Highlights
-
•
We developed a transgenic mouse model expressing porcine APN that susceptible to porcine coronavirus infection.
-
•
We generated two transgenic mouse lines expressing porcine APN in various organs.
-
•
As they expressed porcine APN, the mice became susceptible to infection by porcine epidemic diarrhea virus, one of the porcine coronaviruses.
-
•
These transgenic mice will be an important tool for research into the porcine coronaviruses.
Keywords: Porcine coronaviruses, Porcine APN, Pathogenesis, Porcine epidemic diarrhea virus, Transgenic mouse model
Abstract
Porcine coronavirus infections have known as they are specific to pigs with predominantly enteric or respiratory diseases. No laboratory animal model is yet been developed in porcine coronaviruses study. Here, we report that development of a transgenic mouse model expressing porcine APN which is susceptible to porcine coronavirus infection. The porcine APN transgene was constructed by fusing with mouse proximal APN promoter at 5′ terminus and bovine growth hormone polyadenylation site at its 3′ terminus. After screen on pubs from the microinjected mice, we confirmed two transgenic lines expressing porcine APN in various organs. We confirmed the susceptibility to porcine epidemic diarrhea virus, one of the porcine coronaviruses. These transgenic mice will be an important tool for research into the porcine coronaviruses.
1. Introduction
The coronaviruses belong to the family Coronaviridae within the order Nidovirales. They are classified into four genera, Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus, based on genetic similarities (Adams et al., 2014). The primary replication of the coronaviruses is often confined to respiratory- or gastrointestinal-tract epithelial cells, so they usually induce respiratory or enteric diseases, but also hepatic, renal and neuronal infections (Lednicky et al., 2013, Masters, 2006, Weiss and Navas-Martin, 2005).
The pathogenesis of several porcine coronaviruses, including transmissible gastroenteritis virus (TGEV) and porcine respiratory coronavirus (PRCoV), has been broadly studied (Enjuanes et al., 1995, Saif, 2004a, Saif, 2004b, Saif, 2004a, Saif, 2004b, Weiss and Navas-Martin, 2005). TGEV is a major cause of viral enteritis and fetal diarrhea in swine, most severely in neonates and with a high mortality rate, which causes significant economic losses worldwide (Enjuanes et al., 1995). PRCoV is reported to be an attenuated variant of TGEV. PRCoV infects lung epithelial cells, and PRCoV antigen has been found in type I and type II pneumocytes and alveolar macrophages, and infection is followed by interstitial pneumonia (Halbur et al., 1993, Saif, 2004a, Saif, 2004b). Porcine epidemic diarrhea virus (PEDV) is the causative agent of porcine epidemic diarrhea, which is characterized by watery diarrhea, as is TGEV infection (Chasey and Cartwright, 1978, Pensaert and de Bouck, 1978, Turgeon et al., 1980). PEDV-infected piglets usually show typical enteric signs, including profuse watery diarrhea, weight loss, and loss of milk uptake, entailing high mortality. PEDV has been reported in Europe and Asia, and also recently in the United States (Hess et al., 1980, Huang et al., 2013, Pan et al., 2012, Park et al., 2013, Wang et al., 2014). All these porcine coronaviruses belong to the genus Alphacoronavirus. A hemagglutinating enteric coronavirus, a member of the genus Betacoronavirus, is antigenically unrelated to the other porcine coronaviruses and uses a 5-N-acetyl-9-O-acetylneuraminic-acid-containing moiety as its cellular receptor.
Coronavirus infections are mediated by the spike (S) glycoprotein, a large surface glycoprotein on the viral envelope (Delmas and Laude, 1990, Masters, 2006). Coronavirus S glycoproteins recognize cellular receptors and mediate virus-cell fusion (Masters, 2006, Peng et al., 2011). Most Alphacoronavirus, including TGEV, PEDV, and PRCoV, use aminopeptidase N (APN/CD13) as their cellular receptor (Delmas et al., 1992, Li et al., 2007, Ren et al., 2010, Tresnan and Holmes, 1998, Yeager et al., 1992). APN/CD13 is a 150-kDa, zinc-dependent metalloprotease consisting of 967 amino acids (Rawlings and Barrett, 1995). Mammalian APN is ubiquitously expressed as a glycosylated homodimer on the surfaces of epithelial cells in the liver, intestine, kidney, and respiratory tract, and in fibroblasts and leukocytes, and plays multiple roles in many physiological processes, including coronavirus entry (Barnes et al., 1994, Luan and Xu, 2007, Mina-Osorio et al., 2008, Miura et al., 1983).
The natural hosts of porcine coronaviruses are young piglets, and clinical illness has only been observed in sucking piglets. However, in vivo studies using suckling piglets have many disadvantages, including their high cost, difficulty in handling the piglets, limited reagents, etc. Because the use of laboratory animal models (e.g., mouse models) can circumvent these limitations, several transgenic animal models have been generated to study porcine viruses (Benbacer et al., 1998, Ono et al., 2006). Here, we generated transgenic mice expressing porcine APN under the control of the mouse proximal APN promoter. We tested their susceptibility to PEDV with reverse transcription-polymerase chain reaction (RT-PCR) and immunochemical analyses, and found that the porcine APN transgenic mice were susceptible to PEDV infection.
2. Materials and methods
2.1. Cells and viruses
Vero monkey kidney cells were maintained in minimal essential medium containing 10% fetal bovine serum (FBS), 100 U/ml penicillin G, 100 μg/ml streptomycin, and 250 μg/ml amphotericin B in a 5% CO2 atmosphere at 37 °C. 293T human embryonic kidney cells were maintained in Dulbecco‘s modified Eagle's medium containing 10% FBS, 100 U/ml penicillin G, 100 μg/ml streptomycin, and 250 μg/ml amphotericin B. All tissue culture reagents were purchased from Gibco (Carlsbad, CA, USA). KPEDV-9, a cell-adapted vaccine strain of PEDV, was grown and titrated in the Vero cells, as described previously, and stored at −80 °C until use (Cruz and Shin, 2007, Hofmann and Wyler, 1988).
