Highlights
► Infectious bronchitis virus is one of the most important coronavirus causing respiratory disease in chicken. ► Analysis of protein epitope in virus could provide important insights that could facilitate the development of specific diagnostics and further understanding of the antigenic structure of protein. ► Two linear B-cell epitopes that were recognized by the mAbs 6D10 and 4F10, which corresponded to the amino acid sequences 242FGPRTK247 and 195DLIARAAKI230, respectively, in the IBV N protein. ► The two epitopes are very conserved among IBV serotypes.
Keywords: Avian infectious, Bronchitis, Coronavirus, Epitope, Monoclonal antibody, Nucleocapsid protein, Phylogenetic analysis
Abstract
The nucleocapsid (N) protein of the infectious bronchitis virus (IBV) may play an essential role in the replication and translation of viral RNA. The N protein can also induce high titers of cross-reactive antibodies and cell-mediated immunity, which protects chickens from acute infection. In this study, we generated two monoclonal antibodies (mAbs), designated as 6D10 and 4F10, which were directed against the N protein of IBV using the whole viral particles as immunogens. Both of the mAbs do not cross react with Newcastle disease virus (NDV), infectious laryngotracheitis virus (ILTV) and subtype H9 avian influenza virus (AIV). After screening a phage display peptide library and peptide scanning, we identified two linear B-cell epitopes that were recognized by the mAbs 6D10 and 4F10, which corresponded to the amino acid sequences 242FGPRTK247 and 195DLIARAAKI203, respectively, in the IBV N protein. Alignments of amino acid sequences from a large number of IBV isolates indicated that the two epitopes, especially 242FGPRTK247, were well conserved among IBV strains. This conclusion was further confirmed by the relationships of 18 heterologous sequences to the 2 mAbs. The novel mAbs and the epitopes identified will be useful for developing diagnostic assays for IBV infections.
1. Introduction
Coronaviruses (CoVs) are found in a wide variety of animals where they cause respiratory, enteric, and neurological diseases with variable severity. Based on genotypic and serological analyses, CoVs are divides into 3 genera; alpha-, beta- and gamma-coronaviruses (Carstens, 2009). Alpha- and beta-coronaviruses have been isolated from mammals, while gamma-coronaviruses cause avian infectious bronchitis (IBV), as well as the genetically closely related Turkey coronavirus (Cao et al., 2008, Cavanagh et al., 2001, Gomaa et al., 2008, Guy, 2000) and pheasant coronavirus (Cavanagh et al., 2002). Numerous variants and serotypes of IBV continue to be discovered in poultry flocks worldwide (Cavanagh et al., 1988, Cavanagh et al., 1992, Dolz et al., 2006, Farsang et al., 2002, Gelb et al., 1991, Han et al., 2011, Wang and Huang, 2000) that cause infectious bronchitis (IB), which is responsible for mortality in young chickens, economic losses due to poor weight gain, and a reduction in the egg quality and quantity (Cavanagh and Gelb, 2008).
Like the typical genomic organization found in other gamma-coronaviruses, the 3′ end of the IBV genome contains the main structural genes; the spike glycoprotein (S), the small membrane protein (E), the integral membrane protein (M), and the nucleocapsid protein (N), as well as several accessory genes, usually in the order S-Gene 3-E-M-Gene 5-N (Boursnell et al., 1987). The S1 subunit of the S protein carries virus-neutralizing and serotype-specific determinants, but it exhibits high sequence diversity among different IBV serotypes. By contrast, the N protein is highly conserved with 91.0–96.5% similarity in different IBV strains (William et al., 1992). Its primary function is the formation of the viral ribonucleoprotein complex, but it is also considered that the IBV N protein is multifunctional. Its intracellular localization suggests that it is a likely component of the coronavirus replication and transcription complex. Furthermore, the N protein can induce high titers of cross-reactive antibodies and cell-mediated immunity, which protects chickens from acute infections (Ignjatovic and Galli, 1994, Seo et al., 1997, Tang et al., 2008). Most of the IBV N protein is composed of 409 amino acids with a predicted molecular weight of 45 kDa. The N protein is a phosphoprotein that can bind viral RNA with high affinity (Chen et al., 2005) and it is expressed abundantly during infections (Cavanagh, 2005). Thus, it is a target protein when designing infectious bronchitis (IB) vaccines (Tian et al., 2008) and a frequent target of diagnostic applications (Chen et al., 2003, Gibertoni et al., 2005, Ndifuna et al., 1998). However, most of the diagnostic assays had been focused on the antibody detection using recombinant N proteins. In the sampling practices in poultry farms in China, it is very common to take tracheal swabs to look for respiratory virus infections, though it is also very common to take blood samples to detect antibody. Hence, new assays focusing on virus detection, such as using mAb(s) against N protein, would be an improvement on current available diagnostic assays.
Naïve B-cells, which are the principal agents of humoral immune responses, are stimulated by the specific recognition and binding of B-cell receptors to a region of the antigen known as the epitope. Together with co-stimulation by T-lymphocytes, naïve B-cells become fully activated then proliferate and differentiate into memory and plasma cells, while the latter act as key engines for producing specific antibodies. The identification and mapping of B-cell epitopes on antigens has been a subject of intense research because knowledge of these markers has profound implications for the development of peptide-based diagnostics, therapeutics, and vaccines. B-cell epitopes may consist of linear, contiguous stretches of amino acids in a protein, or they can be discontinuous stretches of amino acids that are brought together spatially via protein folding. The majority of B-cell epitopes are discontinuous in nature, but difficulties in the design of such epitopes have led to an emphasis on the identification of linear B-cell epitopes. Monoclonal antibodies (mAbs) are used widely as powerful tools for identifying linear epitopes, or for mimicking the epitopes of a variety of infectious agents (Deng et al., 2007, Kaverin et al., 2007, Zhang et al., 2011). In this study, we prepared mAbs against the N protein of IBV strain tl/CH/LDT3/03I and used them to screen for linear B-cell epitopes. The results provided important insights that could facilitate the development of possible specific diagnostics for IBV infection and that further our understanding of the antigenic structure of N protein.
2. Materials and methods
2.1. Viruses and their propagation in specific pathogen-free embryonated eggs
IBV strain tl/CH/LDT3/03I was isolated in 2003 from a teal in Guangdong Province, China (Liu et al., 2005), and it was used for the preparation and identification of mAbs, as well as for N gene cloning and expression. To investigate the reactivity of the 2 mAbs, 25 heterogeneous IBV strains, i.e., 20 field strains and 5 vaccine strains, were used as representatives of different IBV types (Liu et al., 2006, Ma et al., 2012). The backgrounds of the 25 heterogeneous IBV strains are shown in Table 1 . All IBV strains were propagated once in 9–11-day-old specific pathogen-free (SPF) embryonated chicken eggs and the presence of viral particles in the allantoic fluids of inoculated eggs was confirmed using a negative contrast electron microscope (JEM-1200, EX) and by RT-PCR, as previously described (Liu and Kong, 2004).
