Highlights
-
•
Feline infectious peritonitis (FIP) is a coronavirus-induced fatal disease in cats.
-
•
We synthesized peptides derived from the S1 domain of the type I FIPV S protein.
-
•
We investigated inhibitory effects of peptides on FIPV infection.
-
•
5 peptides significantly inhibited type I FIPV.
-
•
2 of 5 peptides significantly inhibited not only type I, but also type II FIPV.
Keywords: Feline coronavirus, Feline infectious peritonitis, Peptide
Abstract
Feline infectious peritonitis virus (FIPV) can cause a lethal disease in cats, feline infectious peritonitis (FIP). A therapeutic drug that is effective against FIP has not yet been developed. Peptides based on viral protein amino acid sequences have recently been attracting attention as new antiviral drugs. In the present study, we synthesized 30 overlapping peptides based on the amino acid sequence of the S1 domain of the type I FIPV strain KU-2 S protein, and investigated their inhibitory effects on FIPV infection. To evaluate the inhibitory effects on type I FIPV infection of these peptides, we investigated a method to increase the infection efficiency of poorly replicative type I FIPV. The efficiency of type I FIPV infection was increased by diluting the virus with medium containing a polycation. Of the 30 peptides, I-S1-8 (S461-S480), I-S1-9 (S471-S490), I-S1-10 (S481-S500), I-S1-16 (S541-S560), and I-S1-22 (S601-S620) significantly decreased the infectivity of FIPV strain KU-2 while I-S1-9 and I-S1-16 exhibited marked inhibitory effects on FIPV infection. The inhibitory effects on FIPV infection of these 2 peptides on other type I and type II FIPV strains, feline herpesvirus (FHV), and feline calicivirus (FCV) were also examined. These 2 peptides specifically inhibited type I and type II FIPV, but did FHV or FCV infection. In conclusion, the possibility of peptides derived from the S protein of type I FIPV strain KU-2 as anti-FIPV agents effective not only for type I, but also type II FIPV was demonstrated in vitro.
1. Introduction
Feline coronavirus (FCoV) belongs to the alpha coronaviruses of the family Coronaviridae. FCoV is classified into serotypes I and II according to Spike (S) protein properties (Hohdatsu et al., 1991b, Motokawa et al., 1995, Motokawa et al., 1996). Each of these types consists of two viruses: feline infectious peritonitis (FIP)-causing FIP virus (FIPV) and non-FIP-causing feline enteric coronavirus (FECV) (Pedersen et al., 1981). FIPV and FECV of the same serotype cannot be distinguished by their antigenicity or at the gene level, and differ only in their pathogenicity for cats (Pedersen, 2009). Cats that developed FIP were affected in several organs, including the liver, lungs, spleen, and central nervous system, forming lesions accompanied by necrosis and pyogenic granulomatous inflammation. In some cats, pleural effusion and ascitic fluid accumulated. When anti-FCoV antibody-positive cats are inoculated with FIPV, the onset time of FIP is earlier than that in antibody-negative cats, and symptoms are severer (Baldwin and Scott, 1997, Barlough et al., 1984, Pedersen, 2009, Pedersen et al., 1981, Stoddart et al., 1988, Weiss and Scott, 1981). These results of experimented studies suggest that antibody-dependent enhancement (ADE) of FIPV infection can be a serious obstacle to the prevention of FIP by vaccination.
FCoV S protein is considered as a class I fusion protein, similarly to those of other coronaviruses (severe acute respiratory syndrome-coronavirus, mouse hepatitis virus, and transmissible gastroenteritis coronavirus) (Bosch et al., 2003, Olsen, 1993). The S protein exists as radially protruding trimers on the viral envelope, and can be structurally or functionally divided into two domains, namely the S1 and S2 domains, representing the N-terminal globular head and C-terminal membrane-bound stalk, respectively. The C-terminal S1 domain contains the receptor-binding domain (RBD), neutralizing antibody-binding epitope, and ADE epitope (Corapi et al., 1992, Corapi et al., 1995, Hohdatsu et al., 1991a, Hohdatsu et al., 1993, Hohdatsu et al., 1998b, Kida et al., 1999, Olsen et al., 1992). The N-terminal S2 domain sequentially contains putative fusion peptide and is responsible for driving viral and target cell membrane fusion.
Over the past forty years, several studies have investigated potential treatments for FIP (Hartmann and Ritz, 2008). Antiviral, immunostimulating, and immunosuppressive drugs have been experimentally used for the treatment of FIP, but few of these have exhibited a sufficient therapeutic effect. Several agents that significantly inhibit FCoV replication in vitro have been identified (Balzarini et al., 2006, Barlough and Shacklett, 1994, Hsieh et al., 2010, Kim et al., 2012, Takano et al., 2008, Takano et al., 2013). Peptides based on viral protein amino acid sequences have recently been attracting attention as new antiviral drugs. These peptides bind to viral proteins or receptors on cells and inhibit viral infections. A HIV infection-inhibiting peptide with a HIV-derived sequence is currently used in the human medical field to treat patients with HIV (He, 2013). This peptide specifically binds to the HIV fusion protein and blocks cell membrane fusion induced by the fusion protein. Furthermore, a peptide with a sequence derived from the RBD of Japanese encephalitis virus (JEV) specifically binds to the virus receptor on cells and competitively inhibits JEV infection, clarifying that peptides with viral protein-derived sequences inhibit viral infections by binding to the virus and virus receptor (Li et al., 2012, Li et al., 2014). Liu et al. (2013) reported that an FIPV fusion protein-derived peptide exhibited an antiviral effect. Although an FIPV RBD-derived peptide may exhibit antiviral activity, this has not yet been investigated. If an RBD-derived peptide with antiviral activity can be identified, it may be applicable as an antiviral agent against FIPV.
In the present study, we synthesized overlapping peptides based on the amino acid sequence of the S1 domain of the type I FIPV strain KU-2 S protein, in which the presence of RBD is expected. We also investigated the inhibition of type I FIPV infection by these peptides using a plaque assay. The inhibition of type II FIPV infection by these peptides was also examined.
2. Materials and methods
2.1. Peptide synthesis
Thirty different peptide sequences derived from the S1 domain of type I FIPV strain KU-2 (Table 1 ) were synthesized at Sigma–Aldrich (U.S.A.). Peptides were synthesized as 20-mer fragments with 10-amino-acid overlap. The peptides were dissolved in 100% dimethyl sulfoxide (DMSO) at 10 mM, aliquoted, and stored at −80 °C. As the solvent control, DMSO was diluted with medium using the same method as for the preparation of peptide stock solutions.
