Abstract
Cell entry of severe acute respiratory syndrome coronavirus (SARS-CoV) is mediated by the viral spike (S) protein. Amino acids 319–510 on the S protein have been mapped as the receptor-binding domain (RBD), which mediates binding to the SARS-CoV receptor angiotensin converting enzyme 2 (ACE2) on SARS-CoV susceptible cells. In this study, we expressed a fusion protein containing the human codon-optimized RBD of the SARS-CoV spike protein linked to the Fc portion of human IgG1 (named RBD-Fc) in HEK293 cells. The RBD-Fc protein was purified by affinity chromatography. The flow cytometry assay showed that the purified RBD-Fc protein could bind to ACE2. We demonstrated that the RBD spike protein alone could be internalized into SARS-CoV susceptible cells together with ACE2. We also showed that the removal of N-glycans from the RBD spike protein did not abolish this phenomenon. Our discoveries may have some implications for the development of the SARS vaccine.
Abbreviations: SARS-CoV, severe acute respiratory syndrome coronavirus; RBD, receptor-binding domain; ACE2, angiotensin converting enzyme 2
Keywords: Severe acute respiratory syndrome coronavirus (SARS-CoV), Receptor-binding domain (RBD), Endocytosis, N-Linked glycosylation, Angiotensin converting enzyme 2 (ACE2)
1. Introduction
In 2003, severe acute respiratory syndrome (SARS) emerged as a deadly global threat (Lee et al., 2003, Poutanen et al., 2003, Tsang et al., 2003). The pathogen was identified as severe acute respiratory syndrome coronavirus (SARS-CoV) (Drosten et al., 2003, Ksiazek et al., 2003, Marra et al., 2003, Rota et al., 2003), which is an enveloped, single-strand plus-sense RNA virus. Spike (S), nucleocapsid (N), membrane (M) and envelope (E) are its major structural proteins (Drosten et al., 2003, Marra et al., 2003, Rota et al., 2003).
Like other coronaviruses, SARS-CoV entry is mediated by the S protein (Hofmann et al., 2004, Inoue et al., in press, Simmons et al., 2004, Yang et al., 2004). The S protein consists of 1255 amino acids that forms typical petal-shaped spikes on the surface of SARS-CoV (Ksiazek et al., 2003). There is no direct evidence that the S protein of SARS-CoV is processed proteolytically into the S1 receptor-binding subunit and the S2 membrane fusion subunit, but the two subunits can be predicted by sequence alignment with other coronavirus S proteins (Rota et al., 2003, Spiga et al., 2003). Angiotensin converting enzyme 2 (ACE2) has been demonstrated to be a functional receptor for SARS-CoV in vitro and in vivo (Kuba et al., 2005, Li et al., 2003) by binding to the receptor-binding domain (RBD, amino acids 319–510) of the S protein (Chakraborti et al., 2005, Wong et al., 2004). Additionally, there are 23 potential N-linked glycosylation sites in the SARS-CoV S protein (Rota et al., 2003), and two are in the RBD.
Usually, ligand binding induces endocytosis of the receptors. Our previous study demonstrated that the binding of the S protein to endogenous ACE2 in mice resulted in down-regulation of ACE2 surface expression (Kuba et al., 2005), implying ACE2 internalization. Therefore, we would like to explore whether RBD, the minimal receptor-binding domain on the S protein, could induce endocytosis of the receptor.
To test this hypothesis, we used the recombinant RBD spike protein as a defined model system, which avoided possible effects of other fragments on the S protein. We constructed a new vector using a human codon-optimized RBD DNA sequence, and created a stable RBD-Fc-expressing cell line. The RBD spike protein could then be secreted into culture medium and easily purified by Protein A affinity chromatography. The flow cytometry assay and immunostaining experiments demonstrated the endocytosis of the RBD spike protein by susceptible cells together with ACE2. At the same time, the removal of N-glycans from the RBD spike protein could still induce ACE2 internalization. To our knowledge, this is the first report showing that the receptor-binding domain of SARS-CoV alone can trigger the endocytosis of susceptible cells.
2. Materials and methods
2.1. Construction of the recombinant plasmid
The amino acids 319–510 of the SARS-CoV spike protein are mapped as the minimal ACE2-binding domain (RBD) (Chakraborti et al., 2005, Wong et al., 2004). The cDNA fragment encoding the RBD was amplified by PCR using a plasmid, PUC18-S as the template, which contains the human codon-optimized SARS-CoV (Urbani strain) spike protein (GenBank accession no. AAP13441) coding sequence synthesized by Generay Inc., and the primers (forward: 5′-GGCGCTAGCCATCACCAACCTGTGCCCC-3′, containing NheI recognition site; reverse: 5′-CGCGGATCCGTCACGGTGGCGGGGGCGTTC-3′, containing BamHI recognition site). The PCR product was digested with NheI and BamHI, and then cloned in-frame downstream of the leader peptide of human CD5 antigen (CD5L), and upstream of the Fc portion of human IgG1 (Fc) in the Peak13 expression vector (provided by B. Seed, Harvard Medical School, Boston, MA), which was also digested by NheI and BamHI. The resulting recombinant plasmid was named Peak13-RBD-Fc.
