Abstract
Sarcoidosis is a multisystemic granulomatous disease of unknown etiology with various clinical presentations depending on the organs involved. Since cardiac sarcoidosis (CS) portends a higher risk of morbidity and mortality, early diagnosis and aggressive medical treatment are essential to improve the prognosis. 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET) has emerged as an important tool with practical advantages in assessing disease activity and monitoring the treatment response in patients with CS. While it has high sensitivity, it also has great variability in specificity, probably due to normal physiologic myocardial FDG uptake, which interferes with the evaluation and follow-up of CS using FDG-PET. This review details the technical aspects of FDG-PET imaging for evaluating and diagnosing CS, assessing disease activity, and monitoring therapeutic response.
Keywords: Cardiac sarcoidosis, Multimodality imaging, Myocardial positron emission tomography, Extended fasting, High-fat low-carbohydrate diet
INTRODUCTION
Sarcoidosis is a multisystemic disease of unknown etiology characterized by the formation of noncaseating granulomas in multiple organs. The clinical presentation of sarcoidosis depends on the organs involved. Cardiac sarcoidosis (CS) portends a worse prognosis than other types of sarcoidosis, accounting for substantial mortality and morbidity due to this disease.1),2) Although only 5% of patients with sarcoidosis show clinical manifestations of cardiac involvement, autopsy studies have shown that the prevalence of cardiac involvement among patients with sarcoidosis is at least 25%.2),3)
However, diagnosis of CS remains challenging. Because of the focal nature of this disease, the sensitivity of endomyocardial biopsy is only between 20% and 30%.4),5) Advanced cardiovascular imaging modalities, such as cardiac magnetic resonance (CMR) imaging and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET), have emerged as important tools to improve diagnostic certainty and management of CS. FDG-PET, which can detect active inflammatory lesions in which activated proinflammatory cells show higher metabolic rate and glucose utilization, is increasingly utilized to determine disease activity and guide treatment in patients with CS.2) However, normal physiologic myocardial FDG uptake often interferes with the evaluation of CS on FDG-PET. This review summarizes contemporary evidence regarding the use of FDG-PET for evaluation of CS and establishment of myocardial FDG-PET protocols with optimized diagnostic accuracy.
CURRENT CLINICAL GUIDELINES FOR DIAGNOSIS OF CARDIAC SARCOIDOSIS
The Japanese Ministry of Health and Welfare (JMHW) suggested diagnostic criteria for CS in 1993, which were modified in 2006 in the first consensus document for the diagnosis (Table 1).6) The guidelines require (1) histopathological findings of non-necrotizing granuloma in the myocardium, or (2) histopathological changes in organs other than the heart or clinical signs of cardiac manifestations, in addition to one or more of the 6 primary diagnostic criteria. In 2014, the Heart Rhythm Society (HRS) also provided an expert consensus statement, which was the first international guideline (Table 1).5) Similar to the JMHW guidelines, the HRS guidelines require (1) histological findings of non-caseating granuloma from myocardial tissue or (2) clinical diagnosis, which requires a histological diagnosis of extra-cardiac sarcoidosis and one or more of the 7 criteria for cardiac involvement (Table 1). The HRS guidelines acknowledge the inherent uncertainty related to diagnosing CS in the absence of myocardial tissue histology, and the clinical diagnosis criteria indicate that “it is probable that CS is present.” Nevertheless, the diagnostic yield of the current guidelines is unsatisfactory, mainly because these recommendations require histologic diagnosis from the myocardium or other organs as a mandatory criterion.5)6) The diagnosis of isolated CS is often impossible because of the low sensitivity of endomyocardial biopsy and lack of extracardiac involvement for histologic confirmation. Diagnostic imaging techniques have increasingly attracted attention for their potential to overcome such problems in the diagnosis of CS.
Table 1. Diagnostic criteria for cardiac sarcoidosis from guidelines of Japanese Ministry of Health and Welfare and Heart Rhythm Society expert consensus statement.
| Japanese Ministry of Health and Welfare recommendations6) | ||||
| (1) Patient group diagnosed based on histological findings | ||||
| Histopathological findings include non-necrotizing epithelioid granuloma in the myocardium, and the patient is found to exhibit histopathological changes in organs other than the heart or by clinical signs. | ||||
| (2) Patient group diagnosed based on clinical signs | ||||
| Histopathological findings do not include non-necrotizing epithelioid granuloma in the myocardium. Patients are diagnosed with cardiac sarcoidosis when they have histopathological changes in organs other than the heart or by clinical signs, together with the following conditions and one or more of six primary diagnostic criteria. | ||||
| 1. Two or more major criteria | ||||
| 2. One major and two or more minor criteria | ||||
| 1) Major criteria | ||||
| (a) Severe atrioventricular block | ||||
| (b) Ventricular septal thinning localized at the basal portion | ||||
| (c) Abnormal uptake of 67Ga in the heart on 67Ga scintigraphy | ||||
| (d) Left ventricular contraction failure (left ventricular ejection fraction less than 50%) | ||||
| 2) Minor criteria | ||||
| (a) Abnormal electrocardiogram: ventricular arrhythmia (ventricular tachycardia, multi-origin or frequent ventricular premature beats), right bundle branch block, axis deviation, or abnormal Q waves | ||||
| (b) Echocardiography: localized abnormal left ventricular wall motion, or morphological abnormalities (ventricular aneurysm and/or ventricular wall thickening) | ||||
| (c) Nuclear medicine techniques: abnormal blood flow on myocardial perfusion scintigraphy (thallium-201 chloride or technetium-99m methoxyisobutylisonitrile, technetium-99m tetrofosmin) | ||||
| (d) Abnormal imaging on delayed gadolinium-enhanced CMR | ||||
| (e) Endomyocardial biopsy: Moderate or more severe myocardial interstitial fibrosis and mononuclear cell infiltrates | ||||
| Heart Rhythm Society expert consensus recommendations5) | ||||
| (1) Histological diagnosis from myocardial tissue | ||||
| Cardiac sarcoidosis is diagnosed in the presence of non-caseating granuloma on histological examination of myocardial tissue with no alternative cause identified (including negative organismal stains if applicable). | ||||
| (2) Clinical diagnosis from invasive and non-Invasive studies | ||||
| It is probable* that there is cardiac sarcoidosis if: | ||||
| a) There is a histological diagnosis of extra-cardiac sarcoidosis | ||||
| and | ||||
| b) One or more of following is present | ||||
| • Steroid +/− immunosuppressant responsive cardiomyopathy or heart block | ||||
| • Unexplained reduced left ventricular ejection fraction (< 40%) | ||||
| • Unexplained sustained (spontaneous or induced) ventricular tachycardia | ||||
| • Mobitz type II 2nd degree heart block or 3rd degree heart block | ||||
| • Patchy uptake on dedicated cardiac PET (in a pattern consistent with cardiac sarcoidosis) | ||||
| • Late gadolinium enhancement on CMR (in a pattern consistent with cardiac sarcoidosis) | ||||
| • Positive gallium uptake (in a pattern consistent with cardiac sarcoidosis) | ||||
| and | ||||
| c) Other causes for the cardiac manifestation(s) have been reasonably excluded | ||||
| *In general, ‘probable involvement’ is considered adequate to establish a clinical diagnosis of cardiac sarcoidosis. | ||||
CMR: cardiac magnetic resonance, PET: positron emission tomography.