Table 1.
Amino acid sequence of the peptides derived from the S1 domain of FIPV.
| Peptide no. | Amino acid sequence | Start position | Isoelectric point |
|---|---|---|---|
| I-S1-1 | VTDFQPANNSVSHIPFGKTA | S 391 | 7.37 |
| I-S1-2 | VSHIPFGKTAHFCFANFSHS | S 401 | 8.412 |
| I-S1-3 | HFCFANFSHSIVSRQFLGIL | S 411 | 8.413 |
| I-S1-4 | IVSRQFLGILPPTVREFAFG | S 421 | 10.185 |
| I-S1-5 | PPTVREFAFGRDGSIFVNGY | S 431 | 6.459 |
| I-S1-6 | RDGSIFVNGYKYFSLPAIRS | S 441 | 9.986 |
| I-S1-7 | KYFSLPAIRSVNFSISSVEE | S 451 | 6.517 |
| I-S1-8 | VNFSISSVEEYGFWTIAYTN | S 461 | 3.475 |
| I-S1-9 | YGFWTIAYTNYTDVMVDVNG | S 471 | 3.268 |
| I-S1-10 | YTDVMVDVNGTAITRLFYCD | S 481 | 3.732 |
| I-S1-11 | TAITRLFYCDSPLNRIKCQQ | S 491 | 8.615 |
| I-S1-12 | SPLNRIKCQQLKHELPDGFY | S 501 | 8.338 |
| I-S1-13 | LKHELPDGFYSASMLVKKDL | S 511 | 7.344 |
| I-S1-14 | SASMLVKKDLPKTFVTMPQF | S 521 | 10.324 |
| I-S1-15 | PKTFVTMPQFYHWMNVTLHV | S 531 | 9.219 |
| I-S1-16 | YHWMNVTLHVVLNDTEKKYD | S 541 | 6.259 |
| I-S1-17 | VLNDTEKKYDIILAKAPELA | S 551 | 4.608 |
| I-S1-18 | IILAKAPELAALADVHFEIA | S 561 | 4.483 |
| I-S1-19 | ALADVHFEIAQANGSVTNVT | S 571 | 4.167 |
| I-S1-20 | QANGSVTNVTSLCVQARQLA | S 581 | 8.37 |
| I-S1-21 | SLCVQARQLALFYKYTSLQG | S 591 | 9.243 |
| I-S1-22 | LFYKYTSLQGLYTYSNLVEL | S 601 | 6.34 |
| I-S1-23 | LYTYSNLVELQNYDCPFSPQ | S 611 | 3.355 |
| I-S1-24 | QNYDCPFSPQQFNNYLQFET | S 621 | 3.355 |
| I-S1-25 | QFNNYLQFETLCFDVNPAVA | S 631 | 3.355 |
| I-S1-26 | LCFDVNPAVAGCKWSLVHDV | S 641 | 5.235 |
| I-S1-27 | GCKWSLVHDVQWRTQFATIT | S 651 | 8.38 |
| I-S1-28 | QWRTQFATITVSYKHGSMIT | S 661 | 10.369 |
| I-S1-29 | VSYKHGSMITTHAKGHSWGF | S 671 | 10.156 |
| I-S1-30 | THAKGHSWGFQDTSVLVKDE | S 681 | 6.26 |
2.2. Cell cultures
Felis catus whole fetus-4 (fcwf-4) cells, Crandell feline kidney (CrFK) cells (ATCC CCL-94), and swine kidney (CPK) cells were grown in Eagles’ minimum essential medium containing 50% L-15 medium, 10% fetal calf serum (FCS), 100 unit/mL penicillin and 100 mg/mL streptomycin. The cells were maintained in a humidified 5% CO2 incubator at 37 °C. Fcwf-4 cells were supplied by Dr. M. C. Horzinek of State University Utrecht, The Netherlands. CPK cells were supplied by Kitasato Institute, Tokyo, Japan.
2.3. Monoclonal antibodies (MAbs)
MAb 5-6-2 (IgG1) and MAb F19-1 (IgG1) were used in the present study. MAb 5-6-2 and MAb F19-1 were developed in our laboratory and respectively recognize S protein and M protein of virus, as demonstrated by immunoblotting (Hohdatsu et al., 1991b, Hohdatsu et al., 1991c).
2.4. Viruses
Type I FIPV strain KU-2, strain Black, strain UCD-1, type II FIPV strain 79-1146, TGEV strain To-163, CCoV strain 1-71, feline herpesvirus (FHV) strain C7301, and feline calicivirus (FCV) strain F4 were used in this study. FIPV strain KU-2 was isolated in our laboratory. FIPV strain Black was supplied by Dr. J. K. Yamamoto of the University of Florida. FIPV strain UCD-1 was supplied by Dr. N. C. Pedersen of the University of California, Davis. FIPV strain 79-1146 was supplied by Dr. M. C. Horzinek of State University Utrecht, The Netherlands. TGEV strain To-163 was supplied by National Institute of Animal Health of Japan. FHV strain C7301, and FCV strain F4 were supplied by Dr. E. Takahashi of the University of Tokyo, Japan. FIPV and TGEV, were passaged in fcwf-4 cells and CPK cells, respectively, FHV and FCV were passaged CrFK cells. Virus was passaged two or three times in our laboratory and used in subsequent experiments. Strain KU-2 with a potency of 2.4 × 104 pfu/mL was used in all experiments.
2.5. Influences of polycations and polyanions on FIPV infection
DEAE-dextran (GE Healthcare UK Ltd., England), polybrene (Sigma–Aldrich, U.S.A.), and protamine (Sigma–Aldrich, U.S.A.) were used as polycations. Dextran sulfate (GE Healthcare UK Ltd., England) and heparin (Ajinomoto, Japan) were used as polyanions. FIPV strain KU-2 was diluted with medium containing a polycation or polyanion at various concentrations. fcwf-4 cells seeded on 6-well plates were inoculated with 10 μL of a 1000 pfu/mL viral suspension and incubated at 37 °C for 60 min. After incubation, cells were washed with medium and 1 mL of medium containing 2% FCS and 1.5% carboxymethyl cellulose was added to each well. The cultures were incubated at 37 °C for 2 days, fixed in 10% buffered formalin, and stained with 1% crystal violet.
