Highlights
-
•
PRRSV-nsp1〈alpha〉 degrades CREB-binding protein and suppresses IFN production.
-
•
PRRSV-nsp1〈beta〉 degrades karyopherin-α1 and blocks nuclear import of ISGF3.
-
•
LDV-nsp1〈alpha〉 and SHFV-nsp1〈gamma〉 also degrade CREB-biding protein in the nucleus.
-
•
All subunits of arterivirus nsp1 are nuclear proteins and have capacities of IFN suppression.
Keywords: Arterivirus, nsp1, PRRSV, Innate immunity, Interferon signaling, CREB-binding protein
Abstract
Arteriviruses infect immune cells and may cause persistence in infected hosts. Inefficient induction of pro-inflammatory cytokines and type I IFNs are observed during infection of this group of viruses, suggesting that they may have evolved to escape the host immune surveillance for efficient survival. Recent studies have identified viral proteins regulating the innate immune signaling, and among these, nsp1 (nonstructural protein 1) is the most potent IFN antagonist. For porcine reproductive and respiratory syndrome virus (PRRSV), individual subunits (nsp1α and nsp1β) of nsp1 suppress type I IFN production. In particular, PRRSV-nsp1α degrades CREB (cyclic AMP responsive element binding)-binding protein (CBP), a key component of the IFN enhanceosome, whereas PRRSV-nsp1β degrades karyopherin-α1 which is known to mediate the nuclear import of ISGF3 (interferon-stimulated gene factor 3). All individual subunits of nsp1 of PRRSV, equine arteritis virus (EAV), lactate dehydrogenase-elevating virus (LDV), and simian hemorrhagic fever virus (SHFV) appear to contain IFN suppressive activities. As with PRRSV-nsp1α, CBP degradation is evident by LDV-nsp1α and partly by SHFV-nsp1γ. This review summarizes the biogenesis and the role of individual subunits of nsp1 of arteriviruses for innate immune modulation.
1. Introduction
The family Arteriviridae is grouped in the order Nidovirales together with Coronaviridae, Roniviridae, and Mesoniviridae (Nga et al., 2011, Zirkel et al., 2011). The family Arteriviridae consists of equine arteritis virus (EAV), porcine reproductive and respiratory syndrome virus (PRRSV), lactate dehydrogenase-elevating virus (LDV) of mice, and simian hemorrhagic fever virus (SHFV). The wobbly possum disease virus (WPDV) has recently been discovered to infect Australian brush-tail possums and is believed to be most closely related to the current members of the family Arteriviridae (Dunowska et al., 2012), and three additional SHFV isolates have been identified in Africa that appear to be distantly related to SHFV of Southeast Asia (Lauck et al., 2013). The genome of arteriviruses is a single-stranded positive-sense RNA, and its coding strategy is relatively conserved but its length varies between 12.7 and 15.7 kb (Snijder et al., 2013). Two large overlapping ORFs occupy the 5′ three-quarters of the genome and generate pp1a and pp1ab two polyproteins by the mechanism of −1 frame-shifting translation (den Boon et al., 1991). These polyproteins are self-cleaved to 13 (for EAV) or 14 (for PRRSV and LDV) cleavage products by papain-like proteinases (PLPs) of the nonstructural protein (nsp) 1 and nsp2, and by serine protease reside in nsp4 (Snijder and Meulenberg, 1998, Snijder et al., 1993, Snijder et al., 1994, Snijder et al., 2013). The structural genes are located in the 3′one-quarter of the genome; ORF2a, ORF2b, ORFs 3 through 7, and ORF5a overlapping with ORF5, coding for GP2, E, GP3, GP4, GP5, M, N, and ORF5a proteins, respectively (Firth et al., 2011, Johnson et al., 2011, Meulenberg et al., 1993, Snijder et al., 1999). The SHFV genome contains a duplication of the ORFs 2a, 2b, 3, and 4 downstream of ORF1b, and this gene duplication has been confirmed in the recent African isolates of SHFV (Lauck et al., 2011, Lauck et al., 2013). While pp1a and pp1ab are directly translated from the viral genome, structural genes require the synthesis of subgenomic (sg) mRNAs (Sawicki et al., 2007). A −2 ribosomal frame-shifting translation mechanism has recently been identified in nsp2 for expression of nsp2TF for PRRSV, LDV, and SHFV, but it is absent in EAV (Fang et al., 2012b).
During infection of PRRSV, poor induction of pro-inflammatory cytokines and type I IFNs are observed, and PRRSV seems to have a capacity to escape the immune surveillance for survival. At least six viral proteins have been identified as IFN antagonists regulating the innate immune signaling during infection: nsp1α, nsp1β, nsp2, nsp4, nsp11, and N proteins. Among these proteins, nsp1α and nsp1β are two most potent IFN antagonists (Beura et al., 2010, Chen et al., 2010, Kim et al., 2010, Song et al., 2010, Sun et al., 2012a, Yoo et al., 2010). nsp1 is cleaved into two subunits of nsp1α and nsp1β, and both subunits suppress the type I IFN induction. In order for IFN gene expression, enhanceosome has to be first assembled, and CREB (cyclic AMP responsive element binding)-binding protein (CBP) is a key component of the IFN enhanceosome. CBP has been found to be degraded in the presence of PRRSV-nsp1α (Han et al., 2013), which is likely the basis for IFN suppression by nsp1α. As with PRRSV-nsp1, EAV-nsp1 has recently been found to suppress IFN response (Go et al., 2014), leading us to expand our study to nsp1 of other arteriviruses. We cloned individual subunit genes of nsp1 from PRRSV, EAV, LDV, and SHFV, and examined their IFN modulatory activities. CBP degradation was evident for LDV-nsp1α and to some extent for SHFV-nsp1γ. The current report describes the innate immune signaling modulated by arteriviruses with a particular focus on the function of nsp1.
2. Immune modulation by arteriviruses
The host range of arteriviruses is narrow and their infection is restricted to suids, mice, equids, and non-human primates for PRRSV, LDV, EAV, and SHFV, respectively. Macrophages appear to be primary target cell for their infection (Snijder and Meulenberg, 1998). Arterivirus infection may cause persistence in infected animals. PRRSV may persist up to 6 months in pigs and EAV may persist for life-long in horses. LDV infection is typically asymptomatic and the virus persists for life-long in infected mice (Anderson et al., 1995, Plagemann et al., 1995). For SHFV, fatal hemorrhagic fever occurs in macaques, but asymptomatic persistent infection is observed in baboons (Vatter and Brinton, 2014). PRRSV infection is characterized by poor induction of proinflammatory cytokines and type I interferons (IFN-α/β). Arteriviruses seem to have developed an ability to manipulate a variety of host cell processes related to the innate immunity to facilitate the survival in infected host.
2.1. Arterivirus-mediated type I interferon modulation
The type I IFN system is a key component of the innate immunity and represents a first line of defense against viral infection (Samuel, 2001). PRRSV is sensitive to anti-viral effects of type I IFNs, and the treatment with porcine IFN-α impedes the growth of PRRSV significantly (Albina et al., 1998, Buddaert et al., 1998). The sensitivity of PRRSV to IFN-α varies and dose-dependent (Lee et al., 2004). LDV is resistant to anti-viral effects of IFN-α in mice, which is probably a reason for its life-long persistence (Ammann et al., 2009). Arteriviruses do not trigger effective innate immune responses upon infection, and it may explain the weak adaptive response and viral persistence. Unlike other respiratory viruses such as swine influenza and porcine respiratory coronavirus, whose infections induce high concentrations of IFN-α, PRRS causes minimal expression of IFN-α which is a hallmark for PRRSV infection in cells and pigs (Van Reeth et al., 1999), even though considerable variations are observed for different isolates (Lee et al., 2004, Miller et al., 2004, Nan et al., 2012). Dendritic cells (DCs) play an important role in anti-viral immunity by providing early innate protection against viral replication and by presenting antigens to T cells for initiation of the adaptive immune response (Clark et al., 2000), and thus IFN response in DCs by PRRSV has been studied. Monocyte-derived DCs are susceptible for PRRSV, and the expression of IFN-α/β mRNA is elevated in time-dependent and transient manners. However, a little or no detectable levels of IFNs are found in the supernatants and cell lysates (Loving et al., 2007, Zhang et al., 2012). IFN production is observed in plasmacytoid dendritic cells (pDCs) after infection (Baumann et al., 2013). pDC is the primary cell type that produces IFN-α during LDV infection (Ammann et al., 2009). For EAV infection, poor induction of IFN is seen in pulmonary endothelial cells (Go et al., 2014). For SHFV, IFN response is host cell-dependent. Up-regulation of IFN-β production is detected in myeloid dendritic cells (mDCs) and macrophages in macaques, but a low and no detectable levels of IFN-β response is seen in macrophages and mDCs of baboons, respectively (Vatter and Brinton, 2014).
The suppression of IFN-α is observed in PRRSV-infected macrophages (Albina et al., 1998, Lee et al., 2004), and the suppression occurs likely at the post-transcriptional level since IFN-α mRNA is increased after stimulation (Lee et al., 2004, Miller et al., 2009). On the contrary, a decrease of IFN transcripts is observed in PRRSV-infected MARC-145 cells (Miller et al., 2004). Plasmacytoid DCs (pDCs) are characterized by rapid and mass production of type I IFNs upon infection (Mildner and Jung, 2014), and porcine pDCs appear to be non-susceptible for PRRSV. In these cells, IFN production is blocked in the presence of TLR agonists when incubated with PRRSV, and the inhibitory effect is not altered by UV-inactivated PRRSV, suggesting the inhibition likely occurs on the cell surface (Calzada-Nova et al., 2011). IRF7 is not up-regulated by PRRSV in pDCs compared to other TLR (toll-like receptor) agonists. Another study shows no inhibition of IFN-α in porcine pDCs by PRRSV (Baumann et al., 2013). In that study, both genotypes of PRRSV were used, and all test isolates of both genotypes induced IFN-α production in pDCs by both infectious and non-infectious PRRSV (Baumann et al., 2013). It is possible that PRRSV prevents the paracrine loop of IFN induction and shuts off the IFN-boosted IRF7 expression while still inducing IFN production during the early stage of infection. Besides PRRSV, EAV has also recently been reported to inhibit IFN production with decreased IFN-β transcripts in EAV-infected equine pulmonary artery endothelial cells (Go et al., 2014).