Table 1.
Background information of IBV strains used in Western blotting in the present study.
| IBV strain | Countrya | Yearb | Type | GenBank accession number |
|---|---|---|---|---|
| H120 | Vaccine | – | Mass | AY856349 |
| H94 | Vaccine | – | Mass | EF602438 |
| IBN | Vaccine | – | Mass | AY856349 |
| M41 | US | 1965 | Mass | FJ904720 |
| CK/CH/LHN/00I | China (Henan) | 2000 | N1/62 associated strain | EF602456 |
| JAAS | Vaccine | – | N1/62 associated strain | AY839138 |
| J9 | Vaccine | – | N1/62 associated strain | EF602440 |
| CK/CH/LDL/97I | China (Dalian) | 1997 | CK/CH/LDL/97I | EF602445 |
| tl/CH/LDT3/03 | China (Guangdong) | 2003 | tl/CH/LDT3/03 | AY702975 |
| CK/CH/LHLJ/04V | China (Heilongjiang) | 2004 | LX4 | FJ821744/FJ821725 |
| CK/CH/LSD/03I | China (Shandong) | 2003 | LX4 | EF602457 |
| CK/CH/LTJ/95I | China (Tianjin) | 1995 | LX4 | DQ287917 |
| CK/CH/LGD/04II | China (Guangdong) | 2004 | LX4 | EF602444 |
| CK/CH/LXJ/02I | China (Xinjiang) | 2002 | LX4 | EF602458 |
| CK/CH/LLN/98I | China (Liaoning) | 1998 | LX4 | EF602451 |
| LX4 | China (Xinjiang) | 1999 | LX4 | AY338732 |
| TW2575/98 | China (Taiwan) | 1998 | TW-II | AY606327 |
| CK/CH/LGD/04III | China (Guangdong) | 2004 | Variant | EF602447 |
| CK/CH/LSD/05I | China (Shandong) | 2005 | Variant | EU637854/EU637824 |
Country (province) where the viruses were isolated.
Year when viruses were isolated.
Newcastle disease virus (NDV) La Sota vaccine strain, infectious laryngotracheitis virus (ILTV) (Tong et al., 2001) and subtype H9 avian influenza virus (AIV) (Yu et al., 2008) were used for evaluating the cross-reactivity with the 2 mAbs. All these virus strains were propagated once in 9–11-day-old SPF embryonated chicken eggs and the presence of NDV and subtype H9 AIV viral particles in the allantoic fluids of inoculated eggs was confirmed by HI using specific antibodies, respectively (Majiyagbe and Hitchner, 1977). The ILTV was confirmed by RT-PCR as previously described (Tong et al., 2001).
Fertile white Leghorn embryonated SPF chicken eggs were obtained from the Laboratory Animal Center, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, China.
2.2. Generation and identification of mAbs
Six 8-week-old BALB/c female mice were immunized subcutaneously with condensed IBV tl/CH/LDT3/03I virus-infected allantoic fluids (Yu et al., 2010) mixed with Freund's complete adjuvant, followed by two booster immunizations. The protocols used for the preparation of mAbs and ascetic fluids were as previously described (Ruf et al., 1983, Vilella et al., 1983, Yu et al., 2010). All hybridomas were cloned via at least 3 rounds of limiting dilution. Primary screening of hybridomas was by enzyme-linked immunosorbent assay (ELISA) using a commercial total antibody ELISA kit (IDEXX Corporation, Westbrook, ME, USA), according to the manufacturer's instructions. The mAbs were reacted with both IBV tl/CH/LDT3/03I virus particles and recombinant N protein as coating antigens for ELISA and Western blotting, respectively. The mAb classes and subclasses were determined using an SBA Clonotyping System/HRP kit (Southern Biotechnology Associates, Birmingham, AL, USA). Two mAbs, designated as 6D10 and 4F10, were identified and used for further fine-level epitope mapping.
2.3. Biopanning
A Ph.D.12™ Phage Display Peptide Library Kit was purchased from New England BioLabs Inc. The dodecapeptide library contained 2.7 × 109 electroporated sequences (1.5 × 1013 pfu/ml). The mAbs were purified from the ascites fluids of mice that had been inoculated with hybridoma cells and that secreted the mAbs 6D10 and 4F10, using affinity chromatography with rProtein G (Sigma, USA), according to the manufacturer's instructions, and the concentration was determined. mAbs 6D10 and 4F10 were obtained with high purity (>90%, as determined by SDS-PAGE) and used for biopanning. Three successive rounds of biopanning were carried out according to the manufacturer's instructions. Briefly, one well of a 96-well microtiter plate was coated with 15 μg of each mAb in coating buffer (0.1 M NaHCO3, pH 8.6), followed by blocking with blocking buffer (0.1 M NaHCO3, pH 8.6, 0.02% NaN3, and 5 mg/ml BSA) for 2 h at 4 °C. About 1.5 × 1011 pfu (4 × 1010 phages, i.e., 10 μl from the original library) were added to a well and incubated for 1 h at room temperature. The unbound phages were removed by successive washes with TBS buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl), which contained gradually increasing concentrations (0.1%, 0.3%, and 0.5%) of Tween-20, and the bound phages were eluted using elution buffer (0.2 M glycine–HCl, pH 2.2) containing 1 mg/ml BSA. The eluted phages were amplified in early-log phase Escherichia coli ER2738 strain cells.
2.4. Phage ELISA and the sequencing of DNA inserts displayed by phage clones
After 3 rounds of biopanning, 10 individual phage clones were selected and assayed for target binding using a sandwich ELISA, according to the manufacturer's instructions. Briefly, 96-well microtiter plates were coated overnight with 10 μg of each mAb, while anti-porcine IFN-γ mAb (Sigma, USA) served as the negative control. After 2 h of blocking with blocking buffer at 4 °C, the phage clones were added to the wells (2 × 1011 pfu in 100 μl per well) and incubated with agitation for 2 h at room temperature. Bound phages were reacted with horseradish peroxidase (HRP)-conjugated anti-M13 antibody (Pharmacia, USA), followed by color development with substrate solution containing O-phenylenediamine (OPD). The positive phage clones detected by the phage ELISA were sequenced using the −96 gIII sequencing primer 5′-TGA GCG GAT AAC AAT TTC AC-3′, as described in the manufacturer's instructions.
