Abstract
Two-pore channels (TPCs) are Ca2+-permeable ion channels localised to the endo-lysosomal system where they regulate trafficking of various cargoes including viruses. As a result, TPCs are emerging as important drug targets. However, their pharmacology is ill-defined. There are no approved drugs to target them. And their mechanism of ligand activation is largely unknown. Here, we identify a number of FDA-approved drugs as TPC pore blockers. Using a model of the pore of human TPC2 based on recent structures of mammalian TPCs, we virtually screened a database of ~1500 approved drugs. Because TPCs have recently emerged as novel host factors for Ebola virus entry, we reasoned that Ebola virus entry inhibitors may exert their effects through inhibition of TPCs. Cross-referencing hits from the TPC virtual screen with two recent high throughput anti-Ebola screens yielded approved drugs targeting dopamine and estrogen receptors as common hits. These compounds inhibited endogenous NAADP-evoked Ca2+ release from sea urchin egg homogenates, NAADP-mediated channel activity of TPC2 re-routed to the plasma membrane, and PI(3,5)P2-mediated channel activity of TPC2 expressed in enlarged lysosomes. Mechanistically, single channel analyses showed that the drugs reduced mean open time consistent with a direct action on the pore. Functionally, drug potency in blocking TPC2 activity correlated with inhibition of Ebola virus-like particle entry. Our results expand TPC pharmacology through the identification of approved drugs as novel blockers, support a role for TPCs in Ebola virus entry, and provide insight into the mechanisms underlying channel regulation. This article is part of a Special Issue entitled: ECS Meeting edited by Claus Heizmann, Joachim Krebs and Jacques Haiech.
Keywords: TPC2, NAADP, Ca2+, Lysosomes, Virtual screening, Ebola virus
Highlights
-
•
Identification of approved drugs as TPC2 pore blockers through virtual screening
-
•
Prioritisation of drugs that inhibit Ebola virus entry
-
•
Blockade of endogenous and recombinant TPC2 channel activity by select drugs
-
•
Similar potency for inhibition of channel activity and Ebola virus entry
1. Introduction
NAADP is a potent Ca2+ mobilizing messenger that triggers Ca2+ release from acidic Ca2+ stores such as lysosomes [[1], [2], [3], [4], [5]]. Critical to NAADP action are the endo-lysosomal two-pore channels (TPCs) [[6], [7], [8], [9]]. Local Ca2+ fluxes through TPCs are increasingly implicated in membrane trafficking events [10,11]. These include roles for TPCs in regulating lysosome morphology [12], retrograde transport between late endosomes and the Golgi [13], and endocytic trafficking of receptors for LDL and growth factors [14,15], pigment granules [16] and integrins [17]. They also likely regulate non-vesicular trafficking by strengthening membrane contact sites between late endosomes and the ER [18]. Consequently, TPCs are fast emerging as potential therapeutic targets in diverse disorders such as Parkinson's disease, fatty liver disease and cancer [12,14,17,19]. Moreover, TPCs have been identified as novel host factors for Ebola virus (EBOV) entry [20]. This virus enters cells through macropinocytosis, traffics through the endo-lysosomal system and, following binding to its intracellular receptor NPC1 [21], fuses with late endosomes/lysosomes to release its genome into the cytoplasm. Molecular or chemical inhibition of TPCs prevents EBOV infection likely at a late entry step, although the mechanism is unclear [20,22].
TPCs are dimeric ion channels, comprising a duplicated domain architecture, which are likely a bridge during the evolution of four-domain voltage-gated Ca2+ and Na+ channels [[23], [24], [25]]. Atomic structures of plant [[26], [27], [28]] and mammalian [29,30] channels are emerging providing detailed insight into the architecture of the pore. Two isoforms (TPC1 and TPC2) found in humans localise to distinct compartments within the endo-lysosomal system [9,31]. TPC2 is predominantly localised to late endosomes and lysosomes through an N-terminal targeting motif, which when mutated results in rerouting of the channel to the plasma membrane [32]. The activation mechanisms and ion selectivities of TPCs are areas of active investigation [33,34]. Although many studies have shown TPCs are required for NAADP-mediated Ca2+ signalling, NAADP appears to interact with TPCs indirectly through putative binding proteins [[35], [36], [37], [38]]. In contrast, the endo-lysosomal lipid PI(3,5)P2 has emerged as a direct channel activator that binds within the first domain of TPCs [29,30,39]. Interestingly, residues in the S4–S5 linker within this domain required for NAADP-mediated Ca2+ signals [40] fall within the PI(3,5)P2 binding site [29] indicating a complex interplay between small molecule activators and accessory proteins.
Despite the growing pathophysiological importance of TPCs, there are currently few selective chemical tools to inhibit NAADP/TPC signalling. BZ194 [41], a N-alkylated nicotinic acid derivative, and Ned-19 [42], identified through a ligand-based virtual screen, are cell-permeable inhibitors of NAADP-evoked Ca2+ signals. But their indirect action, coupled with potentially promiscuous actions of their target NAADP-binding proteins on other channels [43], complicates the use of NAADP antagonists in a physiological setting. This underscores the need for direct channel inhibitors. We recently used a combination of homology modelling, molecular docking analyses and Ca2+ measurements to show that TPCs are likely to be direct targets for a number of voltage-gated Ca2+ and Na+ channel modifiers [24]. Tetrandrine [20], another Ca2+ channel antagonist has also recently emerged as a TPC blocker and potent inhibitor of EBOV entry. Finally, the flavonoid naringenin [44] has been shown to block TPCs, but with low affinity. Both tetrandrine and naringenin have multiple targets and are not approved for human use world-wide. There are therefore a limited number of drugs that directly inhibit TPCs.
Here, we apply a combination of structural modelling, virtual screening, Ca2+ measurements, electrophysiology and virus entry assays to identify approved drugs that both inhibit EBOV entry and block TPC activity.
2. Methods
2.1. Virtual screening
The structure of human TPC2 (Uniprot accession: Q8NHX9) in an open conformation was modelled on the cryoEM structure of PI(3,5)P2-bound mouse TPC1 (pdb: 6C9A) [29] using SWISS-MODEL [45]. The first 40 residues were excluded due to lack of appropriate template. The final model was chosen using the default local model quality estimation method (QMEANDisCo) based on a novel version of QMEAN [45]. Additional model refinement was performed using GalaxyRefine [46].
A conformer library for the e-Drugs3D-2017 drug set [47] was prepared using Omega v3.0.1.2 (OpenEye Scientific Software, Santa Fe, NM). MakeReceptor (OEDOCKING 3.2.0.2; OpenEye Scientific Software, Santa Fe, NM) was used to generate the active site for virtual screening. This was centred on the pore vestibule and extended from above the selectivity filter to below the bundle crossing (40 × 40 × 77 μm). The starting pose was based on blind docking of verapamil using AutoDock Vina [48] with an exhaustiveness value of 24. Screening was carried out with FRED (OEDOCKING 3.2.0.2; OpenEye Scientific Software, Santa Fe, NM) using default parameters returning the top 10,000 conformers ranked by the Chemgauss4 score (Table S1). All but the top ranked conformer for each ligand were excluded. This analysis retrieved 1480 unique drugs and their metabolites. The top 200 are listed in Table S1.
Enrichment analysis of the top hits was performed by dividing the proportion of a given drug class within the top 200 hits by the proportion of that class in the screen as a whole. Drugs were classified according to their annotated primary target in e-Drugs3D. This analysis was limited to classes where there were >3 drugs. A class was considered enriched if values were >1.5.
2.2. Ca2+ measurements
Ca2+ release assays using sea urchin egg homogenates were performed as described previously [49]. Briefly, egg homogenates were loaded with Ca2+ by sequential dilution in a cytosol-like medium supplemented with an ATP-regenerating system. Medium Ca2+ concentration was monitored fluorimetrically with Fluo-4.
2.3. Single channel current recording
Patch clamp recording in the excised inside-out configuration was performed as described in [32] using HEK cells expressing human TPC2 tagged with GFP at its C-terminus and where leucines 11 and 12 were replaced with alanine to promote cell surface targeting. The pipette (luminal) solution contained (in mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 10 MES (pH adjusted to 4.6 with NaOH). The bath (cytoplasmic) solution had the same composition except MES was excluded and pH was adjusted to 7.2 with NaOH. Osmolarity of the solutions was adjusted to ~300 mOsm with glucose.
The methods used for recording and analysing currents were reported previously [50]. Pipettes were coated with Sigmacote™, fire polished and minimally filled with the pipette solution. Currents were amplified using an Axopatch 200B amplifier, filtered at 1 kHz (−3 dB) with an internal 4-pole Bessel filter and digitized at 10 kHz with a Digidata 1322A interface and pClamp 10 suite. Unless otherwise stated, all recordings were at a holding potential of +40 mV.
