Highlights
► Assembly of SGs can be dramatically influenced by viruses. ► Viruses have elaborated mechanisms to impose a blockade/induction to SG assembly ► New knowledge on the SG biology may be beneficial in developing new anti-viral drugs.
Keywords: Viruses, Stress granules, P-bodies, eIF2α, RNA granules
Abstract
Following viral infection, the host responds by mounting a robust anti-viral response with the aim of creating an unfavorable environment for viral replication. As a countermeasure, viruses have elaborated mechanisms to subvert the host response in order to maintain viral protein synthesis and production. In the last decade, several reports have shown that viruses modulate the assembly of stress granules (SGs), which are translationally silent ribonucleoproteins (RNPs) and sites of RNA triage. This review discusses recent advances in our understanding of the interactions between viruses and the host response and how virus-induced modulations in SG abundance play fundamental roles in dictating the success of viral replication.
1. Introduction
Exposure of cells to environmental stress (e.g., heat shock, UV irradiation, hypoxia, endoplasmic reticulum (ER) stress and viral infection) trigger a rapid translational arrest generating polysome disassembly (Anderson and Kedersha, 2002). This event triggers a molecular triage, where the affected cell must make a decision on the fate of mRNA that is released from polysomes: decay or silencing (Anderson and Kedersha, 2008). For these events, cells have elaborated different classes of RNA granules named processing P-bodies (PBs) or stress granules (SGs) that contribute to the regulation and lifecycle of mRNAs. Both PBs and SGs contain share proteins and are assembled in cells subjected to stress, but differ in: (i) only PBs are observed in unstressed cells, (ii) SG assembly typically requires phosphorylation of translation initiation factor eIF2α, but not PB assembly (Fig. 1 ), and (iii) PBs contain proteins involved in mRNA decay, whereas SGs contain proteins of translation initiation complex (Eulalio et al., 2007).
Fig. 1.
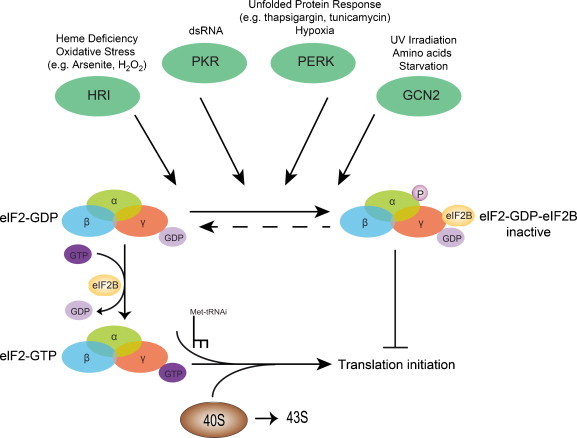
Control of translation by eukaryotic initiation factor 2 (eIF2). eIF2 bound to GDP (eIF2-GDP) is recycled to the active eIF2-GTP by a reaction catalyzed by eIF2B. Once recycled, eIF2-GTP forms a ternary complex with initiator-methionine tRNA (Met-tRNAi) and 40S ribosome resulting in 43S pre-initiation complex. Four kinases activated by hemin deficiency/oxidative stress (HRI), viral infection (PKR), endoplasmic reticulum stress/hypoxia (PERK/PEK) and amino acid starvation/UV irradiation (GCN2); can phosphorylate eIF2 subunit α, stabilize eIF2-GDP–eIF2B complex (inactive) and prevents eIF2 recycling. These events result in a shut-off of the host protein synthesis and subsequently SG assembly (Fig. 2, i).
PBs are cytoplasmic structures that, unlike SGs, are responsible for mRNA decay, RNA-mediated gene silencing (microRNA and siRNA-based gene silencing) and mRNA surveillance (or RNA quality control) (Beckham and Parker, 2008). PBs were discovered by Bashkirov et al. (1997) and they showed that XRN1, a 5′–3′ exoribonuclease, was localized in small granular structures within the cytoplasm. Other proteins related to mRNA degradation were also found to localize to this granules, such as a deadenylase (CCR4), decapping enzymes Dcp1 and Dcp2 as well as the activators of decapping Dhh1/p54/Rck/DDX6, Pat1, Scd6/RAP55, Edc3, Hedls and Lsm1–7 complex (Eulalio et al., 2007, Ingelfinger et al., 2002, van Dijk et al., 2002). Moreover, PBs can contain mRNAs and proteins involved in Nonsense-Mediated Decay (NMD) (e.g., SMG5, SMG7, and UPF1) (Fukuhara et al., 2005, Unterholzner and Izaurralde, 2004) and components of the RNA-induced silencing complexes (RISC) (e.g., argonaute, microRNA and GW182) (Liu et al., 2005, Rehwinkel et al., 2005) (Fig. 2 ).
Fig. 2.
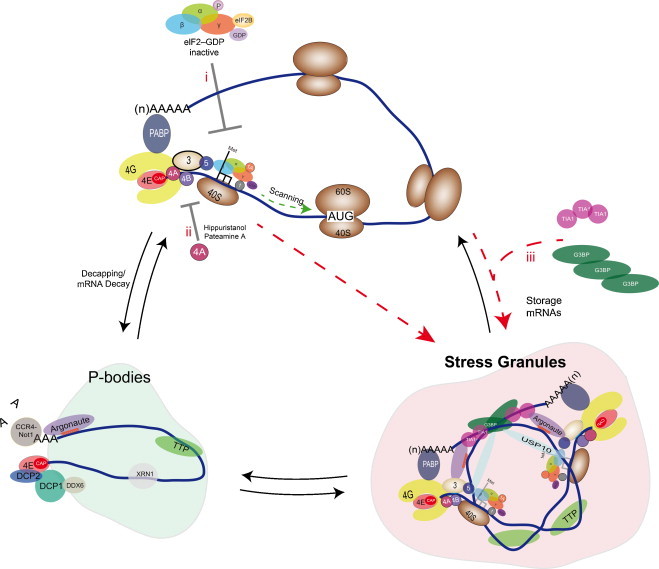
SG assembly pathways. Polysomes disassembly can lead to the assembly of cytoplasmic granules know as processing P-bodies (PBs) or stress granules (SGs). If deadenylation (e.g., CCR4/Not1), destabilization (e.g., TTP/XRN1) and decapping (e.g., DCP1/DCP2) complex; and even RISC (Ago) complex are recruited to mRNA, these will be targeted to PBs. Conversely, if TIA-1/TIAR or proteins such as G3BP/USP10 are recruited to the stalled initiation complexes, these will be directed to SGs. Different pathways in SG assembly are described (in red): (i) phosphorylation of eIF2α induced by the exposure to different stress inducers (e.g., arsenite and thapsigargin) (Fig. 1); (ii) Hippuristanol and Pateamine A, drugs that inhibit the helicase activity of eIF4A altering ATP binding or ATPase activity; and (iii) the overexpression of SG markers, such as G3BP or TIA-1. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
On the other hand, SGs were first observed in the cytoplasm of plant cells exposed to heat shock (Nover et al., 1983). SGs are translationally silent ribonucleoproteins and serve as storage sites of mRNAs and proteins (Anderson and Kedersha, 2006) (Fig. 2), while other functions also have been discussed (Thomas et al., 2011). SGs typically contain poly(A) + mRNA, 40S ribosomal subunits, eIF4E, eIF4G, eIF4A, eIF4B, poly(A)-binding protein (PABP1), eIF3, eIF2, p54/Rck/DDX6, and many other RNA-binding proteins that regulate mRNA structure and function, including human antigen R (HuR), Staufen 1, polysomal ribonuclease 1 (PMR-1), Smaug, tristetraprolin (TTP), T-cell restricted intracellular antigen 1 (TIA-1) and TIA-1-related protein (TIAR), Fragile X Mental Retardation Protein (FXMR/FXR1), Ras-Gap SH3-binding protein (G3BP-1), cytoplasmic polyadenylation binding protein (CPEB) and Survival of Motor Neurons (SMN) protein, although the composition can vary (Anderson and Kedersha, 2006) (listed in Table 1 ).