2.5. Construction of a recombinant plasmid and expression of recombinant proteins
The IBV tl/CH/LDT3/03I N gene and N gene fragments were cloned and sequenced, as previously described (Yu et al., 2010). To allow directional cloning into the expression vector pGEX-6p-1, Bam HI and Sal I recognition sites were introduced to the 5′ ends of the forward and reverse primers (Table 2 ). The directionality of the recombinant plasmid was verified by restriction analysis and nucleotide sequencing. The plasmid was transformed into E. coli BL21 (DE3) cells for expression. A series of fusion proteins with the expected molecular weights were induced by 1 mM IPTG, as previously described (Yu et al., 2010). The cells were harvested by centrifugation and the pellets were suspended in phosphate-buffered saline (PBS; pH 7.4). Recombinant proteins were stained with Coomassie blue after SDS-PAGE, as previously described (Yu et al., 2010). To prepare the purified proteins, the inclusion body proteins were separated by SDS-PAGE, the proteins of interest were excised, and the gel slices were crushed and added to an appropriate volume of sterilized PBS. The extracted proteins were used for Western blotting and ELISA.
Table 2.
Sequences of the primers used in this study.
 |
 |
a Underlining indicates restriction enzyme sites (Bam HI and Sal I) introduced into each primer. The boxed ATGs and TAAs are the start codon and stop codon, respectively, which were introduced into the primers of each N gene fragments.
b The nucleotide positions correspond to those in the sequence of the IBV tl/CH/LDT3/03 N gene with GenBank accession no. AY702975.
2.6. Western blotting and indirect ELISA
In the Western blotting analysis, the IBV-, NDV-, LITV- and AIV H9-infected allantoic fluids, homogenized tracheal swabs or suspended pellets in PBS were electrophoresed by SDS-PAGE using 10% acrylamide gel and transferred onto nitrocellulose membrane using a mini trans-blot system (Bio-Rad, USA), according to the manufacturer's instructions. Nonspecific binding to the membrane was blocked with 5% skim milk in PBS containing 0.05% Tween-20 (PBST) overnight at 4 °C. The membrane was washed three times with PBST and incubated with the 6D10 and 4F10 mAbs for 1 h at 37 °C, respectively. After three washes with PBST, the membrane was incubated with HRP-conjugated goat anti-mouse IgG (1:5000 dilution in PBS, pH 7.4) for 1 h at 37 °C. Following another three washes, the mAb binding to the antigen was detected using 3,3-diaminobenzidine tetrahydrochloride (DAB), which was stopped by rinsing the membrane in deionized water, followed by drying of the membrane. In the indirect ELISA, each microplate well was coated with 400 ng of each recombinant GST fusion proteins, blocked with 5% skim milk in PBST for 2 h at 37 °C, and incubated with the 6D10 and 4F10 mAbs for 1 h at 37 °C, followed by incubation with HRP-conjugated anti-mouse IgG for 1 h at 37 °C. The color was developed with TMB (3,3′,5,5′-tetramethylbenzidine) substrates for 15 min and stopped with 2 M H2SO4. Sterile allantoic fluids and recombinant GST were used as negative controls.
2.7. Phylogenetic analysis of the IBV N protein genes and a comparison of the conservation of the epitope-containing sequences of IBV strains
The N protein genes from the 228 IBV strains available in GenBank were aligned and used to construct the phylogenetic trees. The GenBank accession numbers are shown in Fig. 1 . Nucleotide sequence alignment was conducted using the CLUSTAL method and phylogenetic trees were constructed using the neighboring-joining method. Analysis of the epitope-containing sequences of IBV strains was performed using the MegAlign application in the Lasergene software package.
Fig. 1.
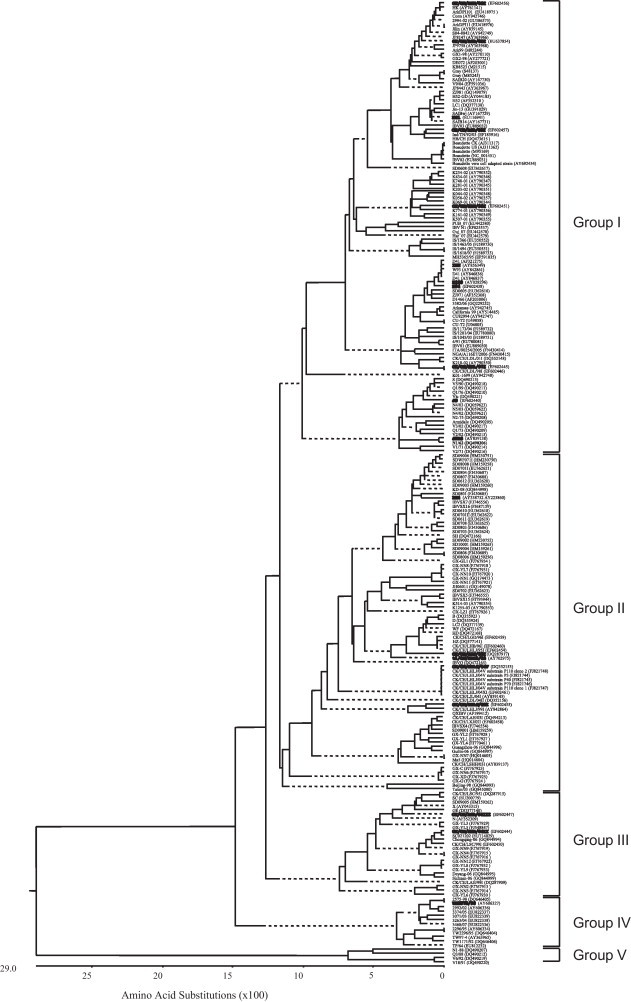
Phylogenetic tree constructed using the N genes from 228 IBV strains in GenBank using the neighbor-joining method. The IBV strains used for reacting with the 2 mAbs, 6D10 and 4F10, are highlighted in bold.
2.8. Virus recovery
The 10 swab samples taken from 8-day-old broilers of a commercial broiler flock and the 5 SPF chicken tracheal swab samples were processed individually and used for virus recovery as previously described (Liu et al., 2005). Briefly, individual samples were homogenized, diluted 1:4 with PBS, clarified by centrifugation at 300 × g for 5 min and filtered with a Teflon membrane. The filtered samples were inoculated into at least four 9-day-old SPF embryonated eggs via the allantoic cavity (0.2 ml per egg). The eggs were candled daily to record embryo mortality. After 7 days, the remaining embryos were chilled at 4 °C and examined for characteristic IBV lesions such as the dwarfing, stunting, or curling of embryos. Embryo mortality recorded in the first 24 h post-inoculation was considered non-specific and a positive sample was recorded if the specific lesions were observed.
3. Results
3.1. The 2 mAbs were active against IBV N protein
Two hybridomas, 6D10 and 4F10, were found to secrete mAbs specifically against the IBV tl/CH/LDT3/03 N protein (Fig. 2 ). The mAbs recognized recombinant N protein and the native IBV tl/CH/LDT3/03 antigen according to Western blotting (Fig. 2A). The reactivity and specificity of the 6D10 and 4F10 mAbs were confirmed using a commercial ELISA and an ELISA where whole IBV tl/CH/LDT3/03 virus particles were used as the coating antigen (Fig. 2B). In addition, the 2 mAbs, 6D10 and 4F10, showed no reactions with other respiratory viruses, such as NDV, ILTV and H9 subtype AIV, using Western blotting. The 2 mAbs were determined to be IgG1 (К) and IgG2b (К).
