Abstract
Feline infectious peritonitis virus (FIPV) causes a fatal disease called FIP in Felidae. The effusion in body cavity is commonly associated with FIP. However, the exact mechanism of accumulation of effusion remains unclear. We investigated vascular endothelial growth factor (VEGF) to examine the relationship between VEGF levels and the amounts of effusion in cats with FIP. Furthermore, we examined VEGF production in FIPV-infected monocytes/macrophages, and we used feline vascular endothelial cells to examine vascular permeability induced by the culture supernatant of FIPV-infected macrophages. In cats with FIP, the production of effusion was related with increasing plasma VEGF levels. In FIPV-infected monocytes/macrophages, the production of VEGF was associated with proliferation of virus. Furthermore, the culture supernatant of FIPV-infected macrophages induced hyperpermeability of feline vascular endothelial cells. It was suggested that vascular permeability factors, including VEGF, produced by FIPV-infected monocytes/macrophages might increase the vascular permeability and the amounts of effusion in cats with FIP.
Keywords: Coronavirus, FIP, VEGF, Monocyte, Macrophage, Effusion
1. Introduction
Feline infectious peritonitis virus (FIPV), a feline coronavirus (FCoV) of the family Coronaviridae, causes a fatal disease called FIP in wild and domestic cat species. FCoV is mainly composed of nucleocapsid (N) protein, envelope protein, membrane protein, and peplomer spike (S) protein (Pedersen, 2009). FCoV is classified into serotypes I and II according to the amino acid sequence of its S protein (Motokawa et al., 1995, Motokawa et al., 1996). Both serotypes consist of two biotypes: FIPV and feline enteric coronavirus (FECV). FECV infection is asymptomatic in cats. In contrast, FIPV infection causes FIP. Stoddart and Scott (1989) reported that the proliferation in macrophages was associated with the virulence of FIPV because FECV exhibited a lower proliferation in macrophages than FIPV. It has been proposed that FIPV arises from FECV by mutation (Chang et al., 2010, Poland et al., 1996, Vennema et al., 1998), but the exact mutation and inducing factors have not yet been clarified. Recently, Brown et al. (2009) theoretically demonstrated that FIP develops via the horizontal infection of FIPV. On the basis of their theory, FIPV and FECV are prevalent among cats.
Macrophages/monocytes play an important role in the pathogenesis of FIP. It has been reported that virus replication in monocytes/macrophages induced interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α production (Regan et al., 2009, Takano et al., 2007b, Takano et al., 2009a). The FIPV replication and cytokine production are enhanced when monocytes/macrophages are inoculated with FIPV in presence of anti-FIPV S antibodies (antibody-dependent enhancement: ADE) (Hohdatsu et al., 1991, Corapi et al., 1992, Olsen et al., 1992). The phenomenon of ADE is involved in aggravation of the FIP pathology (Pedersen and Boyle, 1980, Takano et al., 2007b, Takano et al., 2008, Takano et al., 2009a).
FIP is characterized by granulomatous lesions in several organs, and the effusion in pleural and peritoneal cavities (Pedersen, 2009). It has been suggested that TNF-α, G-CSF, and GM-CSF produced by FIPV-infected monocytes/macrophages are involved in the formation of granulomatous lesions (Takano et al., 2009b). On the other hand, the effusion has been considered to result from increased vascular permeability induced by a type-III hypersensitivity reaction. It was assumed that immune complex-mediated damage to vessels causes increase in permeability of endothelial cells in cats with FIP (Weiss and Scott, 1981a, Weiss and Scott, 1981b). However, it has been reported that there are no immune complexes in the vascular lesions of cats with FIP (Kipar et al., 2005). Specifically, factors other than the type-III hypersensitivity reaction may also cause the effusion in cats with FIP.
It has been reported that vascular endothelial growth factor (VEGF) is increased in blood by systemic vasculitis or granulomatous lesions (Bragado et al., 1999, Li et al., 1998, Maeno et al., 1998, Viac et al., 1999). VEGF is a potent factor involved in vascular permeability and angiogenesis (Dvorak et al., 1999). VEGF-producing cells include monocytes, macrophages, fibroblasts, and keratinocytes. It has been reported that VEGF is involved in effusion in body cavities, for example, plasma leakage in dengue hemorrhagic fever and ascetic fluid accumulation in ovarian cancer (Kraft et al., 1999, Tseng et al., 2005). Based on these findings, VEGF may also be increased in cats with FIP. However, it is unknown whether VEGF is involved in effusion in cats with FIP.
We determined plasma VEGF levels of cats with FIP and investigated their relationship with the amounts of the effusion in body cavity. In addition, we examined the relationship between viral proliferation and VEGF production in FIPV-infected monocytes and macrophages. Furthermore, we used feline vascular endothelial cells to examine vascular permeability induced by the culture supernatant of FIPV-infected macrophages.
2. Result
2.1. Plasma VEGF level of specific pathogen-free (SPF) cats and cats with FIP
We compared the levels of VEGF in plasma from SPF cats and cats with FIP. The VEGF levels were significantly higher in the plasma of cats with FIP than those of SPF cats (Fig. 1A). In cats with FIP, the amounts of effusion per weight were positively correlated with plasma VEGF levels (Fig. 1B).
Fig. 1.
