Abstract
Porcine circovirus type 2 (PCV2) is the essential component of porcine circovirus disease (PCVD) as the disease syndrome is referred to in Europe and porcine circovirus associated disease (PCVAD) as it is referred to in North America. Singular PCV2 infection rarely results in clinical disease; however, PCVAD is often accelerated in onset, enhanced in severity and prolonged in duration by concurrent viral or bacterial infections. Due to its effect on the immune system, PCV2 has also been shown to enhance protozoal, metazoal, and fungal infections. Several retrospective or cross-sectional studies have investigated the presence and prevalence of various infectious agents associated with PCVAD under field conditions. Experimental models confirm that PCV2 replication and associated lesions can be enhanced by concurrent infection with other viruses or bacteria. The exact mechanisms by which concurrent pathogens upregulate PCV2 are unknown. Co-infections may promote PCV2 infection by increasing immune host cell replication and accumulation in tissues thereby enhancing targets for PCV2 replication. It has also been proposed that co-infections interfere with PCV2 clearance by alteration of cytokine production and profiles. The outcome of differences in timing of co-infections in PCV2-infected pigs is also likely very important and is an area where more research is needed. Given the current knowledge base, it is important that veterinarians do a thorough diagnostic investigation on herds where PCVAD is a recurrent problem in order to implement the most appropriate and cost effective intervention strategies.
Keywords: Swine, Porcine circovirus type 2, Co-infection
1. Introduction
Porcine circovirus (PCV) type 2 (PCV2) was initially recognized in 1998 (Allan et al., 1998, Meehan et al., 1998, Morozov et al., 1998) but based on retrospective molecular and serological investigations it is now known to have been present in the global pig population many decades prior to its discovery (Jacobsen et al., 2009). PCV2 is a small, single-stranded, ambisense DNA virus (Tischer et al., 1974, Tischer et al., 1982) that belongs to the genus Circovirus in the family Circoviridae (Todd et al., 2005). PCVs contain two major open reading frames (ORFs) oriented in opposite directions which encode for proteins associated with replication (ORF1) and the virus capsid (ORF2) (Mankertz et al., 1998, Nawagitgul et al., 2000). Recent phylogenic investigations indicate that PCV2 can be further subdivided into several types of which PCV2a and PCV2b are highly prevalent in the pig population and are present worldwide (Cheung et al., 2007, Segalés et al., 2008). The various clinical manifestations of PCV2 infection in pigs across age groups have been summarized as “porcine circovirus disease” (PCVD) in Europe (Segalés et al., 2005) and as “porcine circovirus associated disease” (PCVAD) in North America (Opriessnig et al., 2007). PCVAD includes systemic disease characterized by wasting and growth retardation and better known as postweaning multisystemic wasting syndrome or PMWS (Harding and Clark, 1997), respiratory disease often with other respiratory pathogens as part of the porcine respiratory disease complex or PRDC (Harms et al., 2002), enteric disease characterized by diarrhea in grow-finish pigs (Jensen et al., 2006), reproductive disease associated with small litter sizes and increased numbers of stillborn and mummified fetuses (Madson and Opriessnig, 2011), and porcine dermatitis and nephropathy syndrome or PDNS (Rosell et al., 2000). To the authors’ knowledge, PDNS is the one PCV2-associated clinical manifestation that has never been experimentally reproduced. It has been suggested that the underlying pathogenic mechanism for development of PDNS is a rapid-onset of systemic coagulation that could be induced by any number of combinations of endotheliotropic pathogens or toxins with or without PCV2 (Krakowka et al., 2008).
Several studies have demonstrated that PCV2 is the primary and essential etiological agent in the pathogenesis of PCVAD (Bolin et al., 2001, Ellis et al., 1999, Ladekjær-Mikkelsen et al., 2002). However, experimentally, PCVAD is reproduced most efficiently and consistently when PCV2 inoculation is done in conjunction with other swine pathogens. Several of these PCV2-co-infection models have evolved to nicely mimic what is observed in the field (Allan et al., 1999, Allan et al., 2000a, Ellis et al., 2008, Harms et al., 2001, Jung et al., 2006b, Kennedy et al., 2000, Kim et al., 2003, Krakowka et al., 2000, Opriessnig et al., 2004a, Opriessnig et al., 2004b, Opriessnig et al., 2008, Opriessnig et al., 2009, Opriessnig et al., 2011a, Opriessnig et al., 2011b, Opriessnig et al., 2011c, Rovira et al., 2002, Shen et al., 2010, Sinha et al., 2011) and these models have allowed the body of knowledge on the pathogenesis of PCV2 to continue to advance. It is well known that PCV2 has a tropism for lymphoid tissues where it accumulates in large numbers in the cytoplasm of macrophages in depleted lymphoid follicles (Sorden, 2000). PCV2 is one of the smallest viruses known to mankind (Todd et al., 2005) and lacks its own enzymes so viral DNA intermediates are generated in host cell nuclei and they depend on host cell enzymes present in the synthesis (S) phase of the cell cycle for completion of the replication cycle (Tischer et al., 1987). Virions are assembled in both nuclei and cytoplasm and released from infected cells in the absence of viral cytopathic effects in vitro (Allan and Ellis, 2000).
Co-infections can be frequently identified in field cases of PCVAD (Fig. 1 A). A retrospective analysis of 484 systemic PCVAD cases collected in the Midwestern United States between 2000 and 2001 revealed that singular PCV2 infection was found in only 1.9% (9/484) of the cases investigated (Pallarés et al., 2002). Porcine reproductive and respiratory syndrome virus (PRRSV) was detected in 51.9% (251/484) of the cases, the combination PCV2 and Mycoplasma hyopneumoniae (M. hyopneumoniae) was found in 35.5% (172/484) of the cases, and both PCV2 and swine influenza virus (SIV) were detected in 5.4% (26/484) of the PCVAD cases (Pallarés et al., 2002). A similar study conducted in 2009 and involving 200 systemic PCVAD cases collected in the same region as in the 2000/2001 study revealed the following: PRRSV was detected in 60.0% (120/200) of the cases (Fig. 2 A), the combination of PCV2 and M. hyopneumoniae was found in 15.5% (31/200) of the cases (Fig. 3 A), and both PCV2 and SIV were detected in 13.0% (26/200) of the PCVAD cases (Opriessnig, unpublished data). The main difference between the data obtained in 2000/2001 and those obtained in 2009 was a higher percentage of cases without concurrent pathogen detection and increased isolation of common secondary bacterial invaders such as Arcanobacterium pyogenes (Fig. 4 ).
Fig. 1.
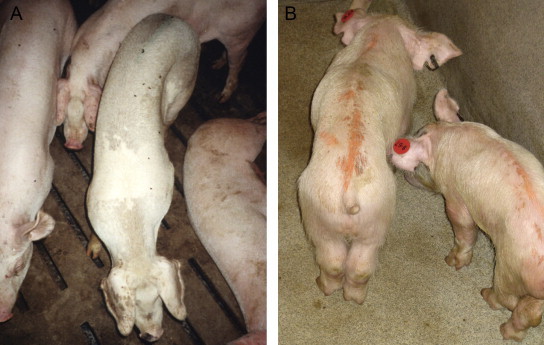
Pigs concurrently infected with PCV2 and PRRSV. (A) Field case. (B) Experimentally inoculated pigs 12 days post infection (Opriessnig et al., unpublished). The pigs had decreased weight gain, rough hair coat and respiratory distress.
Fig. 2.
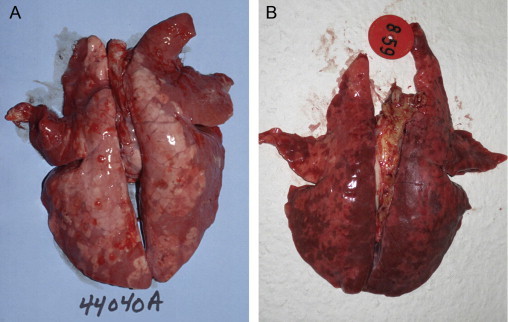
Lungs from pigs concurrently infected with PCV2 and PRRSV. (A) Field case. There is cranioventral tan consolidation, failure of the lung to collapse and diffuse mottling of the caudodorsal lung surface. Pasteurella multocida was isolated from this lung in addition to PRRSV and PCV2. (B) Lungs from pigs experimentally inoculated with PCV2 and PRRSV 12 days after co-infection (Opriessnig et al., unpublished). There is failure of the lung to collapse and severe multifocal-to-diffuse tan mottling and consolidation of most of the lung surface.
Fig. 3.
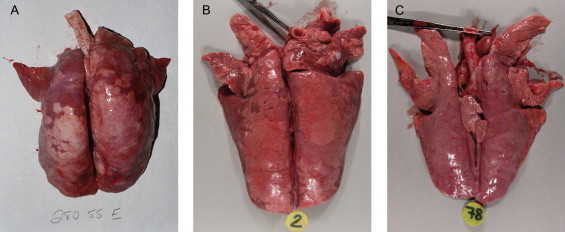
(A) Field case. Lung from a pig concurrently infected with PCV2 and Mycoplasma hyopneumoniae. PRRSV was also present as determined by immunohistochemistry. There are cranioventral dark purple consolidated areas typical of M. hyopneumoniae infection and the lung failed to collapse. (B) Lung from a pig experimentally co-infected with PCV2 and M. hyopneumoniae 21 days post PCV2 inoculation and 35 days post M. hyopneumoniae inoculation (Opriessnig et al., 2004b). The lesions are similar to those described in the field case in Part B. (C) Lungs from a pig experimentally inoculated with PCV2 alone 21 days post infection (Opriessnig et al., 2004b). Note the normal lung surface and the enlarged tracheobronchiolar lymph nodes.
Fig. 4.
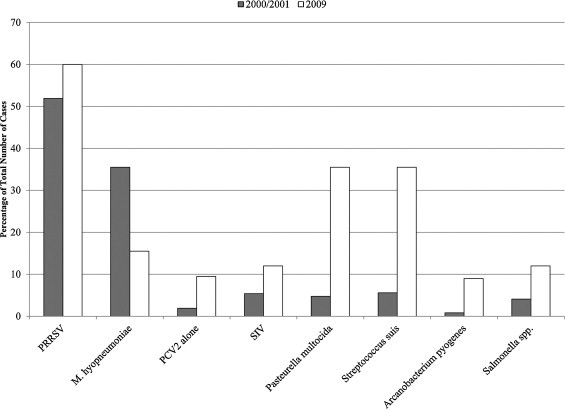
Comparison of pathogen detection in cases of systemic PCVAD collected in the Midwestern United States in 2000/2001 (Pallarés et al., 2002) or in 2009 (Opriessnig, unpublished). Data are presented in percentages of total numbers of cases investigated (n = 485 for 2000/2001 and n = 200 for 2009).
In 2007, the prevalence of PCV2 and co-infecting pathogens in 3-, 9-, 16-, and 24-week-old pigs from 41 different 1-, 2-, and 3-site production systems located in the United States was investigated (Dorr et al., 2007). Compared with PCV2-negative pigs, PCV2-positive pigs were more likely to be infected with SIV and M. hyopneumoniae. Additionally, PCV2-positive pigs had higher serum anti-PRRSV antibody titers and more severe lung lesions. Overall in PCV2-positive pigs, co-infections with SIV, M. hyopneumoniae, and PRRSV were determined to be most important and to have the greatest effect in the early to late nursery phase in 3-site production systems (Dorr et al., 2007).
Under field and experimental conditions, a variety of pathogens have been found to directly enhance PCV2 replication, lesions and disease. To the authors’ knowledge, investigations of PCVAD epidemics in different countries and continents did not reveal a distinct co-pathogen that can be solely attributed to enhancing the severity of PCVAD. It is more likely that co-pathogens in PCVAD vary from region to region and herd to herd but utilize similar mechanisms which result in enhancement of PCVAD.