Table 1.
Stress granule components.
| Protein | Reference |
|---|---|
| 40S | Kedersha et al. (2002) |
| eIF2 | Kedersha et al. (2002) |
| eIF3 | Kedersha et al. (2002) |
| eIF4AI | Kedersha et al. (2002) |
| eIF4E | Kedersha et al. (2002) |
| eIF4G | Kedersha et al. (2002) |
| PABP-1 | Kedersha et al. (1999) |
| p54/RCK/DDX6 | Wilczynska et al. (2005) |
| TIA-1/TIAR | Gilks et al. (2004) |
| TTP | Stoecklin et al. (2004) |
| HuR/HuD | Kedersha et al. (1999) |
| Staufen 1 | Thomas et al. (2009) |
| SMN | Hua and Zhou (2004) |
| G3BP-1 | Tourriere et al. (2003) |
| Smaug | Baez and Boccaccio (2005) |
| FXMR/FXR1 | Mazroui et al. (2006) |
| CPEB | Wilczynska et al. (2005) |
| PMR1 | Yang et al. (2006) |
| RSK2 | Eisinger-Mathason et al. (2008) |
| RACK1 | Arimoto et al. (2008) |
| TRAF2 | Kim et al. (2005) |
| FAST | Kedersha et al. (2005) |
| BRF1 | Kedersha et al. (2005) |
During a stress response, cells induce a shut-off of cellular protein synthesis and subsequently promote SG assembly (Anderson and Kedersha, 2009). Different pathways in SG assembly have been described. The most popular pathway is the phosphorylation of the critical translation initiation factor, eIF2α by a family of four serine/threonine kinases HRI, PKR, PERK/PEK and GCN2. HRI (eIF2αK1) is activated in heme deprivation and oxidative stress (Han et al., 2001); PKR (eIF2αK2) is activated by viral infection (Williams, 2001); PERK/PEK (eIF2αK3) is activated in the presence of unfolded proteins in the endoplasmic reticulum (ER) and during hypoxia (Harding et al., 2000); and GCN2 (eIF2αK4) is activated during amino acid starvation and UV irradiation (Jiang and Wek, 2005). Each kinase causes the phosphorylation of the α-subunit of eIF2 at Ser52, which implies the tight binding with eIF2B, inhibiting the exchange of GDP for GTP (Fig. 1). Therefore, there is a decrease in translation tertiary complex assembly (eIF2/GTP/Met-tRNA) which suppresses the initiation of translation and promotes SG assembly (Fig. 2, step i) (Kedersha et al., 2002). Other mechanisms independent of the phosphorylation of eIF2α have also been explored. Hippuristanol and Pateamine A, drugs that inhibit the helicase activity of eIF4A, are able to induce the assembly of SGs (Fig. 2, step ii) (Dang et al., 2006, Mazroui et al., 2006). As well, the overexpression of SG markers (Anderson and Kedersha, 2008), such as TIA1 (Kedersha et al., 1999) or G3BP-1 (Tourriere et al., 2003), can trigger the assembly of SGs (Fig. 2, step iii).
The activation of eIF2α kinases by viral infection may result in the inhibition of cellular protein synthesis (Walsh and Mohr, 2011) and/or promotion of autophagy, process involving lysosomal-dependent recycling of intracellular components (Talloczy et al., 2002). Moreover, some viral proteins can bind eIF4A (Aoyagi et al., 2010, Page and Read, 2010). All of these mechanisms induce SG assembly (i.e., shut-off of cellular protein synthesis), but the viruses have found ways to bypass the hostile environment generated by the cell to ensure their survival. In the last decade, several studies have also demonstrated that the assembly of SGs can be dramatically influenced by viruses: the induction and blockage of SG assembly mediated by viral infections have both been described as means to promote virus replication (Beckham and Parker, 2008, Montero and Trujillo-Alonso, 2011, White and Lloyd, 2012). In this review we will summarize the current understanding that exists between different virus families and the regulation of stress granules.
2. Virus-mediated blockade of SG assembly
In 2002, the first evidence was reported showing an interaction between viruses and what we understand to be protein components of SGs. Li et al. showed that the negative strand 3′ terminal stem–loop structure present in the genome of West-Nile Virus (WNV) interacts with two SG markers, TIA-1 and TIAR (Li et al., 2002). In support of the necessity for these virus–host interactions, WNV replication was reduced when TIAR−/− cells were infected (Li et al., 2002). WNV is a neurotropic flavivirus responsible for viral meningoencephalitis which has an enzootic cycle between mosquitoes and birds, but can infect amphibians, reptiles, horses and humans (Dauphin et al., 2004). Moreover, Emara et al. expanded these observations to other members of the same viral flaviviridae family, where TIA-1/TIAR were shown to co-localize with viral replication complexes (dsRNA and NS3) in both WNV- and dengue virus-infected cells (Emara and Brinton, 2007). SGs can be induced in mammalian cells by several drugs (Kedersha and Anderson, 2007), apparently as a consequence of the phosphorylation of eIF2α. In order to determine if viral infection would have any effect on SG assembly, Baby Hamster Kidney (BHK) cells were infected with wild-type WNV and subjected to arsenite-mediated oxidative stress. Infected cells were found to be resistant to SG induction (Emara and Brinton, 2007). However, recent studies showed that chimeric WNV produces high levels of an early viral RNA (W956IC), allowing PKR activation and subsequent induction of SG, likely due to translational arrest (Courtney et al., 2012).
