Highlights
-
•
Coronavirus replication induces ER stress and the unfolded protein response (UPR).
-
•
Several coronavirus proteins modulate coronavirus-induced UPR.
-
•
During coronavirus infection, PERK is pro-apoptotic and IRE1 is anti-apoptotic.
-
•
Coronavirus-induced UPR is involved in the regulation of innate immune response.
-
•
UPR cross-talks with other signaling pathways, such as the MAP kinase pathway.
Keywords: Coronavirus, ER stress, Unfolded protein response, Apoptosis, Pro-inflammatory cytokines, Innate immune response
Abstract
Coronavirus replication is structurally and functionally associated with the endoplasmic reticulum (ER), a major site of protein synthesis, folding, modification and sorting in the eukaryotic cells. Disturbance of ER homeostasis may occur under various physiological or pathological conditions. In response to the ER stress, signaling pathways of the unfolded protein response (UPR) are activated. UPR is mediated by three ER transmembrane sensors, namely the PKR-like ER protein kinase (PERK), the inositol-requiring protein 1 (IRE1) and the activating transcriptional factor 6 (ATF6). UPR facilitates adaptation to ER stress by reversible translation attenuation, enhancement of ER protein folding capacity and activation of ER-associated degradation (ERAD). In cells under prolonged and irremediable ER stress, UPR can also trigger apoptotic cell death. Accumulating evidence has shown that coronavirus infection causes ER stress and induces UPR in the infected cells. UPR is closely associated with a number of major signaling pathways, including autophagy, apoptosis, the mitogen-activated protein (MAP) kinase pathways, innate immunity and pro-inflammatory response. Therefore, studies on the UPR are pivotal in elucidating the complicated issue of coronavirus-host interaction. In this paper, we present the up-to-date knowledge on coronavirus-induced UPR and discuss its potential involvement in regulation of innate immunity and apoptosis.
1. Introduction
In eukaryotic cells, ER is the major site for the synthesis, folding, modification and sorting of secreted and transmembrane proteins. The protein influx to the ER can fluctuate substantially in cells under physiological changes (such as cell differentiation) or stimulations from the environment (such as deficiency of amino acid or glucose). When proteins entering ER saturate its folding capacity, unfolded proteins accumulate in the ER and lead to ER stress. In an attempt to re-establish ER homeostasis, signaling pathways known as the unfolded protein response (UPR) are activated, which are mediated by the three ER transmembrane sensors–PKR-like ER protein kinase (PERK), inositol-requiring protein 1 (IRE1) and activating transcriptional factor 6 (ATF6) (Ron and Walter, 2007). UPR resolves ER stress via multiple mechanisms, including translation attenuation, enhancement of ER folding capacity, mRNA degradation and activation of ER-associated degradation (ERAD), etc (Ron and Walter, 2007). However, if ER stress persists, UPR can also signal the over-stressed cells to undergo apoptosis (Tabas and Ron, 2011).
Coronaviruses are a family of enveloped RNA viruses that cause diseases in animals and humans. Coronavirus infection in domestic animals has led to major economic loss worldwide, such as infectious bronchitis virus (IBV) in chickens (Cavanagh, 2007) and porcine epidemic diarrhea virus (PEDV) in pigs (Song and Park, 2012). Traditionally, infection of coronaviruses such as Human Coronavirus 229E (HCoV-229E) and HCoV-OC43 has been associated with mild upper respiratory symptoms and accounts for nearly one third of common cold in human adults (Hamre and Procknow, 1966, Kaye et al., 1972). In 2003, the pandemic of severe acute respiratory syndrome (SARS) caused global panic and led to the identification of a highly pathogenic human coronavirus—SARS-CoV (Ksiazek et al., 2003). Infection with SARS-CoV resulted in extensive tissue damage and respiratory failure, with considerable mortality and morbidity (Perlman and Dandekar, 2005). The virus was proposed to origin from bat, adapt to the intermediate host palm civet and finally to human (Li et al., 2005, Wang and Eaton, 2007); although recent identification of a SARS-like coronavirus from bats suggests that direct human infection by some bat coronavirus may be possible (Ge et al., 2013). The recent emergence and spreading of a novel human coronavirus–the Middle East respiratory syndrome coronavirus (MERS-CoV), has attracted public attention and intensive research (de Groot et al., 2013). Although sustained human-to-human transmission is considered low, MERS-CoV has gradually spread from Saudi Arabia to countries in the Middle East, Europe and the US, with a considerable mortality in patients with comorbidities (Graham et al., 2013). Accumulating evidence has suggested that bats are the natural reservoirs of MERS-CoV (Memish et al., 2013) and the dromedary camels are the intermediate hosts (Alagaili et al., 2014). This further supports that coronaviruses can cross the species barrier and become lethal zoonotic human pathogens. In face of that, research on the pathogenesis and host interaction of coronavirus is essential for identifying antiviral agents and vaccine development.
The replication of coronavirus occurs in the cytoplasm and is closely associated with ER and other cellular membrane organelles. Accumulating evidence has suggested that coronavirus replication causes ER stress and induces UPR in the infected cells. Given the extensive cross-talk between UPR and major cell signaling pathways, UPR induction may modulate host anti-viral response and constitute a major aspect of coronavirus-host interaction. In the following sections, current knowledge on the signaling mechanisms of UPR and its modulation by coronavirus infection will be summarized. The implication of UPR in host response will also be discussed, with emphasis on apoptosis and innate immunity.
2. Induction of ER stress by coronavirus infection
Transcription induction of ER protein chaperones, such as the immunoglobulin heavy chain-binding protein (BiP, also known as glucose regulated protein 78, or GRP78) or glucose-regulated protein 94 (GRP94), is generally accepted as an indicator of ER stress (Samali et al., 2010). In global transcriptome studies, induction of BiP and GRP94, as well as other genes related to ER stress, has been detected in cells infected with SARS-CoV or in cells over-expressing the SARS-CoV spike protein (Tang et al., 2005, Yeung et al., 2008). Consistent with these findings, activation of luciferase reporters under the control of BiP or GRP94 promoters has been observed in cells infected with SARS-CoV (Chan et al., 2006). ER stress has also been observed in cells infected with other coronaviruses such as MHV (Versteeg et al., 2007) or IBV (Liao et al., 2013). Therefore, ER stress induction is likely a common outcome in cells infected with coronaviruses (Fung and Liu, 2014). Although coronavirus-induced ER stress has been mainly attributed to the spike protein, other mechanisms may also be contributing as discussed below.
2.1. Coronavirus proteins and ER stress
As a high molecular weight and highly glycosylated transmembrane protein, the spike protein is massively produced for virion assembly during coronavirus infection (Masters, 2006). Exclusively N-linked glycosylation significantly increases the size of the spike protein, and is previously shown to promote folding and trimerization (Delmas and Laude, 1990). The folding and maturation of spike protein presumably depends heavily on the ER protein chaperones, such as calreticulin and calnexin. In fact, physical interaction between calnexin and the SARS-CoV spike protein has been determined, and inhibition of calnexin function has been shown to reduce the infectivity of pseudotyped lentivirus bearing the SARS-CoV spike protein (Fukushi et al., 2012). It is therefore not unexpected that studies on coronavirus-induced ER stress have been focused on the spike proteins. Indeed, over-expression of the spike protein of SARS-CoV, MHV and HCoV-HKU1 has been shown to induce potent ER stress in cell culture (Chan et al., 2006, Siu et al., 2014, Versteeg et al., 2007). Interestingly, a HCoV-OC43 variant harboring persistence-associated mutations in the spike protein has been found to induce a stronger ER stress and UPR in the infected neurons as compared to the wild type virus (Favreau et al., 2009). Apart from the spike protein, some coronavirus accessory proteins, such as protein 3a, 6 and 8ab of SARS-CoV, have also been shown to induced ER stress when over-expressed in cells (Minakshi et al., 2009, Sung et al., 2009, Ye et al., 2008).
2.2 Membrane modifications and ER stress
Modification of cellular membranes has been observed in cells infected with various RNA viruses (Miller and Krijnse-Locker, 2008). Among them, coronaviruses have been demonstrated to induce the formation of double membrane vesicles (DMVs) in the infected cells (David-Ferreira and Manaker, 1965). Using immunocytochemistry and electron microscopy, these DMVs are found closely associated with the coronavirus replication transcription complexes (RTCs) and the de novo synthesized viral RNAs (Gosert et al., 2002, Snijder et al., 2006). The formation of DMVs is presumably induced by specific coronavirus non-structural proteins (nsp). In fact, DMV formation has been observed in cell co-transfected with the SARS-CoV nsp3, nsp4 and nsp6, all of which are multipass transmembrane proteins (Angelini et al., 2013). Using high resolution electron tomography, Knoops et al. have clearly demonstrated that the DMVs formed in SARS-CoV-infected cells are generated from a reticulovesicular network derived from the ER (Knoops et al., 2008). Moreover, a recent study has revealed a mechanism whereby MHV obtains DMV membrane by hijacking the ER-derived vesicles EDEMosome (dubbed from the protein ER degradation enhancer, mannosidase alpha-like 1; EDEM1), further supporting the ER-origin of the coronavirus-induced DMVs (Reggiori et al., 2010). Apart from DMVs, other membrane rearrangements associated with RNA synthesis have been observed in coronavirus-infected cells. These include convoluted membranes, vesicle packets, paired membranes and spherules, which may also contribute to coronavirus-induced ER stress (Knoops et al., 2008, Maier et al., 2013).
2.3. Membrane depletion and ER stress
Previous studies have established that the intracellular site for coronavirus assembly and budding is the ER-Golgi intermediate compartment (ERGIC), which is a structural and functional extension of the ER (Klumperman et al., 1994, Stertz et al., 2007). Therefore, continuous morphogenesis and budding of virions in the ERGIC in essence deplete the membrane of the ER. It has been shown that depletion of phosphatidylcholine – the lipid component of ER membrane, affects the ER morphology and impairs protein trafficking in the Golgi (Testerink et al., 2009). Moreover, lipid depletion induces ER stress and UPR, and the affected cells respond by increasing lipid biosynthesis and ER membrane biogenesis (Sriburi et al., 2004, van der Saden et al., 2003). In addition to the budding process, coronavirus infection triggers autophagy, and the autophagosome membranes have been shown to be derived from the ER (Cottam et al., 2011). Therefore, it is highly possible that membrane depletion during coronavirus replication contribute to ER stress induction, although further functional studies are required.
In summary, the ER stress induced by coronavirus infection may be attributed to: (1) massive synthesis, modification and folding of coronavirus proteins in the ER; (2) drastic membrane re-organization of the ER to form DMVs for genome replication; and (3) ER membrane depletion due to virion budding and autophagy induction. In response to the ER stress, the infected cells activate the UPR pathways (Fig. 1A). In the following section, signaling of the three UPR branches and their modulation by coronavirus infection will be discussed in detail.
Fig. 1.

Signaling pathways of three branches of UPR and modulations by coronavirus infection. (A) Coronavirus replication causes ER stress by three major mechanisms. The ER stress sensors PERK, IRE1, and ATF6 are activated and trigger UPR in an attempt to counter ER stress. (B) The signaling pathway of integrated stress response and coronavirus intervention. Infection with MHV-A59, SARS-CoV, and IBV has been shown to cause eIF2α phosphorylation, which is most likely mediated by PKR and/or PERK. The phosphorylated eIF2α sequesters eIF2B, inhibits recycling of GTP-bound eIF2α and leads to translation attenuation. (C) The IRE1 signaling pathway and coronavirus intervention. IRE1 mediates splicing of XBP1, which induces UPR genes such as ERdj4 and p58IPK. IRE1 can also recruit TRAF2 and activate JNK-mediated apoptosis. The over-expression of spike proteins of IBV and MHV-A59 has been shown to activate IRE1, whereas the SARS-CoV E protein inhibits XBP1 splicing. (D) The ATF6 signaling pathway and coronavirus intervention. ATF6 protein is cleaved by S1P and S2P under ER stress. The released fragment (ATF6f) translocates to the nucleus and induces UPR genes. Transfection of SARS-CoV accessory protein 8ab or infection with IBV or MHV-A59 has been shown to induce ATF6 cleavage.