2.2. Cell cultures
VeroE6 cells (African green monkey kidney cell line), HEK293 cells (human embryo kidney cell line) and a HEK293 cell line stably expressing RBD-Fc (RBD-Fc-293) or human ACE2-GFP (ACE2-GFP-293) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C with 5% CO2.
2.3. Establishment of a stable RBD-Fc expressing cell line
The day before transfection, HEK293 cells were trypsinized and plated into 6-well plate at a density of 2 × 105 cells per well. The next day, 0.5 μg Peak13-RBD-Fc plasmid linearized by AvrII or an equal volume of H2O was transfected into the HEK293 cells using lipofectamine™ 2000 (Invitrogen) according to the manufacturer's protocol. After 48 h, aliquots of the transfected cells were transferred into selection medium containing increasing concentrations of puromycin (0.3, 0.4, 0.5, 0.6 and 0.7 μg/ml). After 3 days, the transfected HEK293 cells were selected in suitable puromycin concentration, under which some cells transfected with the Peak13-RBD-Fc plasmid survived, but those transfected with H2O all died, and were transferred into 96-well plate using a limited dilution method. After about 10 days, 10 μl of culture supernatant from a single confluent cell colony in the 96-well plate was assayed for RBD-Fc expression by Western blot using anti-human IgG (whole molecule) peroxidase conjugated antibody (Sigma). The highest RBD-Fc expression clone was selected by an ELISA assay for recombinant protein production.
2.4. ELISA
2 × 105 cells from each RBD-Fc-293 cell clone were seeded in 6-well plates and cultured with 2 ml culture medium (DMEM containing 10% FBS) for 72 h. The concentration of the RBD-Fc protein in culture supernatant was quantified with an ELISA assay, which was performed using BD OptEIA™ Reagent Set A (BD Biosciences) as described by the manufacturer. Briefly, 96-well flat bottom EIA/RIA plates (Corning, NY) were coated overnight at 4 °C with 100 μl culture medium, the human IgG standards serial diluted in culture medium (10 ng to 30 μg/ml) or the cell culture supernatant of each RBD-Fc-293 cell clone. Then, wells were washed once with Washing Solution, blocked with Assay Diluent for 1 h at room temperature and washed with Washing Solution three times. Next, anti-human IgG (whole molecule) peroxidase conjugated antibody (Sigma) diluted in Assay Diluent (1: 5000) was added to each well, and gently agitated at room temperature for 1 h. After being washed eight times, 100 μl of Substrate Reagent was added to each well and wells were incubated at room temperature in the dark for 30 min. Finally, the reaction was stopped using Stop Solution and color development was monitored at a wavelength of 450 nm.
2.5. Protein purification
The cell culture supernatant of RBD-Fc-293 cells was harvested and dialyzed with a solution (20 mM sodium phosphate and 1 mM EDTA, pH 7.0) over 8 h. After centrifugation at 4000 rpm for 30 min, the supernatant was filtered through 0.45 μm Durapore membrane filters (Millipore, Ireland). The purification of RBD-Fc protein was performed using HiTrap Protein A HP 5 ml column (GE healthcare Amersham Biosciences AB) and Econo™ Gradient Pump Tubing Kit (Bio-Rad, USA) according to the manufacturer's protocol. In brief, the column was first washed with 10 column volumes of the binding buffer (20 mM sodium phosphate, pH 7.0) at a flow rate of 5 ml/min. Then the cell culture supernatant was pumped onto the column. Next, the column was washed with 20 column volumes of the binding buffer followed by the supernatant. Finally, the RBD-Fc protein was eluted with 2–5 column volumes of the elution buffer (0.1 M glycine, pH 3.1). Neutralization buffer (60–200 μl, 1 M Tris–HCl, pH 9.0) was added to each collection tube. The RBD-Fc protein was further purified using a HiTrap Protein A HP 1 ml column (GE healthcare Amersham Biosciences AB). The protein concentration was determined using Protein Assay Dye Reagent Concentrate (Bio-Rad) according to the manufacturer's protocol, in which bovine serum albumin (BSA) was used as a standard.
2.6. SDS-PAGE and Western blot analysis
Samples were mixed 1:1 with 2× loading buffer (2% SDS, 5% sucrose, 0.1% bromphenol blue and 5% mercaptoethanol) and heated at 95 °C for 5 min. SDS-PAGE was performed using 5% stocking and 10% or 12% separating acrylamide gels (Laemmli, 1970). A broad range of pre-stained protein marker kit (6–175 kDa, New England Biolabs) was used to determine approximate molecular mass. Proteins in gels were visualized using 0.25% Coomassie Brilliant Blue R-250 staining in 45% methanol and 10% acetic acid. The images of gels were captured using Furi FR-200 equipment with Smartview software (China). For Western blot analysis, proteins in gels were electrophoretically transferred onto a nitrocellulose membrane with a buffer of 20 mM Trisbase, 153 mM glycine and 20% (v/v) methanol at 300 mA for 2 h. After being blocked in 2% chicken egg white albumin and 0.1% TritonX-100 at room temperature for 1 h, the membrane was incubated with anti-human IgG (whole molecule) peroxidase conjugated antibody (1:10,000 dilution, Sigma) for 1 h and washed three times with TBST buffer (50 mM Trisbase, 150 mM NaCl and 0.1% TritonX-100, pH 7.4). Finally, the membrane was developed with a Western blotting luminal reagent (Santa Cruz Biotechnology) to visualize positive signals.