Another flavivirus, Hepatitis C Virus (HCV), the major etiologic agent of hepatitis C in humans, is able to disrupt PB assembly but at the same time, promote SG assembly during the course of viral infection (Ariumi et al., 2011). However, late in HCV infection corresponding to 48 h post-infection, G3BP-1 and DDX6, both components of SG (Table 1), are found to co-localize with the HCV core, resulting in the suppression of SG assembly. This blockade to SG assembly was found to be due to an interaction between G3BP-1 and the HCV non-structural protein, (NS)5B and the 5′ end of the HCV minus-strand RNA (Yi et al., 2011). Thus, as shown in the examples above, through sequestration of factors essential for the assembly of SGs, several viruses have elaborated mechanisms to impose a blockade to SG assembly.
Some viruses inhibit cap-dependent translation (hence host cell mRNA translation) to ensure the synthesis of their own proteins. Pelletier et al. discovered that the translation of the uncapped picornaviral mRNA is mediated by an RNA structure known as the internal ribosome entry site, IRES, at the 5′ end of the viral RNA (Pelletier et al., 1988). Infection by poliovirus (PV), the etiologic agent of paralytic disease known as poliomyelitis, induces the inhibition of cap-dependent translation initiation by the cleavage of the translation initiation factors eIF4GI, eIF4GII, and PABP mediated by viral proteinases (Gradi et al., 1998, Kuyumcu-Martinez et al., 2002). SG assembly is induced at a very early time post-PV infection (at approximately 2–4 h), but later, SGs disappear because the same viral 3C proteinase (3Cpro) cleaves G3BP-1, but not TIA-1 or TIAR, and thereby prevents SG assembly (White et al., 2007). The SGs found in PV-infected cells contain viral RNA and TIA-1, but are compositionally distinct since they exclude well-described SG components such as G3BP-1, PABP, and eIF4G, all of which are eventually cleaved by 3Cpro (Piotrowska et al., 2010, White and Lloyd, 2011). Furthermore, PV infection also disrupts the assembly of PBs. Also during PV infection, Xrn1, Dcp1a and Pan3, three factors involved in mRNA decapping, degradation and deadenylation, respectively, undergo degradation or cleavage by the viral 3Cpro (Dougherty et al., 2011).
Likewise, Cricket Paralysis Virus (CrPV) infection in Drosophila cells leads to a rapid shut-off of host protein synthesis concomitant with phosphorylation of eIF2α (Wilson et al., 2000). Because these characteristics are common to the induction of SGs, Khong et al. investigated the assembly of SG after CrPV infection. Through an immunofluorescence assay, the authors showed that Rox8 and Rin, Drosophila SG marker homologs of TIA-1 and G3BP-1, respectively, do not aggregate in CrPV infected cells, even in the presence of SG inducers such as heat shock, oxidative stress and Pateamine A. It was also demonstrated that CrPV viral 3C proteinase is sequestered to SGs under cellular stress but not during virus infection (Khong and Jan, 2011).
Another picornavirus, Theiler's murine encephalomyelitis virus (TMEV) which causes a demyelinating disease similar to multiple sclerosis in the central nervous system, also inhibits SG assembly. Borghese et al. showed that TMEV infection induces SG assembly, but the expression of the leader (L) protein during infection was sufficient to inhibit SG assembly induced by arsenite-mediated oxidative stress or by thapsigargin-mediated ER stress. Unlike the effects induced by PV 3C proteinase, G3BP-1 was not cleaved by TMEV and was in fact found in SGs post-TMEV infection (Borghese and Michiels, 2011).
For efficient protein synthesis, mRNA circularization is required during translation. PABP, that is bound to poly (A) 3′ tail, interacts with eIF4GI at the 5′, causing circularization of the mRNA by linking the 5′ and 3′ mRNA ends, increasing the binding of eIF4E to the cap (Lopez-Lastra et al., 2010). Rotavirus, the causative agent of a common infantile gastroenteritis, subverts the host translation machinery at this step. Because rotavirus mRNAs are capped but lack poly(A) tails, the virus-encoded protein, non-structural (NS) P3, binds to a consensus RNA sequence in the 3′ end of viral mRNA, enabling mRNA circularization by interaction with eIF4GI (Piron et al., 1998). As a consequence, a shut-off of host protein synthesis ensues and thereby provides an advantage for viral protein synthesis. In infected cells, Montero et al. found that eIF2α is phosphorylated during the entire virus replication cycle but this does not have an impact in the formation of viroplasms (cytoplasmic viral factories found in rotavirus-infected cells) or viral replication and surprisingly, SG assembly was not induced. One possibility for explain this observation may be due to PABP, a component of SG (Table 1), is able to translocate from the cytoplasm to the nucleus in rotavirus infected cells in a NSP3-dependent manner (Montero et al., 2008).
Instead, Junin virus (JUNV), that is responsible for Argentine hemorrhagic fever, is able to impair the phosphorylation of eIF2α. Linero et al. showed that in JUNV-infected Vero cells exposed to arsenite-mediated oxidative stress, eIF2α phosphorylation was impaired but this did not lead to the induction of SG assembly (Linero et al., 2011). Furthermore, the JUNV nucleoprotein (N) and/or the glycoprotein precursor (GPC) was responsible for this virus-induced blockade to SG assembly. Rather, when JUNV-infected cells were treated with hippuristanol, an eIF4A-helicase activity inhibitor that induces SGs in an eIF2α-independent manner (Mazroui et al., 2006), SG assembly was observed in 100% of cells indicating that JUNV affects an unidentified event downstream of eIF2α phosphorylation or the integrity of viral mRNAs on polysomes (Linero et al., 2011).