3. The PERK branch of UPR and integrated stress response (ISR)
3.1. ISR signaling pathways
Among the three sensors of UPR, PERK is generally believed to be activated first in response to ER stress (Ron and Walter, 2007, Szegezdi et al., 2006). In unstressed cells, PERK is held inactive by the binding of ER chaperone BiP to its luminal domain. Under ER stress, BiP dissociates from PERK to interact with the excessive influx of unfolded proteins. Dissociation of BiP leads to oligomerization and auto-phosphorylation of PERK, thereby activating its tyrosine kinase activity (Bertolotti et al., 2000). Normally, phosphorylated PERK is inactivated by protein tyrosine phosphatase 1B (PTP1B). However, PTP1B is inhibited by sulfhydration in cells under ER stress, and active PERK is thus protected from dephosphorylation (Krishnan et al., 2011). The substrate for PERK is the α-subunit of eukaryotic initiation factor 2 (eIF2α) (Shi et al., 1998). Once phosphorylated at serine 51, eIF2α forms a stable complex with and inhibits the enzymatic activity of eukaryotic initiation factor 2B (eIF2B). EIF2B is a guanine nucleotide exchange factor that converts the inactive GDP-bound eIF2α to its active GTP-bound form, which mediates the binding of initiator methionine-transfer RNA to the 40S ribosome to form the 43S pre-initiation complex (De Haro et al., 1996). Therefore, phosphorylation of eIF2α results in the inhibition of translation initiation and a global shutdown of cellular protein synthesis (Kimball, 1999). The translation attenuation reduces the influx of newly synthesized proteins into the already stressed ER. Moreover, eIF2α phosphorylation liberates ribosomes and translation factors from mRNA, allowing them to preferentially initiate translation of UPR regulated genes, thereby reprogramming the ER for the stress condition (Harding et al., 2000).
Besides PERK, three other kinases are known to phosphorylate eIF2α, namely the heme-regulated inhibitor kinase (HRI), the general control non-derepressible 2 (GCN2) and the protein kinase RNA-activated (PKR) (Ron and Walter, 2007). HRI is expressed predominantly in erythroid cells and hepatocytes. It is activated by low level of heme and coordinates the synthesis of heme and protein moieties of hemoglobin in red blood cells and P450 cytochromes in hepatocytes (Acharya et al., 2010, McEwen et al., 2005). GCN2 is the only known eIF2α kinase with a homolog in Saccharomyces cerevisiae. Under conditions of amino acid deprivation, uncharged transfer RNA binds to the C-terminal of GCN2 and activates its kinase activity (Sood et al., 2000). From a virological perspective, PKR is perhaps especially important as an eIF2α kinase, because it is induced by interferon and activated by the binding of double-stranded RNA (dsRNA), which is a common byproduct during replication of a wide variety of DNA and RNA viruses (Clemens and Elia, 1997). The binding of dsRNA to the two N-terminal dsRNA binding motifs induces dimerization and auto-phosphorylation of the C-terminal kinase domain, thereby switching on the kinase activity (Sadler and Williams, 2007). Although differing in the upstream stimuli, activation of all four eIF2α kinases results in translation suppression and activates similar downstream signaling pathways, which are collectively known as the integrated stress response (ISR) (Fig. 1B) (Ron and Walter, 2007).
Although translation of a majority of cellular mRNAs is suppressed under ISR, mRNAs of certain genes containing small upstream ORFs (uORFs) in their 5′UTR are indeed preferentially translated when eIF2α is phosphorylated (Dever et al., 1992). One of these genes is the activating transcription factor 4 (ATF4), which is the mammalian homolog of the general control non-derepressible 4 (GCN4) in yeast (Harding et al., 2000, Vattem and Wek, 2004). ATF4 belongs to a large family of transcription factors characterized by a basic-region leucine zipper (bZIP) DNA binding domain (Ameri and Harris, 2008). By binding to the cAMP response element, ATF4 transactivates genes involved in amino acid metabolism (Chen et al., 2004), antioxidant response (He et al., 2001), as well as another critical UPR-regulated bZIP transcription factor – the C/EBP homologous protein (CHOP, also known as growth arrest and DNA damage-inducible protein 153, or GADD153) (Fawcett et al., 1999). Together with ATF4, CHOP activates the growth arrest and DNA damage-inducible protein 34 (GADD34), which is a regulatory subunit of the protein phosphatase 1 (PP1) (Marciniak et al., 2004). GADD34 interacts with and activates PP1, which catalyzes de-phosphorylation of eIF2α and release the translation suppression (Fig. 1B) (Brush et al., 2003). This negative feedback loop ensures that normal translation initiation is resumed after ER stress is resolved. However, if ER stress persists, restoration of protein synthesis can aggravate the ER stress and lead to cell death (Han et al., 2013, Marciniak et al., 2004).
3.2. Activation of ISR during coronavirus infection
There have been discrepancies regarding the activation of ISR during coronavirus infection. For MHV, an early study has detected negligible transcriptional activation of PKR, as well as another interferon-stimulated gene, 2′5′-oligoadenylate synthetase (OAS) in cells infected with MHV-1 (Zorzitto et al., 2006). A latter study using MHV-A59 confirmed that PKR and eIF2α were not phosphorylated and that host protein synthesis was not inhibited in the infected cells (Ye et al., 2007). The failure to activate PKR and OAS in MHV-A59 infected cells was attributed to resistance and inhibition of the IFN response by the virus (Roth-Cross et al., 2007, Ye et al., 2007). However, in a separate study using the same virus MHV-A59, Bechill et al. have detected significant phosphorylation of eIF2α starting from 8 h post infection (hpi) (Bechill et al., 2008). Although the phosphorylation status of the corresponding upstream kinases was not determined, the level of downstream protein ATF4 was up-regulated, whereas no induction of CHOP and GADD34 was observed (Bechill et al., 2008). The authors thus conclude that uncharacterized mechanisms may be adopted by the virus to down-regulate the CHOP-GADD34/PP1 feedback loop, enabling sustained translation suppression of the host proteins (Fig. 1B) (Bechill et al., 2008). Meanwhile, it remains unknown how MHV mRNAs can still be translated normally when eIF2α is phosphorylated.
As for SARS-CoV, Krähling et al. have observed significant phosphorylation of PKR and PERK, but not GCN2 in the infected cells (Krähling et al., 2009). The phosphorylation of eIF2α was also detected, but changes in the host protein synthesis have not been determined. Surprisingly, knock-down of PKR using specific morpholino oligomers did not affect SARS-CoV replication or virus-induced eIF2α phosphorylation (Krähling et al., 2009). These results suggest that SARS-CoV is resistant to the antiviral activity of PKR in vitro and that other kinase(s), most likely PERK, is responsible for the phosphorylation of eIF2α induced by SARS-CoV. Other studies based on transient transfection of SARS-CoV proteins have also implicated the involvement of the PERK branch of UPR. For example, Chan et al. have shown that over-expression of SARS-CoV spike protein up-regulates BiP and GRP98 promoter activities in a dosage dependent manner (Chan et al., 2006). The trans-activation is likely mediated via PERK-eIF2α, because co-transfection of dominant negative forms of PERK or eIF2α significantly suppresses the reporter activities (Chan et al., 2006). Later, the UPR activating domain of SARS-CoV spike protein is mapped to the central region (amino acids 201–400) of the S1 subunit, and seems to function independent of N-linked glycosylation (Siu et al., 2014). Interestingly, the accessory protein 3a of SARS-CoV, which is a small multipass transmembrane protein, also activates the ATF4 and CHOP promoter activities, and thus may also activate the PERK branch of UPR (Minakshi et al., 2009).
Regarding Alphacoronaviruses, TGEV replication has been shown to induce phosphorylation of PKR and eIF2α in the infected cells, although the activation of PERK has not been determined (Cruz et al., 2011). Intriguingly, using a recombinant TGEV virus lacking the accessory gene 7 (rTGEV-Δ7), Cruz et al. have demonstrated that the protein 7 of TGEV physically interacts with PP1 and promotes eIF2α de-phosphorylation. Compared with the wild type virus, cells infected with rTGEV-Δ7 have a much higher level of phosphorylated eIF2α, resulting in significant translation attenuation and drastic induction of GADD34 (Cruz et al., 2011). Although changes in other pathway intermediates such as ATF4 and CHOP have not been determined, the data supports that TGEV activates the PKR-eIF2α-GADD34 pathway, which is modulated by the accessory protein 7.
In terms of Gammacoronaviruses, studies done by this group have shown that IBV triggers phosphorylation of PKR and PERK at early time points of infection (2–8 hpi), which diminished quickly thereafter (Liao et al., 2013, Wang et al., 2009). The effective inhibition of PKR (and likely also PERK) phosphorylation has been attributed to the nsp2 protein, which dosage dependently restore the PKR-mediated translation suppression of a reporter construct (Wang et al., 2009). The kinetic of eIF2α phosphorylation is similar to that of PKR and PERK, which peaks at early infection but drastically reduces afterwards (Liao et al., 2013, Wang et al., 2009). As a result, de novo synthesis of host proteins is not significantly suppressed in IBV-infected cells throughout the course of infection (Wang et al., 2009). Apart from the inactivation of its upstream kinases, the rapid de-phosphorylation of eIF2α has also been ascribed to the induction of GADD34 (Wang et al., 2009). Up-regulation of GADD34 is likely mediated by the transcription factors ATF4 and CHOP, as both are significantly induced in IBV-infected cells starting from 12 h post infection (Fig. 1B) (Liao et al., 2013). Functional studies using RNA interference and specific inhibitors further support the activation of PKR/PERK-eIF2α-ATF4-CHOP pathway and the negative feedback via GADD34/PP1. Knock-down of PKR or PERK, as well as drug inhibition of PKR or eIF2α, greatly reduces the IBV-induced up-regulation of CHOP (Liao et al., 2013), whereas inhibition of PP1 by okadaic acid dosage dependently enhances IBV-induced eIF2α phosphorylation and inhibites IBV replication (Wang et al., 2009). Interestingly, IBV replication is not significantly affected by knock-down of PKR or PERK, indicating that similar to SARS-CoV, IBV is not sensitive to the antiviral activities of PKR or PERK in vitro (Liao et al., 2013).
The different results regarding coronaviruses induced ISR reflect that the signaling pathway may be differentially modulated by individual coronaviruses. One excellent example is the antagonism of the cellular OAS/RNaseL pathway by the non-structural protein 2 (ns2) of MHV (Zhao et al., 2012). Homologous proteins of ns2 are encoded by Beta-coronaviruses of the same lineage, but not Beta-coronaviruses of different lineages (such as SARS-CoV) or in different genera (such as TGEV or IBV) (Zhao et al., 2011). It is possible that other group-specific proteins may similarly interfere with the activation of PKR or other eIF2α kinases, resulting in the differential activation of ISR during infection. On the other hand, the discrepancies may be a result from the different cell lines used, which have distinct tissue origins and genetic backgrounds, and can differ greatly in virus-induced stress response. Therefore, further mechanistic studies using recombinant viruses as well as proper in vivo models are required to better understand the involvement of ISR during coronavirus infection.
3.3. Induction and regulation of ISR by other viruses
Translation attenuation has been generally considered as a defensive mechanism of the host cells to limit virus replication. Therefore, it is not surprising that one or more of the eIF2α kinases is activated during infection of various viruses. For example, GCN2 has been shown to be activated and play antiviral functions in cells infected with human immunodeficiency virus 1 (HIV-1) (Cosnefroy et al., 2013) and in mice infected with mouse cytomegalovirus (Won et al., 2012), Semliki forest virus or Sindbis virus (Berlanga et al., 2006). Similarly, activation of PERK has been observed in cells infected with various DNA and RNA viruses, such as Coxsackievirus B3 (Zhang et al., 2010), vesicular stomatitis virus (VSV) (Baltzis et al., 2004), bovine viral diarrhea virus (Jordan et al., 2002) and herpes simplex virus 1 (HSV1) (Cheng et al., 2005), to name just a few. As for PKR, which is induced by interferon and activated by dsRNA of viral origin, extensive studies in a large number of DNA and RNA viruses have firmly established its antiviral activities (He, 2006, Langland et al., 2006). Nonetheless, it should be noted that the antiviral functions of eIF2α kinases may not be essentially mediated via eIF2α phosphorylation, such as in the case of PERK and GCN2 during VSV infection (Krishnamoorthy et al., 2008).
Given the detrimental effect of translation attenuation on virus replication, it is also not surprising that viruses have evolved counter-defense mechanisms against the ISR. Virus-encoded proteins, such as NS1 of Influenza A virus (Lu et al., 1995) and US11 of HSV1 (Khoo et al., 2002), specifically bind to and sequester dsRNAs to prevent recognition by PKR. The eIF2α kinases can also be inhibited by physical binding of viral proteins. For example, the NS5A protein of hepatitis C virus (HCV) binds to the catalytic site of PKR (Gale et al., 1997), while its E2 protein binds to PERK and inhibit its kinase activity (Pavio et al., 2003). Several viruses have been shown to target eIF2α kinases for degradation, such as the poliovirus (Black et al., 1989), rift valley fever virus (Habjan et al., 2009) and HIV-1 (del Pino et al., 2012). Other viruses have been found to encode an eIF2α phosphatase (γ34.5 protein of HSV1) (He et al., 1997), or stimulate the cellular GADD34/PP1 complex (E6 protein of human papillomavirus Type 18) (Kazemi et al., 2004), in order to dephosphorylate eIF2α and restore protein synthesis. Moreover, the internal ribosomal entry site (IRES) elements, present in the mRNAs of some viruses such as HCV or the classical swine fever virus, have been demonstrated to translate independently of eIF2 (Pestova et al., 2008, Terenin et al., 2008). The fact that viruses of different families employ varieties of mechanisms counteracting every single step of ISR further demonstrates its essential antiviral functions.
