Abstract
Porcine epidemic diarrhea (PED) was identified in the United States in the spring of 2013, and professionals from many parts of the U.S. swine industry responded rapidly to understand and control the newly emerging disease. In less than two months, the disease had spread to more than 200 herds in thirteen states. Experts from the US Department of Agriculture (USDA) engaged in laboratory diagnostics, analytic support, epidemiology expertise, and data management to facilitate the effort. By 2014, a great deal had been learned about the disease; however, the question of how it entered the United States remained unanswered. In 2014, USDA formed an investigative group to address the question and leverage current knowledge with resources and partnerships not readily available to non-federal investigators. The group formed collaborations with other government and non-government organizations and individuals, and followed many avenues of inquiry; ultimately arriving at a small number of scenarios that describe possible mechanisms for PED introduction. For a scenario to be plausible, it had to explain: contamination of a person or product in the source country, its transit and entry to the United States, rapid dispersal across a wide geographic area, and exposure/infection of pigs. It had to be compatible with findings of swine herd investigations and research studies. Potential products had to have been imported legally during the time prior to the beginning of the epidemic, or delivered to the United States through prohibited channels. Follow-up studies were initiated to gather more evidence for the most plausible scenarios. Of the scenarios, flexible intermediate bulk containers (“feed totes”) used to transport bulk feed serving as fomites for movement of PED virus provided the simplest explanation for the accumulated findings of the investigation.
Keywords: Porcine epidemic diarrhea, Outbreak investigation, Flexible intermediate bulk container, Tote, Feed, Virus transmission, Epidemiology
1. Introduction
Cases of porcine epidemic diarrhea (PED) were first diagnosed in the United States (U.S.) beginning in April 2013. The swine industry and associated professionals responded on many fronts with the Veterinary Services (VS) branch of U.S. Department of Agriculture (USDA), Animal and Plant Health Service (APHIS) initially engaging in laboratory diagnostics, analytic support, epidemiology expertise, and data management. Multiple investigations and studies were conducted in an attempt to answer questions about the epidemic; at the top of the list were how the virus arrived in the United States and if there was risk of another disease following the same path.
Feed was initially suspected by veterinarians in several of the first-detected cases, and became a focus for a case-control study. The study included multiple farms with 25 cases and 18 controls. It was completed by June, 2013 and summarized by Dr. Harry Snelson from AASV at the 2014 World Pork Expo (Snelson, 2014). This was conducted as a collaborative effort between the American Association of Swine Veterinarians (AASV), National Pork Board (NPB), National Pork Producers Council (NPPC), VS' Center for Epidemiology and Animal Health, VS' Chief Epidemiologist, and the National Center for Foreign Animal and Zoonotic Disease Defense (currently: Institute for Infectious Animal Diseases (IIAD), a DHS Center of Excellence at Texas A&M University). Univariate regression analysis on the probability of being a case (i.e., presence of PED virus RNA plus animals with clinical signs) revealed feed factors that were associated with statistically significant higher odds of having PED. Feed that was custom mixed off-farm, increased number of meal/mash rations fed to nursery or finisher pigs, and whether vitamins and minerals were in the same as opposed to separate premixes increased the odds of PED on a farm between 1.5 and 3.5 times. These variables suggest the potential for contamination of feed where complete feed mixed off-farm is related to an effect where more ration types could mean more chances to get a contaminated batch. Although the case-control study suggested that feed was associated with the outbreak, subsequent investigations and herd histories failed to show a common feed or ingredient among the farms.
In late spring 2014, the mechanisms by which PED arrived in the United States had not been determined. USDA formed an investigative group to research the root cause of the epidemic; that is, to find the initiating cause or causal chain where an intervention could reasonably be implemented. A Root Cause Investigation Group (RCG) was tasked with revisiting the mass of information that had accumulated following the initial outbreak, and to leverage resources and partnerships not readily available to non-federal investigators. The group's objective was to identify the mechanism or most likely mechanisms by which PED reached the United States and infected U.S. pigs. The RCG reviewed literature; evaluated data from research projects; consulted with swine industry and veterinary specialists familiar with the individual outbreaks; collaborated with U.S. Government partners; reviewed reports published on university, industry, and laboratory websites; examined U.S. Customs and Border Protection (CBP) data on imported products; evaluated data from prohibited product seizures at U.S. ports; and collated testing data from affected herds. Additionally, the RCG initiated new studies as indicated, analyzed data, and conducted follow-up investigations of early-affected farms, and also reached out to international partners that had experienced outbreaks of novel swine enteric coronavirus diseases.
In many instances, information gathering was complicated because records and recall were not available or not collected at the time of the initial veterinarian's herd examinations. Although many people were eager to help solve the problem, some had concerns about sharing intellectual property or individual information with the Federal Government.
2. Methods
2.1. Epidemiology approach
During 2014, APHIS-VS prepared a pathway entry risk assessment entitled, Pathways Assessment: Entry Assessment for Exotic Viral Pathogens of Swine as the first step towards determining whether significant gaps exist in import regulations that may result in infections of U.S. domestic swine with exotic viral pathogens (USDA-APHIS, 2014). The RCG used the conclusions of this report and results of published research studies as a baseline, and followed with an epidemiological approach to narrow the scope and more specifically address the entry of PED virus into the United States. The group applied basic concepts of host, agent, environment, and evaluation of transmission mechanisms to develop scenarios that could explain the epidemic. National epidemic curves and timelines were developed to plot the course of the disease spread. Individual herd data gathered by first-responding veterinarians, and later augmented by revisits by VS officers to farms provided vital information for limiting the number of likely disease introduction mechanisms. Host factors, such as a naïve swine population with an explosive spread of disease, helped to limit the probable time of first introduction. Data from genetic epidemiology, virus survival, and infectious dose studies further narrowed the possibilities. Fig. 1 describes five criteria the RCG considered essential for a scenario to explain PED virus entry to the country.
Fig. 1.
Criteria to narrow the scope of the investigation.
