Highlights
-
•
N protein of TGEV interacted with vimentin was firstly identified in this study.
-
•
Vimentin was required for TGEV replication in host cell.
-
•
The function of vimentin cytoskeleton in ST cells during TGEV infection was examined.
Keywords: Vimentin, Nucleocapsid, Transmissible gastroenteritis virus
Abstract
Nucleocapsid (N) protein of transmissible gastroenteritis virus (TGEV) packages viral RNA genome to form a ribonucleoprotein complex. In addition to its function as a structural protein, N protein is involved in cell apoptosis or cell-cycle regulation. N protein possibly interacts with host factors to modulate cellular functions. To identify cellular proteins that interacted with N protein of TGEV, methods of GST pull-down and Co-IP were utilized to precipitate cellular proteins of swine testicular (ST). Bound cellular proteins were resolved by SDS-PAGE. Analysis of interacting proteins by mass spectrometry allowed identification of 15 cellular protein bands representative of 12 cellular proteins including vimentin that bound to N protein. Furthermore, the function of vimentin cytoskeleton in ST cells during TGEV infection was examined. Vimentin cytoskeleton was required for virus replication. The present study thus provides protein-related information about interaction of TGEV N protein with host cell that should be useful for understanding host cell response to coronavirus pathogenesis infection and the underlying mechanism of coronavirus replication.
1. Introduction
Coronaviruses (CoVs), a genus in the Coronaviridae family, are pleomorphic, enveloped viruses (Perlman and Netland, 2009). CoVs have been clustered in the Cornavirinae subfamily, which includes three approved genera, alpha-, beta- and gammacoronavirus, as well as a tentative new genus, the deltacoronavirus (de Groot et al., 2011, Reguera et al., 2012). Transmissible gastroenteritis virus (TGEV) is a representative CoV in the alphacoronavirus genus; severe acute respiratory syndrome-related coronavirus (SARS-related CoV) is a representative of the betacoronavirus genus; infectious bronchitis virus (IBV) is a representative of the gammacoronavirus genus; and Bulbul-CoV is a representative of the deltacoronavirus genus (de Groot et al., 2011). The Coronaviridae are involved in respiratory, enteric, hepatic and neuronal infectious disease in animals and humans (Perlman and Netland, 2009).
TGEV infection causes severe diarrhea in suckling piglets (about 2 weeks old), which results in enormous economic loss in swine-producing areas in the world (Kim and Chae, 2001, Sestak et al., 1996). About two-thirds of the TGEV genome (28.5 kb) encodes the replicase gene (rep) at the 5′ end, and one-third of the genome encodes other viral genes at the 3′ end in the order 5′-S-3a-3b-E-M-N-7-3′ (Penzes et al., 2001). The genome of the TGEV encodes four structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N). N proteins of CoVs are highly basic with a molecular mass ranging from 40 to 63 kDa, depending on the species and strains. N protein binds to the RNA genome, forming a helical nucleocapsid (Escors et al., 2001, Sturman et al., 1980).
Recently, some reports showed that N protein of TGEV play an important role in host cell for virus replication. N protein of TGEV facilitates template switching and is required for efficient transcription (Zuniga et al., 2010). N protein underwent proteolysis in parallel with the activation of caspases within host cell and N protein of TGEV is a substrate for caspases (Eleouet et al., 2000). TGEV N protein nucleolus localization was found in transfection experiments and might induced a cell cycle delay or arrest to facilitate virus replication (Wurm et al., 2001). In contrast, TGEV N protein was not accumulated in the nucleus in the infection context (Calvo et al., 2005). In addition, the role of TGEV N protein in cell cycle arrest has been recently reported (Ding et al., 2014). N protein of TGEV may function through direct or indirect interaction with cellular proteins.
For better understanding of the mechanisms associated with pleiotropic functions of N protein, cellular proteins of swine testicular (ST) cells were pulled down associated with N protein using glutathione (GST)-tagged full-length N proteins immobilized on GST agarose. And cellular proteins of ST cells were precipitated using N protein mAb. By SDS-PAGE coupled with mass spectrometry (MS), a total of 12 cellular proteins interacting with N protein were successfully identified. Information on the expanded repertoire of cellular proteins interacting with N protein will provide a framework for future biochemical analyses of these protein functions in TGEV infection.
2. Materials and methods
2.1. Cells and virus
ST cells were grown in RPMI-1640 medium supplemented with 10% fetal calf serum under standard culture conditions (5% CO2, 37 °C). TGEV infectious strain H (Accession No. FJ755618) (Wang et al., 2010) was propagated on an ST cell monolayer.