4. The IRE1 branch of UPR
4.1. The IRE1 signaling pathway
The IRE1 branch of UPR is a highly conserved pathway from yeast to humans (Korennykh and Walter, 2012). Initial studies have suggested that IRE1 is activated via a similar mechanism as PERK, which involved ER stress induced dissociation of BiP followed by oligomerization and trans-phosphorylation (Bertolotti et al., 2000, Zhou et al., 2006). This notion is further strengthened by the high sequence homology (∼47%) of the two proteins’ N-terminal luminal domains (NLDs), as well as the fact that their NLDs are even interchangeable in vivo (Liu et al., 2000). However, recent studies have demonstrated that the NLD of IRE1 can directly bind unfolded proteins (Credle et al., 2005). Moreover, the dissociation of BiP may not be the primary switch for IRE1 activation, but rather serves as a buffer to modulate the sensitivity and dynamics of IRE1 activity (Pincus et al., 2010).
Phosphorylation of IRE1 activates its cytosolic RNase domain, which results in the unconventional splicing of the mRNA of homologous to Atf/Creb1 (HAC1) in yeast and X-box binding protein 1 (XBP1) in Metazoans (Sidrauski and Walter, 1997, Yoshida et al., 2001a). The sequence flanking the spliced intron is highly conserved, which forms secondary structures recognized by IRE1 (Hooks and Griffiths-Jones, 2011). In human, splicing of the 26-nucleotide intron leads to a frame-shift transcript, which encodes the spliced XBP1 protein (XBP1s) (Yoshida et al., 2001a). XBP1s is a potent bZIP transcription factor that induces expression of genes harboring the UPR element (UPRE) or the ER stress response element (ERSE) in the promoter sequences (Yamamoto et al., 2004). To counteract ER stress, XBP1s regulates genes involved in protein entry into ER, folding, glycosylation, ER-associated degradation (ERAD), lipid biogenesis and vesicular trafficking (Glimcher, 2010). The expression of at least two genes, the ER DNA J domain-containing protein 4 (ERdj4) and the protein kinase inhibitor of 58 kDa (p58IPK) have been shown to be specifically induced by XBP1s, but not other UPR transcription factors (Lee et al., 2003). XBP1s also induces the E3 ubiquitin ligase synoviolin, which promotes ubiquitination and degradation of IRE1, forming a negative feedback loop (Gao et al., 2008, Yamamoto et al., 2008). The unspliced mRNA (XBP1u) is also translated, which contains only the bZIP domain but not the transactivation domain. XBP1u has been shown to negatively regulate the activity of XBP1s and undergoes rapid proteasome dependent degradation (Fig. 1C) (Tirosh et al., 2006, Yoshida et al., 2006).
Previously, the mRNA of XBP1 has been considered the only splicing substrate for IRE1. Recently, Hollien et al. have identified degradation of ER-localized mRNAs by IRE1 under ER stress, which is termed regulated IRE1-dependent mRNA decay (RIDD) (Hollien et al., 2009, Hollien and Weissman, 2006). Although most RIDD substrates identified harbor XBP1-like consensus sequences (Oikawa et al., 2010), IRE1-mediated XBP1 splicing and RIDD seems to operate via two distinct mechanisms (Han et al., 2009). Whereas basal level of RIDD removes ER-associated mRNA to facilitate ER homeostasis, prolonged RIDD has been shown to degrade mRNAs encoding pro-survival proteins and contribute to ER-stress induced cell death (Maurel et al., 2014).
Apart from mediating XBP1 splicing, the kinase domain of phosphorylated IRE1 has been shown to recruit the tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) (Urano et al., 2000). The IRE1-TRAF2 complex further interacts with the Apoptosis signal-regulating kinase 1 (ASK1), which ultimately activates the c-Jun N-terminal kinase (JNK) and induces ER stress dependent apoptosis (Fig. 1C) (Nishitoh et al., 2002). The IRE1-JNK pathway is also required for autophagy activation after pharmacological induction of ER stress (Ogata et al., 2006). The decision between autophagy and apoptosis in cells under ER stress may be intricately regulated by multiple mechanisms, including the JNK-mediated phosphorylation of Bcl-2 and the stress integrator Bax-inhibitor 1 (Castillo et al., 2011, Wei et al., 2008). In short, IRE1 facilitates the resolution of ER stress by activating the UPR gene master control XBP1 and induction of autophagy, but prolonged activation of IRE1 can also trigger RIDD and apoptosis via the JNK pathway.
4.2. Activation of IRE1-XBP1 pathway by coronavirus and other viruses
Studies have demonstrated that the IRE1-XBP1 pathway is activated in cells infected with RNA viruses, such as Influenza A virus (IAV) (Hassan et al., 2011), Respiratory Syncytial virus (RSV) (Hassan et al., 2014), HCV (Merquiol et al., 2011), Tick-borne encephalitis virus (TBEV) (Yu et al., 2013), Japanese Encephalitis virus (JEV) (Bhattacharyya et al., 2014) and Dengue virus serotype 2 (Yu et al., 2006), to name just a few. Using specific IRE inhibitors or RNAi, IRE1 has been shown to facilitate the replication of IAV and TBEV, suppress the replication of RSV, but is not involved in the replication of HCV or DEN-2 (Hassan et al., 2014, Hassan et al., 2011, Merquiol et al., 2011, Yu et al., 2013, Yu et al., 2006).
As for coronaviruses, MHV infection or over-expression of its spike protein has been shown to induce significant splicing of XBP1 mRNA (Bechill et al., 2008, Versteeg et al., 2007). However, XBP1s protein cannot be detected in the infected cells and downstream genes such as ERdj4, EDEM1 and p58IPK are not up-regulated. It is possible that sustained translation attenuation by MHV-induced eIF2α phosphorylation blocks the translation of XBP1s protein and suppresses the activation of this branch of UPR (Bechill et al., 2008). In contrast to MHV, infection of SARS-CoV or transfection of SARS-CoV spike protein fails to induce XBP1 mRNA splicing (DeDiego et al., 2011, Versteeg et al., 2007). This observation have been attributed to the ability of SARS-CoV E protein to suppress host stress response (DeDiego et al., 2011). A recombinant SARS-CoV with the E gene deleted (rSARS-CoV-ΔE) was generated, and significant XBP1 splicing and higher expression levels of UPR genes were observed in the infected cells, compared with wild type SARS-CoV. Furthermore, transfection of E protein strongly inhibits ER stress induced by chemicals or RSV infection (DeDiego et al., 2011). The UPR modulating function of E protein may explain why SARS-CoV lacking E protein is attenuated in animal models (DeDiego et al., 2007), but its detail mechanisms remain to be investigated.
Regarding Gammacoronaviruses, studies done by this group have shown that the IRE1-XBP1 pathway is activated in mammalian and avian cells infected with IBV (Fung et al., 2014). Significant XBP1 splicing can be observed in cells transfected with IBV S protein or in cells infected with IBV at late stage of infection (∼20 h post infection). Although the expression of XBP1s protein has not been determined due to the lack of a specific antibody, expression of downstream genes (ERdj4 and p58IPK) is significantly elevated in IBV-infected cells. Knockdown of IRE1 abolishes IBV-induced XBP1 splicing, whereas knockdown of either IRE1 or XBP1 drastically reduces IBV-induced ERdj4 and p58IPK expression. Consistently, over-expression of IRE1 but not its kinase or RNase mutants significantly increases IBV-induced XBP1 splicing and UPR genes expression (Fung et al., 2014). Taken together, it seems that the IRE1 branch of UPR is fully activated in cells infected with IBV, whereas its activation is hampered in MHV or SARS-CoV-infected cells through different mechanisms.
5. The ATF6 branch of UPR
5.1. The ATF6 signaling pathway
Unlike PERK or IRE1, the ATF6 protein is a Type-II transmembrane protein with an N-terminal bZIP signaling domain and an ER luminal domain that is modified by glycosylation and disulfide bonding. Similar to PERK, ATF6 is activated by ER-stress induced dissociation of BiP (Shen et al., 2002), although underglycosyltion (Hong et al., 2004) and reduction of disulfide bonds (Nadanaka et al., 2007) have also been suggested as alternative activation mechanisms. Activated ATF6 is translocated to Golgi apparatus and processed by the Site-1 protease (S1P) and Site-2 protease (S2P) (Ye et al., 2000). The intramembrane proteolysis releases the cytosolic DNA binding domain, which translocates into the nucleus and switches on genes harboring ERSE or ERSE-II in the promoters (Yoshida et al., 2001b). The major target genes of ATF6 consist of ER chaperones (such as BiP, GRP94 and calreticulin) and some ERAD components, thus activation of the ATF6 branch promotes protein folding and restoration of ER homeostasis (Fig. 1D) (Adachi et al., 2008).
5.2. Activation of ATF6 pathway during coronavirus infection
Activation of the ATF6 branch of UPR has been observed in cells infected by a handful of viruses including HSV-1 (Burnett et al., 2012), African swine fever virus (ASFV) (Galindo et al., 2012), West Nile virus (WNV) (Ambrose and Mackenzie, 2011) and HCV (Wang et al., 2014). Although ATF6 has been shown to promote the replication of WNV by suppressing interferon signaling (Ambrose and Mackenzie, 2013), the effect of ATF6 activation on replication of other viruses has not been studied in detail.
Compared with PERK and IRE1, there have been limited studies on the activation of ATF6 pathway during coronavirus infection. The cleavage of ATF6 can be observed in cells infected with MHV, but the level of both full length and cleaved ATF6 protein diminish at late stage of infection, with no activation of target genes determined by ERSE reporter constructs (Bechill et al., 2008). Similar to XBP1s, the lack of ATF6 transactivation may be a result of sustained translation suppression mediated by eIF2α phosphorylation. As for SARS-CoV, no significant ATF6 cleavage is observed compared with mock infected cells (DeDiego et al., 2011). Overexpression of the SARS-CoV spike protein also fails to activate ATF6 reporter constructs (Chan et al., 2006). In contrast, ATF6 cleavage and nuclear translocation have been determined in cells transfected with the SARS-CoV accessory protein 8ab, which has also been shown to physically interact with the luminal domain of ATF6 (Sung et al., 2009). The SARS-CoV 8ab protein was only detected in early human isolates during the SARS-CoV pandemic. In the later isolates, genome deletion resulted in the splitting of ORF8 into two smaller ORFs, encoding two truncated protein 8a and 8b respectively (Guan et al., 2003). Therefore, further experiments on the 8a and 8b proteins, as well as studies using recombinant SARS-CoV deletion mutants are required. In terms of Gammacoronaviruses, preliminary data from this group have shown that, similar to MHV, ATF6 cleavage is observed in cells infected with IBV (unpublished observations). Moreover, knockdown of ATF6 significantly reduces the IBV-induced expression of XBP1 and other UPR genes, suggesting that ATF6 contributes to UPR during IBV infection (unpublished observations).
4. UPR and coronavirus-induced apoptosis
Prolonged UPR activation is known to cause apoptotic cell death and has been reviewed in detailed (Sano and Reed, 2013, Szegezdi et al., 2006). It has also been demonstrated that coronavirus infection induces apoptosis in vitro (An et al., 1999, Mizutani et al., 2004) and in vivo (Chau et al., 2004, Haagmans et al., 1996), which is caspase-dependent but p53-independent (Li et al., 2007, Liu et al., 2001). Also, coronavirus-induced apoptosis is dependent on but not essential for virus replication (Bordi et al., 2006, Ren et al., 2005). However, there have been limited studies on the involvement of UPR in coronavirus-induced apoptosis (Zhong et al., 2012). Here, we summarize current knowledge on the mechanisms of ER stress induced apoptosis and the implications of UPR during coronavirus infection.