2.2. Collaborations
Collaborations were established with other government and non-government entities, including Food and Drug Administration (FDA), other APHIS units (Wildlife Services (WS) and Plant Protection and Quarantine (PPQ)), U.S. Department of Homeland Security National Biodefense Analysis and Countermeasures Center (NBACC), and CBP, as well as universities and industry organizations.
2.3. Interviews with swine veterinary consultants
U.S. veterinary consultants were interviewed for their front line perspective on the herd outbreaks and impressions about the disease introduction. Unlike the case control study which had detailed survey questions (Snelson, 2014), the interviews were structured as informal discussions. A framework for topics was formulated to guide the discussions (Fig. 2, Fig. 3 ), but the experts were encouraged to brainstorm and speculate about potential scenarios.
Fig. 2.
General interview topics for swine veterinary consultants and first responders. The veterinarians were encouraged to postulate and expand on each topic.
Fig. 3.
Feed specific interview topics for swine veterinary consultants and first responders. The veterinarians were encouraged to postulate and expand on each topic.
2.4. Herd investigations
Eight infected herds were identified by the testing data or by swine consultants as having occurred in April and May 2013, and were believed to include the earliest affected farms. These were chosen for further investigation. Results from the case control survey (Snelson, 2014) that included the eight farms were evaluated, and the farms re-visited by VS veterinary officials during summer and fall 2014. The veterinarians originally attending the herds were interviewed as well as production managers or owners. In some cases, the follow-up visits extended to the feed mills that formulated rations for the farms.
2.5. Data analysis
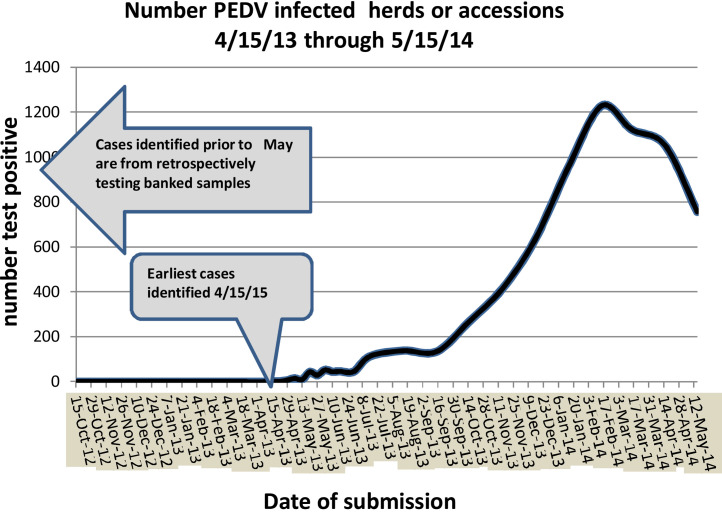
Data from voluntary laboratory submissions through May 2014 were fit to an exponential distribution using the distribution fitting function software in @Risk version 6.3.0 software (copyright Palisade Corporation) to form an epidemiological outbreak curve (Fig. 4 ). There were data limitations in that sample submissions prior to a June 2014 USDA Federal Order were voluntary, but assumed to be reasonably complete. In the initial six weeks following the first identified cases of PED, submissions represented herds, while later data represented laboratory accessions. It is unknown whether the latter data represented individual herds. Univariate regression analysis was conducted on data from an initial case-control study with 25 cases and 18 controls (Snelson, 2014) at USDA's Center for Epidemiology and Animal Health using SAS software (Copyright, SAS Institute Inc.) to determine odds ratios and significance for association of cases with feed related factors. Additionally, Iowa State University Veterinary Diagnostic Laboratory (ISUVDL) conducted retrospective testing on specimens with submission reasons of gastrointestinal (G.I.) disease archived between December 2012 and May 2013. The G.I. samples represent highly targeted samples for finding PED in cases across the laboratory's area of service across the Midwestern region of the United States. USDA epidemiologists qualitatively evaluated metadata data from Custom and Border Patrol Import Tracking Database to identify categories of products that could potentially be associated with swine feed and serve as mechanical vectors of SECD virus. The data were then qualitatively risk-analyzed by USDA trade risk specialists based on five criteria. Methods are described in further detail in Entry Assessment for Exotic Viral Pathogens of Swine (USDA-APHIS, 2014). Criteria included:
-
1
Likelihood of swine, swine tissues, or fluids contact with raw ingredients.
-
2
Likelihood typical processing will not inactivate coronaviruses.
-
3
Likelihood of swine, swine tissues, or fluids will have contact with the ingredient post-processing.
-
4
Likelihood that virus will survive transport from manufacturer to swine farm.
-
5
Likelihood that swine will be exposed to the ingredient.
Fig. 4.
Sample accessions from October 2012 through May 2014 are shown. Sample dates prior to May 2013 were tested retrospectively from laboratory samples banked from previous diarrheal disease outbreaks. The earliest two infected herds developed clinical signs on approximately April 15th. The slope of the epidemic curve is more flat during the summer months of 2013, probably due to summertime environmental conditions.
Definitions of risk estimation terms are presented in Table 1 .
Table 1.
Risk terms used to assess CBP data potential for a feed related product to move SECD viruses.
| Risk term | Definition |
|---|---|
| Negligible | Occurs so rarely that it does not merit consideration |
| Low | Occurs rarely |
| Medium | Occurs sometimes, but not often |
| High | Occurs often |
2.6. Genetic epidemiology
Genetic epidemiology reports from the peer reviewed literature were evaluated to help characterize the virus agent as well as to determine the nearest worldwide relatives. Additional study was initiated with government partners in the Department of Homeland Security, National Biodefense Analysis and Countermeasures Center (NBACC) to further evaluate the virus' nearest relatives and time of divergence from them.
2.7. United States Customs and Border Protection (CBP) data
The RCG reviewed U.S. CBP metadata for products considered to have the potential for carrying PED virus. These were products that could be contaminated in the source country with an ultimate use that might expose U.S. pigs (e.g., organic soybeans, or products that could be repurposed for feed). In collaboration with APHIS Plant Protection and Quarantine (PPQ) data managers, the group accessed detailed CBP data, and risk-evaluated it to further narrow the list of likely products. Those that had non-negligible risk were further assessed to determine if they had been imported to the United States during the three months of 2013 prior to the initial detection of PED.