2.2. Regulation of IFN signaling pathway
Production of type I IFNs is an important component of the host innate immunity against viral infection, and activation of IFN cascade starts from recognition of viral components by two distinct pattern recognition receptors (PRRs); TLRs and retinoic acid inducible gene I (RIG-I)-like receptors (RLRs) including melanoma differentiation associated protein 5 (MDA5) and RIG-I. TLR3, TLR7/8, and TLR9 function at either cell surface or endosomal membranes and are involved in antiviral response (Baccala et al., 2007). When pathogen-associated molecular patterns (PAMPs) are sensed by PRRs, signal transduction is turned on to activate IFN regulatory factor 3 (IRF3) and activating protein (AP)-1, and release of NF-κB from its inhibitor IκB (Baccala et al., 2007). Activated AP-1, IRF3 and NF-κB are translocated to the nucleus and bind their positive regulatory domains (PRDs) within the IFN promoter region and instigate the production of IFNs by recruiting CREB (cyclic AMP responsive element binding)-binding protein (CBP) to form an enhanceosome complex for IFN transcription (Randall and Goodbourn, 2008). Impaired production of type I IFNs has been observed during infection of arteriviruses.
Minimal IFN production is noticeable under arterivirus infection, but PRRs still senses invading arteriviruses. An increased activation of IFN-β promoter has been noticed in PRRSV-infected PAMs and MARC-145 cells, and TLR3 seems to be involved (Miller et al., 2004, Miller et al., 2009, Shi et al., 2010). TLR7 is also thought to be essential for IFN production in LDV- and PRRSV-stimulated IFN response in pDCs (Ammann et al., 2009, Baumann et al., 2013). A study using EAV in specific gene-knockout mouse embryonic fibroblasts suggests that both MDA-5 and RIG-I play a role in counteracting viral infection (van Kasteren et al., 2013). In PRRSV-infected PAMs and DCs, expression of TLR3 and TLR7 is inhibited at early infection but restored later (Chaung et al., 2010). The TLR3 and TLR7 expressions are delayed in PRRSV-infected tracheobronchial lymph nodes (Liu et al., 2009), but no difference is observed in PAMs among different isolates of PRRSV for transcription of TLR3, TLR7, and TLR9 (Kuzemtseva et al., 2014).
To investigate the modulation of IFN signaling by arteriviruses, poly(I:C) as a dsRNA analog or Sendai virus (SeV) has been used for IFN activation. The dsRNA analog induced full activation of IRF3 in MARC-145 cells. When these cells were infected with PRRSV, only a partial activation was seen for Toll/IL-1R (TIR) domain-containing adaptor inducing IFN-β (TRIF) which is an adaptor molecule of TLR3 (Luo et al., 2008). For PRRSV-mediated IFN suppression, the suppression of NF-κB activity seems to be involved during early in infections, even though PRRSV activates NF-κB later in infection (Lee and Kleiboeker, 2005, Song et al., 2013, Sun et al., 2010). Reduction of CBP is observed during infection of PRRSV, and this inhibits the formation of enhanceosome to result in transcription inhibition of IFN expression (Kim et al., 2010). PRRSV also inhibits the JAK-STAT signaling pathway. In PRRSV-infected cells, nuclear translocation of IFN-stimulated gene factor 3 (ISGF3) is blocked (Patel et al., 2010), and this is due to the disruption of nuclear pores by nsp1β. The PRRSV function to inhibit IFN induction seems to be redundant by different viral proteins including nsp1α, nsp1β, nsp2, nsp4, nsp11, and N proteins (Table 1 ). nsp1 and nsp2 of LDV and SHFV also possess IFN suppressive activities. It seems that arteriviruses tend to employ a combination of modulatory function for innate immunity.
Table 1.
Arterivirus proteins modulating innate immune signaling.
| Virus | Protein | Modulatory function for innate immunity | Reference |
|---|---|---|---|
| PRRSV | nsp1α | Inhibits production of type I IFNs and impairs IFN promoter activity | Chen et al. (2010) |
| Suppresses NF-κB activation | Song et al. (2010), Subramaniam et al. (2010) | ||
| Induces CBP degradation | Han et al. (2013), Kim et al. (2010) | ||
| Suppresses TNF-α promoter activity | Subramaniam et al. (2010) | ||
| nsp1β | Inhibits production of type I IFNs and impairs IFN promoter activity | Chen et al. (2010) | |
| Impairs IRF3 phosphorylation and IRF3 nuclear localization | Beura et al. (2010) | ||
| Interferes with IFN-α induction and ISG expression | Patel et al. (2010) | ||
| Blocks nuclear translocation of ISGF3 by inducing KPNA1 degradation | Wang et al. (2013) | ||
| Suppresses TNF-α promoter activity | Subramaniam et al. (2010) | ||
| nsp2 (PLP2) | Antagonizes type I interferon induction Interferes with NF-κB signaling pathway Prevents IκBα degradation by OTU domain |
Sun et al. (2010) | |
| Inhibits ISG15 production and ISGylation | Sun et al. (2012b) | ||
| Activates NF-κB | Fang et al. (2012a) | ||
| nsp4 | Inhibits IFN-β promoter activity Suppresses NF-κB mediated signaling pathway in the nucleus |
Chen et al. (2014) | |
| nsp11 | Impair IFN promoter activity Participates in suppression of RIG-I signaling Degrades mRNA of IPS-1 |
Sun et al. (2012a) | |
| N | Inhibits production of type I IFNs and impairs IFN promoter activity Impairs IRF3 phosphorylation and IRF3 nuclear localization |
Sagong and Lee (2011) | |
| Upregulates IL-10 gene expression | Wongyanin et al. (2012) | ||
| Activates NF-κB | Luo et al. (2011), Pujhari et al. (2014) | ||
| EAV | nsp1 | Inhibits production of type I IFNs and impairs IFN promoter activity | Han et al. (2014), Go et al. (2014) |
| nsp2 (PLP2) | Inhibits RIG-I-mediated innate immune signaling inhibits RIG-I ubiquitination by its DUB activity |
van Kasteren et al., 2012, van Kasteren et al., 2013 | |
| LDV | nsp1α | Inhibits production of type I IFNs and impairs IFN promoter activity Induces CBP degradation |
Han et al. (2014) |
| nsp1β | Inhibits production of type I IFNs and impairs IFN promoter activity | Han et al. (2014) | |
| SHFV | nsp1αβ | Inhibits production of type I IFNs and impairs IFN promoter activity | Han et al. (2014) |
| nsp1γ | Inhibits production of type I IFNs and impairs IFN promoter activity | Han et al. (2014) | |
2.3. Regulation of other cytokines by PRRSV
While type I IFN is suppressed by PRRSV, other cytokines such as TNF-α, IL-6, IL-8, and IL-10 are stimulated (Darwich et al., 2010). Association of three serum cytokines (IL-8, IL-1β, IFN-γ) is significantly correlated with PRRSV persistence (Lunney et al., 2010). For EAV, virulent and avirulent strains differed in the induction of TNF-α, IL-1β, IL-6, and IL-8 (Moore et al., 2003). For SHFV, pro-inflammatory cytokines including IL-1β, IL-6, IL-12/23(p40), and TNF-α are efficiently induced in macaques but not in baboons, which is consistent with the scenario of IFN production (Vatter and Brinton, 2014). TNF-α is produced mainly by monocytes and macrophages. It is a pleiotropic cytokine important for induction and regulation of inflammatory responses (Hawiger, 2001). TNF-α inhibits PRRSV replication in PAMs (Ait-Ali et al., 2007, Lopez-Fuertes et al., 2000), and thus induction of TNF-α by PRRSV is controversial. An early study showed impaired production of TNF-α in PRRSV-infected PAMs, and this was consistent with the findings in bronchoalveolar fluids after infection (Thanawongnuwech et al., 2001, Van Reeth et al., 1999). However, TNF-α is still detectable in PAMs, peripheral blood mononuclear cells (PBMCs), and bronchoalveolar cells (Aasted et al., 2002, Ait-Ali et al., 2007, Johnsen et al., 2002), along with the virus in the lesions of lungs, lymph nodes, and serum of infected pigs (Choi et al., 2002, Miguel et al., 2010, Rowland et al., 2001). Different breeds and ages of pigs also lead to differential expression of TNF-α (Ait-Ali et al., 2007, Johnsen et al., 2002). In a study using 39 different isolates of PRRSV, various patterns of TNF-α expression are observed (Gimeno et al., 2011). Another study also shows that TNF-α expression was strain-dependent; a lower level expression of TNF-α by highly pathogenic (HP)-PRRSV compared to the conventional strains of PRRSV (Hou et al., 2012). TNF-α induction has been linked to activation of the ERK (extracellular signal-regulated kinase), MAPK (p38 mitogen-activated protein kinase), or NF-κB pathway (Mathur et al., 2004, Saccani et al., 2002), and PRRSV has been shown to induce a robust but transient activation of ERK (Lee and Lee, 2010). A later study shows that the ERK pathway, rather than the p38-MAPK and NF-κB pathways, is associated with differential expression of TNF-α in macrophages. HP-PRRSV suppresses the release of TNF-α by inactivating the ERK pathway (Hou et al., 2012). This may explain the reduction of TNF-α by PRRSV (Lopez-Fuertes et al., 2000).