Although echocardiography plays an important role in screening for CS,7) patients with CS may not show abnormal findings on echocardiography, resulting in low sensitivity for the diagnosis of CS.8) Compared to echocardiography, CMR provides higher sensitivity in detecting CS, as it can detect late gadolinium enhancement (LGE). However, diagnosis of CS on CMR is based on the presence of myocardial fibrosis, which can be observed in all pathologic stages of CS (from active inflammatory phase to chronic fibrotic phase).9) Therefore, myocardial FDG-PET is increasingly utilized to determine the necessity for immunosuppressive therapy (e.g. corticosteroids) and to assess treatment response in patients with CS.2)
USE OF MYOCARDIAL FDG-PET FOR DIAGNOSIS OF CARDIAC SARCOIDOSIS
Metabolic activity and myocardial FDG-PET
Because sarcoidosis is an inflammatory disease characterized by aggregation of T-lymphocytes, macrophages, and noncaseating granulomas, the metabolic activity of inflamed myocardial tissue is important in determining treatment strategy. Previously, gallium-67 imaging was used to detect myocardial inflammation, and abnormal uptake of gallium-67 is a major diagnostic criteria in both the JMHW recommendations and the HRS expert consensus.5),6) However, the diagnostic sensitivity of gallium-67 is low due to its low image resolution. Myocardial FDG-PET, which has higher sensitivity and better accuracy than gallium-67 scintigraphy,10),11),12) has been increasingly utilized to evaluate CS. FDG, a glucose analog, is useful in detecting active inflammatory lesions, as activated proinflammatory macrophages show a higher metabolic rate and glucose utilization.2) Due to its sensitivity and accuracy in diagnosing CS, as well as its usefulness in guiding CS therapy, the importance of FDG-PET in patients with CS has been further emphasized.2),12)
Physiologic FDG uptake in the myocardium and detection of inflammatory lesions by PET
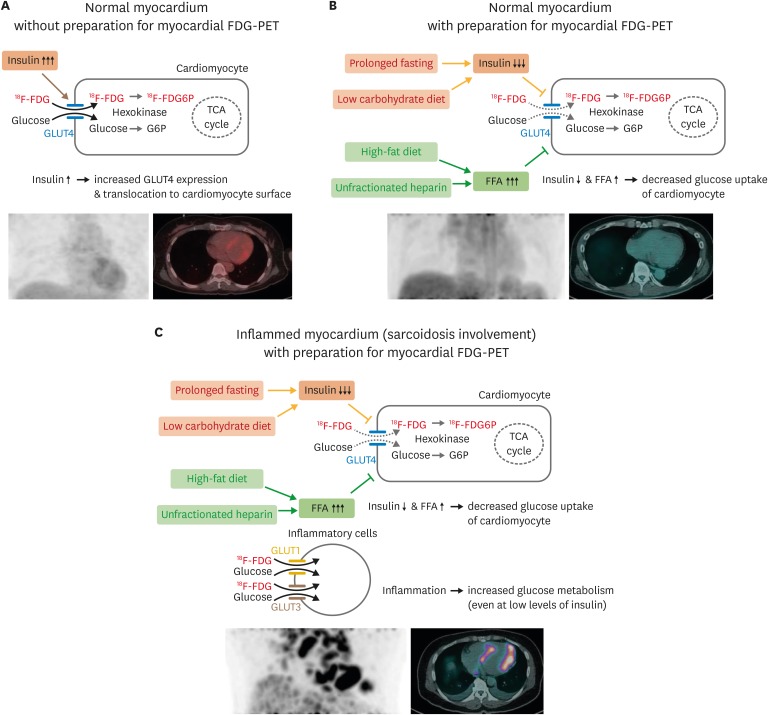
Although FDG-PET is highly useful for diagnosing CS, it should be noted that glucose is used as an energy source by normal cardiomyocytes as well as by inflammatory cells in CS-affected myocardium. However, the mode of action for glucose uptake differs between normal cardiomyocytes and inflammatory cells in CS-affected myocardium (Figure 1). In normal myocardium, more than 90% of fasting energy metabolism is due to fatty acids, and the remaining less than 10% of metabolism involves other substances, including glucose.13) Because the lipid bilayer of the plasma membrane is impermeable to glucose, glucose transport across the plasma membrane is mediated via glucose transporters (GLUT). GLUT4, the main isoform in cardiomyocytes, is located in intracellular membrane compartments and translocated to the cell surface in response to stimuli including increased workload, ischemia, catecholamines, and insulin.14),15) While increased blood glucose and insulin levels enhance glucose utilization in cardiomyocytes (Figure 1A), increased serum fatty acid levels during fasting inhibit glucose uptake via insulin-dependent GLUT4 (Figure 1B).16),17),18),19) In contrast, inflammatory cells, such as neutrophils, monocytes, and macrophages, express high levels of GLUT1 and GLUT3 in cell membranes (Figure 1C).20),21) Increased glucose metabolism is a hallmark of the immune cell activation involved in both innate (e.g., monocytes and macrophages) and adaptive (e.g., T and B cells) immunity.22),23),24) Because glucose metabolism of inflammatory cells is sensitive to proinflammatory cytokines and noxious signals, glucose uptake is enhanced in activated inflammatory cells even in conditions in which blood glucose and insulin levels are not increased.
