Abstract
BACKGROUND
A considerable number of patients with dilated cardiomyopathy (DCM) experience left ventricular reverse remodeling (LVRR). LV global longitudinal strain (LV GLS) offers sensitive and reproducible measurement of myocardial dysfunction. The authors sought to evaluate whether LV GLS at the time of diagnosis may predict LVRR in DCM patients with sinus rhythm and investigate its prognostic role in long-term follow-up in this population.
METHODS
We enrolled 160 DCM patients with sinus rhythm who had been initially diagnosed, evaluated, and followed at our institute. We analyzed their medical records and echocardiographic data.
RESULTS
During the mean follow-up duration of 37.3 ± 21.7 months, LVRR occurred in 28% of patients (n = 45). The initial LV ejection fraction (LVEF) of patients who recovered LV function was 26.1 ± 7.9%, which was not significantly different from the value of 27.1 ± 7.4% (p = 0.49) in those who did not recover. There was a moderate and highly significant correlation between baseline LV GLS (−%) and follow-up LVEF (r = 0.717; p < 0.001). Using multivariate Cox analysis, LV GLS (hazard ratio: 1.474, 95% confidence interval: 1.170-1.856; p = 0.001) was an independent predictor of LVRR.
CONCLUSIONS
We demonstrated that LV GLS was an independent predictor for LVRR and the optimal cut-off point of LV GLS for LVRR was −10% in DCM patients with sinus rhythm. There was a significant correlation between baseline LV GLS and follow-up LVEF.
Keywords: Dilated cardiomyopathy, Echocardiography, Strain, Left ventricle
INTRODUCTION
Dilated cardiomyopathy (DCM) is one of the most common causes of heart failure and the most common indication for heart transplantation worldwide.1) Over the past few years, several studies have reported that 30% to 40% of patients with DCM can experience left ventricular reverse remodeling (LVRR). These findings imply that DCM does not represent an irreversible progressive pathway of myocardial failure but it is rather a dynamic disease with non-linear progression.2),3) Many authors have reported that patients with a marked improvement in LV systolic function have excellent outcomes.2),3),4) Furthermore, the prediction of LV functional recovery is of clinical importance because it affects whether nonpharmacologic interventions such as cardiac transplantation, LV assist device, implantable cardioverter-defibrillator (ICD), and cardiac resynchronization therapy are needed.
Left ventricular ejection fraction (LVEF) is the most widely used parameter for assessing cardiac function and is a predictor of outcomes. However, it has several intrinsic limitations including late decrease only in the advanced stage of heart disease, poor reliability in patients with LV hypertrophy and volume reduction, relatively poor inter-observer and intra-observer variability, and difficult endocardial border detection.
Recent studies have reported that LV global longitudinal strain (GLS) is a more sensitive measure of myocardial dysfunction and is more reproducible than LVEF. Additionally, it is a more powerful predictor of outcomes in patients with heart failure with a reduced ejection fraction.5),6) Most previous studies regarding LVRR in patients with DCM also included patients with atrial fibrillation (AF). However, it is difficult to accurately measure the value of strain by two-dimensional (2D) speckle tracking because of beat-to-beat variation. Furthermore, the LV GLS value is inconsistent. In this study, we aimed to determine whether LV GLS at the time of diagnosis may predict LVRR and to investigate the prognostic role of LV GLS in long-term follow-up in DCM patients with sinus rhythm. Additionally, we evaluated the prognostic role of LV GLS in long-term follow-up in this population. To the best of our knowledge, there are no previous reports regarding the association between initial LV GLS and recovery of LV systolic function in DCM patients with sinus rhythm.
METHODS
Study population
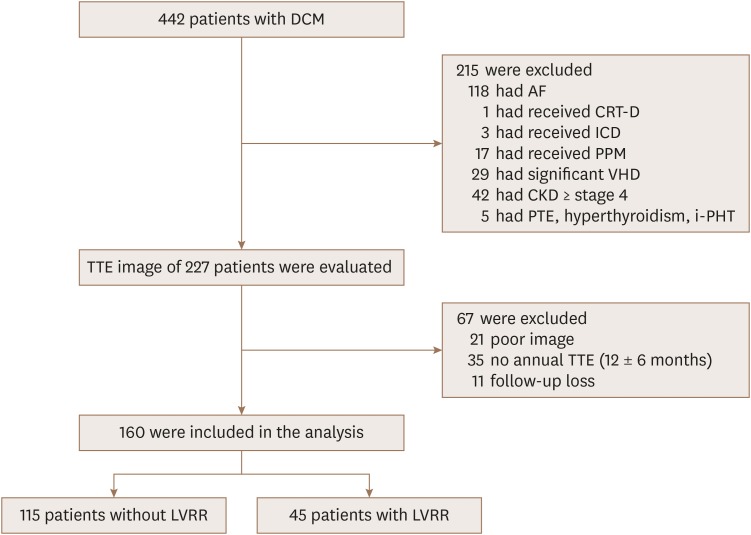
We designed this study as a retrospective analysis. Therefore, we reviewed medical records of 442 patients with heart failure who had been initially diagnosed, evaluated, and followed at Sanggye Paik Hospital, Inje University College of Medicine between March 2013 and February 2018. The diagnosis of DCM was established on the basis of the following criteria: (i) presence of LV dilation (LV end-diastolic diameter ≥ 55 mm); (ii) presence of reduced LV ejection fraction (EF) (all ≤ 45%); (iii) coronary angiographic evidence of absence of coronary artery disease defined as > 50% stenosis of a major epicardial vessel or a history of myocardial infarction; and (iv) absence of cardiac muscle disease secondary to any known systemic disease.7) As shown in Figure 1, patients who received cardiac resynchronization therapy (1), pacemaker (17), implantable cardioverter defibrillator (3), or obtained an LV assist device were excluded. We also excluded patients with AF (118), other major cardiac arrhythmias, pulmonary thromboembolism, idiopathic pulmonary hypertension, hyperthyroidism, more than moderate organic valvular disease except functional mitral regurgitation (MR), and stage 4 or more advanced renal failure or end-stage renal disease requiring renal replacement therapy. We excluded 67 patients who had difficulty in analysis due to poor image on echocardiography or follow-up loss. In total, 160 patients were enrolled in this study. The subjects were in clinically stable condition at the time of enrollment in this study and were undergoing optimal and maximally-tolerated pharmacological therapy. This study complied with the Declaration of Helsinki, and the local ethics committee approved the research protocol. The requirement of informed consent was waived by the committee.
