Abstract
Anti-coronaviral activity of a mixture of oleoresins and essential oils from botanicals, designated QR448(a), was examined in vitro and in vivo. Treatment of avian infectious bronchitis virus (IBV) with QR448(a) reduced the virus titer as measured in two laboratory host systems, Vero E6 cells and embryonating eggs. The effect of QR448(a) on IBV in chickens was also investigated. Administering QR448(a) to chickens at a 1:20 dilution by spray, 2 h before challenge with IBV was determined to be the most effective treatment. Treatment decreased the severity of clinical signs and lesions in the birds, and lowered the amount of viral RNA in the trachea. Treatment with QR448(a) protected chickens for up to 4 days post-treatment from clinical signs of disease (but not from infection) and decreased transmission of IBV over a 14-day period. Anti-IBV activity of QR448(a) was greater prior to virus attachment and entry indicating that the effect is virucidal. In addition, QR448(a) had activity against both Massachusetts and Arkansas type IB viruses, indicating that it can be expected to be effective against IBV regardless of serotype. To our knowledge, this is the first report on the in vivo use of a virucidal mixture of compounds effective against the coronavirus IBV.
Abbreviations: CPE, cytopathic effects; EID50, 50% embryo infectious dose; ELISA, enzyme linked immunosorbent assay; HMA, hexamethylene amiloride; IBV, infectious bronchitis virus; MHV, mouse hepatitis virus; PBS, phosphate buffered saline; RFLP, restriction fragment length polymorphism; RT-PCR, reverse transcriptase-polymerase chain reaction; SARS-CoV, Severe Acute Respiratory Syndrome virus; SPF, specific pathogen free; TCID50, 50% tissue culture infectious dose
Keywords: Infectious bronchitis virus, Botanical extracts, Oleoresins, Essential oils, Natural ingredients, Virus inactivation, Virucidal, Protection, Virus challenge
1. Introduction
Avian infectious bronchitis virus (IBV), a coronavirus, causes a highly contagious upper-respiratory tract disease in chickens. It can also be found in other species of birds including peafowl (Pavo spp.) and teal (Anas crecca) (Cavanagh, 2005). The virus is worldwide in distribution causing huge economic losses to the poultry industry that result from a reduction in feed conversion and weight gain, and condemnations at the processing plant (Cavanagh and Gelb, 2008). Losses are also due to drops in egg production and poor egg quality in layer type chickens, and for some strains of the virus that are nephropathogenic extensive mortality can occur (Cavanagh and Gelb, 2008).
Infectious bronchitis virus, presents unique challenges regarding its control in commercial chickens. Genomic diversity and the ability of IBV to rapidly change have created different serotypes of the virus that do not cross protect (Cavanagh and Gelb, 2008). Consequently, attenuated live vaccines, used to control the disease, must be selected specifically for the serotype of IBV present in the flock. Compounding this situation is the presence of serologically different variant strains for which no vaccines exist.
We were interested in exploring alternative methods of controlling IBV that could potentially be effective across all serotypes. Antiviral compounds have been used to target proteins involved in coronaviral replication. Coronaviruses have a single stranded positive sense RNA genome that is approximately 27.5 kb in length for IBV. During coronavirus replication, a 3′ nested set of subgenomic viral mRNAs are produced by the viral RNA-dependent RNA-polymerase, and four structural proteins are translated by this enveloped virus: spike, envelope, membrane and nucleocapsid (Cavanagh and Gelb, 2008). Two large genes, 1a and 1ab from the genomic viral RNA encode the viral-replication complex. Those genes are translated into polyproteins that are processed by the main protease (Mpro) as well as a papain-like protease 2 (PL2) to form the viral polymerase and associated nonstructural proteins (Ziebuhr et al., 2007). The main protease of coronaviruses appears to be the most attractive target for antiviral compounds (Haagmans and Osterhaus, 2006, Niu et al., 2008, Xue et al., 2008, Yang et al., 2005, Yang et al., 2006). Inhibitors of Mpro include a Michael acceptor inhibitor (designated N3), chloromethyl ketones, epoxides, and AG7088 an inhibitor shown to be effective against the rhinovirus Mpro homolog (Anand et al., 2003, Niu et al., 2008, Xue et al., 2008). Other antiviral compounds like hexamethylene amiloride (HMA) target ion channel conductance, and have been shown to have an antiviral effect against human coronavirus (HCoV)-229E and mouse hepatitis virus (MHV) coronavirus in vitro (Wilson et al., 2006). In addition, specific inhibition of IBV has been reported in vitro using lithium chloride (Harrison et al., 2007). However, lithium chloride is a toxin and its usefulness as an antiviral against IBV in vivo remains to be determined.
A major problem surrounding antiviral compounds targeting specific viral genes or proteins is the ability of the virus to rapidly mutate during replication to become resistant. Some examples include acyclovir resistant human immunodeficiency viruses and herpes simplex viruses (McMahon et al., 2008), oseltamivir resistant influenza viruses (Collins et al., 2008), and nucleoside/nucleotide analog resistant hepatitis B viruses (Delaney and Borroto-Esoda, 2008). Alternatively, compounds that have a virucidal effect work like a disinfectant, and do not require replication to inactivate the virus (Reichling et al., 2005, Schuhmacher et al., 2003). Thus, resistance to virucidal compounds due to mutations generated in the viral genome during replication is unlikely (Schnitzler et al., 2007). Virucidal activity of essential oils from botanicals has been reported for a number of viruses including herpes simplex virus, dengue virus, and Junin virus (Duschatzky et al., 2005, Koch et al., 2008, Schnitzler et al., 2007). Virucidal activity of essential oils, which are lipophilic by nature, is probably due to disruption of the viral membrane or interference with viral envelope proteins involved in host cell attachment. Although selection of resistant mutants is possible, it has been reported that inactivation of virus by lipophilic essential oils is time dependent and that infectious virus remaining after treatment are still sensitive to the essential oils making selection of resistant mutants unlikely (Schnitzler et al., 2007).
In this study we examined the effect of a natural based product consisting of a synergistic blend of botanical oleoresins and essential oils in a liquid emulsion designated QR448(a) against IBV in vitro and in vivo. To our knowledge this is the first report on the virucidal activity of botanical extracts against a coronavirus in vivo. The data are important because these types of compounds might also be useful to control other coronaviruses like the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) as well as other enveloped viruses like avian influenza virus and Newcastle disease virus.