For determining unitary current amplitude and unitary conductance, currents were analysed using the 50% threshold crossing criterion using ClampFit 10 and openings briefer than 700 μs (twice the filter rise time) were excluded from the analysis [51]. Due to uncertainty about the number of channels in most recordings, channel activity was expressed as NP o.
For kinetic analyses of single channel records, a simple two state scheme (C ⇆ O) was initially used to idealize currents at full bandwidth (1 kHz) using the segmental k-means (SKM) hidden-Markov algorithm implemented in QuB [52]. Additional states were then systematically added to the initial scheme with various topologies using the MIL module of QuB. A dead time of 200 μs was retrospectively imposed to correct for missed events [51]. MIL fits a gating scheme to the single channel records and optimises the rate constants based on a maximum likelihood-based Markovian approach. The final gating schemes were derived when further changes in either the number of states or their connectivity did not produce significant (≥10) increment in the log likelihood (LL) ratio [50,52]. Closed time distributions were analysed when channel activity was reasonably high (NP o > 0.15) and when only openings to a single current level were evident over a duration of ≥5 min [53].
Analyses were performed 10–15 s following NAADP/drug application in the bath solution to ensure adequate mixing.
2.4. Whole lysosomal current recording
Lysosomal currents were recorded using a modified patch clamp procedure as described in [54] and COS-7 cells expressing human TPC2 tagged with GFP at its C-terminus [7]. Briefly, cells were treated with 1 μM vacuolin-1, for 1–2 h and the resulting enlarged lysosomes accessed by breaking the plasma membrane with a glass pipette. The pipette (luminal) solution and bath (cytoplasmic) solution contained (in mM): 140 NaCl, 5 KCl, 10 HEPES (pH 7.4 with NaOH). After formation of a GΩ seal with a lysosome, break in was achieved by quick voltage steps of +350 mV. Current was measured with an Axopatch 200B patch clamp amplifier that was controlled by pCLAMP9 software. The current protocol used 400-ms rapid alterations of membrane potential (RAMP) from −100 to +100 mV from a holding potential of 0 mV at 4 s intervals. The currents were filtered at 1 kHz with an internal four-pole Bessel filter, sampled at 5 kHz, and stored to a hard drive by Digidata 1440.
2.5. Ebola virus-like-particle entry assays
Virus-like particles (VLPs) for EBOV were generated as described in [55]. Briefly, HEK293T cells were co-transfected with constructs encoding the EBOV matrix protein, VP40, tagged with a β-lactamase reporter at its N-terminus [56] and the EBOV glycoprotein (GP; strain Zaire) [57], both a kind gift from Adolfo Garcia-Sastre. Cells were maintained in DMEM supplemented with 10% (v/v) FBS, 100 μg/mL streptomycin and 100 units/mL penicillin at 37 °C in a humidified atmosphere containing 5% CO2. Culture media were harvested after 48 h, cleared by centrifugation and VLP-containing supernatants stored at −80 °C until use.
The VLP entry assay [55] was adapted and optimised to a 96-well plate format. HeLa Kyoto cells were seeded at a density of 8 × 103 cells/well and maintained for 40 h prior to drug treatment in DMEM supplemented with 10% (v/v) FBS, 100 μg/mL streptomycin and 100 units/mL penicillin at 37 °C in a humidified atmosphere containing 5% CO2. Cells were incubated with drugs in DMEM +2% (v/v) FBS for 1 h under culture conditions prior to the addition of VLPs (1:2 dilution). Cells were spinoculated at 450 ×g for 1 h at 4 °C, followed by incubation for 3 h under culture conditions to allow VLP entry in the continued presence of drugs. Cells were washed once with CO2-independent medium supplemented with 10% (v/v) FBS and 1% (v/v) l-glutamine and loaded with the β-lactamase substrate, CCF2 (LiveBLAzer FRET–B/G Loading Kit, Invitrogen) according to the manufacturer's instructions using the alternative substrate loading protocol. Cells were trypsinised, fixed with 1% paraformaldehyde for 20 min and analysed by flow cytometry using a LSRII flow cytometer (Becton Dickinson, USA) collecting at least 10,000 events. β-lactamase activity was assessed in live cells with the FlowJo software package by determining the proportion of blue fluorescent cells (cleaved substrate; excitation 405 nm) relative to green fluorescent cells (total substrate; excitation 488 nm) using filter sets for Pacific-blue and FITC, respectively.
2.6. Data analysis
Data are presented as mean values ± standard error of the mean where indicated. Concentrations that caused 50% inhibition (IC50) and Hill coefficients (nH) were derived from fitting of inhibition curves using KaleidaGraph (Synergy software).
3. Results
3.1. Identification of putative TPC2 inhibitors through a structure-based virtual screen of approved drugs based on the TPC2 pore
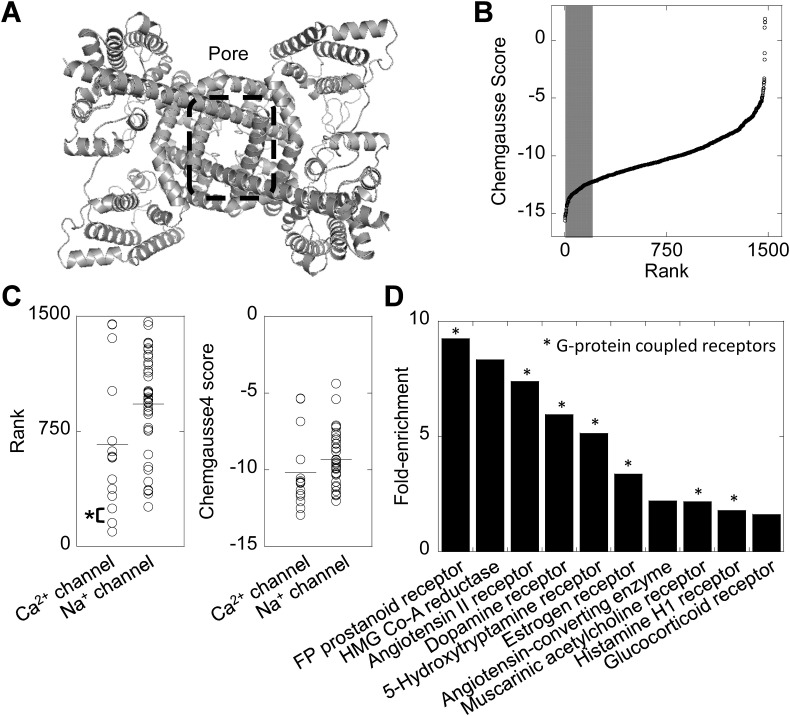
Recent advances in the structural biology of TPCs [28,30] provide novel opportunities to gain insight into TPC pharmacology. We thus applied a structure-based virtual screening approach to identify novel lead compounds targeting the TPC pore. We took advantage of the recent cryo-EM structure of a mammalian TPC [29] to model the pore of human TPC2 (Fig. 1A) and used this model to perform an in silico screen of the e-Drugs3D database, which consists of ~1500 drugs approved by the FDA for use in humans. The results of this screen are depicted schematically in Fig. 1B where each compound is ranked according to its Chemguass4 score. This score is a measure of the predicted strength of interaction whereby more negative values indicate a stronger predicted interaction.
Fig. 1.
A structure-based virtual screen of approved drugs based on the TPC2 pore.
A, structural model of human TPC2 viewed from the cytosolic face. The pore region is highlighted by the dashed box.
B, results of a virtual screen of the e-Drugs3D database against the pore region of TPC2. The top 200 drugs are marked by the shaded region.
C, plots showing the rank of individual drugs from the virtual screen targeting voltage-gated Ca2+ and Na+ channels. The horizontal lines represent the mean. Nicardipine (rank #154) and diltiazem (rank #247) are highlighted*.
D, enrichment analysis of drug classes within the top 200 hits. G-protein coupled receptors are highlighted*.
Our previous targeted docking analyses showed that voltage-gated Ca2+ and Na+ channel blockers likely interact with the TPC pore through a common ‘ancestral’ binding site [24]. Of the Ca2+ channel blockers analysed previously, nicardipine was predicted to interact most strongly with the pore and diltiazem was the most potent functionally [24]. Accordingly, nicardipine and diltiazem were recovered as top ranking blockers in the present screen (Fig. 1C, asterisk). Additionally, the mean rank and mean Chemguass4 scores of all Ca2+ channel blockers in the screen were lower than for Na+ channel blockers (Fig. 1C–D). This accords with our docking and functional analyses showing more avid interaction and more potent inhibition of TPCs by blockers of Ca2+ relative to Na+ channels [24]. Taken together, these analyses support our unbiased approach for identification of TPC inhibitors.