Fig. 2.
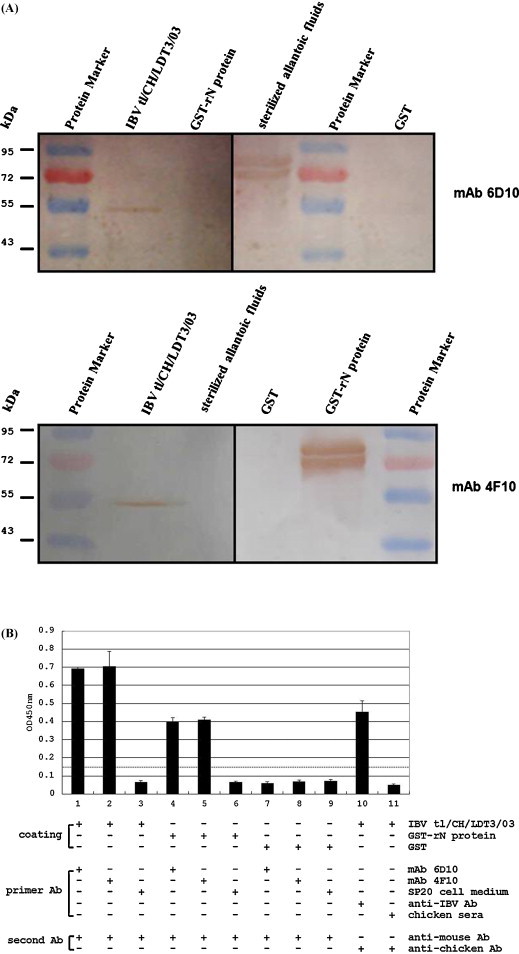
Reactivity of the 6D10 and 4F10 mAbs with IBV strain tl/CH/LDT3/03 and recombinant N protein by Western blotting (A) and indirect ELISA (B). Dashes show the S/P ratios. Samples with S/P ratios equal to or above the dashes were considered positive, whereas those below were considered negative.
3.2. Fine level mapping of the epitope of 6D10 by screening the phage display peptide library
The PhD-12™ Phage display peptide library phage kit (New England Biolabs, USA) was micropanned using the 2 mAbs against the N protein of IBV from the ELISA test. Ten clones were selected randomly from the binding phages of each mAb and sequenced using the dideoxy method. The results showed that that polypeptides displayed by the 10 phage clones using mAb 6D10 were focused on amino acids 242–247 of the N protein (Fig. 3A), which clearly demonstrated that the crucial epitopes for mAb 6D10 were located within amino acids 242–247 of the IBV N protein, designated as 242FGPRTK247. This peptide was expressed and recognized by mAb 6D10 using Western blotting, whereas GPRTK and FGPRT could not be recognized by mAb 6D10 (Fig. 3B). By contrast, the sequences of the phage clones screened by mAb 4F10 were all meaningless sequences and no epitopes of 4F10 were determined by biopanning the phage display peptide library.
Fig. 3.
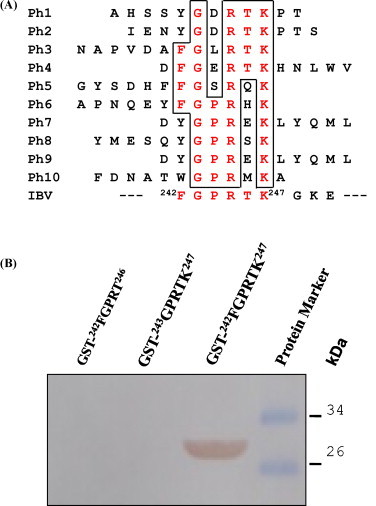
Finely mapping of the epitope for mAb 6D10 in avian infectious bronchitis virus N protein. The sequences of 12 peptides displayed by the selected phage clones were shown. A consensus sequence, 242FGPRTK247, displayed by the 10 phages had a good match with the N protein of IBV at amino acids 242–247 (A). The reactivity of mAb 6D10 with three truncated recombinant N proteins, 242FGPRTK247, 243GPRTK247 and 242FGPRT246, by Western blotting was illustrated (B).
3.3. Fine level mapping of the epitopes of 4F10 by peptide scanning
We did not screen the epitopes of mAb 4F10 using the phage display peptide library in this study. This peptide may not be included in this phage display peptide library, although the exact reason is unknown. Thus, a series of 22 partially overlapping fragments covering the N gene of IBV tl/CH/LDT3/03 were expressed with a GST tag and used to screen for the minimal epitope of mAb 4F10. The epitope of 4F10 was mapped by peptide scanning, as previously described (Yu et al., 2010). The strategy for expressing IBV N and truncated N fragments is shown in Fig. 4A. The entire N gene and its truncated fragments were expressed as GST fusion proteins in E. coli BL21 (DE3). All of the proteins were expressed successfully and they were tested by SDS-PAGE of the cell lysates after induction with IPTG. The GST fusion proteins were used for fine level mapping of the epitope of the IBV tl/CH/LDT3/03 antigen recognized by 4F10 (Fig. 4). Western blotting showed that the minimal recognition sequence was 195DLIARAAKI203, because a deletion of D195 or I203 destroyed the binding of mAb 4F10 to the GST fusion peptides (Fig. 4B). A similar reactivity was observed with ELISA using truncated peptides as coating antigens, which further confirmed that the linear epitope recognized in the N protein of IBV strain tl/CH/LDT3/03 by 4F10 appeared to be localized in 195DLIARAAKI203.
Fig. 4.
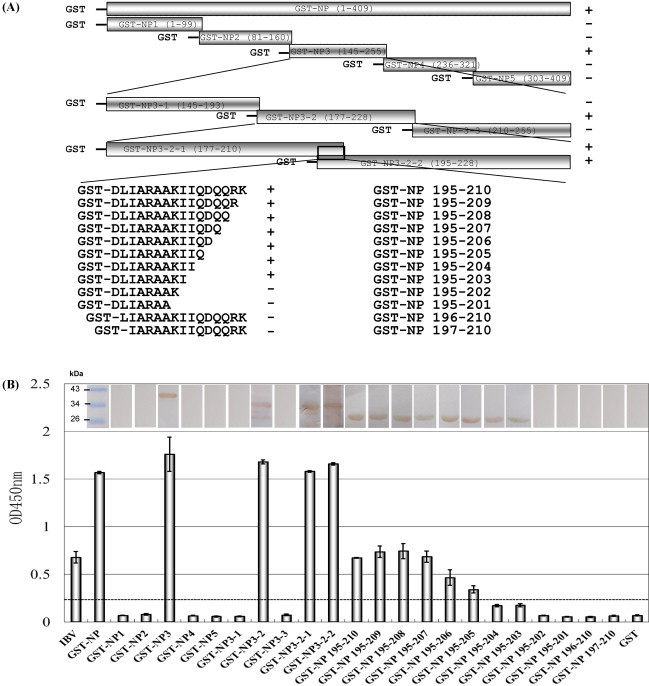
Fine level localization of the mAb 4F10 epitope. The reactivity of mAb 4F10 with truncated recombinant N proteins by Western blotting (upper part of A) and ELISA (lower part of A). The names of the proteins are shown in B. GST was used as a negative control in both assays. The protein molecular markers are indicated by short lines. For the ELISA, the dashed line indicates the S/P ratio. Samples with S/P ratios equal to or above the dashes were considered positive, whereas those below were considered negative.