2.2. Reagents and antibodies
The polyclonal antibodies specific for PEDV and porcine APN were generated in BALB/c mice immunized with KPEDV-9 and purified porcine APN, respectively, as described previously (Cruz et al., 2008). The antibody specificities were confirmed with enzyme-linked immunosorbent assays. The anti-Flag M2 monoclonal antibody (anti-Flag) and Anti-Flag M2 Affinity Gels (gel beads) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.3. Construction of porcine APN transgene
The mouse proximal APN promoter region (starting at nucleotide-1044) was amplified from C57BL/6J genomic DNA by PCR with primers 5′-CCCGCGGCCGCAAGATTTGAAACAGTGGA-3′ and 5′-CCCAAGCTTGATGCCGGTGGACAGGGA-3′, containing flanking NotI and HindIII restriction endonuclease sites, respectively. The PCR product was cloned into the pBluescript KS (+) vector and the pGL3-Basic vector (Promega, Madison, WI, USA), which contains a promoterless luciferase reporter gene. The porcine APN gene was amplified from the total RNA isolated from porcine enterocytes with RT-PCR using specific primers and cloned into the pBluescript KS (+) vector. A sequence encoding the Flag epitope (DYKDDDDK) was fused to the 3′ terminus of porcine APN with PCR with primers 5′-CCCAAGCTTACCATGGCCAAGGGATTCTAC-3′ and 5′-CCCCTCGAGTCACTTGTCGTCATCGTCTTTGTAGTCGCTGTGCTCTATGAACCA-3′, which contain flanking HindIII and XhoI restriction endonuclease sites, respectively. The BGH-polyA sequence was amplified from the pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) with PCR using primers 5′-CCCCTCGAGCGACTGTGCCTTCTAGTT-3′ and 5′-CCCGGTACCCCATAGAGCCCACCGCAT-3′, which contain flanking XhoI and KpnI restriction endonuclease sites, respectively. The PCR product was cloned into the pBluescript KS (+) vector. All PCR products were confirmed with automated sequencing. To generate the porcine APN transgene, porcine APN-Flag and the mouse proximal APN promoter were ligated into pBluescript KS-BGH-polyA after they were digested with HindIII/XhoI and NotI/HindIII, respectively.
2.4. Promoter luciferase assay
293T cells (2.5 × 105 cells/ml) were plated in 24-well plates. After 24 h, the cells were transfected with pGL3-Basic-mouse proximal APN promoter (mAPN-luc) (0.2 μg or 1 μg). The cells were transfected in triplicate using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. After 24 h, the cells were lysed for assay with the luciferase assay system (Promega), according to the manufacture's protocol. All luciferase activities were normalized to β-galactosidase activity.
2.5. Generation and detection of porcine APN transgenic mice
The porcine APN transgene was linearized by restriction with NotI and purified with gel extraction (Qiagen, Valencia, CA, USA). Gain-of-function gene transfer was performed by microinjecting the purified DNA into the pronuclei of ICR mouse zygotes, which were then transferred into the oviducts of female recipient mice. The transgenic mice were identified with PCR analysis of tail genomic DNA with primers: PCR1-F: 5′-CCCAAGCTTACCATGGCCAAGGGATTCTAC-3′ and PCR1-R: 5′-GAAGTTGGAGAGCATCCT-3′; and PCR2-F: 5′-GGCGTCCTACTTGCATGC-3′ and PCR2-R: 5′-CCCCTCGAGTCACTTGTCGTCATCGTCTTTGTAGTCGCTGTGCTCTATGAACCA-3′. The founder mice were backcrossed to the C57BL/6J background for five generations. All the mice used in this study were maintained in a specific-pathogen-free facility at the Biomedical Research Center at the Korea Advanced Institute of Science and Technology, Daejeon, Korea.
2.6. Infection of porcine APN transgenic mice with PEDV
All animals were cared for and the experiments were performed at the animal facility at Chungnam National University (CNU), Korea, with the permission of and according to protocols approved by the Institutional Animal Care and Ethics Committee of CNU (permission number 20110825). The porcine APN transgenic and nontransgenic wild-type mice were orally inoculated with 5 × 106 TCID50 of KPEDV-9 or phosphate-buffered saline (PBS, pH 7.2) as the negative control. Their clinical signs were monitored and their feces collected for 5 days. Two mice from each group were killed on the indicated days after viral infection. The tissues were aseptically collected and prepared for RT-PCR and immunohistopathological analysis.
2.7. RNA extraction and RT-PCR
Total RNAs were extracted from the feces and tissue samples using TRIzol® Reagent (Invitrogen) and transcribed to cDNA using Power cDNA synthesis kit (iNtRON Biotechnology, Korea), according to the manufacturer's protocol. The porcine APN-Flag cDNA was PCR amplified with the specific primers used for the genotyping PCR. As a control, specific primers were used to amplify β-actin (mouse β-Actin-F: 5′-CGGTTCCGATGCCCTGAGGCTCTT-3′ and mouse β-actin-R: 5′-CGTCACACTTCATGATGGAATTGA-3′). The cycling parameters were initial denaturation at 94 °C for 2 min, followed by 30 cycles of denaturation at 94 °C for 15 s, annealing at 60 °C for 40 s, and extension at 72 °C for 90 s, with a final extension step at 72 °C for 5 min.
To detect the viral genome, viral RNA was extracted from tissue homogenates using the Viral Gene-spin Viral DNA/RNA Extraction Kit (iNtRON Biotechnology), according to the manufacturer's instructions. M-MLV reverse transcriptase (iNtRON Biotechnology) was used for first-strand cDNA synthesis, together with 10 μl of extracted viral RNA in a 50 μl randomly primed reaction. The specific primers used for the detection of the N gene were PEDV-N forward (5′-GGTACCATGGCATCTGTCAGCTTT-3′) and PEDV-N reverse (5′-GGATCCTTAATTTCCTGT TCGAA-3′). RT-PCR was performed at 94 °C for 2 min, followed by 30 cycles of 94 °C for 20 s, 54 °C for 10 s, and 72 °C for 2 min, with a final extension at 72 °C for 10 min. The PCR products were analyzed by gel electrophoresis and visualized with ethidium bromide staining and UV transillumination.