4.1. Apoptosis induction and regulation: the PERK branch and ISR
The early phase of ISR, characterized by eIF2α phosphorylation and translation attenuation, aims to restrict ER stress and is pro-survival in nature. However, at late stage of persistent ER stress, with the induction of CHOP and restoration of protein synthesis by GADD34, the pathway becomes pro-apoptotic. First, resuming protein translation in cells under ER stress aggravates the ER burden and allows for the expression of pro-apoptotic proteins, such as CHOP and ER oxidoreductin-1α (ERO1α) (Marciniak et al., 2004). Secondly, CHOP promotes the intrinsic pathway of apoptosis by inhibiting the transcription of anti-apoptotic protein B-cell lymphoma (Bcl-2) (McCullough et al., 2001) and inducing the pro-apoptotic protein Bcl-2-interacting mediator of cell death (Bim) (Puthalakath et al., 2007). Moreover, CHOP also promotes the extrinsic pathway of apoptosis by inducing the cell-surface death receptor 5 (DR5), which mediates apoptosis via caspase-8 cleavage (Yamaguchi and Wang, 2004). Previously we have shown that both the mRNA and protein levels of CHOP are significantly induced in IBV-infected cells at late stage infection (Liao et al., 2013). Knock-down of PKR or PERK significantly reduces IBV-induced CHOP up-regulation and apoptosis. Knock-down of CHOP almost completely abolishes IBV-induced apoptosis in the infected cells, which is associated with the hyper-phosphorylation of the pro-survival extracellular signal-related kinase (ERK) (Fig. 2 ) (Liao et al., 2013). Consistently, further experiments have shown that over-expression of CHOP promotes IBV-induced apoptosis in a dosage-dependent manner (Fig. 3A). Interestingly, mutation experiments have suggested the N-terminal amino acid 36–70 region to be essential for the pro-apoptotic function of CHOP during IBV infection (Fig. 3B and C). This region is distinct from the tribbles-related protein 3 (TRIB3) interaction domain and the DNA-binding bZIP domain described previously (Ohoka et al., 2007), indicating that other uncharacterized mechanisms may be responsible for the phenotype. Induction of CHOP is either not observed or not determined in cells infected with other coronaviruses, although Krähling et al. have shown that PKR is required for SARS-CoV-induced apoptosis, which is independent of eIF2α phosphorylation (Krähling et al., 2009). On the other hand, increased eIF2α phosphorylation has been associated with enhanced cytopathic effect and apoptosis in cells infected with TGEV lacking the accessory gene 7 (Cruz et al., 2011). Although the involvement of CHOP has not been investigated, the data at least suggest that TGEV induced apoptosis may be mediated by the activation of ISR.
Fig. 2.
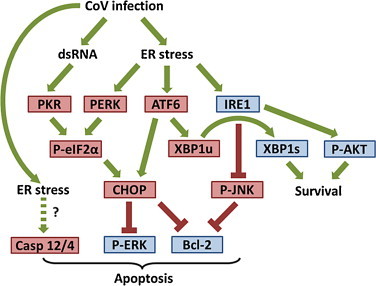
Involvement of UPR in coronavirus-induced apoptosis. Under prolonged ER stress, phosphorylation of eIF2α by PKR/PERK up-regulates CHOP, which has been shown to suppress the pro-survival kinase ERK and the anti-apoptotic mitochondrial protein Bcl-2. IRE1 has been shown to protect IBV-infected cells from apoptosis, by converting the pro-apoptotic unspliced XBP1 to the anti-apoptotic spliced form (XBP1s). Also, in IBV-infected cells, IRE1 activates the pro-survival kinase AKT and suppresses the pro-apoptotic kinase JNK. ATF6 potentially facilitates apoptosis by induction of CHOP and XBP1u. ER-stress induced apoptosis has also been associated with cleavage of caspase 12 (Casp 12) in mouse or caspase 4 in human, although their involvement during coronavirus infection is unknown. Pro-apoptotic factors are shown in red boxes, while pro-survival proteins in blue boxes.
Fig. 3.

Over-expression of CHOP, but not its N-terminal deletion mutant, promotes IBV-induced apoptosis. (A) H1299 cells were transfected with different amount of FLAG-tag CHOP plasmid or empty vector. At 24 h post transfection, cells were infected with IBV or incubated with mock lysate for 22 h. Cell lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane for western blotting using antibodies against FLAG-tag, IBV N protein and the apoptosis marker poly (ADP-ribose) polymerase (PARP). The percentages of PARP cleavage (intensity of cleavage band [Cl] divided by total intensities of full-length [FL] and cleavage bands) were determined and indicated below. The β-tubulin protein was used as loading control. The presented blot is one representative blot from three independent experiments. (B) Schematic diagram showing the known functional domains of CHOP and the two N-terminal deletion mutants (ΔN36 and ΔN70) used in (C). Amino acid 10–18 have been shown to interact with TRIB3. Serine 79 and 82 are phosphorylated by p38, while Leucine 134 and 141 are responsible for DNA binding of the bZIP domain. (C) H1299 cells were transfected with FLAG-tag wild type CHOP or N-terminal deletion mutants. At 24 h post transfection, cells were infected with IBV or incubated with mock lysate. Cell lysates were harvest and subjected to SDS-PAGE and western blot analysis as in (A). Percentage of PARP cleavage is determined as in (A) and indicated below. The β-actin protein was used as loading control. The presented blot is one representative blot from three independent experiments.
4.2. Apoptosis induction and regulation: IRE1, ATF6 and other mechanisms
Similar to PERK, IRE1 can be either pro-survival or pro-apoptotic depending on the strength of activation and the downstream signaling pathways. Enhancement of protein folding and ERAD through the IRE1-XBP1 pathway, as well as basal RIDD of ER-associated mRNAs are considered beneficial for adaptation to ER stress and cell survival (Szegezdi et al., 2006). In contrast, signaling through the IRE-JNK pathway and prolonged RIDD activation are considered detrimental and induce apoptotic cell death (Maurel et al., 2014). As mentioned above, compared with the wild type virus, SARS-CoV lacking the E gene induces XBP1 splicing and a high level of stress response, which is also associated with a higher degree of apoptosis (DeDiego et al., 2011). This may suggest a pro-apoptotic role of IRE1 during SARS-CoV infection, although further functional experiments are required. In contrast, our recent studies have pointed to a pro-survival function of IRE1, which protects cells from apoptosis during IBV infection (Fung et al., 2014). Knockdown of IRE1 does not affect IBV replication but significantly enhances IBV-induced apoptosis. Over-expression of wild type IRE1 or XBP1s, but not IRE1 mutants or XBP1u, reduces apoptosis in cells infected with IBV (Fig. 2). Furthermore, knockdown of IRE1 modulates the phosphorylation status of JNK and RAC-α serine/threonine-protein kinase (AKT). Compared with the control, the pro-apoptotic kinase JNK is hyper-phosphorylated and the anti-apoptotic kinase AKT is hypo-phosphorylated in IBV-infected cells with IRE1 knocked down (Fung et al., 2014). Therefore, it seems the IRE1 branch promotes survival in cells infected with IBV.
The involvement of ATF6 in coronavirus-induced apoptosis has not been characterized. Interestingly, preliminary results from this group have suggested a pro-apoptotic role of ATF6 during IBV infection, which is contrary to the common believe that ATF6 facilitates adaptation to ER stress and promote cell survival (Szegezdi et al., 2006). Moreover, several recent findings have demonstrated that under certain conditions ATF6 activation may also induce apoptosis via transcriptional activation of CHOP and/or suppression of myeloid cell leukemia sequence I (Mcl-1) (Gotoh et al., 2002, Morishima et al., 2011, Nakanishi et al., 2005). Aside from the three UPR branches, ER stress induced apoptosis has been shown to be mediated by other mechanisms, such as fluctuation of ER calcium concentration (Sano and Reed, 2013) and activation of murine caspase 12 (Nakagawa et al., 2000) or human caspase 4 (Hitomi et al., 2004). However, the involvement of these UPR-independent pathways in coronavirus-induced apoptosis remains to be further investigated.
5. UPR and innate immunity during coronavirus infection
The innate immunity constitutes a pivotal anti-viral response against coronavirus infection, although over-activated inflammatory response also causes extensive tissue damage and other immunopathologies associated with SARS-CoV infection (Perlman and Dandekar, 2005). In fact, highly elevated production of pro-inflammatory cytokines/chemokines such as interleukin-1 (IL-1), IL-6, IL-8, Interferon gamma-induced protein 10 (IP-10) and monocyte chemoattractant protein-1 (MCP-1) has been detected in the lung tissues and serum samples from SARS-CoV patients (Huang et al., 2005, Jiang et al., 2005). Recent studies have suggested that the UPR may cross-talk with the innate immune signaling pathways and modulate the production and signaling of type-I interferons and/or pro-inflammatory cytokines in virus infected cells (Liu et al., 2009, Smith, 2014). In the following section, implications of UPR in the innate immunity in the context of coronavirus infection will be briefly discussed.
5.1. UPR and NF-κB activation
For decades, the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) has been well established as the master regulator of innate immunity and pro-inflammatory response (Hayden and Ghosh, 2012). Expression of pro-inflammatory cytokines (such as IL-6 and IL-8) and the early expression of type-I interferon has been shown to be induced by NF-κB together with other transcription factors, such as activator protein 1 (AP-1), interferon regulatory factor 3 (IRF3) and IRF7 (Balachandran and Beg, 2011, Kunsch and Rosen, 1993, Libermann and Baltimore, 1990, Wang et al., 2010). Specifically, the SARS-CoV spike protein dosage-dependently induces the production of TNF-α, IL-6 and IL-8 in transfected cells, which is mediated by the NF-κB pathway (Dosch et al., 2009, Wang et al., 2007).
In its inactive form, NF-κB is sequestered by the inhibitor of NF-κB alpha (IκBα), which prevent the nuclear translocation of NF-κB (Karin and Ben-Neriah, 2000). The protein level of IκBα is determined by the rate of synthesis and its degradation by the proteasome. Degradation of IκBα is in turn facilitated by the IκB kinases (IKK), since phosphorylated IκBα is efficiently modified by poly-ubiquitination and targeted for proteasomal degradation (Kanarek et al., 2010). The PERK and IRE1 branches of UPR have been shown to activate NF-κB (Fig. 4 ). Translation attenuation mediated by eIF2α phosphorylation effectively decreases the synthesis of IκBα protein in cells under various stress conditions (Jiang et al., 2003). On the other hand, IRE1 has been shown to complex with TRAF2 and activate the basal activity of IKK in cells under ER stress, thus facilitating the phosphorylation and degradation of IκBα (Tam et al., 2012). With less synthesis and more degradation, the protein level of IκBα is reduced, and NF-κB is in turn activated.
Fig. 4.

Involvement of UPR in innate immune response during coronavirus infection. The PKR/PERK mediated eIF2α phosphorylation leads to translation attenuation and lower level of IκBα synthesis. On the other hand, IRE1 recruits TRAF2 and activates IKK to phosphorylate IκBα, promoting its ubiquitination and degradation. The outcome is a lower protein level of IκBα, releasing NF-κB for activation of type-I interferons and/or cytokines. The PERK branch also activates NF-κB by up-regulation of CHOP, which forms heterodimer with C/EBPβ and prevent it from activating PPARγ that suppresses NF-κB activation. The MAP kinases p38 and JNK activate AP-1 and promote cytokine production. Under ER stress, JNK is phosphorylated by IRE1/TRAF2 complex, while ATF3 has been shown to induce DUSP1 that de-phosphorylates p38. Several proteins, such as GADD34 or the spliced form of XBP1, have been shown to cross-talk with the innate immune signaling. Refer to text for detailed description.
NF-κB activation in cells under ER stress may be mediated by UPR via other mechanisms. For example, ER stress induces expression of CHOP, which forms a heterodimer with the CCAAT/enhancer binding protein β (C/EBPβ), preventing it from trans-activating another transcription factor called peroxisome proliferator-activated receptor γ (PPARγ) (Park et al., 2010). Because PPARγ is a negative regulator of NF-κB, up-regulation of CHOP in cells under ER stress thus in effect activates NF-κB and induces the expression of IL-8 (Park et al., 2010). Activation of NF-κB has also been associated with the ATF6-dependent phosphorylation of AKT, although the detail mechanisms have not been determined (Yamazaki et al., 2009). Finally, it should be noted that during coronavirus infection, mediators of innate immunity may be targeted and modulated by the virus. Therefore, functional studies need to be performed to validate the involvement of these signaling pathways in the context of coronavirus infection.
5.2. UPR, MAP kinases and cytokine production
It has been well established that the mitogen activated protein (MAP) kinases are key mediators of innate immunity and pro-inflammatory response (Zhang and Dong, 2005). The binding sites of AP-1 are present in the promoters of type-I interferons (Honda et al., 2005) and cytokines such as IL-8 (Hoffmann et al., 2002). Phosphorylation of AP-1 components (such as Jun and ATF2) by the MAP kinases, in particular p38 and JNK, is crucial for gene expression in response to various stimulations (Karin, 1995). In terms of coronavirus, it has been shown that over-expression of the spike, but not other structural proteins of MHV leads to ER stress and induces the expression of IL-8 (Versteeg et al., 2007). Moreover, induction of IL-8 in cells over-expressing the SARS-CoV spike protein has been demonstrated to be dependent on AP-1 but not NF-κB (Chang et al., 2004). However, the mechanistic link between UPR and MAP kinase dependent cytokine induction remain elusive.