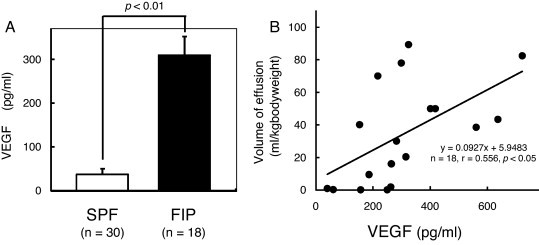
Relationship between plasma VEGF levels and amounts of effusion. (A) Plasma VEGF level of SPF cats and cats with FIP. (B) Plasma VEGF level in cats with FIP correlated with pleural/peritoneal fluid balance.
2.2. Relationship between plasma VEGF levels and the amounts of effusion
Time-dependent changes in the plasma VEGF levels of FIPV-infected cats were investigated. No change was noted for the plasma VEGF levels in cats that developed no FIP symptoms (FIPV-infected non-FIP cats) after FIPV infection until the experiment was completed (for 60 days after FIPV infection) (Fig. 2A; Cat 1–Cat 3). In cats with FIP, plasma VEGF levels increased after FIPV infection (Fig. 2A; Cat 4–Cat 12). In addition, in cats that persistently produced VEGF after FIPV infection until FIP development, the amounts of effusion per weight tended to be increased (Fig. 2A and B; Cat 6, Cat 8, Cat 9, and Cat 12).
Fig. 2.
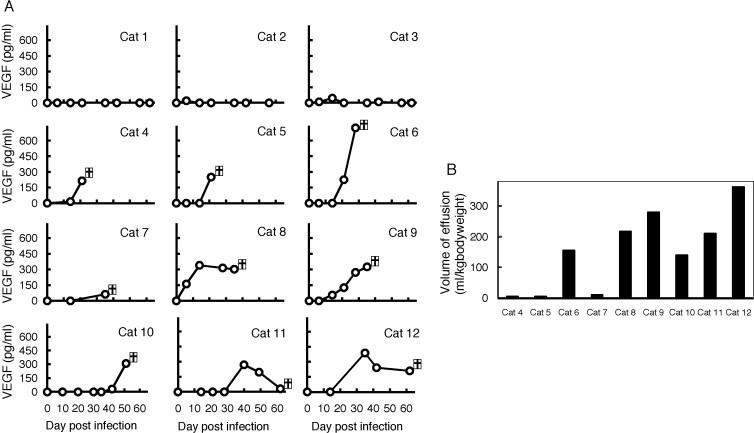
Relationship between plasma VEGF levels and the amounts of effusion after FIPV inoculation. (A) Time-dependent changes in plasma VEGF levels in FIPV-infected cats. Plasma was periodically sampled after the FIPV inoculation of SPF cats until FIP development to measure plasma VEGF levels by ELISA. Cat 1–Cat 3: FIPV-infected non FIP cats. Cat 4–Cat 12: cats with FIP. (B) The amounts of exudate accumulation when cats with FIP were euthanized (Cat 4–Cat 12). The individual numbers on the horizontal axis correspond to those of (A).  in the (A) indicates the day when a cat was euthanized due to worsening of the clinical conditions.
in the (A) indicates the day when a cat was euthanized due to worsening of the clinical conditions.
2.3. VEGF mRNA and FCoV N gene expression levels in monocytes and macrophages of SPF cats and cats with FIP
We compared the levels of VEGF mRNA and FCoV N gene expression in monocytes and alveolar macrophages derived from SPF cats and cats with FIP. VEGF mRNA and FCoV N gene expression levels were increased in monocytes and alveolar macrophages derived from cats with FIP (Fig. 3 ).
Fig. 3.
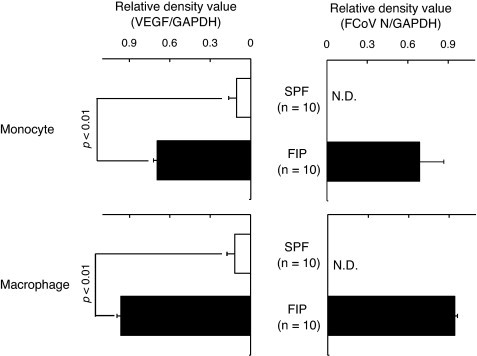
VEGF mRNA and FCoV N gene expression levels in monocytes and macrophages of SPF cats and cats with FIP. Monocytes and macrophages were collected from SPF cats and cats with FIP, and VEGF mRNA and FCoV N gene were detected by RT-PCR. VEGF mRNA and FCoV N gene were quantitatively analyzed in terms of the relative density compared to that of mRNA for the housekeeping gene GAPDH. N.D.: not detected.
2.4. FIPV infection and VEGF production in monocytes and macrophages
To investigate the relationship between FIPV infection and VEGF production in monocytes and alveolar macrophages, SPF cat-derived monocytes and alveolar macrophages were inoculated with medium alone, virus alone, and MAb 6-4-2 alone, and a mixture of FIPV and MAb 6-4-2. After 2 days, the culture supernatant and cells were collected, and FIPV replication and VEGF production were measured. The virus titer and VEGF production were significantly higher in the culture supernatant of monocytes and alveolar macrophages inoculated with a mixture of FIPV and MAb 6-4-2, than in that of monocytes and alveolar macrophages cultured with medium alone, virus alone, and MAb 6-4-2 alone (Fig. 4, Fig. 5 ). The intracellular FCoV gene and VEGF mRNA expression levels were significantly increased in monocytes and alveolar macrophages inoculated with a mixture of FIPV and MAb 6-4-2 (Fig. 4, Fig. 5), showing that VEGF production was increased as virus replication in monocytes and alveolar macrophages.