2.2. Antibodies
Mouse mAb to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ab9484) and Rabbit poloclonal antibody vimentin (ab92547) were purchased from Abcam. FITC-labeled goat anti-mouse IgG was purchased from Kirkegaard and Perry Laboratories (KPL). TRITC-labeled goat anti-rabbit IgG was purchased from Sigma. The mAb to N protein of TGEV were donated from Mr. Wang Shao (Institute of Animal Husbandry and Veterinary Science, Fujian Academy of Agricultural Science, China).
2.3. Cell infection
ST cells were plated in six-well plates 1 day prior to infection with TGEV infectious strain H at a multiplicity of infection (MOI) of 1. TGEV not-infected samples were exposed to culture medium alone. After adsorption for 1 h, cells were washed twice and incubated in fresh RPMI-1640.
2.4. GST pull-down assay
The prokaryotic expression plasmid pGEX-TGEV-N was constructed previously (Zhang et al., 2014). Escherichia coli BL21 (DE3) strain containing pGEX-TGEV-N plasmid was expressed under induction of 1 mM isopropyl-β-d-thiogalactopyranoside. GST pull-down assay was performed as previously described (Zhang et al., 2014). Expressed GST protein was used as a control.
2.5. Co-immunoprecipitation (Co-IP) assay
The lysate of TGEV-infected ST cells was prepared with RIPA lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% deoxycholate) containing a protease inhibitor phenylmethanesulfonyl fluoride (PMSF) (1 mM). After centrifugation at 12,000 × g for 15 min, lysate supernatant was pretreated with 2 μL mouse IgG control (Beyotime) and protein A/G plus-agarose (Santa Cruz Biotechnology) for 30 min at 4 °C to eliminate non-specific binding to agarose gel. The lysate supernatant (500 μg) was incubated with 1 μg of mAb to N protein of TGEV for overnight at 4 °C. Then, 20 μL resuspended protein A/G plus-agarose was added to this mixture and incubated at 4 °C on a rocker platform for 2 h. After washing four times with lysis buffer, isolated immunoprecipitated proteins (boiling 10 min with PAGE sample loading buffer) were then analyzed by 12% PAGE analysis. The lysate of TGEV not-infected ST cells was used as a control.
2.6. Protein identification by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF/TOF) MS
The gels described above stained with PhastGel Blue R (GE Healthcare). Protein bands of interest were manually excised from gels. MALDI-TOF/TOF was performed as previously described (Zhang et al., 2009). Data were searched by GPS Explorer (ver. 3.6) with the search engine MASCOT (ver. 2.1). The search parameters were as follows: National Center for Biotechnology Information non-redundant (NCBInr) database (release date, July 2011), and the database Sus (41,373 sequences; 16,019,616 residues); a trypsin digest was performed with one missing cleavage, MS tolerance was set at 100 ppm and MS/MS tolerance at 0.6 Da. Known contaminant ions (tryptic autodigest peptides) were excluded. MASCOT protein scores (based on combined MS and MS/MS spectra) >59 were considered statistically significant (p ≤ 0.05). Individual MS/MS spectrum, with a statistically significant (confidence interval ≥ 95%) ion score (based on MS/MS spectra), was accepted. To eliminate redundancy of proteins that appeared in database under different names and accession numbers, single protein member belonging to species Sus or with the highest protein score (top rank) was separated from multi-protein family.
2.7. Western blotting
Equivalent amounts of cell lysates were subjected to 12% PAGE and then transferred to 0.22 μm nitrocellulose membranes (Hybond-C Extra, Amersham Biosciences). After blotting, membranes were incubated with mouse pAb to vimentin or with mouse mAb to N protein (1:2000) at 37 °C for 1 h. After washing three times with PBST, membranes were inoculated with DyLight™ 800-labeled antibody to mouse IgG (H+L) (1:10,000, KPL, USA) or DyLight™ 800-labeled antibody to rabbit IgG (H+L) (1:5000, KPL, USA) at 37 °C for 45 min. Images were visualized by Odyssey Infrared Imaging System (LI-COR).
2.8. Immunofluorescence assay
ST cells inoculated with TGEV were cultured for 0, 1, 2, 4, 8, and 16 h. Cells were washed twice with PBS and fixed with paraformaldehyde (4%) for 30 min at 4 °C, and then allowed to air dry. After blotting with 5% skimmed milk powder, the fixed cells were incubated with mAb to TGEV N protein (1:200) and rabbit pAb to vimentin (1:100, Abcam) for 1 h at 37 °C in a humidified chamber. After washing three times with PBST, the fixed cells were incubated with FITC-labeled goat anti-mouse IgG (1:100, KPL) and TRITC-labeled goat anti-rabbit IgG (1:200, Sigma). Additional nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) was performed as described previously (Jungmann et al., 2001). The triple-stained cells were washed three times with PBST and subsequently examined under a Leica TCS SP5 laser confocal microscopy.