Another virus that efficiently shuts off host protein synthesis is influenza A virus (IAV) (Kash et al., 2006). IAV is an animal pathogen that causes severe respiratory disease and pandemics in humans around the world. Viral transcription involves a cap-snatching mechanism during which a nucleotide sequence between 10 and 20 nt, including the 5′ cap structure, is cleaved from the 5′ end of cellular mRNAs. This sequence is used to prime transcription on the viral genome and is ultimately used during translation initiation of viral mRNAs (Lopez-Lastra et al., 2010). Additionally, IAV encodes cap-binding proteins that are able to preferentially recognize capped viral mRNAs. The influenza non-structural protein 1 (NS1) binds eIF4GI and PABP-1, thus stimulating the assembly of the translation initiation complex on capped IAV mRNAs (Lopez-Lastra et al., 2010). IAV actively suppresses SG assembly during viral infection, thereby allowing translation of viral mRNAs. Complete inhibition of SG assembly is dependent on the function of NS1 and its ability to inhibit PKR, the double-stranded RNA-activated protein kinase (Khaperskyy et al., 2011).
Recently, retroviruses such as the human immunodeficiency virus type-1 (HIV-1) and human T-cell lymphotropic virus type-1 (HTLV-1) were shown to impose a blockade to SG assembly in infected cells. Recent work from the authors’ laboratory showed that HIV-1 preferably assembles ribonucleoprotein complexes to which Staufen1, the viral genomic RNA and the structural protein Gag are recruited, called Staufen1 HIV-1-dependent RNPs (SHRNPs). These were compositionally different than SGs since they did not contain many of the classical SG marker proteins G3BP-1, eIF3, TIA-1, TIAR, HuR, PABP-1, but contained Staufen1. The assembly SHRNPs during the late stages of viral replication is believed to impose a blockade to the assembly of SGs but to favor the encapsidation of HIV-1 genomic RNA into assembling virus (Abrahamyan et al., 2010, White and Lloyd, 2012). Follow-up work, reported at the last International Nucleocapsid (NC) Meeting in Barcelona, Spain in September 2011, now demonstrates that the viral Gag protein controls the kinetics of SG assembly and interferes with the cellular stress response pathway (Valiente-Echeverría et al., unpublished). The oncoretrovirus, HTLV-1 elicits a blockade to SG assembly in a different manner and this was found to be mediated by the viral regulatory protein, Tax. Legros et al. observed that Tax relocated from the nucleus to the cytoplasm in response to environmental stress. While Tax is present in the cytoplasm, it interacts with histone deacetylase 6 (HDAC6), a critical component of SGs (Kwon et al., 2007), and thereby impairs SG assembly (Legros et al., 2011). While the details on the mechanisms by which viruses elicit favorable environments in which to replicate will require further work, the sequestration of critical factors for the induction of SGs by viral proteins appears to be an increasingly studied area of research and should yield important new information on how viruses gain control over host cell biology.
While all of the examples described above belong to RNA viruses, Herpes simplex virus (HSV) and Cytomegalovirus (HCMV) are the only members of the DNA virus family that have been shown to regulate SG assembly. HSV-1 causes a shut-off of host cell protein synthesis by the virion host shutoff (Vhs) protein and subsequently induces degradation of cellular RNAs (Kwong and Frenkel, 1987). Several Adenosine–Uracil (AU)-rich binding proteins that promote mRNA stability, such as TIA-1/TIAR, and TTP (Bevilacqua et al., 2003), were upregulated in HSV-1 infected cells (Esclatine et al., 2004). TTP and TIA-1/TIAR were activated during the infection and accumulated in the cytoplasm, but only TTP was able to interact with Vhs. As a consequence, SGs were not observed after infection (Esclatine et al., 2004). More recently, Finnen et al. have shown that HSV-2 infection blocks SG accumulation in cells exposed to arsenite-mediated oxidative stress, but not in cells exposed to Pateamine A, a drug that induces SG assembly in an eIF2α-independent manner (Finnen et al., 2012). These results were similar to those found in JUNV infected cells described above (Linero et al., 2011). On the other hand, HCMV infection induces an unfolded protein response (UPR), activates PERK, but eIF2α phosphorylation levels were limited and viral RNA translation was maintained (Isler et al., 2005b). Likewise, the same group showed that SG assembly was suppressed in HCMV infected cells treated with the ER stressor, thapsigargin (Isler et al., 2005a). As discussed in the previous section, viruses have chosen different mechanisms to inhibit the SG assembly to ensure efficient and unmitigated replication.
3. Virus-mediated induction of SG assembly
Some studies have demonstrated that the SG assembly is not always correlated with a shut-off of host protein synthesis (Kimball et al., 2003, Loschi et al., 2009). Moreover, other authors have showed that SGs could sequester apoptotic molecules favoring cell survival upon exposure to certain types of stress such as heat shock (Kim et al., 2005, Tsai and Wei, 2010). Thus, a virus-mediated induction of SG assembly also represents a strategy employed by some viruses to ensure replication.
Respiratory Syncytial Virus (RSV), which is responsible for lower respiratory tract illnesses in both infants and the elderly, induces SGs during the course of infection (Lindquist et al., 2010). Lindquist et al. showed the correlation between higher viral protein levels and the presence of SGs in infected cells. In addition, G3BP−/− cells, that are unable to generate SGs because of a disrupted g3bp gene locus, exhibited diminished RSV replication (Lindquist et al., 2010). However, a later study by the same group concluded that the stress response may not play an important role in viral replication. They did not see a difference in viral replication in cells that were not able to elicit a stress response because PKR was depleted by siRNA (Lindquist et al., 2011). This later study also noted that RSV infection does cause eIF2α phosphorylation and PKR is needed to induce SGs during viral infection. These results indicate that the assembly of SG neither aids nor interferes with the replication of this virus.
The role of the stress response involving SGs in the Reoviridiae family of viruses has been shown to be implicated in viral replication. Mammalian orthoreovirus (MRV) infection in humans is usually asymptomatic or associated with symptoms of a common cold. During the early stages of infection, MRV induces SG assembly and the expression of ATF4, a transcription factor, through eIF2α phosphorylation (Smith et al., 2006). The assembly of SGs creates a competitive advantage for the viral mRNA to be translated because cellular mRNAs are sequestered in SG. When ATF4 is expressed in MRV infected cells, viral production increases by up to 100-fold (Smith et al., 2006). A later study implicated a role for SG assembly in viral replication since SG formation occurs after viral uncoating but before viral mRNA transcription (Qin et al., 2009). Qin et al. (2011) found that viral mRNAs escape translational inhibition when SGs are disrupted and viral translation occurs in the presence of high levels of phosphorylated eIF2α in a manner that is independent of PKR inhibition. This study also mentions that MRV-infected Cos7 cells are able to block the assembly of SGs induced by arsenite-mediated oxidative stress later in infection (Qin et al., 2011). The implication of these findings is that the stress response and the resulting assembly of SGs must be involved in the early stages of the viral replication cycle but is ultimately detrimental to the virus if it is not able to disassemble SG during later stages of infection.