2.8. Scenario development
By combining information learned through the avenues described above, the RCG developed possible scenarios where the product could have facilitated virus transit through four segments of travel from the origin country to end up in the locations where PED virus was initially detected (Fig. 1). The four segments include contamination in the source country, transit and entry to the United States, dispersal across the nation, and exposure of pigs. Product shipments were considered less likely to have caused the U.S. epidemic if the quantity was very small (e.g., less than five kilograms), or if the product was consigned to companies in the western part of the United States, specifically those without nationwide distribution networks. The group considered products more likely when the consignor was located in the swine-dense geographic area of China near where the closest known ancestors of the U.S. viruses were reported in the international literature, and less likely if they originated in more distant areas. Products were considered less likely if not identified as a ration component in the herd investigations, or processing steps would eliminate the virus and post-processing contamination was unlikely.
Scenarios that were generated explained the evidence from the different avenues of inquiry to greater or lesser degrees. The resulting hypotheses led to further questions, studies, and collaborations, and finally to a small number of possible candidates. Several potential scenarios were ruled out if they were identified as having negligible risk of entry in the Pathways Assessment: Entry Assessment for Exotic Viral Pathogens of Swine (USDA-APHIS, 2014) and not further investigated.
Scenarios that were further evaluated included mechanisms involving: flexible intermediate bulk containers (aka: FIBC or “feed tote bags”), food salvage warehousing and transport networks, imported pet treats, imported organic soybeans, a reservoir in feral swine, intentional or accidental introduction by humans, transport by birds, semen and live animals, spray dried porcine plasma (SDPP), accidental release from a laboratory or diagnostic facility, contaminated biological, plant material used as antibiotic filler, prohibited product importation, amino acid supplements, and vitamin and mineral premixes.
2.9. Follow-up studies
-
1)
Imported organic soybean testing. Between March and September 2015, samples from shipments of organic soybeans originating China were collected at three U.S. Ports of Entry and submitted to South Dakota State University Diagnostic Laboratory for testing for six viruses. The laboratory followed standard diagnostic methodology (http://www.sdstate.edu/vs/adrdl/) for PED virus, Porcine Delta coronavirus (PDCoV), transmissible gastroenteritis (TGE), porcine circovirus type 2 (PCV2), and North American and European type porcine reproductive and respiratory syndrome viruses (PRRS).
-
2)
During 2012–2013 FDA collected and archived samples of pet jerky treats imported from China that had been submitted in association with pet deaths. Methods for extraction and specimen testing by polymerase chain reaction (PCR) were developed and tests conducted for PED virus RNA by Dr. Haile Yancy's group at the FDA's Center for Veterinary Medicine.
-
3)
Stability of PED virus in FIBC material was evaluated using a cell culture model; 1.5″ by 1.5″ pieces of polypropylene tote material were placed in sterile, plastic petri dishes. 1 ml of cell culture adapted PED stock virus diluted in MEM was added to the center of each piece of tote material and allowed to dry overnight at room temperature in a biocontainment hood. Petri dishes were covered and placed in closed containers (30 dishes) at each temperature. After each time-point was reached, replicates for each temperature were removed, rehydrated in 1 ml MEM and titered on confluent Vero-76 cells in 96-well plates. Each titration was conducted in triplicate, resulting in 9 replicates/time-point/temperature variables. At 20 h post-infection, the virus titration plates were fixed in 80% acetone, and then stained with PED virus SD6-29 FITC conjugated monoclonal antibody. Individual foci of PED VIRUS infected cells were counted at appropriate dilutions (generally at the highest virus dilution showing 10–30 individual foci/well). Infectious virus titers for each time point and temperature were presented graphically as mean fluorescent focus units (FFU)/ml.
-
4)
Testing for evidence of PED virus circulation in feral swine. Detecting PED virus in feral swine samples archived prior to April 2013 would suggest that the initial introduction into the United States was earlier than indicated by reported test data, and that the path of introduction differed significantly than what might be expected in commercial swine. The purpose of retrospective testing of feral swine was to produce evidence to inform the hypothesis that SECD-related virus was circulating in feral swine prior to the initial detection of clinical signs in domestic swine in April 2013. Testing of the samples was conducted with an ELISA test (Whole Virus ELISA for PED virus) recently developed by ISUVDL. Initial performance testing on domestic swine indicated that the diagnostic specificity was 98.5% and the sensitivity was greater than the indirect immunofluorescence assay (IFA) from 2 to 7 weeks post inoculation (diagnostic sensitivity point estimate 99.2%). Serum samples from 368 feral swine were provided from the Wildlife Services Wildlife Disease Program archive for fiscal years 2011–2013. The samples were collected opportunistically from various locations in Iowa, Indiana, Michigan, Ohio, Illinois, and Hawaii.
3. Results
3.1. Epidemiology research summary
PED virus is highly transmissible with an infectious dose that is likely only a few dozen virions (Goyal, 2013, Schumacher et al., 2015). It appears to remain viable longer at cooler temperatures (Verma et al., 2014), and has been shown to spread through various mechanisms including trucks, feed, animals, manure, and other fomites (Lowe et al., 2014, McCluskey, 2014, Snelson, 2014, Verma et al., 2014, Yeske, 2014, Nugent, 2015, Sampedro et al., 2015). Genetic epidemiology investigations identify the closest known relatives of the U.S. outbreak to be viruses associated with the 2010–2013 outbreaks in China with the exact date of divergence from a common ancestor unknown (NBACC 2015-unpublished data); (Huang et al., 2013, Stevenson et al., 2013, Chen et al., 2014, Wang et al., 2014a, Wang et al., 2014b). Transpacific transport of products by ocean travel from China and distribution within the United States would likely require three or more weeks and viability of the virus for at least that amount time. This would assume some degree of protection from the environment as well as cool temperatures that might be expected in the spring months of a temperate climate.