2.8. Immunoblotting
293T cells were lysed with cell lysis buffer (25 mM Tris–Cl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1 mM sodium orthovanadate, 1 mM PMSF, 5% glycerol, 0.5% Triton X-100, and protease inhibitors [Roche, Indianapolis, IN, USA]). The small intestines were rinsed with PBS and homogenized in lysis buffer (1% NP40, 150 mM Tris–Cl, 50 mM NaCl, 1 mM EDTA). The cell or tissue lysates were separated with 8–15% gradient SDS-PAGE and transferred into PVDF membrane (Amersham-Pharmacia Biotech, Piscataway, NJ, USA). The proteins were probed with an anti-Flag monoclonal antibody diluted 1:4000 and an anti-β-actin antibody diluted 1:10,000. The immune complexes were detected with peroxidase-conjugated goat anti-mouse IgG antibody (Santa Cruz Biotechnology) and visualized with Amersham ECL western blotting detection reagent (Amersham-Pharmacia Biotech).
2.9. Flow cytometry
293T cells were transfected with the porcine APN transgene. After 24 h, the cells were rinsed with PBS and detached with Non-Enzymatic Cell Dissociation Solution (Sigma–Aldrich) for 2 min. The detached cells were harvested and resuspended in fluorescence-activated cell sorting (FACS) staining buffer (PBS containing 2% FBS). The cells were stained with the anti-Flag M2 monoclonal antibody (1:200) for 15 min at 4 °C and then with FITC-conjugated anti-mouse IgG secondary antibody (1:500) for 15 min at 4 °C. The antibody-stained cells were analyzed with flow cytometry (FACSCalibur, Becton-Dickinson Biosciences, Franklin Lakes, NJ, USA). Kidney and liver tissues collected from mice were rinsed with PBS, and the renal or hepatic cells were isolated from the tissues with a 70 μm mesh filter (Becton-Dickinson Biosciences), resuspended in FACS staining buffer, and analyzed as described above.
2.10. Immunochemistry (IHC)
Small intestine samples were fixed in 10% buffered formalin and embedded in paraffin. Serial 5 μm sections were mounted on silane-coated slides. The sections were deparaffinized for immunohistochemical analysis, and the endogenous peroxidases were blocked with 0.6% H2O2 in methanol for 30 min. Antigens were retrieved with microwave heating in citrate buffer for 10 min, and the slides were incubated with 1.5% horse serum for 30 min at room temperature. Polyclonal anti-porcine APN antiserum, monoclonal anti-Flag antibody, or polyclonal anti-PEDV antiserum were applied overnight at 4 °C. The biotinylated anti-mouse IgG secondary antibody was detected with an avidin-biotin-peroxidase kit (Vectastain ABC kit, Vector Laboratories, Burlingame, CA, USA). Antibody binding was detected with the chromogen diaminobenzidine (Vector Laboratories), and the cells were counterstained with hematoxylin and eosin.
2.11. PEDV replication in the small intestines of porcine APN transgenic mice
Both wild type and porcine APN transgenic mice were infected with PEDV orally on day 0. Viral titer in inoculum was 5X TCID50106. Two mice from each group were killed daily up to 5 days, and small intestine samples from all mice were collected in MEM and homogenized. Samples were centrifuged and supernatants were kept in −80 °C until processed for titration. Titration was performed using Vero cells. The PEDV titer was measured and described in log value.
3. Results
3.1. Construction and evaluation of the mouse APN proximal promoter
The transcription of mouse APN is regulated by two different promoters, as shown in Fig. 1A (Bhagwat et al., 2001). The distal promoter is located 8 kb upstream from the transcription start site and controls APN expression in myeloid and fibroblast cells, whereas the proximal promoter regulates APN expression in epithelial cells (Olsen et al., 1991, Shapiro et al., 1991). Following references and preliminary studies, we choose the mouse proximal APN promoter to express porcine APN in mouse epithelial cells. To test the promoter's activity, the sequence encoding the promoter region was amplified and cloned into the pGL3-Basic promoterless vector (Fig. 1B), and the promoter activity was measured by luciferase assay. As shown in Fig. 1B, the luciferase activity was about eight-fold higher in 293T human embryonic kidney cells transfected with the vector containing the mouse proximal APN promoter (mAPN-luc) than in cells transfected with the empty vector.
Fig. 1.
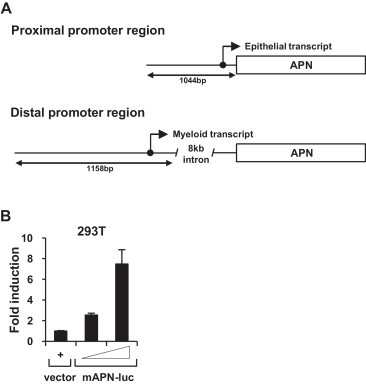
Structure and activity of the mouse proximal APN promoter. (A) Proximal and distal APN promoter regions. Black circle: transcription start site. (B) Activity of the proximal APN promoter in 293T human embryonic kidney cells. 293T cells were transfected with the serially diluted pGL3-Basic vector encoding the mouse proximal APN promoter (mAPN-luc, left panel). After 24 h, the cells were lysed and assayed for luciferase activity. The promoter activity is expressed relative to the luciferase activity measured in cells transfected with the pGL3-Basic vector. The error bars represent the standard deviations of three replicates.
3.2. Construction and characterization of the porcine APN transgene
We constructed a vector encoding porcine APN, which was regulated by the mouse proximal APN promoter (porcine APN transgene) (Fig. 2A). The porcine APN cDNA was amplified from porcine enterocytes and tagged at the C-terminus with the Flag epitope to distinguish exogenously expressed porcine APN from endogenously expressed murine APN. The 2.9-kb Flag-epitope-tagged porcine APN cDNA was cloned into the pBluescript KS (+) vector under the control of a 1.1-kb genomic sequence containing the mouse proximal APN promoter. The splice sites and polyadenylation signal for the cDNA were provided by a 0.2-kb bovine growth hormone polyadenylation signal (BGH-PolyA). The expression of the porcine APN protein was detected with immunoprecipitation and immunoblotting in 293T cells transfected with the porcine APN transgene. The Flag-tagged recombinant protein, with a molecular-weight of about 150 kDa (the expected molecular mass for porcine APN), was confirmed with immunoprecipitation (Fig. 2B). We also measured the surface expression of porcine APN with flow cytometry using an anti-Flag antibody, and found that 21.3% of cells were Flag-positive (Fig. 2C). Our data demonstrate that the mouse proximal APN promoter efficiently induced porcine APN expression from our construct.