2.9. Transfection of siRNA against vimentin
siRNA against vimentin (GenePharma) was used for transfection. Sequence of the siRNA strands (two rounds of silencing) were as follows: 5′-GCUAACUACCAAGACACUATT-3′ (sense) and 5′-UAGUGUCUUGGUAGUUAGCTT-3′ (antisense); 5′-CCUCUGGUUGACACCCAUUTT-3′ (sense) and 5′-AAUGGGUGUCAACCAGAGGTT-3′ (antisense). Negative control siRNA strands were as follows: 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense), 5′-ACGUGACACGUUCGGAGAATT-3′ (antisense). Transfection with siRNA was performed with Lipofectamine 2000 reagent (Invitrogen) by following the manufacturer's instructions. ST cells were cultured overnight in six-well tissue culture plates. The siRNA (20 nM) was complexed with Lipofectamine 2000 reagent by incubating together at room temperature for 30 min. After removing the cell culture supernatant, the complex was added. After incubation for 36 h, the cells were infected with TGEV.
2.10. Real time RT-PCR
Total RNA was extracted from the ST cells transfected with siRNA against vimentin and negative control siRNA, using the RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol. cDNA synthesis was performed with total cellular RNA using a Reverse Transcriptase M-MLV kit (TaKaRa), according to the manufacturer's protocol. Real time RT-PCR was performed using a LightCycler 480 II (Roche) in a total volume of 25 μL containing 10 ng of cDNA template, 1× SYBR® Premix Ex Taq™ II (Perfect Real Time, TaKaRa), and a 0.4 μM concentration of each primer. After initial denaturation at 95 °C for 2 min, the amplification was performed for 40 cycles, each consisting of denaturation at 95 °C for 5 s and primer annealing at 60 °C for 30 s. Melting curves were obtained, and quantitative analysis of the data was performed in a relative quantification (2−ΔΔCT) study model. Parallel TGEV not-infected ST cells were used as control (relative expression = 1) and GAPDH as an internal reference gene.
2.11. Virus titer assay
ST cells were re-plated 1 day before infection in 96 well plates for the 50% infectious dose (TCID50) assays. Treated samples and their paired controls were thawed as described and immediately serially diluted. Cell cultures were then infected for 1 h. After 48 h of incubation, CPE was observed. TCID50 is calculated using the method of Reed and Munch. Virus titer assay were performed three times for each condition and were performed using the Student's t-test.
3. Results
3.1. SDS-PAGE and Co-IP of cellular proteins interacting with N protein
The expressed GST-N protein immobilized on GST-agarose beads was used as a bait to pull down cellular proteins of TGEV-infected ST cells that form a complex with N protein. GST protein was used as control to eliminate non-specifically binding proteins. After extensive washing the resins with RIPA buffer, cellular proteins bound to N protein were analyzed by SDS-PAGE and stained with PhastGel Blue R (GE Healthcare). As shown in Fig. 1 , 10 cellular protein bands that were not seen in GST control gel. The 10 protein bands of interest were manually excised from gels and plated into 96-well microplates for MS identification.
Fig. 1.
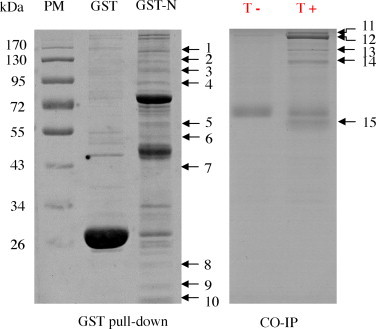
SDS-PAGE analysis of cellular proteins that interact with TGEV N protein. Cellular proteins binding to GST-N agarose or to GST protein were resolved by SDS-PAGE. PM, protein marker. T+ and T− represent the TGEV-infected and not-infected ST cells, respectively.
The mAb to N protein was used as a bait to precipitate cellular proteins of TGEV-infected ST cells that form a complex with N protein. After extensive washing resins with RIPA buffer, cellular proteins bound to N protein were analyzed by SDS-PAGE and stained with PhastGel Blue R (GE Healthcare). As shown in Fig. 1, 5 cellular protein bands that were not seen in TGEV not-infected ST cells. The 5 protein bands of interest were manually excised from the gels and plated into 96-well microplates for MS identification.