2. Materials and methods
2.1. Viruses and virus propagation
Strains of IBV used in this study are; the Vero cell adapted Beaudette strain (American Type Culture Collection (ATCC, Rockville, MD) VR-22), the pathogenic Massachusetts 41 (Mass41) strain (ATCC, VR-21), and the Arkansas DPI (ArkDPI) vaccine strain (Intervet, Schering-Plough Animal Health, Millsboro DE). The Beaudette strain of IBV was propagated in Vero E6 cells (CRL-1586), and the Mass41 and ArkDPI strains of IBV were propagated by injecting the chorioallantoic sac of specific pathogen free (SPF) 10-day-old embryonating chicken eggs (Sunrise Farms Inc., Catskill, NY) using standard methods (Gelb and Jackwood, 2008). The Vero E6 cells were obtained from the American Type Culture Collection (Rockdale, MD) and propagated in Minimum Essential Medium (Eagle) with 2 mM l-glutamine, 1.5 g/l sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, and 10% fetal bovine serum (Invitrogen Corp [Gibco], Carlsbad, CA) at 37 °C and 5% CO2.
2.2. RNA extraction and real-time RT-PCR for IBV
Viral RNA was extracted with the MagMAX-96 RNA Isolation Kit (Ambion Inc., Austin, TX) according to the manufacturer's protocol, using a KingFisher magnetic particle processor (Thermo Scientific, Waltham, MA). Real-time RT-PCR was conducted using a Smart Cycler II (Cepheid, Sunnyvale, CA) and the AgPath-ID™ One-Step RT-PCR kit (Ambion Inc.) according to the manufacturer's recommendations. The real-time RT-PCR primers and probe were previously published (Callison et al., 2006) and consist of a forward primer IBV5′GU391 (5′-GCT TTT GAG CCT AGC GTT-3′), a reverse primer IBV5′GL533 (5′-GCC ATG TTG TCA CTG TCT ATT G-3′) and a Taqman® dual-labled probe IBV5′G probe (5′-FAM-CAC CAC CAG AAC CTG TCA CCT C-BHQ1-3′). The RT-PCR primers were synthesized by Integrated DNA Technologies (Coralville, IA), and the Taqman® probe was obtained from BioSearch Technologies (Novato, CA). Components of the real-time RT-PCR test and the thermocycler parameters were previously described, and a standard curve for the assay, was used to calculate the approximate genome copy number for each sample (Callison et al., 2006).
2.3. Birds and housing
Chicks were hatched from SPF fertile leghorn chicken eggs (Sunrise Farms, Inc.) at the Poultry Diagnostic and Research Center (College of Veterinary Medicine, University of Georgia, Athens, GA). Meat-type commercial broiler chickens were obtained from Harrison Poultry (Bethlehem, GA). For each experiment, the chicks were randomly divided into different groups, and housed in positive-pressure Horsfal isolation units or on litter in 10′ × 10′ colony houses. Feed and water were given ad libitum.
2.4. Experimental design
The experiments described below were designed to determine the effect of QR448(a) obtained from Quigley Pharma, Inc. a subsidiary of the Quigley Corporation (Doylestown, PA), on IBV in vitro and in vivo. QR448(a) is a synergistic blend of botanical oleoresins and essential oils in a liquid emulsion of pharmaceutical grade excipients. The exact composition of QR448(a) is available from Quigley Pharma, Inc.
2.4.1. Experiment 1: effect of QR448(a) on IBV in vitro
Toxicity of QR448(a) on Vero E6 cells, and embryonating eggs was examined. Then, the minimum effective dose of QR448(a) against IBV propagated in those laboratory systems was determined. The Vero E6 cells were grown to 85% confluence in a T75 flask (BD Biosciences, Franklin Lakes, NJ) and transferred to two 96-well plates (BD Biosciences). When the cells reached 90% confluence the media was removed and 100 μl of undiluted and each of the10-fold dilutions of QR448(a) or the diluent (Quigley Pharma, Inc.) prepared in cell culture maintenance medium (containing 1% fetal bovine serum) was added to each of 4 different wells. Control wells receiving cell culture maintenance medium only were also included in the experiment. The cells were incubated for 7 days at 37 °C and 5% CO2 and examined twice daily for cytopathic effects (CPE). The experiment was repeated 3 times.
Specific pathogen free fertile chicken eggs were obtained from Sunrise farms (Catskill, NY) and incubated at 37 °C for 10 days. The embryonated eggs were inoculated in the chorioallantoic sac with 100 μl of undiluted and each of the 10-fold dilutions of QR448(a) or the diluent prepared in PBS (pH 7.4). Four eggs were inoculated with each dilution and the experiment was repeated 3 times. Negative control eggs that received PBS only were also included. The eggs were incubated at 37 °C and candled daily for 7 days to record mortality. Any mortality occurring within the first 24 h was considered to be due to trauma associated with inoculation and disregarded. On the 7th day, all the remaining eggs were chilled to 4 °C and opened to examine the embryos for clinical signs of toxicity.
To test the effect of QR448(a) on IBV propagated in Vero E6 cells, a constant amount of virus, 1 × 103 50% tissue culture infectious dose (TCID50) of the Beaudette strain of IBV was added to seven 10-fold serial dilutions beginning with a dilution of 1 × 10−3 (determined to be nontoxic for the Vero E6 cells) of QR448(a) or the diluent prepared in cell culture maintenance medium. The mixtures were incubated at room temperature for 30 min then nine 10-fold serial dilutions (in maintenance medium) of each mixture were prepared for inoculation onto cells. The cell culture media was removed from the cells in a 96-well tissue culture plate and the mixtures were inoculated onto the monolayers. Negative control wells receiving cell culture maintenance medium only were also included in the experiment. The cells were incubated for 7 days at 37 °C and 5% CO2 and examined twice daily for cytopathic effects (CPE). The experiment was repeated 3 times.
The experimental design to test the effect of QR448(a) on IBV grown in embryonating eggs was the same as above, except that the beginning concentration of QR448(a) and the diluent was undiluted since neither compound was toxic for the embryos. A constant amount of virus, 1 × 104 50% embryo infectious dose (EID50) of the IBV Beaudette strain, was mixed with undiluted and each of the 10-fold dilutions of QR448(a) or the diluent prepared in PBS. Negative control eggs that received PBS only were also included. The mixtures were incubated at room temperature for 30 min then nine 10-fold serial dilutions (in PBS) of each mixture were prepared and 200 μl of each dilution was inoculated into the chorioallantoic sac. Four eggs were inoculated for each dilution incubated at 37 °C and candled daily for 7 days to record mortality. Since mortality occurring within the first 24 h could be due to trauma associated with inoculation, they were disregarded. On the 7th day, all the remaining eggs were chilled to 4 °C and opened to examine the embryos for lesions, and the EID50 titer was calculated by the Reed and Muench method (Villegas, 2008). The experiment was repeated 2 times.