2.7. Cell binding and internalization assay by flow cytometry
We detached 1 × 106 VeroE6 cells using a 2 mM mixture of EDTA and PBS, and then incubated them with 350 nM purified RBD-Fc or Fc protein diluted in DMEM for 3 h at 4 °C or 37 °C, respectively. Next, the cells were washed with ice-cold PBS and incubated with a FITC-conjugated affinipure goat anti-human IgG (H + L) antibody (1: 100, Jackson ImmunoResearch) at 4 °C in the dark for 30 min. After being washed with ice-cold PBS, the cells were resuspended in PBS for flow cytometry using a flow cytometer (Beckman Coulter Epics Elite ESP). The results were analyzed by Expo32 v1.2 analysis software.
2.8. Deglycosylation of the RBD-Fc
Removal of N-glycans from the RBD-Fc protein was performed using PNGase F (New England Biolabs). To maintain the natural conformation of the deglycosylated molecule, 2 μl PNGase F (1000 U) was directly added to 100 μg purified RBD-Fc protein in 200 μl protein store buffer (1 M Tris and 0.1 M glycine, pH 7.0) and then incubated for 10 h at 25 °C. As the completely deglycosylated control, purified RBD-Fc protein was first denatured and then deglycosylated using methods described by the manufacturer. Briefly, the protein was first denatured in 5% SDS and 10% β-mercaptoethanol at 100 °C for 10 min, then mixed with 1/10 volume concentrated PNGase F reaction buffer (0.5 M sodium phosphate, pH 7.5) and 10% NP-40. The sample was digested with 2 μl PNGase F (1000 U) for 2 h at 37 °C. A 2 μg portion was analyzed on a SDS-PAGE gel by Coomassie Brilliant Blue staining. The image of the gel was captured using Furi FR-200 equipment with Smartview software (China).
2.9. Microscopy
The ACE2-GFP-293 cells were digested with trypsin and seeded in 24-well plates at a density of 2 × 104 cells per well. After 24 h, the Fc or the RBD-Fc, the deglycosylated RBD-Fc under nondenatured conditions (dg-RBD-Fc) or the mixture of PNGase F and RBD-Fc were added into the cultures to reach a final concentration of 1.5 μM. They were then incubated for 3 h at 37 °C with 5% CO2. Results were determined using fluorescence microscopy (Nikon Eclipse TE 2000-U). The images were captured with RS image software using a Nikon digital camera Dxm1200F and then adjusted with Adobe Photoshop 7.0. For each sample, over 30 optical fields were chosen randomly.
For the immunostaining experiment, ACE2-GFP-293 cells were seeded on coverslips in the 24-well plate. After incubation with Fc or RBD-Fc as described above, the cells were washed with phosphate-buffered saline (PBS) three times, and then fixed with 4% paraformaldehyde for 15 min and permearized with 0.1% TritonX-100/PBS for 30 min. After being blocked with 1% BSA in 1× PBS, samples were incubated with Alexa Fluor 568-conjugated goat anti-human IgG (H + L) (1:200, Molecular Probes) at room temperature for 1 h and washed with 0.5% Tween20/PBS three times. Results were determined using confocal laser-scanning microscopy (Leica, DMIRE2), and the images were analyzed by Leica confocal software.
2.10. Statistical analysis
The statistical significance of differences between the RBD-Fc and the Fc treatments was accessed by Chi-square test. Differences with P < 0.05 were considered to be significant.
3. Results
3.1. Construction of recombinant plasmid Peak13-RBD-Fc
To get the cDNA fragment encoding the RBD of S protein, PCR was performed using plasmid PUC18-S as the template, which contains the human codon-optimized SARS-CoV spike gene. The PCR product was cloned into the Peak13 expression vector. The leader peptide of human CD5 antigen (CD5L), which efficiently directs the synthesis and export of secreted and membrane-bound proteins (Aruffo et al., 1990), was placed in-frame upstream of RBD. The Fc portion of the human IgG1 (Fc) at the C-terminus of the RBD can be used to monitor protein expression and allow for natural recombinant protein purification. The recombinant plasmid, Peak13-RBD-Fc, was verified by restriction endonuclease analysis and DNA sequencing.
To confirm recombinant protein expression, Peak13-RBD-Fc was transiently transfected into HEK293 cells. At 3 days post-transfection, 10 μl of culture supernatant was harvested for a Western blot using a human IgG-specific antibody. The result of Western blot revealed the expression of a human Fc-tagged protein at approximately 64 kDa (Fig. 1 ), which was slightly higher than the predicted size of the RBD-Fc fusion protein based on amino acid composition due to the glycosylations.
Fig. 1.