3.4. Reactivity of the identified epitopes with the anti-IBV antibody
The 2 peptides, 195DLIARAAKI203 and 242FGPRTK247, which corresponded to the epitopes defined for mAbs 4F10 and 6D10, were used as antigens in Western blotting, which demonstrated that these peptides were also recognized by a chicken anti-IBV antibody. Fig. 5A and B show the results for 195DLIARAAKI203 and 242FGPRTK247, respectively.
Fig. 5.
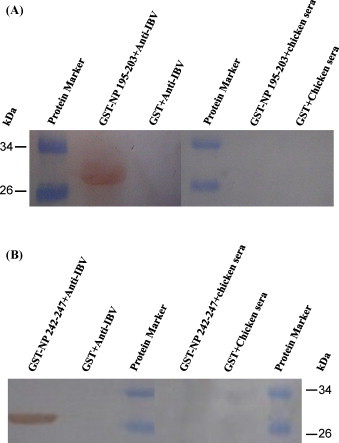
Reactivity of epitopes against mAbs 4F10 and 6D10 using a chicken antibody against IBV by Western blotting. The GST fusion proteins were used to react with chicken antibody against IBV, respectively, and SPF chicken sera was used negative control. The recombinant GST was used as a negative antigen control in both assays.
3.5. Phylogenetic analysis of the N genes
Phylogenetic analysis based on the nucleotide sequences of the N gene were performed to select heterogeneous IBV strains that reacted with the 2 mAbs. The 228 IBV strains were clustered into five distinct groups based on the N gene (Fig. 1). Most of the viruses were clustered into group I, which contained most of the IBV strains isolated from all over the world, such as IBV strains from China, US, Japan, South Korea, Israel, European countries, South Africa, and Australia. Viruses in this group could be clustered into several subgroups. All viruses in group II were from China, except 2 IBVs from South Korea. Group III included viruses isolated from the Southwest region of China. Viruses in group IV were all isolated from Taiwan province, China, while all of the viruses in group V were isolated from Australia. The viruses in each group or subgroup were highly genetically related as well as geographically related. Thus, the phylogenetic pattern based on the N genes suggested a common origin for these viruses.
3.6. Conservation of the epitope-containing sequences in IBV strains
A comparison was made to determine the conservation of epitope-containing and flank sequences of the 6D10 and 4F10 mAbs in N genes of all IBV strains used in the phylogenetic analysis. As shown in Fig. 6A, only 4/228 showed two amino acid changes and seven had one amino acid change, whereas the remaining 221 had identical sequences to the epitope of mAb 6D10, indicating the high conservation of the epitope in IBV genes. With the epitope of mAb 4F10, 59 sequences had one amino acid substitution, one sequence had two, while another had three substitutions the 228 sequences examined in this study (Fig. 6B), which also showed that this epitope was conserved in the N gene of most IBVs.
Fig. 6.
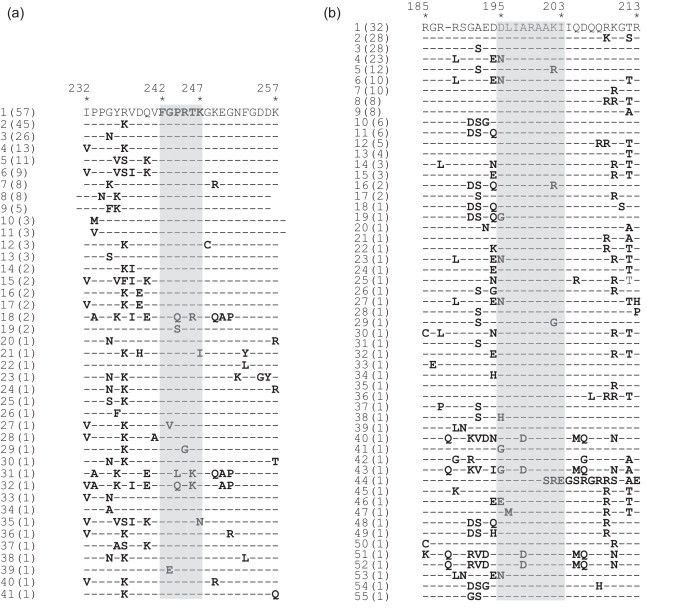
Alignment of the epitope motifs and flanking sequences of the 6D10 (A) and 4F10 (B) mAbs for the 228 strains. Epitope sequences are highlighted in grey. Deleted amino acid residues are represented by dashes. The number of viruses that shared the same motif are shown in parentheses. The viruses selected for comparison were the same as those shown in Fig. 1.
3.7. Reactivity with heterologous IBV strains
Eighteen heterogeneous IBV strains were selected to investigate their reactivity with the 6D10 and 4F10 mAbs, based on the phylogenetic analysis of N genes. The respective sequences in the N genes of the 18 IBV strains were identical to those recognized by the mAbs. All of the viruses react with the 2 mAbs in the Western blotting analysis, which further confirmed that the epitopes recognized by the 2 mAbs were conserved among IBVs. Fig. 7 shows the results for mAb 6D10 and a similar reactivity was observed for mAb 4F10 (data not shown), which suggests that the epitope sequences, and/or mAbs, would be useful in the development of diagnostic reagents to differentiate between IBV and other respiratory viruses.
Fig. 7.
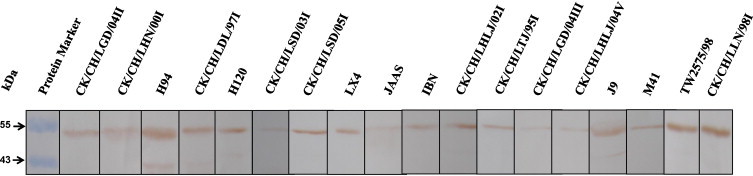
Reactivity of mAb 6D10 with 18 heterogeneous IBV strains by Western blotting. Of the 18 heterogeneous IBV strains, 12 are field isolates and the remaining 6 are vaccine strains. The protein molecular markers were also indicated (in arrowheads).