3.2. Herd investigations and interviews with swine consultants
Initial herd investigations were conducted in 2013 by each company's veterinarian and accompanied by a detailed epidemiological survey questionnaire (Snelson, 2014); VS acquired the survey results and reviewed details prior to visiting farms. In 2014 VS officials revisited eight farms that were identified as having PED at or near the beginning of the outbreak between April 15 and May 22 2013, as well as other farms with epidemiological circumstances that were unusual or suggestive of a specific source of introduction. Additionally, VS interviewed herd veterinarians, managers, owners, and feed mills. Aggregated information is described below.
The consultant interviews and individual herd investigations were unable to identify the source of the epidemic, but in combination with other accrued information, provided valuable insight for the RCG. Affected farms used different brands of feed from different source companies. There were no common veterinarians, and the herds were owned by different companies. The age and production types of the pigs were different between cases, as were ration formulations. Most of the farms employed good if not best practices for biosecurity; in several cases, they were farms that had been able to exclude endemic contagious viruses such as porcine reproductive and respiratory syndrome (PRRS). There was no single brand or type of biological or vaccine that was used in the different farms. No farm reported visitors from other countries or suspicious activities that suggested an intentional infection of their pigs. The farms were not associated geographically and were not proximate to research or laboratory facilities. Semen came from different sources, all within the United States. None of the herds that were reported through laboratory testing data in the first month of the outbreak were small herds; i.e., all affected herds were of size greater than 1000 animals. Operations with less than 1000 head compose approximately 80% of all U.S. swine operations (USDA-NASS, 2012), and small farms in general have been shown less likely to have stringent biosecurity practices (USDA-APHIS-VS-CEAH, 2012). Although possible that small farms may use veterinary or diagnostic services and report less frequently than larger commercial farms, smaller farms may not have been affected because of different management practices such as receiving feed in bulk quantities. Of the consultants interviewed, three believed the virus introduction was associated with feed, one thought it unlikely that PED virus would remain viable for the length of time required for transit to the United States in feed, and others believed feed as a possibility but were non-committal as to mechanisms. Other alternatives varied widely between experts.
Documentation was not available for every herd, but several had record of feed deliveries within 24 h of initial signs. No two farms were found with feed products or supplements having common lot numbers. In some cases, trucks were dedicated to the farm and feed mixed at on-site company owned feed mills. Rations and ingredients varied between farms; however, almost all feed mills used various repurposed products in some of their ration formulations. These included dried distillers grains, soybean hulls, pet food, recycled human food, dairy products, and bakery products.
3.3. Epidemiology data analysis
The first date that PED virus infected herds were detected in the United States was April 15, 2013. Although it is possible that other earlier cases could have existed, PED was unlikely to have been present more than a few days or weeks prior to this date for multiple reasons. Iowa State University Veterinary Diagnostic Laboratory (ISUVDL) tested diagnostic samples from nearly all swine raising regions of the Midwest that had been submitted for gastrointestinal disease. The samples, archived in 2012 and 2013, were found negative for PED virus RNA (personal communication, approximately 800 samples). Because they were collected from pigs with clinical signs compatible with PED, these represented highly targeted samples for detection of PED if it had been present in the herds. Although data were not available from other laboratories, no positive results were reported for PED virus.
The epidemiology curve for PED shows eight herds initially infected between April 15 and May 5 2013, followed by rapid expansion of disease (Fig. 4, Fig. 5 ). Each point on the curve in Fig. 4 represents a laboratory accession from one herd or one associated group of herds from one company. The slope of the curve in Fig. 4 is lower for the months of June–September, which may be the result of summer time high temperatures, followed by steeper exponential increase over the winter months. The curve suggests one or a small number introductions of PED virus followed by a rapidly propagating epidemic typical of a highly contagious disease (Smith, 1995).
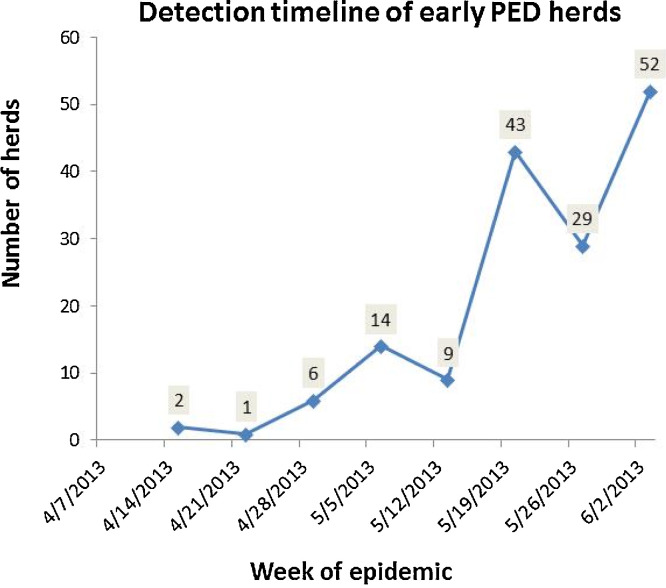
Fig. 5.
Timeline of known PED infected herds showing number of herds detected during each week. The first six cases were geographically dispersed in the states of Ohio, Indiana, and Iowa and followed shortly by herds in Colorado and Minnesota and then other states.
Fig. 5 shows a time line of the first cases that were identified. Each point on the curve represents the number of herds identified during the week, and dates are adjusted to best represent the first day that clinical signs were reported to be present. A case is defined as one or more herds in a facility or geographically associated facilities belonging to one company. The first cases show that herds in at least six separated companies became infected within approximately two weeks in geographically dispersed locations across Ohio, Indiana, and Iowa (Fig. 5). These were shortly followed by outbreaks in Colorado and Minnesota, and other states.
Finally, because the initial PED virus was very virulent causing dramatically overt signs in farrowing sows and young piglets, it would likely have been identified quickly by private veterinarians through passive disease surveillance unless in an isolated population such as feral swine.