Semliki Forest Virus (SFV), which causes lethal encephalitis in rodents, seems to modulate the cellular stress response in a similar fashion than MRV. Upon infection, SFV is able to induce the phosphorylation of eIF2α and promote SG assembly in mouse embryo fibroblasts (MEF) (McInerney et al., 2005). Despite a shut-off of host protein synthesis during these events, SFV is still able to translate its mRNA due to a translational enhancer element present in the viral genome. This study also indicated that areas around viral RNA in the cytoplasm were devoid of SGs. This observation likely indicates that viral proteins or viral RNA could locally disassemble SG to favor viral translation and this was shown to correlate with increased vRNA levels (McInerney et al., 2005).
The theme of utilizing the stress response to shut-off of host protein synthesis appears once again in Coronaviridae. The mouse hepatitis coronavirus (MHV), which is closely related to the SARS coronavirus, has been shown to subvert the host translation machinery through eIF2α phosphorylation (Raaben et al., 2007). eIF2α phosphorylation also leads to the assembly of SG and PB. A genome wide microarray analysis of regulated mRNAs in MHV-infected LR7 cells revealed the decrease in the expression of many cellular mRNAs, which may be due to an increase in PBs activity and function (Raaben et al., 2007). Likewise, viral RNAs transcripts make up 40% of total RNA in the cell, so the virus may be overloading the host cell cytoplasm to ensure that its transcripts will be translated (Raaben et al., 2007). However, the authors come to the conclusion that the inhibition of cellular translation is not beneficial to the virus since in systems lacking the ability to inhibit cellular translation, viral production did not change and thus, the assembly of SG in MHV-infected cells does not appear to dramatically favor viral replication (Raaben et al., 2007).
Finally, Rubella virus (RUBV) infection generates aggregates of G3BP-1 in the cytoplasm (Matthews and Frey, 2012). These aggregates differ from typical SG because they do not contain proteins such as PABP and TIA-1 (Table 1). RUBV is a positive strand RNA where viral replication is mediated for intermediary double stranded RNA (dsRNA). Matthews et al. found that G3BP-1 does not overlap with dsRNA, but rather colocalizes with viral ssRNA in perinuclear clusters (Matthews and Frey, 2012), suggesting that these may represent sites of encapsidation (Beatch and Hobman, 2000).
4. Conclusions and future directions
Despite an intensifying research focus to understand the relationships between the cytoplasmic RNPs called SG and virus replication (refer to Table 2 ), many questions remain to be answered in this growing field of virology. The roles for many SG components (Table 1) that have been found to participate in viral replication either by inclusion or exclusion still remain incompletely defined in host cell biology. As well, the literature has only touched the surface as to how viruses hijack and commandeer SG components. In several cases in which SG assembly is shown to be inhibited, it remains unclear if viruses block the assembly or induce the disassembly of SG. There is also a need to determine at what level viruses are hijacking or co-opting the host cell stress responses that exhibit SG. There is also a need to understand how SG may lead to deleterious effects if they remain present during viral infection. Indeed, further characterization of a virus’ ability to overcome the inhibition of SG assembly or induce their assembly to prevent translation of host mRNAs may be beneficial in developing new anti-viral drugs that could be useful against multiple viruses. Anti-cancer drugs such as etoposide, bortezomib and doxorubicin, do induce SG assembly, however their roles as anti-virals are not known (Arimoto et al., 2008, Fournier et al., 2010, Morita et al., 2012). The many mechanisms by which viruses inhibit or induce SG may pose a problem to developing a broad anti-viral drug targeting SG. Viruses such as PV, which inhibit SG formation through cleavage, would likely be unaffected by drugs that activate the stress response upstream of these cleaved factors. Another caveat to the potential use of these drugs is that SG formation may help the replication of certain viruses which induce SG to create a better environment for viral replication. The knowledge gained on the biology of SG and how it is influenced by viral infections will play a role in further characterizing innate responses to infection and how this system can be taken advantage of to curb viral infections.
Table 2.
SG assembly induction/inhibition by different viruses.
| Virus family | Common name | SG induction | SG blockage | Mechanism | Reference |
|---|---|---|---|---|---|
| Herpesviridae | Herpes Simplex virus-1 (HSV-1) | No | nda | Vhs interact with TTP | Esclatine et al. (2004) |
| Cytomegalovirus (HCMV) | No | Yes | Induce UPR but viral translation is maintained | Isler et al., 2005a, Isler et al., 2005b | |
| Reoviridae | Rotavirus | No | Yes | May be due by PABP is relocates from the cytoplasm to the nucleus | Montero et al. (2008) |
| Mammalian orthoreovirus (MRV) | Yesb | Yesc | Induce SG by eIF2α phosphorylation | Smith et al. (2006) | |
| Flaviviridae | West Nile virus (WNV) | No | Yes | 3′end viral genome interact with TIA-1/TIAR | Li et al. (2002) |
| Dengue virus (DV) | No | Yes | TIA-1/TIAR colocalize with replication complex | Emara and Brinton (2007) | |
| Hepatitis C Virus (HCV) | Yesb | Yesc | G3BP-1 interact with NS5B and 5′end viral genome | Yi et al. (2011) | |
| Picornaviridae | Poliovirus (PV) | Yesb | Yesc | 3Cpro cleaves G3BP-1 | White et al. (2007) |
| Theiler's murine encephalomyelitis (TMEV) | No | Yes | Leader (L) protein inhibit SG assembly | Borghese and Michiels (2011) | |
| Dicistroviridae | Cricket paralysis virus (CrPV) | No | Yes | 3Cpro is sequestered to SG | Khong and Jan (2011) |
| Togaviridae | Semliki Forest Virus (SFV) | Yesb | Yesc | Induce SG by eIF2α phosphorylation | McInerney et al. (2005) |
| Rubella virus (RUBV) | Yes | nda | Accumulation of G3BP | Matthews and Frey (2012) | |
| Coronaviridae | Mouse hepatitis coronavirus (MHV) | Yes | nda | Induce SG by eIF2α phosphorylation | Raaben et al. (2007) |
| Arenaviridae | Junin virus (JUNV) | No | Yes | N and GPC proteins block SG assembly by eIF2α phosphorylation | Linero et al. (2011) |
| Orthomyxoviridae | Influenza (IAV) | No | Yes | NS1 protein inhibit PKR | Khaperskyy et al. (2011) |
| Paramyxoviridae | Respiratory Syncitial virus (RSV) | Yes | nda | Induction PKR dependent | Lindquist et al. (2011) |
| Retroviridae | Human T cell Leukemia virus type-1 (HTLV-1) | No | Yes | Tax interact with HDAC6 | Legros et al. (2011) |
| Human immunodeficiency virus type-1 (HIV-1) | No | Yes | Staufen 1 and Gag block SG assembly | Abrahamyan et al. (2010) |
Not determined.