Fig. 4.
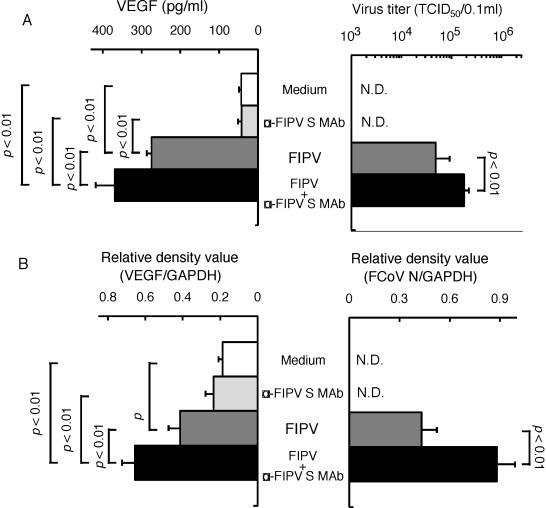
Relationship between FIPV infection and VEGF production in monocytes. SPF cat-derived monocytes were cultured with medium alone, FIPV, FIPV + α-FIPV S MAb, and α-FIPV MAb alone for 2 days, and the cells and culture supernatant were collected. The virus titer in the culture supernatant was measured (A, right), and VEGF production was measured using ELISA (A, left). In addition, the FCoV N gene (B, right) and VEGF mRNA (B, left) in the cells were detected by RT-PCR. The FCoV N gene and VEGF mRNA were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. n = 7, N.D.: not detected.
Fig. 5.
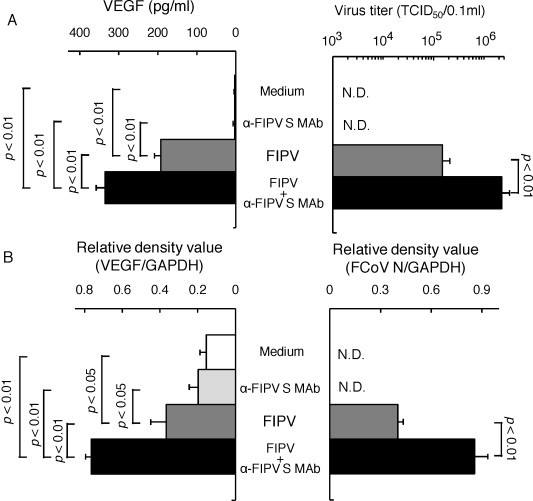
Relationship between FIPV infection and VEGF production in macrophages. SPF cat-derived alveolar macrophages were cultured with medium alone, FIPV, FIPV + α-FIPV S MAb, and α-FIPV MAb alone for 2 days, and the cells and culture supernatant were collected. The virus titer in the culture supernatant was measured (A, right), and VEGF production was measured using ELISA (A, left). In addition, the FCoV N gene (B, right) and VEGF mRNA (B, left) in the cells were detected by RT-PCR. The FCoV N gene and VEGF mRNA were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. n = 7, N.D.: not detected.
2.5. Identification of feline vascular endothelial cells
Vascular endothelial cells were collected from the vessels of SPF cats (see Section 4). The incorporation efficiency of DiL-labelled acetylated low-density lipoprotein (Dil-Ac-LDL), a characteristic function of endothelial cells, was compared between CrFK cells (non-vascular endothelial cells) and human umbilical vein endothelial cell (HUVEC; human vascular endothelial cells). The cells collected from the vessels of SPF cats showed an epithelial cell-like morphology and incorporated DiL-Ac-LDL (Fig. 6 ). Similar results were observed for feline endothelial cells and HUVEC. CrFK cells showed no Dil-Ac-LDL incorporation.
Fig. 6.
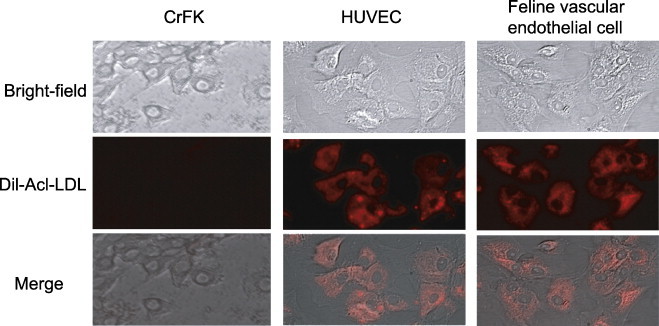
Identification of feline vascular endothelial cells. To demonstrate that the cells isolated from the vessels of SPF cats were vascular endothelial cells, their morphology and Dil-Ac-LDL incorporation were investigated. Human vascular endothelial cells (HUVEC) were used as a positive control and feline endothelial cells (CrFK cells) as a negative control.
2.6. The presence of permeability factor in FIPV-infected macrophage culture supernatant
The presence of permeability factor in the FIPV-infected macrophage culture supernatant was investigated. Feline vascular endothelial cells were exposed for 24 h to recombinant feline VEGF (rfVEGF) and the culture supernatant of macrophages inoculated with a mixture of FIPV and MAb 6-4-2. The culture supernatant was heated at 56 °C to inactivate viruses. As the control, alveolar macrophages were cultured with heat-inactivated culture supernatant of uninfected macrophages. Endothelial permeability was increased by rfVEGF and the culture supernatant of macrophages inoculated with a mixture of FIPV and MAb 6-4-2 compared to those in cells cultured with the control supernatant (Fig. 7 ). Particularly, feline vascular endothelial cells, exposed to the culture supernatant of macrophages inoculated with a mixture of FIPV and MAb 6-4-2, showed a markedly increased endothelial permeability.