3.4. U.S. CBP data
Because the number of products shipped to the United States is large, the RCG filtered the number of plausible ones as described. Products that are not shipped to the United States from Asia such as spray dried porcine plasma and live swine, or products that were not shipped during the first three months of 2013 were excluded. Products identified from the CBP data that met these criteria as candidates with greater than negligible risk were organic grains (i.e., soybean), pet treats, lysine, or contaminated Flexible Intermediate Bulk containers (FIBC) that could have cross-contaminated feed ingredients.
3.5. Results summary of follow-up studies
-
1)
Imported organic soybean testing. As of 9/22/15, samples from 30 shipments of imported soybeans had been received with no detection of any of the assayed viruses (PEDv, PDCoV, TGE, PRRS, PCV2).
-
2)
Testing archived pet jerky treats from China. No virus was detected from 40 samples of the imported jerky pet treats archived prior to April 2013.
-
3)
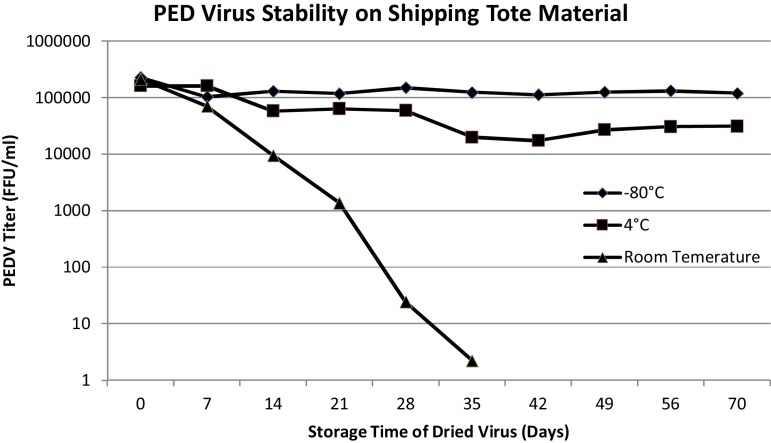
Stability of PED virus in FIBC material. Results for survival of PED virus on FIBC material are suggestive that the FIBC scenario has merit. The woven fabric was treated with a preset amount of cultured PED virus. The virus concentrations remained stable through the 10-week time point for both the 4 °C or −80 °C temperatures. Viable virus was detected after five weeks but not six weeks at room temperature (Fig. 6 ).
-
4)
PED virus circulation in feral swine from a set of feral swine serum samples archived prior to the PED outbreak. Although considerable uncertainty exists in estimating feral pig population numbers, six post-hoc groups of pigs were identified by VS and WS biologists. With the assumption that sampling was proportional to population size and the sampled pig units represented 25 to 90% of the population in each group, the estimates of minimum detection levels ranged from 5 to 30%. All results were negative, and provide no evidence to support the hypothesis that PED virus was present in the United States prior to the first detection in domestic swine in April 2013.
Fig. 6.
After 10 weeks of storage, PED virus remained stable at −80 °C at a concentration of approximately 2 × 105 FFU/ml. In addition, PED virus stored at 4 °C demonstrated similar stability over time. In contrast, PEDV held at room temperature demonstrated a drop in titer of approximately 1 log per week. No viable PEDV was detected after 5 weeks of storage at room temperature.
4. Discussion
The SECD Root Cause investigation evaluated a comprehensive set of empirical data from swine testing, U.S. import data, and research findings as well as published reports and non-structured data from interviews with consultants and first responders. The group also revisited the first identified farms and initiated follow-up testing and studies to address specific questions. Each data source had inherent limitations. Prior the U.S. Federal Order of June 5 2014, laboratories submitting test results did not identify whether the result was for a herd or accession. The group assumed that the shape of the epidemic curve would not vary significantly due to those accessions that could have represented more than a single herd. The customs import data provide very limited description of products through a Harmonized Tariff Code and a brief product description (e.g., “SOYBEANS W/N BROKEN, ORGANIC”). Importer and consignee data represent the port of origin and destination but do not identify the final disposition of a product, and the possibility exists for misidentification of products. Recognizing potential limitations, the Root Cause investigators met with APHIS port inspectors and data managers to help clarify questions about the customs data definitions and inspection processes. Information from farm visits and interviews were subject to recall bias because the outbreak began 15–18 months earlier than the investigation. Some farms had records of ration components, invoices, and deliveries while others had partial records or recollection available. Although no single data source could supply definitive answers, the aggregated information led to several possible scenarios to explain the source of the outbreak.
Scenarios were developed to explain the accumulated data as well as to meet four essential pathway criteria of: (1) the carrier became contaminated at its origin, (2) traveled to the United States, (3) became dispersed across multiple states in a very short time and (4) infected pigs at farms. Although many scenarios could be possible, movement associated with feed containers (FIBC) best fit the data, met the criteria, and was the simplest explanation requiring the fewest assumptions about the findings.
4.1. Flexible Intermediate Bulk Containers (FIBC) as fomites
Feed totes (FIBC) are large container sacks that are commonly used to transport bulk animal feed as well as many other products. A commonly used variety is made of woven polypropylene and may also have an internal liner. The interior of the FIBCs are designed with reinforcing material, various folds, and exit chutes as well as area between the woven fibers that could provide protection from environmental conditions such as flushing by product, desiccation, heat, and ultraviolet radiation from sunlight. In the United States prior to the SECD epidemic, they were frequently reused, and are not likely to have been cleaned or disinfected in a manner to eliminate viruses. In addition to reuse at feed mills, recycled FIBCs are available for sale and may be purchased online for use with any number of products. One retailer of the repurposed FIBCs suggests examples of possible use as: “Landscape Debris–Compost Carriers–Large Sand and Bags for Levees” (http://www.repurposedmaterialsinc.com/store/products/used-tote-bags-bulk-bags/). Similarly, reusable FIBCs are advertised for sale in other countries for a multitude of uses.