3.2. MS identification of cellular proteins interacting with N protein
TGEV N protein-associated cellular proteins were identified using in-gel tryptic digestion, and MALDI-TOF/TOF identification. 15 protein bands binding to N protein were subjected to in-gel trypsin digestion, and peptides were analyzed by MS. Database searches with the peptide masses resulted in positive identification for 8 different cellular proteins (Table 1 , Table S1, and Fig. S1). Two ATP-dependent RNA helicase proteins were identified as interacting with N protein. Protein band 1 was identified as ATP-dependent RNA helicase A (RHA) and protein band 4 was identified as ATP-dependent RNA helicase DDX1 (DBP-RB). Four cytoskeleton proteins were identified as interacting with N protein. Protein band 3 and band 14 were identified as α-actinin-4 (ACTN4). Protein band 5 and band 15 were identified as vimentin (VIM). Protein band 11 and band 12 were identified as myosin. Four ribosome-associated proteins were identified as interacting with N protein: heterogeneous nuclear ribonucleoprotein U (hnRNP U) (band 2 and band 13); 40S ribosomal protein S9 (band 8); 60S ribosomal protein L26 (band 9); and 40S ribosomal protein S13 (band 10). One protein-biosynthesis-associated protein (elongation factor 1-α, band 6) and one cell-cycle-arrest protein (vasohibin-1, band 7) were identified as interacting with N protein. MALDI-TOF MS spectra and TOF/TOF spectra of vimentin are shown in Fig. 2 .
Table 1.
TGEV N protein-associated cellular proteins identified by MALDI-TOF MS.
| Band no.a | Protein name | Accession no. b | Molecular mass (kDa) | No. of peptide matchedc | Sequence coverage (%) | Protein score/best ion scored |
|---|---|---|---|---|---|---|
| 1 | ATP-dependent RNA helicase A | gi|335306989 | 141.7 | 38 | 28 | 562/87 |
| 2 | Heterogeneous nuclear ribonucleoprotein U | gi|335296158 | 104.8 | 17 | 15 | 330/131 |
| 3 | Alpha-actinin-4 | gi|335289614 | 104.3 | 20 | 19 | 96/58 |
| 4 | ATP-dependent RNA helicase | gi|335285861 | 78.9 | 24 | 27 | 324/95 |
| 5 | Vimentin | gi|335296459 | 53.6 | 18 | 36 | 107/20 |
| 6 | Eongation factor 1-alpha | gi|68124034 | 50.1 | 6 | 15 | 60/32 |
| 7 | Vasohibin-1 | gi|335292809 | 40.7 | 11 | 25 | 60/20 |
| 8 | 40S ribosomal protein S9 | gi|335290197 | 24.1 | 11 | 41 | 226/55 |
| 9 | 60S ribosomal protein L26 | gi|213983067 | 17.3 | 8 | 37 | 138/63 |
| 10 | 40S ribosomal protein S13 | gi|311248161 | 17.2 | 9 | 46 | 137/73 |
| 11 | Myosin-9 | gi|335287642 | 228.9 | 44 | 42 | 388/140 |
| 12 | Myosin-10 | gi|335298584 | 165.6 | 26 | 35 | 72/35 |
| 13 | Heterogeneous nuclear ribonucleoprotein U | gi|335296158 | 104.8 | 14 | 18 | 92/39 |
| 14 | Alpha-actinin-4 | gi|335289608 | 102.2 | 27 | 32 | 274/85 |
| 15 | Vimentin | gi|335296459 | 53.6 | 23 | 39 | 389/176 |
Protein band number as in Fig. 1.
Accession numbers according to NCBInr database.
Number of peptides identified by MALDI-TOF/TOF is given by MASCOT. The number of peaks which match to the trypsin peptides.
Protein score (based on combined MS and MS/MS spectra) and best ion score (based on MS/MS spectra) were from MALDITOF/TOF identification. The proteins had statistically significant protein score of great than 59 (p ≤ 0.05) were considered successfully identified.
Fig. 2.

MALDI-TOF MS spectra and TOF/TOF spectra of vimentin.
Three cellular proteins, hnRNP U, ACTN4, and vimentin, were identified both by GST-N pull down and Co-IP in TGEV-infected cells, which should have more biological importance in the context of infection.
3.3. Western blotting confirmation of MS results
To verify the proteins identified by MS, western blotting was performed. Many cytoskeleton associated proteins play important roles in virus infection (Dohner and Sodeik, 2005). Vimentin protein was identified not only in GST pull-down assay but also in Co-IP assay. Therefore, the identified protein vimentin was selected for western blot analysis. Cellular proteins immobilized on GST-agarose beads in GST pull-down assay were examined with specific antibodies to vimentin (Fig. 3 ). Results validated the MALDI-TOF/TOF identification of cellular proteins.
Fig. 3.

Western blot confirmation of representative proteins interacted with TGEV N protein identified by MS. PM, protein marker. GST-N and GST proteins were visualized using mAb to GST; Cellular vimentin of ST cells was visualized using pAb to vimentin.