2. PCV2 and concurrent viral infections
2.1. Porcine parvovirus
Porcine parvovirus (PPV), a linear, single-stranded, negative-sense, non-enveloped DNA virus of about 5 kb in genome size, belongs to the family Parvoviridae (Molitor et al., 1984) and is widespread in the pig population. Similar to PCV2, PPV has a strong cellular tropism for mitotically active tissues like lymph nodes or heart muscle (Allan and Ellis, 2000, Oraveerakul et al., 1993). Based on pathogenicity and tissue tropism, PPV-isolates have been classified as non-pathogenic (Cutlip and Mengeling, 1975, Paul and Mengeling, 1980), pathogenic to non-immunocompetent fetuses leading to death (Mengeling, 1979), pathogenic to immunocompetent fetuses and inducing dermatitis (Choi et al., 1987, Kresse et al., 1985, Lager and Mengeling, 1994, Lager et al., 1992), or enteric (Dea et al., 1985, Duhamel et al., 1991). While incoming gilts, and less often sows, are routinely vaccinated to protect against PPV-associated reproductive losses, infection of growing pigs with PPV is typically not associated with clinical disease (Allan et al., 1999, Brown et al., 1980, Kennedy et al., 2000, Krakowka et al., 2000, Opriessnig et al., 2004a) and producers rarely monitor for or implement control strategies for PPV in growing pigs.
In 2000, a Canadian field investigation was conducted which involved 106 pigs ranging in age from 4 to 12 weeks (Ellis et al., 2000). PPV and PCV2 co-infection was demonstrated in 17.4% (12/69) of pigs with naturally acquired PCVAD. In contrast, there was no evidence of the presence of PCV2 DNA in age-matched control animals suffering from Streptococcus suis infection (Ellis et al., 2000). This study provided important early evidence that PPV is likely a significant co-factor in PCVAD under field conditions. In further support of the importance of PPV in PCVAD, PPV DNA was demonstrated by in situ hybridization in 25.6% (34/133) of Korean PCVAD cases (Kim et al., 2002). When 142 skin sections obtained from pigs diagnosed with exudative epidermitis were further investigated, PCV2 alone, PPV alone, or concurrent PCV2 and PPV infection were demonstrated in 8.5% (12/142), 11.3% (16/142) and 42.3% (60/142) of the tissues, respectively (Kim and Chae, 2004). Distinct positive PCV2 and PPV labeling was found throughout the inflammatory areas in the dermoepidermal junction, the superficial dermis, and occasionally in macrophages in lymphoid tissues further emphasizing the high prevalence of concurrent PCV2 and PPV infection in growing pigs, especially in pigs with exudative epidermitis (Kim and Chae, 2004).
The first experimental study demonstrating the potentiating effect of another pathogen, in this case PPV, on PCV2 was conducted in 1999 (Ellis et al., 1999). The investigators were able to reproduce most of the lesions typical of systemic PCVAD in gnotobiotic pigs inoculated with filtered cell culture material and filtered lymphoid tissues from pigs with naturally acquired disease. Both, PCV2 and PPV and antibodies to these viruses were detected in the experimentally-inoculated pigs (Ellis et al., 1999). Several groups have since demonstrated that pigs dually-inoculated with PCV2 and PPV develop more severe disease and lesions than pigs infected with PCV2 alone (Allan et al., 1999, Hasslung et al., 2005, Kennedy et al., 2000, Kim et al., 2003, Kim et al., 2006, Opriessnig et al., 2004a, Ostanello et al., 2005). Interestingly, PCV2 potentiation by PPV appears to be independent of sequence of infection. When piglets born to sows exposed to PCV2 21 days before the expected farrowing date were infected with PPV intranasally at 4 weeks of age, they developed microscopic lesions consistent with PCVAD and very similar to the lesions in pigs with concurrent PPV–PCV2 inoculation at 4 weeks of age further highlighting the complex interaction of these two small DNA viruses (Ha et al., 2008).
Some believe that initial viral entry in co-infected animals occurs through tonsillar macrophages after which PCV2 and PPV replicate to some extent in circulating macrophages, contributing to the cell-associated viremia and to viral distribution throughout the lymphoid tissues (Kim et al., 2003). Early on, it was suggested that PPV-induced immune dysfunction promotes enhanced replication of PCV2 (Allan et al., 1999). In support of this, it was demonstrated that PPV replication occurred prior to PCV2 replication. In co-infected pigs, PPV antigen predominated in the tissues between 3 and 14 days post inoculation (dpi) with a maximum antigen load observed between 6 and 14 dpi whereas PCV2 antigen was first observed in minimal amounts in mesenteric lymph nodes at 10 dpi with increasing intensity and distribution of PCV2 antigen at 14, 17, 21, and 26 dpi (Allan et al., 2000b).
While combined PPV–PCV2 infection in naïve animals can result in clinical PCVAD, it has been shown that the presence of passive immunity against PCV2 can prevent PCVAD, but may not prevent subclinical PCV2 infection (Ostanello et al., 2005). Three-week-old conventional pigs confirmed to have passively acquired antibodies to PPV and PCV2 were co-infected with PPV and PCV2 and half of the pigs (4/8) were vaccinated with a commercial Actinobacillus pleuropneumoniae vaccine at 3 dpi. Although PCV2 viremia was detected, none of the pigs developed clinical disease through termination of the study at 42 dpi indicating that the pigs with passively-derived immunity became PCV2 infected but remained subclinical (Ostanello et al., 2005).
The family Parvoviridae is very diverse and the subfamily Parvovirinae contains several genera known to infect pigs including the genus Bocavirus (Hao et al., 2011, Zhang et al., 2011a, Zhang et al., 2011b). In 2009, a novel porcine boca-like virus (Pbo-likeV) was identified in lymph nodes obtained from two Swedish pigs suffering from systemic PCVAD using random multiple displacement amplification and large-scale sequencing (Blomström et al., 2009). Following the identification of Pbo-likeV in lymphoid tissues, additional samples from a healthy and a clinically affected pig were characterized and Pbo-likeV was identified in the PCVAD pig but not in the non-PCVAD pig (Blomström et al., 2009). In a follow-up study, 34 PCVAD and 24 non-PCVAD pigs were screened by PCR for the presence of PCV2, torque teno sus virus species 1 (TTSuV1) and 2 (TTSuV2), and Pbo-likeV (Blomström et al., 2010). The prevalence of these viruses in affected animals was 100% for PCV2, 77% for TTSuV-1, 94% for TTSuV-2, and 88% for Pbo-likeV. In non-PCVAD pigs the detection rates were as follows: 80% for PCV2, 79% for TTSuV1, 83% for TTSuV2, and 46% for Pbo-likeV leading to the conclusion that polyviral infections are common in PCVAD pigs and the synergistic effect of concurrent viral infection could contribute to expression of clinical disease (Blomström et al., 2010). Recently, a novel PPV, PPV4, was identified in the lung lavage of a diseased pig co-infected with PCV2 in the United States (Cheung et al., 2010). The importance of this finding is unknown at this point in time.
Although there is sufficient evidence that combined PPV and PCV2 infection is common under field conditions and it is well known that PPV–PCV2 co-infection can be detrimental based on experimental studies, to the authors’ knowledge most veterinary diagnostic laboratories do not test growing pigs routinely for presence of PPV or other PPV-like viruses such as Pbo-likeV. PPV and PPV-like virus co-infections with PCV2 may be substantially underdiagnosed and perhaps more important than they are given credit for.
2.2. Porcine reproductive and respiratory syndrome virus
PRRSV is an enveloped RNA virus containing a positive-sense, single-stranded genome belonging to the family Arteriviridae and the genus Arterivirus of the order Nidovirales (Cavanagh, 1997, Meulenberg et al., 1993). PRRSV was implicated very early as being important in the pathogenesis of PCVAD. Not surprisingly, several field studies conducted in Spain (Grau-Roma and Segalés, 2007, Segalés et al., 2002), the United States (Dorr et al., 2007, Pallarés et al., 2002, Pogranichniy et al., 2002), the Netherlands (Wellenberg et al., 2004), Canada (Drolet et al., 2003), and Italy (Morandi et al., 2010) confirmed the interaction of PRRSV and PCV2. For example, a case-control study in the Netherlands, which included 60 clinically affected pigs and 180 pigs without clinical signs, identified combined PCV2 and PRRSV infection in 83% of the clinically-affected pigs and in 35% of the pigs from PCVAD-free herds (Wellenberg et al., 2004). A case-control study in the United States, which included 31 pigs with a clinical history of wasting and microscopic lesions characteristic of PCVAD and 56 control pigs without PCVAD, revealed that among 10 viruses tested, PCV2 had the strongest association with PCVAD, but the risk for clinical PCVAD was much higher if the pig was concurrently infected with PRRSV (Pogranichniy et al., 2002). Moreover, when proliferative and necrotizing pneumonia cases were tested, concurrent PRRSV and PCV2 infection was confirmed in 40.5% (30/74) of cases in Spain (Grau-Roma and Segalés, 2007), in 41.7% (25/60) in cases in Canada (Drolet et al., 2003) and in 28.6% (8/28) of cases in Italy (Morandi et al., 2010).
Colostrum-deprived (CD) pigs were co-infected with PCV2 and PRRSV at 1–2 days of age resulting in upregulation of PCV2 and presence of higher levels of PCV2 antigen in tissues compared to pigs singularly infected with PCV2 (Allan et al., 2000a). However, the replication and distribution of PRRSV in concurrently infected pigs was not enhanced compared to that of pigs infected singularly with PRRSV (Allan et al., 2000a). To further investigate the high prevalence of combined PRRSV and PCV2 infection in the United States (Harms et al., 2002), cesarean-derived colostrum-deprived (CDCD) pigs were co-infected with PCV2 and PRRSV at 3 weeks of age. It was demonstrated that combined PRRSV and PCV2 infection substantially increased the severity of clinical disease and lesions (Harms et al., 2001). Similarly, when conventional pigs were inoculated with PRRSV at 5 weeks of age and seven days later with PCV2, 14.3% (1/7) of the co-infected pigs died after developing wasting and respiratory disease, and PCV2 DNA was found in higher amounts in co-infected pigs compared to pigs infected with PCV2 alone, confirming that PRRSV infection enhances PCV2 replication (Rovira et al., 2002).
In an attempt to further understand the role of PRRSV in PCVAD, 20 specific-pathogen-free (SPF) pigs were randomly divided into four groups with five pigs in each group and inoculated intranasally with PCV2, PRRSV, or PCV2 and PRRSV isolated from lymphoid and lung tissues from a clinically affected PCVAD herd with an on-farm mortality of 30% post placement in finishers (Opriessnig, unpublished observation). Pigs inoculated with PRRSV only and those co-infected with PRRSV and PCV2 developed moderate-to-severe clinical disease (Fig. 1B) characterized by high and persistent fevers, sneezing, decreased weight gain, and lethargy. By 14 dpi, macroscopically the lungs failed to collapse, were congested and had diffuse and severe mottled-tan areas of consolidation (Fig. 2B). Microscopic lesions in the PCV2–PRRSV co-infected group were characterized by severe lymphoid depletion of lymphoid follicles, moderate-to-severe bronchointerstitial pneumonia, and moderate-to-severe perivascular lymphohistiocytic cuffing in brain tissues which was not observed in singularly-PCV2 or singularly-PRRSV inoculated pigs (Opriessnig, unpublished observation). Besides being upregulated by PRRSV when administered around the same time or after PRRSV infection, initial PCV2 infection prior to PRRSV vaccination has previously shown to have a negative impact on PRRSV vaccine efficacy. Subclinical infection of pigs with PCV2 14 days before vaccination with a modified-live PRRSV vaccine resulted in significantly more severe PRRSV-induced macroscopic lung lesions after PRRSV challenge (Opriessnig et al., 2006). Evidence of interaction of PCV2 with the attenuated vaccine strain of PRRSV was lacking in that study.
In addition to several in vitro studies using the live pig model, a few in vitro experiments have been conducted to investigate the interaction of PRRSV and PCV2. In a co-infection study using pulmonary alveolar macrophages, which are the target cells for PRRSV in vivo, PCV2-induced interferon (IFN) α, a type I interferon which mainly acts by activating natural killer cells, reduced PRRSV infection and the PRRSV-associated cytopathic effect (Chang et al., 2005). In another in vitro study, pulmonary alveolar macrophages were infected singularly or with combinations of PCV2b, PCV2a, chimeric PCV2aORF1–2bORF2 (ORF1 from PCV2a and ORF2 from PCV2b), and chimeric PCV2bORF1–2aORF2 (ORF1 from PCV2b and ORF2 from PCV2a) and one of five PRRSV isolates (Lin et al., 2011. In vitro interaction of PRRSV and PCV2. In: 50th Ann. North Central Conf. Vet. Lab. Diagn., Brookings, South Dakota, p. 21). PCV2 replication, which was measured by detection of spliced PCV2 mRNA, was observed in all groups after 24 h; however, after 48 h, it was limited to groups inoculated with PCV2 strains containing ORF1 of PCV2a (PCV2a, chimeric PCV2aORF1–2bORF2) concurrently infected with PRRSV (Lin et al., 2011. In vitro interaction of PRRSV and PCV2. In: 50th Ann. North Central Conf. Vet. Lab. Diagn., Brookings, South Dakota, p. 21) implying that the PRRSV isolates used in the study provided some selective replication advantage on certain PCV2a isolates. Combined PCV2–PRRSV infection and the expression of Fas and Fas ligand (FasL), a type-II transmembrane protein that belongs to the TNF family and induces apoptosis after binding with its receptor, was investigated on the surface of splenic lymphocytes, macrophages, and peripheral blood lymphocytes (Chang et al., 2007). Splenic lymphocytes and macrophages in the dually infected group displayed a significantly higher amount of Fas and FasL expression on the cell surfaces compared to the group singularly infected with PCV2. Therefore, the investigators believe it seems reasonable that lymphocyte depletion in vivo is in part the result of apoptosis due to increased Fas and FasL expression (Chang et al., 2007).