Fig. 7.
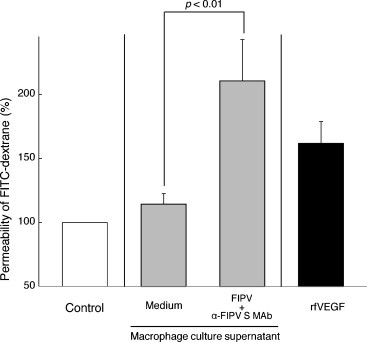
The effects of the culture supernatant of FIPV-infected macrophages on the permeability of feline vascular endothelial cells. Control: medium without supernatant and rfVEGF. n = 5.
3. Discussion
VEGF is a major factor of vascular permeability. It has been reported that the potency of VEGF of vascular permeability is 50,000 times stronger than that of histamine (Dvorak et al., 1999). In patients with inflammatory diseases and cancer, the production of effusion in body cavity was related with increasing plasma VEGF levels and the vascular permeability. For example, in patients with rheumatoid arthritis, the amounts of synovial fluid related with plasma VEGF level (Fava et al., 1994). In addition, it has been demonstrated that VEGF is involved in the ascites of patients with ovarian cancer (Hu et al., 2002, Mesiano et al., 1998). In this study, we observed significantly increased plasma VEGF levels in cats with FIP. We also demonstrated a correlation between plasma VEGF levels and the amounts of effusion in cats with FIP. Specifically, it was strongly suggested that VEGF is involved in effusion in cats with FIP.
We investigated FIPV-infected monocytes/macrophages as VEGF-producing cells in cats with FIP. FIPV-infected monocytes/macrophages are major component cells that produce cytokines (TNF-α, IL-1, IL-6, etc.) involved in pathological deterioration (Regan et al., 2009, Takano et al., 2007b, Takano et al., 2009a). Here, we demonstrated that the production of VEGF associated with FIPV proliferation in monocytes/macrophages. The mechanism of FIPV-induced VEGF production is unknown. Regan et al. (2009) reported that FIPV activates p38-MAP kinase (p38-MAPK) to induce TNF-α production in FIPV-infected monocytes. In addition, p38-MAPK activation induces VEGF production (Yoshino et al., 2006). In the future, the relationship between p38-MAPK activation and the proliferation of FIPV in monocytes/macrophages should be examined.
We demonstrated that vascular permeability-associated factors existed in the culture supernatant of FIPV-infected macrophages, and that the factors were thermostable. It was reported that VEGF was thermostable (Clauss et al., 1990). Based on these facts, VEGF may be a vascular permeability-associated factor existing in the culture supernatant of FIPV-infected macrophages. To verify this concept, we should examine whether an anti-feline VEGF antibody can inhibit the permeability of feline vascular endothelial cells, induced by the culture supernatant of FIPV-infected macrophages. However, to the best of our knowledge, there are no antibodies that neutralizes feline VEGF activity. We will create a monoclonal antibody to neutralize feline VEGF and examine whether this antibody can inhibit the vascular permeability induced by the culture supernatant of FIPV-infected macrophages. The culture supernatant of FIPV-infected macrophages contained VEGF in a smaller amount (about 20 pg/ml VEGF) than rfVEGF (400 pg/ml). However, it showed increased vascular permeability. Thus, the culture supernatant of FIPV-infected macrophages contained vascular permeability factors other than VEGF. According to a recent report, TNF-α increases the vascular permeability induced by VEGF (Stathopoulos et al., 2007). Like VEGF, TNF-α is thermostable (Takano et al., 2007b). We should also examine whether TNF-α, like VEGF, is involved in the vascular permeability induced by the culture supernatant of FIPV-infected macrophages.
In some cats, the volume of effusion was low in spite of the plasma VEGF level was high. These cats were immediately sacrificed from a humane viewpoint because of dyspnea associated with pneumonia. On the autopsy of these cats, slight effusion was present in the thoracic and abdominal regions. If pneumonia had not developed in these cats, effusive fluid may have accumulated, as observed in the other cats.
A balance between T helper (Th)-1/Th-2 in cats after FIPV infection is critical in examining whether FIPV-infected cats develop FIP. The induction of interferon (IFN)-γ, a Th-1 cytokine, inhibits FIP development. In contrast, IL-6, a Th-2 cytokine, promotes FIP development (Gelain et al., 2006, Goitsuka et al., 1990, Gunn-Moore et al., 1998, Kiss et al., 2004, Takano et al., 2009a). Interestingly, VEGF production is inhibited by IFN-γ, but increased by IL-6 (Wen et al., 2003, Feurino et al., 2007). In this study, plasma VEGF levels were not increased in cats that did not develop FIP. In contrast, plasma VEGF levels were increased in cats that developed FIP. Based on these findings, plasma VEGF levels may reflect the immune status of FIPV-infected cats.