Previously, the induction of IL-6 in cells infected with MHV has been shown to be mediated by the MAP kinase p38 (Banerjee et al., 2002). Studies from this group have also demonstrated that induction of IL-6 and IL-8 in IBV-infected cells is dependent on p38 phosphorylation (Liao et al., 2011). Also, as a negative feedback mechanism, IBV induces the expression of the dual specificity protein phosphatase 1 (DUSP1), which de-phosphorylates p38 and suppresses cytokine production (Liao et al., 2011). Previous studies have indicated that DUSP1 is up-regulated in cells under ER stress (Boutros et al., 2008, Li et al., 2011), possibly via the action of ATF3 in the PERK branch of UPR (Gora et al., 2010). In fact, one recent study has detected a reduced activation of the PERK-eIF2α pathway in cystic fibrosis airway cells, resulting in a higher level of phosphorylated p38 and increased production of IL-6 (Blohmke et al., 2012). Therefore, the signaling through PERK-ATF3-DUSP1 may be used by coronavirus to down-regulate p38 activation and cytokine induction in the infected cells (Fig. 4).
Finally, the MAP kinase JNK is known to regulate cytokine expression by directly activating the AP-1 via phosphorylation of c-Jun (Karin, 1995). In fact, it has been shown that the induction of pro-inflammatory cytokines TNF-α and IL-6 in primary mouse astrocytes infected with MHV-A59 is dependent on the activation of JNK, but not NF-κB, ERK or p38 (Yu et al., 2009). Preliminary data from this group have also confirmed the important role of JNK and its upstream MAP kinase kinase 4/7 (MKK4/7) in the induction of IL-8 during IBV infection. In cells under ER stress, JNK activation has been associated with the IRE1-TRAF2 complex and apoptosis (Urano et al., 2000). However, the involvement of UPR in JNK mediated cytokine production during coronavirus infection remained unexplored.
5.3. GADD34, XBP1, RIDD and innate immunity
Besides the well characterized involvement of UPR in NF-κB and MAP kinase activation, UPR has also been shown to modulate innate immune response by other mechanisms. In particular, the UPR proteins GADD34 and XBP1, as well as the RIDD activity of IRE1, have been implicated in the production of type-I interferons and pro-inflammatory cytokines.
As mentioned above, GADD34 is a co-factor of PP1 that mediates the de-phosphorylation of eIF2α to reverse translation attenuation. Interestingly, when dendritic cells (DCs) are treated with polyriboinosinic:polyribocytidylic acid (polyI:C), expression of GADD34 is induced via the PKR-eIF2α-ATF4 pathway (Clavarino et al., 2012b). Although GADD34 does not significantly affect protein synthesis, it is required for the polyI:C induced production of interferon β (IFN-β) and IL-6 (Clavarino et al., 2012b). Importantly, similar GADD34-dependent induction of IFN-β and IL-6 has also been observed in mouse embryonic fibroblasts and mouse neonates infected with Chikungunya virus (CHIKV) (Clavarino et al., 2012a). In a separate study, however, GADD34 is shown to suppress the production of pro-inflammatory cytokines TNF-α and IL-6 in macrophages triggered by toll-like receptor 3 (TLR3), TLR4, and TLR9 (Gu et al., 2014). This inhibition is mediated by the GADD34/PP1 dependent de-phosphorylation of TGF-β-activated kinase 1 (TAK) in the TLR signaling pathway. Therefore, the activity of GADD34 in innate immune response seems to be cell type and stimulus-specific. Since induction of GADD34 has been observed in IBV-infected cells (Wang et al., 2009), it would be desirable to examine its involvement in cytokine production induced by IBV and other coronaviruses.
The transcription factor XBP1 downstream of IRE1 pathway has also been implicated in innate immune response (Fig. 4). In mouse macrophages treated with TLR2 agonist Pam3CSK4 or TLR4 agonist lipopolysaccharide (LPS), significant IRE1 activation and XBP1 mRNA splicing has been detected (Martinon et al., 2010). Moreover, TLR-activated XBP1 is required for the optimum and sustained production of IFN-β and pro-inflammatory cytokines (such as IL-6 and TNF-α) (Martinon et al., 2010). Similar XBP1-dependent induction of IFN-β has also been observed in murine DCs treated with polyI:C (Hu et al., 2011). Notably, recent studies have identified putative XBP1 binding to the promoter/enhancer sequences of IL-6, TNF-α and IFN-β (Martinon et al., 2010, Zeng et al., 2010). In terms of coronavirus infection, induction of IL-8 has been associated with ER stress and XBP1 splicing in cells infected with MHV or in cells over-expressing the MHV spike protein (Versteeg et al., 2007). Moreover, preliminary data from this group have also shown that over-expression of XBP1 up-regulates the IBV-induced expression of IFN-β and IL-8 at the mRNA level.
Intriguingly, one recent study has pointed to the innate immune signaling function of IRE1, which is mediated via RIDD and is independent of XBP1. In their study, Cho et al. have shown that the A subunit of Cholera toxin (CTA) induces ER stress and activates the RIDD activity of IRE1, which degrades endogenous mRNA into small fragments (Cho et al., 2013). These mRNA fragments are recognized by the cytosolic sensor retinoic acid-inducible gene 1 (RIG-I), which activates NF-κB and induces the production of pro-inflammatory cytokines such as IL-6 and IL-8. Similar observations have been obtained with Shiga toxin and SV40 virus, both of which also enter the ER to induce disease (Cho et al., 2013). Therefore, the IRE1-RIDD dependent signaling may be a general mechanism to bridge ER stress and innate immune response, and its implication during coronavirus infection deserves further investigation.
6. Conclusion
Accumulating evidence suggests that coronavirus infection induces ER stress in the host cells. In response to the disturbance of ER homeostasis, the stressed cells activate UPR via the three ER transmembrane sensors: PERK, IRE1 and ATF6. The adaptation to ER stress is characterized by translation attenuation, selected mRNA degradation (RIDD), enhanced protein folding, ER membrane expansion and ERAD. If prolonged ER stress is not resolved, UPR triggers apoptosis via translation recovery, activation of pro-apoptotic transcription factors (CHOP) and kinases (ASK1 and JNK) and other mechanisms.
At the cellular level, the effect of UPR on coronavirus replication may be multifaceted. Degradation of membrane associated mRNAs via RIDD and translation attenuation reduces the abundance of viral transcripts and viral proteins, and thus serve as potent antiviral strategies. However, certain coronaviruses are shown to be resistant to these mechanisms and may even benefit from the preferential expression of viral proteins under such situations (Bechill et al., 2008). Enhanced protein folding most likely promotes coronavirus replication, as increased amounts of chaperones may be beneficial to the massive production of highly glycosylated spike protein. The expansion of ER membrane may also be a plus for coronavirus, by providing additional membrane source for DMV formation and virion budding. Although ERAD may target ER-localized viral proteins for degradation, the significance of ERAD as an antiviral mechanism during coronavirus replication has not been investigated. In contrast, an ERAD tuning organelle called EDEMosome has been shown to be hijacked by MHV to generate DMVs for replication (Reggiori et al., 2010). Finally, early induction of apoptosis is considered anti-viral because the host cell disintegrates before infectious virus particles are released. Moreover, viral components wrapped in apoptotic bodies may be taken up by DCs for antigen presentation and adaptive immune response (Schulz et al., 2005). On the other hand, apoptosis induced at late stage of infection may be beneficial for the virus. Mature virions can be enclosed by apoptotic bodies and engulfed by neighboring cells or phagocytes, allowing the virus to spread without initiating an immune response (Hay and Kannourakis, 2002).
At the organism level, UPR activation may be a “double-edged sword”. On the good side, certain outcomes of UPR are antiviral in nature, such as the translation attenuation and the synergistic activation of innate immunity described above. The importance of these mechanisms in host antiviral response can be manifested by the numerous counter measures evolved by coronaviruses and other viruses (He, 2006). However, over-activation of the innate immune response is also associated with extensive tissue damage and immunopathogenesis associated with SARS-CoV infection (Perlman and Dandekar, 2005). Indeed, aberrations in UPR activation are associated with the pathogenesis of multiple autoimmune diseases, such as rheumatoid arthritis and inflammatory colitis (Todd et al., 2008). Future studies using appropriate in vivo models are required to elucidate the involvement of UPR in the pro-inflammatory response and innate immunity against coronavirus infection.
Notably, previous studies on coronavirus-induced UPR have been mainly focusing on individual branches of the UPR. It is important to note that the three branches of UPR are not functionally independent, but rather operate as an integrated signaling network (Ron and Walter, 2007). For instance, apart from being a splicing substrate of IRE1, XBP1 is also transcriptionally activated by PERK and ATF6 in cells under ER stress (Calfon et al., 2002, Yoshida et al., 2001a). Also, the phosphatase p58IPK, a downstream UPR gene induced by XBP1s, has been shown to inactivate both PERK and PKR, thereby promoting the translation recovery (Lee et al., 1994, Yan et al., 2002). Moreover, the PERK pathway facilitates expression and activation of ATF6 (Teske et al., 2011), whereas the protein disulfide isomerase A6 (PDIA6) induced by ATF6 has been shown to modulate IRE1 signaling by controlling its degradation (Eletto et al., 2014, Vekich et al., 2012). Therefore, the cross-control of activation and feedback regulations among the three UPR braches gives rise to a fine-tuned temporal program in response to ER stress. In coronavirus-infected cells, this is characterized by an early pro-survival adaptation phase, followed by a rapid trigger of apoptosis at late stage of infection (Fig. 5 ).
Fig. 5.

The cross-talks between three UPR branches and temporal control of UPR during coronavirus infection, using IBV as an example. At the early stage (1–8 h) of infection, the PERK/PKR triggers translational block by eIF2α phosphorylation. The activation of ATF4 and its downstream signaling leads to translation recovery and accumulation of CHOP. The ATF6 and IRE1 branches possibly activate at a much later time of IBV infection (12–16 h). Enhanced ER folding and activation of ERAD may promote adaptation to the ER stress and cell survival. Finally, prolonged ER stress due to continuous IBV replication and budding led to the dead phase of UPR, characterized by caspase cleavage and other apoptosis mediated cell demolitions.
To conclude, studies from the past decade have shown that coronavirus replication causes ER stress and induces UPR in the infected cells. As an evolutionarily conserved stress response, UPR cross-talks with major signaling pathways and constitutes a major aspect of coronavirus-host interaction. The involvement of UPR in apoptosis and innate immune response may be an important factor in the virulence and pathogenesis of coronavirus infection. Therefore, further investigation on coronavirus-induced UPR may identify new targets for anti-viral agents and facilitate development of more effective vaccines against coronavirus.