2.4.2. Experiment 2: route of administration and timing of treatment in vivo
Two-week-old SPF leghorn chickens were divided into 11 groups of 10 birds each and housed in Horsfal isolation units. The undiluted QR448(a) compound was given intranasally (200 μl per bird), by spray (1 ml per bird), or in the drinking water (1 ml mixed with an equal volume of reconstituted non-fat dry milk per bird after 1 h of water starvation to promote drinking). The spray was delivered with a Preval® sprayer (Precision Valve Corp., Yonkers, NY) while blocking the fresh air delivery to the isolator. The Preval® spray applicator consists of a 475 ml refillable reservoir and a compressed air power unit. Although not directly measured, the average particle size for this type of sprayer is in the range that would be deposited in the naso-oro-pharyngo-laryngeal region of the respiratory tract (Phalen and Mendez, 2009). The spray treatment lasted approximately 10 s and fresh air was resumed 10 min following treatment. For each of the delivery methods, QR448(a) was administered to the birds at either 6 h before challenge, 2 h before challenge, or 2 h after challenge. Two non-treated groups of birds served as a challenged control and a non-challenged negative control.
All of the treated birds and one of the non-treated groups were challenged with 1 × 104 EID50 of the pathogenic Mass41 strain of IBV per bird. The challenge virus was given intranasally and the birds were examined twice daily for clinical signs of the disease. At 5 days post-challenge the birds were killed and necropsied. At necropsy, tracheal swabs were collected and placed in ice cold PBS. The presence of virus was determined by extracting RNA directly from the PBS, and quantitative real-time RT-PCR was conducted on the RNA. Clinical signs and gross lesions were recorded and tracheas were harvested for histopathology. Clinical signs were scored as follows: 1 = normal, 2 = watery eyes or mucus in the nares, 3 = watery eyes and mucus in the nares, and 4 = watery eyes, mucus in the nares and tracheal rales. The tracheas were collected in 10% neutral buffered formalin, routinely processed into paraffin, and 5-μm sections were cut for hematoxylin and eosin staining. Epithelial hyperplasia, lymphocyte infiltration, and the severity of epithelial deciliation were scored for each trachea with 1 being normal and 4 being severe.
2.4.3. Experiment 3: minimum effective dose in vivo
For this experiment, 2-week-old SPF leghorn chickens were divided into 20 groups of 10 birds each and housed in Horsfal isolation units. Half of the birds were treated 2 h before challenge and the other half were treated 2 h after challenge. The birds were treated with either undiluted or a 2-fold serial dilution of QR448(a) starting at a 1:5 dilution to a dilution of 1:1280. Dilutions were prepared in sterile distilled deionized water, and 1 ml/bird was delivered via spray with a Preval® sprayer (Precision Valve Corp.) as described in Experiment 2. Additional control groups of 10 birds each included in the experiment were birds that were not treated and challenged (challenge control group), birds treated by the spray route with undiluted QR448(a) and not challenged, and birds that were not treated and not challenged (negative control group).
All of the treated birds unless otherwise indicated above and one of the non-treated groups were challenged with 1 × 104 EID50 of the pathogenic Mass41 strain of IBV per bird as described in Experiment 2. All of the birds were examined for clinical signs, and necropsied 5 days post-challenge as described for Experiment 2. Clinical signs were scored as described above.
2.4.4. Experiment 4: duration of the effect in vivo
For this experiment 290 1-day-old commercial broiler chickens that were not vaccinated, were divided into different groups and housed in Horsfal isolation units. Serum was collected from several birds at the beginning of the experiment to determine the level of maternal antibodies to IBV. Antibodies were detected using a commercial enzyme linked immunosorbent assay (ELISA) test (IDEXX, Inc., Westbrook, ME). The birds were treated with a 1:20 dilution of QR448(a) in sterile distilled deionized water by spray with a Preval® sprayer (Precision Valve Corp.) then challenged with 1 × 103.5 EID50 of the Mass41 strain of IBV at 2 h, 6 h, 1 day, 2 days, 4 days and 7 days after treatment. The birds were challenged intranasally as described in Experiment 2. Controls included birds that were not treated and challenged at those times, and a negative group that was not treated or challenged.
Birds challenged at 2, 4, 6, and 12 h were necropsied on the same day, 5 days after challenge, along with one non-treated/non-challenged control group. Birds challenged at 1, 2, 4, and 7 days after treatment were necropsied along with a negative (non-challenged) control group at 5 days after each of those challenge times. All of the birds were scored for clinical signs, and necropsied as described above.
2.4.5. Experiment 5: effect of QR448(a) on transmission of IBV in vivo
In this experiment, 240 commercial broiler chickens that were not vaccinated, were equally divided and housed in two 10′ × 10′ colony houses on pine shavings on the floor. Colony houses were used so an adequate number of birds could be maintained together for the transmission experiment and to mimic as closely as possible commercial chicken house conditions. At 2 weeks of age 20 birds from each house were removed and wing-banded (for future identification) and given a full dose of modified live IBV Arkansas type vaccine in the eye according to the manufacturers recommendations (Intervet, Schering-Plough Animal Health, Millsburo, DE). The birds were held in isolation for 2 h before reintroducing them back into the colony houses. Immediately after reintroducing the birds into the colony houses, all of the birds in one house were treated by spray using a Preval® sprayer (Precision Valve Corp.) with 1 ml of a 1:20 dilution (in sterile deionized-distilled water) of QR448(a) per bird. We monitored the transmission of the virus to contact exposed birds by removing and swabbing the trachea of 25 birds at 3, 7, 10 and 14 days post-treatment. We also removed and swabbed 5 birds given the vaccine at each sample time. Birds that were swabbed were removed from the experiment to avoid promoting the spread of the virus by inducing damage to the trachea and to prevent swabbing the same bird twice during the experiment. Virus detection in the tracheal swabs was determined by real-time RT-PCR.
Serum was collected from 10 birds at the beginning of the experiment (2 weeks of age) to determine the level of maternal antibodies to IBV. In addition, we collected sera from all of the birds (n = 12) necropsied on the last day of the experiment and tested them for antibodies to IBV, Newcastle disease virus, and infectious bursal disease virus. Antibodies were tested using a commercial ELISA test (IDEXX, Inc.).
2.5. Statistical analysis
Virus titers were calculated by the method of Reed and Muench (Villegas, 2008). Means and standard deviations were calculated for Ct values and analyzed statistically with Student's t-test, and the histopathological scores were analyzed by the Kruskal–Wallis test coupled with Dunn's post-test, and transmission experiment data was analyzed with Fisher's exact test using JMP Statistical Discovery Software (SAS Institute, Inc., Cary, NC).
3. Results
3.1. Experiment 1: toxicity of QR448(a) in cell culture and in embryonating eggs, and effect on IBV in vitro
Undiluted and a 1 × 10−1 dilution of QR448(a) was toxic to Vero E6 cells and cells inoculated with a 1 × 10−2 dilution of QR448(a) had CPE affecting about 50% of the monolayer. Cells inoculated with a 1 × 10−3 dilution or higher of QR448(a) were unaffected at 7 days post-treatment. The Vero E6 cells inoculated with undiluted and a 1 × 10−1 dilution of the diluent had CPE in >90% of the cells, whereas cells inoculated with higher dilutions of diluent did not have any CPE following 7 days of incubation. None of the negative control cells had CPE. Disregarding embryo deaths within 24 h of inoculation, none of the embryonating eggs died during the course of the experiment. At 7 days post-treatment, none of the embryos that received QR448(a) or the embryos that received diluent had gross lesions. As expected, none of the negative control embryos had lesions.