Showed in early stage of infection;
Showed in late stage of infection.
Acknowledgments
We thank Marie-Joelle Miron for critical reading of the manuscript. This work is supported by grants from the CIHR (MOP-38111) to A.J.M.; F.V.E. is supported by a Postdoctoral Fellowship from Becas Chile-Conicyt.
References
- Abrahamyan L.G., Chatel-Chaix L., Ajamian L., Milev M.P., Monette A., Clement J.F., Song R., Lehmann M., DesGroseillers L., Laughrea M., Boccaccio G., Mouland A.J. Novel Staufen1 ribonucleoproteins prevent formation of stress granules but favour encapsidation of HIV-1 genomic RNA. Journal of Cell Science. 2010;123(Pt 3):369–383. doi: 10.1242/jcs.055897. [DOI] [PubMed] [Google Scholar]
- Anderson P., Kedersha N. Stressful initiations. Journal of Cell Science. 2002;115(Pt 16):3227–3234. doi: 10.1242/jcs.115.16.3227. [DOI] [PubMed] [Google Scholar]
- Anderson P., Kedersha N. RNA granules. Journal of Cell Biology. 2006;172(6):803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Kedersha N. Stress granules: the Tao of RNA triage. Trends in Biochemical Sciences. 2008;33(3):141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Anderson P., Kedersha N. Stress granules. Current Biology. 2009;19(10):R397–R398. doi: 10.1016/j.cub.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Aoyagi M., Gaspar M., Shenk T.E. Human cytomegalovirus UL69 protein facilitates translation by associating with the mRNA cap-binding complex and excluding 4EBP1. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(6):2640–2645. doi: 10.1073/pnas.0914856107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto K., Fukuda H., Imajoh-Ohmi S., Saito H., Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nature Cell Biology. 2008;10(11):1324–1332. doi: 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- Ariumi Y., Kuroki M., Kushima Y., Osugi K., Hijikata M., Maki M., Ikeda M., Kato N. Hepatitis C virus hijacks P-body and stress granule components around lipid droplets. Journal of Virology. 2011;85(14):6882–6892. doi: 10.1128/JVI.02418-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez M.V., Boccaccio G.L. Mammalian Smaug is a translational repressor that forms cytoplasmic foci similar to stress granules. The Journal of Biological Chemistry. 2005;280(52):43131–43140. doi: 10.1074/jbc.M508374200. [DOI] [PubMed] [Google Scholar]
- Bashkirov V.I., Scherthan H., Solinger J.A., Buerstedde J.M., Heyer W.D. A mouse cytoplasmic exoribonuclease (mXRN1p) with preference for G4 tetraplex substrates. Journal of Cell Biology. 1997;136(4):761–773. doi: 10.1083/jcb.136.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatch M.D., Hobman T.C. Rubella virus capsid associates with host cell protein p32 and localizes to mitochondria. Journal of Virology. 2000;74(12):5569–5576. doi: 10.1128/jvi.74.12.5569-5576.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham C.J., Parker R. P bodies, stress granules, and viral life cycles. Cell Host & Microbe. 2008;3(4):206–212. doi: 10.1016/j.chom.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua A., Ceriani M.C., Capaccioli S., Nicolin A. Post-transcriptional regulation of gene expression by degradation of messenger RNAs. Journal of Cellular Physiology. 2003;195(3):356–372. doi: 10.1002/jcp.10272. [DOI] [PubMed] [Google Scholar]
- Borghese F., Michiels T. The leader protein of cardioviruses inhibits stress granule assembly. Journal of Virology. 2011;85(18):9614–9622. doi: 10.1128/JVI.00480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney S.C., Scherbik S.V., Stockman B.M., Brinton M.A. West Nile virus infections suppress early viral RNA synthesis and avoid inducing the cell stress granule response. Journal of Virology. 2012;86(7):3647–3657. doi: 10.1128/JVI.06549-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y., Kedersha N., Low W., Romo D., Gorospe M., Kaufman R., Anderson P., Liu J. Eukaryotic initiation factor 2alpha-independent pathway of stress granule induction by the natural product pateamine A. The Journal of Biological Chemistry. 2006;281(43):32870–32878. doi: 10.1074/jbc.M606149200. [DOI] [PubMed] [Google Scholar]
- Dauphin G., Zientara S., Zeller H., Murgue B. West Nile: worldwide current situation in animals and humans. Comparative Immunology, Microbiology and Infectious Diseases. 2004;27(5):343–355. doi: 10.1016/j.cimid.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Dougherty J.D., White J.P., Lloyd R.E. Poliovirus-mediated disruption of cytoplasmic processing bodies. Journal of Virology. 2011;85(1):64–75. doi: 10.1128/JVI.01657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger-Mathason T.S., Andrade J., Groehler A.L., Clark D.E., Muratore-Schroeder T.L., Pasic L., Smith J.A., Shabanowitz J., Hunt D.F., Macara I.G., Lannigan D.A. Codependent functions of RSK2 and the apoptosis-promoting factor TIA-1 in stress granule assembly and cell survival. Molecular Cell. 2008;31(5):722–736. doi: 10.1016/j.molcel.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara M.M., Brinton M.A. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(21):9041–9046. doi: 10.1073/pnas.0703348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclatine A., Taddeo B., Roizman B. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. Journal of Virology. 2004;78(16):8582–8592. doi: 10.1128/JVI.78.16.8582-8592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nature Reviews. Molecular Cell Biology. 2007;8(1):9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Finnen R.L., Pangka K.R., Banfield B.W. Herpes simplex virus type 2 infection impacts stress granule accumulation. Journal of Virology. 2012 doi: 10.1128/JVI.00313-12. JVI.00313-12; published ahead of print 23 May 2012, http://dx.doi.org/10.1128/JVI.00313-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M.-J., Gareau C., Mazroui R. The chemotherapeutic agent bortezomib induces the formation of stress granules. Cancer Cell International. 2010;10(1):12. doi: 10.1186/1475-2867-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Ebert J., Unterholzner L., Lindner D., Izaurralde E., Conti E. SMG7 is a 14-3-3-like adaptor in the nonsense-mediated mRNA decay pathway. Molecular Cell. 2005;17(4):537–547. doi: 10.1016/j.molcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Gilks N., Kedersha N., Ayodele M., Shen L., Stoecklin G., Dember L., Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Molecular biology of the cell. 2004;15(12):5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradi A., Imataka H., Svitkin Y.V., Rom E., Raught B., Morino S., Sonenberg N. A novel functional human eukaryotic translation initiation factor 4G. Molecular and Cellular Biology. 1998;18(1):334–342. doi: 10.1128/mcb.18.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A.P., Yu C., Lu L., Fujiwara Y., Browne C., Chin G., Fleming M., Leboulch P., Orkin S.H., Chen J.J. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO Journal. 2001;20(23):6909–6918. doi: 10.1093/emboj/20.23.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H.P., Zhang Y., Bertolotti A., Zeng H., Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Molecular Cell. 2000;5(5):897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Hua Y., Zhou J. Rpp20 interacts with SMN and is re-distributed into SMN granules in response to stress. Biochemical and Biophysical Research Communications. 2004;314(1):268–276. doi: 10.1016/j.bbrc.2003.12.084. [DOI] [PubMed] [Google Scholar]