Figure 1. Study flow chart. AF: atrial fibrillation, CKD: chronic kidney disease, CRT: cardiac resynchronization therapy, DCM: dilated cardiomyopathy, ICD: implantable cardioverter defibrillator, i-PHT: idiopathic pulmonary hypertension, LVRR: left ventricular reverse remodeling, PPM: permanent pacemaker, PTE: pulmonary thromboembolism, TTE: transthoracic echocardiography, VHD: valvular heart disease.
Definition of LVRR
LVRR was defined as the combined presence of: 1) an increase in LVEF of at least 10% points or a follow-up LVEF ≥ 50% (in patients with an LVEF of 40% to 45% at enrolment); and 2) a decrease in indexed left ventricular end-diastolic diameter (LVEDDI) of at least 10% or an LVEDDI ≤ 33 mm/m2.2),3)
Echocardiography
A standardized complete echocardiographic examination was performed at baseline and during follow-up according to the standard method outlined by the American Society of Echocardiography.8) 2D echocardiographic images were obtained using VIVID E9 and E95 (General Electric Medical Systems, Horten, Norway). All conventional echocardiographic parameters were measured and averaged over three cardiac cycles. All echocardiographic data were reanalyzed with commercially available software (EchoPAC PC software, GE Medical Systems, Horten, Norway).
Significant functional MR should fulfill all of the following three criteria: 1) normal mitral valve leaflets and chords, 2) regional or global wall motion abnormalities of LV with tethering of the leaflet, and 3) MR of EROA > 0.1 cm2.9)
2D-speckle tracking measurements of LV GLS were performed in the 2-, 3-, and 4-chamber apical views and aortic valve closure was used for timing of end-systole.10) The endocardial border was traced manually at end-systole. Tracking was adjusted to include the entire myocardial wall from the endocardium to the myoepicardial border. All LV GLS measurements were performed by a single investigator (J. H. Park), with all the images reviewed and validated by a second reader. Reproducibility was performed on a single set of recordings. Variability in the measurement of LV GLS was evaluated in 20 randomly selected patients. To determine intra-observer and inter-observer variability, the investigator measured GLS for each of the selected patients again 2 weeks later. The coefficients of variation of intra- and inter-observer variability for GLS measurements were less than 5%.
Conventional echocardiographic parameters indicating right ventricular (RV) systolic function were measured in a standard manner according to the guidelines of Rudski et al.11) The RV fractional area change was calculated using the percent area change of the end-diastolic and end-systolic areas of the RV in the apical four-chamber views. Tricuspid annular plane systolic excursion was measured as the contraction distance of the RV tricuspid annular plane along its longitudinal plane during systole.
Follow-up for echocardiography and clinical course
There were two different temporal perspectives in this study. One was to see whether LVRR occurred and the other was to see whether an adverse clinical event occurred. First, echocardiographic evaluation was scheduled to be performed annually after diagnosis of DCM, and the mean interval of echocardiography in these patients was 10.7 ± 7.3 months. The interval of echocardiography in patients without LVRR was shorter than in patients with LVRR (9.5 ± 6.4 vs. 13.7 ± 8.8 months, p = 0.027). We analyzed the echocardiographic data at the time DCM was initially diagnosed. Additionally, follow-up echocardiographic data were collected. For patients with LVRR, the results of echocardiography at the time of recovery were analyzed, and for patients who were not recovered, the results of the last performed tests were analyzed. Second, we collected clinical follow-up data for occurrence of adverse clinical events. We recorded the date of last follow-up in patients without adverse events and recorded the date of occurrence of events in patients with events.
Adverse clinical events were defined as all-cause death, death from, cardiac transplantation or hospitalization for deteriorating heart failure, and major ventricular arrhythmias or aborted sudden cardiac death. Major ventricular arrhythmias were considered as ventricular fibrillation/flutter or a sustained ventricular tachycardia (hemodynamically unstable or lasting more than 30 seconds) or appropriate ICD intervention. Long-term follow-up was conducted for 37.3 ± 21.7 months (patients with LVRR vs. without LVRR, 38.1 ± 27.8 vs. 37.0 ± 19.4 months, p = 0.855).
Statistical analysis
Continuous variables with a normal distribution were expressed as mean ± standard deviation or percentages. The parameters of the two subgroups, patients with and without LVRR, were compared using unpaired Student's t-test or Mann-Whitney U test for continuous variables. The χ2 tests (or Fisher's exact tests when any expected count was < 5 for a 2 × 2 table) was used for categorical variables. To evaluate change from baseline, a paired Student's t-test was used. Pearson's correlation was used to calculate the association between baseline LV GLS and follow-up LVEF. We performed a time-to-event analysis using a univariate Cox proportional-hazards model for all patients to determine the independent factors influencing LVRR. Variables with a univariate value of p < 0.05 were incorporated into the stepwise selection, while age, sex, medications, and LV dimensions expected to be highly associated with LVRR were forced into the multivariate analysis regardless of its association on univariate analysis. The receiver-operating characteristic (ROC) curve was constructed to determine the optimal cut-off values of LV GLS for LVRR. We then divided the subjects into two groups based on the cut-off value. Kaplan-Meier curve analysis was conducted to assess cardiovascular event-free survival during the follow-up period and compared it by using log-rank test. A probability value of p < 0.05 was considered significant. All statistical analyses were performed using SPSS software package (version 25, IBM SPSS Statistics, Chicago, IL, USA).