2.6. Plaque inhibition test
A peptide stock solution was diluted with medium containing 2% FCS and DEAE-dextran (25 μg/mL), and 90 μL of this solution was added to fcwf-4 cells seeded on 24-well plates, followed by their incubation at RT for 30 min. Cells were sensitized with DMSO diluted to the same fold as peptide stock solutions as the solvent control. The cells were then inoculated with 10 μL of an 8000 pfu/mL viral suspension without washing and incubated at 37 °C for 30 min. After incubation, cells were washed with medium and 1 mL of medium containing 2% FCS and 1.5% carboxymethyl cellulose was added to each well. The cultures were incubated at 37 °C for 2 days, fixed in 10% buffered formalin, and stained with 1% crystal violet. The percent plaque inhibition was calculated by the following formula: Plaque inhibition (%) = {(plaque number of well containing virus alone − plaque number of well containing peptide or DMSO)/plaque number of well containing virus alone} × 100.
To investigate whether or not changes of peptides influence the effect of polycation, a plaque inhibition test was performed using medium containing DEAE-dextran (0, 25, or 250 μg/mL).
2.7. Reaction conditions for peptides to exhibit inhibitory effect on FIPV infection
To investigate the reaction conditions needed by peptides to exhibit inhibitory effect on FIPV infection, peptide-treated cells were washed and then inoculated with the virus. Cells were combined with a diluted peptide solution (100 μM) or 1% DMSO (solvent control) and reacted at RT for 30 min. These cells were then washed and combined with 90 μL of medium containing 2% FCS and DEAE-dextran, followed by inoculation with 10 μL of the 8000 pfu/mL virus suspension. The procedure thereafter was the same as described above. At the same time, whether the peptide exhibited inhibitory effect on FIPV infection without the pre-sensitization of cells was investigated: Cells were combined with 90 μL of medium containing 2% FCS and DEAE-dextran and kept standing at RT for 30 min. The medium was then removed and the cells were inoculated with a mixture of the virus and peptide or DMSO.
2.8. Flow cytometric analysis
A mixture of the peptide (100 μM) and type II FIPV strain 79-1146 (MOI = 40) was added to 5 × 104 cells and reacted at 4 °C for 1 h. A mixture of 1% DMSO (solvent control) and the virus was added to cells as a control. The cells were washed three times in ice-cold medium containing 0.1% NaN3, and incubated with MAb 5-6-2 and MAb F19-1 at 4 °C for 30 min. The cells washed and resuspended with fluorescein isothiocyanate (FITC)-conjugated F(ab)′2 of goat anti-mouse IgG antibody (MP Biomedicals, LLC-Cappel Products, U.S.A.). After incubation at 4 °C for 30 min, cells were washed and the fluorescein intensity of cells were determined by counting about 5000 cells on a flow cytometer (Cytomics FC500, Beckman Coulter, U.S.A.). The percent inhibition of fluorescein intensity was calculated by the following formula: inhibition of fluorescein intensity (%) = {(mean fluorescein intensity (MFI) of sample containing virus alone − MFI of sample containing peptide or DMSO)/MFI of sample containing virus alone} × 100.
2.9. Protein sequence
The amino acid sequences of the coronavirus S protein were referred to Genbank. The accession numbers were AAB47503.1 (type I FIPV strain KU-2), AB088223.1 (strain Black), AB088222.1 (strain UCD-1), and AGZ84516.1 (type II FIPV strain 79-1146). An alignment comparison of the amino acid sequence of the full-length S protein was performed using ClustalW (http://clustalw.ddbj.nig.ac.jp/). The results of the alignment comparison are shown in Fig. 1 .
Fig. 1.
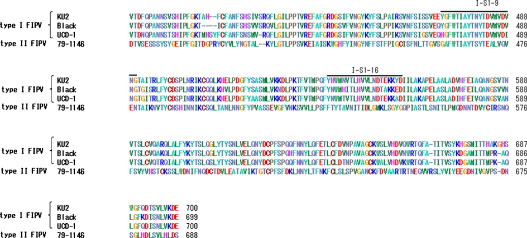
Amino acid sequence homology of the alpha coronavirus S protein. An alignment comparison of the S protein amino acid sequences among type I FIPV strains (KU-2, Black, and UCD-1) and type II FIPV strain 79-1146 was performed using ClustalW.
2.10. Statistical analysis
Data from two groups were analyzed by the Student's t test and multiple groups were analyzed by Tukey–Kramer method or Dunnett's test. The Tukey–Kramer method was only used to investigate the reaction conditions needed by peptides to exhibit inhibitory effect on FIPV infection. Multiple groups in other experiments were analyzed using Dunnett's test.
3. Result
3.1. Influences of polyanions and polycations on Type I FIPV infection
The influences of the polyanions and polycations on Type I FIPV infection were investigated. When the polyanions, dextran sulfate and heparin, were added to culture medium, the infection efficiency of FIPV strain KU-2 was decreased (Fig. 2 ). In contrast, when the polycations, DEAE-dextran, polybrene, and protamine, were added, its infection efficiency was markedly enhanced as the polycation concentration was increased.
Fig. 2.
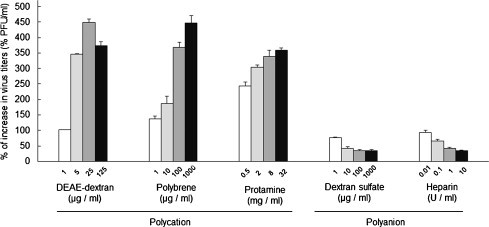
Influences of polycations and polyanions on type I FIPV infection efficiency. Serial dilutions of polycations or polyanions were added to culture medium and used to dilute type I FIPV strain KU-2. fcwf-4 cells were then inoculated with these viral suspensions. After allowing 1 h for viral adsorption, the cells were washed, and medium containing 2% FCS and 1.5% carboxymethyl cellulose was added to each well. The cells were cultured at 37 °C for 2 days and then stained with 1% crystal violet. The plaque formation rate was calculated by the following equation: Percentage of the plaque = {(Number of plaques in a well inoculated with a viral suspension diluted with polycation- or polyanion-containing medium/number of plaques in a well inoculated with a viral suspension diluted with medium containing no polycation or polyanion)} × 100. Experiments were performed in triplicate, and the figure shows mean ± standard errors.