Figure 1. Physiologic myocardium FDG uptake and detection of inflammatory lesions on FDG-PET. Schematic figures detecting physiologic glucose uptake in normal myocardium (A) and suppression of glucose uptake by dietary preparations, prolonged fasting, and unfractionated heparin administration (B). (C) Appropriate preparation for myocardial FDG-PET can suppress physiologic glucose uptake in cardiomyocytes while maintaining glucose uptake of inflammatory cells in the myocardium with sarcoidosis involvement. FDG: fluorodeoxyglucose, FDG6P: fluorodeoxyglucose-6-phosphate, FFA: free fatty acid, G6P: glucose-6-phosphate, GLUT: glucose transporter, PET: positron emission tomography, TCA: tricarboxylic acid.
Because glucose uptake mechanisms differ in cardiomyocytes and inflammatory cells, myocardial FDG-PET can be utilized in diagnosis of cardiac inflammatory diseases, such as CS.12),25),26) Of note, it is important to differentiate pathologic FDG uptake of affected myocardium from physiologic FDG uptake of normal myocardium. Numerous studies have aimed to differentiate cardiac involvement of sarcoidosis using heterogeneous myocardial uptake patterns of CS. For example, Tahara et al.27) compared FDG-PET images between 24 patients with CS and 8 age-matched patients with dilated cardiomyopathy. They found that FDG uptake is distinctly heterogeneous in patients with CS, and patients with CS had a significantly higher coefficient of variation (standard deviation divided by the mean) for myocardial FDG uptake than patients with dilated cardiomyopathy. The authors also reported that the value of the coefficient of variation in myocardial FDG uptake decreased after corticosteroid therapy, suggesting that heterogeneity in myocardial FDG uptake indicates the disease activity of CS. Sperry et al.28) retrospectively analyzed 203 patients who underwent FDG PET imaging to evaluate CS and showed that heterogeneity in FDG uptake assessed by coefficient of variation provides an incremental prognostic advantage. However, the degree of heterogeneity in FDG uptake is not intuitive and is less suitable for quantitative assessments of treatment response than other quantitative measures, such as maximal standardized uptake value (SUVmax) or volume of FDG uptake.29),30) Therefore, effective suppression of physiologic glucose uptake in normal myocardium is a key factor in the evaluation of CS using FDG-PET.
EFFORTS TO IMPROVE DIAGNOSTIC YIELD OF MYOCARDIAL FDG-PET
Physiologic FDG uptake of the normal myocardium is considered the most important issue contributing to low and variable specificity of myocardial FDG-PET for CS evaluation.12) Suppression of physiologic myocardial FDG uptake is crucial for reliable detection of abnormal FDG uptake in the affected myocardium. To date, the following three methods have been proposed to minimize physiologic FDG uptake of normal myocardium (via GLUT4) and better visualize FDG uptake in the affected myocardium (via GLUT1/GLUT3 in inflammatory cells): (1) prolonged fasting, (2) administration of unfractionated heparin, and (3) dietary modifications.
Prolonged fasting
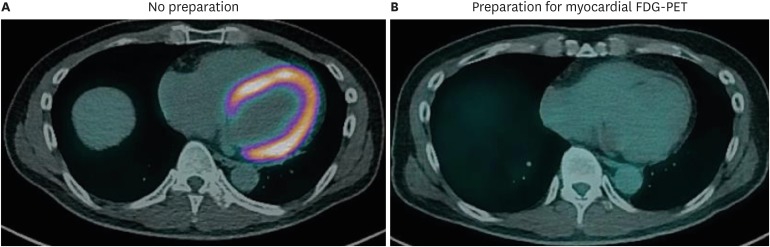
Typical FDG-PET imaging in patients with known or suspected malignancy requires at least 4 hours of fasting to ensure low serum levels of glucose and insulin, as elevated glucose or insulin is directly responsible for glucose uptake by non-tumor cells and low diagnostic yield.31),32) In patients with suspected CS, the contrast in FDG uptake between affected and normal myocardium must be distinct to allow identification of inflammatory lesions in the myocardium. Thus, prolonged fasting prior to imaging is recommended for patients with suspected CS.9),12),33) While many previous studies utilized 12 hours of fasting,10),12),27),34),35),36) more recent studies suggest that fasting for more than 18 hours further suppresses physiologic myocardial glucose uptake and improves the specificity of FDG-PET for CS. Langah et al.37) assessed the diagnostic accuracy of an 18-hour fasting protocol in 76 patients with suspected CS and reported sensitivity of 85% and specificity of 90%.37) They also noted that the FDG myocardial-to-blood pool ratio decreased more significantly after 18-hour fasting than after shorter fasting durations. However, imaging after prolonged fasting remains limited due to variation in suppression of glucose utilization by normal cardiomyocytes (Figure 2).
Figure 2. Suppression of physiologic glucose uptake in normal myocardium. FDG-PET/CT images of patient with suspected cardiac sarcoidosis. (A) This patient underwent initial PET/CT scan with 18 hours of fasting, without a dietary preparation, and showed diffusely increased metabolism in the whole myocardium. (B) During the same hospitalization, this patient underwent PET/CT scanning again after a low-carbohydrate high-fat diet and 18 hours of fasting. PET/CT image after appropriate preparation showed no abnormal uptake in the myocardium, as it was successfully suppressed by the dietary preparation. CT: computed tomography, FDG: fluorodeoxyglucose: PET: positron emission tomography.
