291x(92)92405-m. [DOI] [PubMed] [Google Scholar]
- 34.Aguilera G., Millan M.A., Harwood J.P. Angiotensin II receptors in the gonads. Am J Hypertens. 1989;2 doi: 10.1093/ajh/2.5.395. t 1395–402. [DOI] [PubMed] [Google Scholar]
- 35.Parmentier M., Inagami T., Pochet R., Desclin J.C. Pituitary dependent renin-like immunoreactivity in rat testis. Endocrinology. 1983;112:1318–1323. doi: 10.1210/endo-112-4-1318. [DOI] [PubMed] [Google Scholar]
- 36.Inagami T., Mizuno K., Nakamaru M., Pandey K.N., Naruse M., Naruse K.A.U.M., Okamura T., Kawamura M., Higashimori K. The renin-angiotensin system: an overview of its intracellular function. Cardiovasc Drugs Ther. 1988;2:453–458. doi: 10.1007/BF00051182. [DOI] [PubMed] [Google Scholar]
- 37.Leal M.C., Pinheiro S.V., Ferreira A.J., Santos R.A., Bordoni L.S., Alenina N., Bader M., Franca L.R. The role of angiotensin-(1–7) receptor Mas in spermatogenesis in mice and rats. J Anat. 2009;214:736–743. doi: 10.1111/j.1469-7580.2009.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh U.S., Kumar M.V., Panda J.N. Angiotensin converting enzyme in semen and its possible role in capacitation. Andrologia. 1985;17:472–475. doi: 10.1111/j.1439-0272.1985.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 39.Foresta C., Mioni R., Rossato M., Varotto A., Zorzi M. Evidence for the involvement of sperm angiotensin converting enzyme in fertilization. Int J Androl. 1991;14:333–339. doi: 10.1111/j.1365-2605.1991.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 40.Foresta C., Indino M., Manoni F., Scandellari C. Angiotensin-converting enzyme content of human spermatozoa and its release during capacitation. Fertil Steril. 1987;47:1000–1003. doi: 10.1016/s0015-0282(16)59236-x. [DOI] [PubMed] [Google Scholar]
- 41.Kohn K.M., Miska W., Schill W.B. Release of angiotensin-converting enzyme (ACE) from human spermatozoa during capacitation and acrosome reaction. J Androl. 1995;16:259–265. [PubMed] [Google Scholar]
- 42.Kondoh G., Tojo H., Nakatani Y., Komazawa N., Murata C., Yamagata K., Maeda Y., Kinoshita T., Okabe M., Taguchi R., Takeda J. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat Med. 2005;11:160–166. doi: 10.1038/nm1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speth R.C., Daubert D.L., Grove K.L. Angiotensin II: a reproductive hormone too? Regul Pept. 1999;79:25–40. doi: 10.1016/s0167-0115(98)00141-4. [DOI] [PubMed] [Google Scholar]
- 44.Fyhrquist F., Saijonmaa O. Renin–angiotensin system revisited. J Intern Med. 2008;264:224–236. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki F., Lindpaintner K., Keuneke C., Hellmann W., Takahasi S., Nakamura Y., Ohkubo H., Nakanishi S., Murakami K., Ganten D. Tissue-specific regulation of gene expression for renin and angiotensinogen. Clin Exp Hypertens. 1988;10:1317–1319. doi: 10.1080/07300077.1988.11878929. [DOI] [PubMed] [Google Scholar]
- 46.Ekker M., Tronik D., Rougeon F. Extra-renal transcription of the renin genes in multiple tissues of mice and rats. Proc Natl Acad Sci U S A. 1989;86:5155–5158. doi: 10.1073/pnas.86.13.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castren E., Kurihara M., Saavedra J.M. Autoradiographic localization and characterization of angiotensin II binding sites in the spleen of rats and mice. Peptides. 1987;8:737–742. doi: 10.1016/0196-9781(87)90050-7. [DOI] [PubMed] [Google Scholar]
- 48.Tsutsumi K., Stromberg C., Saavedra J.M. Characterization of angiotensin II receptor subtypes in the rat spleen. Peptides. 1992;13:291–296. doi: 10.1016/0196-9781(92)90111-f. [DOI] [PubMed] [Google Scholar]
- 49.Cox S.L., Trendelenburg A.U., Starke K. Prejunctional angiotensin receptors involved in the facilitation of noradrenaline release in mouse tissues. Br J Pharmacol. 1999;127:1256–1262. doi: 10.1038/sj.bjp.0702652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada K., Yazaki Y. Binding sites for angiotensin II in human mononuclear leucocytes. J Biochem. 1978;84:1013–1015. doi: 10.1093/oxfordjournals.jbchem.a132183. [DOI] [PubMed] [Google Scholar]