Subsequent efforts were focused on the top 200 hits recovered in the virtual screen. We performed enrichment analysis according to the primary target where annotated. As shown in Fig. 1D, this analysis revealed a number of drugs targeting different G-protein coupled receptors, HMG-CoA reductase, the estrogen receptor and the angiotensin II converting enzyme. This unbiased approach thus returns a number of drugs within distinct drug classes that potentially target the TPC2 pore.
3.2. Prioritisation of approved drugs that inhibit Ebola virus entry as putative TPC2 inhibitors
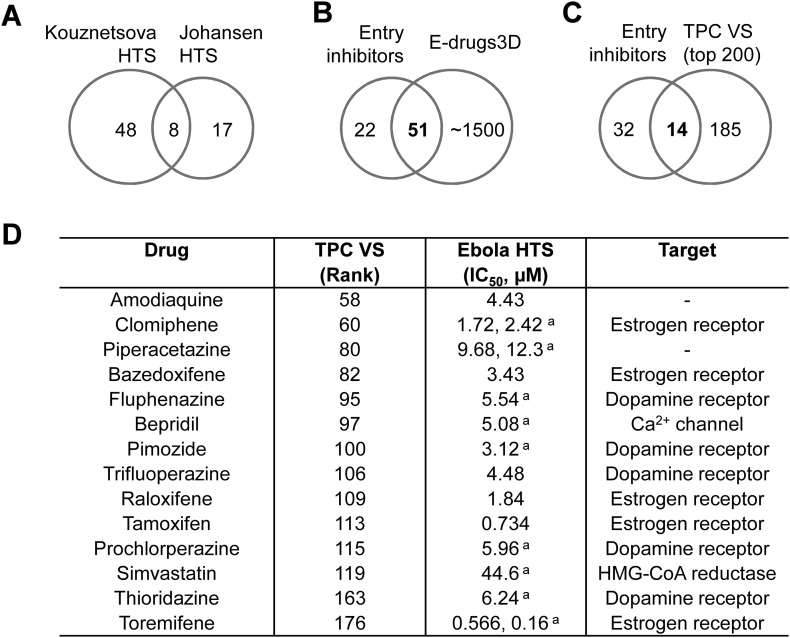
Because TPCs have emerged as novel host factors for EBOV infection [20], we reasoned that some of the FDA-approved drugs recently highlighted in high-throughput screens as EBOV entry inhibitors might function as TPC blockers. We thus compared our top 200 hits with two recent anti-EBOV screens [58,59] that together identified 73 unique EBOV entry inhibitors (Fig. 2A). Of these, 51 were present in e-Drugs3D and all but 5 were recovered in the TPC virtual screen (Fig. 2B). Notably, approximately, a third of these (14) ranked within the top 200 of the TPC screen (Fig. 2C). Inspection of the prioritised hit compounds (Fig. 2D) revealed the Ca2+ channel blocker bepridil, further underscoring this class of compounds as TPC blockers. Importantly, this analysis also returned 4 dopamine receptor antagonists and 5 selective estrogen receptor modulators (SERMs). The prevalence of drugs in these two classes is consistent with our enrichment analysis from the TPC screen (Fig. 1D). Thus, virtual screening against the TPC2 pore and physical screening for EBOV entry inhibitors converge on dopamine receptor antagonists and SERMs as candidate TPC inhibitors.
Fig. 2.
Identification of drugs targeting dopamine and estrogen receptors as putative TPC2 inhibitors.
A, Venn diagram depicting the relationship between approved drugs identified as Ebola virus entry inhibitors in independent high through put screens by Kouznetsova [58] and Johansen et al. [59].
B, Venn diagram depicting the relationships between the entry inhibitors identified in A with the e-Drugs3D database used for the TPC virtual screen.
C, Venn diagram depicting the relationship between entry inhibitors present in the e-Drugs3-D database and the top 200 hits from the TPC virtual screen.
D, prioritised set of 14 drugs identified from C listing their rank in the TPC virtual screen, their reported IC50 values for inhibiting virus entry (from Kouznetsova [58] and/or aJohansen et al. [59]) and their primary target (annotated in e-Drugs3D).
3.3. Dopamine antagonists and selective estrogen receptor modulators block endogenous NAADP-mediated Ca2+ signals
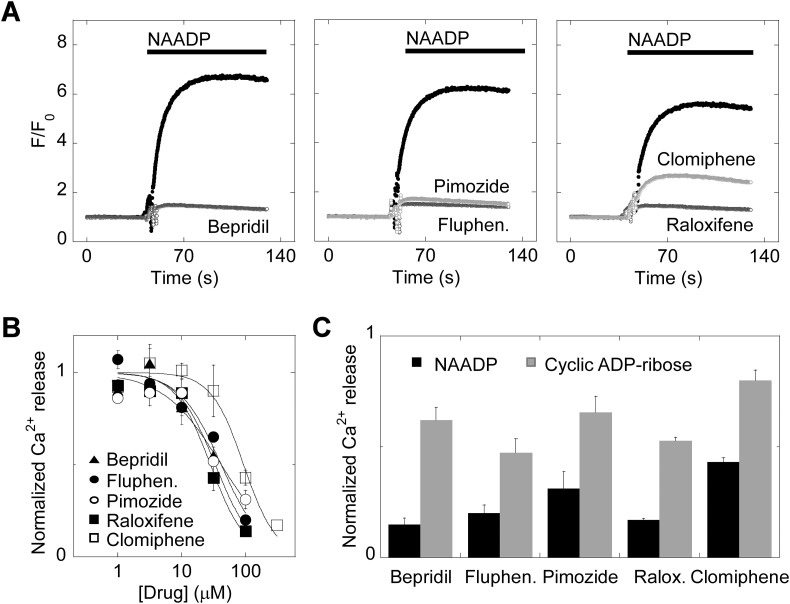
To examine the functionality of the drugs as TPC inhibitors, we first tested their effects on Ca2+ signals evoked by NAADP. We used sea urchin egg homogenates for this analysis as this preparation is considered the ‘gold standard’ for assessing NAADP-mediated Ca2+ signalling [60]. As shown in Fig. 3A, NAADP mediated a robust Ca2+ signal that was largely blocked by bepridil (100 μM). Similar results were obtained with fluphenazine and pimozide, both dopamine receptor antagonists (Fig. 3A). We also tested the effects of the SERMs, raloxifene and clomiphene on NAADP responsiveness. Raloxifene almost completely blocked NAADP-evoked Ca2+ release whereas clomiphene effected a partial block (Fig. 3A).
Fig. 3.
Ca2+ channel blockers, dopamine antagonists and selective estrogen receptor modulators block endogenous NAADP-mediated Ca2+ signals.
A, exemplar Ca2+ signals, recorded using Fluo-4 from sea urchin egg homogenates pre-incubated with the indicated drug (100 μM) or DMSO (black traces) for ~1 min prior to stimulation with NAADP (1 μM).
B, concentration-effect relationships for blockade of NAADP responses by the various drugs (n = 3). Data are normalised to the NAADP response in the presence of DMSO.
C, summary data quantifying the effect of the drugs (100 μM) on Ca2+ response to NAADP versus cyclic ADP-ribose (n = 3). Data are normalised to the NAADP and cyclic ADP-ribose responses in the presence of DMSO.
The inhibitory effects of all these drugs were concentration dependent (Fig. 3B) with the following IC50 and nH values: bepridil (33 μM, 1.5), fluphenazine (42 μM, 1.3), pimozide (42 μM, 0.96), raloxifene (28 μM, 1.6) and clomiphene (94 μM, 1.7). To determine selectivity, we examined the effects of the drugs on Ca2+ release by cyclic ADP-ribose, which mediates its effects through the ryanodine receptor. As summarized in Fig. 3C, all drugs were less effective at inhibiting responses to cyclic ADP-ribose than NAADP, attesting to specificity.
Taken together, these data identify dopamine receptor antagonists and SERMs as novel inhibitors of NAADP action consistent with their predicted interaction with TPCs.