VEGF is reportedly involved in the increased vascular permeability of Orf virus and Hantavirus as well as FIPV (Lyttle et al., 1994, Gavrilovskaya et al., 2008). It has been demonstrated that Orf virus carries a gene encoding a VEGF homolog, and that VEGF produced by the virus increases the permeability of vascular endothelial cells (Lyttle et al., 1994). No gene encoding a VEGF-like protein could be found in the FIPV viral genome (data not shown). Specifically, unlike Orf virus, FIPV may not produce VEGF. On the other hand, Hantavirus reportedly shows increased susceptibility to VEGF and increased vascular permeability in virus-infected vascular endothelial cells (Gavrilovskaya et al., 2008). It is unknown whether FIPV infects vascular endothelial cells. No report has been published on FIPV antigen detection from the vascular endothelial cells of cats with FIP. However, we demonstrated that FIPV proliferated in vascular endothelial cells into which a high-titer FIPV 79–1146 strain was inoculated (Takano and Hohdatsu, unpublished data). The effects of FIPV infection on vascular endothelial cells should be investigated in the future.
FIP is characterized by vasculitis (Hayashi et al., 1976, Pedersen, 2009). To elucidate the pathology of FIP, it is desirable to conduct experiments using feline vascular endothelial cells. However, no report has been published on the use of feline vascular endothelial cells in FIP research. One of the reasons for this, no feline vascular endothelial cell line is available. In addition, as experienced in this study, a method to isolate vascular endothelial cells from SPF cats requires many cats and skills. Even if cells are successfully isolated, their use in experiments is limited by the number of passages. When a feline vascular endothelial cell line is established, it will allow the easy handling of cells and may facilitate further elucidation of the mechanism of pathological deterioration in FIP.
The disease types of FIP are divided into wet (effusive) and dry (non-effusive) forms based on the presence of effusion (Pedersen, 2009). The cat with FIP that we experimentally generated showed various disease types; some cats showed the typical wet-form FIP, and others showed the form of pyogranulomatous lesions in various organs accompanying effusion in body cavity. However, no dry-form FIP was noted. The reason for this is unknown. In the future, the relationship with plasma VEGF levels should be investigated using dry-form FIP.
4. Materials and methods
4.1. Experimental animals
Type II FIPV strain 79–1146 (105 TCID50/ml) was administered orally to 12-month-old SPF cats. Cats with developed FIP symptoms (cats with FIP), such as fever, weight loss, peritoneal or pleural effusion, dyspnea, ocular lesions, and neural symptoms were used in this study. FIP diagnoses were confirmed upon postmortem examination, revealing peritoneal and pleural effusions, and pyogranuloma in major organs. Age-matched SPF cats were used as controls. The protocol for the experiments in the present study using cats was approved by the Ethics Committee of Kitasato University, School of Veterinary Medicine (No. 10-015).
4.2. Plasma sample
Blood collected from cats using a heparinized syringe was centrifuged at 3000 rpm for 10 min, and the supernatant was used as a plasma sample. The plasma samples were stored at −30 °C until day of analysis.
4.3. Measurement of plasma VEGF concentration
Plasma concentrations of VEGF were determined with human VEGF ELISA kit (R & D Systems, U.K.), according to the manufacturer's protocol. The ELISA kit detects primarily the feline VEGF isoform 164 (Koga et al., 2002).
4.4. Cell cultures and virus
Felis catus whole fetus-4 (Fcwf-4) cells was grown in Eagles’ minimum essential medium containing 50% L-15 medium, 5% fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin. Feline monocytes and alveolar macrophages were maintained in RPMI 1640 growth medium supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol. Feline vascular endothelial cells and HUVEC (Cell Systems, U.S.A.) were cultured in endothelial growth medium (Cell systems, U.S.A.) containing 10% FCS, defined cell boost (recombinant growth factors; Cell systems, U.S.A.), 100 U/ml penicillin, and 100 μg/ml streptomycin. Type II FIPV strain 79–1146 was grown in Fcwf-4 cells at 37 °C. FIPV strain 79–1146 was supplied by Dr. M. C. Horzinek of State University Utrecht, The Netherlands.
4.5. Recovery of feline monocytes, alveolar macrophages and vascular endothelial cells
Feline monocytes were isolated from SPF cats as previously described by Dewerchin et al. (2005). Feline alveolar macrophages were obtained by broncho-alveolar lavage with Hanks's balanced salt solution (HBSS) from anti-FCoV antibody-negative SPF cats, as previously described by Hohdatsu et al. (1991).
Feline vascular endothelial cells were obtained from feline aorta of SPF cats by the modified methods based on the report described by Ryan (1984). The aorta was rinsed three times with HBSS. After treatment with collagenase II (Wako, Japan), the intimal surface was lightly scraped with a sterile scalpel blade. The scraped cells were washed twice, and placed into collagen-coated 24-well plastic plate. After three days, the supernatant was removed and was replaced with fresh medium. When the cells became confluent, they were washed twice and are placed into collagen-coated 25 cm2 culture flasks. The endothelial specificity of the cells was verified by the uptake of DiL-Ac-LDL (Invitrogen, U.S.A.).
4.6. Antibodies
MAb 6-4-2 (IgG2a) used in the present study recognizes S protein of type II FIPV, as demonstrated by immunoblotting. It has been reported that MAb 6-4-2 exhibits a neutralizing activity in Fcwf-4 and CrFK cells, but an enhancing activity in feline macrophages depending on the reaction conditions (Hohdatsu et al., 1993).
4.7. RNA isolation and cDNA preparation
RNA isolation and cDNA preparation were performed employing the method of Takano et al. (2007a).
4.8. Determination of levels of feline GAPDH mRNA, VEGF mRNA, and FCoV N gene expression
cDNA was amplified by PCR using specific primers for feline GAPDH mRNA, VEGF mRNA, and FCoV N genes. The primer sequences are shown in Table 1 . PCR was performed using the method of Takano et al. (2007a). The band density was quantified under appropriate UV exposure by video densitometry using Scion Image software (Scion Corporation, U.S.A.). VEGF mRNA and FCoV N genes were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH.