We can only speculate on how or if FIBCs may have been contaminated, and do not know whether FIBCs used for importing products are new or used. There were no federal regulations or requirements in 2013 that precluded importing products in previously used FIBCs as long as they passed visual inspection on entry to U.S. ports. In September 2015, the FDA enacted the Food Safety and Modernization Act (FSMA) which requires companies to conduct a hazard analysis and mitigate potential risks for products and would include hazards associated with food or feed such as containers.
FIBCs are designed for reuse, so it is plausible for an FIBC to carry contaminated material and later be used to carry other products. The FIBCs are commonly used to transport a variety of products including grain, sand for flood control, fertilizer, compost, and wood shavings. Products may become contaminated in transit in the open topped FIBCs. For example, Blomme (2014) describes transmission of PED virus by European starling droppings that may contaminate any materials that are in unprotected containers (Blomme, 2014). An additional opportunity for FIBCs or products to become contaminated is through untreated water either used for washing or exposure to waste or flood water from a crop or animal farm. Fig. 7 shows a picture of FIBCs on pallets in an open field under a tree used to transport fertilizer.
Fig. 7.

FIBC “totes” are reusable, tough, versatile bags made to transport many bulk products but are not specifically designed to exclude environmental contaminants that could harbor viruses. They are commercially available for purchase from many retailers worldwide. This image shows totes containing fertilizer under a tree in an open field. Products carried in them could easily be exposed to contaminated content, bird traffic, flood water, or other sources of virus.
An alternative pathway for FIBC contamination would be via contaminated products imported into the United States transported in FIBCs. The RCG investigated two products that could fit this scenario. Prior to 2013, a large number of pet treats sold in the United States were processed in China, and were associated with pet deaths (FDA-CVM, 2013). Evaluation of the processing and biosecurity practices of the treat manufacturers was reported by FDA, and the inspection reports describe sanitary conditions and processes in the manufacturing plants (FDA-CVM, 2012a, FDA-CVM, 2012b). Viruses are unlikely to remain viable through cooking, but post-cooking biosecurity between cooked and raw product as described in the FDA reports may not be adequate to prevent cross-contamination with the highly contagious PED virus. Pre-shipping procedures include a voluntary cold sterilization irradiation process, which appears be effective against bacterial contaminants, but would not be not adequate to prevent virus carriage. It is unknown whether pet treats in the United States are repurposed and incorporated with other pet food into swine rations, but if so, repurposed pet food is transported in FIBCs and may provide opportunity for virus movement (VS field officers-personal communication). Incorporation of pet food or pet treats is uncommon in swine rations in either Canada or European Union countries due to regulations prohibiting the practice (USDA-FAS, 2010, CFIA, 2012).
A second product with the potential for contamination is organic soybean. The United States is a major soybean exporter, but due to high demand for organically grown and non-genetically modified organism (GMO) beans, the United States imports organic soybeans from several countries including China. For the period in 2013 between January 1st and April 15th, imports from China totaled approximately 31,000 metric tons of soybean and 1000 tons of soy flours (CBP import data). The soybeans are frequently transported to the United States in FIBCs. Since the first PED virus herds detected were not organic farms, transmission of virus would have to occur via an intermediate fomite such as reused FIBCs. After a contaminated product is removed, the FIBC would remain contaminated, and its reuse would potentially transmit virus to the next product it contained. The second product or the container would then arrive at a feed mill or farm to infect pigs.
4.1.1. Recycle/transport/warehousing networks; dispersion within the United States
Several companies in the Midwestern United States provide valuable services to swine producers by recycling various products and by-products used in formulating rations. In this scenario, contaminated food or feed products are warehoused temporarily, and then shipped to feed mills for repurposing into swine feed. The contamination source may be either the product or its container. Because of the efficient network, this may occur within days.
Although no fault was identified or suspected during the evaluation of company operations, they may have inadvertently provided a mechanism that quickly moved the highly contagious PED viruses to multiple locations. Company websites advertise trucking networks that service areas having a radius of several hundred miles; areas that easily encompasses all of the early PED affected farms. In addition to trucking, they often provide services for trading grain, feed ingredients, by-products, and recycled human food products. Warehousing facilities are available as well as multiple kinds of trucks, trailers, and rail delivery. With a large volume of trucks and trade, they have the opportunity to visit many locations in a short time.
4.1.2. Pig exposure
Investigations have suggested feed to be a source of PED virus, and feed mills a possible transit point (Dee et al., 2015, McCluskey, 2014, Yeske, 2014). Feed mills that formulate swine rations receive and process ingredients using various types of equipment, such as grinders and mixers, and send the final mixed ration to farms. Exposure in the FIBC scenario would happen when a finished ration becomes contaminated by FIBC or processing equipment and is delivered to the farm.
The early-herd outbreak case control study (Snelson, 2014) and several herd investigations demonstrated a significant association of outbreaks with feed, but the investigations failed to show a common feed type, brand, company, or component between the infected herds. The FIBC scenario would explain this apparent contradiction as being an association with feed delivery and transport containers rather than the actual feed product.
Results for survival of PED virus on FIBC material are further supportive that this scenario has merit. The woven fabric was treated with a preset amount of cultured PED virus. Virus capable of infecting Vero cells was present in samples taken after five weeks but not six at room temperature. After 10 weeks there was negligible reduction in virus concentration in samples that were stored at either 4 °C or in the −80 °C controls. Although the stability study was conducted in-vitro, it is highly suggestive that PED virus would remain stable through transit of several weeks at temperatures likely present during the early spring months of 2013.
4.2. Feral swine PED virus reservoir
A second scenario that was tested was that PED virus had been circulating in a feral swine reservoir prior to introduction in domestic pigs. Because the first PED virus proved to be very virulent, its unnoticed circulation in domestic or feral swine prior to April 2013 would be unlikely unless in an isolated population. Detecting PED virus in feral swine samples archived between January 2010 and April 2013 would suggest that the initial introduction into the United States was earlier than indicated by reported test data, and that the path of introduction differed significantly than what might be expected in commercial swine.