IL-10 is a pleiotropic cytokine with a potent immune-suppressive function (Conti et al., 2003, Darwich et al., 2010), and thus its expression during PRRSV infection has been studied (Thanawongnuwech et al., 2001, Wang et al., 2007). No response or minimal response is reported in some studies but a significant up-regulation of IL-10 expression is also reported in other studies. The up-regulation of IL-10 is observed in PBMCs, bronchoalveolar cells, and tissues including lungs and lymph nodes in PRRSV-infected pigs of different ages (Feng et al., 2003, Johnsen et al., 2002, Rowland et al., 2001, Suradhat et al., 2003). A significant increase of IL-10 is also observed in bone marrow-derived immature DCs (BM-imDCs) of PRRSV-infected pigs (Chang et al., 2008) and in PRRSV-infected monocyte derived mature DCs isolated from pigs (Flores-Mendoza et al., 2008). In PAMs, the induction of IL10 is time-dependent and dose-dependent (Genini et al., 2008, Song et al., 2013). During PRRSV infection, stress-activated protein kinases (SAPKs) including p38 MAPK and c-Jun N-terminal kinases (JNK) are activated, probably through a post-entry process leading to activation of transcription factors such as activator protein-1 (AP-1) (Lee and Lee, 2012). A later study confirms that the p38 MAPK and NF-κB pathways are responsible for IL-10 up-regulation in PAMs (Song et al., 2013). For NF-κB activation, MyD88 is essential and thus the TLR-MyD88-NF-κB signaling cascade is speculated to be involved in PRRSV-induced IL-10 expression. The N (nucleocapsid) protein is able to trigger NF-κB activation and has been demonstrated to up-regulate the IL-10 expression in PAMs, and thus N-mediated IL-10 induction may rely on NF-κB activation (Luo et al., 2011, Wongyanin et al., 2012). Indeed, NF-κB activation by the N protein has been demonstrated (Luo et al., 2011, Fu et al., 2012, Pujhari et al., 2014).
3. nsp1 proteins of arteriviruses
3.1. Multifunctional nature of PLPs
nsp1 is the first viral protein synthesized during infection of arteriviruses and it functions in viral genome replication. Important domains have been identified for PRRSV-nsp1, which includes papain-like proteinase (PLP), two zinc finger (ZF) motifs, and a nuclease motif (Fang and Snijder, 2010, Sun et al., 2009, Xue et al., 2010). The PLP-like domain is found in the N-terminal region of viral polyproteins of many other positive-sense RNA viruses including picornaviruses, coronaviruses, arteriviruses, and pestiviruses, The PLP activity is essential for polyprotein processing (Chen et al., 1996, den Boon et al., 1995, Gorbalenya et al., 1989, Gorbalenya et al., 1991, Guarne et al., 2000, Harcourt et al., 2004, Karpe and Lole, 2011, Lim et al., 2000, Mielech et al., 2014a, Snijder et al., 1994). The PLP motifs exist in nsp1 of all arteriviruses and are responsible for cleaving nsp1 off from pp1a and pp1ab, and also for internal cleavage of nsp1 to generate nsp1α and nsp1β in PRRSV and LDV. Depending on the number of PLP motifs in nsp1, PLP is designated as PLP1α, PLP1β, or PLP1γ (Fig. 1 ). Correct processing of nsp1 by PLP1 is essential for viral genomic RNA and mRNA syntheses, and an impaired activity of PLP is lethal for PRRSV replication (Kroese et al., 2008). Unlike the leader proteinase (Lpro) of foot-and-mouth disease virus (FMDV), which cleaves the host cell eukaryotic initiation factor 4G (eIF4G) as well as itself from the nascent viral polypeptide, PLPs in arteriviruses hardly maintain their protease activities after self-cleavage (Guarne et al., 1998, Sun et al., 2009, Xue et al., 2010). The structural studies for PRRSV-nsp1α and PRRSV-nsp1β indicate stable interactions between the C-terminal extension (CTE) and the PLP1α and PLP1β domains, hence further proteolytic processing is hardly conducted due to the inhibition of access to other potential substrates (Sun et al., 2009, Xue et al., 2010). In comparison with FMDV Lpro, a large interaction surface is observed for PRRSV-PLP1α and CTE, which enables to stabilize the intramolecular complex in PRRSV-nsp1α (Steinberger et al., 2013).
Fig. 1.
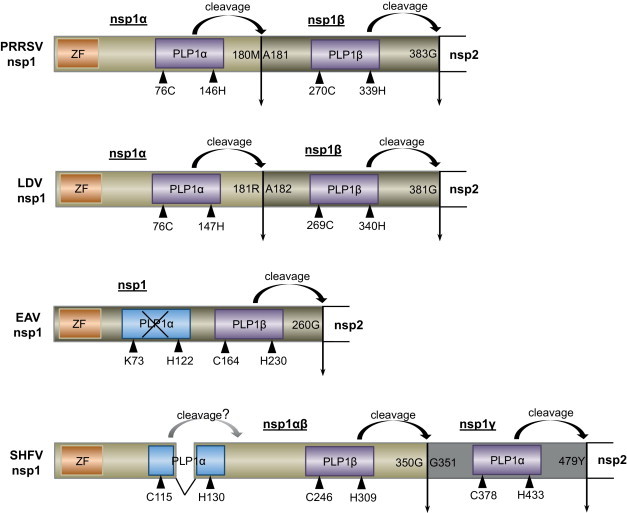
Schematic presentation of nsp1 of arteriviruses. nsp1 protein of PRRSV, LDV, EAV, and SHFV is constituted of 384, 381, 260, and 479 amino acids, respectively. PRRSV-nsp1 is cleaved into PRRSV-nsp1α and PRRSV-nsp1β, and similarly, LDV-nsp1 is cleaved into LDV-nsp1α and LDV-nsp1β. For SHFV, two subunits of SHFV-nsp1αβ and SHFV-nsp1γ are produced from SHFV-nsp1. EAV-nsp1 remains uncleaved. Biologically active PLP domains are indicated in yellow and inactive PLP domains in EAV is shown in blue with crossing-outs. A question mark is labeled for SHFV PLP1α due to its unclear function. Catalytic residues for PLPs are indicated by blue triangles. Vertical arrows represent PLP-mediated cleavage sites (Han et al., 2014). Numbers indicate amino acid positions. Cleavage site 180M/181A is for the internal cleavage of type II PRRSV nsp1. Sequences from PRRSV PA8 strain (GenBank accession no. AF176348), LDV Plagemann strain (GenBank accession no. U15146.1), EAV Bucyrus strain (GenBank accession no. DQ846750), and SHFV (GenBank accession no. AF180391) are used to make the figure.
Besides acting as a protease, PLP2 in nsp2 functions as a deubiquitinating (DUB) enzyme which removes ubiquitin modification from a cellular target. The DUB activity is identified in the PLP of coronaviruses and the DUB activity overlaps with a de-ISGylating activity in some of these protease domains (Chen et al., 2007, Clementz et al., 2010, Lindner et al., 2005, Mielech et al., 2014b, Wojdyla et al., 2010, Xing et al., 2013, Zheng et al., 2008). For PRRSV and EAV, DUB and de-ISGylating activities are identified in the PLP2 domain, and these activities are essential for viral modulation on innate immunity (Frias-Staheli et al., 2007, Sun et al., 2010, Sun et al., 2012b, van Kasteren et al., 2013, van Kasteren et al., 2012). There is no report about the association of PLP1 with the DUB and de-ISGylating activities.
Computer-based sequence alignments of nsp1 from different arteriviruses reveal a conserved zinc finger (ZF) motif in the N-terminal region, and the crystal structure of PRRSV-nsp1α suggests that the topology of the N-terminal ZF domain is generally similar to that of the ββα ZF family of over 1000 known transcription factors (Sun et al., 2009, Tijms et al., 2001). The zinc ion coordinating the C-terminal zinc motif is identified in PRRSV-nsp1α but the biological function of this motif is unknown. Mutations in the N-terminal zinc finger domain of nsp1α selectively abolish the viral transcription whereas the genome replication is not affected (Tijms et al., 2001, Tijms et al., 2007). For PRRSV-nsp1β, no ZF is found according to the crystal structure. Instead, a nuclease activity is identified to degrade either double-stranded (ds) DNA or ssRNA (Xue et al., 2010). Recent studies show the involvement of arterivirus nsp1 in viral gene transcription and translation (Li et al., 2014, Nedialkova et al., 2010). EAV-nsp1 involves in controlling the accumulation of genome-length and subgenome-length minus-strand RNA for mRNA synthesis (Nedialkova et al., 2010), whereas PRRSV-nsp1β functions as a transactivator to induce −1/−2 frameshifting in nsp2 region to produce nsp2TF and nsp2N (Li et al., 2014).
3.2. Biogenesis of nsp1 subunits in arteriviruses
For EAV, the PLP motif in the N-terminal region of nsp1 was initially predicted to somewhere between amino acid positions 158 and 178 (den Boon et al., 1991). Later by in vitro translation and mutagenesis studies, the PLP motif was confirmed to mediate a self-cleavage of nsp1 from nsp2 (Snijder et al., 1993). Cys164 and His230 of the EAV polyprotein are catalytic residues for PLP, and Gly260↓Gly261 was determined as the cleavage site (Snijder et al., 1993). Unlike EAV-nsp1, two adjacent PLP domains, PLP1α and PLP1β, are found in nsp1 of PRRSV and LDV. PLP1α mediates the internal cleavage of nsp1 to release nsp1α, and PLP1β mediates the cleavage between nsp1β and nsp2 to release nsp1β. Their catalytic sites are predicted to Cys76 and His146 for PRRSV-PLP1α, and Cys76 and His147 for LDV-LP1α (Fig. 1) (den Boon et al., 1995). The presence of two PLP motifs may reflect an ancient duplication during viral evolution (Snijder et al., 2013). The cleavage between PRRSV-nsp1α and PRRSV-nsp1β was initially predicted to occur near residues 164–168 (den Boon et al., 1995), and later shown to be Gln166↓Arg167 based on the prediction from sequence alignments (Allende et al., 1999). Recent studies by X-ray crystallography (Sun et al., 2009) and mass spectrometric analysis (Chen et al., 2010) have corrected the cleavage site to Met180↓Ala181, and this cleavage site of Met180/Ala181 is conserved in type II PRRSV. The corresponding cleavage site of H180/S181 in type I PRRSV still needs to be confirmed experimentally. The cleavage site of Gly203↓Ala204 between nsp1β and nsp2 was also determined by protein sequencing (Xue et al., 2010).