The results from the studies above highlight the interaction between PRRSV and PCV2 in potentiating clinical manifestation of disease and the importance of controlling PRRSV infection should be emphasized to swine veterinarians and producers in order to reduce PCV2 virus loads in pig populations.
2.3. Torque teno sus virus
Torque teno sus virus (TTSuV) contains a single-stranded, circular-arranged, non-enveloped DNA genome of about 2.8 kb, but unlike PCV2, it is larger, lies in a negative sense array, and is currently classified into the genus Iotatorquevirus in the family Anelloviriade by the International Committee on Taxonomy of Viruses (ICTV) with two main TTSuV species including TTSuV1 and TTSuV2 (Huang et al., 2011). In a field study conducted in Spain, a significantly higher prevalence of TTSuV DNA was found in sera from PCVAD-affected animals (97%) than in sera from non-PCVAD-affected animals (78%) (Kekarainen et al., 2006). Overall, this indicated a high prevalence of both TTSuV species with a higher frequency in clinically affected animals than in clinically normal animals and with an apparent association between PCVAD and TTSuV2 (Kekarainen et al., 2006). These findings were further confirmed by a different Spanish study which found that TTSuV2 viral loads were significantly higher in PCVAD pigs; however, TTSuV1 prevalence and loads were not related with PCVAD (Aramouni et al., 2011). In contrast, in a Swedish study TTSuV DNA of both species was identified in tissues from clinically affected PCVAD pigs but also in tissues from a pig not suffering from PCVAD without differences between groups (Blomström et al., 2009). In support of the Swedish findings, similar amounts of TTSuV DNA were identified in PCVAD and non-PCVAD pigs in Korea suggesting that TTSuV may not exacerbate PCVAD in all instances (Lee et al., 2010).
Recently, the presence of TTSuV1, TTSuV2 and PCV2 DNA was demonstrated in ovaries and semen samples from naturally infected sows and boars; however, there was no association of presence of PCV2 DNA or TTSuV DNA with any abnormalities of semen morphology or endometritis (Ritterbusch et al., 2011). When livers from conventional Serbian pigs with microscopic lesions consistent with hepatitis were investigated for pathogens by using PCR, concurrent infection of PCV2, hepatitis E virus (HEV), and TTSuV was demonstrated (Savic et al., 2010). Specifically, TTSuV DNA was detected in a total of 86% (25/29) of livers with hepatitis but only in 57% (12/21) of normal livers. Pigs which suffered from infectious hepatitis caused by HEV, PCV2, or both had a 1.5 times higher likelihood of TTSuV detection than pigs with normal liver tissues (Savic et al., 2010).
TTSuV currently cannot be cultured in vitro as permissible cells lines have not been identified. Therefore, experimental co-infection studies of pigs with TTSuV and PCV2 often rely on TTSuV contaminated tissues or sera as inoculum. Interestingly, TTSuV1 was shown to promote PCV2 infection and disease in gnotobiotic pigs; however, it was found that the sequence of infection was important as clinical disease was only observed when TTSuV1 infection preceded PCV2 challenge by seven days (Ellis et al., 2008). The relevance of this finding is unknown as both PCV2 and TTSuV are widely distributed in swine populations. Based on the limited data available from field and experimental studies much more needs to be learned about this virus and its importance in PCVAD.
2.4. Porcine epidemic diarrhea virus
Porcine epidemic diarrhea virus (PEDV), a positive sense, single-stranded RNA virus, is a member of the genus Coronavirus, family Coronaviridae, order Nidovirales (Cavanagh, 1997, Pensaert et al., 1981, Zhou et al., 1988). PEDV can induce severe acute enteritis in pigs of all ages and PEDV infection is often fatal in neonatal piglets (Kim and Chae, 2000, Pensaert and de Bouck, 1978). Korean pigs naturally infected with PEDV were recently investigated for presence of PCV2 (Jung et al., 2006a). Among 107 small intestinal samples, PCV2 DNA was identified in 32.7% (35/107) and PCV2 antigen was found in 29.9% (32/107). Since most of the pigs in that study were less than three days old, prenatal in utero PCV2 infection was suspected (Jung et al., 2006a). To further confirm the possible link between PEDV and PCV2, six pregnant sows were randomly allocated to an infected or a control group and three pregnant sows were inoculated intranasally with PCV2 three weeks before the expected farrowing date (Jung et al., 2006b). Thirty piglets from the PCV2-infected sows and negative control sows were randomly assigned to two groups of 15 piglets each, respectively. The piglets in two of the groups, derived from PCV2-infected or negative control sows, were inoculated orally at 3 days of age with PEDV. In PEDV-infected piglets derived from PCV2-infected sows, significantly more PEDV RNA was detected in the jejunal tissues at 24 h after PEDV inoculation than in PEDV-infected piglets from PCV2 negative sows (Jung et al., 2006b). This is highly suggestive of an influence of transplacental PCV2 infection on PEDV and possibly also on other neonatal viral infections.
2.5. Swine influenza virus
Swine influenza virus (SIV), an enveloped, negative-sense, segmented RNA virus belonging to the family Orthomyxoviridae (Kuntz-Simon and Madec, 2009), infects the epithelium of the respiratory tract of pigs typically causing an acute disease of variable severity of coughing, fever, lethargy, and anorexia. There is field evidence indicating that SIV and PCV2 act synergistically in pigs with PRDC and both, PCV2 and SIV, can be identified frequently in the same pig under field conditions (Dorr et al., 2007, Harms et al., 2002, Pallarés et al., 2002). Despite the available field evidence supporting an interaction between these viruses, it has been shown recently that SIV did not influence PCV2 replication in experimentally infected CDCD pigs (Wei et al., 2010). In this study, the pigs were inoculated intratracheally with cell culture medium only, PCV2 alone, or PCV2 followed by SIV H1N1 infection one week later. Co-infection with SIV did not increase the severity of clinical disease or gross or microscopic lesions associated with PCV2 in lungs or lymph nodes (Wei et al., 2010).
2.6. Porcine endogenous retrovirus
Porcine endogenous retrovirus (PERV) infection has been linked with poor health and disease in pigs. Conversely, in a recent study where serum samples from clinically affected PCVAD pigs and from healthy pigs obtained from the same farm were compared, no difference in prevalence of pigs infected with PERV subtypes A and C or recombinant PERV-A/C was identified (Pal et al., 2011).
2.7. Porcine circovirus type 1
Similar to PCV2, PCV type 1 (PCV1) is a circular arranged, single-stranded, ambisense, non-enveloped DNA virus with a genome size of 1,759 in the Circoviridae family (Todd et al., 2005). When pigs were infected with PCV1 alone under experimental conditions, no clinical disease was observed (Allan et al., 1995, Fenaux et al., 2003, Tischer et al., 1986) and today PCV1 is considered to be non-pathogenic. In a Canadian field study involving 106 age matched PCVAD and non-PCVAD pigs, concurrent PCV1 and PCV2 infection was demonstrated in 13.0% (9/69) of the PCVAD cases indicating a potential role of PCV1 in PCV2 enhancement (Ellis et al., 2000). To the authors’ knowledge, an experimental co-infection with PCV1 and PCV2 has not been conducted to date.
2.8. Pseudorabies virus
Pseudorabies virus (PRV) is a linear enveloped double-stranded DNA virus of about 145 kb in size belonging to the Alphaherpesvirinae which is a subfamily of the Herpesviridae family (Mettenleiter, 2000). PRV is the cause of Aujeszky's disease which may manifest as abortions, central nervous system signs, and respiratory disease in pigs causing high death loss when infection of naïve herds occurs. Under field conditions, PRV infection was demonstrated in PCVAD pigs and associated with multifocal necrotizing tonsilitis and lymphadenitis (Rodríguez-Arrioja et al., 1999). In addition, clinical PCVAD has been reported to occur in farms endemically infected with PRV (Quintana et al., 2001) and wild boars in Spain had a high seroprevalence for PRV-associated disease (60.6%) which was found to be linked with PPV and PCV2 seroprevalence (Ruiz-Fons et al., 2006). To the authors’ knowledge, an experimental co-infection with PRV and PCV2 has not been conducted to date.
3. PCV2 and concurrent bacterial infections
3.1. Mycoplasma hyopneumoniae
M. hyopneumoniae is associated with porcine enzootic pneumonia (Goodwin et al., 1968, Mare and Switzer, 1966) characterized by high morbidity but low mortality in affected herds. Microscopically, the hallmark lesion associated with M. hyopneumoniae is hyperplasia of the bronchus-associated lymphoid tissue (Kwon et al., 2002, Sarradell et al., 2003; Fig. 5 A). Mycoplasma hyopneumoniae colonizes the luminal surface of bronchial and bronchiolar epithelial cells (Fig. 5C) without invading epithelial cells (Kwon et al., 2002, Sarradell et al., 2003). Typically, PCV2 antigen in dual-infected pigs can be readily demonstrated in these areas (Fig. 5B). The immune response induced by M. hyopneumoniae is complex with reports of both immunostimulation and immunosuppression (Kishima and Ross, 1985, Maes et al., 1996, Messier and Ross, 1991), non-specific mitogenic effects on swine lymphocytes (Messier and Ross, 1991), and alteration in cytokine profiles secreted by activated macrophages (Asai et al., 1993, Asai et al., 1994, Thacker et al., 2000, Thanawongnuwech et al., 2001). In a recent field investigation involving 147 pig farms across England, one of the identified factors associated with increased PCVAD severity was seropositivity to M. hyopneumoniae (Alarcon et al., 2011).
Fig. 5.
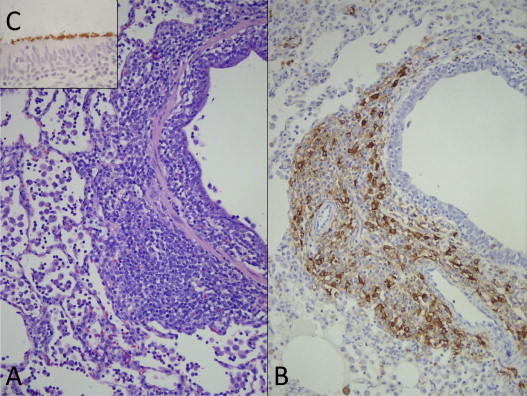
Lung; pig, 35 days after inoculation with Mycoplasma hyopneumoniae and 21 days after inoculation with PCV2 (Opriessnig et al., 2004b). (A) There is peribronchiolar lymphoid hyperplasia typical of M. hyopneumoniae infection. HE. (B) Abundant amounts of PCV2-antigen positive cells (brown staining) are associated with the M. hyopneumoniae-induced peribronchial lymphoid hyperplasia. Immunohistochemical staining with anti-PCV2 polyclonal antibody. Streptavidin–biotin–peroxidase complex method counterstained with hematoxylin. C. M. hyopneumoniae-specific brown staining at the luminal surface of the bronchus epithelial cells. Immunohistochemical staining with anti-M. hyopneumoniae monoclonal antibody. Streptavidin–biotin complex method counterstained with hematoxylin.