To determine the decrease in titer of infectious virus, we examined the effect of QR448(a) on IBV (Beaudette strain) as determined by titration in Vero E6 cells and the data are presented in Fig. 1 . Treatment with QR448(a) at a 1 × 10−3 dilution reduced the titer of IBV from an average of 1 × 103.0 to 1 × 100.8 TCID50/ml. Treatment at a dilution of 1 × 10−4 reduced the IBV titer from 1 × 103.0 to 5 × 102.0 TCID50/ml. None of the dilutions of QR448(a) higher than 1 × 10−4 reduced the titer of IBV. No reduction in virus titer was observed for the diluent at any dilution and, none of the cells in the negative control wells (cell culture maintenance medium alone) had CPE (data not shown).
Fig. 1.
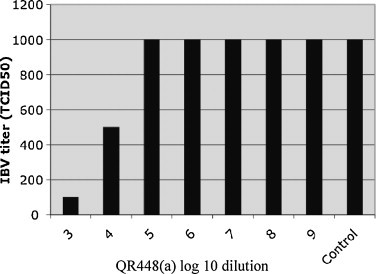
Titration of IBV Beaudette strain in Vero E6 cells following treatment with 10-fold serial dilutions of QR448(a) starting at 1 × 10−3 to 1 × 10−9 in cell culture maintenance medium and cell culture maintenance medium plus IBV alone (control) are presented. The 50% tissue culture infectious dose (TCID50) titers reflect the average of three replicates. No reduction in virus titer was observed for IBV treated with the diluent (data not shown).
Data showing the effect of QR448(a) on IBV (Beaudette strain) as determined by titration in embryonating eggs also measured the decrease of infectious virus and the data are presented in Fig. 2 . Treatment with undiluted QR448(a) reduced the titer of IBV from 1 × 104 to 1 × 102 EID50/ml. Dilutions of QR448(a) at 1 × 10−1 and 1 × 10−2 reduced the titer of IBV from 1 × 104 to 1 × 101.7 and 1 × 103 EID50/ml respectively. No reduction in IBV titer was observed when dilutions of QR448(a) from 1 × 10−3 to 1 × 10−7 were used or when the diluent at any dilution was used. The lesions in the embryos, were typical of those reported for IBV (Gelb and Jackwood, 2008).
Fig. 2.
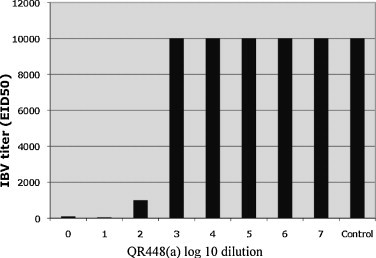
Titration of IBV Beaudette strain in embryonating eggs following treatment with 10-fold serial dilutions of QR448(a) starting at 0 (no dilution) to 1 × 10−7 in PBS, and PBS plus IBV (control) are presented. The 50% embryo infectious dose (EID50) titers reflect the average of two replicates. No reduction in virus titer was observed for IBV treated with the diluent (data not shown).
3.2. Experiment 2: route of administration and timing of QR448(a) treatment against IBV in vivo
Clinical signs were observed in all of the Mass41 virus challenged groups of birds regardless of treatment but in the intranasal and spray treated groups, fewer birds had signs and the signs were milder, as reflected by lower average scores (Table 1 ). Virus was detected in the birds treated by the intranasal route 6 h before challenge but no virus was detected in the other intranasal treatment groups or in the spray treated groups. Clinical signs were observed in all of the birds treated with QR448(a) in the water, and the clinical sign scores were higher than the intranasal and spray treated birds. Virus was detected in all but one bird (6 h before challenge group, see Table 1) receiving QR448(a) in the water. Severe clinical signs were observed and virus was detected in all of the IBV challenged birds that did not receive treatment. In addition, all of the negative control birds remained negative for clinical signs and virus detection.
Table 1.
Clinical signs and virus detection in 2-week-old SPF chickens following treatment with QR448(a) before or after IBV challengea.
| Treatment | Clinical signs (average score)b | Virus detectionc | Average Ct valued | Average histopathology scoree |
|---|---|---|---|---|
| Intranasal 6 h before challenge | 9/10 (2.8) | 10/10 | 23.48 | 3.32a |
| Intranasal 2 h before challenge | 6/10 (1.7) | 0/10 | Negf | 3.29a |
| Intranasal 2 h after challenge | 7/10 (1.9) | 0/10 | Neg | 3.58a |
| Spray 6 h before challenge | 7/10 (1.7) | 0/10 | Neg | 3.28a |
| Spray 2 h before challenge | 7/10 (1.7) | 0/10 | Neg | 3.32a |
| Spray 2 h after challenge | 7/10 (1.7) | 0/10 | Neg | 3.24a |
| Water 6 h before challenge | 10/10 (3.5) | 9/10 | 23.13 | 3.50a |
| Water 2 h before challenge | 10/10 (3.2) | 10/10 | 23.31 | 3.40a |
| Water 2 h after challenge | 10/10 (3.5) | 10/10 | 24.72 | 3.39a |
| Challenge controlg | 10/10 (4.0) | 10/10 | 26.01 | 3.52a |
| Negative controlh | 0/10 (1.0) | 0/10 | Neg | 1.06b |
The birds were intranasally challenged with 3.1 × 104 embryo infectious dose50/bird of pathogenic IBV strain Mass41.
Clinical signs were recorded 5 days following challenge and were scored as follows: 1 = normal, 2 = watery eyes or mucus in the nares, 3 = watery eyes and mucus in the nares, and 4 = watery eyes, mucus in the nares and tracheal rales.
Virus was detected directly from tracheal swabs collected 5 days following challenge by real-time RT-PCR.
The average cycle threshold (Ct) value for only the positive samples was calculated and indicates the relative amount of virus detected in the trachea (higher numbers = less virus).
The average histopathology score for only the birds with clinical signs was based on epithelial hyperplasia, lymphocyte infiltration, and epithelial deciliation with 1 being normal and 4 being severe. Numbers with different superscripts are statistically different (Kruskal–Wallis test, p ≤ 0.05).
Negative.
Challenge control birds were not treated and were challenged.
Negative control birds were not treated or challenged.
Tracheas were scored as described in Section 2 with 1 being normal and 4 being severe, and the birds that did not receive challenge virus had an average lesion score of 1.06. Histopathology scores for all of the treated and challenged birds that had clinical signs were statistically different from the non-challenged controls but not from the challenged controls (p ≤ 0.05). Birds that did not have clinical signs regardless of the group were not statistically different from the negative control birds (data not shown).