3.4. Dopamine antagonists and selective estrogen receptor modulators inhibit single channel activity of TPC2 by reducing open time
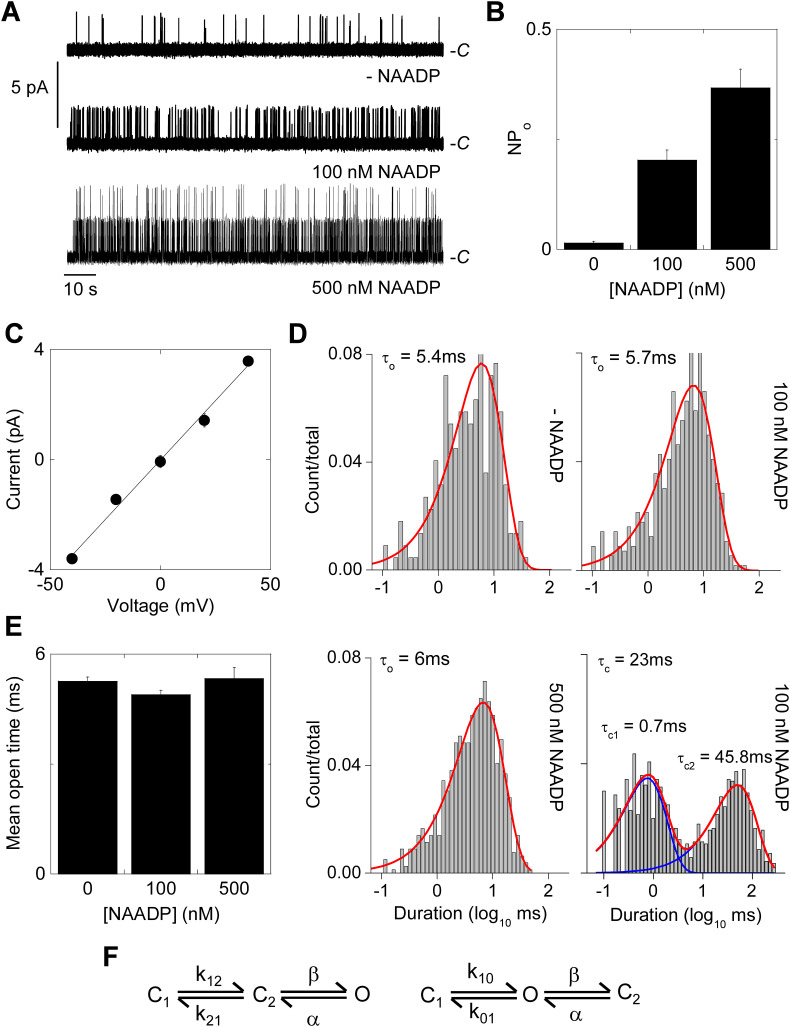
To provide more direct evidence that these compounds are TPC blockers and to extend our analyses to mammalian systems, we analysed NAADP-mediated channel activity of human TPC2. We did this using cell surface targeted TPC2 to facilitate single channel recording in a null background. Na+ was used as the major cation to complement our previous analyses using Ca2+ as the permeant ion [32]. As shown in Fig. 4A, some spontaneous activity was noted in most of the excised patches. NAADP addition to the bath solution robustly enhanced channel activity in a concentration dependent manner. Current amplitude histograms are shown in Fig. S1A–C. Summary data quantifying the normalised open probability NP O is shown in Fig. 4B. Analysis of current-voltage relationships indicated a unitary Na+ conductance (γNa) of 86 ± 0.7 pS (Fig. 4C; n = 3). Single channel openings in the absence and presence of NAADP were best fit by a single exponential with a mean open time of ~5 ms (Fig. 4D). For a sub-set of recordings where we were confident that the openings represented a single active channel (Po ≥ 0.15, ≥5 min recording duration) [50], closed time distribution was also analysed (Fig. 4D). The distribution was best fit by a double exponential with time constants of 0.7 ± 0.4 ms and 47 ± 3.5 ms, constituting 49 ± 1% and 51 ± 1% of the distribution, respectively (n = 4). Kinetic modelling yielded two topologies with similar LL ratios that linearly connected the closed states (C1 and C2) with the open state (O) (Fig. 4F). Collectively, these data affirm the sensitivity of TPC2 to NAADP and its permeability to Na+ and provide a framework for assessing drug action at the single channel level.
Fig. 4.
NAADP stimulates single channel activity of TPC2 rerouted to the plasma membrane.
A, exemplar single channel recordings from inside-out patches excised from HEK cells expressing plasma membrane-targeted TPC2 in response to the indicated concentrations of NAADP. Na+ was the charge carrier and the holding potential was +40 mV. C denotes the closed state.
B, summary data quantifying open probability (NPo; n = 3–28).
C, current-voltage relationships derived from records similar to those shown in A (n = 3).
D, exemplar open and closed time distributions at the indicated NAADP concentration.
E, summary data quantifying mean open time (n = 3–28).
F, likely gating schemes for TPC2.
Fig. S1.
Current amplitude histograms of single channel records from inside-out patches expressing plasma membrane-targeted TPC2.
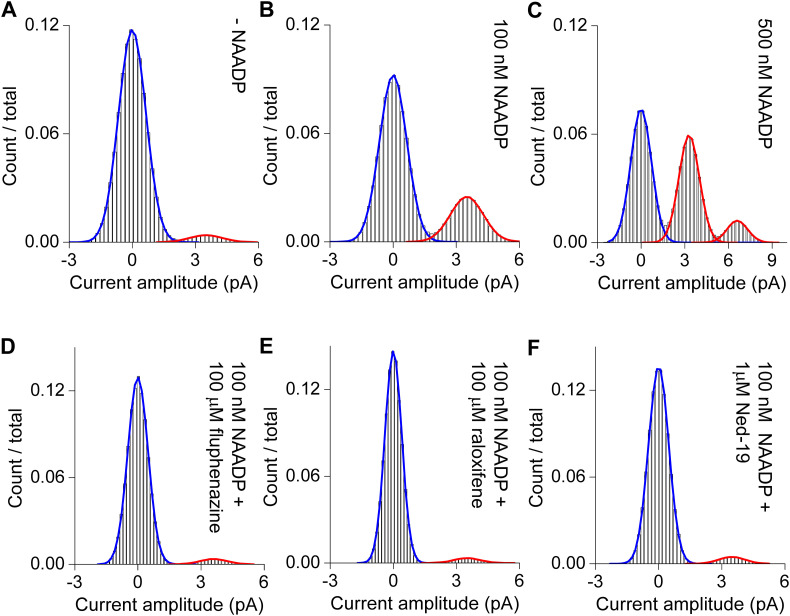
A–C, Histograms in the absence or presence of the indicated concentration of NAADP.
D–E, Histograms in the presence NAADP (100 nM) and the indicated drug.
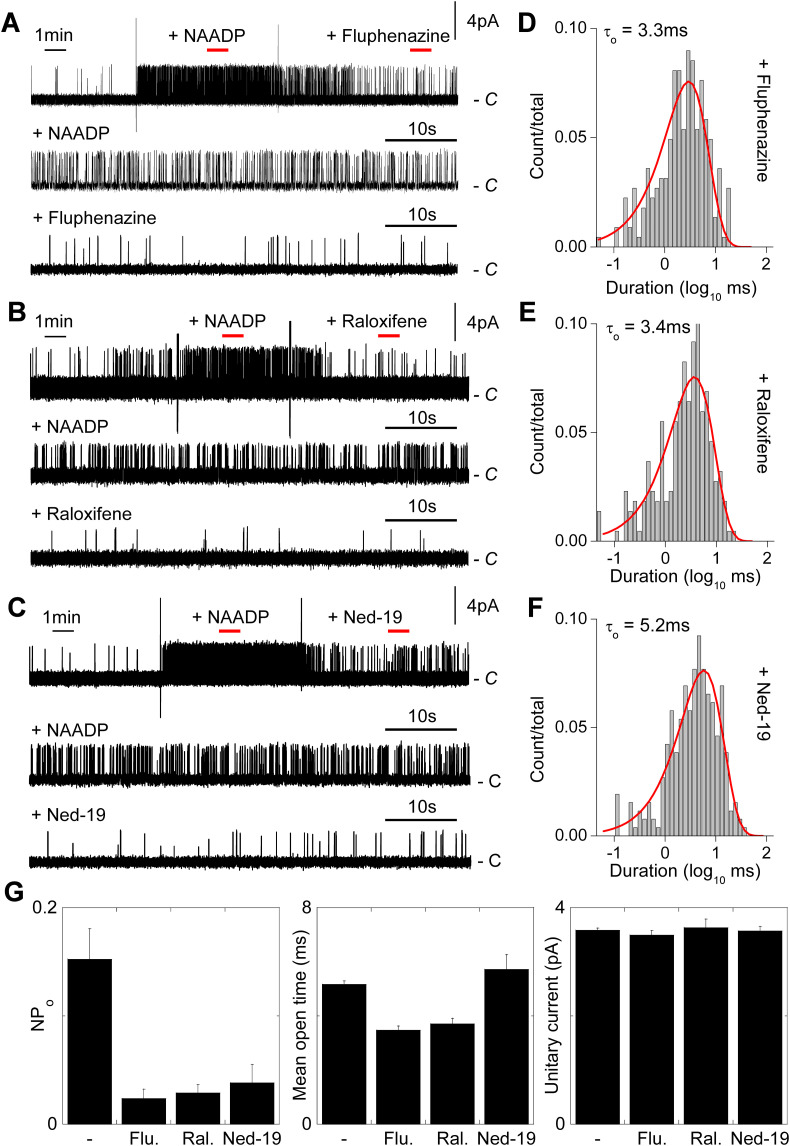
We used this experimental setting to assess the effects of fluphenazine and raloxifene (representative of the two identified drug classes) on TPC2 channel activity. Fig. 5 shows continuous uninterrupted recordings of TPC2 channel activity in response to NAADP and subsequent addition of the drugs. Both drugs acutely inhibited NAADP-mediated channel activity (Fig. 5A–B). We also tested the effects of the NAADP antagonist Ned-19. As shown in Fig. 5C, Ned-19 also similarly inhibited channel activity. To gain mechanistic insight into drug block, we analysed dwell time distributions. As shown in Fig. 5D–E, both fluphenazine and raloxifene reduced the mean open time from ~5 ms to ~3 ms without affecting the unitary current sizes. Current amplitude histograms are shown in Fig. S1D–F. These data are consistent with a direct interaction of the drugs with the pore. In contrast, Ned-19 affected neither the mean open time nor unitary current size (Fig. 5F) suggesting a distinct mechanism of action. Data summarizing the effects of the drugs on NP o, open times and unitary conductance are shown in Fig. 5G. Taken together, these data suggest that fluphenazine and raloxifene effectively behave as pore blockers.