3.2. Search for type I FIPV S protein-derived peptides inhibiting FIPV infection
We synthesized overlapping peptides based on the amino acid sequence of the S1 domain of the type I FIPV strain KU-2 S protein, in which the presence of RBD is expected. The inhibitory effects of peptides on infections with homologous strain KU-2 were investigated using the plaque assay. Based on the polycation-induced enhancement of infection observed above, MEM containing 25 μg/mL of DEAE-dextran was used. fcwf-4 cells seeded on 24-well plates were combined with 90 μL of peptide solution diluted to 100 μM and reacted at RT for 30 min. The cells were then inoculated with 10 μL of an 80 pfu/10 μL viral suspension without washing. Of the 30 peptides tested, I-S1-8, I-S1-9, I-S1-10, I-S1-16, and I-S1-22 significantly inhibited the infectivity of FIPV strain KU-2 (Fig. 3 A) in a dose-dependent manner (Fig. 3B and C).
Fig. 3.
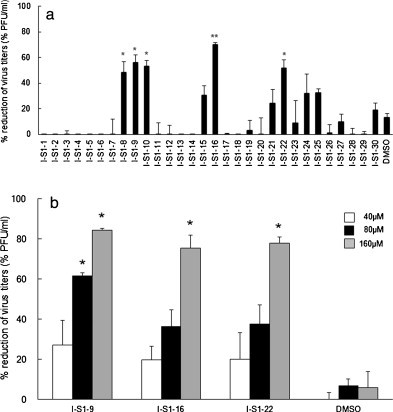
Search for type I FIPV S protein-derived peptides that inhibit FIPV infection. (A) Rates of plaque reductions by type I FIPV S protein-derived peptides. fcwf-4 cells were incubated with peptide (100 μM) or 1% DMSO (solvent control) for 30 min at RT. Then, cells were infected with type I FIPV strain KU-2 in the presence of peptide or 1% DMSO (solvent control). After washing, the number of plaque reduction was calculated. Experiments were performed in triplicate, and the figure shows mean ± standard errors. (B) Morphology of type I FIPV strain KU-2-induced plaques in fcwf-4 cells infected with the virus in the presence of the peptide adjusted to 160, 80, and 40 μM or DMSO (solvent control). (C) Rates of plaque reductions by serial dilutions of peptides. fcwf-4 cells were reacted with peptides adjusted to 160, 80, and 40 μM and then inoculated with type I FIPV strain KU-2. Experiments were performed in triplicate, and the figure shows mean ± standard errors. *p < 0.05 vs. DMSO **p < 0.01 vs. DMSO.
The isoelectric points of peptides that inhibited FIPV infection were acidic, particularly, I-S1-8, 9, and 10, suggesting that viral infection was inhibited through reduction of the effect of polycation (DEAE-dextran) by the peptides. Thus, we changed the DEAE-dextran concentration and investigated the infection-inhibitory effects of the peptides (Fig. 4 ). I-S1-9 and I-S1-16 inhibited viral infection regardless of the DEAE-dextran concentration. In contrast, I-S1-18 did not inhibit infection at any concentration of DEAE-dextran, although its isoelectric point was as low as that of I-S1-8.
Fig. 4.
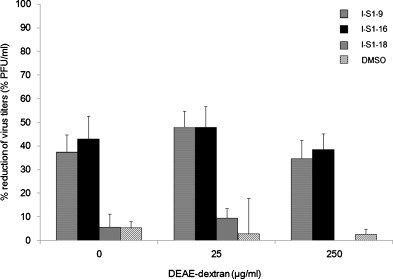
Influence of charge of medium on infection-inhibitory effect of peptide. Peptides and the virus were diluted with medium containing DEAE-dextran at various concentrations (0, 25, or 250 μg/mL). Fcwf-4 cells were incubated with peptide (100 μM) or 1% DMSO (solvent control) for 30 min at RT. Then, cells were infected with type I FIPV strain KU-2 in the presence of peptide or 1% DMSO (solvent control). After washing, the number of plaque reduction was calculated. horizontal striped bar; I-S1-9, black bar; I-S1-16, gray bar; I-S1-18, hatched bar; DMSO .Experiments were performed in triplicate, and the figure shows mean ± standard errors.
3.3. Reaction conditions for peptides to exhibit inhibitory effect on FIPV infection
The reaction conditions needed by peptides to exhibit inhibitory effect on FIPV strain KU-2 infection were investigated. When cells were washed after the reaction with the peptide and then inoculated with the virus, the inhibitory effect of I-S1-9, I-S1-16, and I-S1-22 were significantly decreased (Fig. 5 ). When cells were simultaneously reacted with the peptide and virus without the peptide pretreatment, all peptides inhibited infection by 40% or higher, and the inhibitory effects of I-S1-16 were equivalent to those observed in cells pretreated with the peptide before the inoculation with the virus.
Fig. 5.
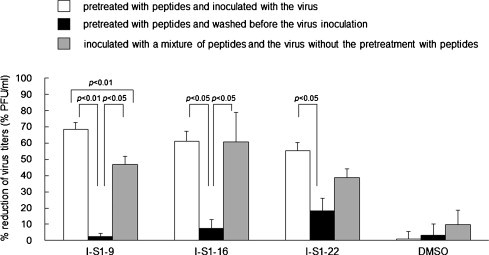
Reaction conditions for peptides to exhibit an inhibitory effect on type I FIPV infection. White bar; cells were pretreated with peptides and inoculated with the virus, black bar; cells were pretreated with peptides and washed before the virus inoculation, gray bar; cells were inoculated with a mixture of peptides and the virus without the pretreatment with peptides. Experiments were performed in triplicate, and the figure shows mean ± standard errors.
3.4. Antiviral effects of peptides on type I and II FIPV, feline herpesvirus, and feline calicivirus
The inhibition of infections by other FIPV strains, FIV, and FCV using I-S1-9 and I-S1-16, which exhibited marked inhibitory effects on type I FIPV strain KU-2 infections, was investigated. I-S1-9 and I-S1-16 significantly inhibited infections by the type I FIPV strains UCD-1 and Black (Fig. 6 ). These also significantly inhibited infections by type II FIPV strain 79-1146. The inhibitory effects of I-S1-9 on FIPV strain 79-1146 infection were equivalent to that on FIPV strain KU-2. In contrast, no inhibitory effects on infection were observed on FHV strain C7301 and FCV strain F4 by any of these peptides.
Fig. 6.