Heparin
The use of unfractionated heparin enhances plasma lipolytic activity, and increased lipoprotein lipase and hepatic lipase activity leads to increased plasma free fatty acids.38),39) The consequent elevation in serum free fatty acid inhibits glucose uptake via GLUT4.17) Based on these findings, a heparin pre-administration protocol has been suggested for diagnosing CS by FDG-PET: unfractionated heparin is administered 15 minutes before FDG administration at a dose of 50 IU/kg.35),36) However, it should also be noted that heparin pre-administration effectively suppresses physiologic myocardial FDG uptake in only some patients, and the relationship between heparin dose and degree of physiologic myocardial FDG uptake has not been established.12) Although pre-administration of unfractionated heparin prior to FDG-PET imaging is theoretically appropriate, further investigations are required to establish an appropriate FDG-PET protocol for the evaluation of CS.
Low-carbohydrate, high-fat diet
In addition to prolonged fasting and pre-administration of unfractionated heparin, dietary modifications have been suggested to suppress physiologic myocardial FDG uptake. As FDG uptake in normal myocardium is dependent on serum levels of glucose and free fatty acid, a low-carbohydrate and high-fat diet prior to FDG-PET imaging would theoretically improve diagnostic yield. Consistent with this, Lum et al.40) reported that clinically significant myocardial FDG uptake on FDG-PET was more commonly observed in patients who consumed carbohydrates compared to those who did not consume carbohydrates prior to scanning. A small randomized trial by Cheng et al.41) further clarified the effect of dietary modifications: compared to patients with an unrestricted diet, a low-carbohydrate diet resulted in significant suppression of physiologic myocardial FDG uptake. Although no well-established study has reported improved diagnostic accuracy with a low-carbohydrate diet, many institutions advocate carbohydrate content of less than 5 g per meal prior to scanning.41),42),43)
Furthermore, adding a high-fat diet to a low-carbohydrate diet 3-6 hours prior to FDG-PET scanning has been suggested to significantly reduce FDG uptake in the entire myocardium.43),44) Consistently, a prospective study by Harisankar et al.45) demonstrated consistent and significant suppression of myocardial FDG uptake in most patients who maintained a low-carbohydrate high-fat protein-permitted diet the night before and 4 hours prior to scanning. However, in a small randomized trial conducted by Cheng et al.41), the addition of a high-fat diet 1 hour prior to FDG injection did not result in greater reduction of physiologic myocardial FDG uptake compared with a low-carbohydrate diet alone. In a similar randomized trial by Demeure et al.46), volunteers who consumed a low-carbohydrate high-fat diet followed by 12 hours of fasting demonstrated effective suppression of physiologic myocardial FDG uptake, whereas those who consumed additional free fatty acids 1 hour prior to FDG injection failed to show additional suppression of myocardial FDG uptake over that achieved in those who did not undergo this additional preparation. Considering these findings, a low-carbohydrate high-fat diet followed by prolonged fasting may be expected to effectively suppress the physiologic glucose uptake of normal myocardium and improve specificity for diagnosing CS (Figure 2).
Specialized FDG-PET protocols for evaluation of CS
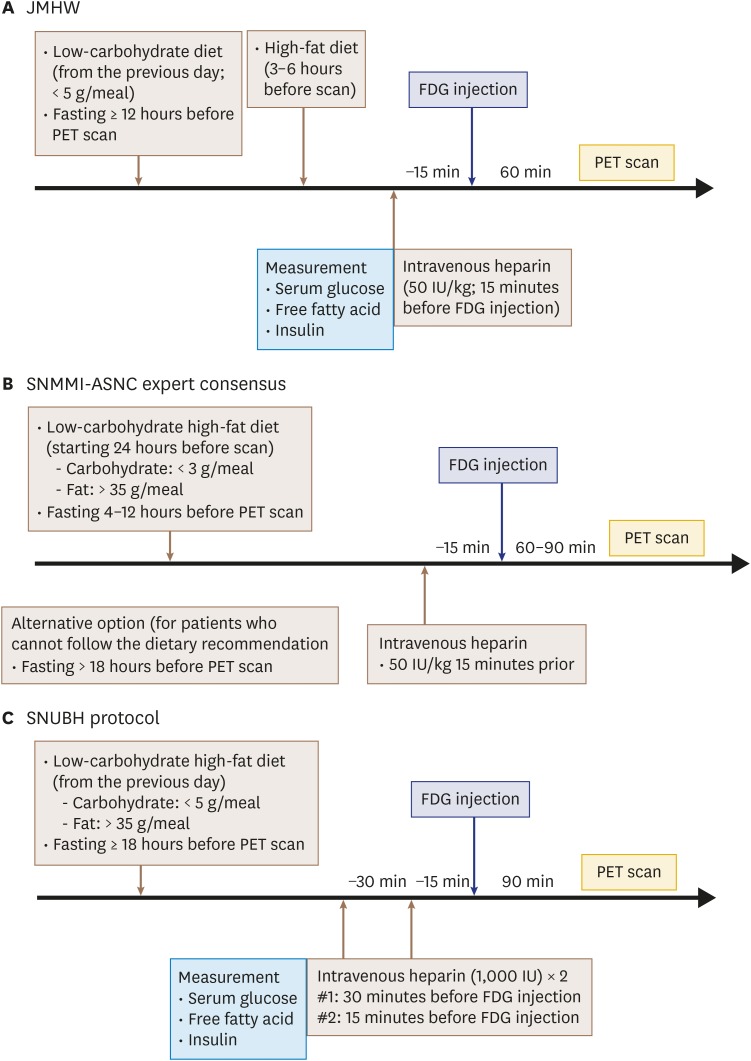
Although the clinical usefulness of FDG-PET for patients with known or suspected CS is well-known, a detailed FDG-PET protocol has not been established. The various protocols utilized by previous studies differ from each other in terms of composition and calorie content of the dietary preparation, duration of fasting, and dosage and timing of heparin pre-administration. For example, the Japanese Society of Nuclear Cardiology Recommendations suggested that patients have a low-carbohydrate diet the night before FDG-PET (< 5 g) followed by at least 12 hours of fasting and a high-fat diet 3-6 hours before the FDG injection, but also recognized that 18 hours of fasting would improve the specificity of CS diagnosis (Figure 3A).12) These recommendations did not include heparin pre-administration. The Society of Nuclear Medicine and Molecular Imaging (SNMMI) and American Society of Nuclear Cardiology (ASNC) expert consensus statement of 2017 recommended starting a low-carbohydrate (< 3 g/meal), high-fat (> 35 g/meal) diet about 24 hours prior to scanning followed by 4–12 hours of fasting (> 18 hours for patients who cannot follow the dietary recommendations) (Figure 3B).33),47) Additionally, the Joint SNMMI-ASNC expert consensus statement suggested the use of unfractionated heparin (50 IU/kg) 15 minutes prior to FDG administration.