Fig. 5.
Dopamine antagonists and selective estrogen receptor modulators inhibit single channel activity of TPC2 by reducing open time.
A–C, exemplar single channel records from inside-out patches expressing plasma membrane-targeted TPC2. Patches were sequentially challenged with 100 nM NAADP and either fluphenazine (100 μM; A), raloxifene (100 μM; B) or Ned-19 (1 μM; C). Large vertical deflections are addition artefacts. Red bars indicate the sections of the upper trace in the presence of NAADP before and after drug addition that were expanded in the corresponding lower traces.
D–F, exemplar open time distributions from records similar to A–C in the presence of the indicated drug.
G, summary data quantifying open probability, mean closed time and unitary conductance for TPC2 activity in response to 100 nM NAADP in the absence (n = 10) and presence of the indicated drug (n = 3–4). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Dopamine antagonists and selective estrogen receptor modulators inhibit lysosomal TPC2 currents and entry of Ebola virus-like particles with similar potency
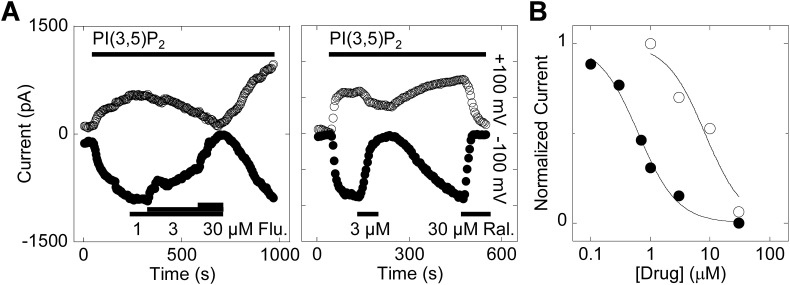
Because TPC2 in the plasma membrane may not faithfully reflect the properties of the channel in its native environment, we performed electrophysiological analysis of enlarged lysosomes expressing wildtype TPC2 (Fig. 6 ). For these experiments, TPC2 was stimulated with a saturating concentration of PI(3,5)P2, which gives larger currents than NAADP in this experimental setting [54] most likely due to a direct interaction with the channel [61]. As shown in Fig. 6A, PI(3,5)P2 stimulated robust currents in TPC2-expressing lysosomes. These responses were inhibited by fluphenazine in a concentration-dependent manner with near full block achieved at 30 μM (Fig. 6A). Raloxifene also inhibited PI(3,5)P2-mediated currents with maximal effects at 3 μM (Fig. 6A). The inhibitory effects of both drugs were reversed upon washout (Fig. 6A). Concentration-effect relationships showed that raloxifene was particularly potent at blocking TPC2 currents (IC50 = 0.63 μM, nH = 1.3) in this setting compared to fluphenazine (IC50 = 8.2 μM, nH = 1.3), respectively (Fig. 6B).
Fig. 6.
Dopamine antagonists and selective estrogen receptor modulators inhibit lysosomal TPC2 currents.
A, exemplar currents recorded from enlarged lysosomes isolated from HEK cells expressing lysosome-targeted TPC2 in response to PI(3,5)P2 (1 μM). The holding potentials were +100 mV (upper traces) and −100 mV (lower traces). Lysosomes were challenged with fluphenazine (Flu.) and raloxifene (Ral.) at the concentrations and for the times indicated.
B, concentration-effect relationships for blockade of PI(3,5)P2 currents by fluphenazine (open circles) and raloxifene (closed circles;n = 2). Data are normalised to the PI(3,5)P2 response in the absence of the drugs.
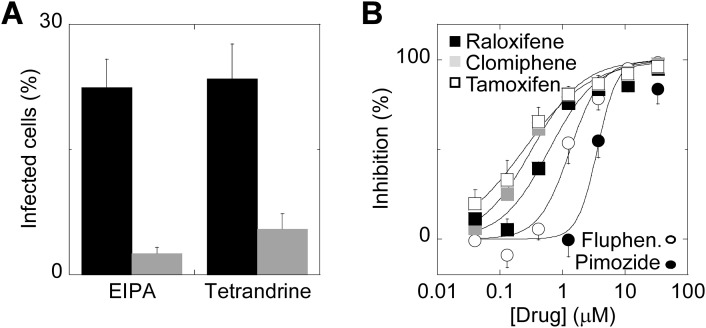
Finally, we examined the potency of dopamine receptor antagonists and SERMs on EBOV entry. As a surrogate for EBOV, we used Ebola VLPs carrying β-lactamase. Following fusion mediated by the EBOV glycoprotein, β-lactamase is delivered to the target cell cytoplasm where it mediates cleavage of a fluorescent substrate. Fig. 7A quantifies infection of HeLa cells by the VLPs. Infection was blocked by pre-treatment with EIPA (11.1 μM), an inhibitor of macropinocytosis, and by tetrandrine (1.2 μM; Fig. 7A). As shown in Fig. 7B, fluphenazine and pimozide inhibited Ebola VLP entry with IC50 values of 1.3 μM (nH = 1.7) and 3.6 μM (nH = 2.8), respectively. Raloxifene, clomiphene and a third SERM, tamoxifen, also inhibited entry with IC50 values of 0.61 μM (nH = 1.1, 0.32 μM (nH = 1.1) and 0.21 μM (nH = 0.82), respectively. Enhanced potency of SERMs relative to dopamine receptor antagonists in blocking VLP entry matches that for inhibition of TPC2 currents (Fig. 6).
Fig. 7.
Dopamine antagonists and selective estrogen receptor modulators inhibit entry of Ebola virus-like particles.
A, infection of HeLa cells with Ebola virus-like particles in the absence and presence of EIPA (11.1 μM; n = 3) or tetrandrine (1.2 μM; n = 4).
B, concentration-effect relationships for blockade of virus-like particle entry by the indicated dopamine antagonist (squares) or SERMs (circles). n = 3–4.
4. Discussion
TPCs are channels of physiological, and increasingly, patho-physiological importance [19]. However, their pharmacology is poorly defined and there are currently no approved drugs to target them. To begin to fill this gap, we performed a virtual structure-based screen using a database of approved drugs and the pore region of TPC2 (Fig. 1). Key to our strategy was i) the availability of structural information for mammalian TPCs, ii) the finding that TPCs are novel host factors for EBOV entry and iii) repurposing screens initiated in the wake of the 2014 Ebola epidemic, which yielded a number of approved drugs as novel EBOV entry inhibitors. To prioritise hits, cross-referencing the latter ‘wet’ data with our ‘dry’ modelling highlighted drugs targeting dopamine and estrogen receptors (Fig. 2). These drugs demonstrably inhibited TPCs in three independent assays (Fig. 3, Fig. 5, Fig. 6), and we correlated their potency with inhibiting Ebola VLP entry (Fig. 7). Single channel analyses confirmed an action of these drugs on the TPC2 pore (Fig. 5).