RESULTS
Baseline characteristics
A total of 160 patients who fulfilled all inclusion and exclusion criteria were enrolled. The mean age was 64.5 years, and 67.5% were male. Forty-five patients (28%) experienced LVRR within 14.7 ± 10.0 months after medical therapy. Clinical parameters are summarized in Table 1. As compared with patients who did not develop LVRR, patents with LVRR had fewer left bundle branch blocks (LBBB) and more dyslipidemia, and more patients used statins. However, no significant differences between the subgroups were noted in terms of age, gender distribution, body mass index, or blood pressures. A comparison of echocardiographic data between patients with LVRR and those without LVRR is shown in Table 2. The initial LVEF of patients who recovered LV function was 26.1 ± 7.9%, which was not significantly different from the value of 27.1 ± 7.4% (p = 0.49) for those who did not recover LV function. However, patients with LVRR had a larger LV, smaller left atrium (LA), and more frequent significant functional MR than those who did not have LVRR. The E/e′ ratio was higher and the values of LV GLS were lower in patients without LVRR.
Table 1. Baseline clinical characteristics.
| All patients (n = 160) | Patients without LVRR (n = 115) | Patients with LVRR (n = 45) | p-value | |||
|---|---|---|---|---|---|---|
| Age (years) | 64.5 ± 14.9 | 65.6 ± 14.9 | 61.9 ± 14.6 | 0.166 | ||
| Male sex | 108 (67.5) | 78 (67.8) | 30 (66.7) | 1.000 | ||
| BSA (m2) | 1.8 ± 0.5 | 1.9 ± 0.6 | 1.7 ± 0.2 | 0.064 | ||
| BMI (kg/m2) | 24.5 ± 4.2 | 24.4 ± 4.3 | 24.7 ± 4.2 | 0.681 | ||
| Systolic BP (mmHg) | 125.0 ± 19.8 | 124.3 ± 21.8 | 125.5 ± 16.2 | 0.859 | ||
| Diastolic BP (mmHg) | 76.7 ± 13.0 | 76.7 ± 13.9 | 76.9 ± 10.1 | 0.937 | ||
| Heart rate (bpm) | 76.9 ± 16.4 | 77.5 ± 16.7 | 75.9 ± 10.1 | 0.499 | ||
| LBBB | 26 (16.3) | 23 (20.0) | 3 (6.7) | 0.040 | ||
| Comorbidities | ||||||
| Hypertension | 71 (44.4) | 46 (40.0) | 25 (55.6) | 0.075 | ||
| Diabetes mellitus | 50 (31.3) | 37 (32.2) | 13 (28.9) | 0.687 | ||
| Dyslipidemia | 62 (38.8) | 38 (33.0) | 24 (53.3) | 0.018 | ||
| CKD | ||||||
| Normal to stage 1 | 137 (85.6) | 96 (83.5) | 41 (91.1) | 0.454 | ||
| Stage 2 | 16 (10.0) | 13 (11.3) | 3 (6.7) | 0.454 | ||
| Stage 3 | 7 (4.4) | 6 (5.2) | 1 (2.2) | 0.454 | ||
| Smoking | 36 (22.5) | 26 (22.6) | 10 (22.2) | 0.958 | ||
| Medication | ||||||
| ACE-I/ARBs | 132 (82.5) | 91 (79.1) | 41 (91.1) | 0.073 | ||
| β-Blockers | 99 (61.9) | 71 (61.7) | 28 (62.2) | 0.955 | ||
| Ivabradine | 61 (38.1) | 44 (38.2) | 17 (37.8) | 0.955 | ||
| MRA | 84 (52.5) | 64 (55.7) | 20 (44.4) | 0.202 | ||
| Statin | 72 (45.0) | 46 (40.0) | 26 (57.8) | 0.042 | ||
Data are presented as mean ± SD, n (%), or median (inter-quartile range).
ACE-I: angiotensin-converting enzyme-inhibitor, ARB: angiotensin II receptor blocker, BMI: body mass index, BP: blood pressure, BSA: body surface area, CKD: chronic kidney disease, LBBB: left bundle branch block, LVRR: left ventricular reverse remodeling, MRA: mineralocorticoid receptor antagonist.
Table 2. Baseline echocardiographic characteristics.