Figure 3. Suggested protocols for diagnosing cardiac sarcoidosis. Suggested myocardial FDG-PET protocols for diagnosing cardiac sarcoidosis provided by the Japanese Ministry of Health and Welfare (JMHW) (A), and the Joint Society of Nuclear Medicine and Molecular Imaging (SNMMI) and American Society of Nuclear Cardiology (ASNC) expert consensus statement (B) are summarized. (C) Protocol used by Seoul National University Bundang Hospital (SNUBH) is also shown. FDG: fluorodeoxyglucose, PET: positron emission tomography.
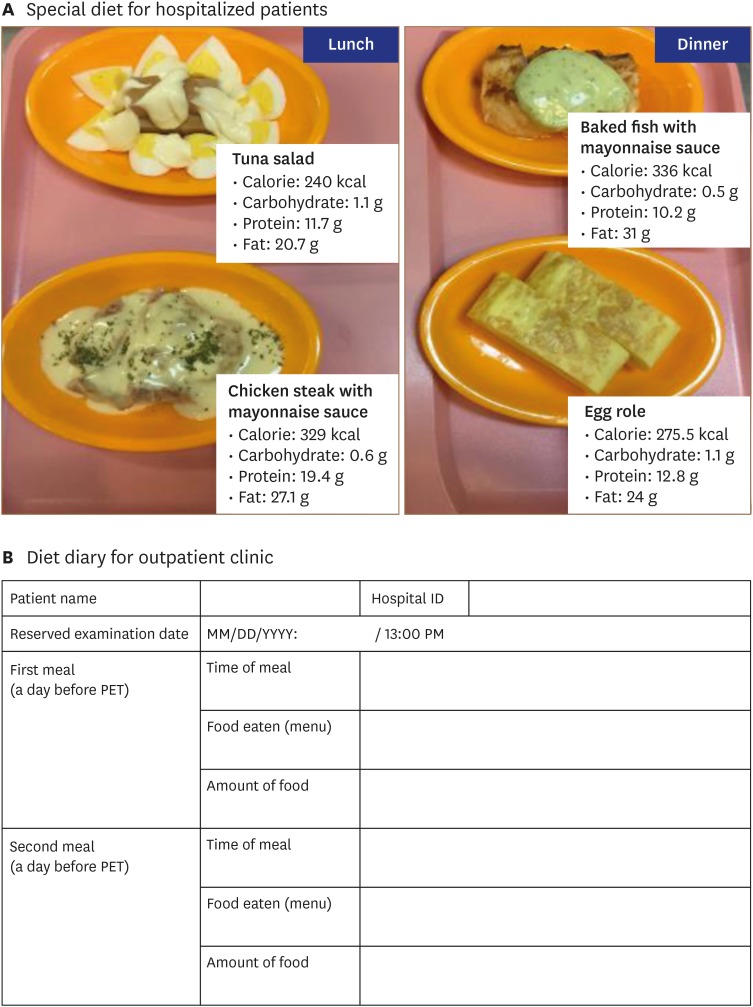
Our institution has adopted a protocol with a low-carbohydrate, high-fat diet followed by at least 18 hours of fasting in addition to intravenous pre-administration of unfractionated heparin prior to FDG injection (Figure 3C). Specifically, hospitalized patients receive a low-carbohydrate (< 5 g/meal), high-fat (> 35 g/meal) lunch and dinner developed by specialists from the nutritional support team (Figure 4A). For those in outpatient clinics, a diet modification manual explaining food types and caloric content is provided, and patients are requested to submit a detailed diet diary (Figure 4B). For patient convenience, myocardial FDG-PET scans are usually scheduled around 1 PM for hospitalized patients. Patients undergo 18 hours of fasting starting at 6 PM immediately after consuming a low-carbohydrate high-fat dinner. Subsequently, patients are given two 1,000 IU doses of unfractionated heparin at 30 minutes and 15 minutes before FDG administration. Although there is limited head-to-head evidence comparing protocols, it is assumed that stricter patient preparation results in better diagnostic performance of myocardial FDG-PET scanning. Indeed, better specificity is easily observed in real-world practice in patients who consume a low-carbohydrate high-fat diet prior to scanning (Figure 2).
Figure 4. Dietary preparations for patients with suspected cardiac sarcoidosis undergoing myocardial PET. (A) Special diets for hospitalized patients with suspected or known cardiac sarcoidosis undergoing myocardial PET. The day before FDG-PET scan, patients are provided lunch (left) and dinner (right) that are low in carbohydrates (< 5 g/meal) and high in fat (> 35 g/meal), as developed by physicians and a nutritional support team. After consuming dinner at 18:00 PM, patients fast for 18 hours, and PET scan is performed at 13:00 PM. (B) Sample diet diary for patient at out-patient clinic. PET: positron emission tomography.
ASSESSMENT OF DISEASE ACTIVITY AND MONITORING TREATMENT RESPONSE
Corticosteroids are the mainstay of CS treatment.2),6),48) The main goal of immunosuppressive therapy is to prevent progression of myocardial inflammation. Suppression of myocardial inflammatory activity can be detected by FDG-PET in a sensitive and quantitative manner. It is well-established that the amount of FDG uptake is associated with disease activity, as well as with prognosis, in patients with CS.49) Additionally, many previous studies have shown a reduction or disappearance of FDG uptake in patients with CS after corticosteroid treatment.27),50),51),52),53) Furthermore, decreased FDG uptake is associated with improved left ventricular ejection fraction and clinical status.53),54) However, in a study by Shelke et al.55), increased intensity and area of FDG uptake on follow-up FDG-PET were noted among non-responders to corticosteroid treatment. Based on these findings, follow-up FDG-PET has been suggested to assess treatment response in patients with CS, especially patients undergoing corticosteroid treatment.2) Repetitive FDG-PET scanning in patients with CS during treatment may enable appropriate assessment of treatment response and early detection of unresponsive patients who require second-line therapy. However, there are no standardized schedules for repeat FDG-PET scans, and it is unclear whether a PET-guided decision strategy improves prognosis. Further studies are required to establish the appropriate treatment algorithm, including timing of follow-up imaging scans.