When conventional pigs were experimentally inoculated intratracheally with M. hyopneumoniae at 4 weeks of age followed by intranasal inoculation with PCV2 at 6 weeks of age, 23.5% (4/17) of the dual-infected pigs had decreased growth rate, severe lung lesions (Fig. 3B) and severe lymphoid depletion and granulomatous lymphadenitis associated with high amounts of PCV2-antigen consistent with PCVAD (Opriessnig et al., 2004b). When boars were challenged with M. hyopneumoniae and PCV2 using an interval of two weeks between inoculations, severe clinical disease was observed in co-infected boars, which was further accompanied by shedding of high levels of PCV2 DNA in semen posing a potential risk for PCV2 infection in downstream breeding herds (Opriessnig et al., 2011b). Overall, these studies documented that M. hyopneumoniae potentiates the severity of PCV2-associated lung and lymphoid lesions by increasing the amount and prolonging the presence of PCV2-antigen thereby increasing the incidence of clinical disease in pigs.
3.2. Lawsonia intracellularis
Proliferative enteropathy or ileitis is associated with Lawsonia intracellularis (L. intracellularis), an obligate intracellular organism which induces very distinct microscopic lesions including proliferation of intestinal epithelium and necrosuppurative enteritis (Jacobson et al., 2010). Clinically, pigs affected by ileitis commonly range from 6 to 20 weeks of age and display diarrhea and decreased growth rates. Initially, PCV2 was indentified in cases of granulomatous enteritis in Iberian pigs naturally infected with both, PCV2 and L. intracellularis (Segalés et al., 2001). In a Danish investigation involving tissues from 64 pigs with enteritis of varying intensity, 53.1% (34/64) were infected with both PCV2 and L. intracellularis whereas the remaining 46.9% (30/64) were infected with PCV2 alone (Jensen et al., 2006). To confirm a possible relationship between these two pathogens, conventional pigs were inoculated with PCV2, L. intracellularis, or both under experimental conditions (Opriessnig et al., 2011a). Individual pigs inoculated with PCV2 regardless of co-infection status developed severe PCVAD characterized clinically by weight loss and microscopically by PCV2-antigen associated severe lymphoid depletion and histiocytic replacement of the majority of lymphoid tissues (Opriessnig et al., 2011a). This is indicative that L. intracellularis does not enhance PCVAD and that co-infection of pigs is likely an incidental finding.
3.3. Salmonella spp.
Salmonella spp. is a common infection of pigs of all ages often resulting in diarrhea of varying degree (Wills, 2000). When the effect of Salmonella typhimurium in PCVAD was investigated under experimental conditions using SPF pigs, Salmonella spp. antigen was detected sporadically along with PCV2 antigen in the same macrophages; however, PCV2, PCVAD or Salmonella typhimurium were not potentiated (Opriessnig et al., 2011a). Interestingly, when CDCD pigs were infected with PCV2 at 5 weeks of age followed by infection with Salmonella choleraesuis at 7 weeks of age, the severity of clinical signs, microscopic lung lesions, and fecal shedding and tissue dissemination of Salmonella choleraesuis was enhanced indicating that prior exposure to PCV2 may increase clinical effects of salmonellosis in the field (Takada-Iwao et al., 2011).
4. PCV2 and concurrent protozoal or metazoal parasites or fungal infections
It is well established that any disease condition that compromises the immune system predisposes to parasitic or fungal infections. Since PCV2 is associated with lymphoid depletion it is not surprising that protozoal, metazoal, and fungal infections have been identified in pigs suffering from PCVAD. Pneumocystis carinii was reported to occur in approximately 5% of cases with PCVAD in Western Canada (Clark, 1997. Post-weaning multisystemic wasting syndrome. In: Proc Am Assoc Swine Pract, Quebec, Canada, vol. 28; pp. 499–501). A more recent study found a prevalence rate of 36.9% for Pneumocystis spp. in Brazilian pigs of which 28% were co-infected with PCV2 (Cavallini-Sanches et al., 2006). Furthermore, 60% (47/78) of wild boars obtained from Southern Brazil had PCVAD-like lesions (Borba et al., 2011). Both, Pneumocystis spp. and PCV2 antigen were detected in 20.5% (16/78) of the wild boars (Borba et al., 2011).
Cryptosporidium parvum, a rare intestinal pathogen in post-weaning and growing pigs, was demonstrated in a three-month-old Iberian pig concurrently infected with PCV2 likely due to PCV2-associated immune-suppression leading to upregulation of the Cryptosporidium parvum (Núñez et al., 2003). Furthermore, and in agreement with the immune-suppression theory, pulmonary aspergillosis was diagnosed in a PCVAD pig (Segalés et al., 2003). In industrialized countries and large indoor pig operations, metazoan parasites play only a limited role in pig production. Nevertheless, a recent case report described the presence of Metastrongylus elongatus and associated fatal bronchopneumonia in a PCVAD pig (Marruchella et al., 2011) further confirming that PCVAD is a multifactorial disease process with PCV2 replication being enhanced by co-infections or vice versa.
5. Polymicrobial infections and their impact on PCV2
Perhaps the best evidence that PCV2 is playing a substantial role in polymicrobial diseases of pigs is provided by the impact of PCV2 vaccines. Since becoming widely commercially available in 2006 (Opriessnig et al., 2007), PCV2 vaccines are among the most commonly used commercial biological products in growing pigs worldwide. In fact, in the United States an estimated 95–98% of pigs that reach market weight are currently being vaccinated against PCV2 at or around weaning age (Edgar Diaz, Boehringer Ingelheim, Vetmedica, personal communication).
Studies carried out under field conditions and in the presence of multiple co-infections, have found a substantial decrease in mortality rate, reduced PCV2 viremia, a reduced back fat depth, reduced number of culls, a reduced time to market and reduced medication cost in pigs vaccinated against PCV2 compared to non-vaccinated pigs raised concurrently in the same herd and under similar conditions (Desrosiers et al., 2009, Fachinger et al., 2008, Horlen et al., 2008, Jacela et al., 2011, Kixmöller et al., 2008, Martelli et al., 2011, Segalés et al., 2009, Takahagi et al., 2010). A recent vaccination study in a clinically affected herd showed that 93% of PCV2 vaccinated pigs but only 79% of non-vaccinated pigs reached the market (Venegas-Vargas et al., 2011).
PCV2 vaccinated pigs had markedly improved average daily gain, a higher percentage of lean meat yield, a better feed conversion rate, a higher number of closeout pigs and a higher carcass weight at slaughter compared to non-vaccinated pigs (Fachinger et al., 2008, Horlen et al., 2008, Jacela et al., 2011, Kixmöller et al., 2008, Martelli et al., 2011, Pejsak et al., 2010, Venegas-Vargas et al., 2011, Young et al., 2011). PCV2 vaccination had a $5.90/pig return on investment in a recent field study conducted in a subclinically infected herd which had greater carcass weight (95 kg versus 94 kg), mean loin depth (65.1 mm versus 63.3 mm) and a greater carcass index (111.6 versus 111.1) in PCV2 vaccinated pigs compared to non-vaccinated pigs (Young et al., 2011). When lung tissues were tested for presence of co-infections, the prevalence of PRRSV and Mycoplasma hyorhinis in lung tissues was significantly reduced in PCV2 vaccinated pigs compared to lung samples of placebo-treated animals (Kixmöller et al., 2008). In a recent meta-analysis investigating the effect of PCV2 vaccination on average daily weight gain in growing pigs, a significant effect was found in all production phases, with a significantly larger average daily gain in herds negative for PRRSV (Kristensen et al., 2011).
To better mimic what is going on under field conditions and to have an improved model to test the efficacy of different treatment strategies for PCV2, polymicrobial experimental inoculations have gained importance in recent years. It is not uncommon to inoculate pigs concurrently with PCV2 and PRRSV (Opriessnig et al., 2008, Sinha et al., 2010), PCV2, PRRSV and SIV (Opriessnig et al., 2009), or PCV2, PRRSV and PPV (Opriessnig et al., 2011c, Shen et al., 2010). The PCV2 and M. hyopneumoniae co-infection model was recently used to investigate the effect of PCV2 vaccination on boars with emphasis on clinical disease and PCV2 shedding in semen (Opriessnig et al., 2011b). When using a PCV2 challenge with one or more co-infecting pathogens, PCV2 vaccination is typically able to reduce or even prevent PCV2 viremia in vaccinated pigs and reduce the prevalence and severity of clinical disease (Opriessnig et al., 2011b, Opriessnig et al., 2011c).
6. Mechanisms by which co-infecting pathogens are thought to enhance PCV2 replication and PCVAD
Some known mechanisms by which pig pathogens interact include damaging of the mucociliary apparatus in respiratory tissues to allow bacterial colonization (Loving et al., 2010, Pol et al., 1997), inducing immune suppression (Renukaradhya et al., 2010), altering cytokine responses (Thanawongnuwech et al., 2004) or affecting macrophage function (Chiou et al., 2000). Several of these mechanisms can potentially be applied to PCV2 and PCVAD.
It has been suggested that PCV2 replication is enhanced by simultaneous infection of target cells and initiation of the host cells replication by the co-pathogen. It has been reported that new cellular DNA synthesis is the only in vivo prerequisite for PCV2 replication (Kennedy et al., 2000) implying that PCV2 requires support for activation of host cell replication. For example, it is possible that new DNA synthesis, mitosis and PPV-induced proliferation of histiocytes and macrophages, cell types in which PCV2 antigen can be identified frequently (Sorden, 2000), provide ideal conditions for PCV2 replication and dissemination (Kim et al., 2003). Similarly, the mechanism involved in potentiation of PCV2 replication in PCV2–PRRSV co-infected pigs may relate to the fact that monocytes and macrophages are common targets of both of these viruses (Allan et al., 2000a).
Alteration of the host cytokine response to create a more favorable environment for PCV2 is another possible mechanism for PCV2 upregulation. It has been demonstrated that treatment of porcine kidney-15 cell cultures with IFN-γ causes a 20 times higher production of PCV2 progeny (Meerts et al., 2005) indicating that any other pathogen which triggers IFN-γ expression in vivo ultimately could increase PCV2 replication. Increase of IFN-γ levels in pulmonary alveolar macrophages and bronchial lavage fluid is a common response to swine respiratory pathogens such as PRRSV and M. hyopneumoniae infection (Thanawongnuwech and Thacker, 2003). Recently, cytokine and chemokine mRNA expression profiles were determined in tracheobronchial lymph nodes from 28 pigs singularly infected with PCV2, M. hyopneumoniae, or dual-infected (Zhang et al., 2011a, Zhang et al., 2011b). Overall, M. hyopneumoniae potentiated PCV2 infection by increasing IFN-γ and IL-10 mRNA expression levels. The increase of IFN-γ and chemokines, and decrease of IFN-α in pigs singularly infected with PCV2 or dual-infected was correlated with increased severity of lymphoid lesions and presence of PCV2 antigen. This likely provides evidence that increased severity of lesions in dual-infected pigs was associated with presence of PCV2 antigen and alterations of cytokine mRNA expression profiles (Zhang et al., 2011a, Zhang et al., 2011b).
Studies using concurrent PPV and PCV2 infection have lead to the speculation that PPV-induced immune dysfunction allows enhanced replication of PCV2 or PPV infection and may activate monocytes which become more susceptible to PCV2 (Allan et al., 1999). Under cell-culture conditions, concurrent PCV2–PPV infection has been shown to decrease the ability of porcine pulmonary macrophages to phagocytose (Liu et al., 2011). Excessive production of tumor necrosis factor (TNF)-α, a cytokine that is involved in systemic inflammation and acute phase reactions, in response to PPV has been implicated as a potential contributor to PCVAD (Kim et al., 2006). An in vitro assay for TNF-α, conducted on alveolar macrophages obtained from pigs co-infected with PCV2 and PPV, showed a significant increase in TNF-α compared to macrophages infected alone with either PCV2 or PPV (Kim et al., 2006). In experimental models, all pigs inoculated with PPV alone or in combination with PCV2, displayed IL-10 responses in serum while only pigs infected with PPV in combination with PCV2 showed TNF-α in serum on repeated occasions (Hasslung et al., 2005). As IFN-γ production was reduced in this model, induction of TNF-α secretion by PPV (Kim et al., 2006) could promote the high levels of PCV2 typically seen with this particular co-infection.
It has also been suggested that pathogens such as PRRSV may interfere with PCV2 clearance, thereby favoring the persistence of PCV2. In this regard, PRRSV infection at the time of PCV2 infection significantly prolonged the presence of PCV2 DNA in serum and increased the amount of PCV2 DNA in oral and nasal secretions and fecal excretions in the later stages of infection (Sinha et al., 2011). The exact mechanisms of this phenomenon are unknown thus far, but PRRSV-induced immunosuppression and immune response modifications have been shown to play a role in the pathogenicity of other respiratory viruses (Jung et al., 2009) and may contribute similarly to PCV2 pathogenesis. The observations of reduction of a PRRSV-associated cytopathic effect in vitro in cells co-infected with PCV2 and PRRSV (Chang et al., 2005) and increased de novo expression and production of Fas ligand thereby increasing apoptosis (Chang et al., 2007) may also be applicable in vivo and may contribute to accelerated and prolonged disease commonly observed in PRRSV–PCV2 co-infected pigs.