| All patients (n = 160) | Patients without LVRR (n = 115) | Patients with LVRR (n = 45) | p-value | |||
|---|---|---|---|---|---|---|
| LV functional parameters | ||||||
| LVEDDI (mm/m2) | 35.6 ± 6.6 | 34.9 ± 6.8 | 37.4 ± 5.5 | 0.028 | ||
| LVESDI (mm/m2) | 30.3 ± 6.1 | 29.5 ± 6.1 | 32.2 ± 5.7 | 0.012 | ||
| LVEDVI (mL/m2) | 95.0 ± 30.7 | 92.0 ± 30.5 | 102.7 ± 30.2 | 0.047 | ||
| LVESVI (mL/m2) | 70.0 ± 24.8 | 67.1 ± 24.4 | 77.3 ± 24.5 | 0.020 | ||
| LVEF (%) | 26.8 ± 7.5 | 27.1 ± 7.4 | 26.1 ± 7.9 | 0.489 | ||
| LV GLS (−%) | 9.2 ± 3.1 | 8.2 ± 2.9 | 11.9 ± 1.6 | < 0.001 | ||
| Transmitral flow parameters | ||||||
| E wave velocity (cm/s) | 60.1 ± 24.1 | 64.6 ± 25.3 | 49.2 ± 16.5 | < 0.001 | ||
| A wave velocity (cm/s) | 69.7 ± 24.6 | 68.6 ± 26.5 | 72.4 ± 19.5 | 0.396 | ||
| E/A ratio (medial) | 1.4 ± 5.3 | 1.7 ± 6.3 | 0.7 ± 0.4 | 0.291 | ||
| Tissue Doppler parameters | ||||||
| Septal s′ (cm/s) | 4.9 ± 1.4 | 4.6 ± 1.3 | 5.8 ± 1.3 | < 0.001 | ||
| Septal e′ (cm/s) | 4.2 ± 1.6 | 3.9 ± 1.5 | 4.9 ± 1.7 | < 0.001 | ||
| Septal a′ (cm/s) | 7.2 ± 5.2 | 6.1 ± 0.6 | 8.0 ± 1.7 | 0.229 | ||
| E/e′ ratio | 15.9 ± 9.7 | 17.9 ± 10.4 | 10.8 ± 4.5 | < 0.001 | ||
| LAVI (mL/m2) | 28.8 ± 11.3 | 31.5 ± 11.4 | 21.8 ± 7.5 | < 0.001 | ||
| Significant MR | 24 (20.9) | 18 (40.0) | 0.013 | |||
| RV functional parameters | ||||||
| RV s′ velocity (cm/s) | 10.6 ± 2.5 | 10.5 ± 2.7 | 10.8 ± 2.3 | 0.563 | ||
| TRVmax (m/s) | 2.5 ± 0.6 | 2.5 ± 0.6 | 2.4 ± 0.5 | 0.138 | ||
| TAPSE (mm) | 1.9 ± 0.4 | 1.9 ± 0.4 | 1.9 ± 0.3 | 0.958 | ||
| RVFAC (%) | 42.1 ± 10.5 | 42.1 ± 11.3 | 42.2 ± 8.2 | 0.956 | ||
Data are presented as mean ± SD, n (%), or median (inter-quartile range).
LAVI: left atrial volume index, LV: left ventricular, LV GLS: LV global longitudinal strain, LVEDDI: LV end-diastolic diameter index, LVEDVI: LV end-diastolic volume index, LVEF: LV ejection fraction, LVESDI: LV end-systolic diameter index, LVESVI: LV end-systolic volume index, LVRR: left ventricular reverse remodeling, MR: mitral regurgitation, RV: right ventricular, RVFAC: RV fractional area change, TAPSE: tricuspid annular plane systolic excursion, TRVmax: tricuspid regurgitation jet maximum velocity.
Comparison of baseline and follow-up echocardiography parameters
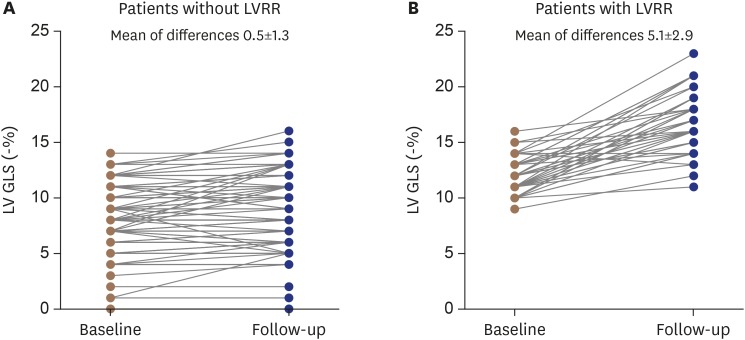
Follow-up LV functional echocardiographic parameters of all patients were improved when compared with baseline data except for the LVEF (27.8 ± 7.4%, ΔLVEF of 0.7%, p = 0.126) of patients without LVRR (Table 3). Patients with LVRR had a final LVEF of 49.4 ± 9.5% (ΔLVEF of 23.9%, p < 0.001) and had a significant decrease in LVEDDI (29.8 ± 5.2 mm/m2; ΔLVEDDI of 7.5 mm/m2, p < 0.001) as well as an increase in the absolute value of LV GLS (16.9 ± 2.7%; ΔLV GLS of 5.1%, p < 0.001). The mean differences of the LV GLS between baseline and follow-up in patients were 0.5 ± 1.3% for patients without LVRR (Figure 2A) and 5.1 ± 2.9% for patients with LVRR (Figure 2B).
Table 3. Comparison of baseline and follow-up LV functional echocardiographic data.
| Baseline | Follow-up | Difference (95% CI) | p-value | ||
|---|---|---|---|---|---|
| All patients (n = 160) | |||||
| LVEDDI (mm/m2) | 35.6 ± 6.6 | 32.9 ± 6.6 | −2.7 (−3.4 to −2.0) | < 0.001 | |
| LVESDI (mm/m2) | 30.3 ± 6.1 | 26.6 ± 6.6 | −3.7 (−4.6 to −2.8) | < 0.001 | |
| LVEDVI (mL/m2) | 95.0 ± 30.7 | 74.3 ± 30.2 | −20.7 (−25.6 to −15.8) | < 0.001 | |
| LVESVI (mL/m2) | 70.0 ± 24.8 | 50.2 ± 26.8 | −19.8 (−24.2 to −15.4) | < 0.001 | |
| LVEF (%) | 26.8 ± 7.5 | 33.9 ± 12.6 | 7.2 (5.2 to 9.2) | < 0.001 | |
| LV GLS (−%) | 9.2 ± 3.1 | 11.0 ± 4.8 | 1.8 (1.3 to 2.2) | < 0.001 | |
| Patients without LVRR (n = 115) | |||||
| LVEDDI (mm/m2) | 34.9 ± 6.8 | 34.1 ± 6.8 | −0.8 (−1.3 to −0.3) | 0.002 | |
| LVESDI (mm/m2) | 29.5 ± 6.1 | 28.4 ± 6.4 | −1.4 (−1.8 to −0.4) | 0.002 | |
| LVEDVI (mL/m2) | 92.0 ± 30.5 | 83.4 ± 29.8 | −8.6 (−12.4 to −4.8) | < 0.001 | |
| LVESVI (mL/m2) | 67.1 ± 24.4 | 59.5 ± 25.3 | −7.6 (−10.9 to −4.3) | < 0.001 | |
| LVEF (%) | 27.1 ± 7.4 | 27.8 ± 7.4 | 0.7 (−0.2 to 1.6) | 0.126 | |
| LV GLS (−%) | 8.2 ± 2.9 | 8.7 ± 3.2 | 0.5 (0.7 to 3.6) | < 0.001 | |
| Patients with LVRR (n = 45) | |||||
| LVEDDI (mm/m2) | 37.4 ± 5.5 | 29.8 ± 5.2 | −7.5 (−9.1 to −6.0) | < 0.001 | |
| LVESDI (mm/m2) | 32.2 ± 5.7 | 21.9 ± 4.4 | −10.3 (−11.9 to −8.6) | < 0.001 | |
| LVEDVI (mL/m2) | 102.7 ± 30.2 | 51.1 ± 15.0 | −51.7 (−61.6 to −41.7) | < 0.001 | |
| LVESVI (mL/m2) | 77.3 ± 24.5 | 26.4 ± 11.3 | −50.9 (−58.8 to −43.1) | < 0.001 | |
| LVEF (%) | 26.1 ± 7.9 | 49.4 ± 9.5 | 23.9 (20.4 to 27.5) | < 0.001 | |
| LV GLS (−%) | 11.9 ± 1.6 | 16.9 ± 2.7 | 5.1 (4.2 to 5.9) | < 0.001 | |
Data are presented as mean ± SD, n (%), or median (inter-quartile range).
