Abstract
Oropouche virus (ORO), family Bunyaviridae, is the second most frequent cause of arboviral febrile illness in Brazil. Studies were conducted to understand ORO entry in HeLa cells. Chlorpromazine inhibited early steps of ORO replication cycle, consistent with entry/uncoating. The data indicate that ORO enters HeLa cells by clathrin-coated vesicles, by a mechanism susceptible to endosomal acidification inhibitors. Transmission electron microscopy and immunofluorescence indicated that ORO associates with clathrin-coated pits and can be found in association with late endosomes in a time shorter than 1 h.
Keywords: Oropouche virus, Clathrin, Endocytosis, Bunyaviridae
Oropouche (ORO) is an emerging zoonotic virus of the family Bunyaviridae, genus Orthobunyavirus, serogroup Simbu (Schmaljohn and Hooper, 2001), which circulates in wild animals, mainly sloths, and causes human epidemics in urban areas of tropical South America. During rainy seasons, proliferating insect vectors, especially the biting midge Culicoides paraensis, facilitate transmission of the virus from animal reservoirs to human hosts, with rapid spread in populations of villages and towns and significant public health impact. Human infection is clinically characterized by fever, myalgia, headache, arthralgia, skin rash and malaise, which may be long lasting (Pinheiro et al., 1981). While ORO fever has been registered almost exclusively in the Amazon, global warming, deforestation and redistribution of vectors and reservoirs increase the risk of ORO spreading to others areas of the Americas.
Many viruses subvert host cell endocytic pathways as entry routes, through a process triggered by receptor binding. Endocytosis within clathrin-coated acidic vesicles (Brodsky et al., 2001) is pivotal for the entry/uncoating of JC and West Nile viruses and human rhinovirus type 2 (Chu and Ng, 2004, Pho et al., 2000, Snyers et al., 2003). Uptake within caveolae has been described for simian virus 40 (SV40) and coronavirus 229E (Nomura et al., 2004, Stang et al., 1997). Little is known about the entry mechanisms of ORO. Here we describe studies conducted to understand how ORO enters HeLa cells.
ORO was inoculated onto monolayers of HeLa cells (MOI = 1), adsorbed at 4 °C for 1 h, washed five times with PBS and incubated at 37 °C. At different times postinfection, cells were fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer pH 7.0, then postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h, dehydrated in ethanol, stained with uranyl acetate and embedded in EMBED 812 (EMS, Hatfield, PA). Thin sections were post-stained with lead citrate and uranyl acetate. ORO-like particles were observed by EM in association with clathrin-coated pits (Fig. 1 ).
Fig. 1.
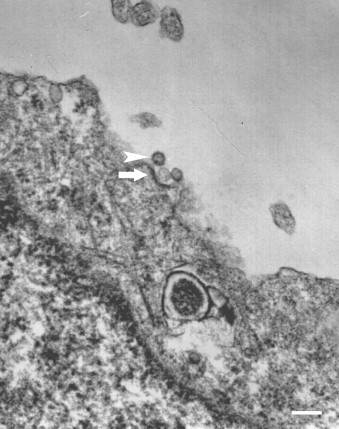
Transmission electron microscopy of ORO in HeLa cells. ORO (arrowhead) is seen in association with clathrin-coated pits (arrow) on the surface of HeLa cells. Bar = 150 nm).
HeLa cell monolayers prepared on 13 mm coverslips were inoculated with ORO (MOI = 1) and absorbed at 4 °C for 1 h. Coverslips were then washed three times with PBS and replenished with MEM with 2% fetal bovine serum (FBS), incubated at 37 °C for 30 min and fixed with 2% paraformaldehyde (Sigma). Cells were permeabilized with 0.3% Triton X-100 (Sigma) in PBS and blocked with 7 μg/mL donkey IgG in PBS with 1%BSA. Coverslips were then incubated with a mixture of goat anti-clathrin heavy chain (Santa Cruz, Santa Cruz, CA) and mouse anti-ORO polyclonal antibody (kind gift from Luiz Tadeu M. Figueiredo). In double-labeling experiments, Alexa-594-conjugated anti-mouse antibody and Alexa-488-conjugated anti-goat secondary antibodies were used (Invitrogen, Carlsbad, CA). The presence of distinctive yellow/orange granular labeling, indicates colocalization of ORO and clathrin heavy chain (Fig. 2 upper pannel). All IF coverslips were examined by confocal microscopy (Leica TCS-SP2-AOBS, Manheim, Germany).