REMAINING ISSUES: STANDARDIZED MEASUREMENT OF METABOLIC ACTIVITY
Metabolic activity and free fatty acid levels
Even with the establishment of a standardized patient preparation protocol, it is not easy to ensure complete suppression of physiologic FDG uptake by the normal myocardium. Because contrast in FDG uptake between normal versus inflamed myocardium is critical for diagnosing CS, it is helpful to monitor the degree of myocardial FDG uptake suppression. Suppression of physiologic FDG uptake in normal myocardium is dependent on increasing free fatty acid level. A randomized trial by Demeure et al.46) compared several protocols for the optimization of FDG-PET, and serum free fatty acid levels were higher in volunteers with better suppression of physiologic FDG uptake.
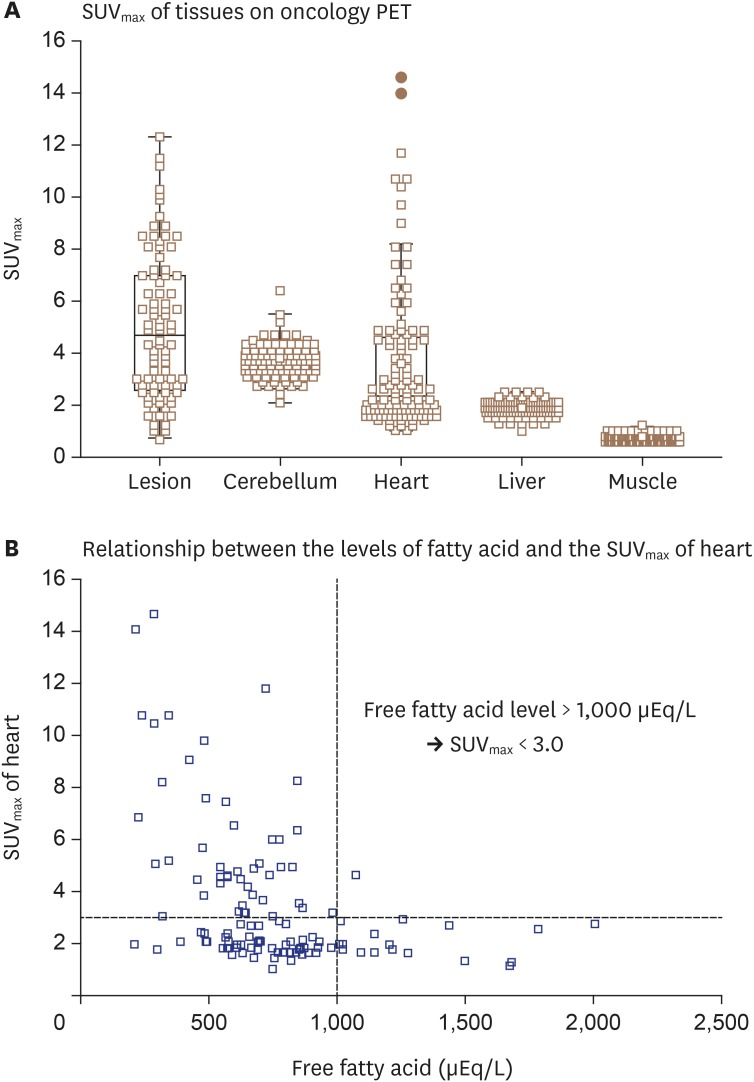
There was also an inverse correlation between serum free fatty acid level and SUVmax, suggesting that serum free fatty acid level predicts the degree of myocardial FDG uptake suppression. Furthermore, our group assessed the relationship between serum free fatty acid level and heart SUVmax in 147 patients who underwent FDG-PET to evaluate malignancy (Figure 5). Because FDG-PET scanning was performed according to the oncology protocol, patients were only required to fast for at least 4 hours without dietary modification or heparin pre-administration. As shown in Figure 5A, the heart and brain demonstrated higher SUVmax values compared to the liver or muscle. Although the heart demonstrated the widest variability in SUVmax values, serum free fatty acid level at the time of FDG-PET scanning showed an inverse correlation with heart SUVmax (Figure 5B). According to preliminary data from oncology patients with normal myocardium, physiologic myocardial FDG uptake was sufficiently suppressed at a serum free fatty acid level of 1,000 μEq/L. This result indicates that patients with suspected CS should have serum fatty acid > 1,000 μEq/L at the time of myocardial FDG-PET, and increased myocardial FDG uptake in patients with a serum free fatty acid level > 1,000 μEq/L at the time of FDG-PET can be regarded as pathologic FDG uptake. Thus, measurement of serum free fatty acid at the time of FDG-PET is expected to be useful to monitor the degree of myocardial FDG suppression and interpret results. Of course, further studies are required to establish an optimal cutoff value.
Figure 5. Myocardial FDG uptake among 147 patients who underwent oncology PET. Serum free fatty acid level and heart SUVmax were measured in 147 patients who underwent FDG-PET for diagnosis of malignancy. (A) On FDG-PET without preparation for sarcoidosis evaluation, the heart and brain demonstrate higher SUVmax values than liver or muscle. (B) Serum free fatty acid level at time of FDG-PET shows inverse correlation with heart SUVmax, suggesting that serum fatty acid level > 1,000 μEq/L is needed at the time of myocardial FDG-PET imaging to suppress physiologic uptake of glucose in normal myocardium. FDG: fluorodeoxyglucose, PET: positron emission tomography, SUVmax: maximal standardized uptake value.