3.4. Cellular vimentin interacts with N protein in TGEV infected cells
To confirm the interaction between N protein of TGEV and cellular vimentin, immunoprecipitation assay was utilized to identify whether TGEV N protein interacted with cellular vimentin in TGEV-infected ST cells. From the immunoprecipitation results (Fig. 4 ), we can see that the N protein of TGEV was precipitated by the pAb to cellular vimentin in TGEV-infected ST cells but not in TGEV not-infected ST cells. Results demonstrate that cellular vimentin interacted with N protein of TGEV.
Fig. 4.
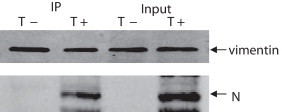
Cellular vimentin interacts with N protein of TGEV in vivo. N protein of TGEV was precipitated by pAb to cellular vimentin in TGEV-infected ST cells but not in TGEV not-infected ST cells (A). T+ and T− represent the TGEV infected and uninfected ST cells, respectively.
3.5. TGEV N protein co-localized with vimentin during infection
Subcellular localization of vimentin was investigated in TGEV-infected ST cells using indirect immunofluorescence confocal microscopy. Results indicated that subcellular localization of vimentin was distributed in cytoplasm with enrichment in the perinuclear region after TGEV infection (Fig. 5 ). Cellular vimentin labeled with TRITC was overlaid with N protein of TGEV labeled with FITC, indicating that cellular vimentin was co-localized with N protein of TGEV within the ST cells during infection.
Fig. 5.
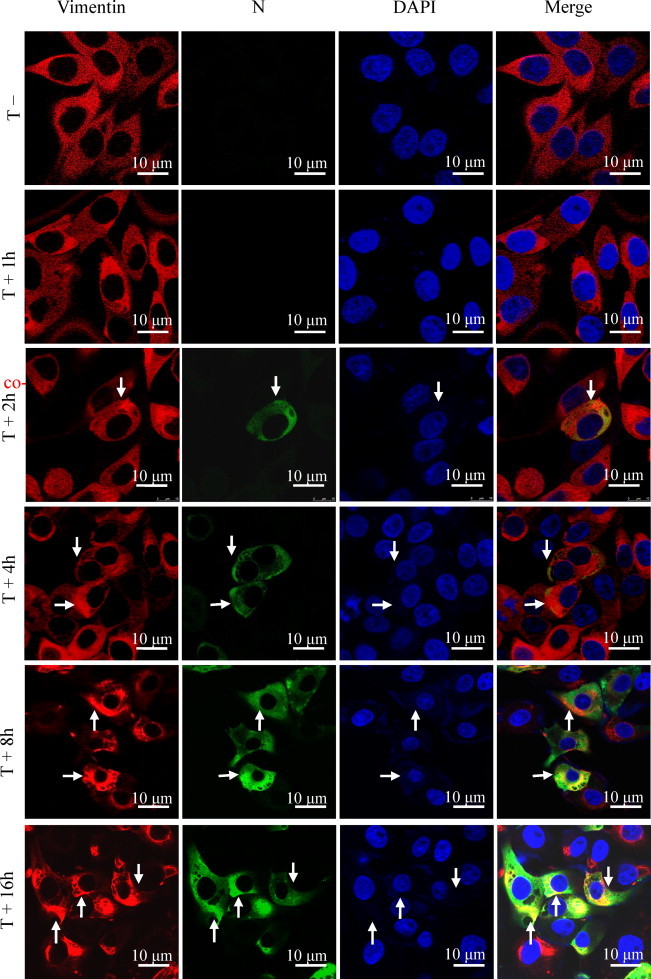
Localization of cellular vimentin and N protein of TGEV. Cells were infected with TGEV. Virus assembly sites were located using antibodies specific for the N protein (green). Vimentin was visualized using antibodies specific for vimentin (red). The nucleus was stained with DAPI (blue). The triple-stained cells were observed by Leica TCS SP5 laser confocal microscopy. The arrows indicate co-localization signal. Bars, 10 μm. (For interpretation of reference to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Knockdown of vimentin impairs TGEV replication in host cell
To further investigate the role of vimentin in virus infection, vimentin protein of ST cells was inhibited using siRNA. The decrease level of vimentin mRNA was validated by RT-PCR (Fig. 6A). ST cells transfected with vimentin-specific siRNA expressed lower level of vimentin by western blot analysis, compared to that transfected with the control siRNA (Fig. 6B). After transfection with siRNA, ST cells were infected with TGEV for another 16 h after transfection with siRNA at an MOI of 1. Virus titer assay were performed three times for each condition and were performed using the Student's t-test. TCID50 of virus in control siRNA group was 105.2/mL and the vimentin siRNA group was 103.1/mL. Fig. 5C shows that knock-down of vimentin resulted in significant reduction of cell-associated virus, which reflected viral replication.
Fig. 6.