References
- Acharya P., Chen J.-J., Correia M.A. Hepatic heme-regulated inhibitor (HRI) eukaryotic initiation factor 2α kinase: a protagonist of heme-mediated translational control of CYP2B enzymes and a modulator of basal endoplasmic reticulum stress tone. Mol. Pharmacol. 2010;77(4):575–592. doi: 10.1124/mol.109.061259. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Adachi Y., Yamamoto K., Okada T., Yoshida H., Harada A., Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell. Struct. Funct. 2008;33(1):75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., de Wit E., Munster V.J., Hensley L.E., Zalmout I.S., Kapoor A. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5(2):00814–884. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose R.L., Mackenzie J.M. West Nile virus differentially modulates the unfolded protein response to facilitate replication and immune evasion. J. Virol. 2011;85(6):2723–2732. doi: 10.1128/JVI.02050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose R.L., Mackenzie J.M. ATF6 signaling is required for efficient West Nile virus replication by promoting cell survival and inhibition of innate immune responses. J. Virol. 2013;87(4):2206–2214. doi: 10.1128/JVI.02097-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri K., Harris A.L. Activating transcription factor 4. Int. J. Biochem. Cell Biol. 2008;40(1):14–21. doi: 10.1016/j.biocel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- An S., Chen C.J., Yu X., Leibowitz J.L., Makino S. Induction of apoptosis in murine coronavirus-infected cultured cells and demonstration of E protein as an apoptosis inducer. J. Virol. 1999;73(9):7853–7859. doi: 10.1128/jvi.73.9.7853-7859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio. 2013;4(4):e00524–00513. doi: 10.1128/mBio.00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S., Beg A.A. Defining emerging roles for NF-κB in antivirus responses: revisiting the interferon-β enhanceosome paradigm. PLoS Pathog. 2011;7(10):e1002165. doi: 10.1371/journal.ppat.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltzis D., Qu L.K., Papadopoulou S., Blais J.D., Bell J.C., Sonenberg N., Koromilas A.E. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2α kinases PERK and PKR. J. Virol. 2004;78(23):12747–12761. doi: 10.1128/JVI.78.23.12747-12761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Narayanan K., Mizutani T., Makino S. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 2002;76(12):5937–5948. doi: 10.1128/JVI.76.12.5937-5948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechill J., Chen Z., Brewer J.W., Baker S.C. Coronavirus infection modulates the unfolded protein response and mediates sustained translational repression. J. Virol. 2008;82(9):4492–4501. doi: 10.1128/JVI.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga J.J., Ventoso I., Harding H.P., Deng J., Ron D., Sonenberg N., Carrasco L., de Haro C. Antiviral effect of the mammalian translation initiation factor 2α kinase GCN2 against RNA viruses. EMBO J. 2006;25(8):1730–1740. doi: 10.1038/sj.emboj.7601073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2(6):326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Sen U., Vrati S. Regulated IRE1-dependent decay pathway is activated during Japanese encephalitis virus-induced unfolded protein response and benefits viral replication. J. Gen. Virol. 2014;95(Pt 1):71–79. doi: 10.1099/vir.0.057265-0. [DOI] [PubMed] [Google Scholar]
- Black T.L., Safer B., Hovanessian A., Katze M.G. The cellular 68,000-Mr protein kinase is highly autophosphorylated and activated yet significantly degraded during poliovirus infection: implications for translational regulation. J. Virol. 1989;63(5):2244–2251. doi: 10.1128/jvi.63.5.2244-2251.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blohmke C.J., Mayer M.L., Tang A.C., Hirschfeld A.F., Fjell C.D., Sze M.A., Falsafi R., Wang S., Hsu K., Chilvers M.A. Atypical activation of the unfolded protein response in cystic fibrosis airway cells contributes to p38 MAPK-mediated innate immune responses. J. Immunol. 2012;189(11):5467–5475. doi: 10.4049/jimmunol.1103661. [DOI] [PubMed] [Google Scholar]
- Bordi L., Castilletti C., Falasca L., Ciccosanti F., Calcaterra S., Rozera G., Di Caro A., Zaniratti S., Rinaldi A., Ippolito G. Bcl-2 inhibits the caspase-dependent apoptosis induced by SARS-CoV without affecting virus replication kinetics. Arch. Virol. 2006;151(2):369–377. doi: 10.1007/s00705-005-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros T., Nantel A., Emadali A., Tzimas G., Conzen S., Chevet E., Metrakos P. The MAP kinase phosphatase-1 MKP-1/DUSP1 is a regulator of human liver response to transplantation. Am. J. Transplant. 2008;8(12):2558–2568. doi: 10.1111/j.1600-6143.2008.02420.x. [DOI] [PubMed] [Google Scholar]
- Brush M.H., Weiser D.C., Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1α to the endoplasmic reticulum and promotes dephosphorylation of the α subunit of eukaryotic translation initiation factor 2. Mol. Cell Biol. 2003;23(4):1292–1303. doi: 10.1128/MCB.23.4.1292-1303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett H.F., Audas T.E., Liang G., Lu R.R. Herpes simplex virus-1 disarms the unfolded protein response in the early stages of infection. Cell Stress Chaperones. 2012;17(4):473–483. doi: 10.1007/s12192-012-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfon M., Zeng H., Urano F., Till J.H., Hubbard S.R., Harding H.P., Clark S.G., Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Castillo K., Rojas-Rivera D., Lisbona F., Caballero B., Nassif M., Court F.A., Schuck S., Ibar C., Walter P., Sierralta J. BAX inhibitor-1 regulates autophagy by controlling the IRE1α branch of the unfolded protein response. EMBO J. 2011;30(21):4465–4478. doi: 10.1038/emboj.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38(2):281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Chan C.P., Siu K.L., Chin K.T., Yuen K.Y., Zheng B., Jin D.Y. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2006;80(18):9279–9287. doi: 10.1128/JVI.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.-J., Liu C.Y.-Y., Chiang B.-L., Chao Y.-C., Chen C.-C. Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J. Immunol. 2004;173(12):7602–7614. doi: 10.4049/jimmunol.173.12.7602. [DOI] [PubMed] [Google Scholar]
- Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C., Choi K.W., Tso Y.K., Lau T., Lai S.T. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Pan Y.-X., Dudenhausen E.E., Kilberg M.S. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J. Biol. Chem. 2004;279(49):50829–50839. doi: 10.1074/jbc.M409173200. [DOI] [PubMed] [Google Scholar]
- Cheng G., Feng Z., He B. Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2α dephosphorylation by the γ134. 5 protein. J. Virol. 2005;79(3):1379–1388. doi: 10.1128/JVI.79.3.1379-1388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.A., Lee A.-H., Platzer B., Cross B., Gardner B.M., De Luca H., Luong P., Harding H.P., Glimcher L.H., Walter P. The unfolded protein response element IRE1α senses bacterial proteins invading the ER to activate RIG-I and innate immune signaling. Cell Host Microbe. 2013;13(5):558–569. doi: 10.1016/j.chom.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Clavarino G., Cláudio N., Couderc T., Dalet A., Judith D., Camosseto V., Schmidt E.K., Wenger T., Lecuit M., Gatti E. Induction of GADD34 is necessary for dsRNA-dependent interferon-β production and participates in the control of chikungunya virus infection. PLoS Pathog. 2012;8(5):e1002708. doi: 10.1371/journal.ppat.1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavarino G., Cláudio N., Dalet A., Terawaki S., Couderc T., Chasson L., Ceppi M., Schmidt E.K., Wenger T., Lecuit M. Protein phosphatase 1 subunit Ppp1r15a/GADD34 regulates cytokine production in polyinosinic: polycytidylic acid-stimulated dendritic cells. Proc. Natl. Acad. Sci. USA. 2012;109(8):3006–3011. doi: 10.1073/pnas.1104491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M.J., Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J. Interferon Cytokine Res. 1997;17(9):503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- Cosnefroy O., Jaspart A., Calmels C., Parissi V., Fleury H., Ventura M., Reigadas S., Andréola M.-L. Activation of GCN2 upon HIV-1 infection and inhibition of translation. Cell. Mol. Life Sci. 2013;70(13):2411–2421. doi: 10.1007/s00018-013-1272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam E.M., Maier H.J., Manifava M., Vaux L.C., Chandra-Schoenfelder P., Gerner W., Britton P., Ktistakis N.T., Wileman T. Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy. 2011;7(11):1335–1347. doi: 10.4161/auto.7.11.16642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credle J.J., Finer-Moore J.S., Papa F.R., Stroud R.M., Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2005;102(52):18773–18784. doi: 10.1073/pnas.0509487102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J.L., Sola I., Becares M., Alberca B., Plana J., Enjuanes L., Zuñiga S. Coronavirus gene 7 counteracts host defenses and modulates virus virulence. PLoS Pathog. 2011;7(6):e1002090. doi: 10.1371/journal.ppat.1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Ferreira J., Manaker R. An electron microscope study of the development of a mouse hepatitis virus in tissue culture cells. J. Cell Biol. 1965;24(1):57–78. doi: 10.1083/jcb.24.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R.J., Baker S.C., Baric R.S., Brown C.S., Drosten C., Enjuanes L., Fouchier R.A., Galiano M., Gorbalenya A.E., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J. Virol. 2013;87(14):7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haro C., Mendez R., Santoyo J. The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 1996;10(12):1378–1387. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- DeDiego M.L., Álvarez E., Almazán F., Rejas M.T., Lamirande E., Roberts A., Shieh W.J., Zaki S.R., Subbarao K., Enjuanes L. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J. Virol. 2007;81(4):1701–1713. doi: 10.1128/JVI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDiego M.L., Nieto-Torres J.L., Jiménez-Guardeño J.M., Regla-Nava J.A., Álvarez E., Oliveros J.C., Zhao J., Fett C., Perlman S., Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein regulates cell stress response and apoptosis. PLoS Pathog. 2011;7(10):e1002315. doi: 10.1371/journal.ppat.1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pino J., Jiménez J.L., Ventoso I., Castelló A., Muñoz-Fernández M.Á., de Haro C., Berlanga J.J. GCN2 has inhibitory effect on human immunodeficiency virus-1 protein synthesis and is cleaved upon viral infection. PLoS One. 2012;7(10):e47272. doi: 10.1371/journal.pone.0047272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J. Virol. 1990;64(11):5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever T.E., Feng L., Wek R.C., Cigan A.M., Donahue T.F., Hinnebusch A.G. Phosphorylation of initiation factor 2α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68(3):585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142(1):19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eletto D., Eletto D., Dersh D., Gidalevitz T., Argon Y. Protein disulfide isomerase A6 controls the decay of IRE1α signaling via disulfide-dependent association. Mol. Cell. 2014;53(4):562–576. doi: 10.1016/j.molcel.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau D.J., Desforges M., St-Jean J.R., Talbot P.J. A human coronavirus OC43 variant harboring persistence-associated mutations in the S glycoprotein differentially induces the unfolded protein response in human neurons as compared to wild-type virus. Virology. 2009;395(2):255–267. doi: 10.1016/j.virol.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett T., Martindale J., Guyton K., Hai T., Holbrook N. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem. J. 1999;339:135–141. [PMC free article] [PubMed] [Google Scholar]
- Fukushi M., Yoshinaka Y., Matsuoka Y., Hatakeyama S., Ishizaka Y., Kirikae T., Sasazuki T., Miyoshi-Akiyama T. Monitoring S protein maturation in the endoplasmic reticulum by calnexin is important for the infectivity of severe acute respiratory syndrome-coronavirus. J. Virol. 2012;86(21):11745–11753. doi: 10.1128/JVI.01250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5(296):1–13. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liao Y., Liu D.X. The ER stress sensor IRE1α protects cells from apoptosis induced by coronavirus infectious bronchitis virus. J. Virol. 2014;88(21):12752–12764. doi: 10.1128/JVI.02138-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M.J., Jr., Korth M.J., Tang N.M., Tan S.L., Hopkins D.A., Dever T.E., Polyak S.J., Gretch D.R., Katze M.G. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 1997;230(2):217. doi: 10.1006/viro.1997.8493. [DOI] [PubMed] [Google Scholar]
- Galindo I., Hernaez B., Munoz-Moreno R., Cuesta-Geijo M., Dalmau-Mena I., Alonso C. The ATF6 branch of unfolded protein response and apoptosis are activated to promote African swine fever virus infection. Cell Death Dis. 2012;3(7):e341. doi: 10.1038/cddis.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Lee S.M., Chen A., Zhang J., Zhang D.D., Kannan K., Ortmann R.A., Fang D. Synoviolin promotes IRE1 ubiquitination and degradation in synovial fibroblasts from mice with collagen-induced arthritis. EMBO Rep. 2008;9(5):480–485. doi: 10.1038/embor.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. XBP1: the last two decades. Ann. Rheum. Dis. 2010;69(Suppl 1):i67–i71. doi: 10.1136/ard.2009.119388. [DOI] [PubMed] [Google Scholar]
- Gora S., Maouche S., Atout R., Wanherdrick K., Lambeau G., Cambien F., Ninio E., Karabina S.-A. Phospholipolyzed LDL induces an inflammatory response in endothelial cells through endoplasmic reticulum stress signaling. FASEB J. 2010;24(9):3284–3297. doi: 10.1096/fj.09-146852. [DOI] [PubMed] [Google Scholar]
- Gosert R., Kanjanahaluethai A., Egger D., Bienz K., Baker S.C. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 2002;76(8):3697–3708. doi: 10.1128/JVI.76.8.3697-3708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh T., Oyadomari S., Mori K., Mori M. Nitric oxide-induced apoptosis in RAW 264.7 macrophages is mediated by endoplasmic reticulum stress pathway involving ATF6 and CHOP. J. Biol. Chem. 2002;277(14):12343–12350. doi: 10.1074/jbc.M107988200. [DOI] [PubMed] [Google Scholar]
- Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M., Ouyang C., Lin W., Zhang T., Cao X., Xia Z., Wang X. Phosphatase holoenzyme PP1/GADD34 negatively regulates TLR response by inhibiting TAK1 serine 412 phosphorylation. J. Immunol. 2014;192(6):2846–2856. doi: 10.4049/jimmunol.1302537. [DOI] [PubMed] [Google Scholar]