Hybrid CMR and FDG-PET
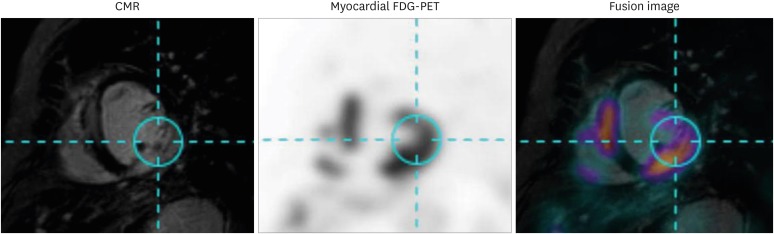
For evaluation of CS, CMR with an LGE protocol can reveal the development of fibrosis, whereas myocardial PET can detect increased metabolic activity of glucose uptake in affected myocardium. Because these two diagnostic modalities characterize different features of CS, hybrid imaging using CMR and myocardial PET is useful for diagnosing CS.56) Combined use of CMR and myocardial PET has distinct strengths, as it co-localizes lesions with increased metabolic activity while also detecting lesions with irreversible scar changes (i.e. presence of LGE without increased FDG uptake) and early forms of myocardial inflammation (i.e. increased FDG uptake in absence of LGE). Fusion images of CMR and myocardial PET can be simultaneously obtained using a hybrid system, but can also be reconstructed from separate images using post-processing software, as shown in Figure 6. Thus, combined images from CMR and myocardial PET may further contribute to a higher diagnostic yield for patients suspected to have CS.
Figure 6. Representative case of hybrid CMR-PET imaging. CMR and myocardial FDG-PET images of patient with cardiac sarcoidosis are shown. Images of these two modalities were fused using dedicated software and show co-localization of late gadolinium enhancement on CMR and increased FDG uptake on myocardial FDG-PET. CMR: cardiac magnetic resonance, FDG: fluorodeoxyglucose, PET: positron emission tomography.
CONCLUSION
Myocardial FDG-PET is a useful noninvasive test for the diagnosis of CS, as well as for monitoring treatment response. Because glucose is an energy source of cardiomyocytes, myocardial PET imaging requires specific preparation protocols to suppress physiologic FDG uptake of the normal myocardium and better detect the increased metabolic activity of inflammatory cells in the affected myocardium. Prolonged fasting, low-carbohydrate and high-fat dietary preparations, and pre-administration of unfractionated heparin can improve the diagnostic yield of myocardial PET in patients with suspected CS. However, standardization of these protocols in terms of fasting duration, appropriate foods and caloric contents of dietary preparations, heparin dose, and measurement of serum free fatty acid is required.
Acknowledgments
We thank Hae Sun Yeom, a nutritional specialist, for her contribution to developing the special diet protocol for appropriate preparation of myocardial FDG-PET.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Porter GH. Sarcoid heart disease. N Engl J Med. 1960;263:1350–1357. doi: 10.1056/NEJM196012292632608. [DOI] [PubMed] [Google Scholar]
- 2.Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac Sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 3.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MK, Gilotra NA, Harrington C, et al. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000-2009. Circ Heart Fail. 2013;6:676–684. doi: 10.1161/CIRCHEARTFAILURE.112.000087. [DOI] [PubMed] [Google Scholar]
- 5.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 6.JCS Joint Working Group. Guidelines for diagnosis and treatment of myocarditis (JCS 2009): digest version. Circ J. 2011;75:734–743. doi: 10.1253/circj.cj-88-0008. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal NR, Snipelisky D, Young PM, Gersh BJ, Cooper LT, Chareonthaitawee P. Advances in imaging for diagnosis and management of cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2015;16:949–958. doi: 10.1093/ehjci/jev142. [DOI] [PubMed] [Google Scholar]
- 8.Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9:e000867. doi: 10.1161/CIRCIMAGING.113.000867. [DOI] [PubMed] [Google Scholar]
- 9.Ohira H, Yoshinaga K, Manabe O, et al. Clinical application of 18F-fluorodeoxyglucose PET and LGE CMR in cardiac sarcoidosis. Ann Nucl Cardiol. 2017;3:125–130. [Google Scholar]
- 10.Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–1998. [PubMed] [Google Scholar]
- 11.Nishiyama Y, Yamamoto Y, Fukunaga K, et al. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006;47:1571–1576. [PubMed] [Google Scholar]
- 12.Ishida Y, Yoshinaga K, Miyagawa M, et al. Recommendations for (18)F-fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations. Ann Nucl Med. 2014;28:393–403. doi: 10.1007/s12149-014-0806-0. [DOI] [PubMed] [Google Scholar]
- 13.Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. 1987;79:359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santalucía T, Camps M, Castelló A, et al. Developmental regulation of GLUT-1 (erythroid/Hep G2) and GLUT-4 (muscle/fat) glucose transporter expression in rat heart, skeletal muscle, and brown adipose tissue. Endocrinology. 1992;130:837–846. doi: 10.1210/endo.130.2.1370797. [DOI] [PubMed] [Google Scholar]
- 15.Montessuit C, Lerch R. Regulation and dysregulation of glucose transport in cardiomyocytes. Biochim Biophys Acta. 2013;1833:848–856. doi: 10.1016/j.bbamcr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 17.Nuutila P, Koivisto VA, Knuuti J, et al. Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo. J Clin Invest. 1992;89:1767–1774. doi: 10.1172/JCI115780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota K, Kubota R, Yamada S, Tada M, Takahashi T, Iwata R. Re-evaluation of myocardial FDG uptake in hyperglycemia. J Nucl Med. 1996;37:1713–1717. [PubMed] [Google Scholar]
- 19.Yamada K, Endo S, Fukuda H, et al. Experimental studies on myocardial glucose metabolism of rats with 18F-2-fluoro-2-deoxy-D-glucose. Eur J Nucl Med. 1985;10:341–345. doi: 10.1007/BF00251308. [DOI] [PubMed] [Google Scholar]
- 20.Miyagawa M, Yokoyama R, Nishiyama Y, Ogimoto A, Higaki J, Mochizuki T. Positron emission tomography-computed tomography for imaging of inflammatory cardiovascular diseases. Circ J. 2014;78:1302–1310. doi: 10.1253/circj.cj-14-0250. [DOI] [PubMed] [Google Scholar]