It was found that the origin of replication of PCV2 genome contains an interferon-stimulated response element (ISRE)-like sequence (CTTCTTTCGTTTTCAGCT) (Mankertz et al., 2004). To assess the role of ISRE in PCV2 pathogenesis during co-infection, an ISRE-mutant PCV2 was constructed and used to experimentally infect pigs singularly or in combination PRRSV (Ramamoorthy et al., 2011). It was determined that mutation of the PCV2 ISRE caused an apparent increase of virulence in pigs co-infected with PCV2 and PRRSV at 14 dpi. The authors speculated that in the context of co-infections, IFN-γ induced transcription factors were unable to bind to the ISRE mutant, thus resulting in increased replication or, alternatively, that IFN-γ played a protective role during the early stages of PCV2–PRRSV co-infection (Ramamoorthy et al., 2011). To further investigate the role of PRRSV on PCV2, the PCV2 genomes recovered from clinically affected PCV2–PRRSV co-infected pigs were sequenced and compared (Yi and Liu, 2010) indicating that PCV2 ORF1 is highly conserved with only four polymorphic sites. In contrast, polymorphic sites were clustered in the capsid protein, and overlapped with the immunoreactive epitopes (Yi and Liu, 2010). Although in general, this approach could give some useful hints on differences in disease expression among pigs, unfortunately, PCV2 genomes from non-PRRSV infected pigs were not included so the importance of this finding and the role of PRRSV remain undetermined.
7. Conclusions
Since the initial recognition of PCV2 in 1998, this virus and its effect on the pig have been well characterized in the field and experimentally. Several experimental models capable of reproducing clinical PCVAD are now available. Comparison of outcomes between experiments is somewhat challenging as this work has been done by several groups across the world using different PCV2 isolates, different virus inocula doses, different routes of inoculation, different pig types (gnotobiotic, CDCD, CD, SPF, conventional) and ages, different sequences of infection and different co-infecting agents. However, based on this growing body of evidence from the field and research community it is clear that PCV2 infection can be upregulated and PCVAD can be enhanced by concurrent infection with a variety of pathogens. Moreover, in most controlled experiments, concurrent infection increased levels of PCV2 while the opposite, upregulation of the concurrent pathogen, was an inconsistent finding.
As demonstrated with TTSuV–PCV2 co-infection (Ellis et al., 2008), timing and sequence of co-infections may be an important factor and needs to be further investigated in order to gain more insight into the pathogenicity of PCV2 infection. It is of interest, that in one study where PCV2 was given first (subclinical PCV2 infection in sows and their piglets), the co-infecting pathogen (PEDV), and not PCV2, was up-regulated (Jung et al., 2006b).
The impact and regimens for control of PCVAD in growing pigs have been well studied and disseminated but the impact and most effective approach to control PCV2-infection in breeding herds still needs more research. Based on current knowledge, it is important that veterinary practitioners and diagnosticians do a thorough diagnostic investigation on herds where PCVAD is a recurrent problem. Prevention efforts should not only focus on efforts to eliminate or reduce PCV2 via vaccination but also to reduce the farm-specific pathogen load in general.
Acknowledgements
We thank previous and current members of the Iowa State University PCV2 research team including Joe Bender, Phil Gauger, Luis Giménez-Lirola, Michelle Hemann, Marlin Hoogland, Scott Jobgen, Kathy Lin, Darin Madson, Kevin O’Neill, Narinder Pal, Abby Patterson, Peter Thomas, Shayleen Schalk, Huigang Shen, Avanti Sinha, Chaoting Xiao and others.
References
- Alarcon P., Velasova M., Mastin A., Nevel A., Stärk K.D., Wieland B. Farm level risk factors associated with severity of post-weaning multi-systemic wasting syndrome. Prev. Vet. Med. 2011;101:182–191. doi: 10.1016/j.prevetmed.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Allan G., Meehan B., Todd D., Kennedy S., McNeilly F., Ellis J., Clark E.G., Harding J., Espuna E., Botner A., Charreyre C. Novel porcine circoviruses from pigs with wasting disease syndromes. Vet. Rec. 1998;142:467–468. [PubMed] [Google Scholar]
- Allan G.M., Ellis J.A. Porcine circoviruses: a review. J. Vet. Diagn. Invest. 2000;12:3–14. doi: 10.1177/104063870001200102. [DOI] [PubMed] [Google Scholar]
- Allan G.M., Kennedy S., McNeilly F., Foster J.C., Ellis J.A., Krakowka S.J., Meehan B.M., Adair B.M. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J. Comp. Pathol. 1999;121:1–11. doi: 10.1053/jcpa.1998.0295. [DOI] [PubMed] [Google Scholar]
- Allan G.M., McNeilly F., Cassidy J.P., Reilly G.A., Adair B., Ellis W.A., McNulty M.S. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet. Microbiol. 1995;44:49–64. doi: 10.1016/0378-1135(94)00136-k. [DOI] [PubMed] [Google Scholar]
- Allan G.M., McNeilly F., Ellis J., Krakowka S., Meehan B., McNair I., Walker I., Kennedy S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch. Virol. 2000;145:2421–2429. doi: 10.1007/s007050070031. [DOI] [PubMed] [Google Scholar]
- Allan G.M., McNeilly F., Meehan B.M., Ellis J.A., Connor T.J., McNair I., Krakowka S., Kennedy S. A sequential study of experimental infection of pigs with porcine circovirus and porcine parvovirus: immunostaining of cryostat sections and virus isolation. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2000;47:81–94. doi: 10.1046/j.1439-0450.2000.00364.x. [DOI] [PubMed] [Google Scholar]
- Aramouni M., Segales J., Sibila M., Martin-Valls G.E., Nieto D., Kekarainen T. Torque teno sus virus 1 and 2 viral loads in postweaning multisystemic wasting syndrome (PMWS) and porcine dermatitis and nephropathy syndrome (PDNS) affected pigs. Vet. Microbiol. 2011 doi: 10.1016/j.vetmic.2011.05.046. (Jun 12, Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Asai T., Okada M., Ono M., Irisawa T., Mori Y., Yokomizo Y., Sato S. Increased levels of tumor necrosis factor and interleukin 1 in bronchoalveolar lavage fluids from pigs infected with Mycoplasma hyopneumoniae. Vet. Immunol. Immunopathol. 1993;38:253–260. doi: 10.1016/0165-2427(93)90085-i. [DOI] [PubMed] [Google Scholar]
- Asai T., Okada M., Ono M., Mori Y., Yokomizo Y., Sato S. Detection of interleukin-6 and prostaglandin E2 in bronchoalveolar lavage fluids of pigs experimentally infected with Mycoplasma hyponeumoniae. Vet. Immunol. Immunopathol. 1994;44:97–102. doi: 10.1016/0165-2427(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Blomström A.L., Belák S., Fossum C., Fuxler L., Wallgren P., Berg M. Studies of porcine circovirus type 2, porcine boca-like virus and torque teno virus indicate the presence of multiple viral infections in postweaning multisystemic wasting syndrome pigs. Virus Res. 2010;152:59–64. doi: 10.1016/j.virusres.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Blomström A.L., Belák S., Fossum C., McKillen J., Allan G., Wallgren P., Berg M. Detection of a novel porcine boca-like virus in the background of porcine circovirus type 2 induced postweaning multisystemic wasting syndrome. Virus Res. 2009;146:125–129. doi: 10.1016/j.virusres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Bolin S.R., Stoffregen W.C., Nayar G.P., Hamel A.L. Postweaning multisystemic wasting syndrome induced after experimental inoculation of cesarean-derived, colostrum-deprived piglets with type 2 porcine circovirus. J. Vet. Diagn. Invest. 2001;13:185–194. doi: 10.1177/104063870101300301. [DOI] [PubMed] [Google Scholar]
- Borba M.R., Sanches E.M., Correa A.M., Spanamberg A., de Souza L.J., Soares M.P., Guillot J., Driemeier D., Ferreiro L. Immunohistochemical and ultra-structural detection of Pneumocystis in wild boars (Sus scrofa) co-infected with porcine circovirus type 2 (PCV2) in Southern Brazil. Med. Mycol. 2011;49:172–175. doi: 10.3109/13693786.2010.510540. [DOI] [PubMed] [Google Scholar]
- Brown T.T., Jr., Paul P.S., Mengeling W.L. Response of conventionally raised weanling pigs to experimental infection with a virulent strain of porcine parvovirus. Am. J. Vet. Res. 1980;41:1221–1224. [PubMed] [Google Scholar]
- Cavallini-Sanches E.M., Borba M.R., Spanamberg A., Pescador C., Corbellini L.G., Ravazzolo A.P., Driemeier D., Ferreiro L. Co-infection of Pneumocystis carinii f. sp. suis and porcine circovirus-2 (PCV2) in pig lungs obtained from slaughterhouses in Southern and Midwestern regions of Brazil. J. Eukaryot. Microbiol. 2006;53:92–94. doi: 10.1111/j.1550-7408.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Chang H.W., Jeng C.R., Lin C.M., Liu J.J., Chang C.C., Tsai Y.C., Chia M.Y., Pang V.F. The involvement of Fas/FasL interaction in porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus co-inoculation-associated lymphocyte apoptosis in vitro. Vet. Microbiol. 2007;122:72–82. doi: 10.1016/j.vetmic.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Chang H.W., Jeng C.R., Liu J.J., Lin T.L., Chang C.C., Chia M.Y., Tsai Y.C., Pang V.F. Reduction of porcine reproductive and respiratory syndrome virus (PRRSV) infection in swine alveolar macrophages by porcine circovirus 2 (PCV2)-induced interferon-alpha. Vet. Microbiol. 2005;108:167–177. doi: 10.1016/j.vetmic.2005.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.K., Lager K.M., Kohutyuk O.I., Vincent A.L., Henry S.C., Baker R.B., Rowland R.R., Dunham A.G. Detection of two porcine circovirus type 2 genotypic groups in United States swine herds. Arch. Virol. 2007;152:1035–1044. doi: 10.1007/s00705-006-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.K., Wu G., Wang D., Bayles D.O., Lager K.M., Vincent A.L. Identification and molecular cloning of a novel porcine parvovirus. Arch. Virol. 2010;155:801–806. doi: 10.1007/s00705-010-0646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou M.T., Jeng C.R., Chueh L.L., Cheng C.H., Pang V.F. Effects of porcine reproductive and respiratory syndrome virus (isolate tw91) on porcine alveolar macrophages in vitro. Vet. Microbiol. 2000;71:9–25. doi: 10.1016/s0378-1135(99)00159-5. [DOI] [PubMed] [Google Scholar]
- Choi C.S., Molitor T.W., Joo H.S., Gunther R. Pathogenicity of a skin isolate of porcine parvovirus in swine fetuses. Vet. Microbiol. 1987;15:19–29. doi: 10.1016/0378-1135(87)90125-8. [DOI] [PubMed] [Google Scholar]
- Cutlip R.C., Mengeling W.L. Experimentally induced infection of neonatal swine with porcine parvovirus. Am. J. Vet. Res. 1975;36:1179–1182. [PubMed] [Google Scholar]
- Dea S., Elazhary M.A., Martineau G.P., Vaillancourt J. Parvovirus-like particles associated with diarrhea in unweaned piglets. Can. J. Comp. Med. 1985;49:343–345. [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R., Clark E., Tremblay D., Tremblay R., Polson D. Use of a one-dose subunit vaccine to prevent losses associated with porcine circovirus type 2. J. Swine Health Prod. 2009;17:148–154. [Google Scholar]
- Dorr P.M., Baker R.B., Almond G.W., Wayne S.R., Gebreyes W.A. Epidemiologic assessment of porcine circovirus type 2 coinfection with other pathogens in swine. J. Am. Vet. Med. Assoc. 2007;230:244–250. doi: 10.2460/javma.230.2.244. [DOI] [PubMed] [Google Scholar]