Results from the enrichment analysis of our virtual screen identified a number of drugs targeting distinct G-protein coupled receptors (Fig. 1D). Notably, a high throughput screen for drugs that block EBOV and Marburg virus (both filoviruses) infection also highlighted numerous drugs targeting G-protein coupled receptors [62]. Common drug classes in that screen and ours include those against 5HT, muscarinic and histamine receptors. This further links TPCs to EBOV infection. We focused our functional analyses on dopamine receptor antagonist and SERMs based on our cross-referencing strategy (Fig. 2). But these compounds represent only a fraction of our top 200 hits. Thus, our screen offers considerable scope for identifying additional TPC blockers in the future.
The dopamine receptor drugs identified here as TPC blockers included several phenothiazines (fluphenazine, trifluoperazine, prochlorperazine and thioridazine) that are used as anti-psychotics (Fig. 2). Our data is consistent with a recent screening campaign published while this work was in progress, which also identified phenothiazines as inhibitors of NAADP-induced Ca2+ release from sea urchin egg homogenates [63]. Indeed, the IC50 values for fluphenazine, 42 μM (Fig. 3B) and ~11 μM [63], are comparable. This congruence highlights the power of our in silico strategy for drug identification which, together with our electrophysiological analyses, points mechanistically to drug action on the TPC pore. We also identified the structurally distinct dopamine receptor drug, pimozide, a diphenylbutylpiperidine. As well as targeting the dopamine receptor, pimozide also targets voltage-gated Ca2+ channels [64]. The common action of pimozide on TPCs and voltage-gated Ca2+ channels likely reflects an evolutionary kindred between these two members of the voltage-gated ion channel superfamily [24]. Although voltage-gated Ca2+ channels have been implicated in viral entry [65], their functional absence in non-excitable cells (such as HeLa cells used in this study) points to TPCs as the likely targets.
Our analyses identifying drugs targeting estrogen receptors as additional TPC blockers converged on first (clomiphene, tamoxifen, toremifene), second (raloxifene) and third (bazedoxifene) generation SERMs (Fig. 2). These drugs act as estrogen receptor agonists or antagonists, depending on the tissue, and are used for treating breast cancer and osteoporosis [66]. Off-target effects of these drugs are more than likely related to well-known rapid non-genomic actions of estrogen [67]. Indeed, inhibition of both viral entry and TPC activity by sub-micromolar concentrations of raloxifene is striking. Additional effects of SERMs such as destabilisation of either the EBOV glycoprotein [68] or lysosomal membranes due to their cationic amphiphilic nature [69] are possible. Nevertheless, both dopamine receptor antagonists and SERMs emerge as novel TPC blockers and provide independent scaffolds for small molecule development.
Estrogen receptor drugs appear more potent in blocking TPC activity (Fig. 6) and Ebola VLP entry (Fig. 7) than dopamine receptor drugs. This correlation points to a common mechanism of action (Fig. 6, Fig. 7). The half maximal inhibitory concentrations by raloxifene were similar but fluphenazine was more effective at inhibiting VLP entry (Fig. 7) than blocking the channel (Fig. 6). This could reflect additional effects of fluphenazine underlying entry inhibition or the very different conditions under which the two assays were performed (acute drug treatment versus pre-incubation; broken versus intact cells). One other notable difference was the relative poor potency of the drugs in blocking Ca2+ signals in egg homogenates (Fig. 3) compared to lysosomal currents (Fig. 6). Again, this could relate to the way in which drugs were administered or the activating ligand. Alternatively, this could point to species differences in drug block. Nevertheless, our study suggests that TPCs may be the target for a number of EBOV entry inhibitors described previously for which mechanistic information was lacking. How TPCs promote EBOV entry is unclear at present. A recent study provided evidence that TPCs may also regulate entry of the MERS corona virus [70]. Pharmacological inhibition with tetrandrine analogues or molecular knockdown of TPCs was shown to reduce Furin activity. This protease activity is required for priming of the envelope protein [71]. Thus, compromised activity upon TPC blockade could underlie reduced MERS corona virus entry. Such a mechanism is unlikely to regulate entry of EBOV because it is processed by endo-lysosomal cathepsins [72]. Moreover, entry of pre-cleaved EBOV is also blocked by tetrandrine [20] suggesting that TPCs act at a step distal to proteolytic processing. Clearly, further work is required to understand the mechanisms of TPC involvement in EBOV entry.
Although cellular studies repeatedly link TPCs to NAADP-mediated Ca2+ signalling, electrophysiological analyses of TPCs are less congruent with some studies failing to demonstrate appreciable Ca2+ permeability or NAADP activation [39,73]. Our previous whole cell recordings and single channel analyses demonstrated robust activation of Ca2+ and Cs+ currents in response to NAADP [32]. Here, we used Na+ as the major permeant ion. Comparing single channel conductances in this study (87 pS, Fig. 4) with those for Ca2+ (40 pS) and Cs+ (128 pS) reported previously [32] points strongly to TPC2 as a non-selective cation channel under these experimental conditions. This conclusion concurs with bilayer studies of TPC2 [74,75] and electrophysiological analyses of endogenous NAADP-activated TPC2 currents recorded from enlarged vacuoles (Na:Ca permeability ratio close to unity) [76]. TPCs interact with many other proteins [16] some of which likely act to effect and modify gating. Loss of accessory factors might well explain NAADP insensitivity in some studies. Indeed, such loss might also affect the ion selectivity of TPCs as accessory proteins are known to dictate the ion selectivity of other channels such as Orai [77] and MCU [78].
Our single channel analyses provide evidence that the drugs target the pore region of TPCs (Fig. 5). The reduction in open time (~2-fold) was modest relative to the reduction in open probability (~10-fold) pointing to an open channel block mechanism. This is compatible with effects produced by inhibitors known to interact directly with the pore of other channels [[79], [80], [81]]. Of note, phenothiazines have long been known to antagonize calmodulin [82] and previous studies with trifluoperazine have suggested a role for calmodulin in NAADP-mediated Ca2+ signalling [83]. Our data, with fluphenazine at least, suggests an additional more direct action of phenothiazines on TPCs likely unrelated to calmodulin (Fig. 5). Clearly, further structure-function analyses are warranted.
In contrast to fluphenazine and raloxifene, Ned-19 did not affect open time (Fig. 5). It thus effectively acts as a gating modifier by reducing the overall frequency of channel openings through directly or indirectly influencing channel regions distal to the pore. Thus, our analyses uncover fundamentally distinct mechanisms for channel inhibition by the drugs and Ned-19. The lack of effect of Ned-19 (and also NAADP; Fig. 4) on open time support the notion that they act more remotely through associated NAADP-binding proteins. [38]. Uncertainty about the number of channels as well as the limited number of events in patches with low activity prevented us from performing close time distribution analysis upon channel inhibition. But further analysis is warranted not least due to the presence of a multiple closed times (Fig. 4F) which could reflect desensitized states.
In sum, we have identified approved drugs as TPC blockers. We used a novel strategy that affirmed the role of TPCs in EBOV entry and which should significantly aid in expanding the pharmacology of this ubiquitous class of endo-lysosomal ion channels.
The following are the supplementary data related to this article.
Virtual screening outputs.
First tab, raw output from the virtual screen.
Second tab, top 200 hits excluding repeat conformers.
Transparency document
Transparency document.
Acknowledgments
This work was supported by PhD studentships from the BBSRC (to CJP and KV), IDB-Cambridge Trust (to XC) and Parkinson's UK (to EY; grant H-1202). MMaz and MMar are supported by UK Medical Research Council funding to the MRC-UCL LMCB University Unit (MC_UU_12018/1). BSK and SP were supported by BBSRC grant BB/N01524X/1. TR was supported by a fellowship and research grant from the Royal Society. We thank Steve Bolsover for useful discussion and Adolfo Garcia Sastre for the gift of plasmids.
Footnotes
This article is part of a Special Issue entitled: ECS Meeting edited by Claus Heizmann, Joachim Krebs and Jacques Haiech.
The Transparency document associated this article can be found in the online version.
Contributor Information
Taufiq Rahman, Email: mtur2@cam.ac.uk.
Sandip Patel, Email: patel.s@ucl.ac.uk.