CI: confidence interval, LV: left ventricular, LV GLS: LV global longitudinal strain, LVEDDI: LV end-diastolic diameter index, LVEDVI: LV end-diastolic volume index, LVEF: LV ejection fraction, LVESDI: LV end-systolic diameter index, LVESVI: LV end-systolic volume index, LVRR: left ventricular reverse remodeling.
Figure 2. Changes in LV GLS of the patients without LVRR (A) and with LVRR (B). The mean difference of LV GLS between baseline and follow-up in both groups were significant; however, LV GLS was more prominent in patients with LVRR. The mean of differences of LV GLS (−%) of patients without LVRR vs. with LVRR were 0.5 ± 1.3% vs. 5.1 ± 2.9% (p < 0.001). LV GLS: left ventricular global longitudinal strain, LVRR: left ventricular reverse remodeling.
Correlation between baseline LV GLS and follow-up echocardiographic parameters
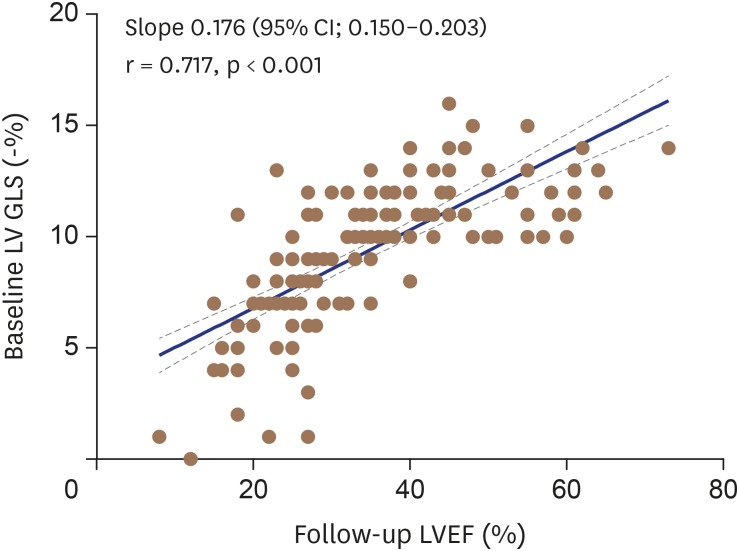
Baseline LV GLS had a significant negative correlation between follow-up LV dimensions and positive correlation with follow-up LVEF and Δ EF (i.e., difference between baseline and follow-up LVEF) (Table 4). Although baseline LV GLS was widely distributed at the follow-up LVEF levels, there was a moderate and highly significant correlation between baseline LV GLS and follow-up LVEF (r = 0.717; p < 0.001) (Figure 3).
Table 4. Correlation between baseline LV GLS and follow-up echocardiographic parameters.
| Echocardiographic parameters | r | p-value |
|---|---|---|
| Follow-up LVEDDI | −0.515 | < 0.001 |
| Follow-up LVESDI | −0.612 | < 0.001 |
| Follow-up LVEDVI | −0.572 | < 0.001 |
| Follow-up LVESVI | −0.639 | < 0.001 |
| Follow-up LVEF | 0.717 | < 0.001 |
| Δ EF | 0.376 | < 0.001 |
LV: left ventricular, LV GLS: LV global longitudinal strain, LVEDDI: LV end-diastolic diameter index, LVEDVI: LV end-diastolic volume index, LVEF: LV ejection fraction, LVESDI: LV end-systolic diameter index, LVESVI: LV end-systolic volume index, r: correlation coefficient, Δ EF: difference between baseline and follow-up LVEF.
Figure 3. There was a moderate but highly significant correlation (r = 0.717, p < 0.001) between baseline LV GLS and follow-up LVEF. LV GLS: left ventricular global longitudinal strain, LVEF: left ventricular ejection fraction.
Adverse events
During follow-up, 25 patients (15.6%) had serious adverse events including 3 cardiac deaths and 1 cardiac transplantation. Among patients without LVRR, 13 patients were admitted for worsening heart failure, and 4 patients experienced major ventricular arrhythmia or aborted sudden cardiac death. In the group that recovered LV function, there were 2 patients who were admitted for worsening heart failure and 2 patients who experienced major arrhythmia, but there were no deaths (Table 5). There was no significant difference in the incidence of adverse events between the two groups (p = 0.142).