For LDV, cleavage sites and PLP domains in nsp1 were predicted on the basis of sequence alignments with other arteriviruses. In our study, a consensus sequence CPFxxAxAT(N)V was identified in the adjacent region between nsp1α and nsp1β of both PRRSV and LDV, and the cleavage by LDV-PLP1α was predicted to Arg181↓Ala182. The sequence alignments also suggest that the cleavage for nsp1 and nsp2 locates at Gly381↓Tyr382 according to comparisons with PRRSV and EAV sequences (Han et al., 2014).
The structural characteristic of SHFV-nsp1 is rather complicated. SHFV-nsp1 contains an array of three potential PLP domains tentatively designated as PLP1α, PLP1β, and PLP1γ, and each domain is presumed to generate nsp1α, nsp1β, and nsp1γ, respectively (Fig. 1; Snijder et al., 2013). SHFV-PLP1α contains Cys115 and His130 and these residues constitute a putative PLP1α by comparisons with PRRSV, LDV, and the remnants of inactive PLP1α in EAV. Interestingly, the SHFV-PLP1β sequence is rather similar to that of SHFV-PLP1γ, suggesting the evolutionary gene duplication.
Both SHFV-PLP1β and SHFV-PLP1γ sequences are well aligned with the sequences of PRRSV-PLP1β, LDV-PLP1β, and EAV-PLP1. For cleavage of SHFV-nsp1, a 39 kDa band is identified in SHFV-nsp1 gene-transfected cells. This band is much larger than the prediction of SHFV-nsp1α and is rather similar to the sum of nsp1α and nsp1β. It suggests that SHFV-PLP1α may be non-functional. To verify this premise, a set of deletion constructs for nsp1 were made using a tag at the N-terminus of each construct. SHFV-PLP1α appears to be inactive, whereas SHFV-PLP1β is functional and cleaves off nsp1β, thus producing nsp1a and nsp1β as a single protein (Han et al., 2014). We have termed this protein SHFV-nsp1αβ. SHFV-PLP1γ appears to be normally functional and cleaves off nsp1γ from nsp2. Compared to PRRSV, discontiguous deletions of 55 amino acids between two catalytic residues of Cys115 and His130 are noticeable in the SHFV-nsp1α region. These deletions in the relatively conserved region of nsp1α may likely contribute to the functional impairment of SHFV-PLP1α. Another study shows that PLP1α is predicted to constitute Cys63 and His130, and is functional to cleave off nsp1α at Gly164↓Gly165 (Vatter et al., 2014). This study was conducted in the cell-free translation system, and the reason for difference remains to be clarified. PLP1γ is functional and generates SHFV-nsp1γ by cleaving at either Tyr479↓Gly480 or Gly484↓Gly485 depending on the experimental system (Han et al., 2014, Vatter et al., 2014). The cleavage between SHFV-nsp1β and SHFV-nsp1γ mediated by SHFV-PLP1β occurs at Gly350↓Gly351 (Han et al., 2014).
3.3. Subcellular localization of individual nsp1 subunits in arteriviruses
Cellular localization of nsp1 of arteriviruses has been investigated in virus-infected cells and gene-transfected cells. For EAV, nsp1 is localized in the nucleus in addition to the cytoplasmic distribution during infection (Tijms et al., 2002). For PRRSV, both nsp1α and nsp1β localize in the nucleus and cytoplasm with distinct patterns (Song et al., 2010). PRRSV-nsp1β shows two different distribution patterns; a punctate, perinuclear localization early in infection and predominantly a nuclear distribution later in infection (Li et al., 2012). In gene-transfected cells, both EAV-nsp1 and PRRSV-nsp1β are predominantly nuclear, whereas PRRSV-nsp1α shows the nuclear-cytoplasmic distributions (Chen et al., 2010, Han et al., 2013, Song et al., 2010, Tijms et al., 2002).
In our study, the subcellular localization of LDV-nsp1 and SHFV-nsp1 has been investigated (Fig. 2A). LDV-nsp1α localizes in the both nucleus and cytoplasm, and distribution patterns are similar to those of PRRSV-nsp1α in gene-transfected cells. Identical to PRRSV-nsp1β, predominantly nuclear distribution appears in cells expressing LDV-nsp1β, SHFV-nsp1αβ, and SHFV-nsp1γ. Three different types of perinuclear staining, nuclear aggregation, and predominantly nuclear staining are observed for SHFV-nsp1αβ and SHFV-nsp1γ, and the proportion of each type varies depending on the cell types (Han et al., 2014). Sequence analysis of nsp1 does not show the presence of nuclear localization signal (NLS), suggesting that the nuclear transport of nsp1 may be mediated through the interaction with a cellular protein containing such a signal or by the passive transport.
Fig. 2.
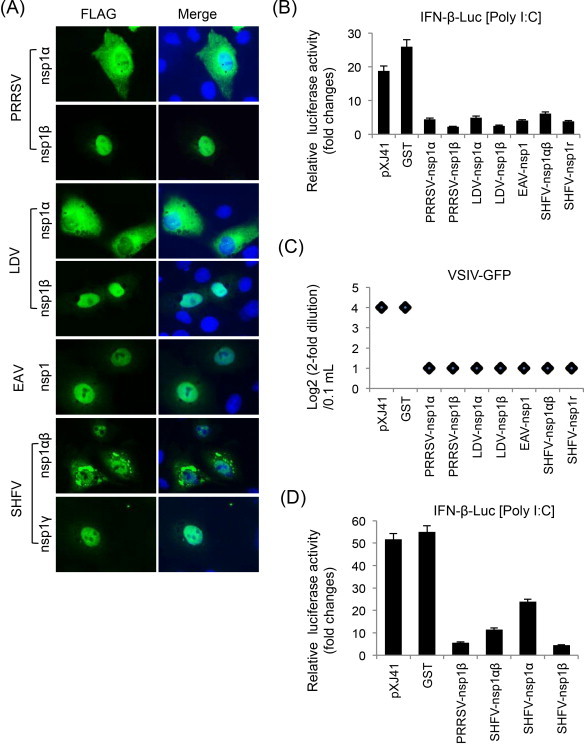
Suppression of IFN-β production by individual subunits of nsp1 of arteriviruses. (A) Subcellular localization of individual nsp1 subunits of arteriviruses in MARC-145 cells. Cells were grown to 40% confluency and transfected with indicated genes for 24 h. Cells were stained with anti-FLAG Ab followed by staining with Alexa 488-labeled anti-mouse Ab and DAPI. Cellular localization of individual subunits was examined by fluorescent microscopy. (B) HeLa cells were seeded in 12-well plates and co-transfected with pIFN-β-Luc along with individual genes and pTK-RL as an internal control at a ratio of 1:1:0.1. At 24 h post-transfection, cells were stimulated with 1 μg/ml of poly(I:C) for 12 h, followed by lysis and determination for reporter activity using the dual luciferase assay system (Promega). Relative luciferase activities were calculated by normalizing the firefly luciferase to renilla luciferase according to the manufacturer's protocol. The data represent the means of three independent experiments, each experiment in triplicate. (C) IFN bioassays using VSIV-GFP (vesicular stomatitis Indiana virus expressing GFP). HeLa cells in 6-well plates were transfected with individual genes for 24 h, and stimulated with poly(I:C) for 12 h. Cell culture supernatants were collected and diluted serially by twofolds. MARC-145 cells were grown in 96-well plates and incubated with each dilution of supernatants for 24 h, and then infected with VSIV-GFP at an MOI of 0.1 for 16 h. VSIV replication was measured by monitoring the fluorescence by GFP expression. (D) Identification of the functional region for IFN-β suppression in SHFV-nsp1αβ. SHFV-nsp1α and SHFV-nsp1β were separated according to Vatter et al. (2014) to make SHFV-nsp1α as 164 aa in length and SHFV-nsp1β as 186 aa.
3.4. Arterivirus nsp1-mediated innate immune modulation
Among IFN antagonists of PRRSV, nsp1 and its two subunits are potent modulators for IFN production and signaling (Fang and Snijder, 2010, Sun et al., 2012a). The nsp1α and nsp1β subunits of PRRSV suppress IFN-β activation (Beura et al., 2010, Chen et al., 2010, Kim et al., 2010, Song et al., 2010). Individual elements in the IFN production pathway including RIG-1, IPS-1, MDA-5, TBK1, IKKɛ, and IRF3 are unaffected, suggesting that inhibition occurs downstream of IRF3 activation possibly in the nucleus (Chen et al., 2010). The total amount of IRF3 and its nuclear localization are not affected by nsp1. Instead, CBP is degraded in the nucleus and the CBP degradation is proteasome-dependent (Kim et al., 2010). Further studies show that the nsp1α subunit is responsible for CBP degradation (Fig. 3B) (Han et al., 2013). PRRSV-nsp1α also reduces NF-κB activation (Song et al., 2010). For LDV, EAV, and SHFV, nsp1 subunits suppress IFN production by inhibiting the IFN promoter activities (Fig. 2B and C; Go et al., 2014). The motif for IFN down-regulation in SHFV-nsp1αβ resides in the nsp1β portion (Fig. 2D). The CBP degradation is also true for LDV-nsp1α but is not seen in cells expressing nsp1β. The molecular basis for IFN suppression by nsp1β or EAV-nsp1 remains to be determined (Han et al., 2014). Besides, all subunits of arterivirus nsp1 possess the suppressive activity for ISRE promoter. PRRSV-nsp1β interrupts the phosphorylation of STAT1 and the nuclear translocation of ISGF3 of the JAK (Janus kinase)-STAT (signal transducer and activator of transcription) pathway (Chen et al., 2010, Patel et al., 2010). The inhibition of ISGF3 nuclear localization by PRRSV-nsp1β is due to the degradation of karyopherin-α1 (KPNA1) which is a nuclear import protein (Wang et al., 2013). Both PRRSV-nsp1α and PRRSV-nsp1β involve in the suppression of TNF-α promoter activity through inhibiting the NF-κB activation and Sp1 transactivation (Subramaniam et al., 2010). The functional domains in PRRSV-nsp1 for IFN regulation have been identified by mutational studies. Critical residues have been identified in nsp1α and nsp1β by alanine scanning (Beura et al., 2012, Subramaniam et al., 2012), and a highly conserved motif in PRRSV-nsp1β appears to be important for IFN suppression (Li et al., 2013). In our studies, PRRSV-PLP1α and the C-terminal ZF motif in PRRSV-nsp1α are not important for IFN suppression, but the N-terminal ZF motif in PRRSV-nsp1α is critical for this activity (Fig. 3A). This is consistent with the finding that some of the N-terminal ZF mutants of PRRSV-nsp1α are not able to induce CBP degradation (Fig. 3B) (Han et al., 2013).