References
- 1.Churchill G.C., Okada Y., Thomas J.M., Genazzani A.A., Patel S., Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 2.Lee H.C. NAADP-mediated calcium signaling. J. Biol. Chem. 2005;280(40):33693–33696. doi: 10.1074/jbc.R500012200. [DOI] [PubMed] [Google Scholar]
- 3.Patel S., Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan A.J., Platt F.M., Lloyd-Evans E., Galione A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 2011;439:349–374. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- 5.Patel S., Cai X. Evolution of acid Ca2+ stores and their resident Ca2+-permeable channels. Cell Calcium. 2015;57:222–230. doi: 10.1016/j.ceca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Calcraft P.J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K.T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C.N., Parrington J., Ma J., Evans A.M., Galione A., Zhu M.X. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brailoiu E., Churamani D., Cai X., Schrlau M.G., Brailoiu G.C., Gao X., Hooper R., Boulware M.J., Dun N.J., Marchant J.S., Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rotzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel S. Function and dysfunction of two-pore channels. Sci. Signal. 2015;8 doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- 10.Marchant J.S., Patel S. Two-pore channels at the intersection of endolysosomal membrane traffic. Biochem. Soc. Trans. 2015;43:434–441. doi: 10.1042/BST20140303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm C., Chen C.C., Wahl-Schott C., Biel M. Two-pore channels: catalyzers of endolysosomal transport and function. Front. Pharmacol. 2017;8:45. doi: 10.3389/fphar.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hockey L.N., Kilpatrick B.S., Eden E.R., Lin-Moshier Y., Brailoiu G.C., Brailoiu E., Futter C., Schapira A.H., Marchant J.S., Patel S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 2015;128:232–238. doi: 10.1242/jcs.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruas M., Rietdorf K., Arredouani A., Davis L.C., Lloyd-Evans E., Koegel H., Funnell T.M., Morgan A.J., Ward J.A., Watanabe K., Cheng X., Churchill G.C., Zhu M.X., Platt F.M., Wessel G.M., Parrington J., Galione A. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr. Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm C., Holdt L.M., Chen C.C., Hassan S., Muller C., Jors S., Cuny H., Kissing S., Schroder B., Butz E., Northoff B., Castonguay J., Luber C.A., Moser M., Spahn S., Lullmann-Rauch R., Fendel C., Klugbauer N., Griesbeck O., Haas A., Mann M., Bracher F., Teupser D., Saftig P., Biel M., Wahl-Schott C. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat. Commun. 2014;5:4699. doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- 15.Ruas M., Chuang K.T., Davis L.C., Al-Douri A., Tynan P.W., Tunn R., Teboul L., Galione A., Parrington J. TPC1 has two variant isoforms and their removal has different effects on endo-lysosomal functions compared to loss of TPC2. Mol. Cell. Biol. 2014;34:3981–3992. doi: 10.1128/MCB.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin-Moshier Y., Keebler M.V., Hooper R., Boulware M.J., Liu X., Churamani D., Abood M.E., Walseth T.F., Brailoiu E., Patel S., Marchant J.S. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. PNAS. 2014;111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen O.N., Grimm C., Schneider L.S., Chao Y.K., Atzberger C., Bartel K., Watermann A., Ulrich M., Mayr D., Wahl-Schott C., Biel M., Vollmar A.M. Two-pore channel function is crucial for the migration of invasive cancer cells. Cancer Res. 2017;77:1427–1438. doi: 10.1158/0008-5472.CAN-16-0852. [DOI] [PubMed] [Google Scholar]
- 18.Kilpatrick B.S., Eden E.R., Hockey L.N., Yates E., Futter C.E., Patel S. An endosomal NAADP-sensitive two-pore Ca2+ channel regulates ER-endosome membrane contact sites to control growth factor signaling. Cell Rep. 2017;18:1636–1645. doi: 10.1016/j.celrep.2017.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel S., Kilpatrick B.S. Two-pore channels and disease. Biochim. Biophys. Acta. November 2018;1865(11):1678–1686. doi: 10.1016/j.bbamcr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakurai Y., Kolokolstov A.A., Chen C.C., Tidwell M.W., Bauta W.E., Klugbauer N., Grimm C., Wahl-Schott C., Biel M., Davey R.A. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:6225. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cote M., Misasi J., Ren T., Bruchez A., Lee K., Filone C.M., Hensley L., Li Q., Ory D., Chandran K., Cunningham J. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477:344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmons J.A., D'Souza R.S., Ruas M., Galione A., Casanova J.E., White J.M. Ebolavirus glycoprotein directs fusion through NPC1+ endolysosomes. J. Virol. 2016;90:605–610. doi: 10.1128/JVI.01828-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churamani D., Hooper R., Brailoiu E., Patel S. Domain assembly of NAADP-gated two-pore channels. Biochem. J. 2012;441:317–323. doi: 10.1042/BJ20111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman T., Cai X., Brailoiu G.C., Abood M.E., Brailoiu E., Patel S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 2014;7 doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penny C.J., Rahman T., Sula A., Miles A.J., Wallace B.A., Patel S. Isolated pores dissected from human two-pore channel 2 are functional. Sci. Rep. 2016;6:38426. doi: 10.1038/srep38426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo J., Zeng W., Chen Q., Lee C., Chen L., Yang Y., Cang C., Ren D., Jiang Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:196–201. doi: 10.1038/nature16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kintzer A.F., Stroud R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:258–264. doi: 10.1038/nature17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S., Penny C.J., Rahman T. Two-pore channels enter the atomic era. Structure of plant TPC revealed. Trends Biochem. Sci. 2016;41:475–477. doi: 10.1016/j.tibs.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.She J., Guo J., Chen Q., Zeng W., Jiang Y., Bai X.C. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature. 2018;556:130–134. doi: 10.1038/nature26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel S. Two-pore channels open up. Nature. 2018;556:38–40. doi: 10.1038/d41586-018-02783-8. [DOI] [PubMed] [Google Scholar]
- 31.Cai X., Patel S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol. Biol. Evol. 2010;27:2352–2359. doi: 10.1093/molbev/msq122. [DOI] [PubMed] [Google Scholar]
- 32.Brailoiu E., Rahman T., Churamani D., Prole D.L., Brailoiu G.C., Hooper R., Taylor C.W., Patel S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 2010;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchant J.S., Patel S. Questioning regulation of two-pore channels by NAADP. Messenger (Los Angel.) 2013;2:113–119. doi: 10.1166/msr.2013.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan A.J., Galione A. Two-pore channels (TPCs): current controversies. BioEssays. 2013;36:173–183. doi: 10.1002/bies.201300118. [DOI] [PubMed] [Google Scholar]
- 35.Lin-Moshier Y., Walseth T.F., Churamani D., Davidson S.M., Slama J.T., Hooper R., Brailoiu E., Patel S., Marchant J.S. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walseth T.F., Lin-Moshier Y., Jain P., Ruas M., Parrington J., Galione A., Marchant J.S., Slama J.T. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide 2′-phosphate (NAADP) proteins in sea urchin egg. J. Biol. Chem. 2012;287:2308–2315. doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walseth T.F., Lin-Moshier Y., Weber K., Marchant J.S., Slama J.T., Guse A.H. Nicotinic acid adenine dinucleotide 2′-phosphate (NAADP) binding proteins in T-lymphocytes. Messenger (Los Angel.) 2012;1:86–94. doi: 10.1166/msr.2012.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchant J.S., Lin-Moshier Y., Walseth T.F., Patel S. The molecular basis for Ca2+ Signalling by NAADP: two-pore channels in a complex? Messenger (Los Angel.) 2012;1:63–76. doi: 10.1166/msr.2012.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., Zhang X., Dong X.P., Samie M., Li X., Cheng X., Goschka A., Shen D., Zhou Y., Harlow J., Zhu M.X., Clapham D.E., Ren D., Xu H. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel S., Churamani D., Brailoiu E. NAADP-evoked Ca2+ signals through two-pore channel-1 require arginine residues in the first S4–S5 linker. Cell Calcium. 2017;68:1–4. doi: 10.1016/j.ceca.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dammermann W., Zhang B., Nebel M., Cordiglieri C., Odoardi F., Kirchberger T., Kawakami N., Dowden J., Schmid F., Dornmair K., Hohenegger M., Flugel A., Guse A.H., Potter B.V. NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10678–10683. doi: 10.1073/pnas.0809997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naylor E., Arredouani A., Vasudevan S.R., Lewis A.M., Parkesh R., Mizote A., Rosen D., Thomas J.M., Izumi M., Ganesan A., Galione A., Churchill G.C. Identification of a chemical probe for NAADP by virtual screening, Nat. Chem. Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guse A.H. Linking NAADP to ion channel activity: a unifying hypothesis. Sci. Signal. 2012;5 doi: 10.1126/scisignal.2002890. [DOI] [PubMed] [Google Scholar]