In addition, sequence comparison showed that all 18 N proteins used for reactivity with mAb 6D10 are of the same sequence over the epitopes. But for the epitope-containing sequence of mAb 4F10, strains CK/CH/LGD/04II and CK/CH/LGD/04II were Asn at position 195, and strains M41 and CK/CH/LSD/03I were Arg at position 202, respectively. The remaining 14 N proteins are of the same sequence over the epitopes.
3.8. Detection of viruses from tracheal swabs using Western blotting and virus recovery
Of the 10 tracheal swab samples collected from H120 vaccinated broilers, 5 and 6 can be detected by the mAbs 6D10 and 4F10, respectively, by using Western blotting. However, 9 of these samples are positive in virus recovery. The results are showed in Fig. 8 . No virus had been detected from tracheal swabs of SPF chickens by either Western blotting using the 2 mAbs or by virus recovery.
Fig. 8.
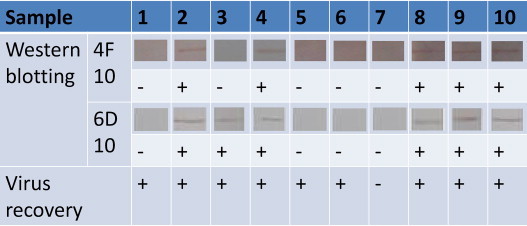
Detection of viruses from tracheal swabs using western blotting and virus recovery. Of the 10 tracheal swab samples collected from H120 vaccinated broilers, 5 and 6 can be detected by the mAbs 6D10 and 4F10, respectively, by using Western blotting, however, 9 of these samples are positive in virus recovery.
4. Discussion
The identification of B-cell antigenic epitopes for the IBV structural N protein has been limited (Ignjatovic and Sapats, 2005, Seah et al., 2000, Yu et al., 2010). In this study, we generated 2 mAbs against the N protein of IBV and we finely mapped two linear B-cell epitopes, i.e., 242FGPRTK247 and 195DLIARAAKI203, of the 2 mAbs by screening a phage display peptide library and peptide scanning, respectively. To the best of our knowledge, these 2 epitopes are the first finely mapped B-cell epitopes for the IBV N protein. In a previous study, Seah et al. (2000) used 12 fragments of the N gene expressed by E. coli, which were coupled with chicken convalescent sera against Australia T, China Ch5, Singapore P4, USA M41, and China T3 isolates, to map the linear immunodominant epitopes of N protein into 3 regions that covered amino acid residues 175–241, 310–370, and 360–409, although these epitopes were not finely mapped. The epitope 195DLIARAAKI203 recognized by mAb 4F10 was included in the first region identified by Seah et al. (2000) but the 242FGPRTK247 region recognized by mAb 6D10 was not included in these 3 regions. This discrepancy may be because different viruses were used as the antigens for generating convalescent sera or mAbs. The serotype tl/CH/LDT3/03 (Liu et al., 2005) used in our study was also a different serotype from those used by Seah et al. (2000).
Nucleocapsid protein is produced abundantly in infections and it readily induces antibody so it is the preferred choice for the development of a group-specific diagnostic tool for IBV infections (Ndifuna et al., 1998). This is also true of other various RNA viruses, such as mumps, rabies, vesicular stomatitis, measles, and Newcastle disease viruses, which have been used as targeted antigens in the development of diagnostic agents (Linde et al., 1987, Reid-Sanden et al., 1990, Hummel et al., 1992, Ahmad et al., 1993, Errington et al., 1995). The N protein of IBV contains 409 amino acids with a predicted molecular mass of 50 kDa (Boursnell et al., 1987). Previous work has shown that the N gene of IBVs is highly conserved and they share 94–99% identity among various strains (Sapats et al., 1996, William et al., 1992), but genetic diversity has also been observed in IBV strains isolated during recent years (Liu et al., 2008a). To comprehensively analyze the degree of conservation, we carefully compared the two epitope-containing sequences in the N gene of all IBV strains (228 strains) available in GenBank. This showed that the two epitope-containing sequences were conserved among IBV strains, which further confirmed our conclusions.
We selected 18 heterologous IBV strains to test whether they could be recognized by the 2 mAbs by Western blotting, which further highlighted their suitability for the development of a diagnostic method using the mAbs and/or the epitope-containing sequences in the N gene. A large number of IBV N gene sequences were available in GenBank and a phylogenetic tree was constructed, which was used as a criterion for the selection of IBV strains. Remarkably, the phylogenetic relationship among IBV strains based on the N gene did not parallel those based on the S1 genes (Han et al., 2011), which may be explained by recombination events in the genomes of IBV strains (Jackwood et al., 2010). There was a lack of IBV strains in group V in our laboratory (this group only contained viruses isolated in Australia) and the 18 viruses selected in this study only came from the four remaining groups (groups I–IV). Nevertheless, all of the heterologous IBV strains selected were well recognized by the 2 mAbs, which suggested that the epitopes recognized by the 2 mAbs were conserved among the heterologous IBV strains. However, by using Western blotting, IB viruses can not be detected from some of the tracheal swabs of chickens which recently vaccinated with H120 vaccines, which may be due to the limited viruses.
Viral upper respiratory diseases such as IBV (Liu and Kong, 2004), NDV (Liu et al., 2008b), subtype H9 AIV infection (Ji et al., 2010), and ILTV (Pang et al., 2002) are a serious problem in farms in China. The detection of IBV infections of poultry flocks is a major challenge because of the difficulty in differentiating this condition from other upper respiratory diseases. Thus, appropriate diagnostic methods are needed and they are an important tool for taking appropriate preventive steps. Another problem that complicates IBV detection and diagnosis is the existence of multiple serotypes of IBV, which co-circulate in vaccinated and non-vaccinated chicken flocks (Liu and Kong, 2004). The astonishing diversity of IBV in chickens is probably a result of the higher mutation rate of RNA viruses due to the infidelity of their polymerases and higher opportunity for recombination because of their unique replication mechanism, which suggests that there are probably many other unknown types and that many more will emerge in chickens.
The availability of the mAbs and their corresponding epitope-containing sequences identified in this study will facilitate the development of diagnostic molecular assays to differentiate between IBV and other respiratory viruses, and to identify unknown types of IBVs or future IBV strains. However, because the 2 mAbs can recognize both field isolates and commonly used vaccine strains, they can not be used for developing diagnostic molecular assays to differentiate between field isolates and vaccines. In addition, we have identified a small amount of variations across both epitopes by comparing IBV N gene sequences in the GenBank database. However, because of the limited IBV strains available in our laboratory, we only tested cross-reactivity of 18 heterogeneous IBV strains which contained identical sequences over the epitope regions. In the future study, we will generate a series recombinant N proteins by mutating the GST-N constructs according to the sequence variations across both epitopes to identify the reactivity of the two antibodies to non-identical sequences of N proteins. Nevertheless, the development of these diagnostic assays will be very challenging.