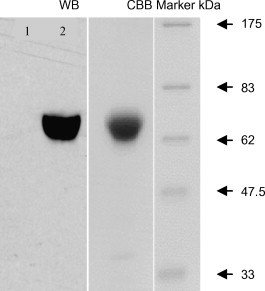
Expression and purification of the RBD spike protein of SARS-CoV. Western blot (WB) analysis of RBD-Fc protein expression using a human IgG-specific antibody. HEK293 cell culture supernatant (lane 1), the culture supernatant of HEK293 cells transiently transfected with Peak13-RBD-Fc (lane 2). Coomassie Brilliant Blue (CBB) staining of 10% SDS-PAGE analysis of the purified recombinant RBD-Fc protein.
3.2. The expression and purification of recombinant RBD-Fc protein
In order to express the RBD protein permanently in mammalian cells for future research, the stable RBD-Fc-expressing cell lines (RBD-Fc-293) were established. After screening using puromycin, seven positive monoclones were obtained by Western blot analysis. To compare the protein yield, 2 × 105 cells from each clone were cultured for 72 h and the culture supernatant was harvested for an ELISA assay using a human IgG-specific antibody. The highest concentration of RBD-Fc expression clones in the cell supernatant reached 14 mg/l.
Due to the high, specific affinity of the Fc portion of the human IgG1 with protein A, two-step affinity chromatography was performed using HiTrap Protein A HP 5 and 1 ml columns in succession. The two chromatographic steps isolated the recombinant RBD-Fc protein, which migrated on 10% SDS-PAGE at approximately 64 kDa (Fig. 1), consistent with the result of Western blot. Coomassie brilliant blue staining indicated over 90% purity of the RBD-Fc protein (Fig. 1).
3.3. Endocytosis of SARS-CoV RBD spike protein by susceptible cells together with ACE2
Our previous study demonstrated that SARS-CoV S protein encoded by a synthesized, human codon-optimized gene bound specifically to human ACE2 over-expressed in HEK293 cells or endogenous ACE2 in VeroE6 cells (Kuba et al., 2005). To verify whether the independent RBD spike protein we obtained above can bind to ACE2, we performed a flow cytometry binding assay of RBD-Fc to VeroE6 cells at 4 °C using a FITC-conjugated human IgG-specific antibody. In comparison to VeroE6 cells incubated with Fc, the fluorescence intensity on the surface of those incubated with RBD-Fc at 4 °C obviously increased (Fig. 2A), which indicated that the purified RBD spike protein could bind to endogenous ACE2 on the surface of VeroE6 cells. Our results demonstrated that the purified RBD spike protein was properly folded.
Fig. 2.
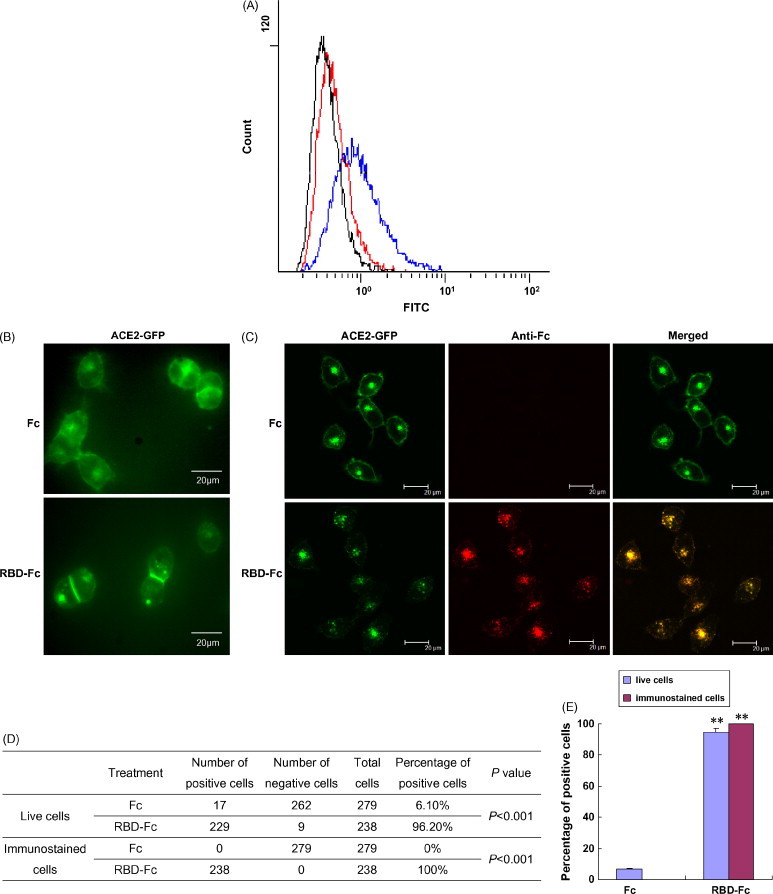
SARS-CoV RBD spike protein was internalized by the susceptible cells together with ACE2. (A) Decreased cell surface-bound RBD-Fc protein following incubation with VeroE6 cells at 37 °C compared to 4 °C. After incubation with VeroE6 cells for 3 h, cell surface-bound Fc or RBD-Fc proteins were detected using a FITC-conjugated human IgG-specific antibody. Representative FACS histograms are shown for three independent experiments. Blue and black histograms represent the results from the cells incubated with the RBD-Fc or Fc at 4 °C, respectively. The red histogram represents the results from the cells incubated with the RBD-Fc at 37 °C. (B) ACE2 internalization is the RBD spike protein-dependent. After ACE2-GFP-293 cells were incubated with the RBD-Fc or Fc protein at 37 °C for 3 h, the distribution of ACE2-GFP molecules in live cells was observed under a fluorescence microscope. (C) Co-localization of ACE2 and the RBD of SARS-CoV in ACE2-GFP-293 cells under a confocal laser-scanning microscope. After incubation with the RBD-Fc or Fc protein at 37 °C for 3 h, ACE2-GFP-293 cells were washed to remove excess RBD-Fc or Fc protein and then fixed with 4% paraformaldehyde. Immunostaining was performed using an Alexa Fluor 568-conjugated human IgG-specific antibody to detect the distribution of the Fc or RBD-Fc protein in the cells. (D) and (E) Statistical analysis of the differences between the RBD-Fc and Fc protein treatments (Chi-square test). **P < 0.001. “Live cells” means that the treated cells were directly observed under a fluorescence microscope. “Immunostaining cells” means that the treated cells were first fixed and immunostained, and then observed under a confocal laser-scanning microscope.