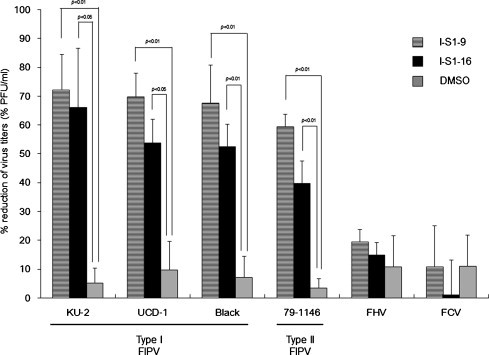
Antiviral effects of peptides against type I and II FIPV, feline herpesvirus, and feline calicivirus. fcwf-4 cells were incubated with peptide (100 μM) or 1% DMSO (solvent control) for 30 min at RT. Then, cells were infected with type I FCoV strains KU-2, UCD-1, or Black, typeII FCoV strains 79-1146, FHV strain C7301, or FCV strain F4, respectively. After staining, reductions in the number of plaques were calculated. horizontal striped bar; I-S1-9, black bar; I-S1-16, gray bar; DMSO. Experiments were performed in triplicate, and the figure shows mean ± standard errors.
3.5. Inhibitory effects of peptides on FIPV adsorption to fcwf-4 cells
We determined whether the peptides examined decreases viral adsorption efficiency using flow cytometric analysis. The peptide, I-S1-9, which exhibited the most potent inhibitory effects on FIPV infection, was used.
A peak was present at 420 on a fluorescence intensity (FL1 Lin) histogram of fcwf-4 cells without viral adsorption. When the virus was adsorbed to fcwf-4 cells, the peak of the fluorescence intensity shifted to 620 (Fig. 7A). Similarly, when fcwf-4 cells were inoculated with a mixture of DMSO and the virus, the peak of the histogram shifted to 575 (Fig. 7A). In contrast, when cells were inoculated with a mixture of the virus and I-S1-9, the peak was present at 470 (Fig. 7B), i.e., the peak after inoculation with the virus/-S1-9 mixture shifted left to a greater extent than those after the inoculation with the virus alone and virus/DMSO mixture.
Fig. 7.
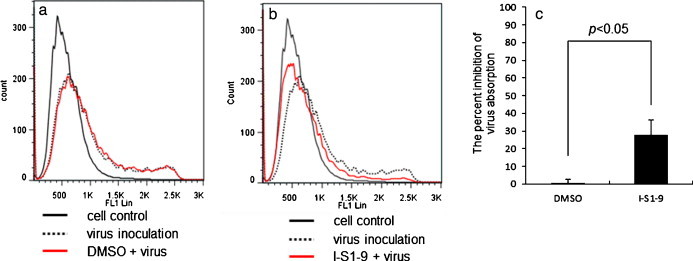
Inhibitory effects of a peptide on FIPV adsorption to fcwf-4 cells. A mixture of a peptide (I-SI-9, 100 μM) and type II FIPV strain 79-1146 (MOI = 40) was added to 5 × 104 fcwf-4 cells and reacted at 4 °C for 1 h. A mixture of 1% DMSO (solvent control) and the virus was added to cells as the control. The cells were washed and incubated with MAb 5-6-2 and MAb F19-1 at 4 °C for 30 min. The cells washed and reacted with FITC-conjugated F(ab)′2 of goat anti-mouse IgG antibody. After incubation at 4 °C for 30 min, cells were washed and the fluorescein intensity of cells were determined by counting about 5000 cells on a flow cytometer. (A and B) Fluorescence intensity histograms of fcwf-4 cells inoculated with a mixture of DMSO or a peptide and the virus Black line; cells treated with medium, dotted line; cells inoculated with virus, red line; Cells inoculated with a mixture of DMSO or a peptide and the virus. (C) The percent inhibition of virus absorption. The percentage inhibition was calculated from the mean fluorescein intensity (MFI) regarded as a level of viral adsorption to fcwf-4 cells in each sample. The inhibition of viral adsorption by DMSO or a peptide was calculated using the following equation: inhibition of virus absorption (%) = {(MFI of sample containing virus alone − MFI of sample containing peptide or DMSO)/MFI of sample containing virus alone} × 100. Experiments were performed in triplicate, and the figure shows mean ± standard errors.
The level of viral adsorption to fcwf-4 cells was evaluated based on the mean fluorescence intensity (MFI), i.e., the rate of inhibition of viral adsorption by the peptide was calculated. The viral adsorption level was significantly lower on fcwf-4 cells inoculated with the I-S1-9/virus mixture than on those inoculated with the DMSO/virus mixture (Fig. 7C).
4. Discussion
We synthesized overlapping peptides based on the amino acid sequence of the type I FIPV strain KU-2 S protein, and investigated the inhibitory effects on type I FIPV and type II FIPV infection of these peptides.
Type I FIPV poorly replicates in cells (Hohdatsu et al., 1995). To evaluate the inhibitory effects on type I FIPV infection of these peptides, we initially investigated a method to increase the infection efficiency of type I FIPV. To accurately evaluate inhibitory effect on type I FIPV infection, we examined the addition of a polycation or polyanion to a diluted viral suspension. When a viral suspension was diluted with medium containing a polycation and subjected to the infection of fcwf-4 cells, the number of Type I FIPV plaques increased in a manner dependent on the polycation concentration. In contrast, when a viral suspension was diluted with polyanion-containing medium, the number of plaques decreased in a manner dependent on the polyanion concentration. A polycation-induced increase and polyanion-induced decrease in viral infection efficiency have been reported for other viruses, such as TGEV, MHV, and vesicular stomatitis virus (VSV) (Bailey et al., 1984, Hirano et al., 1978, Nguyen et al., 1987). Bailey et al. (1984) demonstrated that positively charged polycations increased electrostatic interactions between viral particles and cell surfaces, promoting the binding of viral particles to the cells. Similarly, the polycation may have enhanced electrostatic interactions between the virus and cell surface and increased type I FIPV infection efficiency.
Of the 30 overlapping peptides synthesized, I-S1-8, I-S1-9, I-S1-10, I-S1-16, and I-S1-22 significantly decreased the type I FIPV strain KU-2 infection rate. The isoelectric points of these peptides were acidic, suggesting that the peptides reduced the effect of the polycation and decreased the number of plaques. However, I-S1-9 and I-S1-16 significantly inhibited type I FIPV strain KU-2 infection regardless of the polycation concentration. Moreover, I-S1-18 showed no infection-inhibitory effect although its isoelectric point was as low as that of I-S1-9. Based on these findings, the infection-inhibitory effect was exhibited regardless of the charge of the peptides. In addition, I-S1-8 and I-S1-10 partially overlapped the amino acid sequence of I-S1-9, and these peptides have similar inhibitory effect as I-S19. In contrast, I-S1-7 (partially overlapping I-S1-8) and I-S1-11 (partially over lapping I-S1-10) did not show inhibitory effect. These findings suggested that the amino acid sequence of I-S1-9 was very important for exhibiting inhibitory effects on FIPV infection.