Table 5. Events in the patients with LVRR compared with the patients without LVRR.
| All patients (n = 160) | Patients without LVRR (n = 115) | Patients with LVRR (n = 45) | p-value | ||
|---|---|---|---|---|---|
| Events | 25 (15.6) | 21 (18.3) | 4 (8.9) | 0.142 | |
| Hospitalization for worsening HF | 15 (9.4) | 13 (11.3) | 2 (4.4) | NS | |
| MVA or aborted SCD | 6 (3.8) | 4 (3.5) | 2 (4.4) | NS | |
| Death or heart transplantation | 4 (2.5) | 4 (3.5) | 0 (0) | NS | |
Data are presented as n (%).
HF: heart failure, LVRR: left ventricular reverse remodeling, MVA: major ventricular arrhythmia, SCD: sudden cardiac death.
Predictor for LVRR
The hazard ratio (HR) of each variable is shown in Table 6. By univariate analysis using a Cox proportional hazard model, no LBBB, higher value of LV GLS (−%), lower LA volume index, and E/e′ ratio were significantly associated with LVRR. Using multivariate Cox analysis, LV GLS (HR: 1.474, p = 0.001) was an independent predictor of LVRR in DCM patients with sinus rhythm. Among the medications, ACE-I/ARB was not a valid variable for LVRR in univariate analysis (HR: 1.686, p = 0.332), but it was a significant variable in multivariate analysis (HR: 4.031, p = 0.029).
Table 6. Cox regression analysis for LV reverse remodeling.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age | 0.993 | 0.973–1.013 | 0.465 | 1.000 | 0.974–1.026 | 0.974 |
| Female sex | 1.054 | 0.567–1.959 | 0.869 | 0.859 | 0.380–1.944 | 0.716 |
| LBBB | 5.066 | 1.567–16.376 | 0.007 | 1.808 | 0.519–6.300 | 0.352 |
| ACE-I/ARBs | 1.686 | 0.586–4.849 | 0.332 | 4.031 | 1.155–14.075 | 0.029 |
| β-Blockers | 1.285 | 0.188–1.927 | 0.337 | 1.692 | 0.839–3.697 | 0.173 |
| Ivabradine | 0.579 | 0.290–1.159 | 0.123 | 1.328 | 0.147–1.733 | 0.107 |
| MRA | 1.273 | 0.705–2.297 | 0.423 | 1.607 | 0.869–3.196 | 0.136 |
| LVEDDI | 0.994 | 0.948–1.042 | 0.798 | 1.329 | 0.985–1.792 | 0.062 |
| LVESDI | 0.993 | 0.944–1.045 | 0.782 | 0.689 | 0.494–0.960 | 0.028 |
| LVEF | 0.972 | 0.928–1.018 | 0.227 | 1.058 | 0.983–1.240 | 0.178 |
| LV GLS | 1.411 | 1.240–1.605 | <0.001 | 1.474 | 1.170–1.856 | 0.001 |
| LA volume index | 0.918 | 0.880–0.957 | <0.001 | 0.966 | 0.922–1.012 | 0.143 |
| E/e′ ratio | 0.921 | 0.874–0.971 | 0.002 | 0.958 | 0.890–1.031 | 0.255 |
| Significant MR | 0.851 | 0.466–1.551 | 0.598 | |||
| RV FWLS | 1.002 | 0.959–1.048 | 0.919 | |||
ACE-I: angiotensin-converting enzyme-inhibitor, ARB: angiotensin II receptor blocker, CI: confidence interval, GLS: global longitudinal strain, HR: hazard ratio, LA: left atrium, LBBB: left bundle branch block, LV: left ventricular, LVEDDI: LV end-diastolic diameter index, LVEF: LV ejection fraction, LVESDI: LV end-systolic diameter index, MR: mitral regurgitation, MRA: mineralocorticoid receptor antagonist, RV: right ventricular, RV FWLS: RV free-wall longitudinal strain.
LVRR and adverse clinical events
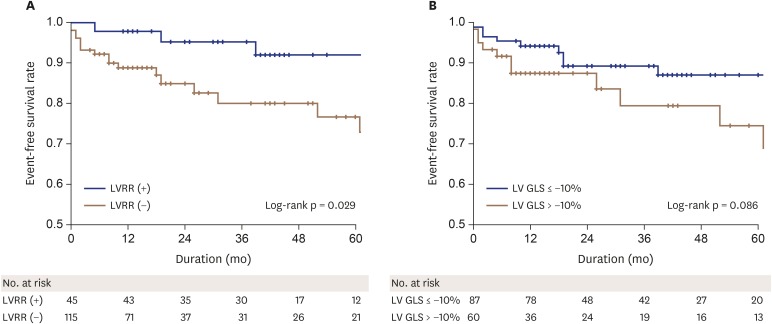
The survival curves of the study population classified according to LVRR are shown in Figure 4A. DCM patients with LVRR had a significantly better long-term prognosis with respect to the others (Log-rank test, p = 0.029).
Figure 4. Kaplan-Meier curves for event-free survival according to the patients with and without LVRR (A), and two groups divided by LV GLS of −10% (B). LV GLS: left ventricular global longitudinal strain, LVRR: left ventricular reverse remodeling.
By analyzing the ROC curve, LV GLS ≤ −10% was selected as the best cut-off value to discriminate between patients with and without LVRR with 98% sensitivity and 63% specificity (area under the curve = 0.854, p < 0.001). However, patients with LV GLS ≤ −10% was not lower in occurrence of adverse event compared to those with LV GLS > −10% (Log-rank test, p = 0.086) when we divided the subjects into two groups based on the cut-off value for LV GLS of −10% (Figure 4B).