- Guan Y., Zheng B., He Y., Liu X., Zhuang Z., Cheung C., Luo S., Li P., Zhang L., Guan Y. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Haagmans B.L., Egberink H.F., Horzinek M.C. Apoptosis and T-cell depletion during feline infectious peritonitis. J. Virol. 1996;70(12):8977–8983. doi: 10.1128/jvi.70.12.8977-8983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habjan M., Pichlmair A., Elliott R.M., Överby A.K., Glatter T., Gstaiger M., Superti-Furga G., Unger H., Weber F. NSs protein of rift valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J. Virol. 2009;83(9):4365–4375. doi: 10.1128/JVI.02148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Exp. Biol. Med. 1966;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Han D., Lerner A.G., Vande Walle L., Upton J.-P., Xu W., Hagen A., Backes B.J., Oakes S.A., Papa F.R. IRE1α kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Back S.H., Hur J., Lin Y.-H., Gildersleeve R., Shan J., Yuan C.L., Krokowski D., Wang S., Hatzoglou M. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013;15(5):481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Hassan I.H., Zhang M.S., Powers L.S., Shao J.Q., Baltrusaitis J., Rutkowski D.T., Legge K., Monick M.M. Influenza A viral replication is blocked by inhibition of the inositol requiring enzyme 1 (IRE1) stress pathway. J. Biol. Chem. 2011;287(7):4679–4689. doi: 10.1074/jbc.M111.284695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan I., Gaines K.S., Hottel W.J., Wishy R.M., Miller S.E., Powers L.S., Rutkowski D.T., Monick M.M. Inositol-requiring Enzyme 1 Inhibits Respiratory Syncytial Virus Replication. J. Biol. Chem. 2014;289(11):7537–7546. doi: 10.1074/jbc.M113.510594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay S., Kannourakis G. A time to kill: viral manipulation of the cell death program. J. Gen. Virol. 2002;83(7):1547–1564. doi: 10.1099/0022-1317-83-7-1547. [DOI] [PubMed] [Google Scholar]
- Hayden M.S., Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13(3):393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- He B., Chou J., Brandimarti R., Mohr I., Gluzman Y., Roizman B. Suppression of the phenotype of gamma (1) 34.5-herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J. Virol. 1997;71(8):6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.H., Gong P., Hu B., Stewart D., Choi M.E., Choi A.M., Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001;276(24):20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- Hitomi J., Katayama T., Eguchi Y., Kudo T., Taniguchi M., Koyama Y., Manabe T., Yamagishi S., Bando Y., Imaizumi K. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J. Cell Biol. 2004;165(3):347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E., Dittrich-Breiholz O., Holtmann H., Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72(5):847–855. [PubMed] [Google Scholar]
- Hollien J., Weissman J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- Hollien J., Lin J.H., Li H., Stevens N., Walter P., Weissman J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009;186(3):323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Yanai H., Takaoka A., Taniguchi T. Regulation of the type I IFN induction: a current view. Int. Immunol. 2005;17(11):1367–1378. doi: 10.1093/intimm/dxh318. [DOI] [PubMed] [Google Scholar]
- Hong M., Luo S., Baumeister P., Huang J.-M., Gogia R.K., Li M., Lee A.S. Underglycosylation of ATF6 as a novel sensing mechanism for activation of the unfolded protein response. J. Biol. Chem. 2004;279(12):11354–11363. doi: 10.1074/jbc.M309804200. [DOI] [PubMed] [Google Scholar]
- Hooks K.B., Griffiths-Jones S. Conserved RNA structures in the non-canonical Hac1/Xbp1 intron. RNA Biol. 2011;8(4):552–556. doi: 10.4161/rna.8.4.15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Yu X., Wang H., Zuo D., Guo C., Yi H., Tirosh B., Subjeck J.R., Qiu X., Wang X.Y. ER stress and its regulator X-box-binding protein-1 enhance polyIC-induced innate immune response in dendritic cells. Eur. J. Immunol. 2011;41(4):1086–1097. doi: 10.1002/eji.201040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., Lei H.Y. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.-Y., Wek S.A., McGrath B.C., Scheuner D., Kaufman R.J., Cavener D.R., Wek R.C. Phosphorylation of the α subunit of eukaryotic initiation factor 2 is required for activation of NF-κB in response to diverse cellular stresses. Mol. Cell Biol. 2003;23(16):5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171(8):850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- Jordan R., Wang L., Graczyk T.M., Block T.M., Romano P.R. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 2002;76(19):9588–9599. doi: 10.1128/JVI.76.19.9588-9599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek N., London N., Schueler-Furman O., Ben-Neriah Y. Ubiquitination and Degradation of the Inhibitors of NF-κB. Cold Spring Harb. Perspect. Biol. 2010;2(2):a000166. doi: 10.1101/cshperspect.a000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;270(28):16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 2000;18(1):621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kaye H.S., Ong S.B., Dowdle W.R. Detection of coronavirus 229E antibody by indirect hemagglutination. Appl. Microbiol. 1972;24(5):703–707. doi: 10.1128/am.24.5.703-707.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi S., Papadopoulou S., Li S., Su Q., Wang S., Yoshimura A., Matlashewski G., Dever T.E., Koromilas A.E. Control of α subunit of eukaryotic translation initiation factor 2 (eIF2α) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2α-dependent gene expression and cell death. Mol. Cell Biol. 2004;24(8):3415–3429. doi: 10.1128/MCB.24.8.3415-3429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo D., Perez C., Mohr I. Characterization of RNA determinants recognized by the arginine-and proline-rich region of Us11, a herpes simplex virus type 1-encoded double-stranded RNA binding protein that prevents PKR activation. J. Virol. 2002;76(23):11971–11981. doi: 10.1128/JVI.76.23.11971-11981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S.R. Eukaryotic initiation factor eIF2. Int. J. Biochem. Cell Biol. 1999;31(1):25–29. doi: 10.1016/s1357-2725(98)00128-9. [DOI] [PubMed] [Google Scholar]
- Klumperman J., Locker J.K., Meijer A., Horzinek M.C., Geuze H.J., Rottier P. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J. Virol. 1994;68(10):6523–6534. doi: 10.1128/jvi.68.10.6523-6534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops K., Kikkert M., Van Den Worm S.H.E., Zevenhoven-Dobbe J.C., Van Der Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6(9):e226. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korennykh A., Walter P. Structural basis of the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2012;28:251–277. doi: 10.1146/annurev-cellbio-101011-155826. [DOI] [PubMed] [Google Scholar]
- Krähling V., Stein D.A., Spiegel M., Weber F., Mühlberger E. Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity. J. Virol. 2009;83(5):2298–2309. doi: 10.1128/JVI.01245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy J., Mounir Z., Raven J.F., Koromilas A.E. The eIF2alpha kinases inhibit vesicular stomatitis virus replication independently of eIF2alpha phosphorylation. Cell Cycle. 2008;7(15):2346–2351. doi: 10.4161/cc.6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N., Fu C., Pappin D.J., Tonks N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011;4(203):ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kunsch C., Rosen C.A. NF-κB subunit-specific regulation of the interleukin-8 promoter. Mol. Cell Biol. 1993;13(10):6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langland J.O., Cameron J.M., Heck M.C., Jancovich J.K., Jacobs B.L. Inhibition of PKR by RNA and DNA viruses. Virus Res. 2006;119(1):100–110. doi: 10.1016/j.virusres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Lee T.G., Tang N., Thompson S., Miller J., Katze M.G. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol. Cell Biol. 1994;14(4):2331–2342. doi: 10.1128/mcb.14.4.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol. 2003;23(21):7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Li F.Q., Tam J.P., Liu D.X. Cell cycle arrest and apoptosis induced by the coronavirus infectious bronchitis virus in the absence of p53. Virology. 2007;365(2):435–445. doi: 10.1016/j.virol.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Yi P., Zhang B., Xu C., Liu Q., Pi Z., Xu X., Chevet E., Liu J. Differences in endoplasmic reticulum stress signalling kinetics determine cell survival outcome through activation of MKP-1. Cell. Signal. 2011;23(1):35–45. doi: 10.1016/j.cellsig.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Liao Y., Wang X., Huang M., Tam J.P., Liu D.X. Regulation of the p38 mitogen-activated protein kinase and dual-specificity phosphatase 1 feedback loop modulates the induction of interleukin 6 and 8 in cells infected with coronavirus infectious bronchitis virus. Virology. 2011;420(2):106–116. doi: 10.1016/j.virol.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Fung T.S., Huang M., Fang S.G., Zhong Y., Liu D.X. Up-regulation of CHOP/GADD153 during coronavirus infectious bronchitis virus infection modulates apoptosis by restricting activation of the extracellular signal-regulated kinase pathway. J. Virol. 2013;87(14):8124–8134. doi: 10.1128/JVI.00626-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann T.A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.Y., Schröder M., Kaufman R.J. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 2000;275(32):24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- Liu C., Xu H.Y., Liu D.X. Induction of caspase-dependent apoptosis in cultured cells by the avian coronavirus infectious bronchitis virus. J. Virol. 2001;75(14):6402–6409. doi: 10.1128/JVI.75.14.6402-6409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., HuangFu W.-C., Kumar K., Qian J., Casey J.P., Hamanaka R.B., Grigoriadou C., Aldabe R., Diehl J.A., Fuchs S.Y. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe. 2009;5(1):72–83. doi: 10.1016/j.chom.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Wambach M., Katze M.G., Krug R.M. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology. 1995;214(1):222. doi: 10.1006/viro.1995.9937. [DOI] [PubMed] [Google Scholar]
- Maier H.J., Hawes P.C., Cottam E.M., Mantell J., Verkade P., Monaghan P., Wileman T., Britton P. Infectious bronchitis virus generates spherules from zippered endoplasmic reticulum membranes. mBio. 2013;4(5):e00801–00813. doi: 10.1128/mBio.00801-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak S.J., Yun C.Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H.P., Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F., Chen X., Lee A.-H., Glimcher L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010;11(5):411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel M., Chevet E., Tavernier J., Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014;39(5):245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- McCullough K.D., Martindale J.L., Klotz L.-O., Aw T.-Y., Holbrook N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell Biol. 2001;21(4):1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen E., Kedersha N., Song B., Scheuner D., Gilks N., Han A., Chen J.J., Anderson P., Kaufman R.J. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J. Biol. Chem. 2005;280(17):16925–16933. doi: 10.1074/jbc.M412882200. [DOI] [PubMed] [Google Scholar]
- Memish Z.A., Mishra N., Olival K.J., Fagbo S.F., Kapoor V., Epstein J.H., AlHakeem R., Durosinloun A., Al Asmari M., Islam A. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 2013;19(11):1819. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merquiol E., Uzi D., Mueller T., Goldenberg D., Nahmias Y., Xavier R.J., Tirosh B., Shibolet O. HCV causes chronic endoplasmic reticulum stress leading to adaptation and interference with the unfolded protein response. PLoS One. 2011;6(9):e24660. doi: 10.1371/journal.pone.0024660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S., Krijnse-Locker J. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 2008;6(5):363–374. doi: 10.1038/nrmicro1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakshi R., Padhan K., Rani M., Khan N., Ahmad F., Jameel S. The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PLoS One. 2009;4(12):e8342. doi: 10.1371/journal.pone.0008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. Phosphorylation of p38 MAPK and its downstream targets in SARS coronavirus-infected cells. Biochem. Biophys. Res. Commun. 2004;319(4):1228–1234. doi: 10.1016/j.bbrc.2004.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima N., Nakanishi K., Nakano A. Activating transcription factor-6 (ATF6) mediates apoptosis with reduction of myeloid cell leukemia sequence 1 (Mcl-1) protein via induction of WW domain binding protein 1. J. Biol. Chem. 2011;286(40):35227–35235. doi: 10.1074/jbc.M111.233502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaka S., Okada T., Yoshida H., Mori K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol. Cell Biol. 2007;27(3):1027–1043. doi: 10.1128/MCB.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A., Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403(6765):98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Sudo T., Morishima N. Endoplasmic reticulum stress signaling transmitted by ATF6 mediates apoptosis during muscle development. J. Cell Biol. 2005;169(4):555–560. doi: 10.1083/jcb.200412024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16(11):1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Hino S., Saito A., Morikawa K., Kondo S., Kanemoto S., Murakami T., Taniguchi M., Tanii I., Yoshinaga K., Shiosaka S., Hammarback J.A., Urano F., Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell Biol. 2006;26(24):9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka N., Hattori T., Kitagawa M., Onozaki K., Hayashi H. Critical and functional regulation of CHOP (C/EBP homologous protein) through the N-terminal portion. J. Biol. Chem. 2007;282(49):35687–35694. doi: 10.1074/jbc.M703735200. [DOI] [PubMed] [Google Scholar]