3.3. Experiment 3: minimum effective dose of QR448(a) against IBV in vivo
A dose response was observed in the birds treated 2 h before Mass41 virus challenge (Fig. 3 ). A less pronounced response was observed in birds treated 2 h after virus challenge. Virus was detected in the trachea of all of the challenged birds, but treatment with the lower dilutions of QR448(a) 2 h before challenge, had a more pronounced effect on lowering the amount of virus detected in the trachea. In general, the clinical signs were more severe and involved more birds at the higher dilutions or QR448(a) (see average scores, Table 2 ). Tracheas were scored as described in Section 2 with 1 being normal and 4 being severe, and all of the birds given undiluted QR448(a) were scored as normal (average score = 1.10). Statistically lower (p ≤ 0.05) lesion scores (Table 2) were observed for QR448(a) at a 1:20 dilution or lower when it was administered by spray treatment 2 h before challenge. Spray treatment with QR448(a) did not statistically lower the tracheal lesion scores when given 2 h after challenge. Birds that were not treated and received the challenge virus had an average tracheal lesion score of 3.00.
Fig. 3.
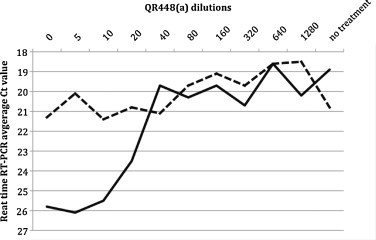
Dose titration of QR448(a) in SPF chickens challenged with 1 × 104 EID50 of the pathogenic Mass41 strain of IBV per bird. Solid line = treatment 2 h before challenge and dashed line = treatment 2 h after challenge. Detection of viral RNA in tracheal swab samples by real-time RT-PCR is presented as the average cycle threshold value (higher numbers equal less virus) for each group (n = 10). Dilutions of QR448(a) represent no dilution (0), or doubling dilutions beginning with a 1:5 dilution. No treatment are non-treated and challenged birds.
Table 2.
Experiment 3: dose titration of QR448(a) spray treatment in 2-week-old specific pathogen free chickens 2 h before or 2 h after challenge with pathogenic IBV.
| Treatmenta | Treatment 2 h before challenge |
Treatment 2 h after challenge |
||
|---|---|---|---|---|
| Number of birds with clinical signs/total (average score)b | Histopathology scoresc | Number of birds with clinical signs/total (average score) | Histopathology scoresc | |
| Undiluted + IBV | 6/10 (1.6) | 2.43a | 3/10 (1.4) | 2.52a |
| 1:5 + IBV | 6/10 (1.6) | 2.58a | 6/10 (1.7) | 2.55a,b |
| 1:10 + IBV | 3/10 (1.3) | 2.68a | 7/10 (1.7) | 2.45a |
| 1:20 + IBV | 4/10 (1.4) | 2.72a | 6/10 (1.6) | 2.45a |
| 1:40 + IBV | 8/10 (2.0) | 3.05b | 5/10 (1.8) | 2.44a |
| 1:80 + IBV | 6/10 (2.0) | 3.18b | 6/10 (2.0) | 2.45a |
| 1:160 + IBV | 4/10 (2.0) | 2.95b | 9/10 (2.8) | 2.67a,b |
| 1:320 + IBV | 5/10 (2.2) | 2.89b | 6/10 (2.5) | 2.74a,b |
| 1:640 + IBV | 7/10 (2.7) | 2.83b | 9/10 (3.7) | 2.78a,b |
| 1:1280 + IBV | 8/10 (3.2) | 3.45c | 8/10 (3.2) | 2.95b |
| IBV | 9/10 (3.7) | 3.21c | 7/7 (4.0) | 3.00b |
| Undiluted | 0/10 (1.0) | 1.10d | NDd | 1.10c |
| Negative control | 0/10 (1.0) | 1.05d | ND | 1.05c |
Treatment = QR448(a) sprayed undiluted or diluted at a dose of 1 ml/bird followed by challenge with 1 × 104.5 EID50 of pathogenic IBV, Mass41 strain.
Clinical signs were scored as follows: 1 = normal, 2 = watery eyes or mucus in the nares, 3 = watery eyes and mucus in the nares, and 4 = watery eyes, mucus in the nares and tracheal rales.
Tracheas were scored for epithelial hyperplasia, lymphocyte infiltration, and epithelial deciliation with 1 being normal and 4 being severe. Histopathology scores within a column with different superscripts are statistically different at p ≤ 0.05 (Kruskal–Wallis test).
Not done.
3.4. Experiment 4: duration of the effect against IBV in vivo
Ten birds were bled at 1 day of age and as expected all of the nonvaccinated commercial broilers were positive for maternal antibodies to IBV (average titer = 2542) by ELISA (IDEXX Laboratories). The numbers of birds showing clinical signs of IBV infection and the average clinical sign scores are shown in Table 3 . The number of birds with clinical signs that were treated with QR448(a) and challenged with Mass41 within 4 days of treatment ranged from 0 to 50%. In birds challenged at 7 days after treatment, 90% of the birds had clinical signs. At each of the sample days, we observed 80–100% of the birds (78% at day 1) in the non-treated Mass41 challenge control group with clinical signs indicating that maternal antibodies did not prevent the disease. None of the non-challenged birds had clinical signs. In general, clinical signs in the treated challenged birds consisted of watery eyes or mucus in the nares with an occasional bird having both. The signs were not unlike a vaccine reaction, and were considered to be extremely mild as evidenced by the average clinical sign scores. In comparison, the non-treated challenged birds had more severe signs consisting of watery eyes, mucus in the nares, swollen sinuses, and excessive mucus in the trachea resulting in tracheal rales.
Table 3.
Experiment 4: clinical signsa in broiler chickens challenged with IBV at various times after treatment with QR448(a) at 1 day of age.
| Treatment group | Challengeb times post-treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 12 h | 1 day | 2 days | 4 days | 7 days | |
| QR448(a) + IBV | 3/10 (1.3A)c | 5/10 (1.5A) | 0/9 (1.0A) | 3/10 (1.3A) | 0/10 (1.0A) | 3/10 (1.3A) | 1/10 (1.1A) | 9/10 (3.6B) |
| QR448(a) | 0/10 (1.0A) | 0/9 (1.0A) | 0/10 (1.0A) | 0/10 (1.0A) | 0/10 (1.0A) | 0/10 (1.0A) | 0/7 (1.0A) | 0/10 (1.0A) |
| IBV | 8/10 (3.1B) | 8/10 (3.1B) | 9/10 (3.6B) | 9/10 (3.6B) | 7/9 (3.3B) | 5/6 (3.5B) | 8/8 (4.0B) | 8/8 (4.0B) |
| Negative controld | 0/10 | 0/9 | 0/6 | 0/7 | 0/9 | |||
Birds were necropsied and clinical signs recorded 5 days post-challenge. Clinical signs consisted of watery eyes, tracheal rales, and mucus in the nares and the trachea.
Each bird was given 1 × 103.5 EID50 of the Mass41 strain of IBV intranasally.