To test the hypothesis of whether there was a reservoir of PED virus in feral swine, VS conducted a study in collaboration with APHIS-Wildlife Services (WS) and ISUVDL to gather additional information. The purpose of retrospective testing of feral swine was to produce evidence to inform the hypothesis that PED virus was circulating in feral swine prior to the initial detection of clinical signs in domestic swine in April 2013. A total of 368 serum samples had been collected and archived from 2010 through April 2013 by WS from feral swine control activities; no positive serological results were found. The samples were collected opportunistically from various locations in Iowa, Indiana, Michigan, Ohio, Illinois, and Hawaii. Although considerable uncertainty exists in estimating feral pig population numbers, six post-hoc groups of pigs were identified by VS and WS biologists. With the assumption that sampling was proportional to population size and the sampled pig units represented 25–90% of the population in each group, estimates of minimum detection levels ranged from 5 to 30%. The scenario was not ruled out, but considered unlikely.
4.3. Introduction by humans
Postulating introduction of PED viruses into the United States by people provides several compelling scenarios; however, there is little data to support this actually happening. International movement of people in the swine industry and veterinary professions has been substantial for the last decade as China modernizes its large swine industry, but there was no known change in numbers of travelers before or after April 2013. It is likely that veterinary consultants and swine industry professionals coming to meetings in the United States, or individuals buying swine breeding stock were in contact with affected pigs before traveling to the United States because of the high prevalence of the disease in China (Feng, 2014). Swine consultants that have worked in China for many years acknowledged the risk, but explained that travel biosecurity measures have been standard and adequate to prevent disease introduction in the past. Practices in the U.S. commercial swine industry for visitors or employees include down time after travel, isolation of visitors from pigs, and segregation of clothing (personal communication swine consultants).
PED virus was able to enter farms that were considered to have excellent biosecurity through unknown mechanisms (Stevenson et al., 2013), and infect farms that had been able to exclude other contagious viruses such as PRRS (personal communication swine consultants). There was no history of visitors or travel to foreign countries by personnel, managers, or other company employees in any of the PED virus herds investigated and no human link between the herds. These findings are not definitive, but indicate that accidental introduction by people on clothing is probably not likely.
Intentional introduction is a possible scenario, but there is no evidence of a person directly infecting herds. None of the investigations or consultant interviews identified visitors or unusual events associated with outbreaks that might suggest intentional exposure of swine, while much of the collected evidence suggests an association with feed or feed delivery. Although the sudden appearance of disease in multiple farms at close to the same time might suggest an intentional introduction, an access route for direct introduction to the farms by a person was not identified. While someone contaminating a central location such as a feed distribution network as described above is possible, employing Occam's razor would suggest that simpler explanations are more likely (https://en.wikipedia.org/wiki/Occam%27s_razor).
4.4. Other scenarios considered
Other scenarios were evaluated including birds as carriers, contaminated semen, live animals, spray dried porcine plasma (SDPP), release from diagnostic laboratory or research facility, contaminated biological, antibiotic filler (e.g., rice hulls), prohibited product importation, vitamin and mineral premixes, and amino acid supplements. Many were ruled as unlikely because investigation data from the earliest detected herds did not support them or were considered less likely because of other data. Evaluation of the entire set of information required a number of assumptions for these scenarios to be feasible. Birds were considered unlikely because none of the herds had exposure to migratory birds, and no evidence has been reported that the current strains of SEC viruses can cross species to infect birds then back to pigs again. Different sources of semen, supplies, and biologicals were reported between the farms, and none of the operations were located proximate to research facilities or laboratories. Although some were fed antibiotic products, others were not. Further, the plant material fillers like rice hulls are very dry and likely not hospitable to virus stability. SDPP was used in some rations but not in others, and is a prohibited import from China. Use of prohibited products was feasible, but unlikely that all of the diverse farms received the same product and none reported using any products likely to contain prohibited material. While not available from all farms, lot and product number from vitamin, mineral, and amino acids were from different sources.
5. Conclusion
The specific route and travel of PED viruses that came to the United States may never be uncovered; however, a common theme runs through the most plausible scenarios described above. The viruses must have been carried through four segments of the journey: contamination in the country of origin, entry to the United States, dispersion to multiple locations, and exposure and infection of pigs. The FIBC scenario explains a mechanism for each and is compatible with findings of herd investigations as well as other information gathered with few assumptions. It is plausible that pet treats entered the United States contaminated by PED virus, but unlikely that they infected pigs without a secondary fomite such as the FIBCs and unknown if they were ever fed to pigs. Similarly, organic soybeans could be contaminated with viruses, but they too must have a secondary carrier to achieve the dispersal and exposure parts of the journey to infect pigs. The same conclusion, that a secondary fomite is necessary, exists for almost any of the plausible scenarios that the RCG investigated.
Breaking any one of the four segments of virus transit would suffice to mitigate the risks of this type of event. Contamination of products in an origin country is largely out of U.S. Government regulatory control and likely outside the realm of industry management. Inspections at entry ports are vital, but visual inspections or even empirical testing would be unlikely to identify products contaminated with miniscule amounts of infectious virus. If the fomite that moved the virus was indeed the FIBC, sanitary management prior to reusing the bags may be an effective mitigation. Further research is necessary to identify appropriate cleaning and disinfection procedures and parameters, but the answer could be as simple as not reusing the bags, or a to-be-determined, protocol of dry heat or disinfection prior to reusing the containers.