Fig. 2.

Upper pannel: confocal microscopy of ORO (green) and clathrin (CLAT) heavy chain (red). Image of one representative slice of HeLa cells is shown 30 min post-ORO adsorption with XY and XZ projections of slice stacks. Colocalization of ORO and clathrin is shown in yellow/orange tones. Bar = 10 μm. Middle panel: confocal microscopy of ORO (green) and EEA1 (red). Image of one representative slice of HeLa cells is shown 40 min post-ORO adsorption with XY and XZ projections of slice stacks. The lack of yellow tones indicates absence of colocalization. Bar = 30 μm. Down panel: confocal microscopy of ORO (green) and Rab7 (red). Image of one representative slice of HeLa cells is shown 40 min post-ORO adsorption with XY and XZ projections of slice stacks. Colocalization of ORO and late endosomes is shown in yellow/orange tones (arrowheads). Bar = 10 μm.
Chlorpromazine (Sigma, St. Louis, MO), an agent that blocks clathrin-dependent endocytosis (Pho et al., 2000, Subtil et al., 1994, Wang et al., 1993), inhibited ORO replication in HeLa cells in a dose-dependent fashion and, at 4 μg/ml, significantly reduced virus replication by more than 90% without significant cytotoxicity (Figure S1). Transferrin uptake is known to be clathrin-dependent and therefore, transferrin conjugated with Texas Red (Invitrogen, Carlsbad, CA) was used as control for the effect of chlorpromazine (Figure S2).
For chlorpromazine timing of addition assays, ORO was inoculated onto HeLa cell monolayers (MOI = 0.1), adsorbed at 4 °C for 1 h, then cells were washed five times with cold PBS, replenished with fresh MEM with 2% FBS and incubated at 37 °C for 24 h. Chlorpromazine was added to HeLa cell cultures at 4 μg/ml 1 h before (−1), at the time of virus inoculation (0), and at 1 and 2 h postinfection. After 24 h, cells and supernatants were harvested separately for virus titration by CPE detection in quadruplicate monolayers of Vero cells inoculated with decimal serial dilutions (TCID50).
Chlorpromazine inhibited ORO production when added at −1 h and 0 h, but accumulation of progeny virus was close to the control level when the drug was added at 1 or 2 h postinfection, indicating that chlorpromazine inhibits ORO production at an initial phase of the virus cycle, consistent with a clathrin-dependent mechanism of entry/uncoating (Fig. 3A).
Fig. 3.
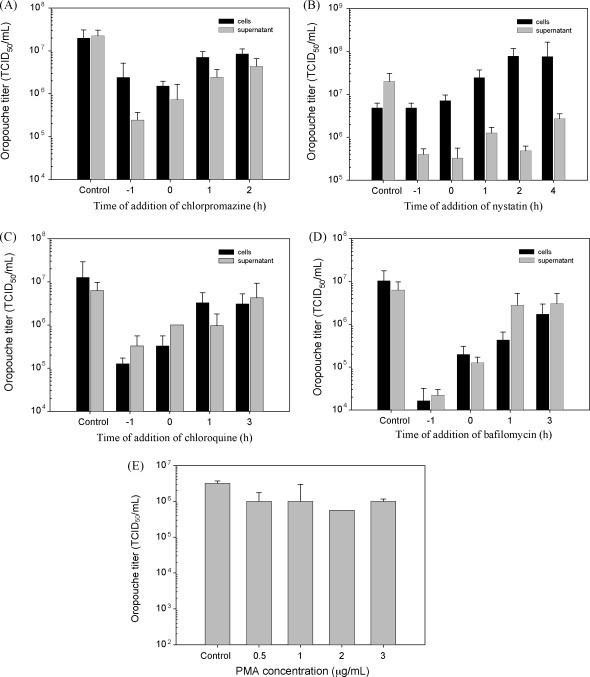
Effects of drugs on the replication of ORO: 4 μg/mL of chlorpromazine (A), 40 μg/mL of nystatin (B), 4 μg/mL of chloroquine (C) or 8 nM of bafilomycin (D) were added to HeLa monolayers, at different times relative to the infection, starting 1 h before (−1) and continuing at different times post-infection with ORO. PMA did not inhibit ORO replication (E), therefore, timing of addition assay was not carried out with this drug.