- Drolet R., Larochelle R., Morin M., Delisle B., Magar R. Detection rates of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and swine influenza virus in porcine proliferative and necrotizing pneumonia. Vet. Pathol. 2003;40:143–148. doi: 10.1354/vp.40-2-143. [DOI] [PubMed] [Google Scholar]
- Duhamel G.E., Bargar T.W., Schmitt B.J., Molitor T.W., Lu W. Identification of parvovirus-like virus particles in intestinal crypt epithelial cells of pigs with diarrhea. J. Vet. Diagn. Invest. 1991;3:96–98. doi: 10.1177/104063879100300126. [DOI] [PubMed] [Google Scholar]
- Ellis J., Krakowka S., Lairmore M., Haines D., Bratanich A., Clark E., Allan G., Konoby C., Hassard L., Meehan B., Martin K., Harding J., Kennedy S., McNeilly F. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. J. Vet. Diagn. Invest. 1999;11:3–14. doi: 10.1177/104063879901100101. [DOI] [PubMed] [Google Scholar]
- Ellis J.A., Allan G., Krakowka S. Effect of coinfection with genogroup 1 porcine torque teno virus on porcine circovirus type 2-associated postweaning multisystemic wasting syndrome in gnotobiotic pigs. Am. J. Vet. Res. 2008;69:1608–1614. doi: 10.2460/ajvr.69.12.1608. [DOI] [PubMed] [Google Scholar]
- Ellis J.A., Bratanich A., Clark E.G., Allan G., Meehan B., Haines D.M., Harding J., West K.H., Krakowka S., Konoby C., Hassard L., Martin K., McNeilly F. Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J. Vet. Diagn. Invest. 2000;12:21–27. doi: 10.1177/104063870001200104. [DOI] [PubMed] [Google Scholar]
- Fachinger V., Bischoff R., Jedidia S.B., Saalmüller A., Elbers K. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine. 2008;26:1488–1499. doi: 10.1016/j.vaccine.2007.11.053. [DOI] [PubMed] [Google Scholar]
- Fenaux M., Opriessnig T., Halbur P.G., Meng X.J. Immunogenicity and pathogenicity of chimeric infectious DNA clones of pathogenic porcine circovirus type 2 (PCV2) and nonpathogenic PCV1 in weanling pigs. J. Virol. 2003;77:11232–11243. doi: 10.1128/JVI.77.20.11232-11243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R.F., Pomeroy A.P., Whittlestone P. Attempts to recover Mycoplasma suipneumoniae from experimental and natural cases of enzootic pneumonia in pigs. J. Hyg. 1968;66:595–603. doi: 10.1017/s0022172400028333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau-Roma L., Segalés J. Detection of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, swine influenza virus and Aujeszky's disease virus in cases of porcine proliferative and necrotizing pneumonia (PNP) in Spain. Vet. Microbiol. 2007;119:144–151. doi: 10.1016/j.vetmic.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Ha Y., Lee Y.H., Ahn K.K., Kim B., Chae C. Reproduction of postweaning multisystemic wasting syndrome in pigs by prenatal porcine circovirus 2 infection and postnatal porcine parvovirus infection or immunostimulation. Vet. Pathol. 2008;45:842–848. doi: 10.1354/vp.45-6-842. [DOI] [PubMed] [Google Scholar]
- Hao X., Lu Z., Sun P., Fu Y., Cao Y., Li P., Bai X., Bao H., Xie B., Chen Y., Li D., Liu Z. Phylogenetic analysis of porcine parvoviruses from swine samples in China. Virol. J. 2011;8:320. doi: 10.1186/1743-422X-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J., Clark E. Recognizing and diagnosing postweaning multisystemic wasting syndrome (PMWS) J. Swine Health Prod. 1997;5:201–203. [Google Scholar]
- Harms P.A., Halbur P.G., Sorden S.D. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. J. Swine Health Prod. 2002;10:27–30. [Google Scholar]
- Harms P.A., Sorden S.D., Halbur P.G., Bolin S.R., Lager K.M., Morozov I., Paul P.S. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet. Pathol. 2001;38:528–539. doi: 10.1354/vp.38-5-528. [DOI] [PubMed] [Google Scholar]
- Hasslung F., Wallgren P., Ladekjær-Hansen A.S., Bøtner A., Nielsen J., Wattrang E., Allan G.M., McNeilly F., Ellis J., Timmusk S., Belák K., Segall T., Melin L., Berg M., Fossum C. Experimental reproduction of postweaning multisystemic wasting syndrome (PMWS) in pigs in Sweden and Denmark with a Swedish isolate of porcine circovirus type 2. Vet. Microbiol. 2005;106:49–60. doi: 10.1016/j.vetmic.2004.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlen K.P., Dritz S.S., Nietfeld J.C., Henry S.C., Hesse R.A., Oberst R., Hays M., Anderson J., Rowland R.R. A field evaluation of mortality rate and growth performance in pigs vaccinated against porcine circovirus type 2. J. Am. Vet. Med. Assoc. 2008;232:906–912. doi: 10.2460/javma.232.6.906. [DOI] [PubMed] [Google Scholar]
- Huang Y.W., Harrall K.K., Dryman B.A., Beach N.M., Kenney S.P., Opriessnig T., Vaughn E.M., Roof M.B., Meng X.J. Expression of the putative ORF1 capsid protein of Torque teno sus virus 2 (TTSuV2) and development of Western blot and ELISA serodiagnostic assays: correlation between TTSuV2 viral load and IgG antibody level in pigs. Virus Res. 2011;158:79–88. doi: 10.1016/j.virusres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Jacela J.Y., Dritz S.S., DeRouchey J.M., Tokach M.D., Goodband R., Nelssen J.L. Field evaluation of the effects of a porcine circovirus type 2 vaccine on finishing pig growth performance, carcass characteristics, and mortality rate in a herd with a history of porcine circovirus-associated disease. J. Swine Health Prod. 2011;19:10–18. [Google Scholar]
- Jacobsen B., Krueger L., Seeliger F., Bruegmann M., Segalés J., Baumgaertner W. Retrospective study on the occurrence of porcine circovirus 2 infection and associated entities in Northern Germany. Vet. Microbiol. 2009;138:27–33. doi: 10.1016/j.vetmic.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Fellström C., Jensen-Waern M. Porcine proliferative enteropathy: an important disease with questions remaining to be solved. Vet. J. 2010;184:264–268. doi: 10.1016/j.tvjl.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Jensen T.K., Vigre H., Svensmark B., Bille-Hansen V. Distinction between porcine circovirus type 2 enteritis and porcine proliferative enteropathy caused by Lawsonia intracellularis. J. Comp. Pathol. 2006;135:176–182. doi: 10.1016/j.jcpa.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Jung K., Ha Y., Ha S.K., Kim J., Choi C., Park H.K., Kim S.H., Chae C. Identification of porcine circovirus type 2 in retrospective cases of pigs naturally infected with porcine epidemic diarrhoea virus. Vet. J. 2006;171:166–168. doi: 10.1016/j.tvjl.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Kim J., Ha Y., Choi C., Chae C. The effects of transplacental porcine circovirus type 2 infection on porcine epidemic diarrhoea virus-induced enteritis in preweaning piglets. Vet. J. 2006;171:445–450. doi: 10.1016/j.tvjl.2005.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Renukaradhya G.J., Alekseev K.P., Fang Y., Tang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J. Gen. Virol. 2009;90:2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekarainen T., Sibila M., Segalés J. Prevalence of swine torque teno virus in post-weaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected pigs in Spain. J. Gen. Virol. 2006;87:833–837. doi: 10.1099/vir.0.81586-0. [DOI] [PubMed] [Google Scholar]
- Kennedy S., Moffett D., McNeilly F., Meehan B., Ellis J., Krakowka S., Allan G.M. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J. Comp. Pathol. 2000;122:9–24. doi: 10.1053/jcpa.1999.0337. [DOI] [PubMed] [Google Scholar]
- Kim J., Chae C. Concurrent presence of porcine circovirus type 2 and porcine parvovirus in retrospective cases of exudative epidermitis in pigs. Vet. J. 2004;167:104–106. doi: 10.1016/j.tvjl.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kim J., Choi C., Chae C. Pathogenesis of postweaning multisystemic wasting syndrome reproduced by co-infection with Korean isolates of porcine circovirus 2 and porcine parvovirus. J. Comp. Pathol. 2003;128:52–59. doi: 10.1053/jcpa.2002.0605. [DOI] [PubMed] [Google Scholar]
- Kim J., Chung H.K., Jung T., Cho W.S., Choi C., Chae C. Postweaning multisystemic wasting syndrome of pigs in Korea: prevalence, microscopic lesions and coexisting microorganisms. J. Vet. Med. Sci. 2002;64:57–62. doi: 10.1292/jvms.64.57. [DOI] [PubMed] [Google Scholar]
- Kim J., Ha Y., Chae C. Potentiation of porcine circovirus 2-induced postweaning multisystemic wasting syndrome by porcine parvovirus is associated with excessive production of tumor necrosis factor-alpha. Vet. Pathol. 2006;43:718–725. doi: 10.1354/vp.43-5-718. [DOI] [PubMed] [Google Scholar]
- Kim O., Chae C. In situ hybridization for the detection and localization of porcine epidemic diarrhea virus in the intestinal tissues from naturally infected piglets. Vet. Pathol. 2000;37:62–67. doi: 10.1354/vp.37-1-62. [DOI] [PubMed] [Google Scholar]
- Kishima M., Ross R.F. Suppressive effect of nonviable Mycoplasma hyopneumoniae on phytohemagglutinin-induced transformation of swine lymphocytes. Am. J. Vet. Res. 1985;46:2366–2368. [PubMed] [Google Scholar]
- Kixmöller M., Ritzmann M., Eddicks M., Saalmüller A., Elbers K., Fachinger V. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine. 2008;26:3443–3451. doi: 10.1016/j.vaccine.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Ellis J.A., Meehan B., Kennedy S., McNeilly F., Allan G. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet. Pathol. 2000;37:254–263. doi: 10.1354/vp.37-3-254. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Hartunian C., Hamberg A., Shoup D., Rings M., Zhang Y., Allan G., Ellis J.A. Evaluation of induction of porcine dermatitis and nephropathy syndrome in gnotobiotic pigs with negative results for porcine circovirus type 2. Am. J. Vet. Res. 2008;69:1615–1622. doi: 10.2460/ajvr.69.12.1615. [DOI] [PubMed] [Google Scholar]
- Kresse J.I., Taylor W.D., Stewart W.W., Eernisse K.A. Parvovirus infection in pigs with necrotic and vesicle-like lesions. Vet. Microbiol. 1985;10:525–531. doi: 10.1016/0378-1135(85)90061-6. [DOI] [PubMed] [Google Scholar]
- Kristensen C.S., Baadsgaard N.P., Toft N. A meta-analysis comparing the effect of PCV2 vaccines on average daily weight gain and mortality rate in pigs from weaning to slaughter. Prev. Vet. Med. 2011;98:250–258. doi: 10.1016/j.prevetmed.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Kuntz-Simon G., Madec F. Genetic and antigenic evolution of swine influenza viruses in Europe and evaluation of their zoonotic potential. Zoonoses Public Health. 2009;56:310–325. doi: 10.1111/j.1863-2378.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- Kwon D., Choi C., Chae C. Chronologic localization of mycoplasma hyopneumoniae in experimentally infected pigs. Vet. Pathol. 2002;39:584–587. doi: 10.1354/vp.39-5-584. [DOI] [PubMed] [Google Scholar]
- Ladekjær-Mikkelsen A.-S., Nielsen J., Stadejek T., Storgaard T., Krakowka S., Ellis J., McNeilly F., Allan G., Bøtner A. Reproduction of postweaning multisystemic wasting syndrome (PMWS) in immunostimulated and non-immunostimulated 3-week-old piglets experimentally infected with porcine circovirus type 2 (PCV2) Vet. Microbiol. 2002;89:97–114. doi: 10.1016/S0378-1135(02)00174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lager K.M., Mengeling W.L. Porcine parvovirus associated with cutaneous lesions in piglets. J. Vet. Diagn. Invest. 1994;6:357–359. doi: 10.1177/104063879400600313. [DOI] [PubMed] [Google Scholar]
- Lager K.M., Mengeling W.L., Liu W. Comparison of the virulence of two isolates of porcine parvovirus in 72-day-old porcine fetuses. J. Vet. Diagn. Invest. 1992;4:245–248. doi: 10.1177/104063879200400303. [DOI] [PubMed] [Google Scholar]