Fig. 2.
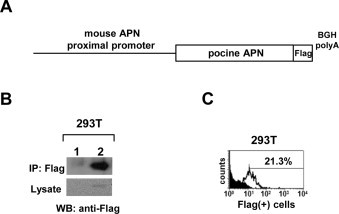
Construction and characterization of porcine APN transgenic vector. (A) Schematic structure of the porcine APN transgene. (B) Epithelial expression of the porcine APN transgene. 293T cells were transfected with the porcine APN transgene. After 24 h, the cells were lysed and Flag-tagged porcine APN was immunoprecipitated using Anti-Flag M2 Affinity Gels (gel beads), and immunoblotted with an anti-Flag monoclonal antibody. 1: pcDNA3.1 vector; 2: porcine APN transgene. (C) Extracellular expression of porcine APN transgene. 293T cells were transfected with the porcine APN transgene. After 24 h, the cells were detached, stained with anti-Flag monoclonal antibody and FITC-conjugated anti-mouse IgG secondary antibody, and analyzed with flow cytometry.
3.3. Generation of transgenic mice expressing porcine APN
The porcine APN transgene was linearized with NotI restriction and the DNA microinjected into mouse zygotes. To screen for porcine APN expression, we designed two genomic PCR primers, as indicated in Fig. 3A (arrowheads). The presence of the porcine APN transgene in mouse litters was monitored with PCR analysis on tail genomic DNA (Fig. 3B). Microinjection of the porcine APN transgene produced two transgenic founders, designated CNUPEDm-1 and CNUPEDm-3. The transgenic mice were healthy and showed no negative effects of transgene expression.
Fig. 3.
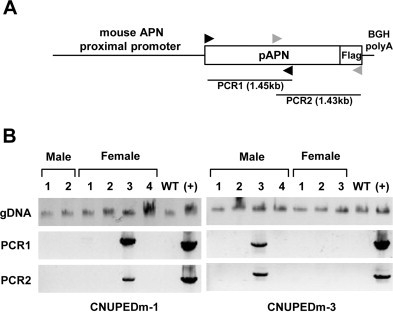
Generation of porcine APN transgenic mice. (A) The positions of the genomic PCR primers used to detect transgene integration are shown. Black arrowheads: PCR primer set 1; gray arrowheads: PCR primer set 2. (B) Screening porcine APN transgenic mice. Genomic DNA of CNUPEDm-1 and CNUPEDm-3 mice was isolated from their tails, and the porcine APN transgene was PCR amplified with two sets of genomic PCR primers. WT: C57BL/6 J nontransgenic mice; (+): positive control.
3.4. Characterization of porcine APN transgenic mice
Because the major pathological changes of the porcine coronaviruses (e.g., TGEV and PEDV) involves enteric diseases, we measured porcine APN expression in the small intestine by RT-PCR, immunoblotting, and IHC. All the tested litters descended from CNUPEDm-1 or CNUPEDm-3 expressed porcine APN mRNA in their small intestines (Fig. 4A). The small intestines expressed a recombinant protein with the molecular mass expected for porcine APN (Fig. 4B). An immunohistochemical analysis, with both anti-Flag and anti-porcine APN antibodies, clearly confirmed porcine APN expression in the brush borders of the absorptive cells in the small intestines of the mouse model (Fig. 4C). These results demonstrate that the porcine APN transgenic mice expressed porcine APN in their small intestines.
Fig. 4.
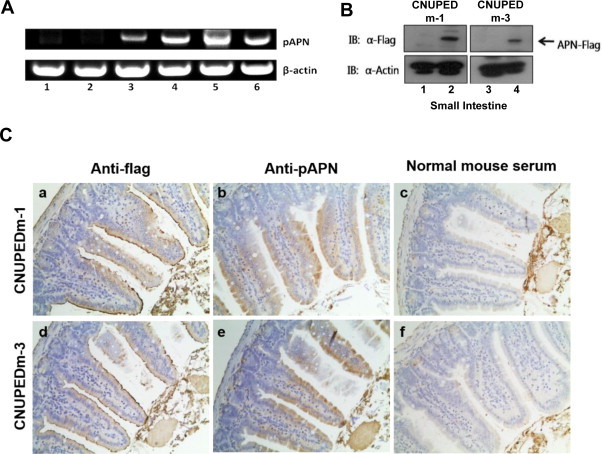
Characterization of porcine APN expression in small intestines of porcine APN transgenic mice. (A) Detection of porcine APN RNA in the small intestines of transgenic mice with RT-PCR. Small intestine samples were collected from nontransgenic wild-type (1 and 2), CNUPEDm-1 (3 and 4), and CNUPEDm-3 mice (5 and 6). Porcine APN RNAs were amplified using specific primers targeting porcine APN, as described in Section 2. β-Actin was detected as the loading control. (B) Expression of porcine APN protein in the small intestines. Small intestine samples were collected from CNUPEDm-1 and CNUPEDm-3 mice. Porcine APN protein was detected in small intestine homogenates with immunoblotting using an anti-Flag monoclonal antibody (2 and 4). No recombinant protein was detected in the small intestine samples derived from nontransgenic wild-type mice (1 and 3). β-Actin was used as the loading control. (C) Immunohistochemical analysis of porcine APN transgenic mice. Small intestine sections were prepared from porcine APN transgenic mice (a–c: CNUPEDm-1; d–f: CNUPEDm-3) and incubated with anti-Flag monoclonal antibody (anti-Flag) (a and d), mouse anti-porcine APN polyclonal antiserum (anti-pAPN) (b and e), or normal mouse serum (c and f). The immune complexes were visualized with avidin-biotin-peroxidase and the cells were counterstained with hematoxylin and eosin. Magnification, ×40.
We also screened for porcine APN expression in various other tissues with RT-PCR. Porcine APN mRNA was strongly expressed in the lung, kidney, and the intestine, and was slightly expressed in the liver (Fig. 5A). The extracellular expression of porcine APN was examined in the kidney and liver by FACS analysis (Fig. 5B). Consistent with the RT-PCR results, the number of Flag-positive renal cells was significantly higher (2–4.3-fold) in the porcine APN transgenic mice than in the nontransgenic wild-type mice, whereas the number of Flag-positive hepatic cells was also slightly higher (1.6–3-fold) In summary, we confirmed the expression of porcine APN in the small intestines, lungs, livers, and kidneys of the porcine APN transgenic mice.