It is known that at both 4 and 37 °C, virions can bind to the surface of host cells. For the B-lymphotropic papovavirus (LPV), the binding capacity is similar at 4 and 37 °C (Haun et al., 1993). For HIV-1, the affinity of virion-bound gpl20 for soluble CD4 at 37 °C is higher than that at 4 °C (Moore et al., 1991). However, internalization only occurs at 37 °C. It is known that cellular endocytosis is a mechanism for SARS-CoV entry (Inoue et al., in press, Wang et al., 2008). Our previous study using a flow cytometry assay showed that SARS-CoV S protein is internalized by VeroE6 cells together with ACE2 at 37 °C (Kuba et al., 2005). The RBD of the S protein binds to ACE2, which is the ligand of ACE2. To determine whether the RBD spike protein binding leads to the endocytosis of SARS-CoV by susceptible cells, a flow cytometry internalization assay was performed. A FITC-conjugated human IgG-specific antibody cannot pass through the plasma membrane of live cells, so it was used to detect the cell surface-bound RBD-Fc protein. The fluorescence intensity on the surface of VeroE6 cells incubated with RBD-Fc at 37 °C was much weaker than those incubated at 4 °C (Fig. 2A). We speculate that this decrease of fluorescence intensity might result from the internalization of RBD-Fc by VeroE6 cells.
To visualize the changes of the receptors on susceptible cells, we incubated ACE2-GFP-293 cells, which stably express human ACE2 fused with GFP at its C-terminus on the surface, with RBD-Fc at 37 °C for 3 h, using Fc protein as a control. We then observed them under a fluorescence microscope. According to Scearce-Levie et al. (2005), the receptor-internalized cells (positive cells) were judged by the following criteria: (a) the green cell boundary became weak and vague; and (b) three or more clear and visible green vesicles existed in the cytoplasm. In the cells incubated with the Fc protein, besides the presumed protein synthesis and trafficking sites in the cytoplasm, strong green fluorescence was predominantly on the plasma membrane (Fig. 2B), which was similar to the fluorescence distribution of the untreated cells (data not shown). However, the intensity of the green fluorescence on the surface of those cells incubated with RBD-Fc was very low, accompanied by clear and visible green vesicles in the cytoplasm of almost all cells (96.2%; Fig. 2B, D and E). In contrast, only 6.1% of cells had visible green vesicles in the cytoplasm in the control (Fig. 2D and E). These results show that after incubation with the RBD spike protein, ACE2 internalization occurred, which suggested that ACE2 internalization was the RBD spike protein-dependent.
To further confirm ACE2 internalization was caused by the RBD spike protein binding, we performed immunostaining experiments to track Fc or RBD-Fc localization in ACE2-GFP-293 cells. After being incubated with RBD-Fc or Fc protein at 37 °C for 3 h, ACE2-GFP-293 cells were washed to remove excess RBD-Fc or Fc protein and then fixed with 4% paraformaldehyde. Next, immunostaining was performed using an Alexa Fluor 568-conjugated human IgG-specific antibody to detect the distribution of Fc or RBD-Fc protein in the cells. As shown in Fig. 2C, there were strong Fc fluorescence signals in the cells incubated with the RBD-Fc, whereas no Fc signal was detected in the cells incubated with the Fc. This indicated that the RBD spike protein was internalized into the cells after incubation. Furthermore, the Fc signals overlapped with the ACE2-GFP signals in the cytoplasm and on the cell surface (Fig. 2C, D and E), which suggests that the RBD spike proteins are co-localized with ACE2 proteins in these cells. The Fc signals especially accumulated in the green vesicles seen in live cells (Fig. 2B and C). The results demonstrated that the binding of the RBD spike protein to ACE2 induced internalization by endocytosis.