- 51.Maeda A., Okazaki T., Inoue M., Kitazono T., Yamasaki M., Lemonnier F.A., Ozaki S. Immunosuppressive effect of angiotensin receptor blocker on stimulation of mice CTLs by angiotensin II. Int Immunopharmacol. 2009;9:1183–1188. doi: 10.1016/j.intimp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Kunert-Radek J., Stepien H., Komorowski J., Pawlikowski M. Stimulatory effect of angiotensin II on the proliferation of mouse spleen lymphocytes in vitro is mediated via both types of angiotensin II receptors. Biochem Biophys Res Commun. 1994;198:1034–1039. doi: 10.1006/bbrc.1994.1147. [DOI] [PubMed] [Google Scholar]
- 53.Uchendu C.N. Renin-like activity in the rat epididymis. Indian J Physiol Pharmacol. 1995;39:204–208. [PubMed] [Google Scholar]
- 54.Esther C.R., Semeniuk D., Marino E.M., Zhou Y.D., Overbeek P.A., Bernstein K.E. Expression of testis angiotensin-converting enzyme is mediated by a cyclic AMP responsive element. Lab Invest. 1997;77:483–488. [PubMed] [Google Scholar]
- 55.Grove K.L., Speth R.C. Rat epididymis contains functional angiotensin II receptors. Endocrinology. 1989;125:223–230. doi: 10.1210/endo-125-1-223. [DOI] [PubMed] [Google Scholar]
- 56.Tahmasebi M., Puddefoot J.R., Inwang E.R., Vinson G.P. The tissue renin–angiotensin system in human pancreas. J Endocrinol. 1999;161:317–322. doi: 10.1677/joe.0.1610317. [DOI] [PubMed] [Google Scholar]
- 57.Chappell M.C., Millsted A., Diz D.I., Brosnihan K.B., Ferrario C.M. Evidence for an intrinsic angiotensin system in the canine pancreas. J Hypertens. 1991;9:751–759. doi: 10.1097/00004872-199108000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Leung P.S., Chan H.C., Wong P.Y. Immunohistochemical localization of angiotensin II in the mouse pancreas. Histochem J. 1998;30:21–25. doi: 10.1023/a:1003210428276. [DOI] [PubMed] [Google Scholar]
- 59.Chappell M.C., Diz D.I., Jacobsen D.W. Pharmacological characterization of angiotensin II binding sites in the canine pancreas. Peptides. 1992;13:313–318. doi: 10.1016/0196-9781(92)90114-i. [DOI] [PubMed] [Google Scholar]
- 60.Ghiani B.U., Masini M.A. Angiotensin II binding sites in the rat pancreas and their modulation after sodium loading and depletion. Comp Biochem Physiol A Physiol. 1995;111:439–444. doi: 10.1016/0300-9629(95)00030-b. [DOI] [PubMed] [Google Scholar]
- 61.Wang X.P., Zhang R., Wu K., Wu L., Dong Y. Angiotensin II mediates acinar cell apoptosis during the development of rat pancreatic fibrosis by AT1R. Pancreas. 2004;29:264–270. doi: 10.1097/00006676-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 62.Carlsson P.O., Berne C., Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41:127–133. doi: 10.1007/s001250050880. [DOI] [PubMed] [Google Scholar]
- 63.Leung P.S., de Gasparo M. Involvement of the pancreatic renin–angiotensin system in insulin resistance and the metabolic syndrome. J Cardiometab Syndr. 2006;1:197–203. doi: 10.1111/j.1559-4564.2006.05460.x. [DOI] [PubMed] [Google Scholar]
- 64.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2009 doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leung P.S., Chappell M.C. A local pancreatic renin–angiotensin system: endocrine and exocrine roles. Int.J. Biochem Cell Biol. 2003;35:838–846. doi: 10.1016/s1357-2725(02)00179-6. [DOI] [PubMed] [Google Scholar]
- 66.Skeggs L.T., Kahn J.R., Shumway N.P. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cushman D.W., Cheung H.S. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochim Biophys Acta. 1971;250:261–265. doi: 10.1016/0005-2744(71)90142-2. [DOI] [PubMed] [Google Scholar]
- 68.Campbell D.J., Habener J.F. Hybridization in situ studies of angiotensinogen gene expression in rat adrenal and lung. Endocrinology. 1989;124:218–222. doi: 10.1210/endo-124-1-218. [DOI] [PubMed] [Google Scholar]
- 69.Entzeroth M., Hadamovsky S. Angiotensin II receptors in the rat lung are of the AII-1 subtype. Eur J Pharmacol. 1991;206:237–241. doi: 10.1016/s0922-4106(05)80024-0. [DOI] [PubMed] [Google Scholar]