- Ingelfinger D., Arndt-Jovin D.J., Luhrmann R., Achsel T. The human LSm1–7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA. 2002;8(12):1489–1501. [PMC free article] [PubMed] [Google Scholar]
- Isler J.A., Maguire T.G., Alwine J.C. Production of infectious human cytomegalovirus virions is inhibited by drugs that disrupt calcium homeostasis in the endoplasmic reticulum. Journal of Virology. 2005;79(24):15388–15397. doi: 10.1128/JVI.79.24.15388-15397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler J.A., Skalet A.H., Alwine J.C. Human cytomegalovirus infection activates and regulates the unfolded protein response. Journal of Virology. 2005;79(11):6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.Y., Wek R.C. GCN2 phosphorylation of eIF2alpha activates NF-kappaB in response to UV irradiation. Biochemical Journal. 2005;385(Pt 2):371–380. doi: 10.1042/BJ20041164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash J.C., Goodman A.G., Korth M.J., Katze M.G. Hijacking of the host-cell response and translational control during influenza virus infection. Virus Research. 2006;119(1):111–120. doi: 10.1016/j.virusres.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Kedersha N., Anderson P. Mammalian stress granules and processing bodies. Methods in Enzymology. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- Kedersha N., Chen S., Gilks N., Li W., Miller I.J., Stahl J., Anderson P. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Molecular Biology of the Cell. 2002;13(1):195–210. doi: 10.1091/mbc.01-05-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N.L., Gupta M., Li W., Miller I., Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. Journal of Cell Biology. 1999;147(7):1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N., Stoecklin G., Ayodele M., Yacono P., Lykke-Andersen J., Fritzler M.J., Scheuner D., Kaufman R.J., Golan D.E., Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. Journal of Cell Biology. 2005;169(6):871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaperskyy D.A., Hatchette T.F., McCormick C. Influenza A virus inhibits cytoplasmic stress granule formation. FASEB Journal. 2011;26(4):1629–1639. doi: 10.1096/fj.11-196915. [DOI] [PubMed] [Google Scholar]
- Khong A., Jan E. Modulation of stress granules and P bodies during dicistrovirus infection. Journal of Virology. 2011;85(4):1439–1451. doi: 10.1128/JVI.02220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Back S., Kim V., Ryu I., Jang S. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Molecular and Cellular Biology. 2005;25(6):2450–2462. doi: 10.1128/MCB.25.6.2450-2462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S.R., Horetsky R.L., Ron D., Jefferson L.S., Harding H.P. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. American Journal of Physiology: Cell Physiology. 2003;284(2):C273–C284. doi: 10.1152/ajpcell.00314.2002. [DOI] [PubMed] [Google Scholar]
- Kuyumcu-Martinez N.M., Joachims M., Lloyd R.E. Efficient cleavage of ribosome-associated poly(A)-binding protein by enterovirus 3C protease. Journal of Virology. 2002;76(5):2062–2074. doi: 10.1128/jvi.76.5.2062-2074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S., Zhang Y., Matthias P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes & Development. 2007;21(24):3381–3394. doi: 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong A.D., Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(7):1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros S., Boxus M., Gatot J.S., Van Lint C., Kruys V., Kettmann R., Twizere J.C., Dequiedt F. The HTLV-1 Tax protein inhibits formation of stress granules by interacting with histone deacetylase 6. Oncogene. 2011;30(38):4050–4062. doi: 10.1038/onc.2011.120. [DOI] [PubMed] [Google Scholar]
- Li W., Li Y., Kedersha N., Anderson P., Emara M., Swiderek K.M., Moreno G.T., Brinton M.A. Cell proteins TIA-1 and TIAR interact with the 3’ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. Journal of Virology. 2002;76(23):11989–12000. doi: 10.1128/JVI.76.23.11989-12000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist M.E., Lifland A.W., Utley T.J., Santangelo P.J., Crowe J.E., Jr. Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication. Journal of Virology. 2010;84(23):12274–12284. doi: 10.1128/JVI.00260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist M.E., Mainou B.A., Dermody T.S., Crowe J.E., Jr. Activation of protein kinase R is required for induction of stress granules by respiratory syncytial virus but dispensable for viral replication. Virology. 2011;413(1):103–110. doi: 10.1016/j.virol.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linero F.N., Thomas M.G., Boccaccio G.L., Scolaro L.A. Junin virus infection impairs stress-granule formation in Vero cells treated with arsenite via inhibition of eIF2alpha phosphorylation. Journal of General Virology. 2011;92(Pt 12):2889–2899. doi: 10.1099/vir.0.033407-0. [DOI] [PubMed] [Google Scholar]
- Liu J., Valencia-Sanchez M.A., Hannon G.J., Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nature Cell Biology. 2005;7(7):719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lastra M., Ramdohr P., Letelier A., Vallejos M., Vera-Otarola J., Valiente-Echeverria F. Translation initiation of viral mRNAs. Reviews in Medical Virology. 2010;20(3):177–195. doi: 10.1002/rmv.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschi M., Leishman C.C., Berardone N., Boccaccio G.L. Dynein and kinesin regulate stress-granule and P-body dynamics. Journal of Cell Science. 2009;122(Pt 21):3973–3982. doi: 10.1242/jcs.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J.D., Frey T.K. Analysis of subcellular G3BP redistribution during rubella virus infection. Journal of General Virology. 2012;93(Pt 2):267–274. doi: 10.1099/vir.0.036780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui R., Sukarieh R., Bordeleau M., Kaufman R., Northcote P., Tanaka J., Gallouzi I., Pelletier J. Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2alpha phosphorylation. Molecular Biology of the Cell. 2006;17(10):4212–4219. doi: 10.1091/mbc.E06-04-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney G.M., Kedersha N.L., Kaufman R.J., Anderson P., Liljestrom P. Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation. Molecular Biology of the Cell. 2005;16(8):3753–3763. doi: 10.1091/mbc.E05-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero H., Rojas M., Arias C.F., Lopez S. Rotavirus infection induces the phosphorylation of eIF2alpha but prevents the formation of stress granules. Journal of Virology. 2008;82(3):1496–1504. doi: 10.1128/JVI.01779-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero H., Trujillo-Alonso V. Stress granules in the viral replication cycle. Viruses. 2011;3(11):2328–2338. doi: 10.3390/v3112328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Satoh R., Umeda N., Kita A., Sugiura R. The stress granule protein Vgl1 and poly(A)-binding protein Pab1 are required for doxorubicin resistance in the fission yeast Schizosaccharomyces pombe. Biochemical and Biophysical Research Communications. 2012;417(1):399–403. doi: 10.1016/j.bbrc.2011.11.127. [DOI] [PubMed] [Google Scholar]