Number of birds with clinical signs per total (average score). Clinical signs were scored as follows: 1 = normal, 2 = watery eyes or mucus in the nares, 3 = watery eyes and mucus in the nares, and 4 = watery eyes, mucus in the nares and tracheal rales. Average scores with different capital letter superscripts are statistically significant at p ≤ 0.01 (Kruskal–Wallis test).
Clinical sign scores for negative control birds were all normal (1.0).
Challenge virus was detected in all of the challenged birds, even in the QR448(a) treated birds (Table 4 ), indicating that there was no protection from infection. Although the data were not statistically different, QR448(a) treated birds that were challenged at 2 h, 6 h, 1 day, 2 days, 4 days, and 7 days had fewer viral genome copies than non-treated challenge groups at those same times respectively. No virus was detected in the tracheas of birds that were not challenged.
Table 4.
Experiment 4: detectable viral load (viral genomic RNA) expressed as average cycle threshold valuesa ± standard error in broiler chickens challenged with IBV at various times after treatment with QR448(a) at 1 day of age.
| Treatment group | Challengeb times post-treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 12 h | 1 day | 2 days | 4 days | 7 days | |
| QR448(a) + IBV | 24.58 ± 0.78 | 24.15 ± 0.61 | 23.42 ± 0.74 | 21.86 ± 0.52 | 22.00 ± 0.79 | 25.43 ± 0.57 | 24.27 ± 0.96 | 25.14 ± 1.16 |
| QR448(a) | Negc | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| IBV | 24.24 ± 0.73 | 24.78 ± 0.52 | 22.28 ± 0.67 | 22.83 ± 0.67 | 21.06 ± 1.25 | 24.19 ± 0.89 | 24.19 ± 0.87 | 23.66 ± 1.07 |
| Negative control | Neg | Neg | Neg | Neg | Neg | |||
Larger numbers = less virus. Average values were not statistically significant (Student's t-test).
Each bird was given 1 × 103.5 EID50 of the Mass41 strain of IBV intranasally, and necropsied 5 days post-challenge.
Negative.
Tracheas were scored as described in Section 2 with 1 being normal and 4 being severe, and the birds that did not receive challenge virus had a normal 1.00 average score (Table 5 ). Birds that were not challenged did not have any appreciable lesions in the trachea, and the tracheal lesion scores in the non-challenged birds that were treated with QR448(a) were not statistically different from the non-treated, non-challenged birds (p ≤ 0.01). The tracheal lesion scores for the treated and challenged group were statistically higher than the non-challenged birds but were not as high as the non-treated challenged birds (p ≤ 0.01). The positive control (non-treated challenged) birds all had tracheal lesion scores statistically higher than the other groups (p ≤ 0.01).
Table 5.
Experiment 4: histopathology scoresa in broiler chickens challenged with IBV at various times after treatment with QR448(a) at 1 day of age.
| Treatment group | Challengeb times post-treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 12 h | 1 day | 2 days | 4 days | 7 days | |
| QR448(a) + IBV | 2.07D | 2.18D | 2.08D | 2.11D | 1.85D | 2.10D | 2.00D | 2.10D |
| QR448(a) | 1.00E | 1.31E | 1.00E | 1.00E | 1.00E | 1.07E | 1.00E | 1.00E |
| IBV | 3.00ABC | 2.67C | 2.78BC | 2.67C | 2.70C | 3.09AB | 3.00ABC | 3.17A |
| Negative control | 1.00E | 1.00E | 1.00E | 1.07E | 1.00E | |||
Birds were necropsied at 5 days post-challenge, and tracheas were collected in 10% neutral buffered formalin and routinely processed and stained (see Section 2), then scored for epithelial hyperplasia, lymphocyte infiltration, and epithelial deciliation with 1 being normal and 4 being severe. Scores with different capital letter superscripts are statistically significant, Kruskal–Wallis test (p ≤ 0.01).
Birds that received challenge virus were given 1 × 103.5 EID50 of the Mass41 strain of IBV intranasally.
3.5. Experiment 5: effect of QR448(a) on transmission of IBV in vivo
No virus was detected in the birds vaccinated with Arkansas type IBV at 3 and 14 days post-infection (Fig. 4 ). Virus was detected in 1 of 5 vaccinated birds in the treated group at 7 days post-vaccination and in 3 of 6 and 2 of 5 vaccinated birds in the treated and non-treated groups respectively at 10 days post-infection.
Fig. 4.
Experiment 5. Percent of contact exposed chickens with IBV detected in the trachea by real-time RT-PCR. Treated birds (n = 25 at each sample time) received 1 ml each of a 1:20 dilution of QR448(a) in distilled water by spray. Not treated birds (n = 25 at each sample time) were sprayed with distilled water. *Statistically different p ≤ 0.03 (Fisher's exact test)
Transmission of IBV from vaccinated to contact exposed birds was monitored by detecting the virus in the trachea using real-time RT-PCR and the percent of contact exposed birds positive for IBV is presented in Fig. 4. In the treated group, virus was not detected in the contact exposed birds at 3 and 7 days post-exposure. In the non-treated group 1 of 25 birds was positive at 7 days post-exposure. At 10 days post-exposure, 1 of 24 contact exposed birds was positive in the treated group whereas 3 of 25 birds were positive in the non-treated group. At 14 days post-exposure, 1 of 25 contact exposed and treated birds was positive for virus. This is in contrast to the non-treated group where 5 of 23 contact exposed birds were positive at 14 days post-exposure. The data were statistically different at 14 days (p ≤ 0.03) but not at any other day post-exposure.
Serum collected from 10 of the nonvaccinated commercial broilers at 1 day of age (these are the same birds bled in Experiment 4) had an average maternal antibody titer against IBV of 2,542. Antibodies to IBV were not detected by ELISA (n = 12) at 2 weeks of age when the birds were vaccinated. Serum was collected from all of the birds necropsied at the end of the experiment and none of the birds were positive for antibodies to IBV, none were positive for antibodies to NDV, and two birds in the treated contact group were positive for IBDV (average titer = 1038.5).
4. Discussion
The virucidal activity of essential oils, which are lipophilic by nature, likely act to disrupt the viral membrane or interfere with viral envelope proteins involved in host cell attachment (Schuhmacher et al., 2003). In this report, we examined the effect of a natural based product consisting of a synergistic blend of botanical oleoresins and essential oils in a liquid emulsion designated QR448(a) on IBV. The minimum effective concentration against IBV was determined in vitro using the Beaudette strain of IBV because it is adapted to grow in cell culture. The optimal route and timing of administration, most effective dose, and the duration of effect on IBV in chickens were investigated using the widespread and pathogenic Mass41 strain of the virus. Because Arkansas vaccine type viruses are frequently found circulating in commercial chicken flocks (Jackwood et al., 2005), we also examined the effect on transmission of modified live Arkansas type IBV vaccine in 2-week-old broiler chickens.