- Lee S.S., Sunyoung S., Jung H., Shin J., Lyoo Y.S. Quantitative detection of porcine torque teno virus in porcine circovirus-2-negative and porcine circovirus-associated disease-affected pigs. J. Vet. Diagn. Invest. 2010;22:261–264. doi: 10.1177/104063871002200217. [DOI] [PubMed] [Google Scholar]
- Liu X., Chen L., Song Q., Yang F., Li Y., Zuo Y., Jiao J., Wang X. Coinfection effects of porcine circovirus type 2 and porcine parvovirus in vivo on phagocytosis and interferon mRNA expression of porcine alveolar macrophages. Wei Sheng Wu Xue Bao. 2011;51:105–114. [PubMed] [Google Scholar]
- Loving C.L., Brockmeier S.L., Vincent A.L., Palmer M.V., Sacco R.E., Nicholson T.L. Influenza virus coinfection with Bordetella bronchiseptica enhances bacterial colonization and host responses exacerbating pulmonary lesions. Microb. Pathog. 2010;49:237–245. doi: 10.1016/j.micpath.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Madson D.M., Opriessnig T. Effect of porcine circovirus type 2 (PCV2) infection on reproduction: disease, vertical transmission, diagnostics and vaccination. Anim. Health Res. Rev. 2011;12:47–65. doi: 10.1017/S1466252311000053. [DOI] [PubMed] [Google Scholar]
- Maes D., Verdonck M., Deluyker H., de K.A. Enzootic pneumonia in pigs. Vet. Q. 1996;18:104–109. doi: 10.1080/01652176.1996.9694628. [DOI] [PubMed] [Google Scholar]
- Mankertz A., Caliskan R., Hattermann K., Hillenbrand B., Kurzendoerfer P., Mueller B., Schmitt C., Steinfeldt T., Finsterbusch T. Molecular biology of porcine circovirus: analyses of gene expression and viral replication. Vet. Microbiol. 2004;98:81–88. doi: 10.1016/j.vetmic.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Mankertz A., Mankertz J., Wolf K., Buhk H.J. Identification of a protein essential for replication of porcine circovirus. J. Gen. Virol. 1998;9:381–384. doi: 10.1099/0022-1317-79-2-381. [DOI] [PubMed] [Google Scholar]
- Mare C.J., Switzer W.P. Virus pneumonia of pigs: propagation and characterization of a causative agent. Am. J. Vet. Res. 1966;27:1687–1693. [PubMed] [Google Scholar]
- Marruchella G., Paoletti B., Speranza R., Di G.G. Fatal bronchopneumonia in a Metastrongylus elongatus and porcine circovirus type 2 co-infected pig. Res. Vet. Sci. 2011 doi: 10.1016/j.rvsc.2011.05.016. (June 11, Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Martelli P., Ferrari L., Morganti M., De Angelis E., Bonilauri P., Guazzetti S., Caleffi A., Borghetti P. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet. Microbiol. 2011;149:339–351. doi: 10.1016/j.vetmic.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Meehan B.M., McNeilly F., Todd D., Kennedy S., Jewhurst V.A., Ellis J.A., Hassard L.E., Clark E.G., Haines D.M., Allan G.M. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J. Gen. Virol. 1998;79:2171–2179. doi: 10.1099/0022-1317-79-9-2171. [DOI] [PubMed] [Google Scholar]
- Meerts P., Misinzo G., Nauwynck H.J. Enhancement of porcine circovirus 2 replication in porcine cell lines by IFN-gamma before and after treatment and by IFN-alpha after treatment. J. Interferon Cytokine Res. 2005;25:684–693. doi: 10.1089/jir.2005.25.684. [DOI] [PubMed] [Google Scholar]
- Mengeling W.L. Prenatal infection following maternal exposure to porcine parvovirus on either the seventh or fourteenth day of gestation. Can. J. Comp. Med. 1979;43:106–109. [PMC free article] [PubMed] [Google Scholar]
- Messier S., Ross R.F. Interactions of Mycoplasma hyopneumoniae membranes with porcine lymphocytes. Am. J. Vet. Res. 1991;52:1497–1502. [PubMed] [Google Scholar]
- Mettenleiter T.C. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis-state of the art, June 1999. Vet. Res. 2000;31:99–115. doi: 10.1051/vetres:2000110. [DOI] [PubMed] [Google Scholar]
- Meulenberg J.J., Hulst M.M., de Meijer E.J., Moonen P.L., den B.A., de Kluyver E.P., Wensvoort G., Moormann R.J. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor T.W., Joo H.S., Collett M.S. Porcine parvovirus DNA: characterization of the genomic and replicative form DNA of two virus isolates. Virology. 1984;137:241–254. doi: 10.1016/0042-6822(84)90216-2. [DOI] [PubMed] [Google Scholar]
- Morandi F., Ostanello F., Fusaro L., Bacci B., Nigrelli A., Alborali L., Dottori M., Vezzoli F., Barigazzi G., Fiorentini L., Sala V., Leotti G., Joisel F., Sarli G. Immunohistochemical detection of aetiological agents of proliferative and necrotizing pneumonia in Italian pigs. J. Comp. Pathol. 2010;142:74–78. doi: 10.1016/j.jcpa.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Morozov I., Sirinarumitr T., Sorden S.D., Halbur P.G., Morgan M.K., Yoon K.J., Paul P.S. Detection of a novel strain of porcine circovirus in pigs with postweaning multisystemic wasting syndrome. J. Clin. Microbiol. 1998;36:2535–2541. doi: 10.1128/jcm.36.9.2535-2541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawagitgul P., Morozov I., Bolin S.R., Harms P.A., Sorden S.D., Paul P.S. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 2000;81:2281–2287. doi: 10.1099/0022-1317-81-9-2281. [DOI] [PubMed] [Google Scholar]
- Núñez A., McNeilly F., Perea A., Sánchez-Cordón P.J., Huerta B., Allan G., Carrasco L. Coinfection by Cryptosporidium parvum and porcine circovirus type 2 in weaned pigs. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2003;50:255–258. doi: 10.1046/j.1439-0450.2003.00664.x. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Fenaux M., Yu S., Evans R.B., Cavanaugh D., Gallup J.M., Pallarés F.J., Thacker E.L., Lager K.M., Meng X.J., Halbur P.G. Effect of porcine parvovirus vaccination on the development of PMWS in segregated early weaned pigs coinfected with type 2 porcine circovirus and porcine parvovirus. Vet. Microbiol. 2004;98:209–220. doi: 10.1016/j.vetmic.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Madson D.M., Prickett J.R., Kuhar D., Lunney J.K., Elsener J., Halbur P.G. Effect of porcine circovirus type 2 (PCV2) vaccination on porcine reproductive and respiratory syndrome virus (PRRSV) and PCV2 coinfection. Vet. Microbiol. 2008;131:103–114. doi: 10.1016/j.vetmic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Madson D.M., Roof M., Layton S.M., Ramamoorthy S., Meng X.J., Halbur P.G. Experimental reproduction of porcine circovirus type 2 (PCV2)-associated enteritis in pigs infected with PCV2 alone or concurrently with Lawsonia intracellularis or Salmonella typhimurium. J. Comp. Pathol. 2011;145:261–270. doi: 10.1016/j.jcpa.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Madson D.M., Schalk S., Brockmeier S., Shen H.G., Beach N.M., Meng X.J., Baker R.B., Zanella E.L., Halbur P.G. Porcine circovirus type 2 (PCV2) vaccination is effective in reducing disease and PCV2 shedding in semen of boars concurrently infected with PCV2 and Mycoplasma hyopneumoniae. Theriogenology. 2011;76:351–360. doi: 10.1016/j.theriogenology.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., McKeown N.E., Harmon K.L., Meng X.J., Halbur P.G. Porcine circovirus type 2 infection decreases the efficacy of a modified live porcine reproductive and respiratory syndrome virus vaccine. Clin. Vaccine Immunol. 2006;13:923–929. doi: 10.1128/CVI.00074-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opriessnig T., Meng X.J., Halbur P.G. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Invest. 2007;19:591–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Shen H.G., Pal N., Ramamoorthy S., Huang Y.W., Lager K.M., Beach N.M., Halbur P.G., Meng X.J. A live-attenuated chimeric porcine circovirus type 2 (PCV2) vaccine is transmitted to contact pigs but is not upregulated by concurrent infection with porcine parvovirus (PPV) and porcine reproductive and respiratory syndrome virus (PRRSV) and is efficacious in a PCV2b–PRRSV–PPV challenge model. Clin. Vaccine Immunol. 2011;18:1261–1268. doi: 10.1128/CVI.05057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opriessnig T., Thacker E.L., Yu S., Fenaux M., Meng X.J., Halbur P.G. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet. Pathol. 2004;41:624–640. doi: 10.1354/vp.41-6-624. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Patterson A.R., Madson D.M., Pal N., Halbur P.G. Comparison of efficacy of commercial one dose and two dose PCV2 vaccines using a mixed PRRSV-PCV2-SIV clinical infection model 2–3-months post vaccination. Vaccine. 2009;27:1002–1007. doi: 10.1016/j.vaccine.2008.11.105. [DOI] [PubMed] [Google Scholar]
- Oraveerakul K., Choi C.S., Molitor T.W. Tissue tropisms of porcine parvovirus in swine. Arch. Virol. 1993;130:377–389. doi: 10.1007/BF01309668. [DOI] [PubMed] [Google Scholar]
- Ostanello F., Caprioli A., Di F.A., Battilani M., Sala G., Sarli G., Mandrioli L., McNeilly F., Allan G.M., Prosperi S. Experimental infection of 3-week-old conventional colostrum-fed pigs with porcine circovirus type 2 and porcine parvovirus. Vet. Microbiol. 2005;108:179–186. doi: 10.1016/j.vetmic.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Pal N., Baker R., Schalk S., Scobie L., Tucker A.W., Opriessnig T. Detection of porcine endogenous retrovirus (PERV) viremia in diseased versus healthy US pigs by qualitative and quantitative real-time RT-PCR. Transbound Emerg. Dis. 2011;58:344–351. doi: 10.1111/j.1865-1682.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- Pallarés F.J., Halbur P.G., Opriessnig T., Sorden S.D., Villar D., Janke B.H., Yaeger M.J., Larson D.J., Schwartz K.J., Yoon K.J., Hoffman L.J. Porcine circovirus type 2 (PCV-2) coinfections in US field cases of postweaning multisystemic wasting syndrome (PMWS) J. Vet. Diagn. Invest. 2002;14:515–519. doi: 10.1177/104063870201400614. [DOI] [PubMed] [Google Scholar]
- Paul P.S., Mengeling W.L. Evaluation of a modified live-virus vaccine for the prevention of porcine parvovirus-induced reproductive disease in swine. Am. J. Vet. Res. 1980;41:2007–2011. [PubMed] [Google Scholar]
- Pejsak Z., Podgórska K., Truszczyński M., Karbowiak P., Stadejek T. Efficacy of different protocols of vaccination against porcine circovirus type 2 (PCV2) in a farm affected by postweaning multisystemic wasting syndrome (PMWS) Comp. Immunol. Microbiol. Infect. Dis. 2010;33:e1–e5. doi: 10.1016/j.cimid.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Pensaert M.B., de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B., Debouck P., Reynolds D.J. An immunoelectron microscopic and immunofluorescent study on the antigenic relationship between the coronavirus-like agent, CV 777, and several coronaviruses. Arch. Virol. 1981;68:45–52. doi: 10.1007/BF01315166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogranichniy R.M., Yoon K.J., Harms P.A., Sorden S.D., Daniels M. Case-control study on the association of porcine circovirus type 2 and other swine viral pathogens with postweaning multisystemic wasting syndrome. J. Vet. Diagn. Invest. 2002;14:449–456. doi: 10.1177/104063870201400601. [DOI] [PubMed] [Google Scholar]
- Pol J.M., van Leengoed L.A., Stockhofe N., Kok G., Wensvoort G. Dual infections of PRRSV/influenza or PRRSV/Actinobacillus pleuropneumoniae in the respiratory tract. Vet. Microbiol. 1997;55:259–264. doi: 10.1016/s0378-1135(96)01323-5. [DOI] [PubMed] [Google Scholar]