Fig. 5.
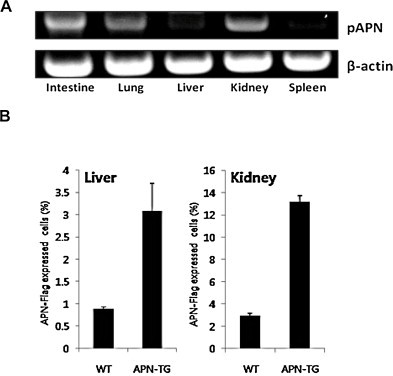
Porcine APN expression in various tissues of porcine APN transgenic mice. (A) Detection of porcine APN RNA in various tissues of transgenic mice with RT-PCR. Various tissue samples were collected from CNUPEDm-3 mice. Porcine APN RNA was amplified using specific primers targeting porcine APN. β-Actin was detected as the loading control. (B) Detection of porcine APN-positive cells in the kidneys and livers of transgenic mice. The kidney and liver tissues were collected from CNUPEDm-3 mice, and cells were isolated with a mesh filter. Porcine APN-positive cells were stained with anti-Flag monoclonal antibody and FITC-conjugated anti-mouse IgG secondary antibody, and analyzed with flow cytometry. WT: C57BL/6J nontransgenic mice; APN-TG: porcine APN transgenic mice.
3.5. PEDV infection of porcine APN transgenic mice
We examined the susceptibility of the porcine APN transgenic mice to PEDV, one of the enteropathogenic porcine coronaviruses. Porcine APN transgenic or nontransgenic wild-type mice were inoculated orally with KPEDV-9, a Vero-cell-adapted Korean strain of PEDV. In infected transgenic mice, other than slightly watery feces observed at 3 days after inoculation (Fig. 6A), whereas no clinical signs (e.g., watery diarrhea, vomiting, fever, weight loss, or death) were observed for 5 days. Viral replication was detected in various tissue extracts with RT-PCR. Viral RNA was confirmed in the small intestines (for at least 5 days), kidneys (for at least 2 days), and spleen (for at least 3 days) only in the porcine APN transgenic mice (Table 1 ), and not in the nontransgenic mice. An immunohistochemical analysis detected PEDV antigen in the brush borders of the small intestines, at the same location at which porcine APN is expressed (Fig. 6B), which has been demonstrated in PEDV-infected piglets (Debouck and Pensaert, 1980, Pensaert et al., 1981). However, no histopathological changes were observed in the small intestines that shown in PEDV infected piglets. Overall, these data indicate that porcine APN transgenic mice are susceptible to PEDV infection, although they showed none of the enteric disease symptoms typical of PEDV-infected piglets (Fig. 7 ).
Fig. 6.
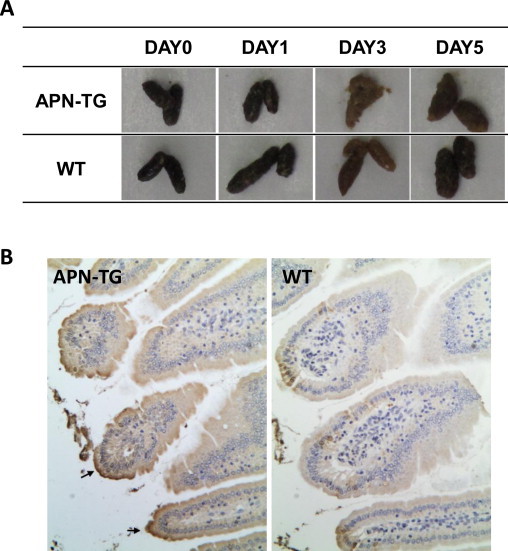
Immunohistochemical analysis of the small intestines of porcine APN transgenic mice infected with PEDV. (A) Feces were collected from the mice on the indicated days after KPEDV-9 inoculation. (B) Porcine APN transgenic mice (APN-TG) and nontransgenic wild-type mice (WT) were orally inoculated with KPEDV-9. Three days after inoculation, the small intestines were collected and tissue sections were incubated with mouse anti-PEDV polyclonal antiserum. Arrows indicate PEDV-infected epithelial cells. Magnification, ×40.
Table 1.
RT-PCR analysis of various tissues of porcine APN transgenic (TG) or nontransgenic wild-type (WT) mice infected with PEDV.
| Days after inoculation | Small intestine |
Lung |
Liver |
Kidney |
Spleen |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT | TG | WT | TG | WT | TG | WT | TG | WT | TG | |
| 1 | − | + | − | − | − | + | − | − | − | + |
| 2 | − | + | − | − | − | + | − | − | − | + |
| 3 | − | + | − | − | − | − | − | − | − | + |
| 5 | − | + | ND | ND | ND | ND | ND | ND | ND | ND |
ND: not determined.
Fig. 7.
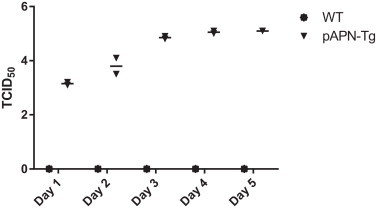
PEDV replication in the small intestines of porcine APN transgenic mice. Both wild type and porcine APN transgenic mice were infected with PEDV (5X TCID50106) orally on day 0. Two mice from each group were killed daily up to 5 days, and intestine samples were collected and prepared for titration. Titration was done using Vero cells. Y value is PEDV titer in TCID50 in log value.
3.6. PEDV replication in the small intestines of porcine APN transgenic mice
PEDV replication in both wild type and porcine APN transgenic mice were confirmed and compared by viral load in the small intestines. There were no detectable PEDV in any of wild type mice samples. In porcine APN transgenic mice, the mean value of PEDV was TCID50-103.1 on day 1. It increased to TCID50-103.8, TCID50-104.8, TCID50-105.0, TCID50-105.1, on day 2, 3, 4, and 5, respectively. There was no big change in titer between 3 and 5 days PI. We could confirm clear PEDV replication in the small intestines of porcine APN transgenic mice.