- 44.Pafumi I., Festa M., Papacci F., Lagostena L., Giunta C., Gutla V., Cornara L., Favia A., Palombi F., Gambale F., Filippini A., Carpaneto A. Naringenin impairs two-pore channel 2 activity and inhibits VEGF-induced angiogenesis. Sci. Rep. 2017;7:5121. doi: 10.1038/s41598-017-04974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L., Lepore R., Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ko J., Park H., Heo L., Seok C. GalaxyWEB server for protein structure prediction and refinement. Nucleic Acids Res. 2012;40:W294–W297. doi: 10.1093/nar/gks493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pihan E., Colliandre L., Guichou J.F., Douguet D. e-Drug3D: 3D structure collections dedicated to drug repurposing and fragment-based drug design. Bioinformatics. 2012;28:1540–1541. doi: 10.1093/bioinformatics/bts186. [DOI] [PubMed] [Google Scholar]
- 48.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickinson G.D., Patel S. Modulation of NAADP receptors by K+ ions: evidence for multiple NAADP receptor conformations. Biochem. J. 2003;375:805–812. doi: 10.1042/BJ20030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taufiq U.R., Skupin A., Falcke M., Taylor C.W. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+ Nature. 2009;458:655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colquhoun D. Practical analysis of single channel records. In: Standen N.B., editor. Microelectrode Techniques: The Plymouth Workshop Handbook. 1994. [Google Scholar]
- 52.Qin F., Auerbach A., Sachs F. A direct optimization approach to hidden Markov modeling for single channel kinetics. Biophys. J. 2000;79:1915–1927. doi: 10.1016/S0006-3495(00)76441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ionescu L., Cheung K.H., Vais H., Mak D.O., White C., Foskett J.K. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal Ca2+ release. J. Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jha A., Ahuja M., Patel S., Brailoiu E., Muallem S. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J. 2014;33:501–511. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tscherne D.M., Manicassamy B., Garcia-Sastre A. An enzymatic virus-like particle assay for sensitive detection of virus entry. J. Virol. Methods. 2010;163:336–343. doi: 10.1016/j.jviromet.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manicassamy B., Rong L. Expression of Ebolavirus glycoprotein on the target cells enhances viral entry. Virol. J. 2009;6:75. doi: 10.1186/1743-422X-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manicassamy B., Wang J., Jiang H., Rong L. Comprehensive analysis of Ebola virus GP1 in viral entry. J. Virol. 2005;79:4793–4805. doi: 10.1128/JVI.79.8.4793-4805.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kouznetsova J., Sun W., Martinez-Romero C., Tawa G., Shinn P., Chen C.Z., Schimmer A., Sanderson P., McKew J.C., Zheng W., Garcia-Sastre A. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg. Microbes. Infect. 2014;3 doi: 10.1038/emi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johansen L.M., DeWald L.E., Shoemaker C.J., Hoffstrom B.G., Lear-Rooney C.M., Stossel A., Nelson E., Delos S.E., Simmons J.A., Grenier J.M., Pierce L.T., Pajouhesh H., Lehar J., Hensley L.E., Glass P.J., White J.M., Olinger G.G. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa5597. [DOI] [PubMed] [Google Scholar]
- 60.Galione A., Chuang K.T., Funnell T.M., Davis L.C., Morgan A.J., Ruas M., Parrington J., Churchill G.C. Preparation and use of sea urchin egg homogenates for studying NAADP-mediated Ca2+ release. Cold Spring Harb Protoc. 2014;2014(9):988–992. doi: 10.1101/pdb.prot076901. [DOI] [PubMed] [Google Scholar]
- 61.Kirsch S.A., Kugemann A., Carpaneto A., Bockmann R.A., Dietrich P. Phosphatidylinositol-3,5-bisphosphate lipid-binding-induced activation of the human two-pore channel 2. Cell. Mol. Life Sci. 2018;75(20):3803–3815. doi: 10.1007/s00018-018-2829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng H., Lear-Rooney C.M., Johansen L., Varhegyi E., Chen Z.W., Olinger G.G., Rong L. Inhibition of Ebola and Marburg virus entry by G protein-coupled receptor antagonists. J. Virol. 2015;89:9932–9938. doi: 10.1128/JVI.01337-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gunaratne G.S., Johns M.E., Hintz H.M., Walseth T.F., Marchant J.S. A screening campaign in sea urchin egg homogenate as a platform for discovering modulators of NAADP-dependent Ca2+ signaling in human cells. Cell Calcium. 2018;75:42–52. doi: 10.1016/j.ceca.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enyeart J.J., Dirksen R.T., Sharma V.K., Williford D.J., Sheu S.S. Antipsychotic pimozide is a potent Ca2+ channel blocker in heart. Mol. Pharmacol. 1990;37:752–757. [PubMed] [Google Scholar]
- 65.Lavanya M., Cuevas C.D., Thomas M., Cherry S., Ross S.R. siRNA screen for genes that affect Junin virus entry uncovers voltage-gated calcium channels as a therapeutic target. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3006827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dutertre M., Smith C.L. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J. Pharmacol. Exp. Ther. 2000;295:431–437. [PubMed] [Google Scholar]
- 67.Kow L.M., Pfaff D.W. Rapid estrogen actions on ion channels: a survey in search for mechanisms. Steroids. 2016;111:46–53. doi: 10.1016/j.steroids.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y., Ren J., Harlos K., Jones D.M., Zeltina A., Bowden T.A., Padilla-Parra S., Fry E.E., Stuart D.I. Toremifene interacts with and destabilizes the Ebola virus glycoprotein. Nature. 2016;535:169–172. doi: 10.1038/nature18615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shoemaker C.J., Schornberg K.L., Delos S.E., Scully C., Pajouhesh H., Olinger G.G., Johansen L.M., White J.M. Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gunaratne G.S., Yang Y., Li F., Walseth T.F., Marchant J.S. NAADP-dependent Ca2+ signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium. 2018;75:30–41. doi: 10.1016/j.ceca.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. U. S. A. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandran K., Sullivan N.J., Felbor U., Whelan S.P., Cunningham J.M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo J., Zeng W., Jiang Y. Tuning the ion selectivity of two-pore channels. Proc. Natl. Acad. Sci. U. S. A. 2017;114:1009–1014. doi: 10.1073/pnas.1616191114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pitt S.J., Funnell T., Sitsapesan M., Venturi E., Rietdorf K., Ruas M., Ganesan A., Gosain R., Churchill G.C., Zhu M.X., Parrington J., Galione A., Sitsapesan R. TPC2 is a novel NAADP-sensitive Ca2+-release channel, operating as a dual sensor of luminal pH and Ca2+ J. Biol. Chem. 2010;285:24925–24932. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee C.S., Tong B.C., Cheng C.W., Hung H.C., Cheung K.H. Characterization of two-pore channel 2 by nuclear membrane electrophysiology. Sci. Rep. 2016;6:20282. doi: 10.1038/srep20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruas M., Davis L.C., Chen C.C., Morgan A.J., Chuang K.T., Walseth T.F., Grimm C., Garnham C., Powell T., Platt N., Platt F.M., Biel M., Wahl-Schott C., Parrington J., Galione A. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li P., Miao Y., Dani A., Vig M. Alpha-SNAP regulates dynamic, on-site assembly and calcium selectivity of Orai1 channels. Mol. Biol. Cell. 2016;27:2542–2553. doi: 10.1091/mbc.E16-03-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamer K.J., Sancak Y., Fomina Y., Meisel J.D., Chaudhuri D., Grabarek Z., Mootha V.K. MICU1 imparts the mitochondrial uniporter with the ability to discriminate between Ca2+ and Mn2+ Proc. Natl. Acad. Sci. U. S. A. 2018;115(34):E7960–E7969. doi: 10.1073/pnas.1807811115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barber M.J., Wendt D.J., Starmer C.F., Grant A.O. Blockade of cardiac sodium channels. Competition between the permeant ion and antiarrhythmic drugs. J. Clin. Invest. 1992;90:368–381. doi: 10.1172/JCI115871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Avdonin V., Shibata E.F., Hoshi T. Dihydropyridine action on voltage-dependent potassium channels expressed in Xenopus oocytes. J. Gen. Physiol. 1997;109:169–180. doi: 10.1085/jgp.109.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harper A.A., Catacuzzeno L., Trequattrini C., Petris A., Franciolini F. Verapamil block of large-conductance Ca-activated K channels in rat aortic myocytes. J. Membr. Biol. 2001;179:103–111. doi: 10.1007/s002320010041. [DOI] [PubMed] [Google Scholar]
- 82.Levin R.M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol. Pharmacol. 1977;13:690–697. [PubMed] [Google Scholar]
- 83.Genazzani A.A., Mezna M., Dickey D.M., Michelangeli F., Walseth T.F., Galione A. Pharmacological properties of the Ca2+-release mechanism sensitive to NAADP in the sea urchin egg. Br. J. Pharmacol. 1997;121:1489–1495. doi: 10.1038/sj.bjp.0701295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Virtual screening outputs.
First tab, raw output from the virtual screen.
Second tab, top 200 hits excluding repeat conformers.
Transparency document.