Gene silencing of vimentin reduced TGEV replication in ST cells. Vimentin-knockdown cells, negative control knockdown cells and untreated cells were adsorbed with TGEV (MOI = 1) at 37 °C for 1 h. Cells were washed and further incubated with TGEV. The levels of mRNA for vimentin in vimentin-knockdown cells were confirmed by RT-PCR compared with the corresponding control (A). The cell lysates were harvested for western blotting with antibodies against vimentin, TGEV N protein and GAPDH, as indicated (B). The culture supernatants of cells infected with TGEV were collected for viral titration (C). The virus titers shown here are the averages and standard deviations of three independent samples. *p < 0.05.
4. Discussion
Identifying host cell protein interacting with viral protein is a critical step for understanding protein function and viral replication. Recently, affinity purification followed by MS has been widely used to find protein–protein interactions (Dunham et al., 2012). In the present study, using GST pull-down and Co-IP coupled with MS identification, 15 cellular protein bands representative of 12 cellular proteins interacting with TGEV N protein were identified.
Several cellular proteins found in this study were identical to those previously described interacting with CoV N protein. EF1A, DDX1 and hnRNP U were also observed in infectious bronchitis virus (IBV) N protein using quantitative proteomic approaches (Emmott et al., 2013). Interaction between EF1A and N protein of TGEV (Zhang et al., 2014) and SARS-CoV were founded (Zhou et al., 2008). The hnRNP U protein was identified as binding preferentially TGEV 3′-end (Galan et al., 2009). A very recent work describes the key role of IBV N protein interaction with DDX1 in viral transcription (Wu et al., 2014).
Four cytoskeleton-associated proteins were identified that associated with N protein in this study. The cytoskeleton consists of a filament scaffold within cell. Filaments are dynamic and divided into three types: microfilaments (actin filaments), microtubules, and intermediate filament (Naghavi and Goff, 2007). During infection, viruses interact with components of the cytoskeleton to reach their appropriate intracellular sites of replication (Radtke et al., 2006). In previous study, differentially expressed cytoskeletal proteins were found in TGEV infected ST cells using two-dimensional difference gel electrophoresis (Zhang et al., 2013). Cytoskeletal proteins found in this study provides new insights for understanding the mechanisms of TGEV replication.
CoV replication uses complex mechanisms that involve viral and cellular proteins. Similar to other positive-strand RNA viruses, CoV genome replication takes place in the cytoplasm in a membrane-protected microenvironment that contains all the protein required for viral RNA synthesis (Enjuanes et al., 2006). Electron microscopy studies of mouse-hepatitis-virus-infected cells have shown that replication complexes of virus consist of double-membrane vesicles (Gosert et al., 2002). CoV replication complex formation possibly utilizes components of cellular autophagy (Prentice et al., 2004). Although CoV replication essentially takes place within the cytoplasm, the virus may control cell machinery by locating some of its proteins in host cell nucleus (Enjuanes et al., 2006). In the present study, vimentin was identified in pull-down assay and Co-IP assay, suggesting that it plays an important role in TGEV infection. Co-staining of cellular vimentin and N protein on immunofluorescence microscopy also supported an association between proteins. Based on these findings, we speculate that vimentin plays a role in TGEV replication.
Some studies demonstrate that vimentin plays important role in virus infection cycle. Vimentin binding is critical for virus entry, such as human cytomegalovirus (CMV) (Miller and Hertel, 2009), Japanese encephalitis virus (JEV) (Liang et al., 2011), and cowpea mosaic virus (CPMV) (Koudelka et al., 2009). Vimentin is required for virus replication, such as bluetongue virus (BTV) (Bhattacharya et al., 2007), and dengue virus (DENV) (Kanlaya et al., 2010). In this study, vimentin is required for TGEV replication. Therefore, these may potentially serve as novel therapeutic targets for controlling TGEV infection.
The efficiency of TGEV infection depends on the presence and integrity of vimentin cytoskeleton, which may facilitate virus infection at different steps. Vimentin may promote viral replication by interaction with N protein, which may help virions to transport through a functional Golgi complex for viral maturation (Risco et al., 1998). Additional work will be needed to pinpoint the exact steps during viral infection.