In the present study, we synthesized overlapping peptides based on the amino acid sequence of the S1 domain of the type I FIPV strain KU-2 S protein, in which the presence of RBD is expected. On the basis of previous reports, the S1 domain of FIPV is expected to involve in RBD (Breslin et al., 2003, Reguera et al., 2012, Regan et al., 2012). Li et al. reported that a JEV RBD-derived peptide inhibited JEV infection by competitively blocking the binding of JEV to cells (Li et al., 2012, Li et al., 2014). Reguera et al. (2012) reported TGEV RBD. I-S1-9 and I-S1-16 regions, which exhibited inhibitory effects on type I and II FIPV infection, were included in TGEV RBD. In the inhibition test of FIPV adsorption to fcwf-4 cells by I-S1-9, viral binding to fcwf-4 cells was found to be reduced. Based on this result, it was assumed that at least I-S1-9 competitively inhibited viral binding to cells, which significantly decreased FIPV infection efficiency. However, inhibitory effects on FIPV infection were markedly decreased when cells were inoculated with the virus after being pre-sensitized with I-S1-9 and I-S1-16 peptides and washed. These findings indicate that the peptides interact with virus protein, but not with cellular virus receptors. However the data in Fig. 5 could be explained by the internalization of peptide-bound receptor, and replacement by a new population via membrane recycling. Further investigations are beeded to elucidate the action mechanism of these peptides in more detail.
S1-9 and I-S1-16 exhibited marked inhibitory effects on type I FIPV infection in the experiment. We investigated the inhibitory effects of these peptides on other type I and II FIPV strains, FHV, and FCV infection. I-S1-9 significantly inhibited both type I and II FIPV infections by 50% or more. I-S1-16 inhibited infections with all type I FIPV strains by 50% or more, but its effect on type II FIPV infection was less than 50%. In a comparison of the amino acid sequence of the S protein among the coronavirus strains, the I-S1-16 amino acid sequence showed 75-80% identity and 90% similarity to type I FIPV, excluding KU-2, but only 20% identity and 30-35% similarity to type II FIPV (Fig. 1), suggesting that the reduced inhibitory effects of I-S1-16 on type II FIPV infection were attributed to reduced amino acid sequence homology. In contrast, the I-S1-9 amino acid sequence was well conserved between type I and II FIPV. These results suggest that I-S1-9 has broad antiviral activity against feline coronavirus regardless of serotypes. Furthermore, the similarity of the effect between type I and type II FIPV suggests that there is a common receptor binding site for the two viruses. However, previous studies showed that type I and type II FIPV have distinct receptors (Hohdatsu et al., 1998a, Hohdatsu et al., 1998b). The reasons for this discrepancy are unknown. In any case, to elucidate it, identification of the virus receptor for type I FIPV is necessary.
FIPV is serologically classified into types I and II, but both types induce FIP in cats. These 2 types do not serologically cross-react with each other (Pedersen et al., 1984, Shiba et al., 2007). Since type II FIPV more actively replicates in cell lines and is more pathogenic for cats, previous studies on FIPV and anti-FIPV agents were performed using type II FIPV; however, studies have not yet been conducted to search for effective antiviral agents against type I FIPV. Since many cats are infected with type I FIPV in the field, it is important for clinical veterinarians to prepare an anti-FIPV agent effective against type I FIPV (Addie et al., 2003, Hohdatsu et al., 1992, Kummrow et al., 2005, Shiba et al., 2007). In the present study, we identified peptides exhibiting coronavirus-specific infection-inhibitory effects. I-S1-9 and I-S1-16 showed marked inhibitory effects on all type I FIPV strains infection, suggesting that these peptides are also effective for wild strains. In addition, I-S1-9 showed marked inhibitory effects on not only type I, but also type II FIPV infection, suggesting its practical applicability as an anti-FIPV agent.
The blood elimination half-life of peptide preparations is generally short, and modifications with polyethylene glycol are applied to prolong the half-life (Alvarez et al., 2012, Østergaard et al., 2011, Shechter et al., 2005). Marastoni et al. (1994) reported that an intramolecular circularized peptide became more resistant to enzymes in the circulation and brain. A half-life-extending method through the fusion of a peptide with albumin and the immunoglobulin Fc domain has also been reported (Andersen et al., 2011, Sockolosky et al., 2014, Yeh et al., 1992, Zettlmeissl et al., 1990). The applicability of these modification methods needs to be investigated before these modified peptides can be administered to cats.
5. Conclusion
The possibility of peptides derived from the S protein of type I FIPV strain KU-2 as anti-FIPV agents effective not only for type I, but also type II FIPV was demonstrated in vitro. Investigations on peptide modification methods and the in vivo effects of these peptides are desired.