Fig. 3.
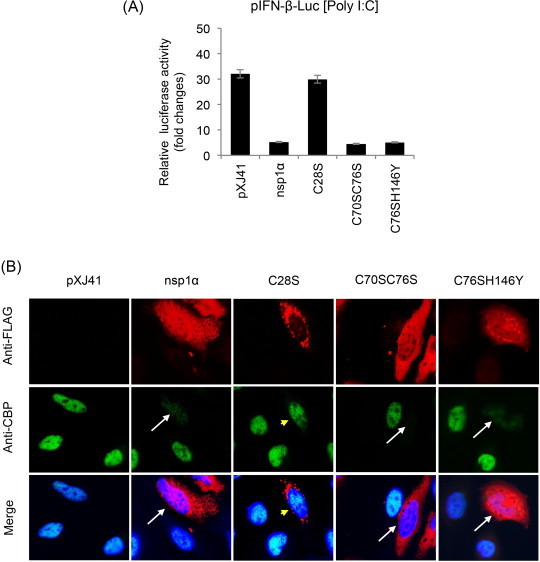
Functional motifs in PRRSV nsp1α for IFN suppression. (A) Mutants of nsp1α were constructed to substitute C28, C70, C76, and H146 to knockout ZF1, PLPα, and ZF2, respectively. C28S, C70SC76S, and C76SH146Y mutants represent three distinct groups; ZF1-destruction mutants, ZF2-destruction mutants, and PLP1α-destruction mutants, respectively. These mutants were expressed in HeLa cells by co-transfection of 500 ng of pIFN-β-Luc along with 50 ng of pTK-RL as an internal control. At 24 h post-transfection, cells were stimulated by 1 μg/ml of poly(I:C) for 12 h followed by determination of reporter expression using the dual luciferase assay system (Promega). Relative luciferase activities were calculated by normalizing the firefly luciferase to renilla luciferase according to the manufacturer's protocol. The data represent the means of three independent experiments, each experiment in triplicate. (B) Degradation of CBP by PRRSV-nsp1α in HeLa cells. Individual mutants of PRRSV-nsp1α were expressed and co-stained with rabbit anti-FLAG Ab and mouse anti-CBP Ab for 2 h, followed by staining with Alexa Fluor 488-conjugated (green) and Alexa Fluor 594-conjugated (red) secondary antibodies, respectively, along with DAPI for nucleus staining (blue). Arrows indicate cells where CBP is degraded, and arrowheads (yellow) indicate no CBP degradation.
4. Conclusion
Arteriviruses have evolved to evade the host innate immune system for better survival and long-term infection in their hosts. PRRSV nsp1 has been studied extensively for its role for innate immune modulation, and accumulating evidence show the alteration of pro-inflammatory cytokines and type I IFNs productions during infection. Type I IFNs are the most potent antiviral cytokines required for both innate and adaptive responses, and PRRSV suppresses the IFN production in pigs. This is probably the most important mechanism for persistence of PRRSV in pigs for a long time. At least six viral proteins have been identified as IFN antagonists for PRRSV, and their mechanisms of action have been studied to a certain extent. Further understanding on the viral strategy for immune modulation and evasion from host immune system is important, and balancing in vivo and in vitro analyses will be needed to affirm each effect.
The nsp1α and nsp1β subunits of PRRSV inhibit the promoter activities of IFN and TNF-α, and the IFN-signaling pathways are suppressed by these subunits, leading to the suppression of IFN-stimulated genes (ISGs) and the establishment of anti-viral state. Such studies have been expanded to nsp1 of other member viruses in the family Arteriviridae, and it is apparent that the nsp1-mediated innate immune modulation is a common strategy for all member viruses in the family. Reverse genetic systems are available for PRRSV and EAV, and the information from structure-function studies will allow us to disable the suppressive viral functions. Such approaches will be useful for developing better vaccines for arteriviruses in the future.
Acknowledgments
This project was supported by US National Pork Board grant no. NPB 13-245 and by AFRI Animal Health competitive grant no. 2013-67015-21243 from the USDA National Institute of Food and Agriculture (NIFA).
References
- Aasted B., Bach P., Nielsen J., Lind P. Cytokine profiles in peripheral blood mononuclear cells and lymph node cells from piglets infected in utero with porcine reproductive and respiratory syndrome virus. Clin. Diagn. Lab. Immunol. 2002;9:1229–1234. doi: 10.1128/CDLI.9.6.1229-1234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Ali T., Wilson A.D., Westcott D.G., Clapperton M., Waterfall M., Mellencamp M.A., Drew T.W., Bishop S.C., Archibald A.L. Innate immune responses to replication of porcine reproductive and respiratory syndrome virus in isolated swine alveolar macrophages. Viral Immunol. 2007;20:105–118. doi: 10.1089/vim.2006.0078. [DOI] [PubMed] [Google Scholar]
- Albina E., Carrat C., Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interferon Cytokine Res. 1998;18:485–490. doi: 10.1089/jir.1998.18.485. [DOI] [PubMed] [Google Scholar]
- Allende R., Lewis T.L., Lu Z., Rock D.L., Kutish G.F., Ali A., Doster A.R., Osorio F.A. North American and European porcine reproductive and respiratory syndrome viruses differ in nonstructural protein coding regions. J. Gen. Virol. 1999;80:307–315. doi: 10.1099/0022-1317-80-2-307. [DOI] [PubMed] [Google Scholar]
- Ammann C.G., Messer R.J., Peterson K.E., Hasenkrug K.J. Lactate dehydrogenase-elevating virus induces systemic lymphocyte activation via TLR7-dependent IFNalpha responses by plasmacytoid dendritic cells. PLoS ONE. 2009;4:e6105. doi: 10.1371/journal.pone.0006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G.W., Rowland R.R.R., Palmer G.A., Even C., Plagemann P.G.W. Lactate dehydrogenase-elevating virus-replication persists in liver, spleen, lymph-node, and testis tissues and results in accumulation of viral-RNA in germinal-centers, concomitant with polyclonal activation of B-cells. J. Virol. 1995;69:5177–5185. doi: 10.1128/jvi.69.8.5177-5185.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccala R., Hoebe K., Kono D.H., Beutler B., Theofilopoulos A.N. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- Baumann A., Mateu E., Murtaugh M.P., Summerfield A. Impact of genotype 1 and 2 of porcine reproductive and respiratory syndrome viruses on interferon-alpha responses by plasmacytoid dendritic cells. Vet. Res. 2013;44:74. doi: 10.1186/1297-9716-44-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura L.K., Sarkar S.N., Kwon B., Subramaniam S., Jones C., Pattnaik A.K., Osorio F.A. Porcine reproductive and respiratory syndrome virus nonstructural protein 1beta modulates host innate immune response by antagonizing IRF3 activation. J. Virol. 2010;84:1574–1584. doi: 10.1128/JVI.01326-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura L.K., Subramaniam S., Vu H.L., Kwon B., Pattnaik A.K., Osorio F.A. Identification of amino acid residues important for anti-IFN activity of porcine reproductive and respiratory syndrome virus nonstructural protein 1. Virology. 2012;433:431–439. doi: 10.1016/j.virol.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddaert W., Van Reeth K., Pensaert M. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV) Adv. Exp. Med. Biol. 1998;440:461–467. doi: 10.1007/978-1-4615-5331-1_59. [DOI] [PubMed] [Google Scholar]
- Calzada-Nova G., Schnitzlein W.M., Husmann R.J., Zuckermann F.A. North American porcine reproductive and respiratory syndrome viruses inhibit type I interferon production by plasmacytoid dendritic cells. J. Virol. 2011;85:2703–2713. doi: 10.1128/JVI.01616-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.C., Peng Y.T., Chang H.L., Chaung H.C., Chung W.B. Phenotypic and functional modulation of bone marrow-derived dendritic cells by porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2008;129:281–293. doi: 10.1016/j.vetmic.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Chaung H.C., Chen C.W., Hsieh B.L., Chung W.B. Toll-like receptor expressions in porcine alveolar macrophages and dendritic cells in responding to poly IC stimulation and porcine reproductive and respiratory syndrome virus (PRRSV) infection. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:197–213. doi: 10.1016/j.cimid.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Chen J.P., Strauss J.H., Strauss E.G., Frey T.K. Characterization of the rubella virus nonstructural protease domain and its cleavage site. J. Virol. 1996;70:4707–4713. doi: 10.1128/jvi.70.7.4707-4713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Li M., He Q., Du J., Zhou L., Ge X., Guo X., Yang H. The amino acid at residue 155 in nonstructural protein 4 of porcine reproductive and respiratory syndrome virus contributes to its inhibitory effect for interferon-beta transcription in vitro. Virus Res. 2014;189:226–234. doi: 10.1016/j.virusres.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Chen Z., Lawson S., Sun Z., Zhou X., Guan X., Christopher-Hennings J., Nelson E.A., Fang Y. Identification of two auto-cleavage products of nonstructural protein 1 (nsp1) in porcine reproductive and respiratory syndrome virus infected cells: nsp1 function as interferon antagonist. Virology. 2010;398:87–97. doi: 10.1016/j.virol.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.B., Wang Y.H., Ratia K., Mesecar A.D., Wilkinson K.D., Baker S.C. Proteolytic processing and deubiquitinating activity of papain-like proteases of human coronavirus NL63. J. Virol. 2007;81:6007–6018. doi: 10.1128/JVI.02747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C., Cho W.S., Kim B., Chae C. Expression of interferon-gamma and tumour necrosis factor-alpha in pigs experimentally infected with Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) J. Comp. Pathol. 2002;127:106–113. doi: 10.1053/jcpa.2002.0566. [DOI] [PubMed] [Google Scholar]
- Clark G.J., Angel N., Kato M., Lopez J.A., MacDonald K., Vuckovic S., Hart D.N. The role of dendritic cells in the innate immune system. Microbes Infect. 2000;2:257–272. doi: 10.1016/s1286-4579(00)00302-6. [DOI] [PubMed] [Google Scholar]
- Clementz M.A., Chen Z.B., Banach B.S., Wang Y.H., Sun L., Ratia K., Baez-Santos Y.M., Wang J., Takayama J., Ghosh A.K., Li K., Mesecar A.D., Baker S.C. Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases. J. Virol. 2010;84:4619–4629. doi: 10.1128/JVI.02406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Kempuraj D., Kandere K., Di Gioacchino M., Barbacane R.C., Castellani M.L., Felaco M., Boucher W., Letourneau R., Theoharides T.C. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol. Lett. 2003;86:123–129. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- Darwich L., Diaz I., Mateu E. Certainties, doubts and hypotheses in porcine reproductive and respiratory syndrome virus immunobiology. Virus Res. 2010;154:123–132. doi: 10.1016/j.virusres.2010.07.017. [DOI] [PubMed] [Google Scholar]