Overall, this study was the first to identified cellular proteins interaction with TGEV N protein using proteomics method. A total of 12 cellular proteins were identified, which provided new information to understand the mechanism of coronavirus replication. The interaction between the cellular vimentin and N protein of TGEV was confirmed in TGEV-infected ST cells. Knockdown of vimentin impairs TGEV replication in ST cells. The present study thus provides insights into interaction of TGEV N protein with host cellular proteins that would be useful for further studying viral replication and pathogenesis.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31172350, 31101823), Heilongjiang Provincial Natural Science Foundation (Grant No. JC201118) and Heilongjiang Provincial Department of Education (2011TD001). We thank Mr. Zhou Xin-Wen (Fudan University, China) for help with MALDI-TOF/TOF mass spectrometry and Mr. Wang Shao (Institute of Animal Husbandry and Veterinary Science, Fujian Academy of Agricultural Science, China) for the donation of mAb to N protein of TGEV.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.virusres.2014.12.013.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Bhattacharya B., Noad R.J., Roy P. Interaction between Bluetongue virus outer capsid protein VP2 and vimentin is necessary for virus egress. Virol. J. 2007;4(1):7–18. doi: 10.1186/1743-422X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo E., Escors D., Lopez J.A., Gonzalez J.M., Alvarez A., Arza E., Enjuanes L. Phosphorylation and subcellular localization of transmissible gastroenteritis virus nucleocapsid protein in infected cells. J. Gen. Virol. 2005;86(8):2255–2267. doi: 10.1099/vir.0.80975-0. [DOI] [PubMed] [Google Scholar]
- de Groot R.J., Baker S.G., Baric R.S., Enjuanes L., Gorbalenya A.E. Coronaviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press; San Diego: 2011. pp. 774–796. [Google Scholar]
- Ding L., Huang Y., Du Q., Dong F., Zhao X., Zhang W., Xu X., Tong D. TGEV nucleocapsid protein induces cell cycle arrest and apoptosis through activation of p53 signaling. Biochem. Biophys. Res. Commun. 2014;445(2):497–503. doi: 10.1016/j.bbrc.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohner K., Sodeik B. The role of the cytoskeleton during viral infection. Curr. Top. Microbiol. Immunol. 2005;285(1):67–108. doi: 10.1007/3-540-26764-6_3. [DOI] [PubMed] [Google Scholar]
- Dunham W.H., Mullin M., Gingras A.C. Affinity-purification coupled to mass spectrometry: basic principles and strategies. Proteomics. 2012;12(10):1576–1590. doi: 10.1002/pmic.201100523. [DOI] [PubMed] [Google Scholar]
- Eleouet J.F., Slee E.A., Saurini F., Castagne N., Poncet D., Garrido C., Solary E., Martin S.J. The viral nucleocapsid protein of transmissible gastroenteritis coronavirus (TGEV) is cleaved by caspase-6 and -7 during TGEV-induced apoptosis. J. Virol. 2000;74(9):3975–3983. doi: 10.1128/jvi.74.9.3975-3983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmott E., Munday D., Bickerton E., Britton P., Rodgers M.A., Whitehouse A., Zhou E.M., Hiscox J.A. The cellular interactome of the coronavirus infectious bronchitis virus nucleocapsid protein and functional implications for virus biology. J. Virol. 2013;87(17):9486–9500. doi: 10.1128/JVI.00321-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Almazan F., Sola I., Zuniga S. Biochemical aspects of coronavirus replication and virus–host interaction. Annu. Rev. Microbiol. 2006;60(1):211–230. doi: 10.1146/annurev.micro.60.080805.142157. [DOI] [PubMed] [Google Scholar]
- Escors D., Camafeita E., Ortego J., Laude H., Enjuanes L. Organization of two transmissible gastroenteritis coronavirus membrane protein topologies within the virion and core. J. Virol. 2001;75(24):12228–12240. doi: 10.1128/JVI.75.24.12228-12240.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan C., Sola I., Nogales A., Thomas B., Akoulitchev A., Enjuanes L., Almazan F. Host cell proteins interacting with the 3′ end of TGEV coronavirus genome influence virus replication. Virology. 2009;391(2):304–314. doi: 10.1016/j.virol.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R., Kanjanahaluethai A., Egger D., Bienz K., Baker S.C. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 2002;76(8):3697–3708. doi: 10.1128/JVI.76.8.3697-3708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann A., Nieper H., Muller H. Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigen-negative cells in their vicinity. J. Gen. Virol. 2001;82(5):1107–1115. doi: 10.1099/0022-1317-82-5-1107. [DOI] [PubMed] [Google Scholar]
- Kanlaya R., Pattanakitsakul S.N., Sinchaikul S., Chen S.T., Thongboonkerd V. Vimentin interacts with heterogeneous nuclear ribonucleoproteins and dengue nonstructural protein 1 and is important for viral replication and release. Mol. BioSyst. 2010;6(5):795–806. doi: 10.1039/b923864f. [DOI] [PubMed] [Google Scholar]
- Kim B., Chae C. In situ hybridization for the detection of transmissible gastroenteritis virus in pigs and comparison with other methods. Can. J. Vet. Res. 2001;65(1):33–37. [PMC free article] [PubMed] [Google Scholar]