Table 1.
Sequence of PCR primers for feline GAPDH, VEGF, and FCoV N.
| Region | Orientation | Nucleotide sequence | Location | Position | Reference |
|---|---|---|---|---|---|
| GAPDH | Forward | 5′-AATTCCACGGCACAGTCAAGG-3′ | 158–178 | 97 | Takano et al. (2007a) |
| Reverse | 5′-CATTTGATGTTGGCGGGATC-3′ | 235–254 | |||
| VEGF | Forward | 5′-CACGGAGGAGTTCAACATCA-3′ | 287–306 | 168 | Genbank accession no. AB071947 |
| Reverse | 5′-AAATGCTTTCTCCGCTCTGA-3′ | 435–454 | |||
| FCoV N | Forward | 5′-CAACTGGGGAGATGAACCTT-3′ | 876–895 | 788 | Genbank accession no. X56496 |
| Reverse | 5′-GGTAGCATTTGGCAGCGTTA-3′ | 1644–1663 |
4.9. Inoculation of feline monocytes and macrophages with FIPV
Viral suspension (FIPV strain 79–1146, 2 × 103 TCID50/0.1 ml) and MAb 6-4-2 solution were mixed at an equivalent volume ratio and reacted at 4 °C for 1 h, and 0.1 ml of this reaction solution was used to inoculate alveolar macrophages (2 × 106 cells) or feline monocytes (2 × 105 cells) cultured in each well of 24-well multi-plates. As the control, medium alone, and virus suspension alone were added to feline alveolar macrophages. After virus adsorption at 37 °C for 1 h, the cells were washed with HBSS and 1 ml of growth medium. The cells and culture supernatant were collected after 48 h. The cells were used for measurement of the VEGF mRNA and FCoV N genes, and the culture supernatant was employed for the quantitative analysis of VEGF and the virus titer.
4.10. Permeability assays
Feline vascular endothelial cells (2 × 104 cells) were seeded on collagen-coated Transwell filters (3 μm pore size; Costar, U.S.A.) in 24-well plates and cultured with 150 μl of the culture medium in the upper chamber and 750 μl of the same growth medium in the lower chamber. When the cells became confluent (24 h after seeding), they were incubated with supernatant of mock-inoculated monocytes/macrophages (diluted 1:20), supernatant of FIPV- and MAb 6-4-2-inoculated monocytes/macrophages (diluted 1:20), rfVEGF (final concentration of 400 pg/ml; R & D Systems, U.K.), and medium without supernatant and rfVEGF as control. After 24 h of incubation, the medium in both of chamber was removed, 150 μl of FITC-dextran (final concentration of 2 mg/ml) was added to the upper chamber and 550 μl of medium was added to the lower chamber. After 2 h, the medium in the lower chamber was removed and the amount of FITC-dextran was determined spectrometrically at 492 nm. Data were the relative permeability percentage (%) of indicated conditions in that treated the medium without supernatant and rfVEGF is defined as 100%, as described by Chen et al. (2008).
4.11. Statistical analysis
Data of two-group were analyzed by Student's t test, and multiple groups were analyzed by one-way ANOVA. Fig. 1B was applied to determine statistical significance using Pearson correlation coefficient. P-values < 0.05 were considered to indicate a significant difference between compared groups.
Acknowledgements
This work was in part supported by KAKENHI (Grants-in-Aid for Young Scientists (B), No. 22780285) from the Ministry of Education, Culture, Sports, Science and Technology, Grant for Encouragement of Young Scientists (No. E0904) from the School of Veterinary Medicine of Kitasato University, and Kitasato University Research Grant for Young Researchers (2010).
References
- Bragado R., Bello E., Requena L., Renedo G., Texeiro E., Alvarez M.V., Castilla M.A., Caramelo C. Increased expression of vascular endothelial growth factor in pyogenic granulomas. Acta Derm. Venereol. 1999;79:422–425. doi: 10.1080/000155599750009834. [DOI] [PubMed] [Google Scholar]
- Brown M.A., Troyer J.L., Pecon-Slattery J., Roelke M.E., O’Brien S.J. Genetics and pathogenesis of feline infectious peritonitis virus. Emerg. Infect. Dis. 2009;15:1445–1452. doi: 10.3201/eid1509.081573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.H., de Groot R.J., Egberink H.F., Rottier P.J. Feline infectious peritonitis:insights into feline coronavirus pathobiogenesis and epidemiology based on genetic analysis of the viral 3c gene. J. Gen. Virol. 2010;91:415–420. doi: 10.1099/vir.0.016485-0. [DOI] [PubMed] [Google Scholar]
- Chen S.U., Chou C.H., Lee H., Ho C.H., Lin C.W., Yang Y.S. Lysophosphatidic acid up-regulates expression of interleukin-8 and -6 in granulosa-lutein cells through its receptors and nuclear factor-kappaB dependent pathways: implications for angiogenesis of corpus luteum and ovarian hyperstimulation syndrome. J. Clin. Endocrinol. Metab. 2008;93:935–943. doi: 10.1210/jc.2007-1512. [DOI] [PubMed] [Google Scholar]