3.4. Removal of N-glycans of RBD-Fc can still induce ACE2 internalization
Glycosylation on spike proteins has been reported to affect virus entry (Fenouillet et al., 1990, Goffard et al., 2005, Ohuchi et al., 1997). For the influenza virus, certain glycans near the receptor-binding site affect the binding of the HA to cellular receptors (Ohuchi et al., 1997). We therefore explored whether glycosylation on the RBD spike protein has an effect on the interaction between SARS-CoV and ACE2. The S protein of SARS-CoV has 23 potential N-linked glycosylation sites (Rota et al., 2003), and a minimal ACE2-binding domain (amino acids 319–510) contains two (amino acids 330 and 357), which are N-linked glycosylated when expressed in HEK293 cells (Chakraborti et al., 2005). The RBD-Fc we expressed migrated at approximately 64 kDa on the SDS-PAGE gel (Fig. 1), which was slightly larger than the molecular mass of the RBD-Fc based on amino acid composition. We speculated that RBD-Fc might therefore be N-linked glycosylated. To confirm this, deglycosylation of the RBD-Fc was performed using PNGase F, which hydrolyzes all types of N-glycan chains. After treatment with PNGase F, the single band of approximately 64 kDa migrated as a 54 kDa band on a 12% SDS-PAGE gel (Fig. 3A, lanes 1 and 2), which indicated that the RBD-Fc was N-linked glycosylated in HEK293 cells. To maintain the natural conformation, the RBD-Fc protein was deglycosylated with PNGase F under nondenaturing conditions. As shown in Fig. 3A (lane 3), the N-glycans in the RBD-Fc were removed under nondenaturing conditions as completely as under denaturing conditions.
Fig. 3.
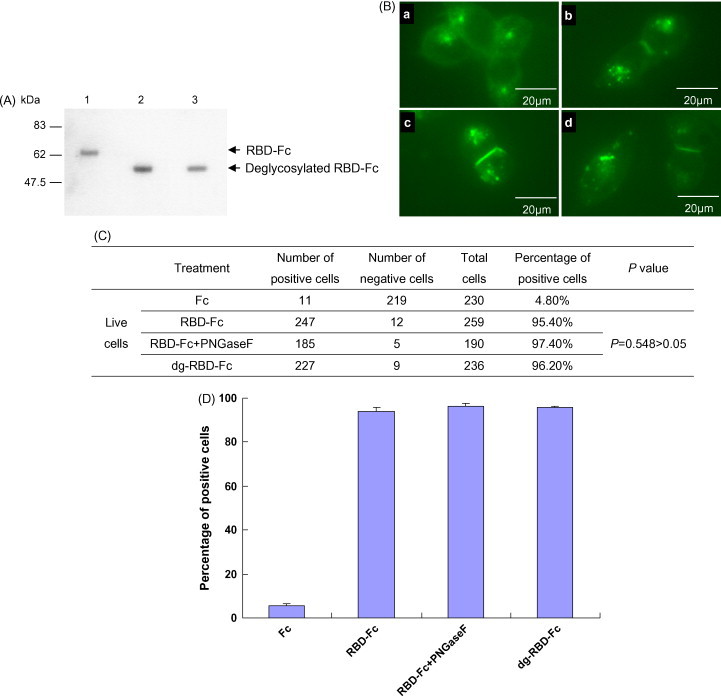
Removal of N-glycans of RBD-Fc can still induce ACE2 internalization (A) the RBD-Fc protein was N-linked glycosylated in HEK293 cells. The purified RBD-Fc (lane 1), the purified RBD-Fc was deglycosylated with PNGase F in the presence of SDS (lane 2), the purified RBD-Fc was deglycosylated with PNGase F in the absence of SDS (lane 3). (B) The deglycosylated RBD-Fc under nondenaturing conditions (dg-RBD-Fc) can still induce ACE2 internalization as the glycosylated RBD-Fc. ACE2-GFP-293 cells were incubated with the Fc protein (a), the RBD-Fc protein (b), the mixture of PNGase F and RBD-Fc (c) and the dg-RBD-Fc (d) at 37 °C for 3 h. The distribution of ACE2-GFP molecules in live cells was observed under a fluorescence microscope. (C) and (D) Statistical analysis of the differences among treatments of the RBD-Fc, the dg-RBD-Fc or the mixture of PNGase F and the RBD-Fc using Chi-square test. P < 0.05 was considered to be significantly different.
Given that the binding of SARS-CoV RBD spike protein induced ACE2 internalization, we wondered whether N-linked glycosylation on the RBD could affect ACE2 internalization. We incubated ACE2-GFP-293 cells with RBD-Fc or dg-RBD-Fc. To eliminate the possible effects of any remaining PNGase F in the deglycosylation reaction, the mixture of PNGase F and RBD-Fc was used as a control. We observed that the deglycosylated RBD-Fc induced ACE2 internalization by susceptible cells as effectively as the glycosylated RBD-Fc (Fig. 3B, C and D), which demonstrated that the removal of N-glycans does not affect the RBD spike protein binding to ACE2, at least under our experimental conditions (see Section 2.8 and Section 2.9).