- den Boon J.A., Faaberg K.S., Meulenberg J.J., Wassenaar A.L., Plagemann P.G., Gorbalenya A.E., Snijder E.J. Processing and evolution of the N-terminal region of the arterivirus replicase ORF1a protein: identification of two papainlike cysteine proteases. J. Virol. 1995;69:4500–4505. doi: 10.1128/jvi.69.7.4500-4505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boon J.A., Snijder E.J., Chirnside E.D., de Vries A.A., Horzinek M.C., Spaan W.J. Equine arteritis virus is not a togavirus but belongs to the coronaviruslike superfamily. J. Virol. 1991;65:2910–2920. doi: 10.1128/jvi.65.6.2910-2920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunowska M., Biggs P.J., Zheng T., Perrott M.R. Identification of a novel nidovirus associated with a neurological disease of the Australian brushtail possum (Trichosurus vulpecula) Vet. Microbiol. 2012;156:418–424. doi: 10.1016/j.vetmic.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Snijder E.J. The PRRSV replicase: exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res. 2010;154:61–76. doi: 10.1016/j.virusres.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Fang L., Wang Y., Lei Y., Luo R., Wang D., Chen H., Xiao S. Porcine reproductive and respiratory syndrome virus nonstructural protein 2 contributes to NF-kappaB activation. Virol. J. 2012;9:83. doi: 10.1186/1743-422X-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Treffers E.E., Li Y., Tas A., Sun Z., van der Meer Y., de Ru A.H., van Veelen P.A., Atkins J.F., Snijder E.J., Firth A.E. Efficient-2 frameshifting by mammalian ribosomes to synthesize an additional arterivirus protein. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2920–E2928. doi: 10.1073/pnas.1211145109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W.H., Tompkins M.B., Xu J.S., Zhang H.X., McCaw M.B. Analysis of constitutive cytokine expression by pigs infected in-utero with porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2003;94:35–45. doi: 10.1016/s0165-2427(03)00059-x. [DOI] [PubMed] [Google Scholar]
- Firth A.E., Zevenhoven-Dobbe J.C., Wills N.M., Go Y.Y., Balasuriya U.B.R., Atkins J.F., Snijder E.J., Posthuma C.C. Discovery of a small arterivirus gene that overlaps the GP5 coding sequence and is important for virus production. J. Gen. Virol. 2011;92:1097–1106. doi: 10.1099/vir.0.029264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Mendoza L., Silva-Campa E., Resendiz M., Osorio F.A., Hernandez J. Porcine reproductive and respiratory syndrome virus infects mature porcine dendritic cells and up-regulates interleukin-10 production. Clin. Vaccine Immunol. 2008;15:720–725. doi: 10.1128/CVI.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias-Staheli N., Giannakopoulos N.V., Kikkert M., Taylor S.L., Bridgen A., Paragas J., Richt J.A., Rowland R.R., Schmaljohn C.S., Lenschow D.J., Snijder E.J., Garcia-Sastre A., Virgin H.W. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbes. 2007;2:404–416. doi: 10.1016/j.chom.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Quan R., Zhang H., Hou J., Tang J., Feng W.H. Porcine reproductive and respiratory syndrome virus induces interleukin-15 through the NF-kB signaling pathway. J. Virol. 2012;86:7625–7636. doi: 10.1128/JVI.00177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genini S., Delputte P.L., Malinverni R., Cecere M., Stella A., Nauwynck H.J., Giuffra E. Genome-wide transcriptional response of primary alveolar macrophages following infection with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2008;89:2550–2564. doi: 10.1099/vir.0.2008/003244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno M., Darwich L., Diaz I., de la Torre E., Pujols J., Martin M., Inumaru S., Cano E., Domingo M., Montoya M., Mateu E. Cytokine profiles and phenotype regulation of antigen presenting cells by genotype-I porcine reproductive and respiratory syndrome virus isolates. Vet. Res. 2011;42:9. doi: 10.1186/1297-9716-42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y.Y., Li Y., Chen Z., Han M., Yoo D., Fang Y., Balasuriya U.B.R. Equine arteritis virus does not induce interferon production in equine endothelial cells: identification of nonstructural protein 1 as a main interferon antagonist. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/420658. Article ID 420658, 13 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Koonin E.V., Donchenko A.P., Blinov V.M. Coronavirus genome: prediction of putative functional domains in the nonstructural polyprotein by comparative amino acid sequence analysis. Nucleic Acids Res. 1989;17:4847–4861. doi: 10.1093/nar/17.12.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Koonin E.V., Lai M.M. Putative papain-related thiol proteases of positive-strand RNA viruses. Identification of rubi- and aphthovirus proteases and delineation of a novel conserved domain associated with proteases of rubi-alpha- and coronaviruses. FEBS Lett. 1991;288:201–205. doi: 10.1016/0014-5793(91)81034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarne A., Hampoelz B., Glaser W., Carpena X., Tormo J., Fita I., Skern T. Structural and biochemical features distinguish the foot-and-mouth disease virus leader proteinase from other papain-like enzymes. J. Mol. Biol. 2000;302:1227–1240. doi: 10.1006/jmbi.2000.4115. [DOI] [PubMed] [Google Scholar]
- Guarne A., Tormo J., Kirchweger R., Pfistermueller D., Fita I., Skern T. Structure of the foot-and-mouth disease virus leader protease: a papain-like fold adapted for self-processing and eIF4G recognition. EMBO J. 1998;17:7469–7479. doi: 10.1093/emboj/17.24.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Kim C.Y., Rowland R.R., Fang Y., Kim D., Yoo D. Biogenesis of nonstructural protein 1 (nsp1) and nsp1-mediated type I interferon modulation in arteriviruses. Virology. 2014;458–459C:136–150. doi: 10.1016/j.virol.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Han M., Du Y., Song C., Yoo D. Degradation of CREB-binding protein and modulation of type I interferon induction by the zinc finger motif of the porcine reproductive and respiratory syndrome virus nsp1 alpha subunit. Virus Res. 2013;172:54–65. doi: 10.1016/j.virusres.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Harcourt B.H., Jukneliene D., Kanjanahaluethai A., Bechill J., Severson K.M., Smith C.M., Rota P.A., Baker S.C. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J. Virol. 2004;78:13600–13612. doi: 10.1128/JVI.78.24.13600-13612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunol. Res. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- Hou J., Wang L., He W., Zhang H., Feng W.H. Highly pathogenic porcine reproductive and respiratory syndrome virus impairs LPS- and poly(I:C)-stimulated tumor necrosis factor-alpha release by inhibiting ERK signaling pathway. Virus Res. 2012;167:106–111. doi: 10.1016/j.virusres.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Johnsen C.K., Botner A., Kamstrup S., Lind P., Nielsen J. Cytokine mRNA profiles in bronchoalveolar cells of piglets experimentally infected in utero with porcine reproductive and respiratory syndrome virus: association of sustained expression of IFN-gamma and IL-10 after viral clearance. Viral Immunol. 2002;15:549–556. doi: 10.1089/088282402320914494. [DOI] [PubMed] [Google Scholar]
- Johnson C.R., Griggs T.F., Gnanandarajah J., Murtaugh M.P. Novel structural protein in porcine reproductive and respiratory syndrome virus encoded by an alternative ORF5 present in all arteriviruses. J. Gen. Virol. 2011;92:1107–1116. doi: 10.1099/vir.0.030213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe Y.A., Lole K.S. Deubiquitination activity associated with hepatitis E virus putative papain-like cysteine protease. J. Gen. Virol. 2011;92:2088–2092. doi: 10.1099/vir.0.033738-0. [DOI] [PubMed] [Google Scholar]
- Kim O., Sun Y., Lai F.W., Song C., Yoo D. Modulation of type I interferon induction by porcine reproductive and respiratory syndrome virus and degradation of CREB-binding protein by nonstructural protein 1 in MARC-145 and HeLa cells. Virology. 2010;402:315–326. doi: 10.1016/j.virol.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese M.V., Zevenhoven-Dobbe J.C., Ruijter J.N.A.B.D., Peeters B.P.H., Meulenberg J.J.M., Cornelissen L.A.H.M., Snijder E.J. The nsp1 alpha and nsp1 beta papain-like autoproteinases are essential for porcine reproductive and respiratory syndrome virus RNA synthesis. J. Gen. Virol. 2008;89:494–499. doi: 10.1099/vir.0.83253-0. [DOI] [PubMed] [Google Scholar]