The lowest dilution of QR448(a) that did not cause toxic effects in Vero E6 cells was capable of reducing the titer of IBV from 1 × 103 to 1 × 100.8 TCID50/ml. Undiluted QR448(a) was not toxic for 10-day-old embryonating eggs and reduced the titer of IBV from 1 × 104 to 1 × 102 EID50/ml. The diluent did not reduce the titer, indicating that the oleoresins and essential oils in QR448(a) were responsible for the effect.
Next we sought to determine if the effects of QR448(a) are biologically significant in chickens infected with IBV. Both positive and negative control groups were included in the in vivo studies however; we did not include a diluent control group because, in the first experiment, the diluent was shown not to have an effect on the virus titer. Based on clinical signs, lesions and virus detection, the best routes of administration were determined to be intranasal and spray inoculation 2 h before challenge. In addition, it appears treatment intranasally or by spray 2 h after challenge was somewhat better than intranasal or spray treatment at 6 h before challenge. Administration of QR448(a) by water had little effect regardless of treatment timing. Based on microscopic lesions, it appears that QR448(a) alone does not adversely affect the epithelium of the trachea. It is interesting that we could not detect virus in some of the groups treated intranasally or by spray in this experiment. It appears that QR448(a) reduced the titer of IBV below an infectious threshold. Infection often does not occur when birds are challenged with ≤1 × 102.5 EID50 of the Mass41 strain of IBV (Jackwood, unpublished data). It is also possible that inhibitors to the real-time RT-PCR reaction prevented detection; however, we were able to detect challenge virus in the other groups suggesting the presence of inhibitors are inconsistent or that another explanation is likely. Since spray is suitable for mass application of commercial chickens, we used that route of administration to examine the minimum effective dose, duration of activity, and effect on transmission of IBV in chickens.
Birds treated by spray with QR448(a) 2 h before challenge showed a clear dose response between undiluted QR448(a) and a dilution of 1:40 (Table 2 and Fig. 3). Treatment 2 h after challenge had a less pronounced dose response, indicating that QR448(a) is less effective following virus attachment and entry into the host cell. This data and the timing of administration data above suggests that QR448(a) activity is virucidal rather than antiviral in nature.
One-day-old broiler chickens treated with QR448(a) were protected from clinical signs of disease following challenge with pathogenic IBV up to 4 days post-treatment (Table 3). Although maternal antibodies in chicks, which generally last from 1 to 2 weeks of age, and can provide some protection against IBV (Mondal and Naqi, 2001), we observed clinical signs in most of the non-treated virus challenged control birds indicating that maternal antibodies did not play a role in protection from clinical signs. Protection from infection was not observed since challenge virus was detected in all of the QR448(a) treated and challenged birds. However, at all but two challenge times (4 and 12 h) the amount of challenge virus in the trachea was slightly lower in the QR448(a) treated groups but the values were not statistically significant. In a previous study, where different amounts of IBV were given to chickens, it was reported that the amount of virus detected in a clinical sample from trachea correlated with dose at 1 day post-challenge but by 5 days post-challenge all of the birds had similar levels of virus in the trachea regardless of challenge dose (Callison et al., 2006). Treatment with QR448(a) may have lowered the amount of virus in the birds but by 5 days post-challenge when we collected samples, little or no differences in viral load were observed.
Examining birds at 5 days post-challenge is the USDA recommended procedure (USDA, APHIS title 9, code of federal regulations http://www.access.gpo.gov/nara/cfr/waisidx_08/9cfrv1_08.html) for evaluating protection from IBV challenge. Since challenge virus was detected in the trachea of the QR448(a) treated birds it is possible that clinical signs would have been observed in more of the treated birds if they were observed beyond 5 days post-challenge. It is also possible that fewer or no clinical signs would be observed in those birds beyond 5 days post-challenge, since a delay in clinical signs may represent additional time for the birds to mount a protective immune response to the challenge virus.
Protection of broiler chickens for up to 4 days post-treatment from clinical signs of disease but not from infection may provide an opportunity to induce some level of local immunity due to the presence of the challenge virus. Although we did not measure local immunity in these studies, protecting birds from severe upper-respiratory disease with QR448(a) and at the same time allowing field viruses to infect and induce an immune response may provide a mechanism to specifically protect chickens from resident field virus(es) regardless of the virus type.
Transmission of Arkansas type IBV vaccine from infected to contact control pen mates was examined and we observed 22% of the non-treated contact exposed birds positive for IBV whereas only 4% of the treated contact exposed birds were positive for virus at 14 days post-exposure. Clearly QR448(a) treatment diminished transmission of IBV to contact exposed birds and the effect was statistically significant (p ≤ 0.03). To ensure that maternal antibodies did not play a role in this experiment, we vaccinated the birds at 2 weeks of age when maternal antibodies were no longer detected.
Previously reported studies have shown that Arkansas type vaccine is the most prevalent virus in the commercial broilers and is capable of persisting in the flock (Jackwood et al., 2005, McKinley et al., 2008, Nix et al., 2000, van Santen and Toro, 2008). Vaccine virus was used in this experiment because colony houses (necessary to house the number of birds needed for the experiment) are not biologically secure, preventing us from using pathogenic isolates of IBV. Although this experiment was designed to be a model for the prevention of spread of pathogenic IBV isolates, the data directly address an extremely important problem of Arkansas vaccine virus persistence in commercial broiler flocks. Persistence of IBV in a flock can cause a severe vaccine reaction and provides opportunity for the vaccine virus to mutate and revert to pathogenicity as well as change antigenically avoiding the immune response of the bird. A reduction in transmission afforded by treatment with QR448(a) is extremely important since it will limit IBV transmission in the flock, and likely lessen or even prevent persistence of Arkansas vaccine virus in flocks. Since QR448(a) is effective against the pathogenic Mass41 strain of IBV it is expected that it will also limit transmission of pathogenic field strains of IBV in commercial broilers and thereby decrease the severity of the disease.
This is the first report on the use of a mixture of all natural ingredients, specifically botanical oleoresins and essential oils, as a control measure for IBV. Mass delivery of QR448(a) by spray is compatible with application in a commercial setting and it is effective against both Mass and Ark type IB viruses, indicating that it ought to be effective against IBV regardless of serotype. The effect appears to be more pronounced on cell free virus indicating that the activity is likely virucidal, which is important because it may also be effective against other enveloped respiratory viruses like avian influenza virus and Newcastle disease virus in commercial poultry, as well as other coronaviruses in animals and humans. In addition, since virucidal compounds work by inactivating viruses, rather than targeting specific virus genes or proteins, it reduces the likelihood that resistance will develop from mutations during virus replication.