4. Discussion
Laboratory animal models are crucial tools for the study of viral pathogenesis in vivo, especially for highly pathogenic human viruses or viruses with restricted host ranges (Calvert et al., 2014, Chiu et al., 2014, Deruaz and Luster, 2013). Unlike in vitro cell culture systems, laboratory animal models offer researchers invaluable opportunities to study the biological, pathological, and histological characteristics of human and animal diseases. For these purposes, many transgenic mouse models have been developed to study viral pathogenesis, immune responses, and vaccines (Darling et al., 2014, O’Brien et al., 2014, O’Connor and Green, 2014, Reynaud et al., 2014). In the field of coronavirus research, studies of severe acute respiratory syndrome coronavirus (SARS-CoV) and human coronavirus 229E (hCoV-229E) have been conducted with transgenic mouse models (Lassnig et al., 2005, Tseng et al., 2007).
The host range of the porcine coronaviruses is strongly limited to pigs, so pigs are the only animals available in which to study viral pathogenesis. However, in vivo experiments using pigs are relatively challenging because they require special treatments, reagents, and facilities. Because most porcine coronaviruses use porcine APN as their receptor, we generated porcine APN transgenic mice, which are susceptible to porcine coronaviruses. We constructed the porcine APN transgene by attaching the mouse proximal APN promoter to the 5′ terminus of the porcine APN gene and the BGH-polyA signal at its 3′ terminus. We thus generated porcine APN transgenic mice expressing porcine APN in the brush borders of their small intestines and various tissues (lungs and kidneys).
Previous attempts to generate APN transgenic mice susceptible to TGEV or hCoV-229E have been documented (Benbacer et al., 1998, Lassnig et al., 2005). Benbacer et al. generated an RT-PCR-positive lineage, but failed to confirm either the expression of the porcine APN protein in the intestine or TGEV replication in their porcine APN transgenic mouse model (Benbacer et al., 1998). A susceptible mouse model for hCoV-229E infection was successfully developed using comprehensive APN regulatory elements to generate human APN +/+ STAT1 −/−double transgenic mice, and a virus was adapted to grow in primary embryonic fibroblasts from these mice (Lassnig et al., 2005). A transgenic mouse model of SARS was generated by expressing human angiotensin-converting enzyme 2, a functional receptor for the virus, under the regulation of a global promoter (Tseng et al., 2007). The significance of our study is that we successfully generated transgenic mice expressing porcine APN with minimal modifications, by using the mouse APN proximal promoter and the BGH-polyA signal to ensure the strong expression and correct splicing of the gene (Pfarr et al., 1986). Therefore, we could express porcine APN in the brush borders of the mouse small intestines and on the surfaces of their renal cells (Fig. 4, Fig. 5).
Among the porcine coronaviruses, PEDV is relatively poorly studied, and many questions and controversies remain regarding its outbreaks and pathogenesis. Outbreaks of porcine epidemic diarrhea have been limited to few countries in East Asia in the cold season, whereas it has spread to most swine farms in the Asian region (Mole, 2013, Pan et al., 2012, Park et al., 2013, Song and Park, 2012, Stevenson et al., 2013). Recently, PEDV has also spread rapidly among swine farms in the United States, causing high piglet mortality in more than 17 states (Huang et al., 2013, Mole, 2013, Stevenson et al., 2013, Wang et al., 2014). More importantly, although vaccination is practiced on most swine farms in Korea, reported outbreaks are still increasing. Moreover, a method for isolating wild-type PEDV is not well established. For these reasons, we tested the susceptibility of porcine APN transgenic mice to PEDV. Although significant clinical illness was not observed when the transgenic mice were infected with PEDV, their susceptibility to the virus was confirmed by the detection of viral RNA in various organs with RT-PCR and viral proteins in the small intestines with IHC. More clearly, PEDV replication was confirmed by increase in viral titer up to 5 days PI compared to those in wild type mice. We could found clear increase in viral titer in the small intestines of pAPN transgenic mice but not from wild type mice. These results also confirmed that porcine APN plays a role as the cellular receptor for PEDV. However, similar experiments using other porcine coronaviruses are required.
Although we have presented evidence of PEDV infection in the small intestines of porcine APN transgenic mice, no clinical signs were observed, apart from moderately soft feces. It has previously been shown that PEDV can infect cells lacking extracellular trypsin, although trypsin is essential for the generation of a cytopathic effect (CPE) in infected cells (Park et al., 2011). Therefore, we assume that cellular cofactors (e.g., trypsin) other than the primary receptor might be involved in and required for PEDV pathogenesis in vivo. However, these factors are probably present at insufficient levels in mice. Proteases are generally required for efficient viral entry and the CPEs of coronaviruses (Matsuyama et al., 2005, Simmons et al., 2004, Simmons et al., 2013). Although the viral receptors are usually expressed in various organs, the diseases caused by coronavirus infections are limited to the small intestines and/or lungs, which are enriched cellular proteases. This suggests that proteases play a role in the pathogenesis of the coronaviruses in vivo, as is already well established for influenza virus infections (Hatesuer et al., 2013, Sakai et al., 2014, Tarnow et al., 2014).
5. Conclusions
The porcine APN transgenic mouse model should extend our understanding of the pathogenesis of and other studies to porcine coronavirus infections. This laboratory animal model should also circumvent all the disadvantages and difficulties related to the study of porcine coronaviruses in suckling piglets. With the strategy described here, transgenic mouse models expressing the APN genes of other animals could be developed and used for the study of other coronaviruses.
Acknowledgements
This work was supported by a grant from Animal and Plant Quarantine Agency of Korea (grant no. Z-AD-14-2009-10-02), and a grant from the Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, the Korean Research Foundation Grants (grants no. 20120008358, 2011-0023942, 211-2006-2-E00027).