Acknowledgments
This work was supported by grants from the China Agriculture Research Systerm (No. CARS-41-K12) and Special Fund for Agro-scientific Research in the Public Interest (No. 201203056).
References
- Ahmad S., Bassiri M., Banerjee A.K., Yilma T. Immunological characterisation of the VSV nucleocapsid (N) protein expressed by recombinant baculovirus in Spodoptera exigua larva: use in differential diagnosis between vaccinated and infected animals. Virology. 1993;192:207–216. doi: 10.1006/viro.1993.1023. [DOI] [PubMed] [Google Scholar]
- Boursnell M.E., Brown T.D., Foulds I.J., Green P.F., Tomley F.M., Binns M.M. Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. Journal of General Virology. 1987;68:57–77. doi: 10.1099/0022-1317-68-1-57. [DOI] [PubMed] [Google Scholar]
- Carstens E.B. Report from the 40th meeting of the executive committee of the International Committee of Taxonomy of Viruses. Archives of Virology. 2009;154:1571–1574. [Google Scholar]
- Cao J., Wu C.C., Lin T.L. Complete nucleotide sequence of polyprotein gene 1 and genome organization of turkey coronavirus. Virus Research. 2008;136:43–49. doi: 10.1016/j.virusres.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathology. 2005;34:439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Cook J.K., Li D., Kant A., Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathology. 1992;21:33–43. doi: 10.1080/03079459208418816. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Mockett A.P.A. Amino acids within hypervariable region 1 of avian coronavirus IBV (Massachusetts serotype) spike glycoprotein are associated with neutralization epitopes. Virus Research. 1988;11:141–150. doi: 10.1016/0168-1702(88)90039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Gelb J. Infectious bronchitis. In: Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. Diseases of Poultry. 12th ed. Wiley-Blackwell Publishing; Iowa: 2008. pp. 117–135. [Google Scholar]
- Cavanagh D., Mawditt K., Sharma M., Drury S.E., Ainsworth H.L. Detection of a coronavirus from turkey poults in Europe genetically related to infectious bronchitis virus of chickens. Avian Pathology. 2001;30:355–368. doi: 10.1080/03079450120066368. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Mawditt K., Welchman D.B., Britton P., Gough R.E. Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathology. 2002;31:81–93. doi: 10.1080/03079450120106651. [DOI] [PubMed] [Google Scholar]
- Chen H., Coote B., Attree S., Hiscox J.A. Evaluation of a nucleoprotein-based enzyme-linked immunosorbent assay for the detection of antibodies against infectious bronchitis virus. Avian Pathology. 2003;32:519–526. doi: 10.1080/0307945031000154125. [DOI] [PubMed] [Google Scholar]
- Chen H., Gill A., Dove B.K., Emmett S.R., Kemp F.C., Ritchie M.A., Dee M., Hiscox J.A. Mass spectroscopic characterisation of the coronavirus infectious bronchitis virus nucleoprotein and elucidation of the role of phosphorylation in RNA binding using surface plasmon resonance. Journal of Virology. 2005;79:1164–1179. doi: 10.1128/JVI.79.2.1164-1179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Gao Y., Gao H., Qi X., Cheng Y., Wang X., Wang X. Antigenic structure analysis of VP3 of infectious bursal disease virus. Virus Research. 2007;129:35–42. doi: 10.1016/j.virusres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Dolz R., Pujols J., Ordonez G., Porta R., Majó N. Antigenic and molecular characterization of isolates of the Italy 02 infectious bronchitis virus genotype. Avian Pathology. 2006;35:77–85. doi: 10.1080/03079450600597295. [DOI] [PubMed] [Google Scholar]
- Errington W., Steward M., Emmerson P. A diagnostic immunoassay for Newcastle disease virus based on the nucleocapsid protein expressed by a recombinant baculovirus. Journal of Virological Methods. 1995;55:357–365. doi: 10.1016/0166-0934(95)00074-7. [DOI] [PubMed] [Google Scholar]
- Farsang A., Ros C., Renstrom L.H.M., Baule C., Soós T., Belák S. Molecular epizootiology of infectious bronchitis virus in Sweden indicating the involvement of a vaccine strain. Avian Pathology. 2002;31:229–236. doi: 10.1080/03079450220136530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb J., Jr., Wolff J.B., Moran C.A. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens. Avian Diseases. 1991;35:82–87. [PubMed] [Google Scholar]
- Gibertoni A.M., Montassier Mde F., Sena J.A., Givisiez P.E., Furuyama C.R., Montassier H.J. Development and application of a Saccharomyces cerevisiae-expressed nucleocapsid protein-based enzyme-linked immunosorbent assay for detection of antibodies against infectious bronchitis virus. Journal of Clinical Microbiology. 2005;43:1982–1984. doi: 10.1128/JCM.43.4.1982-1984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa M.H., Barta J.R., Ojkic D., Yoo D. Complete genomic sequence of turkey coronavirus. Virus Research. 2008;135:237–246. doi: 10.1016/j.virusres.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J.S. Turkey coronavirus is more closely related to avian infectious bronchitis virus than to mammalian coronaviruses: a review. Avian Pathology. 2000;29:207–212. doi: 10.1080/03079450050045459. [DOI] [PubMed] [Google Scholar]
- Han Z., Sun C., Yan B., Zhang X., Wang Y., Li C., Zhang Q., Ma Y., Shao Y., Liu Q., Kong X., Liu S. A 15-year analysis of molecular epidemiology of avian infectious bronchitis coronavirus in China. Infection, Genetics and Evolution. 2011;11:190–200. doi: 10.1016/j.meegid.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel K.B., Erdman D.D., Heath J., Bellini W.J. Baculovirus expression of the nucleocapsid gene of measles virus and utility of the recombinant proten in diagnostic enzyme immunoassays. Journal of Clinical Microbiology. 1992;30:2280–2874. doi: 10.1128/jcm.30.11.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic J., Galli L. The S1 glycoprotein but not N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Archives of Virology. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic J., Sapats S. Identification of previously unknown antigenic epitopes on the S and N proteins of avian infectious bronchitis virus. Archives of Virology. 2005;150:1813–1831. doi: 10.1007/s00705-005-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W., Boynton T.O., Hilt D.A., McKinley E.T., Kissinger J.C., Paterson A.H., Robertson J., Lemke C., McCall A.W., Williams S.M., Jackwood J.W., Byrd L.A. Emergence of a group 3 coronavirus through recombination. Virology. 2010;398:98–108. doi: 10.1016/j.virol.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K., Jiang W.M., Liu S. Characterization of the hemagglutinin gene of subtype H9 avian influenza viruses isolated in 2007–2009 in China. Journal of Virological Methods. 2010;163:186–189. doi: 10.1016/j.jviromet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Kaverin N.V., Rudneva I.A., Govorkova E.A. Epitope mapping of the hemagglutinin molecule of a highly pathogenic H5N1 influenza virus by using monoclonal antibodies. Journal of Virology. 2007;81:12911–12917. doi: 10.1128/JVI.01522-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde G.A., Granstrom M., Orvell C. Immunoglobiulin class and immunoglobulin G subclass enzyme-linked immunosorbent assays compared with microneutralisation assay for sero-diagnosis of mumps infection and determination of immunity. Journal of Clinical Microbiology. 1987;25:1653–1658. doi: 10.1128/jcm.25.9.1653-1658.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang Z., Wang Y., Sun C., Zheng D., Wu Y. Characterization of Newcastle disease virus isolated from waterfowl in China. Avian Diseases. 2008;52:150–155. doi: 10.1637/8030-061507-Reg. [DOI] [PubMed] [Google Scholar]