- 21.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maratou E, Dimitriadis G, Kollias A, et al. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest. 2007;37:282–290. doi: 10.1111/j.1365-2362.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 24.Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–E253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–248. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama R, Miyagawa M, Okayama H, et al. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int J Cardiol. 2015;195:180–187. doi: 10.1016/j.ijcard.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 27.Tahara N, Tahara A, Nitta Y, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010;3:1219–1228. doi: 10.1016/j.jcmg.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Sperry BW, Tamarappoo BK, Oldan JD, et al. Prognostic impact of extent, severity, and heterogeneity of abnormalities on (18)F-FDG PET scans for suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018;11:336–345. doi: 10.1016/j.jcmg.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Ahmadian A, Brogan A, Berman J, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014;21:925–939. doi: 10.1007/s12350-014-9901-9. [DOI] [PubMed] [Google Scholar]
- 30.Blankstein R, Agarwal V. Maximizing the prognostic value of cardiac PET in patients with suspected cardiac sarcoidosis: a simple semiquantitative score may help. JACC Cardiovasc Imaging. 2018;11:346–348. doi: 10.1016/j.jcmg.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer--a PET study. J Nucl Med. 1993;34:1–6. [PubMed] [Google Scholar]
- 33.Chareonthaitawee P, Beanlands RS, Chen W, et al. Joint SNMMI-ASNC expert consensus document on the role of (18)F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Med. 2017;58:1341–1353. doi: 10.2967/jnumed.117.196287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morooka M, Moroi M, Uno K, et al. Long fasting is effective in inhibiting physiological myocardial 18F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res. 2014;4:1. doi: 10.1186/2191-219X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–1543. doi: 10.1093/eurheartj/ehi180. [DOI] [PubMed] [Google Scholar]
- 36.Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. doi: 10.1007/s00259-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 37.Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16:801–810. doi: 10.1007/s12350-009-9110-0. [DOI] [PubMed] [Google Scholar]
- 38.Persson E, Nordenström J, Hagenfeldt L, Nilsson-Ehle P. Plasma lipolytic activity after subcutaneous administration of heparin and a low molecular weight heparin fragment. Thromb Res. 1987;46:697–704. doi: 10.1016/0049-3848(87)90271-4. [DOI] [PubMed] [Google Scholar]
- 39.Persson E. Lipoprotein lipase, hepatic lipase and plasma lipolytic activity. Effects of heparin and a low molecular weight heparin fragment (Fragmin) Acta Med Scand Suppl. 1988;724:1–56. [PubMed] [Google Scholar]
- 40.Lum DP, Wandell S, Ko J, Coel MN. Reduction of myocardial 2-deoxy-2-[18F]fluoro-D-glucose uptake artifacts in positron emission tomography using dietary carbohydrate restriction. Mol Imaging Biol. 2002;4:232–237. doi: 10.1016/s1095-0397(01)00062-0. [DOI] [PubMed] [Google Scholar]
- 41.Cheng VY, Slomka PJ, Ahlen M, Thomson LE, Waxman AD, Berman DS. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: A randomized controlled trial. J Nucl Cardiol. 2010;17:286–291. doi: 10.1007/s12350-009-9179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohira H, Tsujino I, Yoshinaga K. ¹⁸F-Fluoro-2-deoxyglucose positron emission tomography in cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2011;38:1773–1783. doi: 10.1007/s00259-011-1832-y. [DOI] [PubMed] [Google Scholar]
- 43.Wykrzykowska J, Lehman S, Williams G, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009;50:563–568. doi: 10.2967/jnumed.108.055616. [DOI] [PubMed] [Google Scholar]
- 44.Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR Am J Roentgenol. 2008;190:W151-6. doi: 10.2214/AJR.07.2409. [DOI] [PubMed] [Google Scholar]
- 45.Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011;18:926–936. doi: 10.1007/s12350-011-9422-8. [DOI] [PubMed] [Google Scholar]
- 46.Demeure F, Hanin FX, Bol A, et al. A randomized trial on the optimization of 18F-FDG myocardial uptake suppression: implications for vulnerable coronary plaque imaging. J Nucl Med. 2014;55:1629–1635. doi: 10.2967/jnumed.114.138594. [DOI] [PubMed] [Google Scholar]
- 47.Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 48.Hamzeh N, Steckman DA, Sauer WH, Judson MA. Pathophysiology and clinical management of cardiac sarcoidosis. Nat Rev Cardiol. 2015;12:278–288. doi: 10.1038/nrcardio.2015.22. [DOI] [PubMed] [Google Scholar]
- 49.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–1036. [PubMed] [Google Scholar]
- 51.Tadamura E, Yamamuro M, Kubo S, et al. Images in cardiovascular medicine. Multimodality imaging of cardiac sarcoidosis before and after steroid therapy. Circulation. 2006;113:e771–3. doi: 10.1161/CIRCULATIONAHA.105.594200. [DOI] [PubMed] [Google Scholar]
- 52.Ahmadian A, Pawar S, Govender P, Berman J, Ruberg FL, Miller EJ. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol. 2017;24:413–424. doi: 10.1007/s12350-016-0490-7. [DOI] [PubMed] [Google Scholar]
- 53.Lee PI, Cheng G, Alavi A. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol. 2017;24:19–28. doi: 10.1007/s12350-016-0682-1. [DOI] [PubMed] [Google Scholar]
- 54.Osborne MT, Hulten EA, Singh A, et al. Reduction in ¹⁸F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–174. doi: 10.1007/s12350-013-9828-6. [DOI] [PubMed] [Google Scholar]
- 55.Shelke AB, Aurangabadkar HU, Bradfield JS, Ali Z, Kumar KS, Narasimhan C. Serial FDG-PET scans help to identify steroid resistance in cardiac sarcoidosis. Int J Cardiol. 2017;228:717–722. doi: 10.1016/j.ijcard.2016.11.142. [DOI] [PubMed] [Google Scholar]
- 56.Dweck MR, Abgral R, Trivieri MG, et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018;11:94–107. doi: 10.1016/j.jcmg.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]