4. Discussion
Understanding the biochemistry of SARS-CoV infection is important for the development of a drug or vaccine against SARS-CoV. The receptor-binding domain (RBD) on the S protein can mediate SARS-CoV binding to ACE2 and contains major neutralizing epitopes (Sui et al., 2004), which elicit higher titers of neutralizing antibodies (He et al., 2004). Therefore, it is a key target for inhibiting SARS-CoV infection. Here, we constructed and expressed the human codon-optimized RBD spike protein of SARS-CoV, which several other laboratories have also used (Babcock et al., 2004, Li et al., 2003, Wong et al., 2004, Wang et al., 2005). The recombinant RBD spike protein yield can reach 14 mg/l in cell culture medium.
The VeroE6 cell line contains ACE2 on its surface, which is extensively used to culture SARS-CoV. A flow cytometry binding assay demonstrated that the purified RBD spike protein specially bound to ACE2 on the surface of VeroE6 cells. In addition, immunostaining experiments on ACE2-GFP-293 cells showed that ACE2 co-localized with the RBD spike protein on the cell surface, which further demonstrates the binding of the RBD spike protein and ACE2. These results indicate that the purified recombinant RBD spike protein is properly folded.
Cell entry is the first step in virus infection. Many viruses can activate cellular signal transduction pathways after binding to the plasma membrane (Greber, 2002, Nemerow, 2000, Pelkmans et al., 2002), in order to make use of the cell machinery for entry. Cellular endocytosis is often used by viruses for entry (Marsh and Helenius, 2006, Pelkmans and Helenius, 2003). Viruses may enter different cell types via different endocytotic pathways. SARS-CoV mainly utilizes the clathrin-dependent endocytosis pathway for its entry into HepG2 cells (Inoue et al., in press). However, SARS-CoV can enter VeroE6 cells in a novel clathrin- and caveolae-independent endocytotic pathway (Wang et al., 2008). Our previous study showed that the SARS-CoV S protein could induce the internalization and recycling of ACE2 (Wang et al., 2008). Here, we demonstrate that the RBD spike protein alone can be internalized together with ACE2. We propose that after binding to ACE2, the RBD spike protein activates the ACE2 mediated cellular endocytosis signal pathway, by which SARS-CoV enters the susceptible cells.
Our previous study demonstrated the rennin–angiotensin system (RAS) was involved in SARS pathogenesis (Kuba et al., 2005). ACE2 functions as a carboxypeptidase, which plays a central role in regulating the severity of acute lung failure during the disease process, together with other components of the RAS (Kuba et al., 2005). SARS-CoV S-protein-mediated down-regulation of ACE2 appears to contribute to the severe acute lung injury in SARS (Kuba et al., 2005). Here, we demonstrate that the binding of the RBD spike protein leads to ACE2 internalization. This is consistent with worsened acid-induced acute lung injury by RBD spike protein treatment in wild type mice (Kuba et al., 2005). It has also been reported that in other viruses, the virus receptor is down-regulated by the interaction with the virus ligand. For instance, after binding with HIV gp120, CD4 was internalized and led to the impairment of immune cell functions (Wahl et al., 1989), and the binding of measles hemagglutinin resulted in the down-regulation of its receptor, CD46, to disrupt the complement pathways and immune systems (Oldstone et al., 1999). Therefore, in addition to using receptors for cell entry, many viruses may induce down-regulation of the receptor to impair its normal function, leading to severe disease.
The S protein of SARS-CoV has 23 potential N-linked glycosylation sites (Rota et al., 2003), two of which are in the RBD. Through treatment with PNGase F, we demonstrated that the RBD spike protein was N-linked glycosylated in HEK293 cells, which is consistent with the result obtained by site-directed mutagenesis analysis (Chakraborti et al., 2005). Here, we demonstrated that N-glycans did not affect RBD binding to ACE2 through the ACE2 internalization assay induced by the RBD spike protein, which is in agreement with co-crystal RBD expressed in Sf9 cells bound with ACE2 (Li et al., 2005) and previous mutagenesis analysis (Chakraborti et al., 2005). The role of glycosylation in SARS-CoV infection is to be further investigated.
In conclusion, we created the stable RBD-Fc-expressing cell lines, and we demonstrated SARS-CoV RBD spike protein induced cell endocytosis after binding to ACE2. Additional N-glycans on the RBD spike protein had no effect on its binding to ACE2. Cell endocytosis is an important mechanism for SARS-CoV entry. Our study shows that the RBD spike binding is the trigger for this entry pathway, which further confirms the importance of the RBD spike protein in SARS-CoV infection. Because the RBD spike binding to ACE2 contributes to SARS pathogenesis, the use of subunit vaccines based on the RBD spike should be considered carefully. A RBD spike protein mutant, which diminishes or eliminates its avidity with ACE2, but retains the major neutralizing epitopes, is desirable. Previous study has shown that the replacement of two active-site histidines of ACE2 by asparagines does not affect the syncytia formation mediated by the SARS-CoV S protein (Li et al., 2003). A soluble form of enzyme-inactive ACE2 may be used for SARS therapy.
Acknowledgements
This work was funded by National Natural Science Foundation of China (30625013, 30623009) and 30721063) and Ministry of Science and Technology of China (2006AA02Z152 and 2005CB523000).