- Corapi W.V., Olsen C.W., Scott F.W. Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus. J. Virol. 1992;66:6695–6705. doi: 10.1128/jvi.66.11.6695-6705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss M., Gerlach M., Gerlach H., Brett J., Wang F., Familletti P.C., Pan Y.C., Olander J.V., Connolly D.T., Stern D. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J. Exp. Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H.F., Nagy J.A., Feng D., Brown L.F., Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr. Top. Microbiol. Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- Dewerchin H.L., Cornelissen E., Nauwynck H.J. Replication of feline coronaviruses in peripheral blood monocytes. Arch. Virol. 2005;150:2483–2500. doi: 10.1007/s00705-005-0598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava R.A., Olsen N.J., Spencer-Green G., Yeo K.T., Yeo T.K., Berse B., Jackman R.W., Senger D.R., Dvorak H.F., Brown L.F. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J. Exp. Med. 1994;180:341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurino L.W., Zhang Y., Bharadwaj U., Zhang R., Li F., Fisher W.E., Brunicardi F.C., Chen C., Yao Q., Min L. IL-6 stimulates Th2 type cytokine secretion and upregulates VEGF and NRP-1 expression in pancreatic cancer cells. Cancer Biol. Ther. 2007;6:1096–1100. doi: 10.4161/cbt.6.7.4328. [DOI] [PubMed] [Google Scholar]
- Gavrilovskaya I.N., Gorbunova E.E., Mackow N.A., Mackow E.R. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 2008;82:5797–5806. doi: 10.1128/JVI.02397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelain M.E., Meli M., Paltrinieri S. Whole blood cytokine profiles in cats infected by feline coronavirus and healthy non-FCoV infected specific pathogen-free cats. J. Feline Med. Surg. 2006;8:389–399. doi: 10.1016/j.jfms.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitsuka R., Ohashi T., Ono K., Yasukawa K., Koishibara Y., Fukui H., Ohsugi Y., Hasegawa A. IL-6 activity in feline infectious peritonitis. J. Immunol. 1990;144:2599–2603. [PubMed] [Google Scholar]
- Gunn-Moore D.A., Caney S.M., Gruffydd-Jones T.J., Helps C.R., Harbour D.A. Antibody and cytokine responses in kittens during the development of feline infectious peritonitis (FIP) Vet. Immunol. Immunopathol. 1998;65:221–242. doi: 10.1016/S0165-2427(98)00156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Goto N., Takahashi R., Fujiwara K. Systemic vascular lesions in feline infectious peritonitis. Nippon Juigaku Zasshi. 1976;39:365–377. doi: 10.1292/jvms1939.39.365. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Nakamura M., Ishizuka Y., Yamada H., Koyama H. A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies. Arch. Virol. 1991;120:207–217. doi: 10.1007/BF01310476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Yamada H., Ishizuka Y., Koyama H. Enhancement and neutralization of feline infectious peritonitis virus infection in feline macrophages by neutralizing monoclonal antibodies recognizing different epitopes. Microbiol. Immunol. 1993;37:499–504. doi: 10.1111/j.1348-0421.1993.tb03242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Hofmann J., Zaloudek C., Ferrara N., Hamilton T., Jaffe R.B. Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. Am. J. Pathol. 2002;161:1917–1924. doi: 10.1016/S0002-9440(10)64467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar A., May H., Menger S., Weber M., Leukert W., Reinacher M. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 2005;42:321–330. doi: 10.1354/vp.42-3-321. [DOI] [PubMed] [Google Scholar]
- Kiss I., Poland A.M., Pedersen N.C. Disease outcome and cytokine responses in cats immunized with an avirulent feline infectious peritonitis virus (FIPV)-UCD1 and challenge -exposed with virulent FIPV-UCD8. J. Feline Med. Surg. 2004;6:89–97. doi: 10.1016/j.jfms.2003.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga L., Kobayashi Y., Yazawa M., Maeda S., Masuda K., Ohno K., Tsujimoto H. Nucleotide sequence and expression of the feline vascular endothelial growth factor. J. Vet. Med. Sci. 2002;64:453–456. doi: 10.1292/jvms.64.453. [DOI] [PubMed] [Google Scholar]
- Kraft A., Weindel K., Ochs A., Marth C., Zmija J., Schumacher P., Unger C., Marmé D., Gastl G. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178–187. [PubMed] [Google Scholar]
- Li C.G., Reynolds I., Ponting J.M., Holt P.J., Hillarby M.C., Kumar S. Serum levels of vascular endothelial growth factor (VEGF) are markedly elevated in patients with Wegener's granulomatosis. Br. J. Rheumatol. 1998;37:1303–1306. doi: 10.1093/rheumatology/37.12.1303. [DOI] [PubMed] [Google Scholar]
- Lyttle D.J., Fraser K.M., Fleming S.B., Mercer A.A., Robinson A.J. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J. Virol. 1994;68:84–92. doi: 10.1128/jvi.68.1.84-92.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno N., Takei S., Masuda K., Akaike H., Matsuo K., Kitajima I., Maruyama I., Miyata K. Increased serum levels of vascular endothelial growth factor in Kawasaki disease. Pediatr. Res. 1998;44:596–599. doi: 10.1203/00006450-199810000-00021. [DOI] [PubMed] [Google Scholar]