Acknowledgement
This work was supported by KAKENHI (Grants-in Aid for Scientific Research (B), no. 25292183) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Addie D.D., Schaap I.A., Nicolson L., Jarrett O. Persistence and transmission of natural type I feline coronavirus infection. J. Gen. Virol. 2003;84:2735–2744. doi: 10.1099/vir.0.19129-0. [DOI] [PubMed] [Google Scholar]
- Alvarez H.M., So O.Y., Hsieh S., Shinsky-Bjorde N., Ma H., Song Y., Pang Y., Marian M., Escandón E. Effects of PEGylation and immune complex formation on the pharmacokinetics and biodistribution of recombinant interleukin 10 in mice. Drug Metab. Dispos. 2012;40:360–373. doi: 10.1124/dmd.111.042531. [DOI] [PubMed] [Google Scholar]
- Andersen J.T., Pehrson R., Tolmachev V., Daba M.B., Abrahmsén L., Ekblad C. Extending half-life by indirect targeting of the neonatal Fc receptor (FcRn) using a minimal albumin binding domain. J. Biol. Chem. 2011;286:5234–5241. doi: 10.1074/jbc.M110.164848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C.A., Miller D.K., Lenard J. Effects of DEAE-dextran on infection and hemolysis by VSV. Evidence that nonspecific electrostatic interactions mediate effective binding of VSV to cells. Virology. 1984;133:111–118. doi: 10.1016/0042-6822(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Baldwin C.W., Scott F.W. Attempted immunization of cats with feline infectious peritonitis virus propagated at reduced temperatures. Am. J. Vet. Res. 1997;58:251–256. [PubMed] [Google Scholar]
- Balzarini J., Keyaerts E., Vijgen L., Vandermeer F., Stevens M., De Clercq E., Egberink H., Van Ranst M. Pyridine N-oxide derivatives are inhibitory to the human SARS and feline infectious peritonitis coronavirus in cell culture. J. Antimicrob. Chemother. 2006;57:472–481. doi: 10.1093/jac/dki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlough J.E., Shacklett B.L. Antiviral studies of feline infectious peritonitis virus in vitro. Vet. Rec. 1994;135:177–179. doi: 10.1136/vr.135.8.177. [DOI] [PubMed] [Google Scholar]
- Barlough J.E., Stoddart C.A., Sorresso G.P., Jacobson R.H., Scott F.W. Experimental inoculation of cats with canine coronavirus and subsequent challenge with feline infectious peritonitis virus. Lab. Anim. Sci. 1984;34:592–597. [PubMed] [Google Scholar]
- Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein, structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin J.J., Mørk I., Smith M.K., Vogel L.K., Hemmila E.M., Bonavia A., Talbot P.J., Sjöström H., Norén O., Holmes K.V. Human Coronavirus 229E: receptor binding domain and neutralization by soluble receptor at 37 ̊C. J. Virol. 2003;77:4435–4438. doi: 10.1128/JVI.77.7.4435-4438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corapi W.V., Olsen C.W., Scott F.W. Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus. J. Virol. 1992;66:6695–6705. doi: 10.1128/jvi.66.11.6695-6705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corapi W.V., Darteil R.J., Audonnet J.C., Chappuis G.E. Localization of antigenic sites of the S glycoprotein of feline infectious peritonitis virus involved in neutralization and antibody-dependent enhancement. J. Virol. 1995;69:2858–2862. doi: 10.1128/jvi.69.5.2858-2862.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K., Ritz S. Treatment of cats with feline infectious peritonitis. Vet. Immunol. Immunopathol. 2008;123:172–175. doi: 10.1016/j.vetimm.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. Synthesized peptide inhibitors of HIV-1 gp41-dependent membrane fusion. Curr. Pharm. Des. 2013;19:1800–1809. doi: 10.2174/1381612811319100004. [DOI] [PubMed] [Google Scholar]
- Hirano N., Suda W., Ono K., Murakami T., Matumoto M. Effect of diethylaminoethyl-dextran on plaque formation of mouse hepatitis virus. Jpn. J. Exp. Med. 1978;48:265–267. [PubMed] [Google Scholar]
- Hohdatsu T., Izumiya Y., Yokoyama Y., Kida K., Koyama H. Differences in virus receptor for type I and type II feline infectious peritonitis virus. Arch. Virol. 1998;143:839–850. doi: 10.1007/s007050050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Nakamura M., Ishizuka Y., Yamada H., Koyama H. A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies. Arch. Virol. 1991;120:207–217. doi: 10.1007/BF01310476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Ishizuka Y., Yamada H., Koyama H. The prevalence of types I and II feline coronavirus infections in cats. J. Vet. Med. Sci. 1992;54:557–562. doi: 10.1292/jvms.54.557. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Koyama H. Characterization of monoclonal antibodies against feline infectious peritonitis virus type II and antigenic relationship between feline, porcine, and canine coronaviruses. Arch. Virol. 1991;117:85–95. doi: 10.1007/BF01310494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Sasamoto T., Okada S., Koyama H. Antigenic analysis of feline coronaviruses with monoclonal antibodies (MAbs): preparation of MAbs which discriminate between FIPV strain 79-1146 and FECV strain 79-1683. Vet. Microbiol. 1991;28:13–24. doi: 10.1016/0378-1135(91)90096-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Tatekawa T., Koyama H. Enhancement of feline infectious peritonitis virus type I infection in cell cultures using low-speed centrifugation. J. Virol. Methods. 1995;51:357–362. doi: 10.1016/0166-0934(94)00119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Yamada H., Ishizuka Y., Koyama H. Enhancement and neutralization of feline infectious peritonitis virus infection in feline macrophages by neutralizing monoclonal antibodies recognizing different epitopes. Microbiol. Immunol. 1993;37:499–504. doi: 10.1111/j.1348-0421.1993.tb03242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Yamada M., Tominaga R., Makino K., Kida K., Koyama H. Antibody-dependent enhancement of feline infectious peritonitis virus infection in feline alveolar macrophages and human monocyte cell line U937 by serum of cats experimentally or naturally infected with feline coronavirus. J. Vet. Med. Sci. 1998;60:49–55. doi: 10.1292/jvms.60.49. [DOI] [PubMed] [Google Scholar]
- Hsieh L.E., Lin C.N., Su B.L., Jan T.R., Chen C.M., Wang C.H., Lin D.S., Lin C.T., Chueh L.L. Synergistic antiviral effect of Galanthus nivalis agglutinin and nelfinavir against feline coronavirus. Antiviral Res. 2010;88:25–30. doi: 10.1016/j.antiviral.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida K., Hohdatsu T., Fujii K., Koyama H. Selection of antigenic variants of the S glycoprotein of feline infectious peritonitis virus and analysis of antigenic sites involved in neutralization. J. Vet. Med. Sci. 1999;61:935–938. doi: 10.1292/jvms.61.935. [DOI] [PubMed] [Google Scholar]