- Nover L., Scharf K.D., Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Molecular and Cellular Biology. 1983;3(9):1648–1655. doi: 10.1128/mcb.3.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page H.G., Read G.S. The virion host shutoff endonuclease (UL41) of herpes simplex virus interacts with the cellular cap-binding complex eIF4F. Journal of Virology. 2010;84(13):6886–6890. doi: 10.1128/JVI.00166-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V.R., Sonenberg N. Translational efficiency of poliovirus mRNA: mapping inhibitory cis-acting elements within the 5′ noncoding region. Journal of Virology. 1988;62(7):2219–2227. doi: 10.1128/jvi.62.7.2219-2227.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska J., Hansen S.J., Park N., Jamka K., Sarnow P., Gustin K.E. Stable formation of compositionally unique stress granules in virus-infected cells. Journal of Virology. 2010;84(7):3654–3665. doi: 10.1128/JVI.01320-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piron M., Vende P., Cohen J., Poncet D. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO Journal. 1998;17(19):5811–5821. doi: 10.1093/emboj/17.19.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q., Carroll K., Hastings C., Miller C.L. Mammalian orthoreovirus escape from host translational shutoff correlates with stress granule disruption and is independent of eIF2{alpha} phosphorylation and PKR. Journal of Virology. 2011;85(17):8798–8810. doi: 10.1128/JVI.01831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q., Hastings C., Miller C.L. Mammalian orthoreovirus particles induce and are recruited into stress granules at early times postinfection. Journal of Virology. 2009;83(21):11090–11101. doi: 10.1128/JVI.01239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaben M., Groot Koerkamp M.J., Rottier P.J., de Haan C.A. Mouse hepatitis coronavirus replication induces host translational shutoff and mRNA decay, with concomitant formation of stress granules and processing bodies. Cellular Microbiology. 2007;9(9):2218–2229. doi: 10.1111/j.1462-5822.2007.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J., Behm-Ansmant I., Gatfield D., Izaurralde E. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA. 2005;11(11):1640–1647. doi: 10.1261/rna.2191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.A., Schmechel S.C., Raghavan A., Abelson M., Reilly C., Katze M.G., Kaufman R.J., Bohjanen P.R., Schiff L.A. Reovirus induces and benefits from an integrated cellular stress response. Journal of Virology. 2006;80(4):2019–2033. doi: 10.1128/JVI.80.4.2019-2033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklin G., Stubbs T., Kedersha N., Wax S., Rigby W.F., Blackwell T.K., Anderson P. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO Journal. 2004;23(6):1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talloczy Z., Jiang W., Virgin H.W., 4th, Leib D.A., Scheuner D., Kaufman R.J., Eskelinen E.L., Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proceedings of the National Academy of Sciences of the United States of America U S A. 2002;99(1):190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.G., Loschi M., Desbats M.A., Boccaccio G.L. RNA granules: the good, the bad and the ugly. Cellular Signalling. 2011;23(2):324–334. doi: 10.1016/j.cellsig.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.G., Martinez Tosar L.J., Desbats M.A., Leishman C.C., Boccaccio G.L. Mammalian Staufen 1 is recruited to stress granules and impairs their assembly. Journal of Cell Science. 2009;122(Pt 4):563–573. doi: 10.1242/jcs.038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourriere H., Chebli K., Zekri L., Courselaud B., Blanchard J.M., Bertrand E., Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. Journal of Cell Biology. 2003;160(6):823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tsai N.P., Wei L.N. RhoA/ROCK1 signaling regulates stress granule formation and apoptosis. Cellular Signalling. 2010;22(4):668–675. doi: 10.1016/j.cellsig.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner L., Izaurralde E. SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Molecular Cell. 2004;16(4):587–596. doi: 10.1016/j.molcel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- van Dijk E., Cougot N., Meyer S., Babajko S., Wahle E., Seraphin B. Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO Journal. 2002;21(24):6915–6924. doi: 10.1093/emboj/cdf678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Mohr I. Viral subversion of the host protein synthesis machinery. Nature Reviews. Microbiology. 2011;9(12):860–875. doi: 10.1038/nrmicro2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.P., Cardenas A.M., Marissen W.E., Lloyd R.E. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host & Microbe. 2007;2(5):295–305. doi: 10.1016/j.chom.2007.08.006. [DOI] [PubMed] [Google Scholar]
- White J.P., Lloyd R.E. Poliovirus unlinks TIA1 aggregation and mRNA stress granule formation. Journal of Virology. 2011;85(23):12442–12454. doi: 10.1128/JVI.05888-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.P., Lloyd R.E. Regulation of stress granules in virus systems. Trends in Microbiology. 2012 doi: 10.1016/j.tim.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.E., Powell M.J., Hoover S.E., Sarnow P. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Molecular and Cellular Biology. 2000;20(14):4990–4999. doi: 10.1128/mcb.20.14.4990-4999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.R. Signal integration via PKR. Science's STKE. 2001;2001(89):re2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- Yi Z., Pan T., Wu X., Song W., Wang S., Xu Y., Rice C.M., Macdonald M.R., Yuan Z. Hepatitis C virus co-opts Ras-GTPase-activating protein-binding protein 1 for its genome replication. Journal of Virology. 2011;85(14):6996–7004. doi: 10.1128/JVI.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