- 70.Cassis L., Shenoy U., Lipke D., Baughn J., Fettinger M., Gillespie M. Lung angiotensin receptor binding characteristics during the development of monocrotaline-induced pulmonary hypertension. Biochem Pharmacol. 1997;54:27–31. doi: 10.1016/s0006-2952(97)00142-1. [DOI] [PubMed] [Google Scholar]
- 71.Xu Z.H., Shimakura K., Yamamoto T., Wang L.M., Mineshita S. Pulmonary edema induced by angiotensin I in rats. Jpn J Pharmacol. 1998;76:51–56. doi: 10.1254/jjp.76.51. [DOI] [PubMed] [Google Scholar]
- 72.Alexander J.M., Nyby M.D., Jasberg K.A. Effect of angiotensin on hypoxic pulmonary vasoconstriction in isolated dog lung. J Appl Physiol. 1976;41:84–88. doi: 10.1152/jappl.1976.41.1.84. [DOI] [PubMed] [Google Scholar]
- 73.Marshall R.P., Gohlke P., Chambers R.C., Howell D.C., Bottoms S.E., Unger T., McAnulty R.J., Laurent G.J. Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L156–L164. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- 74.Papp M., Li X., Zhuang J., Wang R., Uhal B.D. Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am J Physiol Lung Cell Mol Physiol. 2002;282:L713–L718. doi: 10.1152/ajplung.00103.2001. [DOI] [PubMed] [Google Scholar]
- 75.Bradding P., Walls A.F., Holgate S.T. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 76.Veerappan A., Reid A.C., Estephan R., O'Connor N., Thadani-Mulero M., Salazar-Rodriguez M., Levi R., Silver R.B. Mast cell renin and a local renin–angiotensin system in the airway: role in bronchoconstriction. Proc Natl Acad Sci U S A. 2008;105:1315–1320. doi: 10.1073/pnas.0709739105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marshall R.P. The pulmonary renin–angiotensin system. Curr Pharm Des. 2003;9:715–722. doi: 10.2174/1381612033455431. [DOI] [PubMed] [Google Scholar]
- 78.Cushman D.W., Ondetti M.A. Design of angiotensin converting enzyme inhibitors. Nat Med. 1999;5:1110–1113. doi: 10.1038/13423. [DOI] [PubMed] [Google Scholar]
- 79.Karamyan V., Arsenault J., Klarskov K., Shariat-Madar Z., Escher E., Speth R. Experimental Biology Meeting April 2009. 2009. Purification and partial characterization of a novel non-AT1, non-AT2 binding site for angiotensins in the rat brain. Program number: 943.8- [Google Scholar]
- 80.Sasaki A., Hinck L., Watanabe K. RumMAGE-D the members: structure and function of a new adaptor family of MAGE-D proteins. J Recept Signal Transduct Res. 2005;25:181–198. doi: 10.1080/10799890500210511. [DOI] [PubMed] [Google Scholar]
- 81.Sekine-Aizawa Y., Hama E., Watanabe K., Tsubuki S., Kanai-Azuma M., Kanai Y., Arai H., Aizawa H., Iwata N., Saido T.C. Matrix metalloproteinase (MMP) system in brain: identification and characterization of brain-specific MMP highly expressed in cerebellum. Eur J NeuroSci. 2001;13:935–948. doi: 10.1046/j.0953-816x.2001.01462.x. [DOI] [PubMed] [Google Scholar]
- 82.McKie N., Dando P.M., Rawlings N.D., Barrett A.J. Thimet oligopeptidase: similarity to 'soluble angiotensin II- binding protein' and some corrections to the published amino acid sequence of the rat testis enzyme. Biochem J. 1993;295:57–60. doi: 10.1042/bj2950057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kato A., Sugiura N., Hagiwara H., Hirose S. Cloning, amino acid sequence and tissue distribution of porcine thimet oligopeptidase. A comparison with soluble angiotensin-binding protein. Eur J Biochem. 1994;221:159–165. doi: 10.1111/j.1432-1033.1994.tb18725.x. [DOI] [PubMed] [Google Scholar]
- 84.Albiston A.L., McDowall S.G., Matsacos D., Sim P., Clune E., Mustafa T., Lee J., Mendelsohn F.A., Simpson R.J., Connolly L.M., Chai S.Y. Evidence that the angiotensin IV (AT4) receptor is the enzyme insulin regulated aminopeptidase. J Biol Chem. 2001;276:48623–48626. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- 85.Vanderheyden P.M. From angiotensin IV binding site to AT4 receptor. Mol Cell Endocrinol. 2009;302:159–166. doi: 10.1016/j.mce.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]