Acknowledgements
The VS Root Cause Investigation team (Drs. Monica Brown-Reid, Rebecca Gordon, Julie Gauthier, Daniel Grear, Charles Haley, Brian McCluskey, Paul Pitcher, Barbara Porter-Spaulding, Greg Ramos, Denise Spencer, and Ms. Mary Foley); Swine Industry groups (National Pork Board, National Pork Producers Council, and American Association of Swine Veterinarians); Iowa State University Veterinary Diagnostic laboratory; National Animal Health Laboratory Network laboratories; Swine consultant veterinarians: Drs. Bill Minton, Terry Specht, Joe Connor, Jim Lowe, Harry Snelson, Max Rodibaugh, Luc Dufresne, Dominican Republic: Dr. Uciel Duran; Puerto Rico: Drs. Jose Urdaz, Carlos Soto, Jose Acosta, Jesus Santiago; Food and Drug Administration (Dr. Haile Yancy laboratory, Mr. Shannon Jordre); USDA-APHIS-Wildlife Services National Wildlife Disease Program; USDA-APHIS National Feral Swine Damage Management Program; USDA-APHIS-PPQ; DHS-CBP; DHS-NBACC; South Dakota State University, Animal Disease and Research Diagnostic Laboratory and Drs. Eric Nelson, Travis Clement, and Aaron Singrey.
References
- Blomme, B., 2014. Swine health monitoring project, PED virus infection associated with bird feces. <http://www.cvm.umn.edu/sdec/prod/groups/cvm/@pub/@cvm/@sdec/documents/content/cvm_content_475778pdf> (accessed: 30.09.15.).
- CFIA, editor. CFIA; Canada: 2012. Regulatory Guidance: Petfood Not Approved for Use in Livestock Feed. [Google Scholar]
- Chen Q., Li G., Stasko J., Thomas J.T., Stensland W.R., Pillatzki A.E., Gauger P.C., Schwartz K.J., Madson D., Yoon K.J., Stevenson G.W., Burrough E.R., Harmon K.M., Main R.G., Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the united states. J. Clin. Microbiol. 2014;52:234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee S., Clement T., Schelkopf A., Nerem J., Knudsen D., Christopher-Hennings J. An evaluation of contaminated complete feed as a vehicle for porcine epidemic diarrhea virus infection of naïve pigs following consumption via natural feeding behavior: proof of concept. BMC Vet. Res. 2015;10 doi: 10.1186/s12917-014-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA-CVM, 2012a. FDA Inspection Report Gambol Pet Products. <www.fda.gov/> (accessed 23.02.15.).
- FDA-CVM, 2012b. FDA Inspection Report: Shandong Honva Food Co. LTD. In: HHS (Ed.), <www.fda.gov/>.
- FDA-CVM, 2013. FDA Update on Jerky Treats. <www.fda.gov/> (accessed 23.02.15.).
- Feng L. The updated epidemic and controls of swine enteric coronavirus in China. International SECD Meeting; USDA, Chicago, IL; 2014. [Google Scholar]
- Goyal, S.M., 2013. Research update: PEDv survival and infectious dose, <www.Pork.org>, Research updates from NPB funded projects.
- Huang Y., Dickerman A.W., Pineyro P., Li L., Fang L., Kiehne R., Opriessnig T., Meng X. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. Mbio. 2013;4 doi: 10.1128/mBio.00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Gauger P.C., Harmon K., Zhang J., Connor J., Yeske P., Loula T., Levis I.L.D., Main R.G. Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerg. Infect. Dis. 2014;20:872–874. doi: 10.3201/eid2005.131628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey B. Results of AASV/USDA rapid response team investigations of swine enteric coronavirus positive herds. MN, U.o. (Ed.), 2014 Allen D. Leman Swine Conference; St. Paul, MN; 2014. [Google Scholar]
- Nugent R. State of the knowledge: the relationships between PEDV/PDCoV transmission and feed. AASV Annual Meeting. American Association of Swine Veterinarians; Orlando, Florida; 2015. [Google Scholar]
- Sampedro F., Snider T., Bueno I., Bergeron J., Urriola P., Davies P. University of Minnesota; 2015. Risk Assessment of Feed Ingredients of Porcine Origin as Vehicles for Transmission of Porcine Epidemic Diarrhea Virus (PEDV) [Google Scholar]
- Schumacher L., Woodworth J., Zhang J., Gauger P.C., Chen Q., Welch M., Salzebrenner H., Thomas J., Main R., Dritz S., Cochrane R., Jones C. Determining the minimum infectious dose of porcine epidemic diarrhea virus in a feed matrix. Midwest Animal Science Annual Conference 2015; Des Moines, IA; 2015. [Google Scholar]
- Smith R. CRC Press Inc.; 1995. Veterinary Clinical Epidemiology, A Problem Oriented Approach. [Google Scholar]
- Snelson, H., 2014. PEDV-Lessons Learned, In, 2014 World Pork Expo, Des Moines, IA.
- Stevenson G.W., Hoang H., Schwartz K.J., Burrough E.R., Sun D., Madson D., Cooper V.L., Pillatzki A., Gauger P., Schmitt B.J., Koster L.G., Killian M.L., Yoon K.J. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- USDA-APHIS-VS-CEAH, 2012. Biosecurity in Smale-scale U.S. Livestock Operations, In: USDA (Ed.).
- USDA-APHIS, 2014. Pathway Assessment: Entry Assessment for Exotic Viral Pathogens of Swine, USDA-APHIS.
- USDA-FAS, 2010. FAS GAIN Report: EU Feed and Pet Food Labeling Requirements, Describes labeling requirments for animal feed—species authorized for ingredients.
- USDA-NASS, 2012. Census of Agriculture, In: Agriculture, U.D.o. (Ed.).
- Verma H., Erber J., Goede D.P., Morrison R.B., Goyal S.M. Survival of Porcine Epidemic Diarrhea virus in environmental samples. Allen D. Leman Swine Conference; University of Minnesota; 2014. p. 20. [Google Scholar]
- Wang L., Byrum B., Yan Z. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014;20:917–919. doi: 10.3201/eid2005.140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Cheng X., Chen S., Lin F., Jiang B., Zhu X., Li Z., Wang J. Classification of emergent U. S. strains of porcine epidemic diarrhea virus by phylogenetic analysis of nucleocapsid and ORF3 genes. Emerg. Infect. Dis. 2014;52:3509–3510. doi: 10.1128/JCM.01708-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeske P. Evidence of transmission in integrated systems. Allen D. Leman Swine Conference; University of Minnesota; 2014. [Google Scholar]