- Mesiano S., Ferrara N., Jaffe R.B. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am. J. Pathol. 1998;153:1249–1256. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Aizawa C., Koyama H., Hashimoto H. Molecular cloning and sequence determination of the peplomer protein gene of feline infectious peritonitis virus type I. Arch. Virol. 1995;140:469–480. doi: 10.1007/BF01718424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Hashimoto H., Koyama H. Comparison of the amino acid sequence and phylogenetic analysis of the peplomer, integral membrane and nucleocapsid proteins of feline, canine and porcine coronaviruses. Microbiol. Immunol. 1996;40:425–433. doi: 10.1111/j.1348-0421.1996.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W., Corapi W.V., Ngichabe C.K., Baines J.D., Scott F.W. Monoclonal antibodies to the spike protein of feline infectious peritonitis virus mediate antibody-dependent enhancement of infection of feline macrophages. J. Virol. 1992;66:956–965. doi: 10.1128/jvi.66.2.956-965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. A review of feline infectious peritonitis virus infection: 1963–2008. J. Feline Med. Surg. 2009;11:225–258. doi: 10.1016/j.jfms.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F. Immunologic phenomena in the effusive form of feline infectious peritonitis. Am. J. Vet. Res. 1980;41:868–876. [PubMed] [Google Scholar]
- Poland A.M., Vennema H., Foley J.E., Pedersen N.C. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. J. Clin. Microbiol. 1996;34:3180–3184. doi: 10.1128/jcm.34.12.3180-3184.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan A.D., Cohen R.D., Whittaker G.R. Activation of p38 MAPK by feline infectious peritonitis virus regulates pro-inflammatory cytokine production in primary blood-derived feline mononuclear cells. Virology. 2009;384:135–143. doi: 10.1016/j.virol.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U.S. Isolation and culture of pulmonary endothelial cells. Environ. Health Perspect. 1984;56:103–114. doi: 10.1289/ehp.8456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos G.T., Kollintza A., Moschos C., Psallidas I., Sherrill T.P., Pitsinos E.N., Vassiliou S., Karatza M., Papiris S.A., Graf D., Orphanidou D., Light R.W., Roussos C., Blackwell T.S., Kalomenidis I. Tumor necrosis factor-alpha promotes malignant pleural effusion. Cancer Res. 2007;67:9825–9834. doi: 10.1158/0008-5472.CAN-07-1064. [DOI] [PubMed] [Google Scholar]
- Stoddart C.A., Scott F.W. Intrinsic resistance of feline peritoneal macrophages to coronavirus infection correlates with in vivo virulence. J. Virol. 1989;63:436–440. doi: 10.1128/jvi.63.1.436-440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Azuma N., Hashida Y., Satoh R., Hohdatsu T. B-cell activation in cats with feline infectious peritonitis (FIP) by FIP-virus-induced B-cell differentiation/survival factors. Arch. Virol. 2009;154:27–35. doi: 10.1007/s00705-008-0265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Azuma N., Satoh M., Toda A., Hashida Y., Satoh R., Hohdatsu T. Neutrophil survival factors (TNF-alpha, GM-CSF, and G-CSF) produced by macrophages in cats infected with feline infectious peritonitis virus contribute to the pathogenesis of granulomatous lesions. Arch. Virol. 2009;154:775–781. doi: 10.1007/s00705-009-0371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Hohdatsu T., Hashida Y., Kaneko Y., Tanabe M., Koyama H. A “possible” involvement of TNF-alpha in apoptosis induction in peripheral blood lymphocytes of cats with feline infectious peritonitis. Vet. Microbiol. 2007;119:121–131. doi: 10.1016/j.vetmic.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Hohdatsu T., Toda A., Tanabe M., Koyama H. TNF-alpha, produced by feline infectious peritonitis virus (FIPV)-induced macrophages, upregulates expression of type II FIPV receptor feline aminopeptidase N in feline macrophages. Virology. 2007;364:64–72. doi: 10.1016/j.virol.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Kawakami C., Yamada S., Satoh R., Hohdatsu T. Antibody-dependent enhancement occurs upon re-infection with the identical serotype virus in feline infectious peritonitis virus infection. J. Vet. Med. Sci. 2008;70:1315–1321. doi: 10.1292/jvms.70.1315. [DOI] [PubMed] [Google Scholar]
- Tseng C.S., Lo H.W., Teng H.C., Lo W.C., Ker C.G. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol. Med. Microbiol. 2005;43:99–102. doi: 10.1016/j.femsim.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150–157. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viac J., Pernet I., Schmitt D., Claudy A. Overexpression of circulating vascular endothelial growth factor (VEGF) in leukocytoclastic vasculitis. Arch. Dermatol. Res. 1999;291:622–623. doi: 10.1007/s004030050464. [DOI] [PubMed] [Google Scholar]
- Weiss R.C., Scott F.W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp. Immunol. Microbiol. Infect. Dis. 1981;4:175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R.C., Scott F.W. Pathogenesis of feline infectious peritonitis: nature and development of viremia. Am. J. Vet. Res. 1981;42:382–390. [PubMed] [Google Scholar]
- Wen F.Q., Liu X., Manda W., Terasaki Y., Kobayashi T., Abe S., Fang Q., Ertl R., Manouilova L., Rennard S.I. TH2 Cytokine-enhanced and TGF-beta-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-gamma and corticosteroids. J. Allergy Clin. Immunol. 2003;111:1307–1318. doi: 10.1067/mai.2003.1455. [DOI] [PubMed] [Google Scholar]
- Yoshino Y., Aoyagi M., Tamaki M., Duan L., Morimoto T., Ohno K. Activation of [38p] MAPK and/or JNK contributes to increased levels of VEGF secretion in human malignant glioma cells. Int. J. Oncol. 2006;29:981–987. [PubMed] [Google Scholar]