- Kuzemtseva L., de la Torre E., Martin G., Soldevila F., Ait-Ali T., Mateu E., Darwich L. Regulation of toll-like receptors 3, 7 and 9 in porcine alveolar macrophages by different genotype 1 strains of porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2014;158:189–198. doi: 10.1016/j.vetimm.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Lauck M., Hyeroba D., Tumukunde A., Weny G., Lank S.M., Chapman C.A., O’Connor D.H., Friedrich T.C., Goldberg T.L. Novel, divergent simian hemorrhagic fever viruses in a wild Ugandan red colobus monkey discovered using direct pyrosequencing. PLoS ONE. 2011;6:e19056. doi: 10.1371/journal.pone.0019056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauck M., Sibley S.D., Hyeroba D., Tumukunde A., Weny G., Chapman C.A., Ting N., Switzer W.M., Kuhn J.H., Friedrich T.C., O’Connor D.H., Goldberg T.L. Exceptional simian hemorrhagic fever virus diversity in a wild African primate community. J. Virol. 2013;87:688–691. doi: 10.1128/JVI.02433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.M., Kleiboeker S.B. Porcine arterivirus activates the NF-kappaB pathway through IkappaB degradation. Virology. 2005;342:47–59. doi: 10.1016/j.virol.2005.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.M., Schommer S.K., Kleiboeker S.B. Porcine reproductive and respiratory syndrome virus field isolates differ in in vitro interferon phenotypes. Vet. Immunol. Immunopathol. 2004;102:217–231. doi: 10.1016/j.vetimm.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Lee C. Porcine reproductive and respiratory syndrome virus replication is suppressed by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. Virus Res. 2010;152:50–58. doi: 10.1016/j.virusres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Lee C. Stress-activated protein kinases are involved in porcine reproductive and respiratory syndrome virus infection and modulate virus-induced cytokine production. Virology. 2012;427:80–89. doi: 10.1016/j.virol.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Li Y., Treffers E.E., Napthine S., Tas A., Zhu L., Sun Z., Bell S., Mark B.L., van Veelen P.A., van Hemert M.J., Firth A.E., Brierley I., Snijder E.J., Fang Y. Transactivation of programmed ribosomal frameshifting by a viral protein. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E2172–E2181. doi: 10.1073/pnas.1321930111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Tas A., Snijder E.J., Fang Y. Identification of porcine reproductive and respiratory syndrome virus ORF1a-encoded nonstructural proteins in virus-infected cells. J. Gen. Virol. 2012;93:829–839. doi: 10.1099/vir.0.039289-0. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhu L., Lawson S.R., Fang Y. Targeted mutations in a highly conserved motif of the nsp1 beta protein impair the interferon antagonizing activity of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2013;94:1972–1983. doi: 10.1099/vir.0.051748-0. [DOI] [PubMed] [Google Scholar]
- Lim K.P., Ng L.F., Liu D.X. Identification of a novel cleavage activity of the first papain-like proteinase domain encoded by open reading frame 1a of the coronavirus Avian infectious bronchitis virus and characterization of the cleavage products. J. Virol. 2000;74:1674–1685. doi: 10.1128/jvi.74.4.1674-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner H.A., Fotouhi-Ardakani N., Lytvyn V., Lachance P., Sulea T., Menard R. The papain-like protease from the severe acute respiratory syndrome coronavirus is a deubiquitinating enzyme. J. Virol. 2005;79:15199–15208. doi: 10.1128/JVI.79.24.15199-15208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.H., Chaung H.C., Chang H.L., Peng Y.T., Chung W.B. Expression of Toll-like receptor mRNA and cytokines in pigs infected with porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2009;136:266–276. doi: 10.1016/j.vetmic.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Lopez-Fuertes L., Campos E., Domenech N., Ezquerra A., Castro J.M., Dominguez J., Alonso F. Porcine reproductive and respiratory syndrome (PRRS) virus down-modulates TNF-alpha production in infected macrophages. Virus Res. 2000;69:41–46. doi: 10.1016/s0168-1702(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Loving C.L., Brockmeier S.L., Sacco R.E. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007;120:217–229. doi: 10.1111/j.1365-2567.2006.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney J.K., Fritz E.R., Reecy J.M., Kuhar D., Prucnal E., Molina R., Christopher-Hennings J., Zimmerman J., Rowland R.R. Interleukin-8, interleukin-1beta, and interferon-gamma levels are linked to PRRS virus clearance. Viral Immunol. 2010;23:127–134. doi: 10.1089/vim.2009.0087. [DOI] [PubMed] [Google Scholar]
- Luo R., Fang L., Jiang Y., Jin H., Wang Y., Wang D., Chen H., Xiao S. Activation of NF-kappaB by nucleocapsid protein of the porcine reproductive and respiratory syndrome virus. Virus Genes. 2011;42:76–81. doi: 10.1007/s11262-010-0548-6. [DOI] [PubMed] [Google Scholar]
- Luo R., Xiao S., Jiang Y., Jin H., Wang D., Liu M., Chen H., Fang L. Porcine reproductive and respiratory syndrome virus (PRRSV) suppresses interferon-beta production by interfering with the RIG-I signaling pathway. Mol. Immunol. 2008;45:2839–2846. doi: 10.1016/j.molimm.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur R.K., Awasthi A., Wadhone P., Ramanamurthy B., Saha B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat. Med. 2004;10:540–544. doi: 10.1038/nm1045. [DOI] [PubMed] [Google Scholar]
- Meulenberg J.J.M., Hulst M.M., Demeijer E.J., Moonen P.L.J.M., Denbesten A., Dekluyver E.P., Wensvoort G., Moormann R.J.M. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielech A.M., Chen Y., Mesecar A.D., Baker S.C. Nidovirus papain-like proteases: multifunctional enzymes with protease, deubiquitinating and deISGylating activities. Virus Res. 2014 doi: 10.1016/j.virusres.2014.01.025. pii: S0168-1702(14)00040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielech A.M., Kilianski A., Baez-Santos Y.M., Mesecar A.D., Baker S.C. MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology. 2014;450–451:64–70. doi: 10.1016/j.virol.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel J.C., Chen J., Van Alstine W.G., Johnson R.W. Expression of inflammatory cytokines and Toll-like receptors in the brain and respiratory tract of pigs infected with porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2010;135:314–319. doi: 10.1016/j.vetimm.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Mildner A., Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Miller L.C., Laegreid W.W., Bono J.L., Chitko-McKown C.G., Fox J.M. Interferon type I response in porcine reproductive and respiratory syndrome virus-infected MARC-145 cells. Arch. Virol. 2004;149:2453–2463. doi: 10.1007/s00705-004-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.C., Lager K.M., Kehrli M.E., Jr. Role of Toll-like receptors in activation of porcine alveolar macrophages by porcine reproductive and respiratory syndrome virus. Clin. Vaccine Immunol. 2009;16:360–365. doi: 10.1128/CVI.00269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B.D., Balasuriya U.B., Watson J.L., Bosio C.M., MacKay R.J., MacLachlan N.J. Virulent and avirulent strains of equine arteritis virus induce different quantities of TNF-alpha and other proinflammatory cytokines in alveolar and blood-derived equine macrophages. Virology. 2003;314:662–670. doi: 10.1016/s0042-6822(03)00506-3. [DOI] [PubMed] [Google Scholar]
- Nan Y., Wang R., Shen M., Faaberg K.S., Samal S.K., Zhang Y.J. Induction of type I interferons by a novel porcine reproductive and respiratory syndrome virus isolate. Virology. 2012;432:261–270. doi: 10.1016/j.virol.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedialkova D.D., Gorbalenya A.E., Snijder E.J. Arterivirus Nsp1 modulates the accumulation of minus-strand templates to control the relative abundance of viral mRNAs. PLoS Pathog. 2010;6:e1000772. doi: 10.1371/journal.ppat.1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nga P.T., Parquet M.D., Lauber C., Parida M., Nabeshima T., Yu F.X., Thuy N.T., Inoue S., Ito T., Okamoto K., Ichinose A., Snijder E.J., Morita K., Gorbalenya A.E. Discovery of the first insect Nidovirus, a missing evolutionary link in the emergence of the largest RNA virus genomes. PLoS Pathog. 2011;7:e1002215. doi: 10.1371/journal.ppat.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D., Nan Y.C., Shen M.Y., Ritthipichai K., Zhu X.P., Zhang Y.J. Porcine reproductive and respiratory syndrome virus inhibits type I interferon signaling by blocking STAT1/STAT2 nuclear translocation. J. Virol. 2010;84:11045–11055. doi: 10.1128/JVI.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P.G.W., Rowland R.R.R., Even C., Faaberg K.S. Lactate dehydrogenase-elevating virus – an ideal persistent virus. Springer Semin. Immunopathol. 1995;17:167–186. doi: 10.1007/BF00196164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujhari S., Baig T.T., Zakhartchouk A.N. Potential role of porcine reproductive and respiratory syndrome virus structural protein GP2 in apoptosis inhibition. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/160505. Article ID 160505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R.E., Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Rowland R.R., Robinson B., Stefanick J., Kim T.S., Guanghua L., Lawson S.R., Benfield D.A. Inhibition of porcine reproductive and respiratory syndrome virus by interferon-gamma and recovery of virus replication with 2-aminopurine. Arch. Virol. 2001;146:539–555. doi: 10.1007/s007050170161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccani S., Pantano S., Natoli G. p38-dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat. Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- Sagong M., Lee C. Porcine reproductive and respiratory syndrome virus nucleocapsid protein modulates interferon-beta production by inhibiting IRF3 activation in immortalized porcine alveolar macrophages. Arch. Virol. 2011;156:2187–2195. doi: 10.1007/s00705-011-1116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S.G., Sawicki D.L., Siddell S.G. A contemporary view of coronavirus transcription. J. Virol. 2007;81:20–29. doi: 10.1128/JVI.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Wang L., Zhi Y., Xing G., Zhao D., Deng R., Zhang G. Porcine reproductive and respiratory syndrome virus (PRRSV) could be sensed by professional beta interferon-producing system and had mechanisms to inhibit this action in MARC-145 cells. Virus Res. 2010;153:151–156. doi: 10.1016/j.virusres.