DISCUSSION
In this study, we showed that LV GLS is an independent predictive factor for LVRR in DCM patients with sinus rhythm. Even though the LV of the patients with LVRR was more dilated and LVEF was similar compared with those without LVRR on baseline echocardiographic data, the absolute value of LV GLS was higher in patients who experienced a reverse LV remodeling. Additionally, we demonstrated a significant correlation between baseline LV GLS and follow-up LVEF in DCM patients with sinus rhythm. Recent studies have shown that tailored medical therapy can lead to a reverse LV remodeling and improve LVEF resulting in reduced heart failure morbidity and mortality.3),4),12),13) In the present study, LVRR occurred in 28% of DCM patients, and the long-term prognosis of patients with LVRR was better than those without LVRR. These results are similar to those reported by other investigators.
It was our principal finding that LVRR occurred more frequently in patients with better LV GLS values of LV GLS even with similar LVEF. LV global strain is an accurate and sensitive measure of myocardial deformation, allowing for angle-independent quantification of myocardial function in 2D based on the LV active shortening in the longitudinal, circumferential, and radial directions. which is more reproducible than EF and does not rely on geometrical assumptions.14) Among the LV global strains, LV GLS has been shown to be more reproducible and more useful clinically than circumferential strain (GCS) and radial strains.15),16) Furthermore, LV GLS reflects the contraction of the subendocardial layer, which is mainly longitudinally oriented. It is well established that the longitudinal cardiac fibers located in the subendocardium are the first to be affected by myocardial injury.17) The absolute value of LV GLS had significant negative correlation with subendocardial percentage area fibrosis in a rat model of hypertensive heart failure with preserved LVEF.18) A recent study demonstrated that LV GLS has a significant association with cardiovascular events in patients with acute heart failure, but GCS and twist value did not.19) DCM is associated with increase in collagen content, and the excessive extracellular matrix turnover may contribute to adverse LV remodeling and poor prognosis.20) The degree of myocardial fibrosis may be associated with reversibility in ventricular function in DCM. The gold standard in clinical practice for in-vivo tissue characterization is late gadolinium enhancement (LGE) detected by cardiac magnetic resonance (CMR); it has been shown to be a reliable method for detecting and quantifying myocardial fibrosis, a pathological expansion of the myocardial interstitium that is a common manifestation of most advanced cardiomyopathies with major prognostic implications.21),22),23) Therefore, LGE is associated with functional recovery of DCM, and the smaller the area of LGE is, the better result for reverse LV remodeling. Several studies have demonstrated an association between LV GLS and areas of LGE by CMR.24) Our study showed that LV GLS is an independent predictor for functional recovery of DCM, and this result supports previous findings that the greater the area of myocardial fibrosis, the lower the likelihood of recovery of LV function.
Similar to previous studies, good clinical outcomes were observed in patients with LVRR compared with those without LVRR in this study. In addition, we demonstrated that the optimal cut-off point of LV GLS for reverse LV remodeling was −10%. In our population, in which LV systolic function was already reduced, there was a relatively small difference in LV GLS between patients with and without LVRR. Although there is a highly significant correlation between LVEF and LV GLS, a recent study showed that the values of LV GLS in patients with acute heart failure could be distributed widely at any given LVEF level. The study's results demonstrated that every 1% decrease in LV GLS was associated with a 5% increased risk for death after adjustment for significant covariates.19) Therefore, we speculated that this subtle difference could affect the recovery of LV function in patients with heart failure. However, we failed to show differences in the event-free survival rate between groups based on the LV GLS cut-off value of −10% for LVRR. We believe that this outcome is likely a result of the events rate being relatively low in the subjects of this study, as described in the study limitation below.
The LA size of our DCM patients was relatively small compared with the population of other studies, and the number of patients with LVRR was smaller than that of those without LVRR. LA size and volume serve as good predictors for clinical outcomes in patients with heart failure, because they correlate well with the severity and chronicity of LV diastolic dysfunction.25) Furthermore, LA enlargement is associated with poor clinical outcomes in DCM patients.26),27) Because of the larger LA and LV sizes, more severe functional MR correlates with more AF. However, we excluded patients with AF, because AF per se may cause impairment of LV systolic dysfunction; AF is not only the most common arrhythmia in heart failure, but it also causes heart failure. Moreover, ventricular filling may be impaired because of loss of active atrial contraction and shortening of the diastolic time in patients with AF, resulting in a 15%–20% reduction of cardiac output.28),29),30) Additionally, paroxysmal tachycardia is frequent in AF patients and may lead to systolic impairment through a mechanism characterized by tachycardia-induced cardiomyopathy, resulting in a further decrease in cardiac output. Therefore, we cannot be certain regarding the initial cause in the relationship between AF and heart failure. Furthermore, it is difficult to accurately measure the value of strain by 2D speckle tracking because of the beat-to-beat variation.
Study limitations
The present study has several limitations. First, this was a single-center observational study of subjects who were registered in our institute and was performed using a single vendor-specific software. Therefore, our study group may not represent all patients with DCM, and our results may not apply to other equipment. Second, only 61.9% of patients enrolled in our study used beta-blockers, which was significantly lower than that reported in previous studies. At the time of enrollment in this study, it is possible that many of the patients with DCM had low blood pressure. And this reason forced them to use a combination of low dose beta blocker and ivabradine (25 patients without LVRR vs. 9 with LVRR, 21.7% vs. 20.0%) but often use ivabradine alone for patients who cannot tolerate beta blocker (19 patients without LVRR vs. 8 with LVRR, 16.5% vs. 17.8%). However, there was no significant difference between the two groups in the frequency of ivabradine alone or beta blocker combination (p = 0.785). Furthermore, Cox regression multivariate analysis revealed that ACE-i / ARB, rather than beta blockers or ivabradine, had a significant effect on the occurrence of LVRR in this study population. Third, the number of patients with cardiovascular events was relatively small, although the mean follow-up period of about 40 months was adequate. This is likely because we excluded subjects with AF and enrolled a relatively small number of patients with significant MR; AF and MR are considered important prognostic factors for clinical outcomes in DCM patients. We think this is one of the reasons why we failed to demonstrate the differences of event-free survival rate between groups based on the cut-off value of LV GLS for LVRR. Finally, because LV GLS values are highly correlated with LVEF, the difference in LV GLS values between patients who have already reduced LVEF, such as DCM patients, is not numerically large. We suggest that these results disprove that LV GLS is a more objective indicator than LVEF in patients with DCM; however, it would be inappropriate to predict the prognosis or a recovery of LV function in DCM patients based on only the LV GLS value. Nevertheless, in this study we showed that recovery of LV function occurred more frequently when DCM patients had better LV GLS values, even in patients with similar LVEF, which was significantly reduced at the time of diagnosis.