- Oikawa D., Tokuda M., Hosoda A., Iwawaki T. Identification of a consensus element recognized and cleaved by IRE1α. Nucleic Acids Res. 2010:gkq452. doi: 10.1093/nar/gkq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-H., Choi H.J., Yang H., Do K.H., Kim J., Lee D.W., Moon Y. Endoplasmic reticulum stress-activated C/EBP homologous protein enhances nuclear factor-kappaB signals via repression of peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2010;285(46):35330–35339. doi: 10.1074/jbc.M110.136259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavio N., Romano P.R., Graczyk T.M., Feinstone S.M., Taylor D.R. Protein synthesis and endoplasmic reticulum stress can be modulated by the hepatitis C virus envelope protein E2 through the eukaryotic initiation factor 2α kinase PERK. J. Virol. 2003;77(6):3578–3585. doi: 10.1128/JVI.77.6.3578-3585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5(12):917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., de Breyne S., Pisarev A.V., Abaeva I.S., Hellen C.U. eIF2-dependent and eIF2-independent modes of initiation on the CSFV IRES: a common role of domain II. Embo. J. 2008;27(7):1060–1072. doi: 10.1038/emboj.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus D., Chevalier M.W., Aragón T., van Anken E., Vidal S.E., El-Samad H., Walter P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8(7):e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H., O’Reilly L.A., Gunn P., Lee L., Kelly P.N., Huntington N.D., Hughes P.D., Michalak E.M., McKimm-Breschkin J., Motoyama N. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Reggiori F., Monastyrska I., Verheije M.H., Cali T., Ulasli M., Bianchi S., Bernasconi R., de Haan C.A., Molinari M. Coronaviruses Hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe. 2010;7(6):500–508. doi: 10.1016/j.chom.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Yang R., Guo L., Qu J., Wang J., Hung T. Apoptosis induced by the SARS-associated coronavirus in Vero cells is replication-dependent and involves caspase. DNA Cell Biol. 2005;24(8):496–502. doi: 10.1089/dna.2005.24.496. [DOI] [PubMed] [Google Scholar]
- Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Roth-Cross J.K., Martínez-Sobrido L., Scott E.P., García-Sastre A., Weiss S.R. Inhibition of the alpha/beta interferon response by mouse hepatitis virus at multiple levels. J. Virol. 2007;81(13):7189–7199. doi: 10.1128/JVI.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler A., Williams B. Interferon: The 50th Anniversary. Springer Berlin Heidelberg; 2007. Structure and Function of the Protein Kinase R; pp. 253–292. [Google Scholar]
- Samali A., Fitzgerald U., Deegan S., Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int. J. Cell Biol. 2010;2010:830307. doi: 10.1155/2010/830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O., Diebold S.S., Chen M., Näslund T.I., Nolte M.A., Alexopoulou L., Azuma Y.-T., Flavell R.A., Liljeström P., e Sousa C.R. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433(7028):887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- Shen J., Chen X., Hendershot L., Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell. 2002;3(1):99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Shi Y., Vattem K.M., Sood R., An J., Liang J., Stramm L., Wek R.C. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell Biol. 1998;18(12):7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C., Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90(6):1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Siu K.-L., Chan C.-P., Kok K.-H., Woo P.C., Jin D.-Y. Comparative analysis of the activation of unfolded protein response by spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus HKU1. Cell Biosci. 2014;4(3):1–9. doi: 10.1186/2045-3701-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.A. A new paradigm: innate immune sensing of viruses via the unfolded protein response. Front. Microbiol. 2014:5. doi: 10.3389/fmicb.2014.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J., van der Meulen J., Koerten H.K., Mommaas A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80(12):5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44(2):167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood R., Porter A.C., Olsen D.A., Cavener D.R., Wek R.C. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2α. Genetics. 2000;154(2):787–801. doi: 10.1093/genetics/154.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriburi R., Jackowski S., Mori K., Brewer J.W. XBP1 a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 2004;167(1):35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stertz S., Reichelt M., Spiegel M., Kuri T., Martínez-Sobrido L., García-Sastre A., Weber F., Kochs G. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361(2):304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S.C., Chao C.Y., Jeng K.S., Yang J.Y., Lai M. The 8ab protein of SARS-CoV is a luminal ER membrane-associated protein and induces the activation of ATF6. Virology. 2009;387(2):402–413. doi: 10.1016/j.virol.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E., Logue S.E., Gorman A.M., Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A.B., Mercado E.L., Hoffmann A., Niwa M. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7(10):e45078. doi: 10.1371/journal.pone.0045078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B.S., Chan K.-h., Cheng V.C., Woo P.C., Lau S.K., Lam C.C., Chan T.-l., Wu A.K., Hung I.F., Leung S.-y. Comparative host gene transcription by microarray analysis early after infection of the Huh7 cell line by severe acute respiratory syndrome coronavirus and human coronavirus 229E. J. Virol. 2005;79(10):6180–6193. doi: 10.1128/JVI.79.10.6180-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenin I.M., Dmitriev S.E., Andreev D.E., Shatsky I.N. Eukaryotic translation initiation machinery can operate in a bacterial-like mode without eIF2. Nat. Struct. Mol. Biol. 2008;15(8):836–841. doi: 10.1038/nsmb.1445. [DOI] [PubMed] [Google Scholar]
- Teske B.F., Wek S.A., Bunpo P., Cundiff J.K., McClintick J.N., Anthony T.G., Wek R.C. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol. Biol. Cell. 2011;22(22):4390–4405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testerink N., van der Sanden M.H., Houweling M., Helms J.B., Vaandrager A.B. Depletion of phosphatidylcholine affects endoplasmic reticulum morphology and protein traffic at the Golgi complex. J. Lipid Res. 2009;50(11):2182–2192. doi: 10.1194/jlr.M800660-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh B., Iwakoshi N.N., Glimcher L.H., Ploegh H.L. Rapid turnover of unspliced Xbp-1 as a factor that modulates the unfolded protein response. J. Biol. Chem. 2006;281(9):5852–5860. doi: 10.1074/jbc.M509061200. [DOI] [PubMed] [Google Scholar]
- Todd D.J., Lee A.-H., Glimcher L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008;8(9):663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- van der Saden M., Houweling M., van Golde L., Vaandrager A. Inhibition of phosphatidylcholine synthesis induces expression of the endoplasmic reticulum stress and apoptosis-related protein CCAAT/enhancer-binding protein-homologous protein (CHOP/GADD153) Biochem. J. 2003;369:643–650. doi: 10.1042/BJ20020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem K.M., Wek R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA. 2004;101(31):11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekich J.A., Belmont P.J., Thuerauf D.J., Glembotski C.C. Protein disulfide isomerase-associated 6 is an ATF6-inducible ER stress response protein that protects cardiac myocytes from ischemia/reperfusion-mediated cell death. J. Mol. Cell. Cardiol. 2012;53(2):259–267. doi: 10.1016/j.yjmcc.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg G.A., Van De Nes P.S., Bredenbeek P.J., Spaan W.J.M. The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations. J. Virol. 2007;81(20):10981–10990. doi: 10.1128/JVI.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Eaton, B., 2007. Bats, civets and the emergence of SARS. Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission, 325–344.
- Wang J., Basagoudanavar S.H., Wang X., Hopewell E., Albrecht R., García-Sastre A., Balachandran S., Beg A.A. NF-κB RelA subunit is crucial for early IFN-β expression and resistance to RNA virus replication. J. Immunol. 2010;185(3):1720–1729. doi: 10.4049/jimmunol.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Kang R., Huang H., Xi X., Wang B., Wang J., Zhao Z. Hepatitis C virus core protein activates autophagy through EIF2AK3 and ATF6 UPR pathway-mediated MAP1LC3B and ATG12 expression. Autophagy. 2014;10(5) doi: 10.4161/auto.27954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Ye L., Ye L., Li B., Gao B., Zeng Y., Kong L., Fang X., Zheng H., Wu Z. Up-regulation of IL-6 and TNF-α induced by SARS-coronavirus spike protein in murine macrophages via NF-κB pathway. Virus Res. 2007;128(1):1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liao Y., Yap P.L., Png K.J., Tam J.P., Liu D.X. Inhibition of protein kinase R activation and upregulation of GADD34 expression play a synergistic role in facilitating coronavirus replication by maintaining de novo protein synthesis in virus-infected cells. J. Virol. 2009;83(23):12462–12472. doi: 10.1128/JVI.01546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Sinha S., Levine B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy. 2008;4(7):949–951. doi: 10.4161/auto.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won S., Eidenschenk C., Arnold C.N., Siggs O.M., Sun L., Brandl K., Mullen T.-M., Nemerow G.R., Moresco E.M.Y., Beutler B. Increased susceptibility to DNA virus infection in mice with a GCN2 mutation. J. Virol. 2012;86(3):1802–1808. doi: 10.1128/JVI.05660-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Wang H.-G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004;279(44):45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Yoshida H., Kokame K., Kaufman R.J., Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J. Biochem. 2004;136(3):343–350. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Suzuki N., Wada T., Okada T., Yoshida H., Kaufman R.J., Mori K. Human HRD1 promoter carries a functional unfolded protein response element to which XBP1 but not ATF6 directly binds. J. Biochem. 2008;144(4):477–486. doi: 10.1093/jb/mvn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H., Hiramatsu N., Hayakawa K., Tagawa Y., Okamura M., Ogata R., Huang T., Nakajima S., Yao J., Paton A.W. Activation of the Akt-NF-κB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 2009;183(2):1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Frank C.L., Korth M.J., Sopher B.L., Novoa I., Ron D., Katze M.G. Control of PERK eIF2α kinase activity by the endoplasmic reticulum stress-induced molecular chaperone p58IPK. Proc. Natl. Acad. Sci. USA. 2002;99(25):15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Rawson R.B., Komuro R., Chen X., Davé U.P., Prywes R., Brown M.S., Goldstein J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell. 2000;6(6):1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- Ye Y., Hauns K., Langland J.O., Jacobs B.L., Hogue B.G. Mouse hepatitis coronavirus A59 nucleocapsid protein is a type I interferon antagonist. J. Virol. 2007;81(6):2554–2563. doi: 10.1128/JVI.01634-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Wong C.K., Li P., Xie Y. A SARS-CoV protein, ORF-6, induces caspase-3 mediated, ER stress and JNK-dependent apoptosis. Biochim. Biophys. Acta. 2008;1780(12):1383–1387. doi: 10.1016/j.bbagen.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung Y.S., Yip C.W., Hon C.C., Chow K.Y.C., Ma I., Zeng F., Leung F.C.C. Transcriptional profiling of Vero E6 cells over-expressing SARS-CoV S2 subunit: Insights on viral regulation of apoptosis and proliferation. Virology. 2008;371(1):32–43. doi: 10.1016/j.virol.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M., Mori K. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6α and 6β that activates the mammalian unfolded protein response. Mol. Cell Biol. 2001;21(4):1239–1248. doi: 10.1128/MCB.21.4.1239-1248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Oku M., Suzuki M., Mori K. pXBP1 (U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1 (S) in mammalian ER stress response. J. Cell Biol. 2006;172(4):565–575. doi: 10.1083/jcb.200508145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.Y., Hsu Y.W., Liao C.L., Lin Y.L. Flavivirus infection activates the XBP1 pathway of the unfolded protein response to cope with endoplasmic reticulum stress. J. Virol. 2006;80(23):11868–11880. doi: 10.1128/JVI.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Zhu H., Liu Y., Cao J., Zhang X. Regulation of proinflammatory cytokine expression in primary mouse astrocytes by coronavirus infection. J. Virol. 2009;83(23):12204–12214. doi: 10.1128/JVI.01103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Achazi K., Niedrig M. Tick-borne encephalitis virus triggers inositol-requiring enzyme 1 (IRE1) and transcription factor 6 (ATF6) pathways of unfolded protein response. Virus Res. 2013;178(2):471–477. doi: 10.1016/j.virusres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Zeng L., Liu Y.-P., Sha H., Chen H., Qi L., Smith J.A. XBP-1 couples endoplasmic reticulum stress to augmented IFN-β induction via a cis-acting enhancer in macrophages. J. Immunol. 2010;185(4):2324–2330. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Dong C. MAP kinases in immune responses. Cell. Mol. Immunol. 2005;2(1):20–27. [PubMed] [Google Scholar]
- Zhang H.M., Ye X., Su Y., Yuan J., Liu Z., Stein D.A., Yang D. Coxsackievirus B3 infection activates the unfolded protein response and induces apoptosis through downregulation of p58IPK and activation of CHOP and SREBP1. J. Virol. 2010;84(17):8446–8459. doi: 10.1128/JVI.01416-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Rose K.M., Elliott R., Van Rooijen N., Weiss S.R. Cell-type-specific type I interferon antagonism influences organ tropism of murine coronavirus. J. Virol. 2011;85(19):10058–10068. doi: 10.1128/JVI.05075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Jha B.K., Wu A., Elliott R., Ziebuhr J., Gorbalenya A.E., Silverman R.H., Weiss S.R. Antagonism of the interferon-induced OAS-RNase L pathway by murine coronavirus ns2 protein is required for virus replication and liver pathology. Cell Host Microbe. 2012;11(6):607–616. doi: 10.1016/j.chom.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Liao Y., Fang S., Tam J.P., Liu D.X. Up-regulation of mcl-1 and bak by coronavirus infection of human, avian and animal cells modulates apoptosis and viral replication. PLoS One. 2012;7(1):e30191. doi: 10.1371/journal.pone.0030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Liu C.Y., Back S.H., Clark R.L., Peisach D., Xu Z., Kaufman R.J. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl. Acad. Sci. USA. 2006;103(39):14343–14348. doi: 10.1073/pnas.0606480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzitto J., Galligan C.L., Ueng J.J., Fish E.N. Characterization of the antiviral effects of interferon-α against a SARS-like coronoavirus infection in vitro. Cell Res. 2006;16(2):220–229. doi: 10.1038/sj.cr.7310030. [DOI] [PMC free article] [PubMed] [Google Scholar]