- Liu S., Chen J., Chen J., Kong X., Shao Y., Han Z., Feng L., Cai X., Gu S., Liu M. Isolation of avian infectious bronchitis coronavirus from domestic peafowl (Pavo cristatus) and teal (Anas) Journal of General Virology. 2005;86:719–725. doi: 10.1099/vir.0.80546-0. [DOI] [PubMed] [Google Scholar]
- Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathology. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang Q., Chen J., Han Z., Liu X., Feng L., Shao Y., Rong J., Kong X., Tong G. Genetic diversity of avian infectious bronchitis coronavirus strains isolated in China between 1995 and 2004. Archives of Virology. 2006;151:1133–1148. doi: 10.1007/s00705-005-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang Q., Chen J., Han Z., Shao Y., Kong X., Tong G. Identification of the avian infectious bronchitis coronaviruses 3 with mutations in gene 3. Gene. 2008;412:12–25. doi: 10.1016/j.gene.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Shao Y., Sun C., Han Z., Liu X., Guo H., Liu X., Kong X., Liu S. Genetic diversity of avian infectious bronchitis coronavirus in recent years in China. Avian Diseases. 2012;56:15–28. doi: 10.1637/9804-052011-Reg.1. [DOI] [PubMed] [Google Scholar]
- Majiyagbe K.A., Hitchner S.B. Antibody response to strain combinations of Newcastle disease virus as measured by hemagglutination–inhibition. Avian Diseases. 1977;21:576–584. [PubMed] [Google Scholar]
- Ndifuna A., Waters A.K., Zhou M., Collisson E.W. Recombinant nucleocapsid protein is potentially an inexpensive, effective serodiagnostic reagent for infectious bronchitis virus. Journal of Virological Methods. 1998;70:37–44. doi: 10.1016/S0166-0934(97)00170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y., Wang H., Girshick T., Xie Z., Khan M.I. Development and application of a multiplex polymerase chain reaction for avian respiratory agents. Avian Diseases. 2002;46:691–699. doi: 10.1637/0005-2086(2002)046[0691:DAAOAM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reid-Sanden F.L., Sumner J.W., Smith J.S., Fekadu M., Shaddock J.H., Bellini W.J. Rabies diagnostic reagents prepared from a rabies N gene recombinant expressed in baculovirus. Journal of Clinical Microbiology. 1990;28:858–863. doi: 10.1128/jcm.28.5.858-863.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf J., Carayon P., Sarles-Philip N., Kourilsky F., Lissitzky S. Specificity of monoclonal antibodies against human thyroglobulin; comparison with autoimmune antibodies. EMBO Journal. 1983;2:1821–1826. doi: 10.1002/j.1460-2075.1983.tb01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapats S., Ashton F., Wright P.J., Ignjatovic J. Novel variation in the N protein of avian infectious bronchitis virus. Virology. 1996;226:217–412. doi: 10.1006/viro.1996.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seah J.N., Yu L., Kwang J. Localization of linear B-cell epitopes on infectious bronchitis virus nucleocapsid protein. Veterinary Microbiology. 2000;75:11–16. doi: 10.1016/s0378-1135(00)00202-9. [DOI] [PubMed] [Google Scholar]
- Seo S.H., Wang L., Smith R., Collisson E.W. The carboxyl-terminal 120-residue polypetide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chickens from acute infection. Journal of Virology. 1997;71:7888–7894. doi: 10.1128/jvi.71.10.7889-7894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Wang H., Zhou S., Tian G. Enhancement of the immunogenicity of an infectious bronchitis virus DNA vaccine by a bicistronic plasmid encoding nucleocapsid protein and interleukin-2. Journal of Virological Methods. 2008;149:42–48. doi: 10.1016/j.jviromet.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Wang H., Lu D., Zhang Y., Wang T., Kang R. The immunoreactivity of a chimeric multi-epitope DNA vaccine against IBV in chickens. Biochemical and Biophysical Research Communications. 2008;377:221–225. doi: 10.1016/j.bbrc.2008.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G.Z., Zhang S.J., Wang L., Qiu H.J., Wang Y.F., Wang M. Protection of chickens from infectious laryngotracheitis with a recombinant fowlpox virus expressing glycoprotein B of infectious laryngotracheitis virus. Avian Pathology. 2001;30:143–148. doi: 10.1080/03079450120044542. [DOI] [PubMed] [Google Scholar]
- Vilella R., Yague J., Vives J. Monoclonal antibody against HLA-Aw32tA25. Is HLA-Aw32 an allele with no unique antigenic determinant? Human Immunology. 1983;6:53–62. doi: 10.1016/0198-8859(83)90073-3. [DOI] [PubMed] [Google Scholar]
- Wang C.H., Huang Y.C. Relationship between serotypes and genotypes based on the hypervariable region of the S1 gene of infectious bronchitis virus. Archives of Virology. 2000;45:291–300. doi: 10.1007/s007050050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William A., Wang L., Sneed L.L.W., Collisson E.W. Comparative analyses of the nucleocapsid genes of several strains of infectious bronchitis virus and other coronavirus. Virus Research. 1992;25:213–222. doi: 10.1016/0168-1702(92)90135-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Han Z., Xu J., Shao Y., Li H., Kong X., Liu S. A novel B-cell epitope of avian infectious bronchitis virus N protein. Viral Immunology. 2010;23:189–199. doi: 10.1089/vim.2009.0094. [DOI] [PubMed] [Google Scholar]
- Yu H., Hua R.H., Wei T.C., Zhou Y.J., Tian Z.J., Li G.X., Liu T.Q., Tong G.Z. Isolation and genetic characterization of avian origin H9N2 influenza viruses from pigs in China. Veterinary Microbiology. 2008;131:82–92. doi: 10.1016/j.vetmic.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Zhang X., Xu J., Sun Y., Li Su Li N., Yang S., He F., Huang J.H., Ling L.J., Qiu H.J. Identification of a linear epitope on the capsid protein of classical swine fever virus. Virus Research. 2011;156:134–140. doi: 10.1016/j.virusres.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