Endocytosis by caveolae is dependent on cholesterol-rich domains (Anderson and Jacobson, 2002, Couet et al., 2001, Nabi and Le, 2003, Shin and Abraham, 2001) and because nystatin (Sigma) binds cholesterol with high affinity (Pucadyil et al., 2004), cells treated with this drug are deficient in caveolin-dependent endocytosis. PMA (Sigma) also inhibits caveolae, by activating phosphokinase C (PKC). The caveolae inhibitory effect of nystatin and PMA in HeLa cells was confirmed using cholera toxin B subunit (CTB) as positive control (Figure S3). Inhibition of ORO by nystatin and PMA was tested following the same protocol used with chlorpromazine, except that PMA incubation was carried out for 4 h. PMA did not inhibit ORO production even when used at concentrations as high as 3 μg/mL (Fig. 3E), suggesting that ORO entry is independent of caveolae. Nystatin inhibited ORO in a dose-dependent fashion and at 40 μg/ml significantly reduced virus production by more than 90%. Neither nystatin or PMA had significant cytotoxicity (Figure S1). Timing of addition assays showed that nystatin inhibited ORO production when added 1 h prior to (−1) and at the time of ORO infection (0). However, ORO titers in supernatants did not return to control levels even when drug was added as late as 4 h postinfection (Fig. 3B). Remarkably, significant accumulation of infectious intracellular virus continued to be observed while ORO titers in supernatants remained low, suggesting that, in addition to cholesterol-rich domains associated with caveolae, nystatin may inhibit other cholesterol-dependent processes, like constitutive exocytosis (Salaun et al., 2004) that may be involved in later steps of the ORO replication cycle.
The inhibitors of endosomal acidification, chloroquine and bafilomycin (Sigma), respectively at 4 μg/ml and 8 nM, reduced ORO production by more than 90% without significant cytotoxicity (Figure S1). Both chloroquine and bafilomycin inhibited endosomal acidification in HeLa cells as confirmed by experiments with Lysotracker (Figure S4). In timing of addition assays, chloroquine and bafilomycin at the above concentrations inhibited ORO production in HeLa cells when added 1 h prior to (−1) and at the time of infection (0), but ORO titers tended to resume control levels when the drugs were added at subsequent times (Fig. 3C and D). This suggests that ORO entry requires acidic compartments, probably for uncoating.
To look further into the ORO entry pathway, HeLa cell monolayers prepared on coverslips were inoculated with ORO (MOI = 1), adsorbed at 4 °C for 1 h, incubated for 40 min at 37 °C and fixed with 2% paraformaldehyde (Sigma). EEA 1 and rab7 are markers for early and late endosomes, respectively. While no colocalization of ORO with EEA1 was observed, the colocalization of ORO with rab7 indicates that the virus reaches late endosomes in less than 1 h (Fig. 2).
Despite its importance for public health in tropical South America, virtually nothing is known about the cell biology of ORO replication. This study showed that ORO enters HeLa cells in association with clathrin-coated pits and that its acidification-dependent endocytic pathway reaches late endosomes in less than 1 h. These results are in agreement with previously published data on the entry mechanisms of other Bunyaviridae, such as Hantaan virus, which also enters cells by clathrin-dependent mechanism and requires low pH (Jin et al., 2002). It has also been suggested that Uukuniemi and California Encephalitis viruses require low pH for entry (Hacker et al., 1995, Ronka et al., 1995). It is reasonable to investigate inhibitors of endosomal acidification as possible adjuvants for therapy of ORO infections.