References
- Adams M.J., Lefkowitz E.J., King A.M., Carstens E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2014) Arch. Virol. 2014;159(10):2831–2841. doi: 10.1007/s00705-014-2114-3. [DOI] [PubMed] [Google Scholar]
- Barnes K., Kenny A.J., Turner A.J. Localization of aminopeptidase N and dipeptidyl peptidase IV in pig striatum and in neuronal and glial cell cultures. Eur. J. Neurosci. 1994;6:531–537. doi: 10.1111/j.1460-9568.1994.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Benbacer L., Stinackre M.G., Laude H., Delmas B. Obtention of porcine aminopeptidase-n transgenic mice and analysis of their susceptibility to transmissible gastroenteritis virus. Adv. Exp. Med. Biol. 1998;440:53–59. doi: 10.1007/978-1-4615-5331-1_7. [DOI] [PubMed] [Google Scholar]
- Bhagwat S.V., Lahdenranta J., Giordano R., Arap W., Pasqualini R., Shapiro L.H. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood. 2001;97:652–659. doi: 10.1182/blood.v97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert A.E., Dixon K.L., Delorey M.J., Blair C.D., Roehrig J.T. Development of a small animal peripheral challenge model of Japanese encephalitis virus using interferon deficient AG129 mice and the SA14-14-2 vaccine virus strain. Vaccine. 2014;32:258–264. doi: 10.1016/j.vaccine.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasey D., Cartwright S.F. Virus-like particles associated with porcine epidemic diarrhoea. Res. Vet. Sci. 1978;25:255–256. doi: 10.1016/S0034-5288(18)32994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S.C., Yang C.L., Chen Y.M., Hu S.C., Chiu K.C., Lin Y.C., Chang C.Y., Wang F.I. Multiple models of porcine teschovirus pathogenesis in endemically infected pigs. Vet. Microbiol. 2014;168:69–77. doi: 10.1016/j.vetmic.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Cruz D.J., Kim C.J., Shin H.J. The GPRLQPY motif located at the carboxy-terminal of the spike protein induces antibodies that neutralize porcine epidemic diarrhea virus. Virus Res. 2008;132:192–196. doi: 10.1016/j.virusres.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz D.J., Shin H.J. Application of a focus formation assay for detection and titration of porcine epidemic diarrhea virus. J. Virol. Methods. 2007;145:56–61. doi: 10.1016/j.jviromet.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling A.R., Freyschmidt E.J., Burton O.T., Koleoglou K.J., Oyoshi M.K., Oettgen H.C. IL-10 suppresses IL-17-mediated dermal inflammation and reduces the systemic burden of Vaccinia virus in a mouse model of eczema vaccinatum. Clin. Immunol. 2014;150:153–160. doi: 10.1016/j.clim.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck P., Pensaert M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am. J. Vet. Res. 1980;41:219–223. [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L’Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J. Virol. 1990;64:5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruaz M., Luster A.D. BLT humanized mice as model to study HIV vaginal transmission. J. Infect. Dis. 2013;208(Suppl. 2):S131–S136. doi: 10.1093/infdis/jit318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Smerdou C., Castilla J., Anton I.M., Torres J.M., Sola I., Golvano J., Sanchez J.M., Pintado B. Development of protection against coronavirus induced diseases: a review. Adv. Exp. Med. Biol. 1995;380:197–211. doi: 10.1007/978-1-4615-1899-0_34. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Paul P.S., Vaughn E.M., Andrews J.J. Experimental reproduction of pneumonia in gnotobiotic pigs with porcine respiratory coronavirus isolate AR310. J. Vet. Diagn. Invest. 1993;5:184–188. doi: 10.1177/104063879300500207. [DOI] [PubMed] [Google Scholar]
- Hatesuer B., Bertram S., Mehnert N., Bahgat M.M., Nelson P.S., Pohlman S., Schughart K. Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice. PLoS Pathog. 2013;9:e1003774. doi: 10.1371/journal.ppat.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R.G., Bollwahn W., Pospischil A., Heinritzi K., Bachmann P.A. Current aspects in the etiology of viral diarrheas of swine: occurrence of infections with the epizootic viral diarrhea (EVD) virus. Berl. Munch. Tierarztl. Wochenschr. 1980;93:445–449. [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26:2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W., Dickerman A.W., Pineyro P., Li L., Fang L., Kiehne R., Opriessnig T., Meng X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. MBio. 2013;4 doi: 10.1128/mBio.00737-13. e00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassnig C., Sanchez C.M., Egerbacher M., Walter I., Majer S., Kolbe T., Pallares P., Enjuanes L., Muller M. Development of a transgenic mouse model susceptible to human coronavirus 229E. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8275–8280. doi: 10.1073/pnas.0408589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Waltzek T.B., McGeehan E., Loeb J.C., Hamilton S.B., Luetke M.C. Isolation and genetic characterization of human coronavirus NL63 in primary human renal proximal tubular epithelial cells obtained from a commercial supplier, and confirmation of its replication in two different types of human primary kidney cells. Virol. J. 2013;10:213. doi: 10.1186/1743-422X-10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.X., Ge J.W., Li Y.J. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology. 2007;365:166–172. doi: 10.1016/j.virol.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y., Xu W. The structure and main functions of aminopeptidase N. Curr. Med. Chem. 2007;14:639–647. doi: 10.2174/092986707780059571. [DOI] [PubMed] [Google Scholar]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Ujike M., Morikawa S., Tashiro M., Taguchi F. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12543–12547. doi: 10.1073/pnas.0503203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina-Osorio P., Winnicka B., O’Conor C., Grant C.L., Vogel L.K., Rodriguez-Pinto D., Holmes K.V., Ortega E., Shapiro L.H. CD13 is a novel mediator of monocytic/endothelial cell adhesion. J. Leukoc. Biol. 2008;84:448–459. doi: 10.1189/jlb.1107802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Song I.S., Morita A., Erickson R.H., Kim Y.S. Distribution and biosynthesis of aminopeptidase N and dipeptidyl aminopeptidase IV in rat small intestine. Biochim. Biophys. Acta. 1983;761:66–75. doi: 10.1016/0304-4165(83)90363-x. [DOI] [PubMed] [Google Scholar]