- Kim Y., Mandadapu S.R., Groutas W.C., Chang K.O. Potent inhibition of feline coronaviruses with peptidyl compounds targeting coronavirus 3C-like protease. Antiviral Res. 2012;97:161–168. doi: 10.1016/j.antiviral.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummrow M., Meli M.L., Haessig M., Goenczi E., Poland A., Pedersen N.C., Hofmann-Lehmann R., Lutz H. Feline coronavirus serotypes 1 and 2: seroprevalence and association with disease in Switzerland. Clin. Diagn. Lab. Immunol. 2005;12:1209–1215. doi: 10.1128/CDLI.12.10.1209-1215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ge L.L., Yu Y.L., Huang L., Wang Y., Sun M.X., Ishag H., Ma L.X., Li X.H., Shen Z.Q., Mao X. A tripeptide (NSK) inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch. Virol. 2014;159:1045–1055. doi: 10.1007/s00705-013-1925-y. [DOI] [PubMed] [Google Scholar]
- Li C., Zhang L.Y., Sun M.X., Li P.P., Huang L., Wei J.C., Yao Y.L., Isahg H., Chen P.Y., Mao X. Inhibition of Japanese encephalitis virus entry into the cells by the envelope glycoprotein domain III (EDIII) and the loop3 peptide derived from EDIII. Antiviral Res. 2012;94:179–183. doi: 10.1016/j.antiviral.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Liu I.J., Tsai W.T., Hsieh L.E., Chueh L.L. Peptides corresponding to the predicted heptad repeat 2 domain of the feline coronavirus spike protein are potent inhibitors of viral infection. PLoS ONE. 2013;8:e82081. doi: 10.1371/journal.pone.0082081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marastoni M., Salvadori S., Scaranari V., Spisani S., Reali E., Traniello S., Tomatis A. Synthesis and activity of new linear and cyclic peptide T derivatives. Arzneimittelforschung. 1994;44:1073–1076. [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Aizawa C., Koyama H., Hashimoto H. Molecular cloning and sequence determination of the peplomer protein gene of feline infectious peritonitis virus type I. Arch. Virol. 1995;140:469–480. doi: 10.1007/BF01718424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Hashimoto H., Koyama H. Comparison of the amino acid sequence and phylogenetic analysis of the peplomer, integral membrane and nucleocapsid proteins of feline, canine and porcine coronaviruses. Microbiol. Immunol. 1996;40:425–433. doi: 10.1111/j.1348-0421.1996.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.D., Bottreau E., Aynaud J.M. Transmissible gastroenteritis (TGE of swine, in vitro virus attachment and effects of polyanions and polycations. Vet. Microbiol. 1987;14:343–354. doi: 10.1016/0378-1135(87)90026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard H., Bjelke J.R., Hansen L., Petersen L.C., Pedersen A.A., Elm T., Møller F., Hermit M.B., Holm P.K., Krogh T.N., Petersen J.M., Ezban M., Sørensen B.B., Andersen M.D., Agersø H., Ahmadian H., Balling K.W., Christiansen M.L., Knobe K., Nichols T.C., Bjørn S.E., Tranholm M. Prolonged half-life and preserved enzymatic properties of factor IX selectively PEGylated on native N-glycans in the activation peptide. Blood. 2011;118:2333–2341. doi: 10.1182/blood-2011-02-336172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W. A review of feline infectious peritonitis virus, molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet. Microbiol. 1993;36:1–37. doi: 10.1016/0378-1135(93)90126-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W., Corapi W.V., Ngichabe C.K., Baines J.D., Scott F.W. Monoclonal antibodies to the spike protein of feline infectious peritonitis virus mediate antibody-dependent enhancement of infection of feline macrophages. J. Virol. 1992;66:956–965. doi: 10.1128/jvi.66.2.956-965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. A review of feline infectious peritonitis virus infection, 1963-2008. J. Feline Med. Surg. 2009;11:225–258. doi: 10.1016/j.jfms.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Black J.W., Boyle J.F., Evermann J.F., McKeirnan A.J., Ott R.L. Pathogenic differences between various feline coronavirus isolates. Adv. Exp. Med. Biol. 1984;173:365–380. doi: 10.1007/978-1-4615-9373-7_36. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F., Floyd K., Fudge A., Barker J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am. J. Vet. Res. 1981;42:368–377. [PubMed] [Google Scholar]
- Regan A.D., Millet J.K., Tse L.P., Chillag Z., Rinaldi V.D., Licitra B.N., Dubovi E.J., Town C.D., Whittaker G.R. Characterization of a recombinant canine coronavirus with a distinct receptor-binding (S1) domain. Virology. 2012;430:90–99. doi: 10.1016/j.virol.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera J., Santiago C., Mudgal G., Ordoño D., Enjuanes L., Casasnovas J.M. Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathog. 2012;8:e1002859. doi: 10.1371/journal.ppat.1002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Tsubery H., Mironchik M., Rubinstein M., Fridkin M. Reversible PEGylation of peptide YY3-36 prolongs its inhibition of food intake in mice. FEBS Lett. 2005;579:2439–2444. doi: 10.1016/j.febslet.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Shiba N., Maeda K., Kato H., Mochizuki M., Iwata H. Differentiation of feline coronavirus type I and II infections by virus neutralization test. Vet. Microbiol. 2007;124.:348–352. doi: 10.1016/j.vetmic.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockolosky J.T., Kivimäe S., Szoka F.C. Fusion of a short peptide that binds immunoglobulin G to a recombinant protein substantially increases its plasma half-life in mice. PLoS ONE. 2014;9:e102566. doi: 10.1371/journal.pone.0102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart C.A., Barlough J.E., Baldwin C.A., Scott F.W. Attempted immunisation of cats against feline infectious peritonitis using canine coronavirus. Res. Vet. Sci. 1988;45:383–388. doi: 10.1016/S0034-5288(18)30970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Katada Y., Moritoh S., Ogasawara M., Satoh K., Tanabe M., Hohdatsu T. Analysis of the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection: aminopeptidase N is not important and a process of acidification of the endosome is necessary. J. Gen. Virol. 2008;89:1025–1029. doi: 10.1099/vir.0.83558-0. [DOI] [PubMed] [Google Scholar]
- Takano T., Katoh Y., Doki T., Hohdatsu T. Effect of chloroquine on feline infectious peritonitis virus infection in vitro and in vivo. Antiviral Res. 2013;99:100–107. doi: 10.1016/j.antiviral.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R.C., Scott F.W. Antibody-mediated enhancement of disease in feline infectious peritonitis, comparisons with dengue hemorrhagic fever. Comp. Immunol. Microbiol. Infect. Dis. 1981;4:175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P., Landais D., Lemaître M., Maury I., Crenne J.Y., Crenne J.Y., Becquart J., Murry-Brelier A., Boucher F., Montay G., Fleer R., Mayaux J.F., Klatzmann D. Design of yeast-secreted albumin derivatives for human therapy, biological and antiviral properties of a serum albumin-CD4 genetic conjugate. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1904–1908. doi: 10.1073/pnas.89.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettlmeissl G., Gregersen J.P., Duport J.M., Mehdi S., Reiner G., Seed B. Expression and characterization of human CD4,immunoglobulin fusion proteins. DNA Cell Biol. 1990;9:347–353. doi: 10.1089/dna.1990.9.347. [DOI] [PubMed] [Google Scholar]