We are indebted to Maria Tereza Pianoto Maglia, Maria Dolores Sealbra Ferreira, José Augusto Maulin and Marcia Sirlene Zardin Graeff for technical support, and to Dr. Joao K. Kajiwara for helpful suggestions.
Footnotes
Financial support: FAPESP Grant No. 2003/03682-3. RIMS was a recipient of a FAPESP M.Sc. scholarship. EA, MAR, CO, and RAM are recipients of Research Scholarships from CNPq, Brazil.
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2008.08.016.
Appendix A. Supplementary data
References
- Anderson R.G., Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296(5574):1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- Brodsky F.M., Chen C.Y., Knuehl C., Towler M.C., Wakeham D.E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Chu J.J., Ng M.L. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 2004;78(19):10543–10555. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couet J., Belanger M.M., Roussel E., Drolet M.C. Cell biology of caveolae and caveolin. Adv. Drug Deliv. Rev. 2001;49(3):223–235. doi: 10.1016/s0169-409x(01)00139-9. [DOI] [PubMed] [Google Scholar]
- Hacker J.K., Volkman L.E., Hardy J.L. Requirement for the G1 protein of California encephalitis virus in infection in vitro and in vivo. Virology. 1995;206(2):945–953. doi: 10.1006/viro.1995.1017. [DOI] [PubMed] [Google Scholar]
- Jin M., Park J., Lee S., Park B., Shin J., Song K.J., Ahn T.I., Hwang S.Y., Ahn B.Y., Ahn K. Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology. 2002;294(1):60–69. doi: 10.1006/viro.2001.1303. [DOI] [PubMed] [Google Scholar]
- Nabi I.R., Le P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003;161(4):673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura R., Kiyota A., Suzaki E., Kataoka K., Ohe Y., Miyamoto K., Senda T., Fujimoto T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004;78(16):8701–8708. doi: 10.1128/JVI.78.16.8701-8708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pho M.T., Ashok A., Atwood W.J. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 2000;74(5):2288–2292. doi: 10.1128/jvi.74.5.2288-2292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro F.P., Travassos da Rosa A.P., Travassos da Rosa J.F., Ishak R., Freitas R.B., Gomes M.L., LeDuc J.W., Oliva O.F. Oropouche virus. I. A review of clinical, epidemiological, and ecological findings. Am. J. Trop. Med. Hyg. 1981;30(1):149–160. [PubMed] [Google Scholar]
- Pucadyil T.J., Shrivastava S., Chattopadhyay A. The sterol-binding antibiotic nystatin differentially modulates ligand binding of the bovine hippocampal serotonin1A receptor. Biochem. Biophys. Res. Commun. 2004;320(2):557–562. doi: 10.1016/j.bbrc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Ronka H., Hilden P., Von Bonsdorff C.H., Kuismanen E. Homodimeric association of the spike glycoproteins G1 and G2 of Uukuniemi virus. Virology. 1995;211(1):241–250. doi: 10.1006/viro.1995.1397. [DOI] [PubMed] [Google Scholar]
- Salaun C., James D.J., Chamberlain L.H. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5(4):255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn C.S., Hooper J.W. Bunyaviridae: the virus and their replication. In: Knipe D.M., Howley P.M., editors. 4th ed. vol. 2.2 vols. Lippincott Williams & wilkins; Philadelphia: 2001. pp. 216–220. (Fields—Virology). [Google Scholar]
- Shin J.S., Abraham S.N. Cell biology. Caveolae—not just craters in the cellular landscape. Science. 2001;293(5534):1447–1448. doi: 10.1126/science.1061079. [DOI] [PubMed] [Google Scholar]
- Snyers L., Zwickl H., Blaas D. Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis. J. Virol. 2003;77(9):5360–5369. doi: 10.1128/JVI.77.9.5360-5369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang E., Kartenbeck J., Parton R.G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell. 1997;8(1):47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A., Hemar A., Dautry-Varsat A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J. Cell Sci. 1994;107(Pt 12):3461–3468. doi: 10.1242/jcs.107.12.3461. [DOI] [PubMed] [Google Scholar]
- Wang L.H., Rothberg K.G., Anderson R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993;123(5):1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