- Mole B. Deadly pig virus slips through US borders. Nature. 2013;499:388. doi: 10.1038/499388a. [DOI] [PubMed] [Google Scholar]
- O’Brien L.M., Stokes M.G., Lonsdale S.G., Maslowski D.R., Smither S.J., Lever M.S., Laws T.R., Perkins S.D. Vaccination with recombinant adenoviruses expressing Ebola virus glycoprotein elicits protection in the interferon alpha/beta receptor knock-out mouse. Virology. 2014;452–453:324–333. doi: 10.1016/j.virol.2013.03.028. [DOI] [PubMed] [Google Scholar]
- O’Connor M.A., Green W.R. Use of IRF-3 and/or IRF-7 knockout mice to study viral pathogenesis: lessons from a murine retrovirus-induced AIDS model. J. Virol. 2014;88:2349–2353. doi: 10.1128/JVI.02960-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J., Laustsen L., Karnstrom U., Sjostrom H., Noren O. Tissue-specific interactions between nuclear proteins and the aminopeptidase N promoter. J. Biol. Chem. 1991;266:18089–18096. [PubMed] [Google Scholar]
- Ono E., Tomioka Y., Watanabe Y., Amagai K., Taharaguchi S., Glenisson J., Cherel P. The first immunoglobulin-like domain of porcine nectin-1 is sufficient to confer resistance to pseudorabies virus infection in transgenic mice. Arch. Virol. 2006;151(9):1827–1839. doi: 10.1007/s00705-006-0747-6. [DOI] [PubMed] [Google Scholar]
- Pan Y., Tian X., Li W., Zhou Q., Wang D., Bi Y., Chen F., Song Y. Isolation and characterization of a variant porcine epidemic diarrhea virus in China. Virol. J. 2012;9:195. doi: 10.1186/1743-422X-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E., Cruz D.J., Shin H.J. Receptor-bound porcine epidemic diarrhea virus spike protein cleaved by trypsin induces membrane fusion. Arch. Virol. 2011;156:1749–1756. doi: 10.1007/s00705-011-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J., Song D.S., Park B.K. Molecular epidemiology and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field isolates in Korea. Arch. Virol. 2013;158:1533–1541. doi: 10.1007/s00705-013-1651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G., Sun D., Rajashankar K.R., Qian Z., Holmes K.V., Li F. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10696–10701. doi: 10.1073/pnas.1104306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B., de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B., Debouck P., Reynolds D.J. An immunoelectron microscopic and immunofluorescent study on the antigenic relationship between the coronavirus-like agent, CV 777, and several coronaviruses. Arch. Virol. 1981;68:45–52. doi: 10.1007/BF01315166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr D.S., Rieser L.A., Woychik R.P., Rottman F.M., Rosenberg M., Reff M.E. Differential effects of polyadenylation regions on gene expression in mammalian cells. DNA. 1986;5:115–122. doi: 10.1089/dna.1986.5.115. [DOI] [PubMed] [Google Scholar]
- Rawlings N.D., Barrett A.J. Families of aspartic peptidases, and those of unknown catalytic mechanism. Methods Enzymol. 1995;248:105–120. doi: 10.1016/0076-6879(95)48009-9. [DOI] [PubMed] [Google Scholar]
- Ren X., Li G., Liu B. Binding characterization of determinants in porcine aminopeptidase N, the cellular receptor for transmissible gastroenteritis virus. J. Biotechnol. 2010;150:202–206. doi: 10.1016/j.jbiotec.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud J.M., Jegou J.F., Welsch J.C., Horvat B. Human herpesvirus 6A infection in CD46 transgenic mice: viral persistence in the brain and increased production of proinflammatory chemokines via Toll-like receptor 9. J. Virol. 2014;88:5421–5436. doi: 10.1128/JVI.03763-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J. Animal coronavirus vaccines: lessons for SARS. Dev. Biol. (Basel) 2004;119:129–140. [PubMed] [Google Scholar]
- Saif L.J. Animal coronaviruses: what can they teach us about the severe acute respiratory syndrome? Rev. Sci. Tech. 2004;23:643–660. doi: 10.20506/rst.23.2.1513. [DOI] [PubMed] [Google Scholar]
- Sakai K., Ami Y., Tahara M., Kubota T., Anraku M., Abe M., Nakajima N., Sekizuka T., Shirato K., Suzaki Y., Ainai A., Nakatsu Y., Kanou K., Nakamura K., Suzuki T., Komase K., Nobusawa E., Maenaka K., Kuroda M., Hasegawa H., Kawaoka Y., Tashiro M., Takeda M. The host protease TMPRSS2 plays a major role in in vivo replication of emerging H7N9 and seasonal influenza viruses. J. Virol. 2014;88:5608–5616. doi: 10.1128/JVI.03677-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L.H., Ashmun R.A., Roberts W.M., Look A.T. Separate promoters control transcription of the human aminopeptidase N gene in myeloid and intestinal epithelial cells. J. Biol. Chem. 1991;266:11999–12007. [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Zmora P., Gierer S., Heurich A., Pohlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antivir. Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44:167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G.W., Hoang H., Schwartz K.J., Burrough E.R., Sun D., Madson D., Cooper V.L., Pillatzki A., Gauger P., Schmitt B.J., Koster L.G., Killian M.L., Yoon K.J. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- Tarnow C., Engels G., Arendt A., Schwalm F., Sediri H., Preuss A., Nelson P.S., Garten W., Klenk H.D., Gabriel G., Bottcher-Friebertshauser E. TMPRSS2 is a host factor that is essential for pneumotropism and pathogenicity of H7N9 influenza A virus in mice. J. Virol. 2014;88:4744–4751. doi: 10.1128/JVI.03799-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresnan D.B., Holmes K.V. Feline aminopeptidase N is a receptor for all group I coronaviruses. Adv. Exp. Med. Biol. 1998;440:69–75. doi: 10.1007/978-1-4615-5331-1_9. [DOI] [PubMed] [Google Scholar]
- Tseng C.T., Huang C., Newman P., Wang N., Narayanan K., Watts D.M., Makino S., Packard M.M., Zaki S.R., Chan T.S., Peters C.J. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human angiotensin-converting enzyme 2 virus receptor. J. Virol. 2007;81:1162–1173. doi: 10.1128/JVI.01702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon D.C., Morin M., Jolette J., Higgins R., Marsolais G., DiFranco E. Coronavirus-like particles associated with diarrhea in baby pigs in Quebec. Can. Vet. J. 1980;21:100–110. [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014;20:917–919. doi: 10.3201/eid2005.140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]