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Kikkert M., Fang Y. Arterivirus molecular biology and pathogenesis. J. Gen. Virol. 2013;94:2141–2163. doi: 10.1099/vir.0.056341-0. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., Meulenberg J.J. The molecular biology of arteriviruses. J. Gen. Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., van Tol H., Pedersen K.W., Raamsman M.J.B., de Vries A.A.F. Identification of a novel structural protein of arteriviruses. J. Virol. 1999;73:6335–6345. doi: 10.1128/jvi.73.8.6335-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Wassenaar A.L., Spaan W.J. Proteolytic processing of the N-terminal region of the equine arteritis virus replicase. Adv. Exp. Med. Biol. 1993;342:227–232. doi: 10.1007/978-1-4615-2996-5_36. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., Wassenaar A.L.M., Spaan W.J.M. Proteolytic processing of the replicase ORF1a protein of equine arteritis virus. J. Virol. 1994;68:5755–5764. doi: 10.1128/jvi.68.9.5755-5764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Krell P., Yoo D. Nonstructural protein 1α subunit-based inhibition of NF-kappaB activation and suppression of interferon-beta production by porcine reproductive and respiratory syndrome virus. Virology. 2010;407:268–280. doi: 10.1016/j.virol.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Song S., Bi J., Wang D., Fang L., Zhang L., Li F., Chen H., Xiao S. Porcine reproductive and respiratory syndrome virus infection activates IL-10 production through NF-kappaB and p38 MAPK pathways in porcine alveolar macrophages. Dev. Comp. Immunol. 2013;39:265–272. doi: 10.1016/j.dci.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Steinberger J., Kontaxis G., Rancan C., Skern T. Comparison of self-processing of foot-and-mouth disease virus leader proteinase and porcine reproductive and respiratory syndrome virus leader proteinase nsp1α. Virology. 2013;443:271–277. doi: 10.1016/j.virol.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Beura L.K., Kwon B., Pattnaik A.K., Osorio F.A. Amino acid residues in the nonstructural protein 1 of porcine reproductive and respiratory syndrome virus involved in down-regulation of TNF-alpha expression in vitro and attenuation in vivo. Virology. 2012;432:241–249. doi: 10.1016/j.virol.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Kwon B., Beura L.K., Kuszynski C.A., Pattnaik A.K., Osorio F.A. Porcine reproductive and respiratory syndrome virus nonstructural protein 1 suppresses tumor necrosis factor-alpha promoter activation by inhibiting NF-kappaB and Sp1. Virology. 2010;406:270–279. doi: 10.1016/j.virol.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Sun Y., Han M.Y., Kim C., Calvert J.G., Yoo D. Interplay between interferon-mediated innate immunity and porcine reproductive and respiratory syndrome virus. Viruses. 2012;4:424–446. doi: 10.3390/v4040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.N., Xue F., Guo Y., Ma M., Hao N., Zhang X.J.C., Lou Z.Y., Li X.M., Rio Z.H. Crystal structure of porcine reproductive and respiratory syndrome virus leader protease Nsp1α. J. Virol. 2009;83:10931–10940. doi: 10.1128/JVI.02579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Chen Z., Lawson S.R., Fang Y. The cysteine protease domain of porcine reproductive and respiratory syndrome virus nonstructural protein 2 possesses deubiquitinating and interferon antagonism functions. J. Virol. 2010;84:7832–7846. doi: 10.1128/JVI.00217-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Li Y., Ransburgh R., Snijder E.J., Fang Y. Nonstructural protein 2 of porcine reproductive and respiratory syndrome virus inhibits the antiviral function of interferon-stimulated gene 15. J. Virol. 2012;86:3839–3850. doi: 10.1128/JVI.06466-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suradhat S., Thanawongnuwech R., Poovorawan Y. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2003;84:453–459. doi: 10.1099/vir.0.18698-0. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R., Young T.F., Thacker B.J., Thacker E.L. Differential production of proinflammatory cytokines: in vitro PRRSV and Mycoplasma hyopneumoniae co-infection model. Vet. Immunol. Immunopathol. 2001;79:115–127. doi: 10.1016/s0165-2427(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Tijms M.A., Nedialkova D.D., Zevenhoven-Dobbe J.C., Gorbalenya A.E., Snijder E.J. Arterivirus subgenomic mRNA synthesis and virion biogenesis depend on the multifunctional nsp1 autoprotease. J. Virol. 2007;81:10496–10505. doi: 10.1128/JVI.00683-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijms M.A., van der Meer Y., Snijder E.J. Nuclear localization of nonstructural protein 1 and nucleocapsid protein of equine arteritis virus. J. Gen. Virol. 2002;83:795–800. doi: 10.1099/0022-1317-83-4-795. [DOI] [PubMed] [Google Scholar]
- Tijms M.A., van Dinten L.C., Gorbalenya A.E., Snijder E.J. A zinc finger-containing papain-like protease couples subgenomic mRNA synthesis to genome translation in a positive-stranded RNA virus. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1889–1894. doi: 10.1073/pnas.041390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kasteren P.B., Bailey-Elkin B.A., James T.W., Ninaber D.K., Beugeling C., Khajehpour M., Snijder E.J., Mark B.L., Kikkert M. Deubiquitinase function of arterivirus papain-like protease 2 suppresses the innate immune response in infected host cells. Proc. Natl. Acad. Sci. U. S. A. 2013;110(9):E838–E847. doi: 10.1073/pnas.1218464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kasteren P.B., Beugeling C., Ninaber D.K., Frias-Staheli N., van Boheemen S., Garcia-Sastre A., Snijder E.J., Kikkert M. Arterivirus and nairovirus ovarian tumor domain-containing deubiquitinases target activated RIG-I to control innate immune signaling. J. Virol. 2012;86:773–785. doi: 10.1128/JVI.06277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Labarque G., Nauwynck H., Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res. Vet. Sci. 1999;67:47–52. doi: 10.1053/rvsc.1998.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatter H.A., Brinton M.A. Differential responses of disease-resistant and disease-susceptible primate macrophages and myeloid dendritic cells to simian hemorrhagic fever virus infection. J. Virol. 2014;88:2095–2106. doi: 10.1128/JVI.02633-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatter H.A., Di H., Donaldson E.F., Radu G.U., Maines T.R., Brinton M.A. Functional analyses of the three simian hemorrhagic fever virus nonstructural protein 1 papain-like proteases. J. Virol. 2014;88:9129–9140. doi: 10.1128/JVI.01020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Nan Y., Yu Y., Zhang Y.J. Porcine reproductive and respiratory syndrome virus Nsp1beta inhibits interferon-activated JAK/STAT signal transduction by inducing karyopherin-alpha1 degradation. J. Virol. 2013;87:5219–5228. doi: 10.1128/JVI.02643-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Eaton M., Mayer M., Li H., He D., Nelson E., Christopher-Hennings J. Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Arch. Virol. 2007;152:289–303. doi: 10.1007/s00705-006-0857-1. [DOI] [PubMed] [Google Scholar]
- Wojdyla J.A., Manolaridis I., van Kasteren P.B., Kikkert M., Snijder E.J., Gorbalenya A.E., Tucker P.A. Papain-like protease 1 from transmissible gastroenteritis virus: crystal structure and enzymatic activity toward viral and cellular substrates. J. Virol. 2010;84:10063–10073. doi: 10.1128/JVI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongyanin P., Buranapraditkul S., Yoo D., Thanawongnuwech R., Roth J.A., Suradhat S. Role of porcine reproductive and respiratory syndrome virus nucleocapsid protein in induction of interleukin-10 and regulatory T-lymphocytes (Treg) J. Gen. Virol. 2012;93:1236–1246. doi: 10.1099/vir.0.040287-0. [DOI] [PubMed] [Google Scholar]
- Xing Y.L., Chen J.F., Tu J., Zhang B.L., Chen X.J., Shi H.Y., Baker S.C., Feng L., Chen Z.B. The papain-like protease of porcine epidemic diarrhea virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase. J. Gen. Virol. 2013;94:1554–1567. doi: 10.1099/vir.0.051169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F., Sun Y.N., Yan L.M., Zhao C., Chen J., Bartlam M., Li X.M., Lou Z.Y., Rao Z.H. The crystal structure of porcine reproductive and respiratory syndrome virus nonstructural protein Nsp1β reveals a novel metal-dependent nuclease. J. Virol. 2010;84:6461–6471. doi: 10.1128/JVI.00301-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo D., Song C., Sun Y., Du Y.J., Kim O., Liu H.C. Modulation of host cell responses and evasion strategies for porcine reproductive and respiratory syndrome virus. Virus Res. 2010;154:48–60. doi: 10.1016/j.virusres.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Guo X., Nelson E., Christopher-Hennings J., Wang X. Porcine reproductive and respiratory syndrome virus activates the transcription of interferon alpha/beta (IFN-alpha/beta) in monocyte-derived dendritic cells (Mo-DC) Vet. Microbiol. 2012;159:494–498. doi: 10.1016/j.vetmic.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D.H., Chen G., Guo B.C., Cheng G.H., Tang H. PLP2, a potent deubiquitinase from murine hepatitis virus, strongly inhibits cellular type I interferon production. Cell Res. 2008;18:1105–1113. doi: 10.1038/cr.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkel F., Kurth A., Quan P.L., Briese T., Ellerbrok H., Pauli G., Leendertz F.H., Lipkin W.I., Ziebuhr J., Drosten C., Junglen S. An insect nidovirus emerging from a primary tropical rainforest. MBio. 2011;2:e00077-11. doi: 10.1128/mBio.00077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