- Quintana J., Segalés J., Rosell C., Calsamiglia M., Rodriguez-Arrioja G.M., Chianini F., Folch J.M., Maldonado J., Canal M., Plana-Duran J., Domingo M. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet. Rec. 2001;149:357–361. doi: 10.1136/vr.149.12.357. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S., Opriessnig T., Pal N., Huang F.F., Meng X.J. Effect of an interferon-stimulated response element (ISRE) mutant of porcine circovirus type 2 (PCV2) on PCV2-induced pathological lesions in a porcine reproductive and respiratory syndrome virus (PRRSV) co-infection model. Vet. Microbiol. 2011;147:49–58. doi: 10.1016/j.vetmic.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Renukaradhya G.J., Alekseev K., Jung K., Fang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunol. 2010;23:457–466. doi: 10.1089/vim.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritterbusch G.A., Sa Rocha C.A., Mores N., Simon N.L., Zanella E.L., Coldebella A., Ciacci-Zanella J.R. Natural co-infection of torque teno virus and porcine circovirus 2 in the reproductive apparatus of swine. Res. Vet. Sci. 2011 doi: 10.1016/j.rvsc.2011.04.001. (April 27, Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Rodríguez-Arrioja G.M., Segalés J., Rosell C., Quintana J., Ayllón S., Camprodón A., Domingo M. Aujeszky's disease virus infection concurrent with postweaning multisystemic wasting syndrome in pigs. Vet. Rec. 1999;144:152–153. doi: 10.1136/vr.144.6.152. [DOI] [PubMed] [Google Scholar]
- Rosell C., Segales J., Ramos-Vara J.A., Folch J.M., Rodriguez-Arrioja G.M., Duran C.O., Balasch M., Plana-Duran J., Domingo M. Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome. Vet. Rec. 2000;146:40–43. doi: 10.1136/vr.146.2.40. [DOI] [PubMed] [Google Scholar]
- Rovira A., Balasch M., Segalés J., García L., Plana-Durán J., Rosell C., Ellerbrok H., Mankertz A., Domingo M. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J. Virol. 2002;76:3232–3239. doi: 10.1128/JVI.76.7.3232-3239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Fons F., Vicente J., Vidal D., Hofle U., Villanua D., Gauss C., Segalés J., Almeria S., Montoro V., Gortazar C. Seroprevalence of six reproductive pathogens in European wild boar (Sus scrofa) from Spain: the effect on wild boar female reproductive performance. Theriogenology. 2006;65:731–743. doi: 10.1016/j.theriogenology.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Sarradell J., Andrada M., Ramirez A.S., Fernandez A., Gomez-Villamandos J.C., Jover A., Lorenzo H., Herraez P., Rodriguez F. A morphologic and immunohistochemical study of the bronchus-associated lymphoid tissue of pigs naturally infected with Mycoplasma hyopneumoniae. Vet. Pathol. 2003;40:395–404. doi: 10.1354/vp.40-4-395. [DOI] [PubMed] [Google Scholar]
- Savic B., Milicevic V., Bojkovski J., Kureljusic B., Ivetic V., Pavlovic I. Detection rates of the swine torque teno viruses (TTVs), porcine circovirus type 2 (PCV2) and hepatitis E virus (HEV) in the livers of pigs with hepatitis. Vet. Res. Commun. 2010;34:641–648. doi: 10.1007/s11259-010-9432-z. [DOI] [PubMed] [Google Scholar]
- Segalés J., Allan G.M., Domingo M. Porcine circovirus diseases. Anim. Health Res. Rev. 2005;6:119–142. doi: 10.1079/ahr2005106. [DOI] [PubMed] [Google Scholar]
- Segalés J., Calsamiglia M., Domingo M. Post-weaning multisystemic wasting syndrome (PMWS) and porcine circovirus type 2 (PCV-2) infection in Spain. Pig J. 2003;51:98–107. [Google Scholar]
- Segalés J., Calsamiglia M., Rosell C., Soler M., Maldonado J., Martin M., Domingo M. Porcine reproductive and respiratory syndrome virus (PRRSV) infection status in pigs naturally affected with post-weaning multisystemic wasting syndrome (PMWS) in Spain. Vet. Microbiol. 2002;85:23–30. doi: 10.1016/s0378-1135(01)00474-6. [DOI] [PubMed] [Google Scholar]
- Segalés J., Fernandez-Salguero J.M., Fructuoso G., Quintana J., Rosell C., Pozo J., De Arriba M.L., Rubio P., Domingo M. Granulomatous enteritis and lymphadenitis in Iberian pigs naturally infected with Lawsonia intracellularis. Vet. Pathol. 2001;38:343–346. doi: 10.1354/vp.38-3-343. [DOI] [PubMed] [Google Scholar]
- Segalés J., Olvera A., Grau-Roma L., Charreyre C., Nauwynck H., Larsen L., Dupont K., McCullough K., Ellis J., Krakowka S., Mankertz A., Fredholm M., Fossum C., Timmusk S., Stockhofe-Zurwieden N., Beattie V., Armstrong D., Grassland B., Baekbo P., Allan G. PCV-2 genotype definition and nomenclature. Vet. Rec. 2008;162:867–868. doi: 10.1136/vr.162.26.867. [DOI] [PubMed] [Google Scholar]
- Segalés J., Urniza A., Alegre A., Bru T., Crisci E., Nofrarías M., López-Soria S., Balasch M., Sibila M., Xu Z., Chu H.J., Fraile L., Plana-Duran J. A genetically engineered chimeric vaccine against porcine circovirus type 2 (PCV2) improves clinical, pathological and virological outcomes in postweaning multisystemic wasting syndrome affected farms. Vaccine. 2009;27:7313–7321. doi: 10.1016/j.vaccine.2009.09.084. [DOI] [PubMed] [Google Scholar]
- Shen H.G., Beach N.M., Huang Y.W., Halbur P.G., Meng X.J., Opriessnig T. Comparison of commercial and experimental porcine circovirus type 2 (PCV2) vaccines using a triple challenge with PCV2, porcine reproductive and respiratory syndrome virus (PRRSV), and porcine parvovirus (PPV) Vaccine. 2010;28:5960–5966. doi: 10.1016/j.vaccine.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Sinha A., Shen H.G., Schalk S., Beach N.M., Huang Y.W., Halbur P.G., Meng X.J., Opriessnig T. Porcine reproductive and respiratory syndrome virus infection at the time of porcine circovirus type 2 vaccination has no impact on vaccine efficacy. Clin. Vaccine Immunol. 2010;17:1940–1945. doi: 10.1128/CVI.00338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A., Shen H.G., Schalk S., Beach N.M., Huang Y.W., Meng X.J., Halbur P.G., Opriessnig T. Porcine reproductive and respiratory syndrome virus (PRRSV) influences infection dynamics of porcine circovirus type 2 (PCV2) subtypes PCV2a and PCV2b by prolonging PCV2 viremia and shedding. Vet. Microbiol. 2011 doi: 10.1016/j.vetmic.2011.05.005. (May 11, Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Sorden S.D. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS) J. Swine Health Prod. 2000;8:133–136. [Google Scholar]
- Takada-Iwao A., Nakanishi M., Souma J., Chikata S., Okuda Y., Imai Y., Sato S. Porcine circovirus type 2 potentiates morbidity of Salmonella enterica serovar choleraesuis in cesarean-derived, colostrum-deprived pigs. Vet. Microbiol. 2011 doi: 10.1016/j.vetmic.2011.06.036. (July 5, Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Takahagi Y., Toki S., Nishiyama Y., Morimatsu F., Murakami H. Differential effects of porcine circovirus type 2 (PCV2) vaccination on PCV2 genotypes at Japanese pig farms. J. Vet. Med. Sci. 2010;72:35–41. doi: 10.1292/jvms.09-0314. [DOI] [PubMed] [Google Scholar]
- Thacker E.L., Thacker B.J., Young T.F., Halbur P.G. Effect of vaccination on the potentiation of porcine reproductive and respiratory syndrome virus (PRRSV)-induced pneumonia by Mycoplasma hyopneumoniae. Vaccine. 2000;18:1244–1252. doi: 10.1016/s0264-410x(99)00395-3. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R., Thacker B., Halbur P., Thacker E.L. Increased production of proinflammatory cytokines following infection with porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Clin. Diagn. Lab. Immunol. 2004;11:901–908. doi: 10.1128/CDLI.11.5.901-908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawongnuwech R., Thacker E.L. Interleukin-10, interleukin-12, and interferon-gamma levels in the respiratory tract following Mycoplasma hyopneumoniae and PRRSV infection in pigs. Viral Immunol. 2003;16:357–367. doi: 10.1089/088282403322396154. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R., Young T.F., Thacker B.J., Thacker E.L. Differential production of proinflammatory cytokines: in vitro PRRSV and Mycoplasma hyopneumoniae co-infection model. Vet. Immunol. Immunopathol. 2001;79:115–127. doi: 10.1016/s0165-2427(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Tischer I., Gelderblom H., Vettermann W., Koch M.A. A very small porcine virus with circular single-stranded DNA. Nature. 1982;295:64–66. doi: 10.1038/295064a0. [DOI] [PubMed] [Google Scholar]
- Tischer I., Mields W., Wolff D., Vagt M., Griem W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 1986;91:271–276. doi: 10.1007/BF01314286. [DOI] [PubMed] [Google Scholar]
- Tischer I., Peters D., Rasch R., Pociuli S. Replication of porcine circovirus: induction by glucosamine and cell cycle dependence. Arch. Virol. 1987;96:39–57. doi: 10.1007/BF01310989. [DOI] [PubMed] [Google Scholar]
- Tischer I., Rasch R., Tochtermann G. Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines. Zentralbl Bakteriol. 1974;226:153–167. [PubMed] [Google Scholar]
- Todd D., Bendinelli M., Biagini P., Hino S., Mankertz A., Mishiro S., Niel C., Okamoto H., Raidal S., Ritchie B.W., Teo G. Circoviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy. Eighth report of the international committee on taxonomy of viruses; 2005. pp. 327–334. [Google Scholar]
- Venegas-Vargas M.C., Bates R., Morrison R., Villani D., Straw B. Effect of porcine circovirus type 2 vaccine on postweaning performance and carcass composition. J. Swine Health Prod. 2011;19:233–237. [Google Scholar]
- Wei H., Lenz S.D., Van Alstine W.G., Stevenson G.W., Langohr I.M., Pogranichniy R.M. Infection of cesarean-derived colostrum-deprived pigs with porcine circovirus type 2 and Swine influenza virus. Comp. Med. 2010;60:45–50. [PMC free article] [PubMed] [Google Scholar]
- Wellenberg G.J., Stockhofe-Zurwieden N., Boersma W.J., de Jong M.F., Elbers A.R. The presence of co-infections in pigs with clinical signs of PMWS in The Netherlands: a case-control study. Res. Vet. Sci. 2004;77:177–184. doi: 10.1016/j.rvsc.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills R.W. Diarrhea in growing-finishing swine. Vet. Clin. North Am. Food Anim. Pract. 2000;16:135–161. doi: 10.1016/s0749-0720(15)30140-7. [DOI] [PubMed] [Google Scholar]
- Yi J., Liu C. Molecular characterization of porcine circovirus 2 isolated from diseased pigs co-infected with porcine reproductive and respiratory syndrome virus. Virol. J. 2010;7:286. doi: 10.1186/1743-422X-7-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.G., Cunningham G.L., Sanford S.E. Circovirus vaccination in pigs with subclinical porcine circovirus type 2 infection complicated by ileitis. J. Swine Health Prod. 2011;19:175–180. [Google Scholar]
- Zhang H., Lunney J.K., Baker R.B., Opriessnig T. Cytokine and chemokine mRNA expression profiles in tracheobronchial lymph nodes from pigs singularly infected or coinfected with porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae (MHYO) Vet. Immunol. Immunopathol. 2011;140:152–158. doi: 10.1016/j.vetimm.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Zhang H.B., Huang L., Liu Y.J., Lin T., Sun C.Q., Deng Y., Wei Z.Z., Cheung A.K., Long J.X., Yuan S.S. Porcine bocaviruses: genetic analysis and prevalence in Chinese swine population. Epidemiol. Infect. 2011;19:1–6. doi: 10.1017/S0950268811000847. [DOI] [PubMed] [Google Scholar]
- Zhou Y.L., Ederveen J., Egberink H., Pensaert M., Horzinek M.C. Porcine epidemic diarrhea virus (CV 777) and feline infectious peritonitis virus (FIPV) are antigenically related. Arch. Virol. 1988;102:63–71. doi: 10.1007/BF01315563. [DOI] [PMC free article] [PubMed] [Google Scholar]