References
- Aruffo A., Stamenkovic I., Melnick M., Underhill C.B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Babcock G.J., Esshaki D.J., Thomas W.D., Jr., Ambrosino D.M. Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J. Virol. 2004;78:4552–4560. doi: 10.1128/JVI.78.9.4552-4560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti S., Prabakaran P., Xiao X., Dimitrov D.S. The SARS coronavirus S glycoprotein receptor binding domain: fine mapping and functional characterization. Virol. J. 2005;2:73–82. doi: 10.1186/1743-422X-2-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., Berger A., Burguière A., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H., Osterhaus A.D.M.E., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Fenouillet E., Gluckman J.C., Bahraoui E. Role of N-linked glycans of envelope glycoproteins in infectivity of human immunodeficiency virus type 1. J. Virol. 1990;64:2841–2848. doi: 10.1128/jvi.64.6.2841-2848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffard A., Callens N., Bartosch B., Wychowski C., Cosset F.L., Montpellier C., Dubuisson J. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J. Virol. 2005;79:8400–8409. doi: 10.1128/JVI.79.13.8400-8409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U.F. Signalling in viral entry. Cell. Mol. Life Sci. 2002;59:608–626. doi: 10.1007/s00018-002-8453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun G., Keppler O.T., Bock C.T., Herrmann M., Zentgraf H., Pawlita M. The cell surface receptor is a major determinant restricting the host range of the B-lymphotropic papovavirus. J. Virol. 1993;67:7482–7492. doi: 10.1128/jvi.67.12.7482-7492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zhou Y., Liu S., Kou Z., Li W., Farzan M., Jiang S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem. Biophys. Res. Commun. 2004;324:773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Hattermann K., Marzi A., Gramberg T., Geier M., Krumbiegel M., Kuate S., Uberla K., Niedrig M., Pöhlmann S. S protein of severe acute respiratory syndrome-associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients. J. Virol. 2004;78:6134–6142. doi: 10.1128/JVI.78.12.6134-6142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, Y., Tanaka, N., Tanaka, Y., Inoue, S., Morita, K., Min, Z., Hattori, T., Sugamura, K. Clathrin-dependent entry of SARS coronavirus into target cells expressing cytoplasmic tail-deleted ACE2. J. Virol. (in press). [DOI] [PMC free article] [PubMed]
- Ksiazek T.G., Erdman D., Goldsmith C., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Nghiem K.H., Dowell A., Ling S., Humphrey C., Shieh W., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J., Cox N., Hughes J., LeDuc J.W., Bellini W.J., Anderson L.J., the SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophase T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J.Y. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.P., McKeating J.A., Norton W.A., Sattentau Q.J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J. Virol. 1991;65:1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G.R. Cell receptors involved in adenovirus entry. Virology. 2000;274:1–4. doi: 10.1006/viro.2000.0468. [DOI] [PubMed] [Google Scholar]
- Ohuchi M., Ohuchi R., Feldmann A., Klenk H.D. Regulation of receptor binding affinity of influenza virus hemagglutinin by its carbohydrate moiety. J. Virol. 1997;71:8377–8384. doi: 10.1128/jvi.71.11.8377-8384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M.B., Lewicki H., Thomas D., Tishon A., Dales S., Patterson J., Manchester M., Homann D., Naniche D., Holz A. Measles virus infection in a transgenic model: virus-induced immunosuppression and central nervous system disease. Cell. 1999;98:629–640. doi: 10.1016/s0092-8674(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Helenius A. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 2003;15:414–422. doi: 10.1016/s0955-0674(03)00081-4. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Puntener D., Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Peñaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Günther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Scearce-Levie K., Lieberman M.D., Elliott H.H., Conklin B.R. Engineered G protein coupled receptors reveal independent regulation of internalization, desensitization and acute signaling. BMC. Biol. 2005;3:3. doi: 10.1186/1741-7007-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoproteinmediated viral entry. Proc. Natl. Acad. Sci. USA. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga O., Bernini A., Ciutti A., Chiellini S., Menciassi N., Finetti F., Causarono V., Anselmi F., Prischi F., Niccolai N. Molecular modeling of S1 and S2 subunits of SARS coronavirus spike glycoprotein. Biochem. Biophys. Res. Commun. 2003;310:78–83. doi: 10.1016/j.bbrc.2003.08.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Li W., Murakami A., Tamin A., Matthews L.J., SWong K., Moore M.J., Tallarico A.S., Olurinde M., Choe H., Anderson L.J., Bellini W.J., Farzan M., Marasco W.A. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. USA. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Wahl S.M., Allen J.B., Gartner S., Orenstein J.M., Popovic M., Chenoweth D.E., Arthur L.O., Farrar W.L., Wahl L.M. HIV-1 and its envelope glycoprotein down-regulate chemotactic ligand recept1ors and chemotactic function of peripheral blood monocytes. J. Immunol. 1989;142:3553–3559. [PubMed] [Google Scholar]
- Wang S., Chou T.H., Sakhatskyy P.V., Huang S., Lawrence J.M., Cao H., Huang X., Lu S. Identification of two neutralizing regions on the severe acute respiratory syndrome coronavirus spike glycoprotein produced from the mammalian expression system. J. Virol. 2005;79:1906–1910. doi: 10.1128/JVI.79.3.1906-1910.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