- Koudelka K.J., Destito G., Plummer E.M., Trauger S.A., Siuzdak G., Manchester M. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog. 2009;5(5):e1000417. doi: 10.1371/journal.ppat.1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J.J., Yu C.Y., Liao C.L., Lin Y.L. Vimentin binding is critical for infection by the virulent strain of Japanese encephalitis virus. Cell. Microbiol. 2011;13(9):1358–1370. doi: 10.1111/j.1462-5822.2011.01624.x. [DOI] [PubMed] [Google Scholar]
- Miller M.S., Hertel L. Onset of human cytomegalovirus replication in fibroblasts requires the presence of an intact vimentin cytoskeleton. J. Virol. 2009;83(14):7015–7028. doi: 10.1128/JVI.00398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi M.H., Goff S.P. Retroviral proteins that interact with the host cell cytoskeleton. Curr. Opin. Immunol. 2007;19(4):402–407. doi: 10.1016/j.coi.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes Z., Gonzalez J.M., Calvo E., Izeta A., Smerdou C., Mendez A., Sanchez C.M., Sola I., Almazan F., Enjuanes L. Complete genome sequence of transmissible gastroenteritis coronavirus PUR46-MAD clone and evolution of the purdue virus cluster. Virus Genes. 2001;23(1):105–118. doi: 10.1023/A:1011147832586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice E., Jerome W.G., Yoshimori T., Mizushima N., Denison M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 2004;279(11):10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke K., Dohner K., Sodeik B. Viral interactions with the cytoskeleton: a hitchhiker's guide to the cell. Cell. Microbiol. 2006;8(3):387–400. doi: 10.1111/j.1462-5822.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- Reguera J., Santiago C., Mudgal G., Ordono D., Enjuanes L., Casasnovas J.M. Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathog. 2012;8(8):e1002859. doi: 10.1371/journal.ppat.1002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco C., Muntion M., Enjuanes L., Carrascosa J.L. Two types of virus-related particles are found during transmissible gastroenteritis virus morphogenesis. J. Virol. 1998;70(7):4773–4777. doi: 10.1128/jvi.72.5.4022-4031.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K., Lanza I., Park S.K., Weilnau P.A., Saif L.J. Contribution of passive immunity to porcine respiratory coronavirus to protection against transmissible gastroenteritis virus challenge exposure in suckling pigs. Am. J. Vet. Res. 1996;57(5):664–671. [PubMed] [Google Scholar]
- Sturman L.S., Holmes K.V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J. Virol. 1980;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chen J., Shi H., Qiu H., Xue F., Liu C., Zhu Y., Liu S., Almazan F., Enjuanes L., Feng L. Molecular characterization of a Chinese vaccine strain of transmissible gastroenteritis virus: mutations that may contribute to attenuation. Virus Genes. 2010;40(3):403–409. doi: 10.1007/s11262-010-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.H., Chen P.J., Yeh S.H. Nucleocapsid phosphorylation and RNA helicase DDX1 recruitment enables coronavirus transition from discontinuous to continuous transcription. Cell Host Microb. 2014;16(4):462–472. doi: 10.1016/j.chom.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm T., Chen H., Hodgson T., Britton P., Brooks G., Hiscox J.A. Localization to the nucleolus is a common feature of coronavirus nucleoproteins, and the protein may disrupt host cell division. J. Virol. 2001;75(19):9345–9356. doi: 10.1128/JVI.75.19.9345-9356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Shi H., Chen J., Shi D., Li C., Feng L. EF1A interacting with nucleocapsid protein of transmissible gastroenteritis coronavirus and plays a role in virus replication. Vet. Microbiol. 2014;172(3–4):443–448. doi: 10.1016/j.vetmic.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Shi H.Y., Chen J.F., Shi D., Lang H.W., Wang Z.T., Feng L. Identification of cellular proteome using two-dimensional difference gel electrophoresis in ST cells infected with transmissible gastroenteritis coronavirus. Proteome Sci. 2013;11(1):31–43. doi: 10.1186/1477-5956-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhou J., Wu Y., Zheng X., Ma G., Wang Z., Jin Y., He J., Yan Y. Differential proteome analysis of host cells infected with porcine circovirus type 2. J. Proteome Res. 2009;8(11):5111–5119. doi: 10.1021/pr900488q. [DOI] [PubMed] [Google Scholar]
- Zhou B., Liu J., Wang Q., Liu X., Li X., Li P., Ma Q., Cao C. The nucleocapsid protein of severe acute respiratory syndrome coronavirus inhibits cell cytokinesis and proliferation by interacting with translation elongation factor 1alpha. J. Virol. 2008;82(14):6962–6971. doi: 10.1128/JVI.00133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga S., Cruz J.L., Sola I., Mateos-Gomez P.A., Palacio L., Enjuanes L. Coronavirus nucleocapsid protein facilitates template switching and is required for efficient transcription. J. Virol. 2010;84(4):2169–2175. doi: 10.1128/JVI.02011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