References
- Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- Callison S.A., Hilt D.A., Boynton T.O., Sample B.F., Robison R., Swayne D.E., Jackwood M.W. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. Journal of Virological Methods. 2006;138:60–65. doi: 10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathology. 2005;34:439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Gelb J., Jr. Infectious bronchitis. In: Saif Y.M., Fadley A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. Diseases of Poultry. 12th ed. Blackwell Publishing; Ames, IA, USA: 2008. pp. 117–135. [Google Scholar]
- Collins P.J., Haire L.F., Lin Y.P., Liu J., Russell R.J., Walker P.A., Skehel J.J., Martin S.R., Hay A.J., Gamblin S.J. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature. 2008;453:1258–1261. doi: 10.1038/nature06956. [DOI] [PubMed] [Google Scholar]
- Delaney W.E.T., Borroto-Esoda K. Therapy of chronic hepatitis B: trends and developments. Current Opinion in Pharmacology. 2008;8:532–540. doi: 10.1016/j.coph.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Duschatzky C.B., Possetto M.L., Talarico L.B., Garcia C.C., Michis F., Almeida N.V., de Lampasona M.P., Schuff C., Damonte E.B. Evaluation of chemical and antiviral properties of essential oils from South American plants. Antiviral Chemistry and Chemotherapy. 2005;16:247–251. doi: 10.1177/095632020501600404. [DOI] [PubMed] [Google Scholar]
- Gelb J., Jr., Jackwood M.W. Infectious bronchitis. In: Dufour-Zavala L., Swayne D.E., Glisson J.R., Pearson J.E., Reed W.M., Jackwood M.W., Woolcock P., editors. A Laboratory Manual for the Isolation, Identification, and Characterization of Avian Pathogens. 5th ed. American Association of Avian Pathologists; Jacksonville, FL, USA: 2008. pp. 146–149. [Google Scholar]
- Haagmans B.L., Osterhaus A.D. Coronaviruses and their therapy. Antiviral Research. 2006;71:397–403. doi: 10.1016/j.antiviral.2006.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.M., Tarpey I., Rothwell L., Kaiser P., Hiscox J.A. Lithium chloride inhibits the coronavirus infectious bronchitis virus in cell culture. Avian Pathology. 2007;36:109–114. doi: 10.1080/03079450601156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W., Hilt D.A., Lee C.-W., Kwon H.M., Callison S.A., Moore K.M., Moscoso H., Sellers H., Thayer S. Data from 11 years of molecular typing infectious bronchitis virus field isolates. Avian Diseases. 2005;49:614–618. doi: 10.1637/7389-052905R.1. [DOI] [PubMed] [Google Scholar]
- Koch C., Reichling J., Kehm R., Sharaf M.M., Zentgraf H., Schneele J., Schnitzler P. Efficacy of anise oil, dwarf-pine oil and chamomile oil against thymidine-kinase-positive and thymidine-kinase-negative herpesviruses. Journal of Pharmacy and Pharmacology. 2008;60:1545–1550. doi: 10.1211/jpp/60.11.0017. [DOI] [PubMed] [Google Scholar]
- McKinley E.T., Hilt D.A., Jackwood M.W. Avian coronavirus infectious bronchitis attenuated live vaccines undergo selection of subpopulations and mutations following vaccination. Vaccine. 2008;26:1274–1284. doi: 10.1016/j.vaccine.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon M.A., Siliciano J.D., Lai J., Liu J.O., Stivers J.T., Siliciano R.F., Kohli R.M. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. Journal of Biological Chemistry. 2008;283:31289–31293. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S.P., Naqi S.A. Maternal antibody to infectious bronchitis virus: its role in protection against infection and development of active immunity to vaccine. Veterinary Immunology and Immunopathology. 2001;79:31–40. doi: 10.1016/S0165-2427(01)00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C., Yin J., Zhang J., Vederas J.C., James M.N. Molecular docking identifies the binding of 3-chloropyridine moieties specifically to the S1 pocket of SARS-CoV Mpro. Bioorganic and Medicinal Chemistry. 2008;16:293–302. doi: 10.1016/j.bmc.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix W.A., Troeber D.S., Kingham B.F., Keeler C.L., Gelb J., Jr. Emergence of subtype strains of the Arkansas serotype of infectious bronchitis virus in Delmarva broiler chickens. Avian Diseases. 2000;44:568–581. [PubMed] [Google Scholar]
- Phalen R.F., Mendez L.B. Dosimetry considerations for animal aerosol inhalation studies. Biomarkers. 2009;14:63–66. doi: 10.1080/13547500902965468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling J., Koch C., Stahl-Biskup E., Sojka C., Schnitzler P. Virucidal activity of a beta-triketone-rich essential oil of Leptospermum scoparium (manuka oil) against HSV-1 and HSV-2 in cell culture. Planta Medica. 2005;71:1123–1127. doi: 10.1055/s-2005-873175. [DOI] [PubMed] [Google Scholar]
- Schnitzler P., Koch C., Reichling J. Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrobial Agents and Chemotherapy. 2007;51:1859–1862. doi: 10.1128/AAC.00426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher A., Reichling J., Schnitzler P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine. 2003;10:504–510. doi: 10.1078/094471103322331467. [DOI] [PubMed] [Google Scholar]
- van Santen V.L., Toro H. Rapid selection in chickens of subpopulations within ArkDPI-derived infectious bronchitis virus vaccines. Avian Pathology. 2008;37:293–306. doi: 10.1080/03079450802043783. [DOI] [PubMed] [Google Scholar]
- Villegas P. Titration of biological suspensions. In: Dufour-Zavala L., Swayne D.E., Glisson J.R., Jackwood M.W., Pearson J.E., Reed W.M., Woolcock P., editors. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. 5th ed. American Association of Avian Pathologists; Jacksonville, FL, USA: 2008. pp. 217–221. [Google Scholar]
- Wilson L., Gage P., Ewart G. Hexamethylene amiloride blocks E protein ion channels and inhibits coronavirus replication. Virology. 2006;353:294–306. doi: 10.1016/j.virol.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X., Yu H., Yang H., Xue F., Wu Z., Shen W., Li J., Zhou Z., Ding Y., Zhao Q., Zhang X.C., Liao M., Bartlam M., Rao Z. Structures of two coronavirus main proteases: implications for substrate binding and antiviral drug design. Journal of Virology. 2008;82:2515–2527. doi: 10.1128/JVI.02114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Bartlam M., Rao Z. Drug design targeting the main protease, the Achilles’ heel of coronaviruses. Current Pharmaceutical Design. 2006;12:4573–4590. doi: 10.2174/138161206779010369. [DOI] [PubMed] [Google Scholar]
- Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., Zhao Q., Zhou Z., Pei D., Ziebuhr J., Hilgenfeld R., Yuen K.Y., Wong L., Gao G., Chen S., Chen Z., Ma D., Bartlam M., Rao Z. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biology. 2005;3:e324. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J., Schelle B., Karl N., Minskaia E., Bayer S., Siddell S.G., Gorbalenya A.E., Thiel V. Human coronavirus 229E papain-like proteases have overlapping specificities but distinct functions in viral replication. Journal of Virology. 2007;81:3922–3932. doi: 10.1128/JVI.02091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