Conclusions
In our single-center study, we demonstrated that LV GLS was an independent predictor for LVRR, and the optimal cut-off point of LV GLS for LVRR was −10% in DCM patients with sinus rhythm. Patients with LVRR showed a significantly better long-term prognosis than those without LVRR. There was a significant correlation between baseline LV GLS and follow-up LVEF in this population. Therefore, we suggest LV GLS should be measured in DCM patients with sinus rhythm at the time of initial diagnosis. To validate the role of LV GLS in LVRR and prognosis in DCM patients with sinus rhythm, however, future studies must include long-term data compiled from a larger patient population.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 2.Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011;57:1468–1476. doi: 10.1016/j.jacc.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Merlo M, Caiffa T, Gobbo M, Adamo L, Sinagra G. Reverse remodeling in dilated cardiomyopathy: Insights and future perspectives. Int J Cardiol Heart Vasc. 2018;18:52–57. doi: 10.1016/j.ijcha.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cicoira M, Zanolla L, Latina L, et al. Frequency, prognosis and predictors of improvement of systolic left ventricular function in patients with ‘classical’ clinical diagnosis of idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2001;3:323–330. doi: 10.1016/s1388-9842(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 5.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196–1207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamo L, Perry A, Novak E, Makan M, Lindman BR, Mann DL. Abnormal global longitudinal strain predicts future deterioration of left ventricular function in heart failure patients with a recovered left ventricular ejection fraction. Circ Heart Fail. 2017;10:e003788. doi: 10.1161/CIRCHEARTFAILURE.116.003788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto K, Tanaka H, Onishi A, et al. Bi-ventricular contractile reserve offers an incremental prognostic value for patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:1213–1223. doi: 10.1093/ehjci/jev069. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Kang DH, Park SJ, Shin SH, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–1365. doi: 10.1161/CIRCULATIONAHA.118.037077. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson LW. Heart failure with better ejection fraction: a modern diagnosis. Circulation. 2014;129:2364–2367. doi: 10.1161/CIRCULATIONAHA.114.010194. [DOI] [PubMed] [Google Scholar]
- 13.Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail. 2011;17:527–532. doi: 10.1016/j.cardfail.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006;47:1313–1327. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 15.Mirea O, Pagourelias ED, Duchenne J, et al. Intervendor differences in the accuracy of detecting regional functional abnormalities: a report from the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging. 2018;11:25–34. doi: 10.1016/j.jcmg.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto T, Dulgheru R, Bernard A, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18:833–840. doi: 10.1093/ehjci/jex140. [DOI] [PubMed] [Google Scholar]
- 17.Yip G, Abraham T, Belohlavek M, Khandheria BK. Clinical applications of strain rate imaging. J Am Soc Echocardiogr. 2003;16:1334–1342. doi: 10.1067/j.echo.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Ishizu T, Seo Y, Kameda Y, et al. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension. 2014;63:500–506. doi: 10.1161/HYPERTENSIONAHA.113.02149. [DOI] [PubMed] [Google Scholar]
- 19.Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71:1947–1957. doi: 10.1016/j.jacc.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 20.Pauschinger M, Knopf D, Petschauer S, et al. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 1999;99:2750–2756. doi: 10.1161/01.cir.99.21.2750. [DOI] [PubMed] [Google Scholar]
- 21.Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2462–2508. doi: 10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stirrat J, White JA. The prognostic role of late gadolinium enhancement magnetic resonance imaging in patients with cardiomyopathy. Can J Cardiol. 2013;29:329–336. doi: 10.1016/j.cjca.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 23.White JA, Patel MR. The role of cardiovascular MRI in heart failure and the cardiomyopathies. Magn Reson Imaging Clin N Am. 2007;15:541–564. vi. doi: 10.1016/j.mric.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann R, Altiok E, Friedman Z, Becker M, Frick M. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography in comparison to late gadolinium enhancement cardiac magnetic resonance for analysis of myocardial fibrosis in severe aortic stenosis. Am J Cardiol. 2014;114:1083–1088. doi: 10.1016/j.amjcard.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 26.Dini FL, Cortigiani L, Baldini U, et al. Prognostic value of left atrial enlargement in patients with idiopathic dilated cardiomyopathy and ischemic cardiomyopathy. Am J Cardiol. 2002;89:518–523. doi: 10.1016/s0002-9149(01)02290-1. [DOI] [PubMed] [Google Scholar]
- 27.Rossi A, Cicoira M, Zanolla L, et al. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1425. doi: 10.1016/s0735-1097(02)02305-7. [DOI] [PubMed] [Google Scholar]
- 28.Lee HH, Lee MK, Lee WH, et al. Atrial fibrillation per se was a major determinant of global left ventricular longitudinal systolic strain. Medicine (Baltimore) 2016;95:e4038. doi: 10.1097/MD.0000000000004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umana E, Solares CA, Alpert MA. Tachycardia-induced cardiomyopathy. Am J Med. 2003;114:51–55. doi: 10.1016/s0002-9343(02)01472-9. [DOI] [PubMed] [Google Scholar]
- 30.Seo J, Jung IH, Park JH, et al. The prognostic value of 2D strain in assessment of the right ventricle in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2019;20:1043–1050. doi: 10.1093/ehjci/jez015. [DOI] [PubMed] [Google Scholar]